Abstract:This study investigates how traumatic injuries alter joint movements in the ankle and foot. We used a brain injury model in rats, focusing on the hippocampus between the CA1 and dentate gyrus. We assessed the dissimilarity factor (DF) and vertical displacement (VD) of the ankle and metatarsus joints before and after the hippocampal lesion. We analyzed joint movements in rats after the injury or in rats treated with resveratrol, exercise, or a combination of both. Resveratrol facilitated the recovery of DF in both legs, showing improvements in the ankle and metatarsus joints on the third- and seventh days post-injury. The hippocampal lesion affected VD in both legs, observed on the third or seventh day after the injury. Both exercise and resveratrol partially recovered VD in the ankle and metatarsus joints on these days. These effects may be linked to increased hippocampal neurogenesis and reduced neuroinflammation. The study highlights the benefits of resveratrol and exercise in motor recovery following brain injury, suggesting their potential to enhance the quality of life for patients with neurological disorders affecting motor function and locomotion. These findings also suggest that resveratrol could offer a promising or complementary alternative in managing chronic pain and inflammation associated with orthopedic conditions, thus improving overall patient management.
1. Introducción
Traumatic brain injury (TBI) is one of the leading causes of death and severe disability and is also associated with mental health disorders in humans [
1]. TBI involves complex human mechanisms, challenging their precise replication in animal models. Therefore, no animal model can mimic human brain injury [
2]. However, these models offer the best alternative for investigating the biomechanical, cellular, and molecular mechanisms involved in the neuropathological progression associated with TBI and time-dependent effects [
3,
4]. The lesion in the hippocampus is associated with motor alterations [
5]. We have developed a murine model to investigate the impact of a penetrating lesion in the hippocampus and its consequences on locomotion speed. This lesion not only alters locomotion kinematics, as reported by Lopez Ruiz [
6], but also induces changes in the vertical displacement of the ankle and metatarsal joints and the dissimilarity factor [
7].
On the other hand, exercise has been considered an indispensable factor in facilitating synaptic plasticity. Exercise promotes neurogenesis, neuronal survival, and regeneration. [
8,
9,
10,
11]. It also modulates the inflammatory response [
12], particularly in two hippocampal regions: the subgranular zone and dentate gyrus. In rats, it has been described to enhance neuronal plasticity [
13,
14] and inhibit apoptosis in several ischemia models [
15]. Other findings from animal studies have contributed to considering exercise as a potential non-pharmacological approach to aid recovery after TBI.
Resveratrol is a flavonoid that benefits health, reduces damage in the hippocampus after ischemia [
16], and decreases epileptic episodes [
17]. This flavonoid possesses antioxidant and anti-inflammatory effects. [
18,
19]. It causes a reduction in cerebral edema with improvement in cognitive processes and a decrease in motor deficit after TBI [
20]. In this study, we implemented a penetrating injury to induce damage in the hippocampus. The effects of exercise and resveratrol in the metatarsus and ankle joint vertical displacement and the dissimilarity factor in rats were analyzed to study the recovery of these joint movements.
2. Materials and Methods
2.1. Animals and Experimental
A total of 25 adult Wistar rats (180–250 g) were handled under EU Directive 2010/63/EU for animal experiments and Mexican Regulation of Animal Care and Maintenance (NOM-062-ZOO-1999, 2001). Animals were kept on a 12-hour light-dark cycle at 22°C ± 2°C and constant humidity, with water and food available ad libitum. The groups were divided into five groups. One group was considered control and only filmed on the third and seventh days without injury and treatment. The second group was lessoned and filmed on the third and seventh day. The third group was injured, administered with resveratrol, and filmed on the third and seventh day. The fourth group was taught and trained in exercise and filmed on the third and seventh days. The fifth group was taught and administered with resveratrol, trained with exercise, filmed on the third and seventh days, and then sacrificed. The same animals were used as the control group. To obtain reliable results, we used a minimal number of animals and minimized their suffering.
2.2. Penetrating Injury Model
The animals were anesthetized with 5% isoflurane for induction, then 3%. A cleft was drilled in the left parietal bone to expose the meninges. A 0.5-mm sterile steel cannula was placed at coordinates from Bregma (ML: -2mm, AP: -5mm) as per Paxinos and Watson. The cannula penetrated 4 mm, displaced roughly 2 mm, and was removed. After the surgery, the animals were given antibiotics (Enroxil 2 mg/kg) and analgesics (meloxicam 2 mg/kg) for three days.
2.3. Resveratrol Treatment
Resveratrol (Santa Cruz # SC-200808) was given at 100 mg/kg dissolved in a 50% ethanol and 50% saline solution (0.9% NaCl) via intraperitoneal injection starting the same day of the injury and continuing daily for seventh days post-injury, taken from Sönmez U et al. (2007) [
20].
2.4. Exercise Training
Exercise training was conducted on a treadmill, starting four weeks before the lesion, followed by three days of rest and a week post-lesion. The training involved 5 days per week, with varying speeds and durations: Days 1-4: 7 min at 11 cm/s; Days 5-7: 7 min at 15 cm/s; Days 8-10: 7 min at 15 cm/s; Days 11-13: 10 min at 15 cm/s; Days 14-16: 10 min at 25 cm/s; Days 17-25: 15 min at 25 cm/s from 10:00 to 16:00 hours.
2.5. Tunnel Walk Recordings
Kinematic recordings were made on day 0 and the third- or seventh-day post-injury. Videos from day 0 were considered as control groups (pre-injury), and those from days 3 and 7 were injured groups. Two synchronized cameras recorded left and right hindlimbs at 240 fps, with a 1,280 × 720 resolution. Post-processing removed spherical distortion using a homographic matrix from four image points, as before. Steps were selected using the manual definition of instants corresponding to the beginning and end of the step. Displacement curves for the ankle and metatarsus points were manually annotated using custom software. Each step was analyzed separately (Supplementary Chapter 1).
2.6. Dissimilarity Factor and Vertical Displacement Analysis
Changes in the Dissimilarity Factor (DF) among groups were determined by comparing displacement curves and calculating differences using Euclidean distance between normalized curve points on horizontal (X) and vertical (Y) axes, as described previously.
Where
DF <a,b> is the squared error between every point of the normalized curves, defined as difference factor (
DF); "
xa (i) − xb (i)" is the difference between the coordinates in
x, and "
ya (i) − yb (i)" in
y of every point in the graph, when comparing two steps
(a and
b); and "
i" is the percent in the step cycle [
7,
21]. We averaged the vector Y components at each end of the normalized displacement curves for each group at 3- and 7-days post-injury (
Supplementary Chapter 2). We compared this pattern comparison analysis by using a locally designed MATLAB script.
2.7. Statistical Analysis
Firstly, the Kolmogorov-Smirnov test was used to determine data normality. All results are expressed as means ± SEM. For DF analysis, we analyzed the differences between the total value of X and Y graphically (Horizontal displacement and Vertical displacement in all groups) using a Kruskal-Wallis test with Dunn post hoc. For the vertical displacement analysis, using the T-test, we compared the control curve point by point versus the curve in the experimental group. We divided each curve into one hundred points. The curve represents the mean of the total steps. A value of p ≤ 0,05 (*), p ≤ 0,01 (**), p ≤ 0,001 (***), and p ≤ 0,0001 (****) were considered statistically significant. We conducted the statistical analysis using the Prism 9.0 software (GraphPad).
3. Results
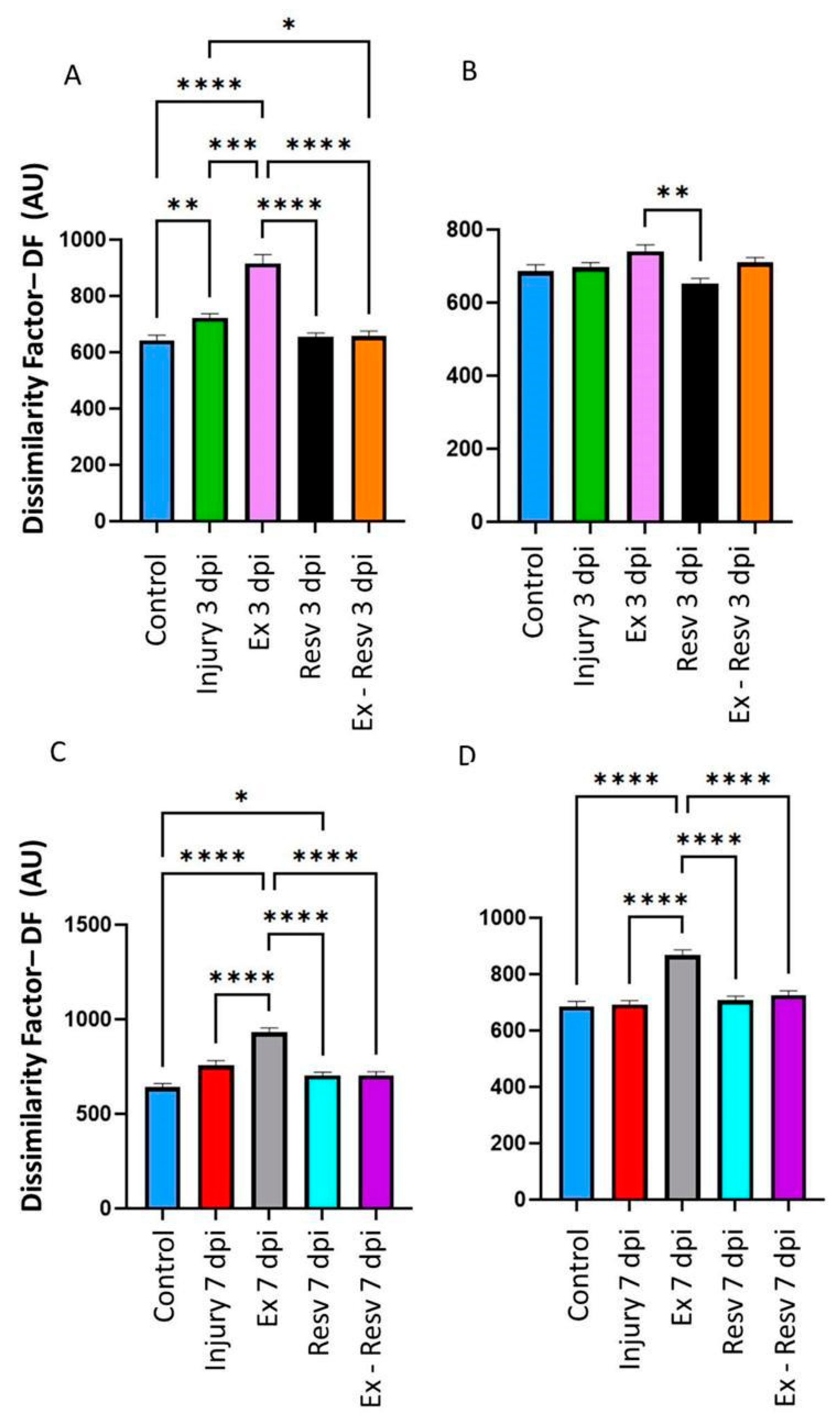
3.1. Dissimilarity Factor (DF) in the Left and Right Metatarsus on the Third- and Seventh-Days Post-Injury with Resveratrol and Exercise
On the third day post-injury (dpi), resveratrol and exercise with resveratrol treatment return the DF to the control value in the left and right metatarsus joints (
Figure 1A and B). The third-day injury rats showed a DF in the left metatarsus compared to the control (
Figure 1A) but not in the right (
Figure 1B). The exercise group showed significant DF in the left metatarsus on the 3 and 7 dpi groups (
Figure 1A,C). On the seventh day, in the Resveratrol group, the decrease was less marked than on the third day, but resveratrol plus exercise returned the DF to the control level (
Figure 1C). The DF was higher in the exercise group in both the left and right metatarsus compared to the control group on the 3 and 7 dpi groups (
Figure 1A,C,D).
3.2. DF in the Ankle Was Treated with Resveratrol and Exercise on the Third- and Seventh-Days Post-Injury
The injury group on the third and seventh days showed an increase in DF compared to the control group. This was more evident in the 3 dpi group (
Figure 2A,C). In the exercise group, the left and right ankle DF differed on the 3 and 7 dpi groups versus the control group, mainly in the left ankle (
Figure 2A,C,D). The resveratrol and exercise group versus the resveratrol treatment group did not show a change in DF in the 3 and 7 dpi groups, but they returned ankle joint movements to the control level (
Figure 2A–D). The resveratrol with the exercise group showed a significant difference compared to the exercise group in both ankles on the third and seventh days (
Figure 2A–D).
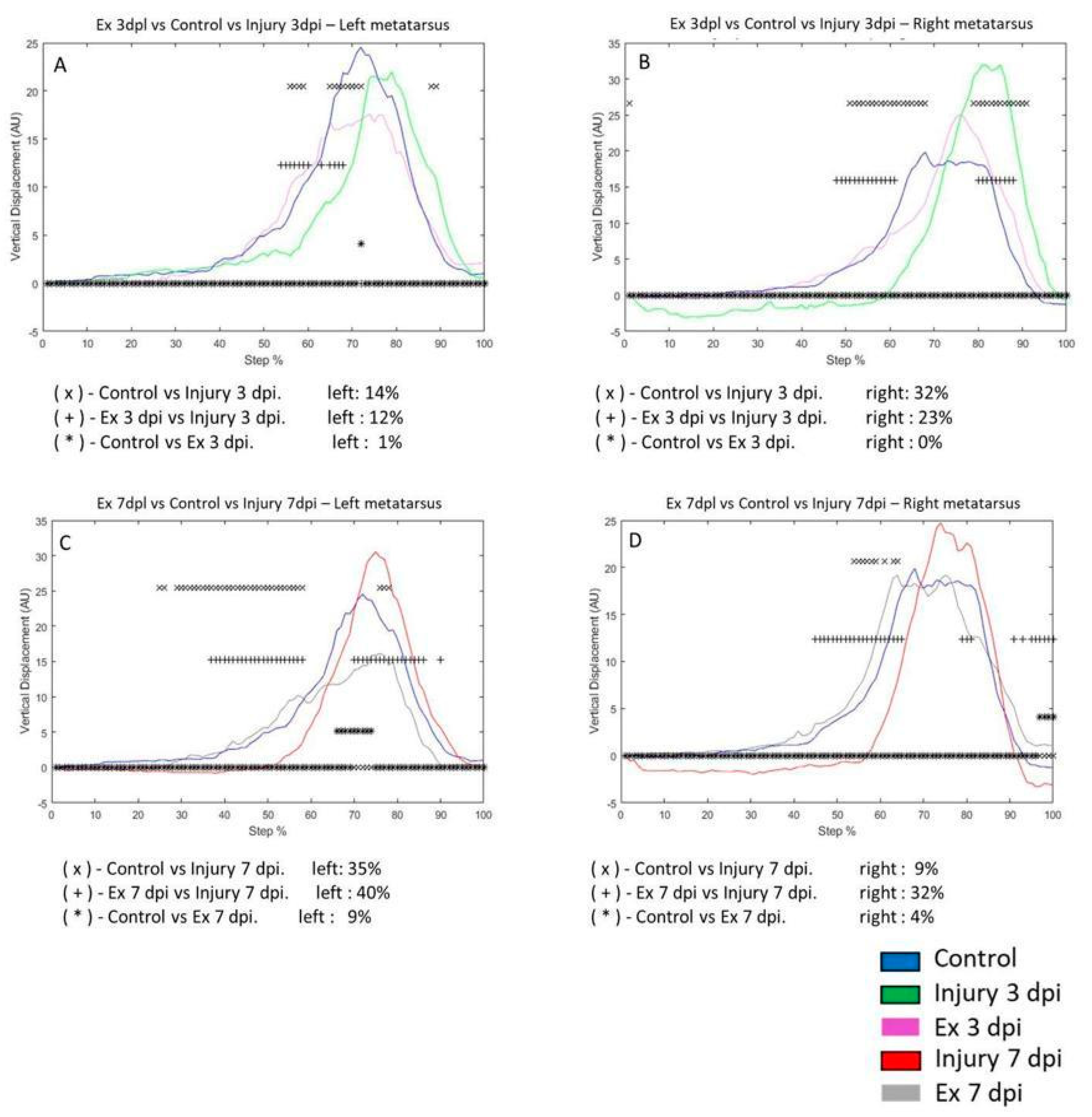
3.3. Exercise Modifies Vertical Displacement (VD) in the Metatarsus and Ankle Post-Injury
We analyzed the displacement of vertical and horizontal steps in the left and right joints. However, we did not find significant differences in Horizontal displacement on the metatarsus, ankle, and knee (
Supplementary Chapter 3). On the third day post-injury, VD differs by 14% of the step cycle in the left metatarsus compared to control and only by 1% after exercise. The Ex 3 dpi group differs by 12% compared to the injury group (
Figure 3A). In the right metatarsus, VD was 32% in the control group and 0% in the Ex 3 dpi group on the third day. In the Ex 3 dpi group, VD was 23% compared to the injury group (
Figure 3B). On the seventh day post-injury, VD changes by 35% in the left metatarsus for the control group and 40% in the Ex 7 dpi group (
Figure 3C). The VD between the control and Ex 7 dpi group was 9%. In the right metatarsus, post-injury changes were 32% versus the control group, but the Ex 3 dpi group showed a difference of 23% (
Figure 3B). On the seventh day post-injury, VD changed by 9% in the control group and 32% after exercise (Ex 7 dpi group); the difference was 4% compared to the control group (
Figure 3D).
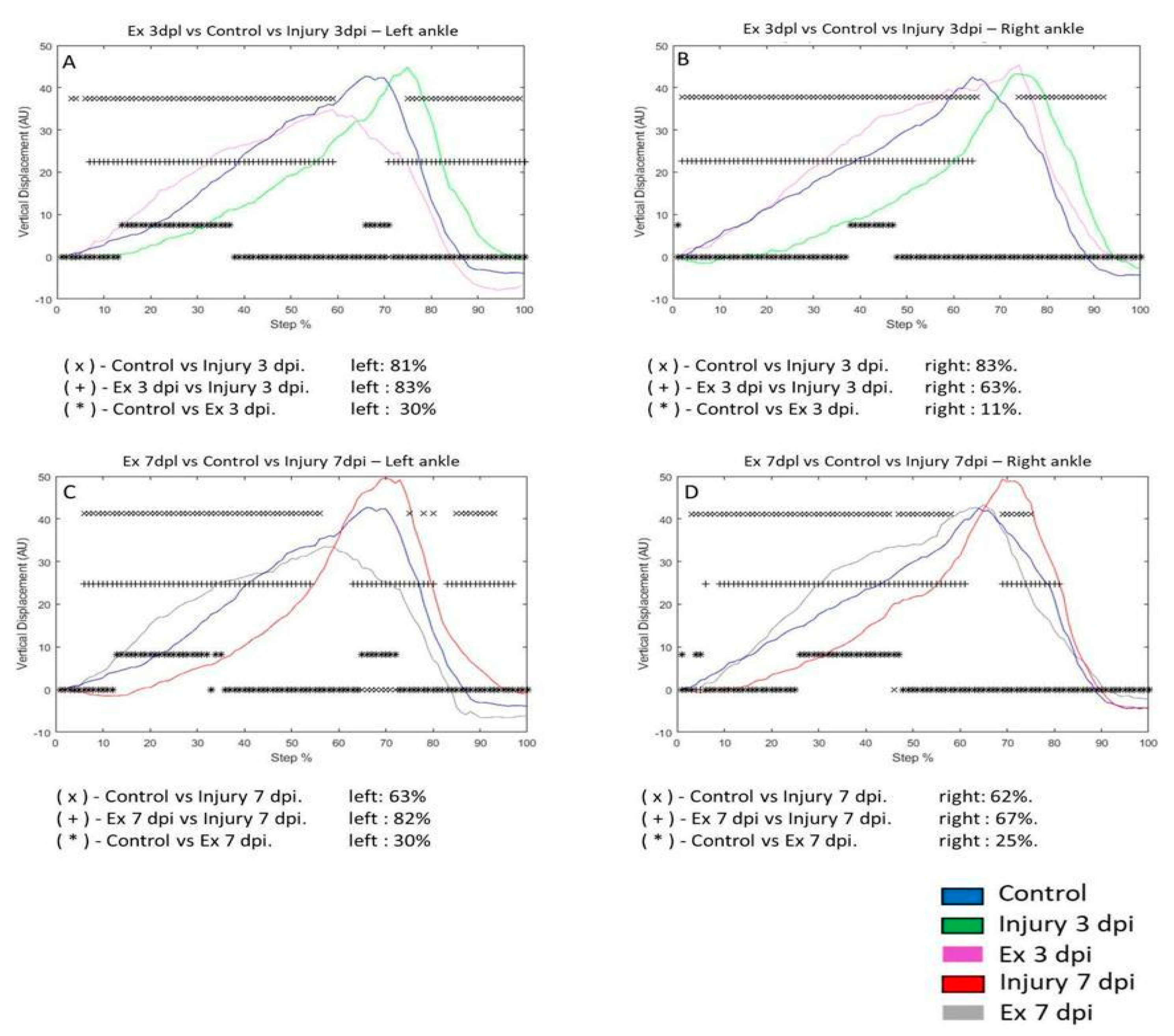
In the injury 3 dpi group, VD of the left ankle was 81% compared to the control group. VD of the control group versus the Ex 3 dpi group was 30%. VD of the 3 dpi group was 83% compared to the Ex 3 dpi group (
Figure 4A). In the 3 dpi group, VD in the right ankle changed by 83% during the step cycle for the control group. VD of the right ankle of the control group versus the Ex 3 dpi group was 11%. VD in the 3 dpi group was 63% compared to the Ex 3 dpi group. (
Figure 4B). On the seventh day post-injury, VD in the left ankle was 63% compared to the control group. VD In the control group, the difference was 30% versus the Ex 7 dpi group. VD in the 3 dpi group was 82% compared to the Ex 7 dpi group (
Figure 4C). VD in the right ankle differed 62% on the 7 dpi post-injury compared to the control group. The change in VD in the control group was 25% compared to the Ex 7 dpi group. VD differed by 67% in the 7 dpi group compared to the Ex 7 dpi injury group (
Figure 4D).
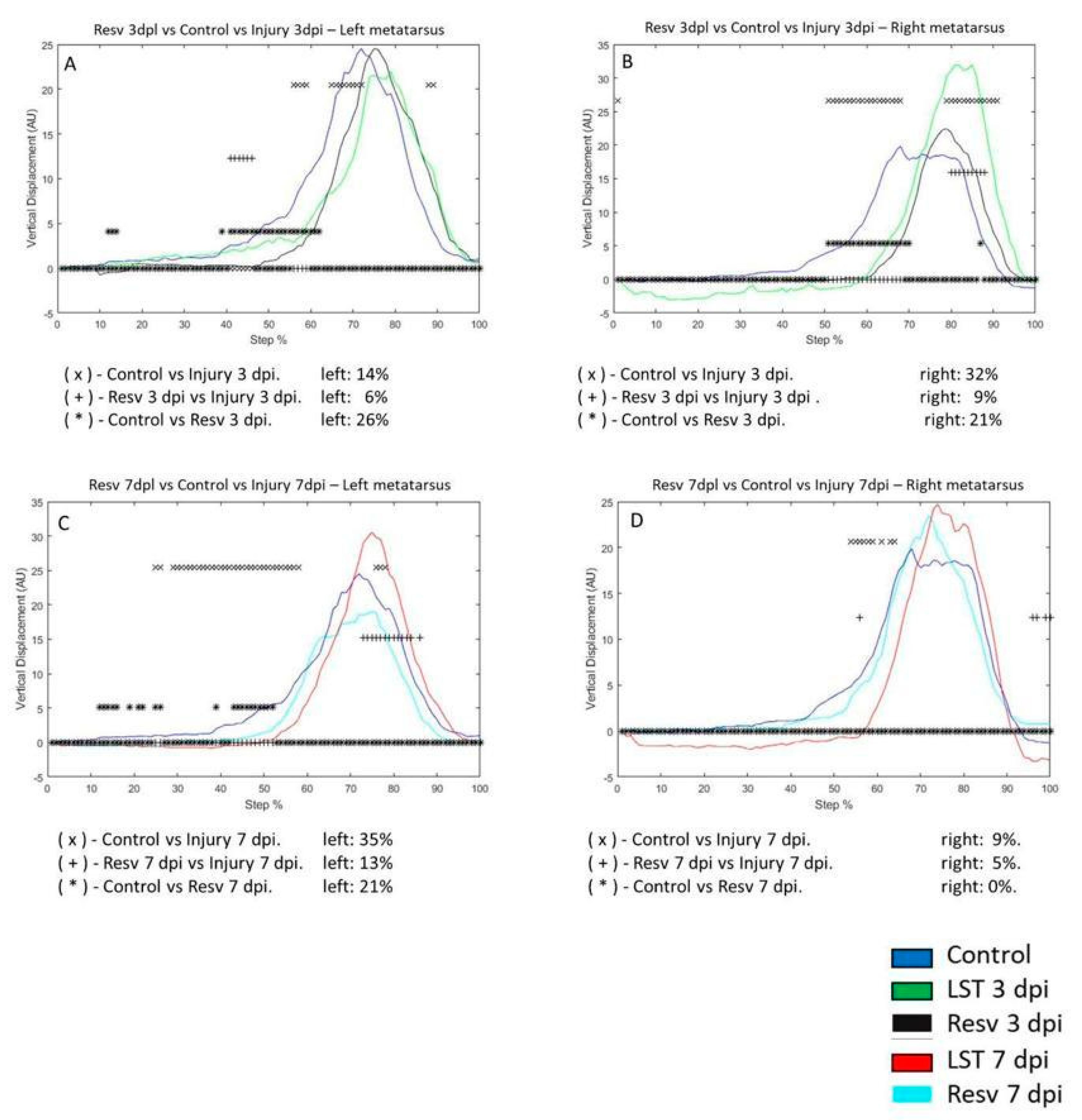
3.4. VD in Metatarsus and Ankle Joints after Resveratrol Treatment in the Penetrating Injury Model
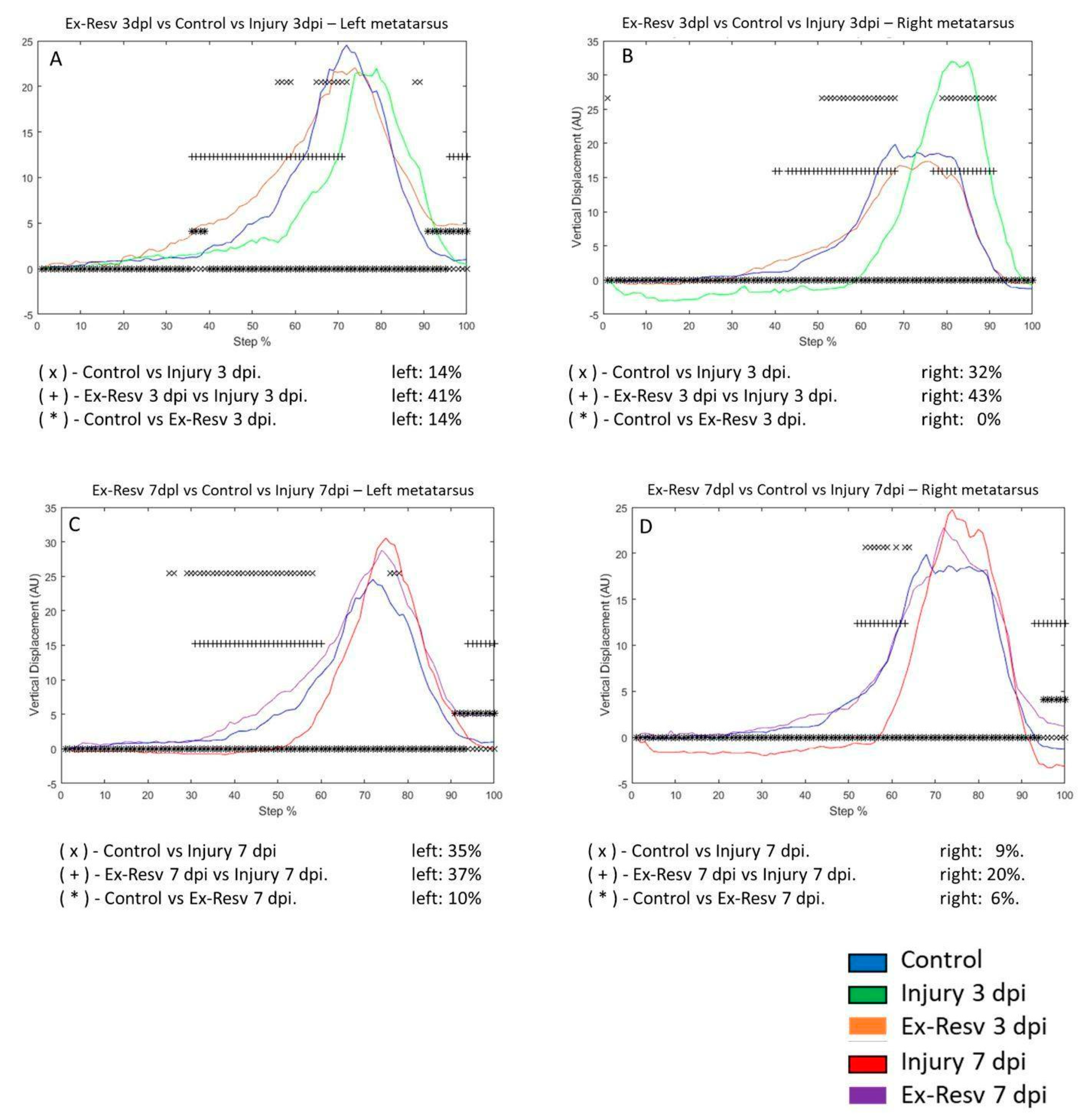
On the injury of 3 dpi in the left metatarsus, VD changed by 14% compared to the control group. In the 3 dpi with resveratrol treatment group differed by 26% compared to the control group. VD in the metatarsus with injury differed by 6% compared to injury and resveratrol treatment (
Figure 5A). VD between the control versus 3 dpi group was 32% in the right metatarsus. Comparing the control and 3 dpi group with resveratrol treatment, VD changed by 21%. Resveratrol treatment on the 3 dpi modified VD by 9% versus 3 dpi post-injury (
Figure 5B). In the 7 dpi group, VD changed by 35% compared to the control group. Resveratrol treatment on the 7 dpi differed by 21% versus the control group. VD of the 7 dpi group versus 7 dpi with resveratrol treatment changed by 13% (
Figure 5C). In the right metatarsus, the change was 9% on the seventh-day post-lesion compared to the control group. VD between control and 7 dpi with resveratrol treatment did not change. In the 7 dpi with resveratrol treatment, VD decreased to 5%, compared to the 7 dpi group (
Figure 5D).
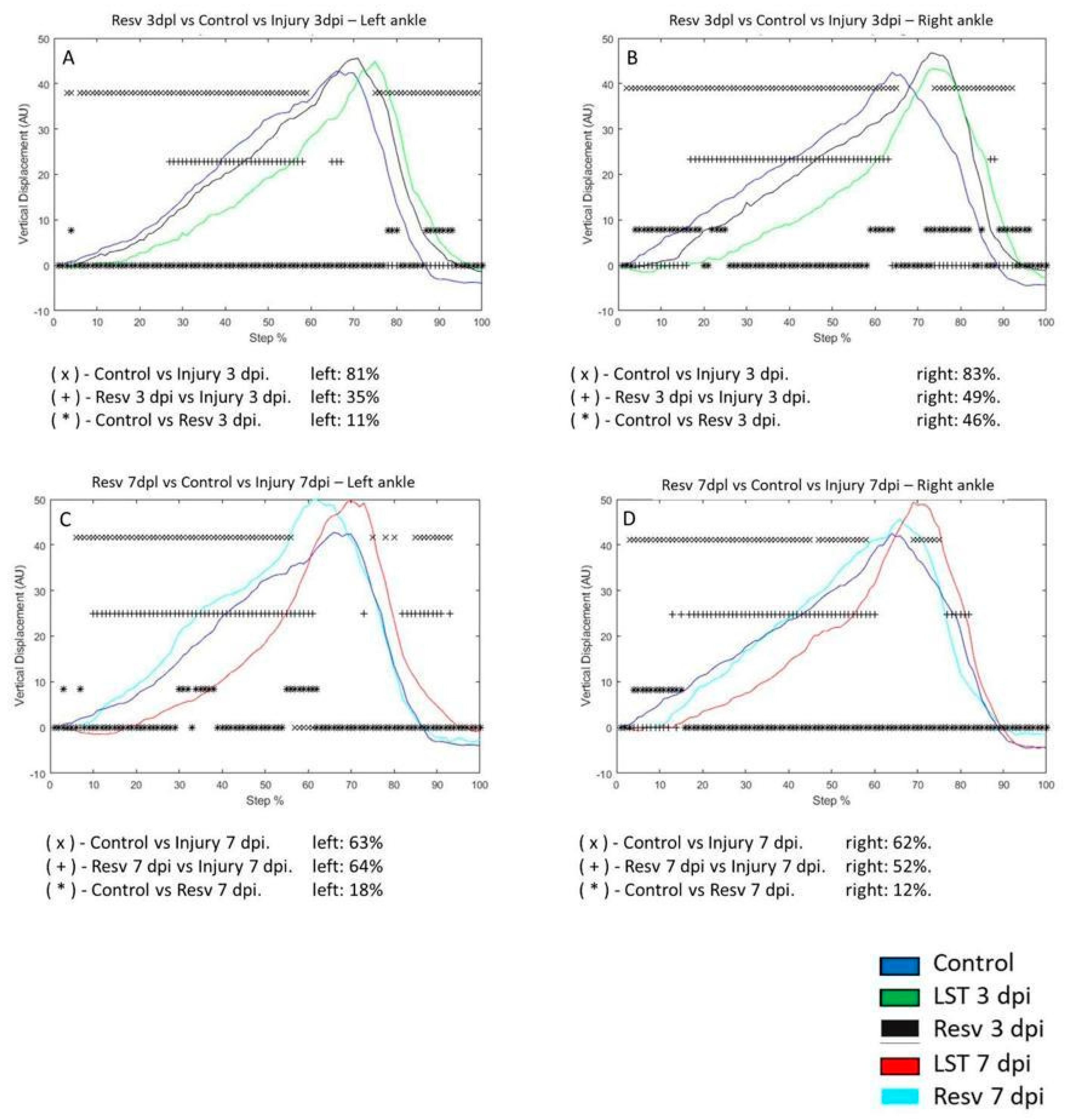
VD of the left ankle in the control group compared to the 3 dpi post-injury changed by 81%, respectively. In the control group, VD was 11% versus 3 dpi with resveratrol treatment. Compared to the 3 dpi group versus the 3 dpi with resveratrol treatment, it changed by 35% (
Figure 6A). VD in the right ankle on the 3 dpi post-injury changed by 83% concerning the control group. In the 3 dpi with resveratrol treatment changes, only 46% versus the control group. In the resveratrol-treated rats after 3 dpi, VD during the step cycle only differed by 49% compared to the 3 dpi group (
Figure 6B). VD of the left ankle in the control group compared to the 7 dpi post-injury changed by 63%, respectively. In the control group, VD was 18% versus 7 dpi with resveratrol treatment. Compared to the 7 dpi group versus the 7 dpi with resveratrol treatment, it changed by 64% (
Figure 6C). VD in the right ankle on the 7 dpi post-injury changed by 62% concerning the control group. In the 7 dpi with resveratrol treatment changes by 12% versus the control group. In the resveratrol-treated rats after 7 dpi, VD during the step cycle only differed by 52% compared to the 7 dpi group (
Figure 6D).
3.5. VD in Metatarsus and Ankle Joints after Exercise and Resveratrol Treatment in Male Rats
VD in the left metatarsus changed by 14% of the step cycle in the control group compared to the 3 dpi group. In the 3 dpi of exercise and resveratrol treatment group, VD differed by 14% versus the control group. Three days post-injury, VD differed by 41% when comparing exercise with the resveratrol treatment group (
Figure 7A). In the right metatarsus, the 3 dpi group change was 32% compared to the control group. In the 3 dpi with exercise and resveratrol treatment group, VD did not change compared to the control group. Comparing the VD of the 3 dpi versus the 3 dpi with exercise and resveratrol-treated rats, the difference was 43% (
Figure 7B).
In the 7 dpi group, VD changed by 35% compared to the control group. In the 7 dpi exercise with resveratrol, the treatment group differed by 10% from the control group. Comparing the VD of exercised rats with resveratrol treatment versus the injury group, the difference was 37% (
Figure 7C). In the right metatarsus, the 7 dpi group, VD changed by 9% compared to the control group. The 7 dpi after exercise with resveratrol treatment changed by 6% compared to the control group. Comparing exercised and resveratrol-treated rats versus injury rats, the difference was 20% (
Figure 7D).
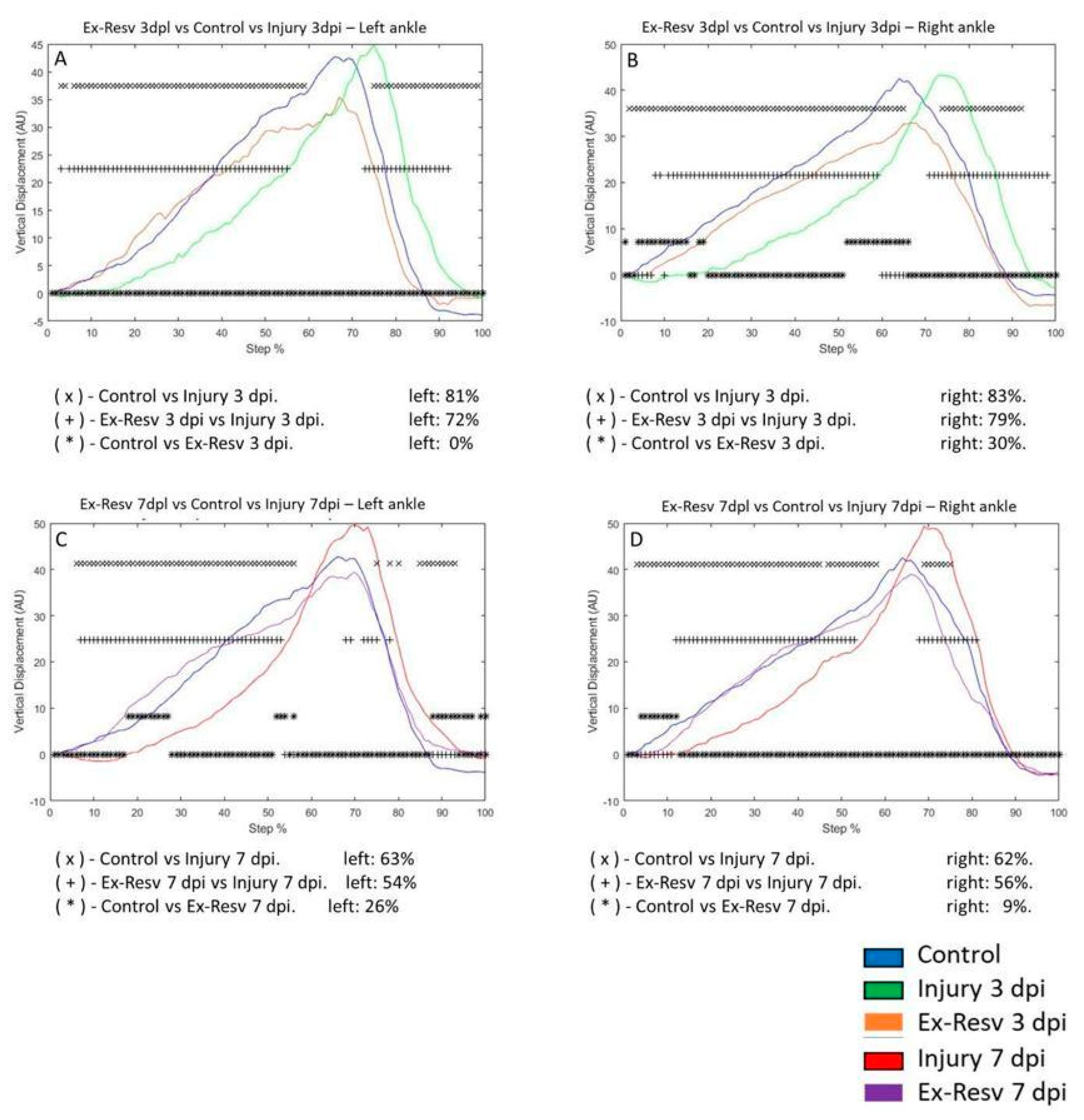
VD in the left ankle joint of the control group compared to the 3 dpi group changed by 81%, respectively. VD was not changed for rats 3 dpi with exercise and resveratrol compared to the control group. The 3 dpi and exercise with resveratrol treatment differed by 72% compared to the 3 dpi group (
Figure 8A). VD in the right ankle on the 3 dpi changed by 83% versus the control group. The 3 dpi exercise and resveratrol group changed by 30% compared to the control group. The 3 dpi exercise and resveratrol group changed by 79% compared to the 3 dpi group (
Figure 8B). On the left ankle, the 7 dpi group was modified by 63% compared to the control group. In the 7 dpi exercise, the resveratrol group changed by 26% compared to the control group. In the 7 dpi exercise, the resveratrol group changed by 54% for the 7 dpi group (
Figure 8C). VD in the right ankle changed 62% in the 7 dpi versus the control group. The exercise and resveratrol treatment group changed by 9% compared to the control group. Comparing injury rats 7 dpi versus exercise and resveratrol-treated rats, VD changed by 56%, respectively (
Figure 8D).
4. Discussion
This study describes changes in the metatarsus and ankle in a rat model of penetrating hippocampus injury and the effects of resveratrol treatment and/or exercise. We report that resveratrol and/or exercise improve metatarsus and ankle kinematics. The ankle and foot play a crucial role in body support, and abnormal movements can impact leg and trunk balance, altering gait. The metatarsal-ankle joint complex's range of motion is critical for locomotion kinematics. Penetrating brain injuries cause gait impairment, making walking restoration a primary goal hetel [
22,
23].
The hippocampus and striatum are linked to locomotion, with some hippocampal neurons reflecting the animal's speed [
24]. Both movement execution and motor imagery share a network, including the striatum [
25]. Our lab's previous studies showed that a penetrating hippocampus injury alters vertical displacement in rat and mouse hind limbs. This suggests the hippocampus's role in locomotion control, similar to human hippocampus focal stroke [
26]. Thus, the hippocampus could benefit significantly from neuroprotection [
2].
This study evaluated exercise and resveratrol individually to improve vertical displacement during the step cycle on the third- and seventh-days post-injury. Improvement patterns appeared over time on both sides. Exercise's effect on locomotion post-TBI or stroke has been reported, even in a single session [
27]. Resveratrol showed a similar improvement, except in the left metatarsus on the third-day post-injury. The right-side injury delayed left metatarsus recovery. This was evident on the seventh day. This warrants further study. Combined exercise and resveratrol also improved vertical displacement in the metatarsal and ankle joints post-injury, suggesting different mechanisms. Exercise may promote neurogenesis, while resveratrol may have anti-inflammatory effects [
28,
29,
30].
Spontaneous recovery is attributed to neuronal plasticity observed in murine injury models, also reported in other murine studies [
31,
32,
33]
. This spontaneous recovery likely contributed to movement improvements in this study. While exercise alone didn't significantly improve the DF compared to the penetrating injury model, resveratrol showed a more significant effect in restoring the DF post-injury [
34]. Resveratrol enhances endurance in high-capacity running rats and, combined with exercise, improves fatigue and exercise intolerance in mice with heart failure [
35].
This study demonstrates the variable effects of resveratrol and exercise, with metatarsus movements showing different patterns. Combined resveratrol and exercise post-injury on the third and seventh days appear to have other effects, possibly due to horizontal or lateral displacement effects, warranting further study. Resveratrol significantly improves functional recovery post-spinal cord injury by promoting axonal regeneration and suppressing apoptosis [
36]. However, changes in different joints and times, or left and right metatarsus, were not made. Spinal cord central pattern generators (CPGs) function individually for each articulation and leg [
37]. Altered muscle activation patterns may result from changes in spinal CPG function or altered higher structure information converging in spinal motor circuits. These CPGs can function autonomously post-denervation, highlighting the hippocampus's role in CPG function [
38,
39,
40,
41].
Resveratrol is a potent neuroprotective compound with mechanisms including antioxidant action [
45], reducing pro-inflammatory cytokines [
43], preventing apoptosis [
44], and decreasing microglial activation [
45]. The hippocampus, heavily affected by injury, ischemia, or neurodegeneration [
46,
47], could benefit significantly from neuroprotection. Exercise is also effective for recovering lower extremity kinematic alterations post-penetrating hippocampus injury. Thus, resveratrol and exercise could be considered for treating hippocampal damage in various pathologies with moto with motor implications.
5. Conclusions
The study shows that exercise and resveratrol contribute to improving locomotion kinematics following a penetrating hippocampal injury. Administration of exercise and resveratrol, individually or in combination, facilitates the recovery of vertical displacement in the metatarsal and ankle joints. The combination of both treatments exhibits a more pronounced synergistic effect, suggesting that exercise and resveratrol are beneficial and that their combined use may offer more comprehensive motor recovery. These findings underscore the potential of combined therapeutic strategies for the rehabilitation of brain injuries, highlighting the importance of exploring multifaceted approaches to enhance motor functionality in brain injury models.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Irene Guadalupe Aguilar García, PhD. Did experiments on rats and wrote a review. manuscript. Jonatan Alpirez, PhD. Students did the experiments on rats and part of the computational program. Rolando Castaneda Arellano PhD: made some images and reviewed the manuscript. Judith Marcela Dueñas Jimenez PhD: reviewed the manuscript. Maria del Carmen Toro Castillo PhD: Reviewed the manuscript. Lilia Carolina León Moreno PhD: reviewed the manuscript. Laura Paulina Osuna Carrasco PhD: reviewed the manuscript. Sergio Horacio Dueñas Jimenez, MD, PhD: wrote the first and final draft of the manuscript, found the grant, and gave the idea. All authors read and approved the final manuscript.
Funding
This study was supported by the Programa de Apoyo a la Mejora en las Condiciones de Producción SNI y SNCA (PROSNI) from the University of Guadalajara, Mexico.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors by request without reservations.
Acknowledgments
No thanks.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Akamatsu Y, Hanafy KA. Cell Death and Recovery in Traumatic Brain Injury. Neurotherapeutics. 2020 17(2):446-456. [CrossRef]
- Briones TL Chapter 3 animal models of traumatic brain injury: is there an optimal model that parallels human brain injury? Annu Rev Nurs Res 2015 33:3173.
- Ma X, Aravind A, Pfister BJ, Chandra N, Haorah J. Animal Models of Traumatic Brain Injury and Assessment of Injury Severity. Mol Neurobiol. 2019 56(8):5332-5345. [CrossRef]
- Najem D, Rennie K, Ribecco-Lutkiewicz M, Ly D, Haukenfrers J, Liu Q, Nzau M, Fraser DD, Bani-Yaghoub M. Traumatic brain injury: classification, models, and markers. Biochem Cell Biol. 2018 96(4):391-406. [CrossRef]
- Bender F, Gorbati M, Cadavieco MC, Denisova N, Gao X, Holman C, Korotkova T, Ponomarenk A. Theta oscillations regulate the speed of locomotion via a hippocampusto lateral septum pathway. Nat Commun. 2015 12;6:8521.
- López Ruiz JR, Osuna Carrasco LP, López Valenzuela CL, Franco Rodríguez NE, de la Torre Valdovinos B, Jiménez Estrada I, Dueñas Jiménez JM, Dueñas Jiménez SH. The hippocampus participates in the control of locomotion speed. Neuroscience. 2015 17; 311:207-15.
- León-Moreno LC, Castañeda-Arellano R, Aguilar-García IG, Desentis-Desentis MF, Torres-Anguiano E, Gutiérrez-Almeida CE, Najar-Acosta LJ, Mendizabal-Ruiz G, Ascencio-Piña CR, Dueñas-Jiménez JM, Rivas-Carrillo JD, Dueñas-Jiménez SH. Kinematic Changes in a Mouse Model of Penetrating Hippocampal Injury and Their Recovery After Intranasal Administration of Endometrial Mesenchymal Stem Cell-Derived Extracellular Vesicles. Front Cell Neurosci. 2020 10;14:579162. [CrossRef]
- Mahalakshmi B, Maurya N, Lee SD, Bharath Kumar V. Possible Neuroprotective Mechanisms of Physical Exercise in Neurodegeneration. Int J Mol Sci. 2020 16;21(16):5895. [CrossRef]
- Monteiro-Junior RS, Cevada T, Oliveira BR, Lattari E, Portugal EM, Carvalho A, Deslandes AC. We need to move more: Neurobiological hypotheses of physical exercise as a treatment for Parkinson's disease. Med Hypotheses. 2015 85(5):537-41. [CrossRef]
- Navarro-Quiroz, E., Navarro-Quiroz, R., España-Puccini, P., Ahmad, M., Díaz-Pérez A.,Villarreal, J. L., Vásquez, L., & Torres, A. Neurogenesis in adult brain. Salud Uninorte, 2018 34(1), 144–159.
- Von Bernhardi R, Bernhardi LE, Eugenín J. What Is Neural Plasticity? Adv Exp Med Biol. 2017; 1015:1-15.
- Piao CS, Stoica BA, Wu J, Sabirzhanov B, Zhao Z, Cabatbat R, Loane DJ, Faden AI. Late exercise reduces neuroinflammation and cognitive dysfunction after traumatic brain injury. Neurobiol Dis. 201354:252-63. [CrossRef]
- Vivar C, Potter MC, van Praag H. All about running: synaptic plasticity, growth factors and adult hippocampal neurogenesis. Curr Top Behav Neurosci. 2013;1 5:189-210.
- Voss MW, Vivar C, Kramer AF, van Praag H. Bridging animal and human models of exercise-induced brain plasticity. Trends Cogn Sci. 2013 17(10):525-44. [CrossRef]
- Zhang P, Zhang Y, Zhang J, Wu Y, Jia J, Wu J, Hu Y. Early Exercise Protects against Cerebral Ischemic Injury through Inhibiting Neuron Apoptosis in Cortex in Rats. Int J Mol Sci. 2013 15;14(3):6074-89. [CrossRef]
- Hong JH, Lee H, Lee SR. Protective effect of resveratrol against neuronal damage following transient global cerebral ischemia in mice. J Nutr Biochem. 2016; 27:146 52. [CrossRef]
- Shetty AK. Promise of resveratrol for easing status epilepticus and epilepsy. Pharmacol Ther. 2011;131(3):269-86. [CrossRef]
- Amirazodi M, Mehrabi A, Rajizadeh MA, Bejeshk MA, Esmaeilpour K, Daryanoosh F, Gaeini A. The effects of combined resveratrol and high intensity interval training on the hippocampus in aged male rats: An investigation into some signaling pathways related to mitochondria. Iran J Basic Med Sci. 2022 (2):254-262. [CrossRef]
- Cui B, Wang Y, Jin J, Yang Z, Guo R, Li X, Yang L, Li Z. Resveratrol Treats UVB-Induced Photoaging by Anti-MMP Expression, through Anti-Inflammatory, Antioxidant, and Antiapoptotic Properties, and Treats Photoaging by Upregulating VEGF-B Expression. Oxid Med Cell Longev. 2022 (4);2022:6037303. [CrossRef]
- Sönmez U, Sönmez A, Erbil G, Tekmen I, Baykara B. Neuroprotective effects of resveratrol agains traumatic brain injury in immature rats. Neurosci Lett. 2007 13;420(2):133-7. [CrossRef]
- Alpirez J, Leon-Moreno LC, Aguilar-García IG, Castañeda-Arellano R, Dueña Jiménez JM, Asencio-Piña CR, Dueñas-Jiménez SH. Walk Locomotion Kinematic Changes in a Mode of Penetrating Hippocampal Injury in Male/Female Mice and Rats. Brain Sci. 2023 2;13(11):1545.
- Hertel J. Functional Anatomy, Pathomechanics, and Pathophysiology of Lateral Ankle Instability. J Athl Train. 2002;37(4):364-375.
- Bonnel F, Toullec E, Mabit C, Tourné Y; Sofcot. Chronic ankle instability: biomechanics and pathomechanics of ligaments injury and associated lesions. Orthop Traumatol Surg Res. 2010; 96(4):424-32. [CrossRef]
- Iwase M, Kitanishi T, Mizuseki K. Cell type, sub-region, and layer-specific speed representation in the hippocampal-entorhinal circuit. Sci Rep. 2020 29;10(1):1407.
- Sauvage C, Jissendi P, Seignan S, Manto M, Habas C. Brain areas involved in the control of speed during a motor sequence of the foot: real movement versus mental imagery. J Neuroradiol. 2013;40(4):267-80. [CrossRef]
- Yamaguchi N, Sawano T, Fukumoto K, Nakatani J, Inoue S, Doe N, Yanagisawa D, Tooyama I, Nakagomi T, Matsuyama T, Tanaka H. Voluntary running exercise after focal cerebral ischemia ameliorates dendritic spine loss and promotes functional recovery. Brain Res. 2021 15;1767:147542. [CrossRef]
- Kim CK, Park JS, Kim E, Oh MK, Lee YT, Yoon KJ, Joo KM, Lee K, Park YS. The effects of early exercise in traumatic brain-injured rats with changes in motor ability, brain tissue, and biomarkers. BMB Rep. 2022;55(10):512-517. [CrossRef]
- Bacanoiu MV, Danoiu M, Rusu L, Marin MI. New Directions to Approach Oxidative Stress Related to Physical Activity and Nutraceuticals in Normal Aging and Neurodegenerative Aging. Antioxidants (Basel). 2023 26;12(5):1008. [CrossRef]
- Durbin SM, Jackson JR, Ryan MJ, Gigliotti JC, Alway SE, Tou JC. Resveratrol supplementation preserves long bone mass, microstructure, and strength in hindlimb suspended old male rats. J Bone Miner Metab. 2014;32(1):38-47.
- Shahcheraghi SH, Salemi F, Small S, Syed S, Salari F, Alam W, Cheang WS, Saso L, Khan H. Resveratrol regulates inflammation and improves oxidative stress via Nrf2 signaling pathway: Therapeutic and biotechnological prospects. Phytother Res. 2023,37(4):1590-1605. [CrossRef]
- Brown, A. R., & Martinez, M. Ipsilesional Motor Cortex Plasticity Participates in Spontaneous Hindlimb Recovery after Lateral Hemisection of the Thoracic Spinal Cord in the Rat. The Journal of Neuroscience. 2018 38(46), 9977–9988.
- Kakuta Y, Adachi A, Yokohama M, Horii T, Mieda T, Iizuka Y, Takagishi K, Chikuda H, Iizuka H, Nakamura K. Spontaneous functional full recovery from motor and sensory deficits in adult mice after mild spinal cord injury. Heliyon. 2019 2;5(6):e01847. [CrossRef]
- Shirota Y, Otani T, Wasada S, Ito S, Mieda T, Nakamura K. Inner and outer penetrating spinal cord injuries lead to distinct overground walking in mice. IBRO Neurosci Rep. 2024 1;16:345-352. [CrossRef]
- Hart N, Sarga L, Csende Z, Koch LG, Britton SL, Davies KJ, Radak Z. Resveratrol attenuates exercise-induced adaptive responses in rats selectively bred for low running performance. Dose Response. 2013 4;12(1):57-71. [CrossRef]
- Sung MM, Byrne NJ, Robertson IM, Kim TT, Samokhvalov V, Levasseur J, Soltys CL, Fung D, Tyreman N, Denou E, Jones KE, Seubert JM, Schertzer JD, Dyck JR. Resveratrol improves exercise performance and skeletal muscle oxidative capacity in heart failure. Am J Physiol Heart Circ Physiol. 2017 1;312(4):H842-H853. [CrossRef]
- Xiang Z, Zhang S, Yao X, Xu L, Hu J, Yin C, Chen J, Xu H. Resveratrol promotes axonal regeneration after spinal cord injury through activating Wnt/β-catenin signaling pathway. Aging (Albany NY). 2021 14;13(20):23603-23619. [CrossRef]
- Taccola G, Ichiyama RM, Edgerton VR, Gad P. Stochastic spinal neuromodulation tunes the intrinsic logic of spinal neural networks. Exp Neurol. 2022 355:114138. [CrossRef]
- Grillner S, Zangger P. How detailed is the central pattern generation for locomotion? Brain Res. 1975 2;88(2):367-71.
- Grillner S. The execution of movement: a spinal affair. J Neurophysiol. 2021 1;125(2):693-698. [CrossRef]
- Grillner S. Interaction between central and peripheral mechanisms in the control locomotion. Prog Brain Res. 1979; 50:227-35.
- Molkov YI, Yu G, Ausborn J, Bouvier J, Danner SM, Rybak IA. Sensory feedback and central neuronal interactions in mouse locomotion. R Soc Open Sci. 2024 11(8):240207. [CrossRef]
- Qi J, Fu LY, Liu KL, Li RJ, Qiao JA, Yu XJ, Yu JY, Li Y, Feng ZP, Yi QY, Jia H, Gao HL, Tan H, Kang YM. Resveratrol in the Hypothalamic Paraventricular Nucleus Attenuates Hypertension by Regulation of ROS and Neurotransmitters. Nutrients. 2022 7;14(19):4177. [CrossRef]
- Nani A, Murtaza B, Sayed Khan A, Khan NA, Hichami A. Antioxidant and Anti-Inflammatory Potential of Polyphenols Contained in Mediterranean Diet in Obesity: Molecular Mechanisms. Molecules. 202112;26(4):985. [CrossRef]
- Hou Y, Wang K, Wan W, Cheng Y, Pu X, Ye X. Resveratrol provides neuroprotection by regulating the JAK2/STAT3/PI3K/AKT/mTOR pathway after stroke in rats. Genes Dis. 2018 15;5(3):245-255.
- Meng T, Xiao D, Muhammed A, Deng J, Chen L, He J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules. 2021 5;26(1):229. [CrossRef]
- Bartsch T, Wulff P. The hippocampus in aging and disease: From plasticity to vulnerability. Neuroscience. 201519; 309:1-16. [CrossRef]
- Callow DD, Won J, Alfini AJ, Purcell JJ, Weiss LR, Zhan W, Smith JC. Microstructural Plasticity in the Hippocampus of Healthy Older Adults after Acute Exercise. Med Sci Sports Exerc. 2021 1;53(9):1928-1936. [CrossRef]
Figure 1.
Bar graphs illustrate the dissimilarity factor (DF) among the control and experimental groups in metatarsus. Control (blue bar); Injury at three days (Injury 3 dpi) and seven days (Injury 7 dpi) post-injury are illustrated as green and red bars, respectively; Exercise (Ex), at 3- and 7-days post-lesion are illustrated in pink and gray bars, respectively; Resveratrol treatment (Resv) at 3- and 7-days post-lesion in black and blue bars respectively; Exercise with Resveratrol treatment (Ex-Resv), at 3- and 7-days post-lesion in orange and cyan bars respectively. The values are expressed as median ± SE. The asterisks illustrate statistical differences between groups utilizing the Kruskal-Wallis test. P ≤ 0,05 (*), P ≤ 0,01 (**), P ≤ 0,001 (***), P ≤ 0,0001 (****).
Figure 1.
Bar graphs illustrate the dissimilarity factor (DF) among the control and experimental groups in metatarsus. Control (blue bar); Injury at three days (Injury 3 dpi) and seven days (Injury 7 dpi) post-injury are illustrated as green and red bars, respectively; Exercise (Ex), at 3- and 7-days post-lesion are illustrated in pink and gray bars, respectively; Resveratrol treatment (Resv) at 3- and 7-days post-lesion in black and blue bars respectively; Exercise with Resveratrol treatment (Ex-Resv), at 3- and 7-days post-lesion in orange and cyan bars respectively. The values are expressed as median ± SE. The asterisks illustrate statistical differences between groups utilizing the Kruskal-Wallis test. P ≤ 0,05 (*), P ≤ 0,01 (**), P ≤ 0,001 (***), P ≤ 0,0001 (****).
Figure 2.
Bar graphs illustrate the dissimilarity factor (DF) among the ankle's control and experimental groups. Control blue bar; Injury at 3- and 7-days post-injury, green and red bars, respectively; Exercise (Ex), at 3- and 7-days post-injury pink and gray bars, respectively; Resveratrol treatment (Resv) at 3- and 7-days post-lesion black and blue bars respectively; Exercise with Resveratrol treatment (Ex-Resv), at 3- and 7-days post-injury orange and Cyan bars respectively. The values are expressed as median ± SE. The asterisks illustrate statistical differences between groups utilizing the Kruskal-Wallis test. p ≤ 0,05 (*), p ≤ 0,01 (**), p ≤ 0,001 (***), p ≤ 0,0001 (****).
Figure 2.
Bar graphs illustrate the dissimilarity factor (DF) among the ankle's control and experimental groups. Control blue bar; Injury at 3- and 7-days post-injury, green and red bars, respectively; Exercise (Ex), at 3- and 7-days post-injury pink and gray bars, respectively; Resveratrol treatment (Resv) at 3- and 7-days post-lesion black and blue bars respectively; Exercise with Resveratrol treatment (Ex-Resv), at 3- and 7-days post-injury orange and Cyan bars respectively. The values are expressed as median ± SE. The asterisks illustrate statistical differences between groups utilizing the Kruskal-Wallis test. p ≤ 0,05 (*), p ≤ 0,01 (**), p ≤ 0,001 (***), p ≤ 0,0001 (****).
Figure 3.
A-B graphs illustrate the vertical displacement (VD) in the left and right metatarsus of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cyan line), respectively. C-D corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercises post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 3.
A-B graphs illustrate the vertical displacement (VD) in the left and right metatarsus of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cyan line), respectively. C-D corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercises post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 4.
A-C graphs illustrate the VD in the left and right ankle of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. G-H corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercise post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 4.
A-C graphs illustrate the VD in the left and right ankle of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. G-H corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercise post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 5.
A-D graphs illustrate the VD in the left and right metatarsus of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. C-D corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercises post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 5.
A-D graphs illustrate the VD in the left and right metatarsus of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. C-D corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercises post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 6.
A-D graphs illustrate the vertical displacement (VD) in the left and right ankle of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. C-D corresponds to VD in the left and right ankle of control (blue line) injury seven days (7 dpi) (red line) and exercises post-lesion groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 6.
A-D graphs illustrate the vertical displacement (VD) in the left and right ankle of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. C-D corresponds to VD in the left and right ankle of control (blue line) injury seven days (7 dpi) (red line) and exercises post-lesion groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 7.
A-D graphs illustrate the vertical displacement (VD) in the left and right metatarsus of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. C-D corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercises post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 7.
A-D graphs illustrate the vertical displacement (VD) in the left and right metatarsus of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. C-D corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercises post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 8.
A-D graphs illustrate the vertical displacement (VD) in the left and right metatarsus of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. C-D corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercises post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
Figure 8.
A-D graphs illustrate the vertical displacement (VD) in the left and right metatarsus of the control (blue line), injury three days (3 dpi) (green line), and exercise post-injury groups (Ex) (Cian line), respectively. C-D corresponds to VD in the left and right metatarsus of control (blue line) injury seven days (7 dpi) (red line) and exercises post-injury groups (Ex) (gray line), respectively. The asterisks illustrate the bins with a statistical difference (*p ≤ 0.05). The percent of change is expressed below the graphs.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).