1. Introduction
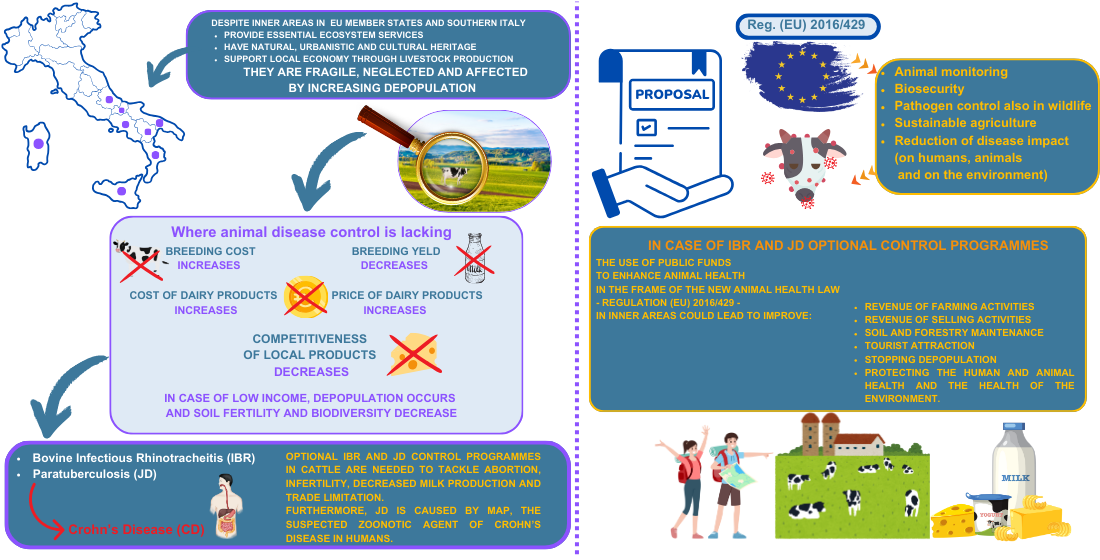
In 2024, the Italian National Institute of Statistics (ISTAT) and the European agency EUROSTAT identified the Italian regions of Abruzzo, Molise, Campania, Puglia, Basilicata, Calabria, Sicily, and Sardinia as comprising Southern Italy, based on more than just geographical criteria. For these regions, the Department for Cohesion Policies and the South [
1] offers development opportunities targeting inner areas. Defined by the former Territorial Cohesion Agency [
2], which was responsible for promoting the National Strategy for Inner Areas (SNAI), [
3], these areas are characterized as fragile, remote from major service centers, and often neglected. They cover 60% of the national territory, include 52% of municipalities, and are home to 22% of the population. The primary objectives for these areas include combating depopulation, revitalizing the local economy, and increasing income by leveraging natural and recreational assets, agri-food vocations, and tourism.
Animal health criteria are crucial to achieving these aims, particularly concerning the presence of infectious diseases in livestock, which decrease productivity and reproductivity and cause economic loss, as well as zoonoses that pose risks to humans and impact food safety.
The European Commission (EC), supported by the European Food Safety Authority (EFSA), conducted a systematic evaluation of diseases, assessing several factors: species susceptibility, disease reservoirs and vectors, prevalence within the European Union (EU), routes of transmission (both among animals and from animals to humans), and the potential impact on human and animal health, including morbidity and mortality rates. Information from the World Organisation for Animal Health (WOAH) was also considered.
The recent enactment of the new animal health legislation, Regulation (EU) 2016/429 [
4], reorganizes and updates veterinary regulations and introduces a modern approach to safeguarding both farmed and wild fauna. It establishes comprehensive guidelines for monitoring, eradicating, and maintaining the disease-free status of animal diseases listed in it. The main goals of these measures are to enhance the health and safety of farm animals, safeguard food products, control zoonotic disease transmission, and prevent wildlife from becoming a source of infection spread. Key aspects of the new regulation are the emphasis on animal traceability, biosecurity practices, and wildlife pathogen control, sustainable farming promotion and reduction of the impact of diseases on both public and animal health and the environment. Vaccination strategies will only be endorsed if proven economically viable.
The Commission Implementing Regulation (EU) 2018/1882 [
5] categorizes diseases into five distinct groups (Categories A through E), each requiring specific actions ranging from immediate eradication to ongoing surveillance, establishing that the prevention and control measures outlined in Article 9, paragraph 1, of Regulation (EU) 2016/429 apply to these Categories for the species and groups listed in its annex.
Furthermore, Commission Delegated Regulation (EU) 2020/689 [
6] supports these efforts by outlining the disease control measures to be taken when Category B or C diseases are detected in areas officially designated as disease-free. Subsequently, Commission Implementing Regulation (EU) 2021/620 defined rules for the approval of the disease-free and non-vaccination status and the approval of eradication programmes [
7].
The growing focus on Infectious Bovine Rhinotracheitis / Infectious Pustular Vulvovaginitis (IBR/IPV), a Category C disease – a disease of significance for certain Member States (MSs), for which measures are necessary to prevent its spread to parts of the EU that are officially disease-free or have ongoing eradication programmes in place - is prompting the expansion of eradication programmes though these are not yet uniformly applied across all EU MSs, leading to trade restrictions [
8]. This situation underscores the urgent need to adopt optional control programmes to bring the zoo-economy of inner areas up to par with more advanced MSs, thereby reducing income loss due to abortion, infertility, decreased milk production, and commercial limitations that IBR/IPV causes.
Additionally, trade restrictions are increasing for animals and products from farms not free of Ruminant Paratuberculosis or Johne’s Disease (JD), a progressive and fatal disease of ruminants, classified among Category E Diseases, meaning a disease listed for monitoring, for which surveillance within the EU is necessary, but it is not required to implement control plans [
5].
Since September 2022, Italy has been aligning with these updated rules, presenting an opportunity to integrate the reassessment of inner areas with the control of animal infectious diseases and zoonoses. This approach can support extensive farming making it economically sustainable while fully aligning with the EU Green Deal [
9] and its relaunched Farm to Fork Strategy [
10], and the overarching One Health approach [
11], aimed at promoting environmental and climate protection, enhancing long-term soil fertility, supporting biodiversity, and ensuring high animal welfare standards. Furthermore, Italy aims to cut antibiotic use in farm animals (and aquaculture) by 50% by 2030 implementing the ClassyFarm system [
12] created for the Italian Farms, that facilitates data collection on biosecurity, animal welfare, health, and antimicrobial use to counter antimicrobial resistance in animals and humans.
2. Infectious Bovine Rhinotracheitis / Infectious Pustular Vulvovaginitis
IBR/IPV is a viral disease having as etiological agent the Bovine herpesvirus 1 (BoAHV-1,
Bovine alphaherpesvirus 1), [
13], worldwide distributed (Americas, Australia, New Zealand, Asia, Africa and many EU MSs) [
14], that affects domestic and wild cattle, but has no zoonotic potential [
15].
IPV, which primarily spreads through sexual transmission and remains latent in the sacral ganglia, seems the original pathology caused by BoHV-1. Over time, this virus increased its virulence for the respiratory tract, due to the selective pressure exerted in feedlot cattle in the United States of America during 1955-1956, where accumulation of animals allowed numerous and rapid passages [
16]. Nowadays, where natural mating is practiced, genital infection can lead to pustular vulvovaginitis or balanoposthitis [
17], whereas the respiratory form leads to IBR.
Transmission routes of IBR include:
- -
vertical transmission (transplacental, transovarian, spermatic, and via colostrum and milk), related to reproductive events;
- -
horizontal transmission, independent of the sex and age of animals. The virus, present in all secretions of infected animals—particularly nasal discharge—is transmitted through direct and indirect contact (aerosol) with infected animals, contaminated surfaces, objects, and confined air (e.g., trucks).
The virus enters through the oro-nasal and oculo-conjunctival mucosa, replicating primarily in the mucosa of the upper respiratory tract and the amygdalae. It then disseminates via cell-mediated viremia and anterograde axonal transport. In the trigeminal ganglia, it establishes a latent phase (during latency, the virus exists as an episome and its genome does not integrate into the host cell’s DNA) that persists for the animal's lifetime.
Reactivation and release of complete virions are triggered by both exogenous and endogenous stress.
Clinical signs of primary infection include:
- -
respiratory symptoms: fever, nasal discharge initially serous and later muco-purulent, confluent erosive-necrotic lesions with exudate that narrows the tracheal lumen;
- -
additional symptoms: hypersalivation, conjunctivitis, enteric pathologies that can cause mortality in young animals, and anorexia.
Reactivation of latent infection typically results in decreased milk production, abortion during the 5th-8th month of pregnancy, and infertility.
Latency, during which viral glycoproteins are produced and displayed on the cellular membrane of infected cells, continuously stimulates the immune system of infected animals. This makes serological methods highly effective for monitoring infected animals and managing the maintenance of IBR-free status in herds [
17].
IBR – Eradication and Control Programmes in Italy
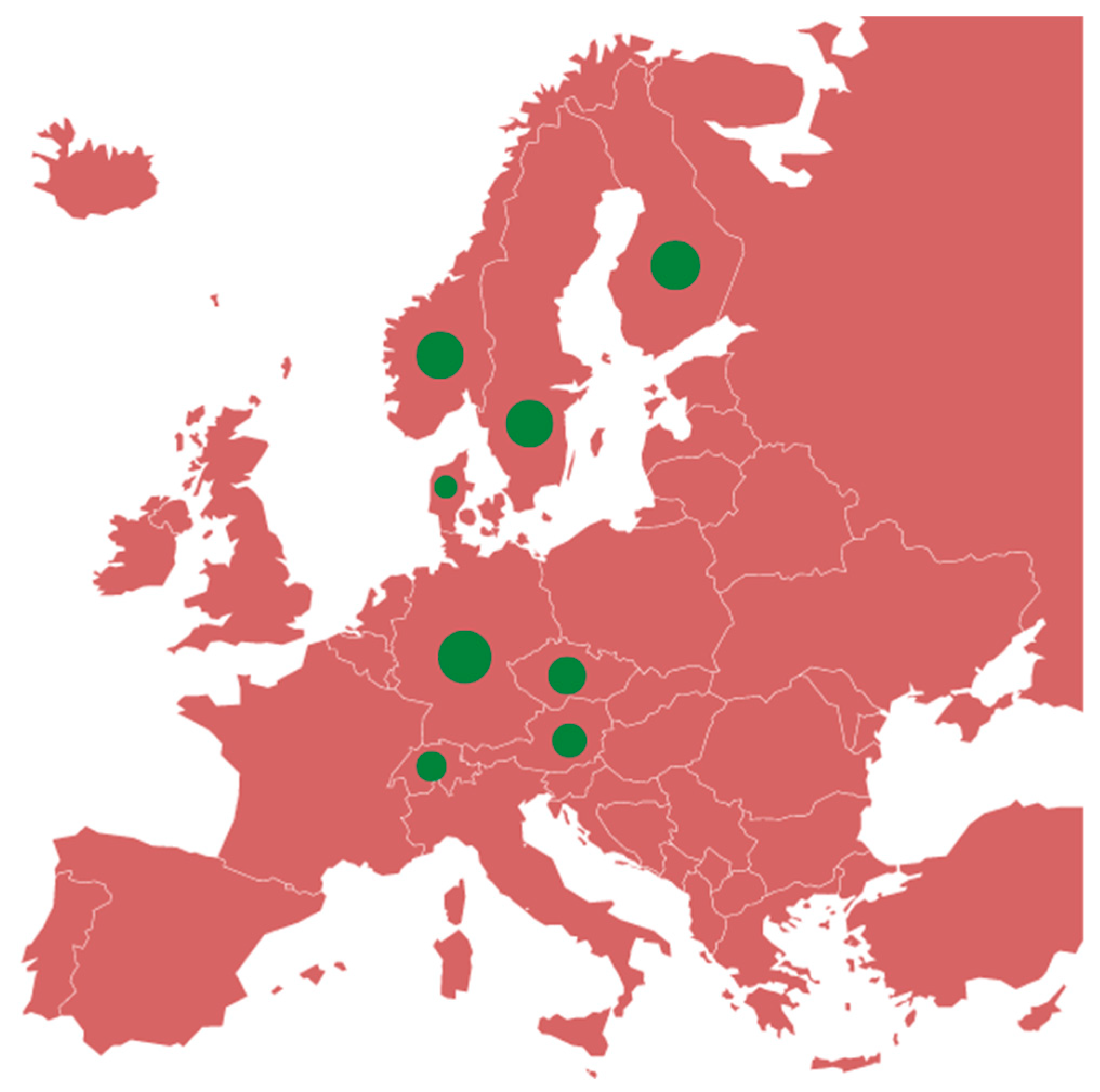
IBR / IPV, subject to the prevention and control measures contained in Article 9 of Regulation (EU) 2016/429, has been classified among the Category C diseases. For these diseases, MSs can optionally adopt national eradication programmes for prevention and control purposes. The geographical distribution of IBR-free countries is characteristically North-South oriented, with EU and European Economic Area (EEA) Member States bordering or neighboring (
figure 1), [
7,
18].
Commission Implementing Regulation (EU) 2018/1882 classifies IBR/IPV as a Category C+D+E infectious disease under control in Bison spp., Bos spp., and Bubalus spp., and as a Category D+E infectious disease under control in Camelidae and Cervidae.
At the national level, according to Article 13, paragraph 1, letter b), of Italian Legislative Decree 136/2022, optional national eradication programmes for category C diseases, where the national territory is not entirely or specifically free of the disease in certain zones or compartments, must be established by the Ministry of Health to ensure a uniform level of animal health protection.
In this regard, as reported on January 31, 2023, the National Center for the Fight and Emergency against Animal Diseases of the Animal Health and Veterinary Medicines Organizational Unit of the Directorate for Prevention, Food Safety, and Veterinary Affairs of the Italian Ministry of Health, decided to prepare a National IBR Eradication Plan for 2024, which is currently being developed.
It is noted that this planning also considers the list of MSs or their individual zones or compartments/establishments that have already obtained the disease-free status or have received approval for an eradication programme under Regulation (EU) 2020/689 and Regulation (EU) 2021/620.
Specifically, regarding IBR, some EU MSs – and some European countries - are already free from IBR: Czekia, Denmark, Germany, Austria, Finland, Sweden [
18].
In Italy, only the Valle d’Aosta Region and the Autonomous Province of Bolzano are IBR-free. The Friuli Venezia Giulia Region and the Autonomous Province of Trento have approved eradication programmes. All these Regions are located in Northern Italy.
Other regions are either implementing IBR control plans or participating in IBR programmes managed by ANABIC (National Association of Italian Beef Cattle Breeders) and ANABORAPI (National Association of Piemontese Cattle Breeders). These programmes, activated in 2015 and 2016 respectively, focus on animals registered in the Herd Books of Italian Beef Breeds (Marchigiana, Chianina, Romagnola, Maremmana, Podolica, and Piemontese), which make up only 7% of Italy's cattle population.
Programmes generally include serological monitoring to detect infected animals and identify negative animals, even vaccinated, when vaccination occurs using a g-E deleted marker vaccine, useful in the Differentiation of Infected and Vaccinated Animals (DIVA) system; occasionally, further analyses could carried out to more accurately confirm the obtained results in case of positive outcomes in IBR-free territories, that could represent false positive results [
19,
20].
3. Ruminant Paratuberculosis / Johne’s Disease (JD)
JD is a progressive chronic infectious inflammatory bowel disease caused by
Mycobacterium avium subspecies
paratuberculosis (MAP) that include as specific genomic element the insertion sequence IS900 [
21,
22]; it survives in the environment for long periods without replicating, being an obligate intracellular pathogen of mammals, which infects many species of susceptible domestic and wild ruminants, including deers.
Transmission routes concern the horizontal transmission through the fecal-oral route in which juvenile animals can ingest MAP contained in the fecal material from infected and shedding animals having significant amount of MAP, that contaminates materials and environments, also inducing fecal contamination of the teats of lactating females. Vertical transmission can occur as transplacental, spermatic, and through colostrum and milk from infected cows. Young animals are highly susceptible. In cattle, the risk of infection decreases after six months of age.
The infection has a long incubation period.
MAP enters orally and targets the tissue of the intestinal mucosa and submucosa in the ileum and jejunum, with a particular focus on the M cells of the Peyer’s patches. This targeting allows MAP to invade and proliferate within the intestinal macrophages. Subsequently, MAP colonizes the entire intestine, causing lymphatic vessels dilatation, and enlargement of mesenteric lymph nodes, chronic granulomatous enteritis, with thickening and inflammation of the terminal ileal mucosa due to lymphocytic infiltrates (granulomatous enteritis). In cattle suffering from Johne's Disease (JD), notable pathological alterations include the thickening and corrugation of the intestinal walls, which impairs nutrient absorption, protein loss, malabsorption syndrome, weight loss, muscle weakness, decreased milk yield, hypoproteinemia, intermandibular edema (bottle jaw). The initially intermittent and unresponsive watery diarrhea becomes chronic. MAP then spreads throughout the organism, causing symptoms of generalized infection: anemia, infertility, emaciation; the animal dies in a cachectic state. The clinical signs appear in the late stages of the infection; consequently, MAP can persist undetected for many years at the herd level, resulting in its spreading among the livestock.
The host immune response is cellular in the initial stage of the disease and so it is detectable highlighting the delayed type IV hypersensitivity through interferon-γ release assay and intradermal test (skin test); the production of antibodies starts later, allowing the detection of infected animals through routine serological tests (indirect-ELISA) [
23,
24,
25,
26,
27,
28].
Recently, a new ELISA method was developed in the aim to differentiate infected animals, even when vaccinated, in accordance with the DIVA system. It uses synthetic lipids, it is not affected by interference from vaccinations, and it is able to detect nearly twice as many PCR-positive cases compared to the commercial serodiagnostic tools, potentially allowing for earlier identification of infections [
29].
In domestic ruminants, JD leads to economic losses (including reduced milk production, increased somatic cell counts that interfere with milk quality parameters, increased incidence of clinical mastitis, reduced fertility, and increased susceptibility to other diseases) and reduced slaughter value [
30,
31,
32].
The economic impact of paratuberculosis in a cattle herd will depend on the number of animals affected, infected and infectious. Animal health has also become a talking point in climate change mitigation strategies such as the European Green Deal as life cycle analysis suggests that greenhouse gas emissions in dairy cows with JD is up to 25% higher than healthy animals [
33].
The point 2 of Annex I of the Directive 2003/99/EC [
34] on the monitoring of zoonoses and zoonotic agents reporting mycobacteria other than in point A - tuberculosis due to
Mycobacterium bovis, should include, specifically, MAP infections.
MAP and Crohn’s Disease
The importance of MAP in the livestock sector in terms of economic losses and animal welfare is further amplified by its putative zoonotic potential.
Crohn’s Disease (CD) is an inflammatory bowel disease in humans, characterized by chronic granulomatous inflammation that can affect the entire gastrointestinal tract. Since the last century, MAP has been suspected of being the causative agent or an initial promoter of CD. Similarities between CD and JD are based on the attitude of MAP to contribute to inflammation and alteration of the gut microbiome triggering inflammatory cascade, caused by the shifting from a Gram-positive aerobic environment to a Gram-negative anaerobic one. The decrease in bacterial diversity leads to dysbiosis and to diseases, ranging from gastrointestinal (specifically associated with CD) to neurologic disorders, since there are evidences suggesting a connection between the brain and the gut microbiome: psychological stressors have been associated with changes in gut microbiota via cytokine release; the pathogenic bacteria can trigger vagal nerve activation; on the other hand, reconstituting the microbiota can normalize Hypothalamic–Pituitary–Adrenal axis levels. For chronically ill patients who are susceptible to psychological stress, then, it is crucial to involve mental health professionals [
35,
36]. A critical role played by bacteria in the human gut is the production of short chain fatty acids (SCFA) - a major player in maintenance of gut and immune homeostasis - through fermentation of polysaccharides that are critical to intestinal inflammation, and poor healing [
36,
37].
MAP is part of the Mycobacterium avium complex (MAC), where bacteria exhibit a spectrum of pathogenic potential, from commensal organisms to opportunistic and obligate pathogens. MAC members have been associated with transient asymptomatic human carriage, and as a result, MAP is not considered a human pathogen by itself, but its infection may lead to colonization, persistence, and latency.
Although some similarity between JD and CD, the precise nature of their association remains unclear, since MAP is typically found in cell-walled, acid-fast forms in ruminants, whereas in humans it exists predominantly as Cell Wall Deficient Mycobacteria (CWDM), also referred to as spheroplasts or L forms.
Furthermore, MAP is categorized under Non-Tuberculous Mycobacteria (NTM), also known as Mycobacteria Other Than tuberculosis (MOTT), in contraposition to Mycobacterium tuberculosis Complex including pathogenic microorganisms as M. tuberculosis, M. bovis and M. caprae. NTM range from environmental saprophytes to obligate parasites that persist within macrophage lysosomes, where they survive by inhibiting phagolysosome maturation and persist as CWDM.
Since MAP exists in a CWDM form in humans, the same culture methods used for the cell-walled JD may not be successful in cultivating it. MAP is the slowest-growing known Mycobacterium, requiring up to 16 weeks to reproduce, and even longer (up to 18 months) in human blood cultures. The requirement to culture microorganisms from human specimens until mycobacterial reversion of CWDM occurs—when CWDM redevelops a cell wall—and the evidence that MAP isolates with a cell wall are rare in CD make it unlikely that cell-walled MAP is a pathogen in humans. Since the gold standard for reliably culturing MAP in humans is lacking, and in the absence of reliable methods for routine bacteriological diagnosis, proving MAP's pathogenicity in CD remains a challenge.
Probes targeting MAP-specific genes and insertion sequences (IS) have been developed to detect and identify MAP more accurately in human specimens. The previously used IS900 was found to be nonspecific, while the F57 sequence is more specific for MAP. However, the F57 gene has only one copy per organism compared to the 15–17 copies of the IS900 sequence, which negatively impacts the PCR detection rate. Studies comparing the detection rates of MAP using PCR in patients with CD versus healthy controls have observed transient carriage of MAP in healthy controls. Furthermore, patients with CD have seven times the odds of harboring MAP in their blood or gut tissues compared to those without the disease. A recently developed Ziehl–Neelsen (ZN) staining method has been introduced to detect CWDM in resected tissues of CD patients. Through this new staining process, CWDM was detected in all 18 tissue samples from CD patients, whereas none of the 15 control samples from individuals without inflammatory bowel disease showed any trace of CWDM [
38].
Both CD and JD share the ability to trigger granuloma formation and T-cell responses, which are central to inflammation in CD and serve as protective mechanisms against intracellular pathogens in humans. Current immunotherapies in CD target the overexpression of cytokines such as interleukins (IL-1, IL-6) and Tumor Necrosis Factor alpha (TNF-α), while underexpression of IL-10 mirrors the immune response to mycobacteria. TNF-α is crucial for clearing intracellular pathogens and controlling mycobacteria by enhancing T-cell responses, promoting macrophage activation, and facilitating CD4+ T-cell immunity. MAP has mechanisms to evade immune responses, such as inducing IL-10 production, which inhibits TNF-α and helps create an intracellular sanctuary. This environment prevents the clearance of mycobacteria from infected macrophages. The similarities between cytokine imbalances in CD and those seen in JD suggest a possible connection between the two diseases, as both show similar immune response patterns. Despite that, studies have shown no significant increase in CD rates among farmers and veterinarians regularly exposed to MAP-infected cattle [
35]. The general prevalence of CD is about 0.3% in developed countries (320 patients / 100.000 inhabitants), with minor increases observed in subgroups with greater exposure to JD. Some studies even report lower rates of CD in farmers and veterinarians compared to the general population, challenging the hypothesis that MAP causes CD. From a microbiological perspective, a defective immune system combined with early exposure to MAP could increases the risk of developing CD. Furthermore, the parallel increase in JD and CD supports the hypothesis that CD may be driven by a genetic susceptibility combined with exposure to an animal pathogen.
In animals, susceptibility to MAP is generally age-dependent, with early exposure being crucial for disease development later in life. The increasing incidence of CD in children may suggest other environmental factors at play, such as increasing virulence of MAP or effects of dysbiosis. Westernized diets and lifestyles, which reduce microbiota diversity, may contribute to immune susceptibility and the rising incidence of CD.
MAP is detectable in rural environments and present in various food and water supplies, making it difficult to distinguish between different modes of exposure. The presence of MAP in retail powdered baby formula and its potential role in early exposure raises interesting epidemiological questions, especially given that breastfeeding appears protective against CD [
35]. It is thus notable that MAP has been detected in raw milk from domestic animals in developed countries including, Czech Republic (2%), Ireland (0.3%), UK (6.9%), USA (0–28.6%). There is debate as to how pasteurisation inactivates MAP [
31,
39], since positive pasteurized milk samples were found positive for cultivable MAP [
40].
Since the supposed multi-step process that consists of MAP infection, dysbiosis of the gut microbiome, and dietary influences, implementing combination therapies are needed, such as antibiotics, vaccination, faecal microbiota transplants (FMT) and dietary plans. Furthermore, gene therapy could be used for remediation of aberrant immune responses [
35,
38,
41]. Despite progress in multimodal medical treatments, the progression of CD often leads to multiple surgeries, which are associated with significant morbidity. Surgical interventions are determined by the prevailing disease pathology. For intra-abdominal cases, common procedures include resection (removal of the diseased bowel section), stricturoplasty (widening of the bowel to alleviate pathological and symptomatic narrowing while maintaining bowel length), and fistulectomy (removal of abnormal connections between the bowel and nearby organs or skin). An important area of interest is understanding how surgery impacts the microbiome and influences disease progression and surgical outcomes. While surgery can be curative in rare cases, particularly when the disease is confined to the ileocecal region, it is generally considered only after medical treatment options have been exhausted. Ultimately, 75% of CD patients will require surgical resection, and half of those who undergo an initial surgical procedure will need additional operations [
36]. Eradication of CWDM remains challenging due to resistant strains and the non-curative nature of Atypical Mycobacterial Antibiotic Therapy (AMAT), with its optimal duration unknown and no placebo-controlled RCTs conducted due to ethical concerns, although AMAT has shown statistical benefit in inducing [
42] and maintaining remission [
35]. Clinical research has produced mixed findings regarding the effectiveness of anti-MAP therapy in improving outcomes for CD, resulting in its exclusion from evidence-based clinical guidelines [
43]. MAP has also been associated with a number of autoimmune diseases in humans, including rheumatoid arthritis, autoimmune thyroiditis, Blau syndrome, multiple sclerosis, autoimmune Type 1 diabetes - associated with early-life dietary exposure to cow’s milk - due to the production of autoantibodies triggered by MAP's heat shock protein (HSP65). These antibodies cross-react with pancreatic glutamic acid decarboxylase (GAD), behaving as anti-GAD antibodies that destroy the insulin-producing cells in the pancreatic islets. [
33,
44,
45]. On the other hand, some researchers call for action: the medical and research communities must move forward collectively, recognizing MAP as a zoonotic mycobacterial pathogen, so that it becomes imperative to eliminate MAP from livestock, reduce its presence in the food supply, develop vaccines and antibiotic treatments for humans [
46].
Despite the lack of conclusive evidence to date, the zoonotic potential of MAP is an ongoing concern [
33].
JD Control Programmes in Italy
Due to the economic importance (
figure 2), [
47], and potential public health threat, most European countries and MSs have established JD control programmes, mainly for cattle.
These programmes are based on a testing and culling strategy along with enhanced management strategies to mitigate risks on farms, avoiding the use of vaccines in cattle that may interfere with both intradermal and serological tests for tuberculosis diagnosis, complicating mandatory tuberculosis control programmes [
48].
In 2013, in Italy the State-Regions Conference approved the “Guidelines for the Control and Attribution of Health Status Regarding Paratuberculosis,” prepared by the National Reference Centre for Paratuberculosis established at the Experimental Zooprophylactic Institue of Lombardia and Emilia Romagna (Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia Romagna - IZS LER) and shared with the Regions, the Autonomous Province (A. P.) of Trento and the A.P of Bolzano, and breeder associations. The approved Guidelines for the control and health certification of cattle herds included: mandatory notification of clinical cases and elimination of infected animals; protection of calves; periodic controls carried out testing of the remaining animals over 36 months old, with sanitary restrictions on seropositive animals; voluntary adoption of the Control Plan.
The Guidelines highlighted the zoonotic nature of the infection, suspected to cause CD. Herds that tested negative in initial controls were classified as PT1 and PT2. Increasing levels of health certification, from PT3 to PT5, were based on continuous seronegativity over time. The certification process was lengthy and complex. In Molise, the JD Control Plan has not been activated, compromising the commercialization of dairy products in foreign disease-free countries that require certifications of herd health.
The aforementioned Guidelines were most recently updated in compliance with the provisions of Regulation (EU) 2016/429 and Implementing Regulation (EU) 2018/1882 that classifies JD as a Category E disease requiring surveillance within the EU in Bison spp., Bos spp., Bubalus spp., Ovis spp., Capra spp., Camelidae, and Cervidae.
In this context, and considering JD endemicity in Italy, more sensitive protocol are needed to reduce the occurrence of unexpected positivity in previously repeatedly negative farms. Since the application of the pre-existing guidelines revealed certain issues (e.g., underreporting of clinical cases and the lower robustness of milk tests compared to blood tests) and the Regions and A. P. of Trento and Bolzano submitted a requested for modification, the Italian Government - after obtaining the opinion of the National Reference Centre for Paratuberculosis - approved the document
Guidelines for the adoption of control plans and for assigning health status regarding paratuberculosis to farms of susceptible species (cattle, buffalo, sheep, goats) [
49].
The objectives pursued through the application of the new guidelines are as follows:
implement surveillance, pursuant to Regulation (EU) 2016/429, on cases of paratuberculosis in Italian establishments of susceptible species (cattle, buffalo, sheep, and goats);
enable certification for the informed trade of animals and their products through a risk-based classification of establishments;
provide breeders with tools to prevent the introduction of Mycobacterium avium subsp. paratuberculosis infection into their establishments;
provide breeders with tools for controlling the infection in infected establishments.
The guidelines include two annexes detailing:
- -
the minimum requirements for drafting a company control plan for paratuberculosis in infected establishments;
- -
the health status of cattle, buffalo, sheep, and goat establishments.
The Control Plan for paratuberculosis in infected establishments is based on the risk assessment of further introduction and spread of the infection within the establishment. It can be drafted in collaboration with the farm veterinarian - which signs it - using the tools and manuals prepared by the National Reference Centre for Paratuberculosis.
4. Impact of Animal Infectious Diseases on Mountain Socio-Ecological Systems
Molise is a predominantly mountainous region, and mountain socio-ecological systems (SES) are crucial for providing essential ecosystem services, such as freshwater supply, carbon storage, and biodiversity preservation. These systems also support local economies through activities as livestock production and forestry, contributing to food production, cultural heritage, and recreational opportunities. The dairy sector, particularly in mountain pastures, plays a significant role, integrating natural and human elements into the milk value chain [
50]. Permanent grasslands are vital for producing high-quality milk, essential for local cheese production.
However, these areas face challenges from climate change, depopulation, soil degradation, and invasive species. Sustainable management of these lands is critical for maintaining ecological balance and supporting mountain livelihoods.
SES in mountain regions involve complex interactions between natural and socio-cultural processes, providing essential services for both upland and lowland communities. These areas require development strategies that balance vulnerability with the value of natural and cultural resources, emphasizing an integrated, asset-based approach.
Despite their ecological and socioeconomic importance, mountain areas are often seen as disadvantaged. Therefore, policies should move beyond agriculture-focused strategies and adopt a place-based approach to development, considering both local specifics and broader opportunities.
The conservation of pastures is key to revitalizing vulnerable mountain areas, which face challenges like the abandonment of agriculture, environmental degradation, economic decline, and depopulation [
51].
The resilience of these regions depends on recovering socio-economic structures rooted in traditional practices, particularly extensive livestock farming. This approach supports high-quality dairy production, tourism, and local economies.
All aspects of this value chain should be managed with the One Health approach, aligning with the EU Green Deal and other European policies, ensuring that animal, human, and environmental health are integrated into sustainable development strategies.
In this scenario, animal health is of utmost importance and central to these strategies, particularly in managing diseases like IBR and JD, which are not yet under mandatory control but significantly impact livestock health and market competition.
5. Discussion
Countries that had previously been isolated from trading ruminant livestock were free from Johne’s disease but faced outbreaks after unknowingly importing infected animals. In these regions, the rise of CD later served as a subtle indicator of MAP infection within the animal population [
44]. In this view, it is hoped that the inner areas of southern regions of Italy, including Molise, which are traditionally involved in dairy cattle farming and typical dairy production, will take advantage of the new EU rules concerning animal health integrating eradication programmes for IBR and JD, which requires a review of control activities. The activation of such programmes in few Italian Regions and A. P. has shown that achieving health certification can be a lengthy process. Their initiation, adopting the One Health approach [
52], is therefore urgent and imperative for the protection of the local economy related to livestock, dairy products and to the environment, beyond the protection of the cultural and traditional heritage. Furthermore, the inclusion of the parameter “Economic Health” in the One Health approach could address the individuation of territories economically unable to conduct eradication and control programmes, in the aim to hasten EU, national and local institutions to finance them [
53,
54,
55]
.
In this scenario, it is important to avoid the dispersion of public funds aimed at increasing livestock activities in inner areas while neglecting the fundamental control of infectious diseases. Control programmes are essential, as infectious diseases limit the profitability of farming and, in some cases, pose a threat to human health, including farmers who have to bear medical care costs. Additionally, strong commercial competition may arise due to the growing number of neighbouring territories implementing optional programmes. These territories will become economically advantaged both through higher farm productivity and through the imposition of trade restrictions outlined in the aforementioned regulations, aimed at preventing the reintroduction of eradicated or under-eradication infectious diseases. All of these factors will inevitably lead to a concerning decline in the profitability of livestock and agri-food businesses that have not promptly initiated optional control programmes.
6. Future Perspectives
The current allocation of funds from the Italian South Funds and the Italian National Recovery and Resilience Plan (Piano Nazionale di Ripresa e Resilienza - PNRR) offers a valuable opportunity to address the complex and costly implementation of animal health programmes. These funds could provide essential financial support to farmers in inner areas, particularly those with smaller herds, who often view the cost associated with JD control programmes as prohibitive due to testing expenses and the requirement for separate calving areas for infected cows [
56,
57,
58]. Similarly, in absence of basic knowledge about IBR infection - which requires farmers to have the ability to correlate a respiratory disease of calves with subsequent abortion and infertility in adult cows - the cost for IBR plans is considered disproportionate.
The PNRR, a strategic initiative developed by Italy - that focuses on critical issues such as green transition, digitalization, infrastructure, education, and social inclusion [
51] - can play a pivotal role in helping farmers initiate and sustain these programmes, with the broader goal of revitalizing inner areas of southern Italy, in full alignment with the One Health approach. As in other inner areas of the EU, which also sustain an agropastoral economy, there is a pressing need to integrate modern scientific advancements and technologies to improve livestock quality—both in terms of animal health and welfare—while also promoting human and environmental health [
48,
56].
Of particular importance for the improvement of animal health in extensive farming in inner areas are the biosecurity measures that need to be implemented, including costly fencing and animal separation structures, as well as specific courses to raise awareness among farmers. The implementation of biosecurity measures requires public funding. Currently, in Italy, no official regulations have been issued regarding the application of biosecurity measures for ruminants.
Currently, science has not yet fully addressed the causes of CD, so it remains without a cure. Raising public awareness on this important topic is particularly crucial. This should be included in the communication efforts of healthcare professionals and universities—through public engagement as part of the university's third mission—to inform the general public about the preventable nature of CD. In fact, the global epidemic could be halted if mothers breastfeed their newborns during the first four weeks of life, and if industries are required to exclusively produce and certify MAP-free infant formula for feeding newborns [
58].
7. Conclusions
Achieving the desired outcomes will require public funds and active involvement from livestock farmers, with support from official veterinarians and professionals in farm veterinary medicine, environmental protection, and other professionals including food technologists, agronomists, economists, and biologists involved in agricultural, food, diagnostic and other related professional and industrial sectors, other than marketing and visual communication experts for the wide dissemination - to the general public - of the effort undertaken and the beneficial ongoing or achieved results.
All these experts must work closely together, following the interdisciplinarity needed in the application of the One Health approach [
59], because once MAP is excreted, it can survive in soil or water for up to 120 weeks. MAP is found in grazing areas, runoff that flows into rivers, and municipal water systems, where it persists in biofilm. Solid and liquid cow manure, often used as fertilizer on agricultural land, also contributes to its spread. Even farms that no longer house ruminants can harbor MAP, which persists in the soil and grass of pastures, including both the root and aerial parts of plants; field studies have shown that the nymphs of
Blattia orientalis may serve as a vector of MAP. Aerosol inhalation has been proposed as another potential transmission route of MAP to animals and possibly humans, as it is detected in river aerosols and domestic showers [
44,
45,
60].
The crucial role of revitalizing inner areas through improved animal health in extensive breeding – where IBR and JD are highly spread [
61] – through the implementation of optional control programmes could then be highlighted as a focal point in advertising campaigns, shifting from a challenge that undermines farm profitability to a valuable asset to promote.
Our proposal could serve as a model that is adaptable for countries beyond the European Union.
Author Contributions
Conceptualisation, A.M., ES. and L.M.; methodology, A.M., E.S., N.R. and V.D.R.; resources, A.M., L.M., V.D.R., S.R., and S.G.; writing—original draft preparation, A.M., E.S. and V.D.R.; writing—review and editing, A.M., E.S., L.M., V.D.R., N.R., S.R., and S.G.; visual, S.R. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
the authors - who organized the first meeting aimed at discussing the opportunity for the implementation of optional control programmes for IBR and JD in Molise, held in April 2024 at Department of Agricultural, Environmental and Food Sciences (DiAAA) of the University of Molise, under the guidance of the former Director, Professor Elena Sorrentino, and at the suggestion of Alessandra Mazzeo, DiAAA Third Mission Delegate - thank Regional Assessor MD Angelo Michele Iorio, the director VD Michele Colitti and other veterinary directors of the Molise Region and of the Regional Health Agency of Molise (ASrEM), the Provincial President dr. Giacinto Ricciuto and the other representatives of Coldiretti Molise, and the representatives of the Italian Farmers' Confederation of Molise, as well as Professors and students of DiAAA, and all those who participated in the meeting giving a fundamental impulse to the realization of this review. In particular, A.M. extends her sincere gratitude to the aforementioned participants, expressing hope for the swift implementation of control programmes for Johne's Disease in Molise. She also hopes that her contribution to organizing the meeting and drafting this review—an effort made in memory of her father, Giovanni Mazzeo, who served diligently as the Director of the Molise Aqueduct in the last century, with a deep commitment to preserving water sources in mountain areas that supported breeding activities—will help foster actions aimed at reducing the prevalence of Crohn’s Disease patients.
References
- Italian Government. Presidency of the Council of Ministers. Department for Cohesion Policies and Sud. https://politichecoesione.governo.it/it/politica-di-coesione/la-programmazione-2021-2027/# (accessed online on 30 August 2024).
- Italian Government. Agency for Territorial Cohesion. https://www.agenziacoesione.gov.it/ (accessed online on 30 August 2024).
- Italian Government. Agency for Territorial Cohesion. National Strategy for Inner Areas. https://www.agenziacoesione.gov.it/strategia-nazionale-aree-interne/ (accessed online on 30 August 2024).
- European Union. EUR-lex. Regulation (EU) 2016/429 of the European Parliament and of the Council of 9 March 2016 on transmissible animal diseases and amending and repealing certain acts in the area of animal health (‘Animal Health Law’). https://eur-lex.europa.eu/eli/reg/2016/429/oj (accessed online on 30 August 2024).
- European Union. EUR-lex. Commission Implementing Regulation (EU) 2018/1882 of 3 December 2018 on the application of certain disease prevention and control rules to categories of listed diseases and establishing a list of species and groups of species posing a considerable risk for the spread of those listed diseases. https://eur-lex.europa.eu/eli/reg_impl/2018/1882/oj#:~:text=Commission%20Implementing%20Regulation%20(EU)%202018,(Text%20with%20EEA%20relevance.) (accessed online on 30 August 2024).
- European Union. EUR-lex. Commission Delegated Regulation (EU) 2020/689 of 17 December 2019 supplementing Regulation (EU) 2016/429 of the European Parliament and of the Council as regards rules for surveillance, eradication programmes, and disease-free status for certain listed and emerging diseases. https://eur-lex.europa.eu/eli/reg_del/2020/689/oj (accessed online on 8 September 2024).
- European Union. EUR-lex. Commission Implementing Regulation (EU) 2021/620 of 15 April 2021 laying down rules for the application of Regulation (EU) 2016/429 of the European Parliament and of the Council as regards the approval of the disease-free and non-vaccination status of certain Member States or zones or compartments thereof as regards certain listed diseases and the approval of eradication programmes for those listed diseases. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32021R0620 (accessed online on 11 September 2024).
- European Commission (EC). Surveillance, eradication programmes and disease-free status. https://food.ec.europa.eu/animals/animal-diseases/surveillance-eradication-programmes-and-disease-free-status_en (accessed online on 30 August 2024).
- European Commission (EC). The European Green Deal. https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal_it (accessed online on 30 August 2024).
- European Commission (EC). Farm to Fork Strategy. https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed online on 2 September 2024).
- World Health Organization (WHO). Quadripartite Secretariat for One Health. https://www.who.int/teams/one-health-initiative/quadripartite-secretariat-for-one-health (accessed online on 8 September 2024).
- Italian Ministry of Health & Istituto Zooprofilattico Sperimentale della Lombardia e dell’Emilia-Romagna “Bruno Ubertini”. Classyfarm. https://www.classyfarm.it/index.php (accessed online on 8 September 2024).
- International Committee of Taxonomy of Virus (ICTV) 2021, https://ictv.global/taxonomy (accessed online on 2 September 2024).
- De Brun, L.; Leites, M.; Furtado, A.; Campos, F.; Roehe, P.; Puentes, R. Field Evaluation of Commercial Vaccines against Infectious Bovine Rhinotracheitis (IBR) Virus Using Different Immunization Protocols. Vaccines 2021, 9, 408. https://www.mdpi.com/2076-393X/9/4/408 (accessed online on 2 September 2024).
- Italian Ministry of Health. Infectious Bovine Rhinotracheitis. https://www.salute.gov.it/portale/sanitaAnimale/dettaglioContenutiSanitaAnimale.jsp?lingua=italiano&id=264 (accessed online on 2 September 2024).
- Straub, O.C. Infectious bovine rhinotracheitis virus. History and recent developments. Dev Biol Stand 1975, 28, 530. https://pubmed.ncbi.nlm.nih.gov/165129/#:~:text=Already%20in%20the%20twenties%20it,the%20USA%20during%201955%2F1956 (accessed online on 12 September 2024).
- World Organisation for Animal Health (WOAH) – Online Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, thirteenth edition 2024. Chapter 3.4.11 Infectious Bovine Rhinotracheitis / Infectious Pustular Vulvovaginitis. https://www.woah.org/fileadmin/Home/eng/Health_standards/tahm/3.04.11_IBR_IPV.pdf 2024 (accessed online on 5 September 2024).
- European Union. EUR-lex. Commission Implementing Regulation (EU) 2024/2032 of 29 July 2024 amending certain Annexes to Implementing Regulation (EU) 2021/620 as regards the approval or withdrawal of the disease-free status of certain Member States or zones thereof as regards certain listed diseases and the approval of eradication programmes for certain listed diseases. OJ L, 2024 (30.07.2024), 1-7. https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=OJ:L_202402032 (accessed online on 18 September 2024).
- Maresca, C.; Scoccia, E.; Dettori A., Felici, A.; Guarcini, R.; Petrini, S.; Quaglia, A.; Filippini, G. National surveillance plan for infectious bovine rhinotracheitis (IBR) in autochthonous Italian cattle breeds: Results of first year of activity. Vet Microbiol 2018, 219, 150-153, https://doi.org/10.1016/j (accessed online on 2 September 2024). [CrossRef]
- Bettini, A.; Stella, M.; Precazzini, F.; Degasperi, M.; Colorio, S.; Tavella, A. Infectious Bovine Rhinotracheitis Post-Eradication Program in the Autonomous Province of Bolzano, Italy: A Retrospective Study on Potential Bovine Herpesvirus Type 2 Cross-Reactivity. Animals 2023, 13, 3502. https://doi.org/10.3390/ani13223502 (accessed online on 2 September 2024). [CrossRef]
- Oren, A.; Arahal, D.R.; Göker, M.; Moore, E.R.B.; Rossello-Mora, R.; Sutcliffe, I.C. International Code of Nomenclature of Prokaryotes. Prokaryotic Code (2022 Revision). Int J Syst Evol Microbiol 2023, 73, 005585 https://www.microbiologyresearch.org/content/journal/ijsem/10.1099/ijsem.0.005585 (accessed online on 14 September 2024).
- van Ingen, J.; Turenne, C.Y.; Tortoli, E.; Wallace, R.J.Jr; Brown-Elliott, B.A. A definition of the Mycobacterium avium complex for taxonomical and clinical purposes, a review. Int J Syst Evol Microbiol 2018, 68, 3666–3677 DOI 10.1099/ijsem.0.003026 https://www.microbiologyresearch.org/docserver/fulltext/ijsem/68/11/3666_ijsem003026.pdf?expires=1728136433&id=id&accname=guest&checksum=19A4AE69FB4474A7FEE26192599E6BC3 (accessed online on 5 October 2024).
- Italian Ministry of Health. Paratuberculosis or Johne’s Disease. https://www.salute.gov.it/portale/sanitaAnimale/dettaglioContenutiSanitaAnimale.jsp?lingua=italiano&id=217&tab=4 (accessed online on 2 September 2024).
- World Organisation for Animal Health (WOAH) – Online Manual of Diagnostic Tests and Vaccines for Terrestrial Animals, thirteenth edition 2024. Chapter 3.1.17 Paratuberculosis (Johne’s Disease) https://www.woah.org/en/disease/paratuberculosis/ (accessed online on 5 September 2024).
- Rathnaiah, G.; Zinniel, D.K.; Bannantine, J.P.; Stabel, J.R.; Gröhn, Y.T.; Collins, M.T.; Barletta, R.G. Pathogenesis, Molecular Genetics, and Genomics of Mycobacterium avium subsp. paratuberculosis, the Etiologic Agent of Johne's Disease. Front Vet Sci 2017, 6, 187. https://pubmed.ncbi.nlm.nih.gov/29164142/ (accessed online on 3 September 2024).
- Filippi, A.; Garbarino, C.; Nava, M.; Russo, S.; Soares Filipe, J.F.; Bianchi, A.; Corlatti, L.; Gugiatti, A.; Buccheri Pederzoli, C.; Pigoli, C.; Pedrotti, L.; Arrigoni, N.; Ricchi, M.; Bertoletti, I.; Luzzago, C. Active surveillance of paratuberculosis in Alpine-dwelling red deer (Cervus elaphus). Front Vet Sci 2024, 25, 1303096. https://pubmed.ncbi.nlm.nih.gov/38332752/ (accessed online on 3 September 2024). [PubMed] [PubMed Central]
- Koets, Ad.P.; Eda, S.; Sreevatsan, S. The within host dynamics of Mycobacterium avium spp. paratuberculosis infection in cattle: where time and place matter. Vet Res 2015, 46, 61. https://doi.org/10.1186/s13567-015-0185-0 (accessed online on 3 September 2024). [CrossRef]
- Spickler, A.R. Paratuberculosis. Retrieved from: The Center for Food Security and Public Health, Iowa State University. 2007. https://www.cfsph.iastate.edu/Factsheets/pdfs/paratuberculosis.pdf (accessed online on 3 September 2024).
- Mason, P.S.; Holder, T.; Robinson, N.; Smith, B.; Hameed, R.T.; Al Dulayymi, J.R.; Hughes, V.; Stevenson, K.; Jones, G.J.; Vordermeier, H.M.; Mc Kenna, S.; Baird, MS. An ELISA Using Synthetic Mycolic Acid-Based Antigens with DIVA Potential for Diagnosing Johne’s Disease in Cattle. Animals 2024, 14, 848. https://doi.org/10.3390/ani14060848 (accessed online on 3 September 2024). [CrossRef]
- Ott, S.L.; Wells, S.J.; Wagner, B.A. Herd-level economic losses associated with Johne's disease on US dairy operations. Prev Vet Med 1999, 11, 179-192. doi: 10.1016/s0167-5877(99)00037-9. PMID: 10423773.https://pubmed.ncbi.nlm.nih.gov/10423773/ (accessed online on 3 September 2024).
- Alonso-Hearn, M.; Badia-Bringué, G.; Canive, M. Genome-wide association studies for the identification of cattle susceptible and resilient to paratuberculosis. Front Vet Sci 2022, 9, 935133. doi: 10.3389/fvets.2022.935133. https://www.frontiersin.org/journals/veterinary-science/articles/10.3389/fvets.2022.935133/full (accessed online on 7 October 2024).
- Griss, S.; Knific, T.; Buzzell, A.; Carmo, L. P.; Schüpbach-Regula, G.; Meylan, M.; Ocepek, M.; Thomann, B. A scoping review on associations between paratuberculosis and productivity in cattle. Front Vet Sci 2024, 11, 1352623. doi: 10.3389/fvets.2024.1352623. PMID: 38756521; PMCID: PMC11097669. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11097669/ (accessed online on 7 October 2024).
- Matthews, C.; Cotter, P.D.; O' Mahony, J. MAP, Johne's disease and the microbiome; current knowledge and future considerations. Anim Microbiome 2021, 7, 34. doi: 10.1186/s42523-021-00089-1. PMC8105914. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8105914/ (accessed online on 3 September 2024).
- European Union. EUR-lex. Directive 2003/99/EC of the European Parliament and of the Council of 17 November 2003 on the monitoring of zoonoses and zoonotic agents, amending Council Decision 90/424/EEC and repealing Council Directive 92/117/EEC. https://eur-lex.europa.eu/eli/dir/2003/99/oj (accessed online on 19 September 2024).
- Agrawal, G.; Aitken, J.; Hamblin, H.; Collins, M.; Borody, T.J. Putting Crohn’s on the MAP: Five Common Questions on the Contribution of Mycobacterium avium subspecies paratuberculosis to the Pathophysiology of Crohn’s Disease. Digestive Dis Sci 2021, 66, 348–358 https://doi.org/10.1007/s10620-020-06653-0 (accessed online on 30 August 2024). [CrossRef]
- Olson, S.; Welton, L.; Jahansouz, C. Perioperative Considerations for the Surgical Treatment of Crohn’s Disease with Discussion on Surgical Antibiotics Practices and Impact on the Gut Microbiome. Antibiotics 2024, 13, 317. https://doi.org/10.3390/antibiotics13040317 (accessed online on 4 September 2024). [CrossRef]
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The role of short-chain fatty acids in health and disease. Adv Immunol 2014, 121, 91-119. doi: 10.1016/B978-0-12-800100-4.00003-9. PMID: 24388214 (accessed online on 4 September 2024).
- Aitken, J.M.; Phan, K.; Bodman, E.S.; Sharma, S.; Watt, A.; George, P.M.; Agrawal, G.; Tie, A.B.M. A Mycobacterium species for Crohn's disease? Pathology 2021, 53, 818-823 https://www.sciencedirect.com/science/article/abs/pii/S0031302521002348 (accessed online on 4 September 2024). [CrossRef]
- McNees, A.L.; Markesich, D.; Zayyani, N.R.; Graham, D.Y. Mycobacterium paratuberculosis as a cause of Crohn’s disease. Expert Rev Gastroent 2015, 9, 1523–1534. https://doi.org/10.1586/17474124.2015.1093931 (accessed online on 5 September 2024). [CrossRef]
- Kuenstner L.; Kuenstner J.T. Mycobacterium avium spp. paratuberculosis in the Food Supply: A Public Health Issue. Front Public Health 2021, 15, 647448. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8319643 (accessed online on 12 September 2024). [CrossRef] [PubMed Central]
- Aitken, J.M.; Aitken, J.E.; Agrawal, G. Mycobacterium avium spp. paratuberculosis and Crohn’s Disease—Diagnostic Microbiological Investigations Can Inform New Therapeutic Approaches. Antibiotics 2024, 13, 158. https://doi.org/10.3390/antibiotics13020158 (accessed online on 4 September 2024). [CrossRef]
- Behr, M.A.; Hanley, J. Antimycobacterial therapy for Crohn’s disease: a reanalysis. Lancet Infect Dis 2008, 8, 344. (accessed online on 30 August 2024). [CrossRef]
- Mintz, M.J.; Lukin, D.J. Mycobacterium avium subspecies paratuberculosis (MAP) and Crohn's disease: the debate continues. Transl Gastroenterol Hepatol 2023, 8, 28. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10432229/ (accessed online on 8 September 2024).
- Dow, C.T.; Alvarez, B.L. Mycobacterium paratuberculosis zoonosis is a One Health emergency. EcoHealth, 2022, 19, 164–174. https://doi.org/10.1007/s10393-022-01602-x (accessed online on 5 September 2024). [CrossRef]
- Dow, C.T.; Sechi, L.A. Cows Get Crohn’s Disease and They’re Giving Us Diabetes. Microorganisms 2019, 7, 466. https://doi.org/10.3390/microorganisms7100466 (accessed online on 5 September 2024). [CrossRef]
- Davis, W. C.; Kuenstner, J. T.; Singh, S. V. Resolution of Crohn’s (Johne’s) disease with antibiotics: what are the next steps? Expert Rev Gastroent 2017, 11, 393–396. https://doi.org/10.1080/17474124.2017.1300529 (accessed online on 6 September 2024).
- World Organisation for Animal Health (WOAH). World Animal Health Information System (WAHIS). https://wahis.woah.org/#/dashboards/country-or-disease-dashboard (accessed online on 7 October 2024).
- Whittington, R.; Donat, K.; Weber, M.F.; Kelton, D.; Nielsen, S.S.; Eisenberg, S.; Arrigoni, N.; Juste, R.; Sáez, J.L.; Dhand, N.; Santi, A.; Michel, A.; Barkema, H.; Kralik, P.; Kostoulas, P.; Citer, L.; Griffin, F.; Barwell, R.; Moreira, M.A.S.; Slana, I.; Koehler, H.; Singh, S.V.; Yoo, H.S.; Chávez-Gris, G.; Goodridge, A.; Ocepek, M.; Garrido, J.; Stevenson, K.; Collins, M.; Alonso, B.; Cirone, K.; Paolicchi, F.; Gavey, L.; Rahman, M.T.; de Marchin, E.; Van Praet, W.; Bauman, C.; Fecteau, G.; McKenna, S.; Salgado, M.; Fernández-Silva, J.; Dziedzinska, R.; Echeverría, G.; Seppänen, J.; Thibault, V.; Fridriksdottir, V.; Derakhshandeh, A.; Haghkhah, M.; Ruocco, L.; Kawaji, S.; Momotani, E.; Heuer, C.; Norton, S.; Cadmus, S.; Agdestein, A.; Kampen, A.; Szteyn, J.; Frössling, J.; Schwan, E.; Caldow, G.; Strain, S.; Carter, M.; Wells, S.; Munyeme, M.; Wolf, R.; Gurung, R.; Verdugo, C.; Fourichon, C.; Yamamoto, T.; Thapaliya, S.; Di Labio, E.; Ekgatat, M.; Gil, A.; Alesandre, A.N.; Piaggio, J.; Suanes, A.; de Waard, J.H. Control of paratuberculosis: who, why and how. A review of 48 countries. BMC Vet Res 2019, 13; 198. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6567393/ (accessed online on 6 September 2024). [CrossRef] [PubMed Central]
- Italian Presidency of the Council of Ministers. Conferenza Permanente per i Rapporti tra lo Stato, le Regioni e le Province Autonome di Trento e Bolzano - accordo 30 novembre 2022 ai sensi dell'articolo 4, comma 1, del decreto legislativo 28 agosto 1997, n. 281, tra il Governo, le regioni e le Province autonome di Trento e Bolzano concernente: «Linee guida per l'adozione dei Piani di controllo e per l'assegnazione della qualifica sanitaria agli allevamenti di specie sensibili (bovini, bufalini, ovini, caprini) nei confronti della paratubercolosi». (Repertorio atti n. 230/CSR del 30 novembre 2022). (23A00089) (GU Serie Generale n. 10 del 13-01-2023). https://www.gazzettaufficiale.it/atto/serie_generale/caricaDettaglioAtto/originario?atto.dataPubblicazioneGazzetta=2023-01-13&atto.codiceRedazionale=23A00089&elenco30giorni=false (accessed online on 15 September 2024).
- Hoang, V.; Nguyen, A.; Hubbard, C.; Nguyen, K.-D. Exploring the Governance and Fairness in the Milk Value Chain: A Case Study in Vietnam. Agriculture 2021, 11, 884. https://doi.org/10.3390/agriculture11090884 (accessed online on 1 September 2024). [CrossRef]
- Moretti, M.; Belliggiano, A.; Grando, S.; Felici, F.; Scotti, I.; Ievoli, C.; Blackstock, K.; Delgado-Serrano, M.M.; Brunori, G. Characterizing value chains’ contribution to resilient and sustainable development in European mountain areas. J Rur Stud 2023, 100, 103022. https://doi.org/10.1016/j.jrurstud.2023.103022 (accessed online on 1 September 2024). [CrossRef]
- Wichert, A.; Kasbohm, E.; Einax, E.; Wehrend, A.; Donat, K. Detection of Low MAP Shedder Prevalence in Large Free-Stall Dairy Herds by Repeated Testing of Environmental Samples and Pooled Milk Samples. Animals 2022, 12, 1343. https://doi.org/10.3390/ani12111343 (accessed online on 7 October 2024). [CrossRef]
- Mazzeo, A.; Tremonte, P.; Lombardi, S.J.; Caturano, C.; Correra, A.; Sorrentino, E. From the Intersection of Food-Borne Zoonoses and EU Green Policies to an In-Embryo One Health Financial Model. Foods 2022, 11, 2736 http://doi.org/10.3390/foods11182736 (accessed online on 8 September 2024). [CrossRef]
- Whittington, R.; Donat, K.; Weber, M.F. et al. Control of paratuberculosis: who, why and how. A review of 48 countries. BMC Vet Res 2019, 15, 198. https://doi.org/10.1186/s12917-019-1943-4 (accessed online on 7 October 2024). [CrossRef]
- Weber, M.F.; Kelton, D.; Eisenberg, S.W.F.; Donat, K. Progress in Paratuberculosis Control Programmes for Dairy Herds. Animals 2024, 14, 1127. https://doi.org/10.3390/ani14071127 (accessed online on 7 October 2024). [CrossRef]
- Italian Government – National Recovery and Resilience Plan (PNRR). https://www.governo.it/sites/governo.it/files/PNRR.pdf (accessed online on 8 September 2024).
- Morrison, R.; Rose, D.C. Factors that influence dairy farmers’ decisions to implement Johne’s Disease control practices: A systematic review. Prev Vet Med 2023, 220, 106053 https://www.sciencedirect.com/science/article/pii/S0167587723002179 (accessed online on 6 September 2024).
- Monif, G.G.R. Crohn’s Disease: The infectious Disease Incorporated’s Perspective. Gastrointest Disord 2021, 3, 138–141. https://doi.org/10.3390/gidisord3030015 (accessed online on 3 September 2024). [CrossRef]
- Mazzeo, A.; Tremonte, P.; Rossi, N.; Ferrara, C.; Mascolo, C.; Lombardi, S.J.; Sorrentino, E. Modulation of the One Health Approach to Tackle Brucellosis in Buffaloes and Cattle in Two Italian Territories with Different Characteristics. J Buffalo Sci 2023, 12, 55-69. https://www.lifescienceglobal.com/pms/index.php/JBS/article/view/9109/4818 (accessed online on 5 October 2024).
- Pal, M.; Rahman, T. Mycobacterium avium subspecies paratuberculosis: an Emerging Bacterial Disease of Global Public Health Significance. Microbes and Health 2015, 4, 4-13 DOI: 10.3329/mh.v4i1.23085. https://www.banglajol.info/index.php/MH/article/view/23085 (accessed online on 5 October 2024).
- Tamba, M.; Pallante, I.; Petrini, S.; Feliziani, F.; Iscaro, C.; Arrigoni, N.; Di Sabatino, D.; Barberio, A.; Cibin, V.; Santi, A.; Ianniello, M.; Ruocco, L.; Pozzato, N. Overview of Control Programs for Twenty-Four Infectious Cattle Diseases in Italy. Front Vet Sci 2021, 8, 818480. https://doi.org/10.3389/fvets.2021.665607 (accessed online on 7 October 2024). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).