Introduction
Turmeric is derived from the dried rhizome of the
Curcuma longa plant and is a member of the
Zingiberaceae (ginger) perennial plant family. For many centuries turmeric has been employed as a spice and traditional medicinal agent across the continent of Asia [
1]. Turmeric consists of a collection of phenolic compounds known as curcuminoids comprising of curcumin, demethoxycurcumin, and bisdemethoxycurcumin, which are essentially responsible for its therapeutic activity and its distinctive orange-yellow pigment. Of these curcuminoids, curcumin is considered the most biologically active and stands as the principal constituent of turmeric, making up approximately 2-5% of the root extract [
1].
Curcumin is a natural compound that falls under the category of diarylheptanoids, a class of organic compounds characterized by two aromatic rings connected by a seven-carbon chain [
2], Curcumin has garnered significant attention in recent years due to its diverse pharmacological properties and potential health benefits [
3,
4]. Extensive research has elucidated its anti-inflammatory, antioxidant, and neuroprotective properties, making curcumin a promising therapeutic agent for a wide range of medical conditions including chronic inflammatory conditions of the brain [
5,
6,
7].
One of the central mechanisms through which curcumin exerts its anti-inflammatory effects is the modulation of pro-inflammatory mediators. Curcumin has been shown to inhibit the activity or decrease the expression of key pro-inflammatory enzymes, such as cyclooxygenase-2 (COX-2) and 5-lipoxygenase (5-LOX), which are responsible for the synthesis of pro-inflammatory prostaglandins and leukotrienes. Curcumin modulates gene expression by interacting with various transcription factors, such as nuclear factor-kappa B (NF-κB), activator protein-1 (AP-1), signal transducer and activator of transcription 3 (STAT3), and peroxisome proliferator-activated receptor gamma (PPAR-γ). Through its interactions with these transcription factors, curcumin can downregulate the expression of inflammatory genes, including cytokines (e.g., TNF-α, IL-1β), chemokines, and adhesion molecules, thereby curtailing the inflammatory cascade [
8,
9].
However, its poor aqueous solubility, limited bioavailability, and rapid metabolism have posed significant challenges in harnessing its full therapeutic potential [
10]. To overcome these limitations, novel curcumin formulations have been introduced, including nanoparticles, liposomes, micelles, and complexation with other compounds, to enhance curcumin’s bioavailability and stability [
11,
12,
13]. Consequently, the assessment of the bioavailability of these formulations in biological matrices, such as blood, is of paramount importance. Such measurements are pivotal for understanding pharmacokinetics, bioavailability, and distribution of novel curcumin preparations
in vivo. One of the more recent developments in this space is AQUATURM
®, a proprietary curcumin extract developed by LODAAT Pharma, which contains water-soluble turmeric extract standardized to contain no less than 23% total curcuminoids. The increased dispersibility of curcuminoids found in AQUATURM
® is attributable to a proprietary formulation and patented manufacturing process that includes comminution of the dried curcuminoid composition to an average particle size of 45-75 nm and blending with a uniquely developed polysaccharide filler.
One of the key unique features of Aquaturm® is its enhanced water solubility due to its proprietary manufacturing process. Increased bioavailability may limit the need for piperine (constituent of black pepper) to enhance curcumin absorption. Additionally, its lack of odor, taste-neutrality, and uniformity in suspension when compared to other curcumin formulations increase the potential applications of the product as a functional food ingredient and supplement.
This study serves to see if this increased water solubility translates into comparably increased bioavailability without a concern of rapid elimination In this pharmacokinetic study, a comprehensive investigation into the measurement of AQUATURM
®, a novel water-soluble curcumin formulation (AQUATURM
®), compared to another commercially available curcumin supplement in form of a tablet [
14] as a control in 12 human subjects was performed. This study aims to evaluate the pharmacokinetics of a water-soluble curcumin formulation, AQUATURM
®, with the goal of determining whether AQUATURM
® offers enhanced bioavailability and therapeutic potential compared to a commercially available curcumin supplement. This research also has the potential to pave the way for the development of more efficacious curcumin-based therapies.
Materials and Methods
Subjects
12 healthy volunteers (7 males and 5 females) were recruited to participate in this study. Subjects were required to meet the following inclusion criteria to take part in the study; any person aged 18-65 years who do not have a history of the following: consuming curcumin-containing supplements (curcumin, turmeric, and curry) or foods (curcumin, turmeric and curry) for 10 days before testing, hyperacidity, gastric/duodenal ulcers, gastrointestinal /gallbladder problems; use of any blood thinners/anti-thrombotic agents or NSAIDs, use of blood sugar-lowering agents, H2 blockers or proton pump inhibitors, hyperglycemic, hemophiliac and diabetic, or any known allergies to soy. All participants were required to avoid pepper at least 2 days before the study and not consume caffeinated drinks on the day of the study. Informed consent was obtained from all subjects involved in the study. This study was approved by the Western Sydney University Human Research Ethics Committee (Approval number H15160).
Study Materials
On two occasions separated by 7 days, participants were asked to ingest one AQUATURM® sachet (corresponding to 450 mg total curcuminoids) orally, that was dissolved in 200 mL of water or a standard commercially available curcumin tablet (“Control Curcumin Supplement”). AQUATURM®, a proprietary water-soluble turmeric extract of Curcuma longa standardized to contains 23% total curcuminoids was studied. The control curcumin supplement, containing Curcumin C3 complex in form of a tablet, and also corresponding to 450 mg total curcuminoids, was purchased from a local health store in Sydney, Australia.
Study Procedure
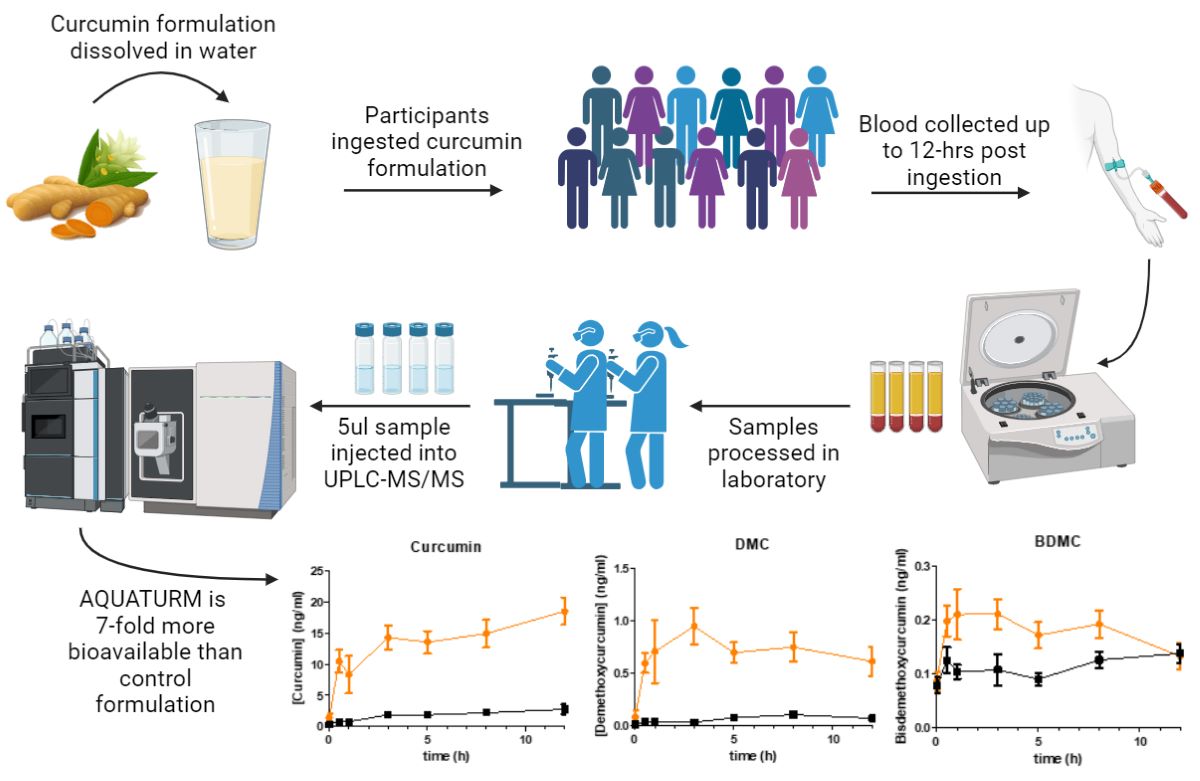
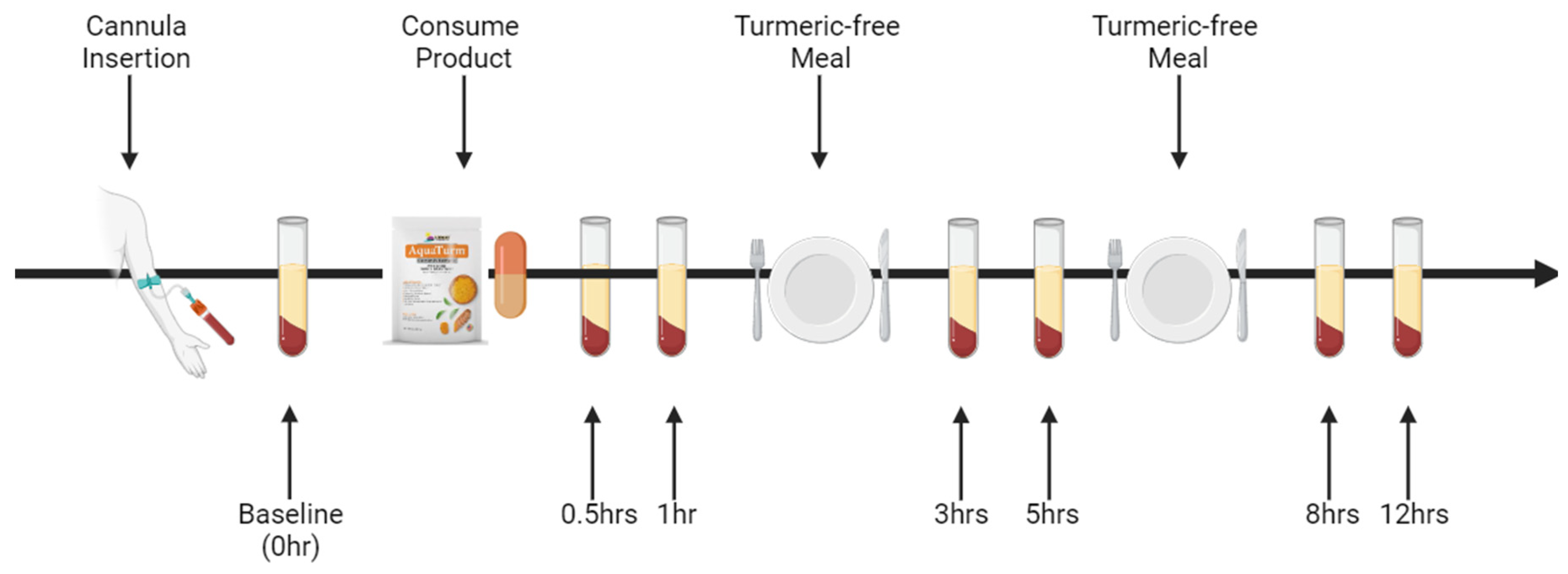
All 12 subjects completed the trials for both formulations, with each subject having nine blood samples drawn in a single day (
Figure 1), under a randomized, double-blind protocol with a seven-day wash-out period between formulations. The curcumin formulations were coded to ensure blinding, so neither the investigators nor the participants knew which formulation was administered during each session. Prior to each trial, subjects arrived at the laboratory in the morning after a 10-hour overnight fast (water allowed). A qualified phlebotomist inserted a catheter into a forearm vein to draw the initial baseline blood sample. Subjects then consumed the designated curcumin formulation, containing 450 mg of total curcuminoids, with water. Subsequent blood samples were collected at 0.5, 1, 3, 5, 8, and 12 hours post-supplementation. These time points were chosen based on previous studies indicating that most digestion and absorption occur within this period [
15,
16]. After each 4-hour and 8-hour blood sample collection, a standardized meal free of curcumin was provided. To ensure full compliance, all subjects stayed in the laboratory for the entire duration of the experiment.
Sample Collection
A cannula was inserted into a suitable vein of the antecubital fossa of each participant by a qualified phlebotomist. Blood was drawn before, 0.5-, 1-, 3-, 5-, 8- and 12-hours following curcumin ingestion into a 6 mL EDTA tube. Samples were centrifuged at 4°C for 10 minutes at (2300 RPM). The upper (plasma) layer was separated and stored at -80°C until further analysis.
Sample Preparation
The plasma samples were prepared according to a modified procedure based on Cuomo et al. [
17]. Plasma samples were thawed at room temperature. Stock solutions of all standards and deuterated internal standards were prepared by dissolving 10 mg of each analyte in 1 mL of methanol. Once thawed, a 200 µL aliquot of plasma was added to an Eppendorf tube along with 1 µL of acetic acid, 2 µL of a 100 ng/mL working solution of deuterated curcumin (CD6) as the internal standard (1 ng/mL final concentration), and 100 µL of a solution containing 1000 U of β-glucuronidase/sulfatase (EC 3.2.1.31) from
Helix pomatia (Sigma, St. Louis, MO) in 0.1 M phosphate buffer (pH ~4.2). The mixture was thoroughly vortexed and incubated at 37°C for 1 hour to enzymatically hydrolyze the phase-2 conjugates of curcuminoids. After incubation, the samples were heated to 70°C for 10 minutes, then 700 µL of ethyl acetate was added as the extraction solvent. The samples were vortexed for 5 minutes, sonicated for 15 minutes, and centrifuged at 14,000 RPM at 4°C for 6 minutes. The upper organic layer was transferred to a new Eppendorf tube. This extraction process was repeated three times, collecting a total of 1500 µL of supernatant. The pooled supernatant was then evaporated to dryness at 30°C under negative pressure in a centrifugal concentrator to remove any residual solvent. Finally, the samples were reconstituted with 200 µL of methanol, transferred to a total recovery glass vial, and injected into the UPLC-MS/MS with a 5 µL injection volume [
17,
18,
19].
Chromatographic Analysis of the Curcuminoids
Blood plasma samples and standards were analyzed by UPLC-MS/MS. The UPLC-MS/MS system utilized was a Waters ACQUITY UPLC I-Class system coupled to a SCIEX triple quadrupole 7500 QTRAP system equipped with an OptiFlow
® Pro Ion Source. A Waters ACQUITY™ UPLC I-Class autosampler was employed to inject 5 μL of sample using the partial loop method. Analyte separation was achieved with a Waters ACQUITY
TM UPLC HSS T3 (1.8 μm, 2.1 X 100 mm) at a temperature of 35 °C and flow rate of 0.200 mL/min. Mobile phase A (Milli-Q water with 0.1% formic acid) and B (acetonitrile with 0.1% formic acid) were run with a linear gradient of 50% B to 65% at 3.0 min and a linear gradient to 100% at 5.0 min, B was held at 100% until 8.0 min at which point the gradient changed to initial conditions (50% B) for equilibration until 10 min. Ionization was achieved by utilizing the OptiFlow
® Pro Ion Source in positive scanning mode at 450°C with an ion spray voltage of 2000 V. The following optimized ion source parameters were utilized: curtain gas 40 psi, ion source gas 1: 50 psi, ion source gas 2: 70 psi, CAD gas 9. Scheduled multiple reaction monitoring (MRM) was performed for analytes using optimized entrance potential (EP), collision energies (CE), and collision cell exit potentials (CXP) (Supplementary
Table 1, 2). The analyte precursor ions, [M+H]
+, and three of their product ion fragments were monitored to quantify and confirm the identity of the analytes (Suppl.
Tables 1, 2). Compound MRM transitions and optimal CE and CXP voltages for each transition was determined through direct injection. The most abundant product ions were selected. The limits of detection (LOD) and limits of quantification (LOQ) were determined using neat standards, with LOD defined as a signal-to-noise ratio greater than 3, and LOQ as a signal-to-noise ratio greater than 10. The LOD values for Curcumin (Curc), Bisdemethoxycurcumin (BDMC), and Demethoxycurcumin (DMC) were found to be 0.00045 ng/mL, 0.00038 ng/mL, and 0.000705 ng/mL, respectively. The corresponding LOQ values were determined to be 0.0015 ng/mL, 0.0012 ng/mL, and 0.00235 ng/mL, respectively. A sample chromatogram is displayed in Supplementary
Figure 1.
Pharmacokinetic Analysis of the Curcuminoid Plasma Concentration
Pharmacokinetic data following the oral administration of the curcumin formulations were analyzed using GraphPad Prism 5. The Cmax, representing the maximum observed plasma concentration, was obtained directly from the mean plasma concentration-time profile. The area under the plasma concentration-time curve (AUC) was calculated using the definite integral from 0 to 12 hours based on the mean plasma concentration-time curves using the additive method. The calculation of t½ was not possible for the curcumin preparations as the concentration did not decline within the 12-hour period. as concentration did not decline in concentration over the 12 hours.
Results
In this study, we compared water-soluble curcumin (AQUATURM
®) with another commercially available product, Control Curcumin Supplement, a standardized extract containing a defined ratio of three curcuminoids (curcumin, demethoxycurcumin, and bisdemethoxycurcumin) that has recently been granted GRAS (Generally Recognized as Safe) status. We analyzed the pharmacokinetic profiles of both formulations in 12 subjects through a randomized, double-blind, crossover study conducted over a 12-hour period. The subjects consumed either 450 mg of total curcuminoids of AQUATURM
® or Control Curcumin Supplement. Treatments were well tolerated, and no adverse events were reported. Curcumin, demethoxycurcumin, and bisdemethoxycurcumin plasma levels were measured by UPLC-MS/MS analysis after
Helix pomatia glucuronidase/sulfatase treatment to liberate the parent compounds from sulfate and glucuronide conjugates (
Figure 2). The glucuronidase/sulfatase treatment has been shown to liberate the parent compounds from the metabolized curcuminoids, but there is evidence that this method might underestimate the amount of curcuminoids due to incomplete hydrolysis of complex metabolites [
20,
21].
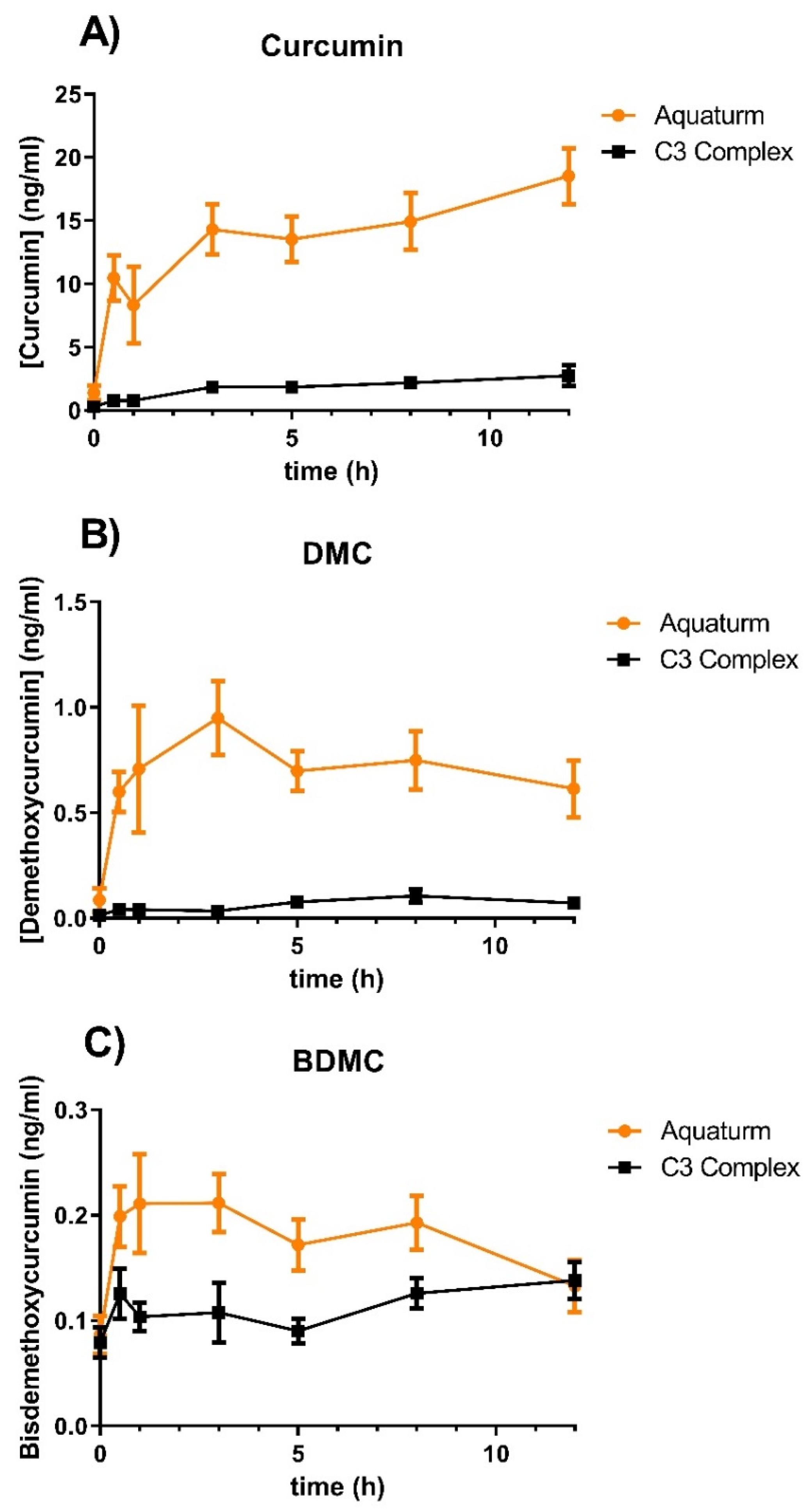
When the AUC was calculated, AQUATURM
® demonstrated a 7.3 times superior bioavailability than that of the control curcumin supplement for curcumin and in the same range for the other minor curcuminoids (
Table 1A). C
max was also higher in AQUATURM
® treated subjects compared to those given the control curcumin supplement tablet (
Table 1B).
Table 1.
A: Comparison of the Area Under the Curve between 0–12 hours (AUC 12h).
Table 1.
A: Comparison of the Area Under the Curve between 0–12 hours (AUC 12h).
| Curcuminoid |
AQUATURM® (AUC12h) |
Control curcumin tablet (AUC12h) |
Ratio of AUCs
(Drug/Control) |
Significance |
| Curcumin |
167.7 |
22.85 |
7.33 |
< 0.001 |
| DMC |
8.70 |
0.85 |
10.2 |
< 0.001 |
| BDMC |
2.17 |
1.36 |
1.59 |
< 0.001 |
| Total Curcuminoids |
178.5 |
25.1 |
7.12 |
<0.001 |
Table 1.
B: Comparison of the maximal concentration (Cmax).
Table 1.
B: Comparison of the maximal concentration (Cmax).
| Curcuminoid |
AQUATURM® (Cmax) |
Control curcumin tablet (Cmax) |
Ratio of Cmax
(Aquaturm/Control) |
| Curcumin |
18.53 |
2.74 |
6.76 |
| DMC |
0.83 |
0.07 |
11.85 |
| BDMC |
0.21 |
0.15 |
1.40 |
Subjects consumed either AQUATURM® or commercially available Control Curcumin Supplement with a content of 450 mg curcuminoids and plasma concentrations of A) Curcumin, B) Demethoxycurcumin (DMC), or C) Bisdemethoxy curcumin (BDMC) were measured at 0-, 0.5-, 1-,3-, 5-, 8- and 12-hours. Data are displayed as mean ± SEM.
Discussion
One of the important aspects of pharmacology is to study the blood and tissue concentrations of drugs and medicines over time to understand if the drug reaches a clinically useful therapeutic concentration at the site of action in the body [
22,
23,
24]. The wide array of curcumin formulations available on the market presents a significant challenge for healthcare professionals when it comes to selecting the most suitable option for their patients [
25,
26,
27]. Curcumin, a natural compound found in turmeric, has gained substantial attention for its potential health benefits, including its anti-inflammatory and antioxidant properties [
28,
29,
30]. However, curcumin’s inherent poor bioavailability—meaning the body struggles to absorb and utilize it effectively—has led to the development of numerous formulations aimed at improving its delivery and effectiveness. These formulations employ various strategies, such as the use of adjuvants, nanoparticles, or special processing techniques, to enhance curcumin’s bioavailability. While some formulations have shown promise in increasing curcumin’s absorption and bioactivity, the sheer diversity and complexity of these products can make it a daunting task for doctors to identify the best option for their patients [
15,
31,
32].
In the study presented, we have compared a novel curcumin formulation (AQUATURM
®) to a well-established and frequently used product, termed “Control Curcumin Supplement”. Our results indicate that AQUATURM
® is more than 7-fold more bioavailable than the control curcumin, reaching concentrations up to 20 ng/mL and maintaining these for up to 12 hours. Interestingly, compared to other formulations like Cavamax or Meriva curcumin, where the plasma concentration peaks as early as 30 minutes, and declines rapidly, one dose of AQUATURM
® leads to a sustained high plasma level over the 12 hours [
15].
One possible explanation for the sustained high plasma concentration could be that the absorption of AQUATURM® is delayed, allowing its elimination to occur simultaneously with its absorption and distribution throughout the body. The sustained concentration in the bloodstream of AQUATURM® may represent a pharmacokinetic advantage over other formulations of bioavailable curcumin which are cleared at much more rapid rates.
Previous pharmacokinetic studies have demonstrated similar plasma concentrations of curcumin, DMC, and BDMC. Purpura et al. 2018, used an 1800mg dose of control curcumin, measuring plasma concentrations of ~2ng/ml of curcumin, DMC and BDMC [
14]. Furthermore, Jager et al. 2014 [
15], used an 1800mg dose of control curcumin, measuring plasma concentrations of ~2ng/mL of total curcuminoids. We measured similar concentrations of total curcuminoids in plasma despite a lower dose of control curcumin (450mg).
Further study by way of clinical trials is warranted to determine specific clinically therapeutic benefits of AQUATURM®’s higher levels of bioavailability.
However, our study has some limitations. For example, the single dose study cannot anticipate the steady-state plasma concentration of a patient on any curcumin supplement taken continuously, as it may increase substantially if the patient takes it continuously and the total volume of distribution is saturated. To account for this, a future study design would include subjects to consume the novel formulation for a week before pharmacokinetic analysis. One other limitation of the study for the interpretation of the therapeutic effect in humans is the use of the de-glucuronidation / desulfatation step. While this step is useful to determine the total bioavailability of curcuminoids, it might be possible that many effects of curcuminoids are only manifested through the free, not the metabolized version, and therefore the total curcuminoid concentration might be misleading, when compared to concentrations used in cell culture studies. One interesting delivery system for drugs undergoing extensive liver metabolism could be the rectal route, as the anal veins drain directly into the systemic circulation [
5].
Conclusions
Curcuminoids are natural compounds found in turmeric that have been shown to have anti-inflammatory and antioxidant effects. The effect that the compounds could have been limited by its inherent poor bioavailability. In this study, it was shown that AQUATURM®, a novel curcumin formulation, is 7 times more bioavailable than another commonly used, commercially available curcumin supplement. Additionally, the component curcuminoids in AQUATURM®, curcumin and demethoxycurcumin, were found to be substantially more bioavailable as well. This is a promising step towards harnessing the therapeutic benefits of curcuminoids, and further study is required to show the specific therapeutic benefit of this enhanced bioavailability. Among the specific aspects which should be explored in future research are long-term safety, efficacy in different populations and different (neuro)inflammatory diseases, or comparisons with other curcumin formulations. AQUATURM® may provide a therapeutic option that allows for clinically effective plasma concentrations that can be sustained for longer periods.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
Conceptualization, G.M. and D.M.; Methodology, L.J.; Software, S.J.; Validation, L.J., S.J. and M.M.; Formal Analysis, L.J.; Investigation, G.N.; Resources, S.J.; Data Curation, L.J; Writing—Original Draft Preparation, G.M.; Writing—Review & Editing, G.N. and E.G; Visualization, G.M.; Supervision, G.M.; Project Administration, G.M.; Funding Acquisition, D.M.
Acknowledgment
The authors thank Reginal Yacob for the preliminary work on this study. The authors extend gratitude to John Sierra for their invaluable assistance in pathology collection during the trial. The PhD fellowship of LJ was covered by the Ainsworth MRFF and the GRS of Western Sydney University. The authors would like to thank the Mass Spectrometry Facility and Animal Facility at Western Sydney University for their assistance. A very special thanks to LODAAT Pharma for the donation of the product and partial support of the consumables cost.
Conflicts of Interest
None
References
- Hewlings, S.J.; Kalman, D.S. Curcumin: A Review of Its Effects on Human Health. Foods 2017, 6. [CrossRef]
- Sudarshan, K.; Yarlagadda, S.; Sengupta, S. Recent Advances in the Synthesis of Diarylheptanoids. Chem. Asian J. 2024, 19, e202400380. [CrossRef]
- Quispe, C.; Cruz-Martins, N.; Manca, M.L.; Manconi, M.; Sytar, O.; Hudz, N.; Shanaida, M.; Kumar, M.; Taheri, Y.; Martorell, M.; et al. Nano-Derived Therapeutic Formulations with Curcumin in Inflammation-Related Diseases. Oxid. Med. Cell. Longev. 2021, 3149223. [CrossRef]
- Slika, L.; Patra, D. Traditional Uses, Therapeutic Effects and Recent Advances of Curcumin: A Mini-Review. Mini-Reviews in Medicinal Chemistry 2020, 20, 1072-1082. [CrossRef]
- Zhou, X.; Venigalla, M.; Raju, R.; Münch, G. Pharmacological considerations for treating neuroinflammation with curcumin in Alzheimer’s disease. J. Neural Transm. 2022, 129, 755-771. [CrossRef]
- Ullah, F.; Gamage, R.; Sen, M.K.; Gyengesi, E. The Effects of Modified Curcumin Preparations on Glial Morphology in Aging and Neuroinflammation. Neurochem. Res. 2022. [CrossRef]
- Perez-Fernandez, V.; Thananjeyan, A.L.; Ullah, F.; Münch, G.; Cameron, M.; Gyengesi, E. The effects of a highly bioavailable curcumin Phytosome(TM) preparation on the retinal architecture and glial reactivity in the GFAP-IL6 mice. Front. Ophthalmol. (Lausanne) 2023, 3, 1205542. [CrossRef]
- Ghosh, S.; Banerjee, S.; Sil, P.C. The beneficial role of curcumin on inflammation, diabetes and neurodegenerative disease: A recent update. Food Chem. Toxicol. 2015, 83, 111-124. [CrossRef]
- Aggarwal, B.B.; Gupta, S.C.; Sung, B. Curcumin: an orally bioavailable blocker of TNF and other pro-inflammatory biomarkers. Br. J. Pharmacol. 2013, 169, 1672-1692. [CrossRef]
- Wahlstrom, B.; Blennow, G. A study on the fate of curcumin in the rat. Acta Pharmacol. Toxicol. (Copenh.) 1978, 43, 86-92.
- Akanchise, T.; Angelova, A. Potential of Nano-Antioxidants and Nanomedicine for Recovery from Neurological Disorders Linked to Long COVID Syndrome. Antioxidants (Basel) 2023, 12. [CrossRef]
- Racz, L.Z.; Racz, C.P.; Pop, L.C.; Tomoaia, G.; Mocanu, A.; Barbu, I.; Sarkozi, M.; Roman, I.; Avram, A.; Tomoaia-Cotisel, M.; et al. Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin. Molecules 2022, 27. [CrossRef]
- Pan-On, S.; Dilokthornsakul, P.; Tiyaboonchai, W. Trends in advanced oral drug delivery system for curcumin: A systematic review. J. Control. Rel. 2022, 348, 335-345. [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G.; et al. Oral curcumin for Alzheimer’s disease: tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer’s Res. Ther. 2012, 4, 43. [CrossRef]
- Purpura, M.; Lowery, R.P.; Wilson, J.M.; Mannan, H.; Münch, G.; Razmovski-Naumovski, V. Analysis of different innovative formulations of curcumin for improved relative oral bioavailability in human subjects. Eur. J. Nutr. 2018, 57, 929-938. [CrossRef]
- Jager, R.; Lowery, R.P.; Calvanese, A.V.; Joy, J.M.; Purpura, M.; Wilson, J.M. Comparative absorption of curcumin formulations. Nutr. J. 2014, 13, 11. [CrossRef]
- Cuomo, J.; Appendino, G.; Dern, A.S.; Schneider, E.; McKinnon, T.P.; Brown, M.J.; Togni, S.; Dixon, B.M. Comparative absorption of a standardized curcuminoid mixture and its lecithin formulation. J. Nat. Prod. 2011, 74, 664-669. [CrossRef]
- Wakabayashi, M.; Fishman, W.H. A method for freeing Helix pomatia beta-glucuronidase of sulfatase by simple heat denaturation. Biochim. Biophys. Acta 1961, 48, 195-197.
- Vareed, S.K.; Kakarala, M.; Ruffin, M.T.; Crowell, J.A.; Normolle, D.P.; Djuric, Z.; Brenner, D.E. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol. Biomarkers Prev. 2008, 17, 1411-1417. [CrossRef]
- Luis, P.B.; Kunihiro, A.G.; Funk, J.L.; Schneider, C. Incomplete Hydrolysis of Curcumin Conjugates by beta-Glucuronidase: Detection of Complex Conjugates in Plasma. Mol Nutr Food Res 2020, 64, e1901037. [CrossRef]
- Stohs, S.J.; Chen, C.Y.O.; Preuss, H.G.; Ray, S.D.; Bucci, L.R.; Ji, J.; Ruff, K.J. The fallacy of enzymatic hydrolysis for the determination of bioactive curcumin in plasma samples as an indication of bioavailability: a comparative study. BMC Complement. Altern. Med. 2019, 19, 293. [CrossRef]
- Steinijans, V.W. Pharmacokinetic characterization of controlled-release formulations. Eur. J. Drug Metab. Pharmacokinet. 1990, 15, 173-181. [CrossRef]
- Evans, W.E. Clinical pharmacodynamics of anticancer drugs: a basis for extending the concept of dose-intensity. Blut 1988, 56, 241-248. [CrossRef]
- Sawe, J. High-dose morphine and methadone in cancer patients. Clinical pharmacokinetic considerations of oral treatment. Clin. Pharmacokinet.1986, 11, 87-106. [CrossRef]
- Matthewman, C.; Krishnakumar, I.M.; Swick, A.G. Review: bioavailability and efficacy of ‘free’ curcuminoids from curcumagalactomannoside (CGM) curcumin formulation. Nutr. Res. Rev. 2023, 1-18. [CrossRef]
- Storka, A.; Vcelar, B.; Klickovic, U.; Gouya, G.; Weisshaar, S.; Aschauer, S.; Bolger, G.; Helson, L.; Wolzt, M. Safety, tolerability and pharmacokinetics of liposomal curcumin in healthy humans. Int. J. Clin. Pharmacol. Ther. 2015, 53, 54-65. [CrossRef]
- Xiao, Y.; Chen, X.; Yang, L.; Zhu, X.; Zou, L.; Meng, F.; Ping, Q. Preparation and oral bioavailability study of curcuminoid-loaded microemulsion. J Agric. Food Chem. 2013, 61, 3654-3660. [CrossRef]
- Vieira, B.M.; Caetano, M.A.F.; de Carvalho, M.T.; Dos Santos Arruda, F.; Tome, F.D.; de Oliveira, J.F.; Soave, D.F.; Pereira, J.X.; Celes, M.R.N. Impacts of Curcumin Treatment on Experimental Sepsis: A Systematic Review. Oxid. Med. Cell. Longev. 2023, 2023, 2252213. [CrossRef]
- Supriyadi, R.; Koswara, M.I.A.; Soelaeman, M.A.; Huang, I. The effect of antioxidants supplementation on oxidative stress and proinflammatory biomarkers in patients with chronic kidney disease: a systematic review and meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 1413-1426. [CrossRef]
- Benameur, T.; Frota Gaban, S.V.; Giacomucci, G.; Filannino, F.M.; Trotta, T.; Polito, R.; Messina, G.; Porro, C.; Panaro, M.A. The Effects of Curcumin on Inflammasome: Latest Update. Molecules 2023, 28. [CrossRef]
- Balakumar, P.; Alqahtani, T.; Alqahtani, A.; Lakshmiraj, R.S.; Singh, G.; Rupeshkumar, M.; Thangathirupathi, A.; Sundram, K. A Unifying Perspective in Blunting the Limited Oral Bioavailability of Curcumin: A Succinct Look. Curr. Drug Metab. 2022, 23, 897-904. [CrossRef]
- Ullah, F.; Liang, A.; Rangel, A.; Gyengesi, E.; Niedermayer, G.; Münch, G. High bioavailability curcumin: an anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch. Toxicol. 2017, 91, 1623-1634. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).