Submitted:
19 September 2024
Posted:
25 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Clinical Case
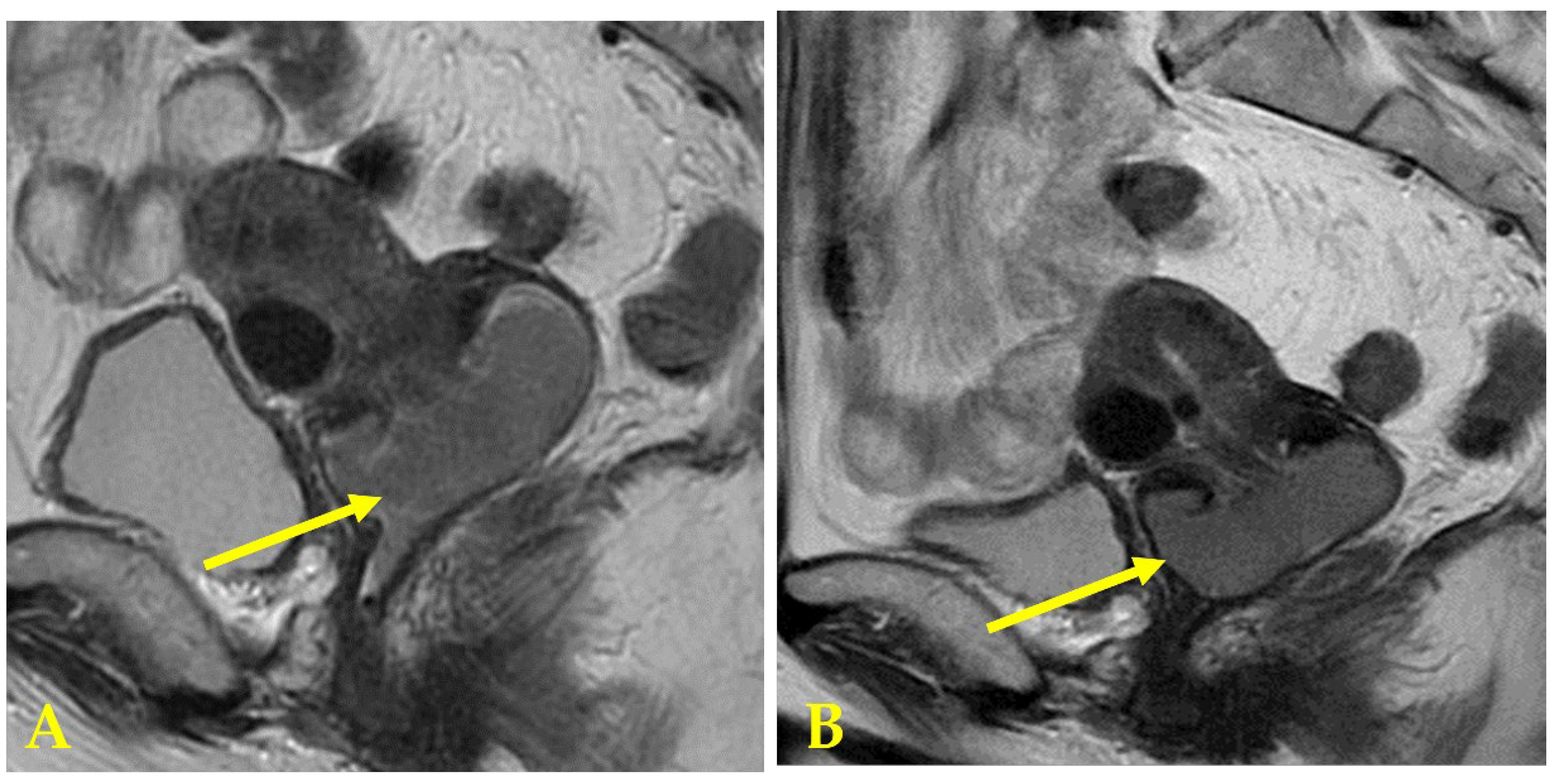
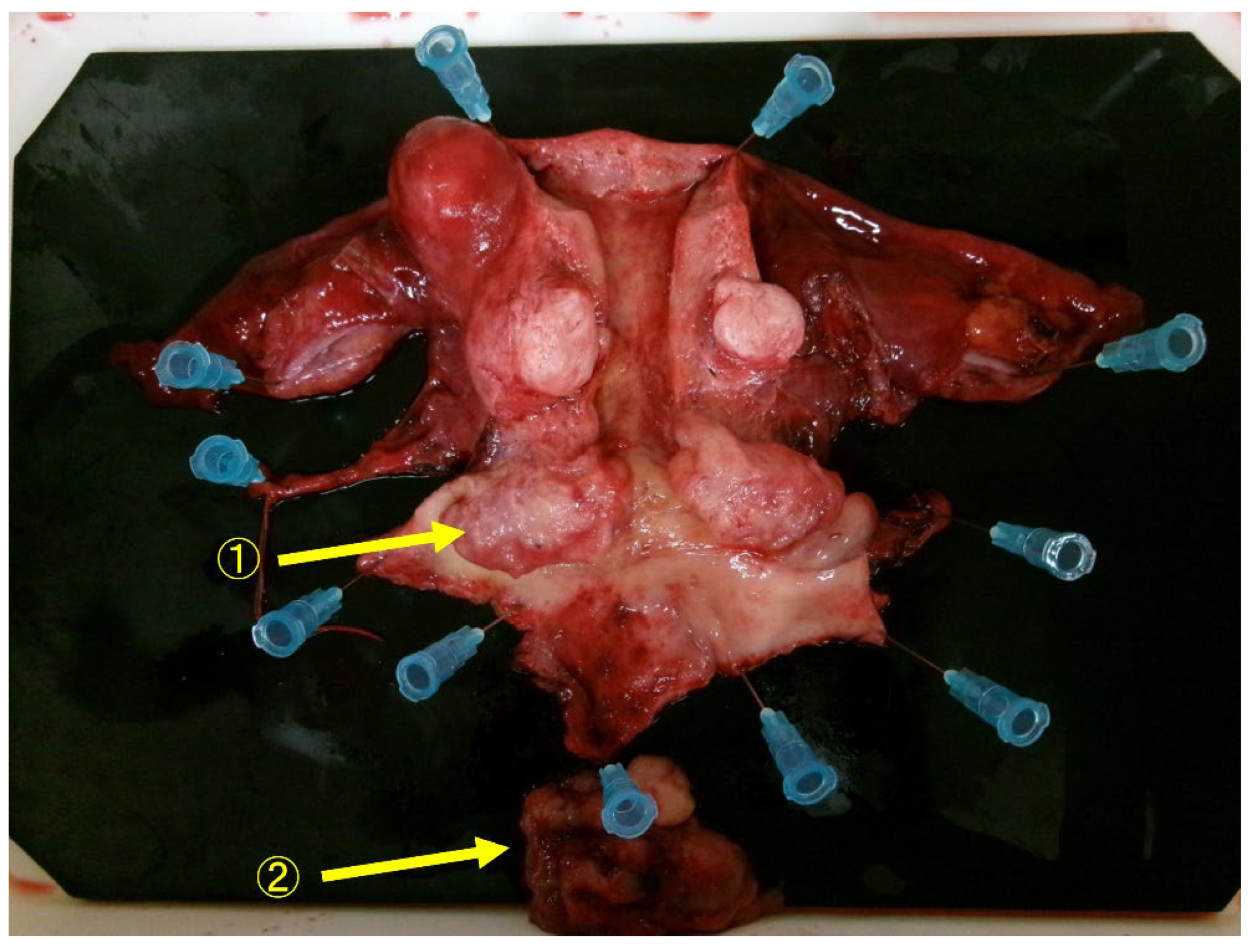
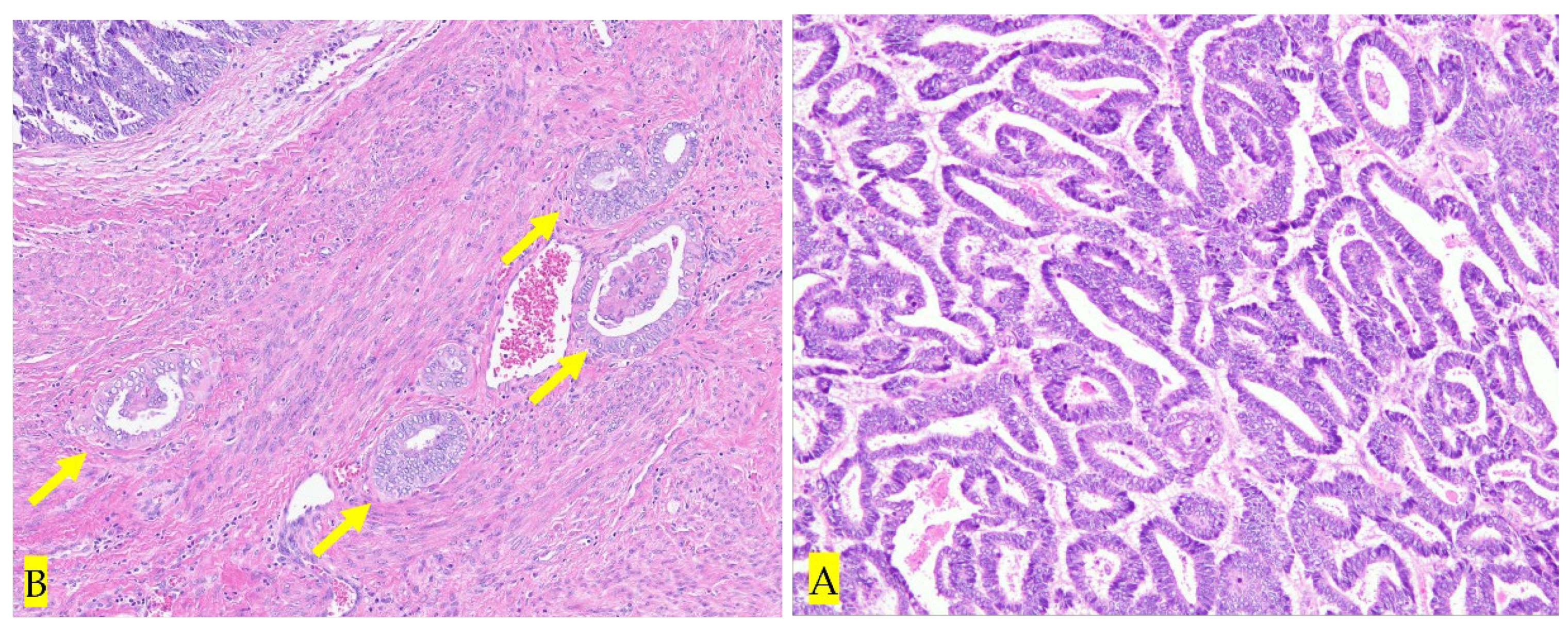
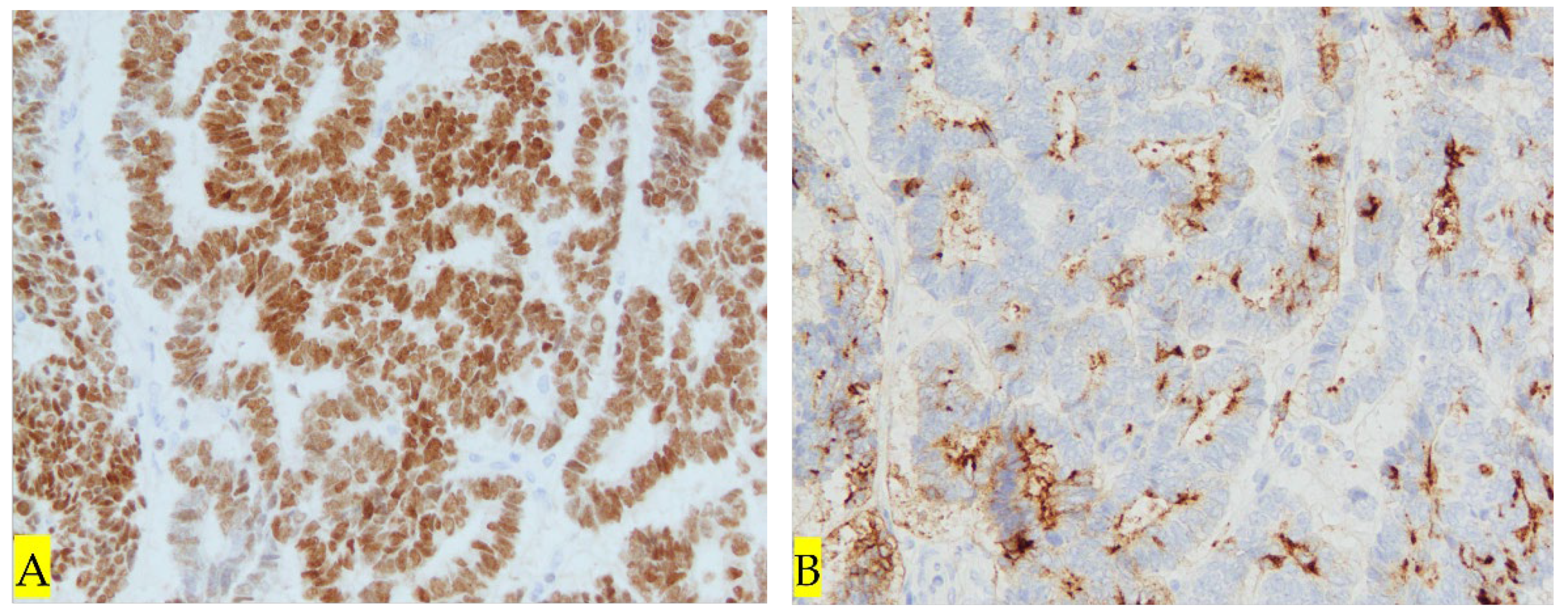
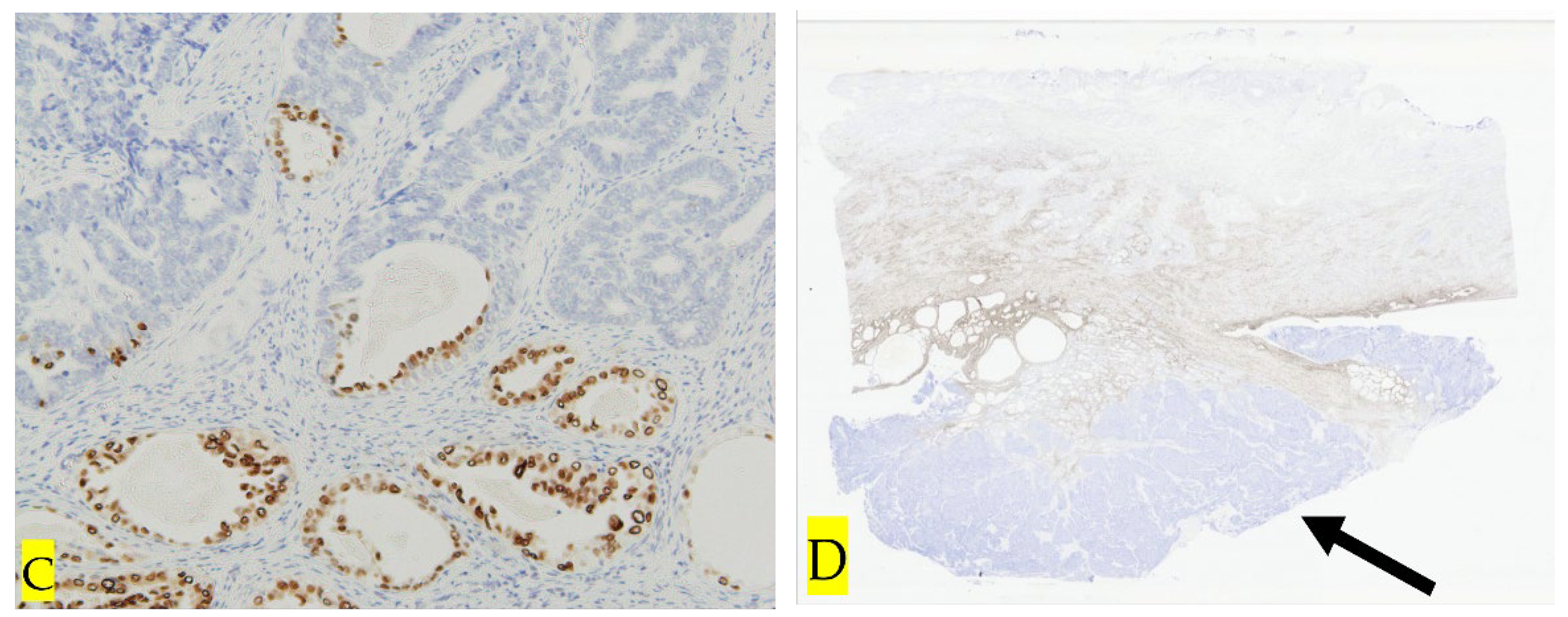
3. Discussion
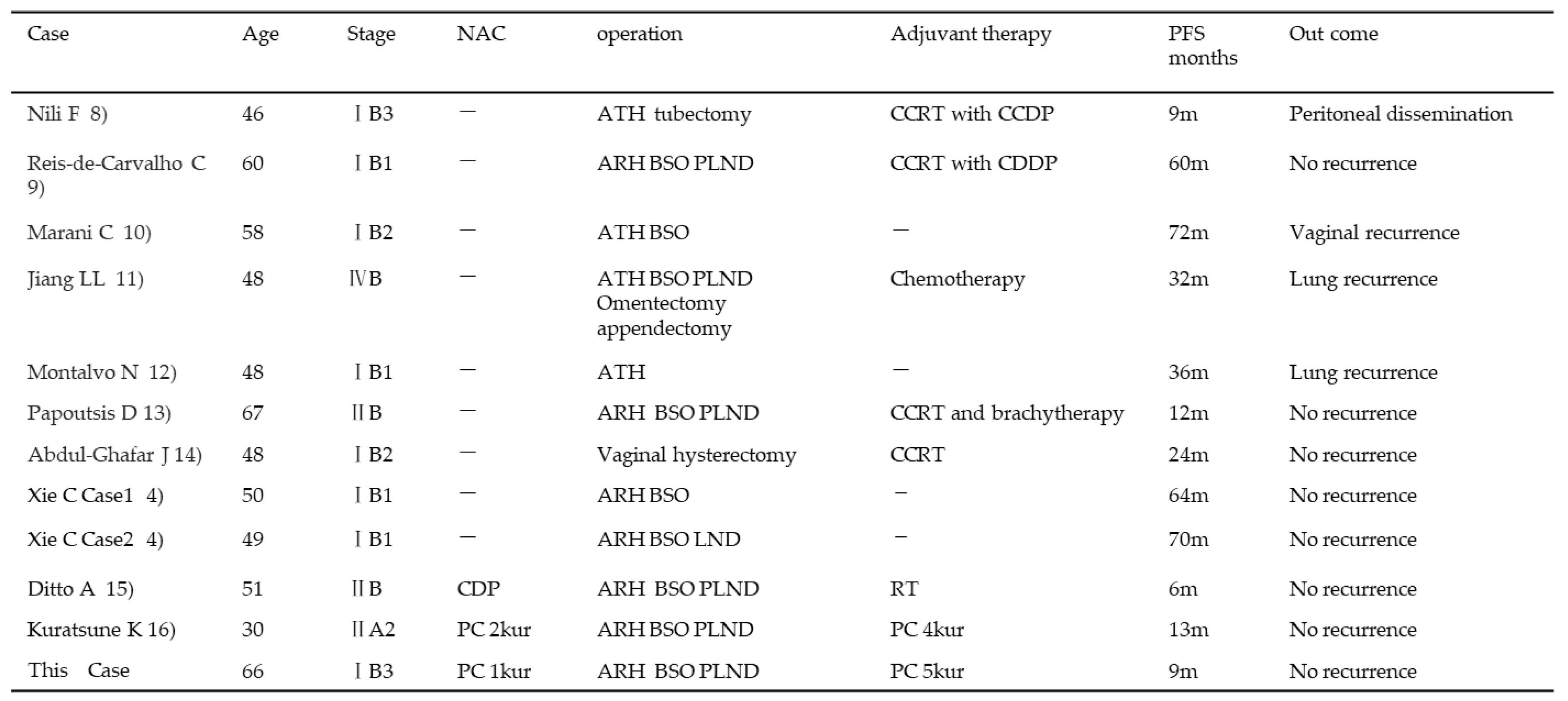 |
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Classification of Tumours Female Genital Tumours[M] (5th ed.), Lyon: IARC Press (2020), pp. 378-379.
- Pors, J.; Segura, S.; Chiu, D.S.; Almadani, N.; Ren, H.; Fix, D.J.; Howitt, B.E.; Kolin, D.; McCluggage, W.G.; Mirkovic, J.; Gilks, B.; Park, K.J.; Hoang, L. Clinicopathologic Characteristics of Mesonephric Adenocarcinomas and Mesonephric-like Adenocarcinomas in the Gynecologic Tract: A Multi-institutional Study. Am J Surg Pathol. 2021, 45, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Howitt, B.E.; Nucci, M.R. Mesonephric proliferations of the female genital tract. Pathology. 2018, 50, 141–150, Epub 2017 Dec 19. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Zhang, L.; Cao, W.; Hu, Y.; Liu, Y. Mesonephric adenocarcinoma of the uterine corpus. Int J Clin Exp Pathol. 2014, 7, 7012–7019. [Google Scholar] [PubMed]
- Xie, C.; Chen, Q.; Shen, Y. Mesonephric adenocarcinomas in female genital tract: A case series. Medicine (Baltimore). 2021, 100, e27174. [Google Scholar] [CrossRef] [PubMed]
- McFarland, M.; Quick, C.M.; McCluggage, W.G. Hormone receptor-negative, thyroid transcription factor 1-positive uterine and ovarian adenocarcinomas: report of a series of mesonephric-like adenocarcinomas. Histopathology. 2016, 68, 1013–1020, Epub 2016 Jan 4. [Google Scholar] [CrossRef] [PubMed]
- Secosan, C.; Balint, O.; Ilian, A.; Balan, L.; Balulescu, L.; Motoc, A.; Zahoi, D.; Grigoras, D.; Pirtea, L. New Insights in the Diagnosis of Rare Adenocarcinoma Variants of the Cervix-Case Report and Review of Literature. Healthcare (Basel). 2022, 10, 1410. [Google Scholar] [CrossRef] [PubMed]
- Nili, F.; Salarvand, S.; Saffar, H.; Kalaghchi, B.; Ghalehtaki, R. Mesonephric Adenocarcinoma of Uterine Cervix: A Case Report and Review of the Literature. Iran J Pathol. 2021, 16, 227–231, Epub 2020 Dec 21. [Google Scholar] [CrossRef] [PubMed]
- Reis-de-Carvalho, C.; Vaz-de-Macedo, C.; Ortiz, S.; Colaço, A.; Calhaz-Jorge, C. Cervical Mesonephric Adenocarcinoma: A Case Report of a Rare Gynecological Tumor from Embryological Remains of the Female Genital Tract. Rev Bras Ginecol Obstet. 2021, 43, 329–333, Epub 2021 Mar 30. [Google Scholar] [CrossRef] [PubMed]
- Marani, C.; Akaev, I.; Yeoh, C.C.; Walsh, E.; Rahimi, S. Cervical malignant mixed mesonephric tumour: A case report with local recurrence after six-years and next-generation sequencing analysis with particular reference to the ataxia telangiectasia mutated gene. Exp Ther Med. 2021, 21, 394, Epub 2021 Feb 24. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.L.; Tong, D.M.; Feng, Z.Y.; Liu, K.R. Mesonephric adenocarcinoma of the uterine cervix with rare lung metastases: A case report and review of the literature. World J Clin Cases. 2020, 8, 1735–1744. [Google Scholar] [CrossRef] [PubMed]
- Montalvo, N.; Redrobán, L.; Galarza, D. Mesonephric adenocarcinoma of the cervix: a case report with a three-year follow-up, lung metastases, and next-generation sequencing analysis. Diagn Pathol. 2019, 14, 71. [Google Scholar] [CrossRef] [PubMed]
- Papoutsis, D.; Sahu, B.; Kelly, J.; Antonakou, A. Perivascular epithelioid cell tumour and mesonephric adenocarcinoma of the uterine cervix: an unknown co-existence. Oxf Med Case Reports. 2019, 2019, omy115. [Google Scholar] [CrossRef] [PubMed]
- Abdul-Ghafar, J.; Chong, Y.; Han, H.D.; Cha, D.S.; Eom, M. Mesonephric Adenocarcinoma of the Uterine Cervix Associated with Florid Mesonephric Hyperplasia: A Case Report. J Lifestyle Med. 2013, 3, 117–120, Epub 2013 Sep 30. [Google Scholar]
- Ditto, A.; Martinelli, F.; Bogani, G.; Gasparri, M.L.; Donato, V.D.; Paolini, B.; Carcangiu, M.L.; Lorusso, D.; Raspagliesi, F. Bulky mesonephric adenocarcinoma of the uterine cervix treated with neoadjuvant chemotherapy and radical surgery: report of the first case. Tumori. 2016, 102 (Suppl. 2). [Google Scholar] [CrossRef] [PubMed]
- Kuratsune, K.; Ueda, T.; Tajiri, R.; Tohyama, A.; Hoshino, K.; Harada, H.; Kurita, T.; Kubo, C.; Komatsu, K.; Shiba, E.; Matsuura, Y.; Yoshino, K. [A Case of Adenocarcinoma, HPV-independent, Mesonephric Type with Significant Response to Neoadjuvant Chemotherapy]. J UOEH. 2024, 46, 45–51, Japanese. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; He, Y.; Yang, S.L.; Zhao, Q.; Wu, Y.M. Neoadjuvant chemotherapy with radical surgery vs radical surgery alone for cervical cancer: a systematic review and meta-analysis. Onco Targets Ther 2019, 12, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, P.T.; Chiva, L.; Eriksson, A.G.Z.; Frumovitz, M.; Fagotti, A.; Gonzalez Martin, A.; Jhingran, A.; Pareja, R. COVID-19 Global Pandemic: Options for Management of Gynecologic Cancers. Int J Gynecol Cancer. 2020, 30, 561–563, Epub 2020 Mar 27. [Google Scholar] [CrossRef] [PubMed]
- Rosa, D.D.; Medeiros, L.R.; Edelweiss, M.I.; Pohlmann, P.R.; Stein, A.T. Adjuvant platinum-based chemotherapy for early stage cervical cancer. Cochrane Database Syst Rev. 2012, 6, CD005342, Update in: Cochrane Database Syst Rev. 2016 Nov 22;11:CD005342. [Google Scholar] [CrossRef] [PubMed]
- Shimada, M.; Nishimura, R.; Hatae, M.; Hiura, M.; Takehara, K.; Tase, T.; Yamada, H.; Kurachis, H.; Sugiyama, T.; Kigawa, J. Comparison of adjuvant chemotherapy and radiotherapy in patients with cervical adenocarcinoma of the uterus after radical hysterectomy: SGSG/TGCU Intergroup surveillance. Eur J Gynaecol Oncol. 2013, 34, 425–428. [Google Scholar] [PubMed]
- Montagut, C.; Mármol, M.; Rey, V.; Ordi, J.; Pahissa, J.; Rovirosa, A.; Gascón, P.; Mellado, B. Activity of chemotherapy with carboplatin plus paclitaxel in a recurrent mesonephric adenocarcinoma of the uterine corpus. Gynecol Oncol. 2003, 90, 458–461. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





