Submitted:
24 September 2024
Posted:
26 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
- To obtain the acetonic extract of J. curcas seeds (JcAE) and identify its major chemical compounds.
- To obtain an enzymatic extract of S. marcescens strain 81 (SmEE) and evaluate the endo- and exokitinase activity of SmEE.
- To test the individual and combined effects of JcAE and SmEE on S. frugiperda
- Determining the lethal concentrations (LC50s and LC90s) of JcAE and SmEE for larval mortality
2.1. Acetonic Extract of Jatropha curcas Seeds
2.1.1. Preparation of the Acetonic Extract of Jatropha curcas Seeds
2.1.2. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis of Jatropha curcas Seeds
2.2. Isolation of an Enzymatic Extract of Serratia marcescens (SmEE)
2.2.1. Serratia marcescens Culture
2.2.2. Obtaining Colloidal Chitin for Chitinase-Producing Media
2.2.3. Preparation of Serratia marcescens Inoculum
2.2.4. Serratia marcescens Enzymatic Extract-Producing Medium 81
2.2.5. Endo and Exo Chitinase Activity Assay
2.3. Spodoptera frugiperda
2.3.1. Insect Breeding
2.3.2. Spodoptera frugiperda Bioassay
2.4. Statistical Analysis
3. Results
3.1. Chemical Compounds in J. curcas Extract
3.2. Endo- and Exochitinase Activity of the Enzymatic Extract of Serratia marcescens
3.3. Insectory and Insecticidal Effects of the Extracts
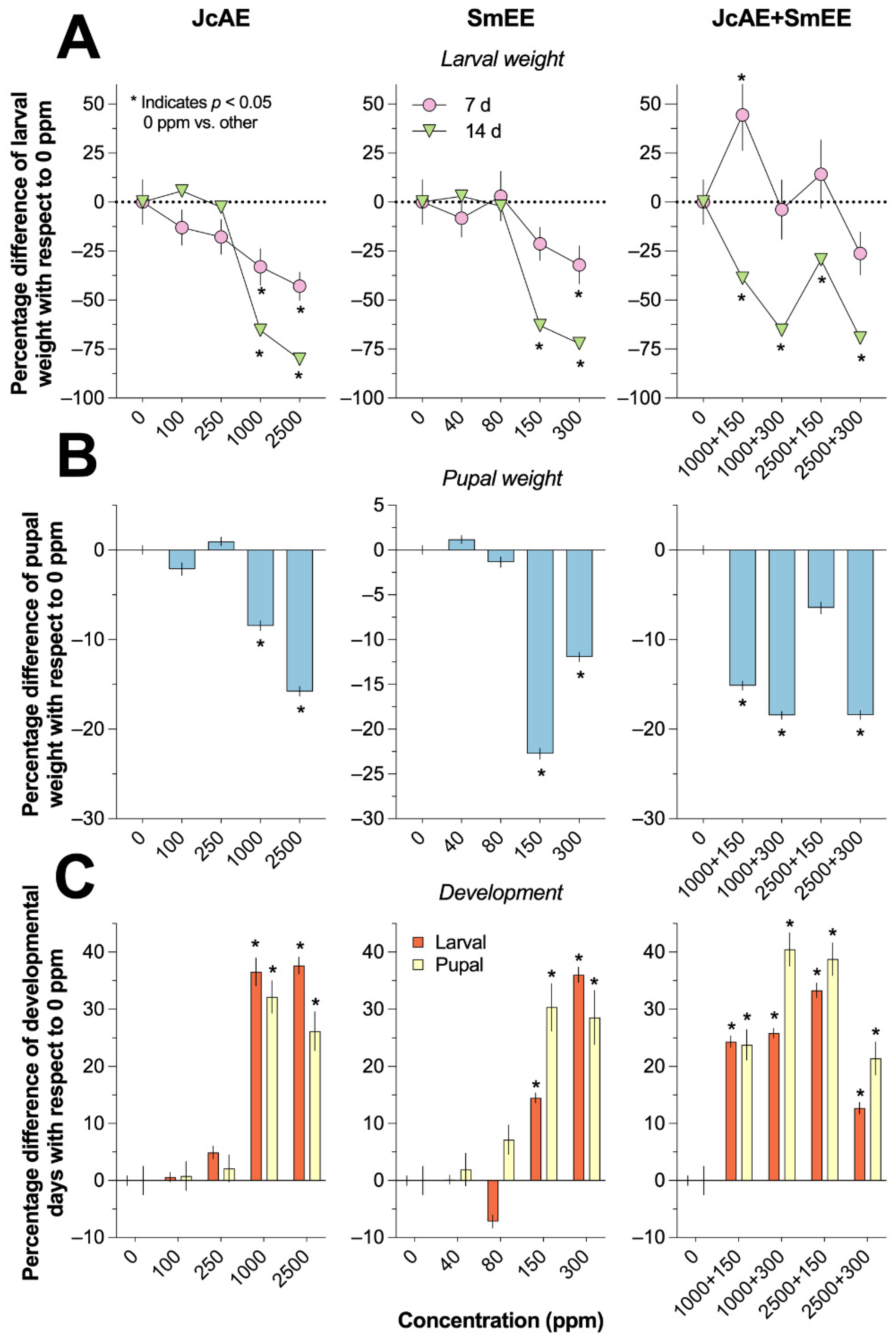
3.3.1. Larval and Pupal Weights
3.3.2. Larval and Pupal Development
3.3.3. Larval and Pupal Mortality
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Overton, K.; Maino, J.L.; Day, R.; Umina, P.A.; Bett, B.; Carnovale, D.; Ekesi, S.; Meagher, R.; Reynolds, O.L. Global crop impacts, yield losses and action thresholds for fall armyworm (Spodoptera frugiperda): A review. Crop Protection 2021, 145, 105641. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Goergen, G.; Koffi, D.; Agboka, K.; Adjevi, A.K.M.; Du Plessis, H.; Van den Berg, J.; Tepa-Yotto, G.T.; Winsou, J.K.; Meagher, R.L.; et al. Genetic studies of fall armyworm indicate a new introduction into Africa and identify limits to its migratory behavior. Sci Rep 2022, 12, 1941. [Google Scholar] [CrossRef] [PubMed]
- Cock, M.J.W.; Beseh, P.K.; Buddie, A.G.; Cafa, G.; Crozier, J. Molecular methods to detect Spodoptera frugiperda in Ghana, and implications for monitoring the spread of invasive species in developing countries. Sci Rep 2017, 7, 4103. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.M.; Gadratagi, B.-G.; Paramesh, V.; Kumar, P.; Madivalar, Y.; Narayanappa, N.; Ullah, F. Sustainable Management of Invasive Fall Armyworm, Spodoptera frugiperda. Agronomy 2022, 12, 2150. [Google Scholar] [CrossRef]
- Ahmed, K.S.; Idrees, A.; Majeed, M.Z.; Majeed, M.I.; Shehzad, M.Z.; Ullah, M.I.; Afzal, A.; Li, J. Synergized Toxicity of Promising Plant Extracts and Synthetic Chemicals against Fall Armyworm Spodoptera frugiperda (JE Smith) (Lepidoptera: Noctuidae) in Pakistan. Agronomy 2022, 12, 1289. [Google Scholar] [CrossRef]
- Gutierrez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Teran-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-Evolved Resistance of the Fall Armyworm (Lepidoptera: Noctuidae) to Synthetic Insecticides in Puerto Rico and Mexico. J Econ Entomol 2019, 112, 792–802. [Google Scholar] [CrossRef]
- Gupta, A. Pesticide residues in food commodities; Agrobios (India): 2006.
- Asghar, U.; Malik, M. Pesticide Exposure and Human Health: A Review. Journal of Ecosystem & Ecography 2016, 01. [Google Scholar] [CrossRef]
- Tavares, W.S.; Costa, M.A.; Cruz, I.; Silveira, R.D.; Serrao, J.E.; Zanuncio, J.C. Selective effects of natural and synthetic insecticides on mortality of Spodoptera frugiperda (Lepidoptera: Noctuidae) and its predator Eriopis connexa (Coleoptera: Coccinellidae). J Environ Sci Health B 2010, 45, 557–561. [Google Scholar] [CrossRef]
- Nunes, C.F.; Santos, D.N.d.; Pasqual, M.; Valente, T.C.T. External morphology of fruits, seeds and seedlings of physic nut. Pesquisa Agropecuária Brasileira 2009, 44, 207–210. [Google Scholar] [CrossRef]
- Pinto, T.L.F.; Marcos Filho, J.; Forti, V.A.; Carvalho, C.d.; Gomes Junior, F.G. Evaluation of the Jatropha curcas L. seed viability by tetrazolium and X-ray tests. Revista Brasileira de Sementes 2009, 31, 195–201. [Google Scholar] [CrossRef]
- Thi, H.T.; Le, B.A.; Le, H.N.T.; Okitsu, K.; Imamura, K.; Takenaka, N.; Luu, B.V.; Maeda, Y. Screening of fatty acids, saccharides, and phytochemicals in Jatropha curcas seed kernel as their trimethylsilyl derivatives using gas chromatography/mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci 2018, 1102-1103, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Valdez-Ramirez, A.; Flores-Macias, A.; Figueroa-Brito, R.; Torre-Hernandez, M.E.d.l.; Ramos-Lopez, M.A.; Beltran-Ontiveros, S.A.; Becerril-Camacho, D.M.; Diaz, D. A Systematic Review of the Bioactivity of Jatropha curcas L. (Euphorbiaceae) Extracts in the Control of Insect Pests. Sustainability 2023, 15, 11637. [Google Scholar] [CrossRef]
- Valdez-Ramírez, A.; Flores-Macías, A.; Ramos-López, M.Á.; Castañeda-Espinoza, J.D.; Rodríguez-González, F.; Herrera-Figueroa, L.E.; Figueroa-Brito, R. Effect of Extracts and Compounds of Jatropha curcas L. Seeds Against the Fall Armyworm Spodoptera frugiperda1. Southwestern Entomologist 2024, 49, 120–132. [Google Scholar] [CrossRef]
- Devappa, R.K.; Angulo-Escalante, M.A.; Makkar, H.P.S.; Becker, K. Potential of using phorbol esters as an insecticide against Spodoptera frugiperda. Industrial Crops and Products 2012, 38, 50–53. [Google Scholar] [CrossRef]
- Aggarwal, C.; Paul, S.; Tripathi, V.; Paul, B.; Khan, M. Chitinolytic activity in Serratia marcescens (strain SEN) and potency against different larval instars of Spodoptera litura with effect of sublethal doses on insect development. BioControl 2015, 60, 631–640. [Google Scholar] [CrossRef]
- Aggarwal, C.; Paul, S.; Tripathi, V.; Paul, B.; Khan, M. Characterization of putative virulence factors of Serratia marcescens strain SEN for pathogenesis in Spodoptera litura. J Invertebr Pathol 2017, 143, 115–123. [Google Scholar] [CrossRef]
- El-Sayed, G.M.; Emam, M.T.H.; Hammad, M.A.; Mahmoud, S.H. Gene Cloning, Heterologous Expression, and In Silico Analysis of Chitinase B from Serratia marcescens for Biocontrol of Spodoptera frugiperda Larvae Infesting Maize Crops. Molecules 2024, 29, 1466. [Google Scholar] [CrossRef]
- Pineda-Castellanos, M.L.; Rodriguez-Segura, Z.; Villalobos, F.J.; Hernandez, L.; Lina, L.; Nunez-Valdez, M.E. Pathogenicity of Isolates of Serratia Marcescens toward Larvae of the Scarab Phyllophaga Blanchardi (Coleoptera). Pathogens 2015, 4, 210–228. [Google Scholar] [CrossRef]
- Figueroa-Brito, R.; Tabarez-Parra, A.S.; Avilés-Montes, D.; Rivas-González, J.M.; Ramos-López, M.Á.; Sotelo-Leyva, C.; Salinas-Sánchez, D.O. Chemical Composition of Jatropha curcas Seed Extracts and Its Bioactivity Against Copitarsia decolora under Laboratory and Greenhouse Conditions. Southwestern Entomologist 2021, 46, 103–114. [Google Scholar] [CrossRef]
- Lovera, A.; Belaich, M.; Villamizar, L.; Patarroyo, M.A.; Barrera, G. Enhanced virulence of Beauveria bassiana against Diatraea saccharalis using a soluble recombinant enzyme with endo- and exochitinase activity. Biological Control 2020, 144, 104211. [Google Scholar] [CrossRef]
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S. Screening of insecticidal activity of Jatropha curcas (L.) against diamond back moth and Helicoverpa armigera. Seed 2017, 5, 20. [Google Scholar]
- Liu, J.; Bai, H.; Song, P.; Nangong, Z.; Dong, Z.; Li, Z.; Wang, Q. Insecticidal Activity of Chitinases from Xenorhabdus nematophila HB310 and Its Relationship with the Toxin Complex. Toxins (Basel) 2022, 14, 646. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, R.; Revathi, K.; Thanigaivel, A.; Kirubakaran, S.A.; Senthil-Nathan, S. Bacillus subtilis chitinase identified by matrix-assisted laser desorption/ionization time-of flight/time of flight mass spectrometry has insecticidal activity against Spodoptera litura Fab. Pestic Biochem Physiol 2014, 116, 1–12. [Google Scholar] [CrossRef] [PubMed]
- López, J.J.; Chirinos, D.T.; Ponce, W.H.; Solórzano, R.F.; Alarcón, J.P. Insecticide activity of botanical formulates on the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). Revista Colombiana De Entomología 2022, 48. [Google Scholar] [CrossRef]
- Diabaté, D.; Gnago, J.A.; Koffi, K.; Tano, Y. The effect of pesticides and aqueous extracts of Azadirachta indica (A. Juss) and Jatropha carcus L. on Bemisia tabaci (Gennadius) (Homoptera: Aleyrididae) and Helicoverpa armigera (Hübner)(Lepidoptera: Noctuidae) found on tomato plants in Côte d’Ivoire. Journal of Applied Biosciences 2014, 80, 7132–7143. [Google Scholar] [CrossRef]
- Ren, Y.; Shi, J.; Mu, Y.; Tao, K.; Jin, H.; Hou, T. AW1 Neuronal Cell Cytotoxicity: The Mode of Action of Insecticidal Fatty Acids. J Agric Food Chem 2019, 67, 12129–12136. [Google Scholar] [CrossRef]
- Ribeiro, S.S.; Silva, T.B.d.; Moraes, V.R.d.S.; Nogueira, P.C.d.L.; Costa, E.V.; Bernardo, A.R.; Matos, A.P.; Fernandes, B.; Silva, M.F.d.G.F.d.; Pessoa, Â.M.d.S. Chemical constituents of methanolic extracts of Jatropha curcas L and effects on spodoptera frugiperda (JE Smith)(Lepidoptera: Noctuidae). Química Nova 2012, 35, 2218–2221. [Google Scholar] [CrossRef]
- Devanand, P.; Rani, P.U. Biological potency of certain plant extracts in management of two lepidopteran pests of Ricinus communis L. Journal of Biopesticides 2008, 1, 170–176. [Google Scholar]
- Ingle, K.P.; Deshmukh, A.G.; Padole, D.A.; Dudhare, M.S. Bioefficacy of crude extracts from Jatropha curcas against Spodoptera litura. Seed 2017, 5, 0. [Google Scholar]
- Danişmazoğlu, M.; DemİR, İ.; Sezen, K.; MuratoĞLu, H.; NalÇAcioĞLu, R. Cloning and expression of chitinase A, B, and C (chiA, chiB, chiC) genes from Serratia marcescens originating from Helicoverpa armigera and determining their activities. Turkish Journal of Biology 2015, 39, 78–87. [Google Scholar] [CrossRef]
- Singh, A.K.; Singh, A.; Joshi, P. Combined application of chitinolytic bacteriumPaenibacillussp. D1 with low doses of chemical pesticides for better control ofHelicoverpa armigera. International Journal of Pest Management 2016, 62, 222–227. [Google Scholar] [CrossRef]
- Zhong, W.; Ding, S.; Guo, H. The chitinase C gene PsChiC from Pseudomonas sp. and its synergistic effects on larvicidal activity. Genet Mol Biol 2015, 38, 366–372. [Google Scholar] [CrossRef] [PubMed]
- Sampson, M.N.; Gooday, G.W. Involvement of chitinases of Bacillus thuringiensis during pathogenesis in insects. Microbiology (Reading) 1998, 144 Pt 8, 2189–2194. [Google Scholar] [CrossRef]
- Martinez-Diaz, Y.; Gonzalez-Rodriguez, A.; Rico-Ponce, H.R.; Rocha-Ramirez, V.; Ovando-Medina, I.; Espinosa-Garcia, F.J. Fatty Acid Diversity is Not Associated with Neutral Genetic Diversity in Native Populations of the Biodiesel Plant Jatropha curcas L. Chem Biodivers 2017, 14, e1600188. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.P.F. Teor e composição do óleo de sementes de Jatropha spp. Bragantia 1987, 46, 151–157. [Google Scholar] [CrossRef]

| Treatment | Acetonic extract of J. curcas seeds (JcAE) ppm | Enzymatic extract of S. marcescens (SmEE) ppm |
|---|---|---|
| Control | 0 | 0 |
| JcAE | 100 | 0 |
| 250 | 0 | |
| 1000 | 0 | |
| 2500 | 0 | |
| SmEE | 0 | 40 |
| 0 | 80 | |
| 0 | 150 | |
| 0 | 300 | |
| JcAE+SmEE | 1000 | 150 |
| 1000 | 300 | |
| 2500 | 150 | |
| 2500 | 300 |
| Elution order | Retention time (minutes) | Compound | KIL | KI | % Area* |
|---|---|---|---|---|---|
| 1 | 8.17 | Capric acid (C10:0) | 1279 | 1231 | 13.1 |
| 2 | 15.91 | Lauric acid (C12:0) | 1562 | 1543 | 9.6 |
| 3 | 17.34 | Myristic acid (C14:0) | 1780 | 1771 | 2.6 |
| 4 | 18.47 | Palmitic acid (C16:0) | 1942 | 1902 | 12.9 |
| 5 | 19.41 | Stearic acid (C18:0) | 2063 | 2053 | 5.2 |
| 6 | 21.85 | Oleic acid (C18:1 (cis-9)) | 2095 | 2087 | 10.4 |
| 7 | 22.91 | Linoleic acid (C18:2(cis-9,12)) | 2175 | 2170 | 15.5 |
| 8 | 24.48 | Unknown | ------ | ------- | 17.1 |
| Treatment | ppm | Larval weight (mg) | Larval development (d) | Larval mortality (%) | |
|---|---|---|---|---|---|
| 7 d | 14 d | ||||
| Control | 0 | 5.16 ± 2.23 | 76.60 ± 2.71 | 25.73 ± 0.77 | 4/30 (13.33) |
| *JcAE | 100 | 4.49 ± 1.51 a | 80.89 ± 4.37 a | 25.89 ± 0.75 b | 3/30 (10.00) |
| 250 | 4.25 ± 1.61 a | 74.62 ± 2.26 b | 27.00 ± 1.27 b | 3/30 (10.00) | |
| 1000 | 3.46 ± 1.93 b | 26.47 ± 2.49 c | 35.13 ± 2.26 a | 15/30 (50.00)* | |
| 2500 | 2.95 ± 1.27 b | 15.14 ± 2.29 c | 35.42 ± 1.08 a | 18/30 (60.00)* | |
| SmEE | 40 | 4.74 ± 1.60 a | 78.85 ± 3.09 a | 25.77 ± 0.66 b | 5/30 (16.67) |
| 80 | 5.32 ± 2.51 a | 74.99 ± 2.95 a | 23.88 ± 1.33 c | 5/30 (16.67) | |
| 150 | 4.06 ± 1.01 a,b | 28.32 ± 2.34 b | 29.46 ± 0.51 a | 17/30 (56.67)* | |
| 300 | 3.51 ± 1.89 b | 21.31 ± 2.76 b | 35.00 ± 0.89 a | 18/30 (60.00)* | |
| JcAE+SmEE | 1000+150 | 7.46 ± 2.96 a | 46.85 ± 2.45 a | 32.00 ± 0.70 b | 15/30 (50.00)* |
| 1000+300 | 4.97 ± 2.83 b | 26.53 ± 2.65 b | 32.39 ± 0.50 b | 20/30 (66.67)* | |
| 2500+150 | 5.90 ± 3.33 a,b | 54.06 ± 2.94 a | 34.30 ± 0.82 a | 15/30 (50.00)* | |
| 2500+300 | 3.81 ± 1.84 b | 23.42 ± 2.81 b | 29.00 ± 0.75 c | 19/30 (63.33)* | |
| Treatment | ppm | Pupal weight (mg) | Pupal development (d) | Pupal mortality (%)+ | Larvae and pupae cumulative mortality (%) |
|---|---|---|---|---|---|
| Control | 0 | 153.6 ± 2.6 | 14.0 ± 1.2 | 6/26 (23.08) | 10/30 (33.33) |
| *JcAE | 100 | 150.3 ± 4.8 a | 14.1 ± 1.3 b | 5/27 (18.52) | 8/30 (26.67) |
| 250 | 155.1 ± 2.4 a | 14.3 ± 1.1 b | 6/27 (22.22) | 9/30 (30.00) | |
| 1000 | 140.6 ± 2.6 b | 18.5 ± 0.7 a | 10/15 (66.67)* | 25/30 (83.33)* | |
| 2500 | 129.4 ± 2.3 b | 17.6 ± 1.2 a | 8/12 (66.67)* | 26/30 (86.67)* | |
| SmEE | 40 | 155.4 ± 2.3 a | 14.2 ± 1.5 c | 4/25 (16.00) | 9/30 (30.00) |
| 80 | 151.5 ± 3.8 a | 15.0 ± 1.2 b,c | 5/25 (20.00) | 10/30 (33.33) | |
| 150 | 118.7 ± 3.0 b | 18.2 ± 0.9 a | 9/13 (69.23)* | 26/30 (86.67)* | |
| 300 | 135.3 ± 2.1 b | 18.0 ± 1.0 a,b | 4/12 (33.33) | 22/30 (73.33)* | |
| JcAE+SmEE | 1000+150 | 130.3 ± 2.5 a | 17.3 ± 0.5 a | 11/15 (73.33)* | 26/30 (86.67)* |
| 1000+300 | 125.3 ± 2.0 b | 19.6 ± 0.5 b | 8/10 (80.00)* | 28/30 (93.33)* | |
| 2500+150 | 143.7 ± 2.7 a | 19.4 ± 0.5 b | 11/15 (73.33)* | 26/30 (86.67)* | |
| 2500+300 | 125.3 ± 2.4 b | 17.0 ± 0.7 a | 8/11 (72.73)* | 27/30 (90.00)* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





