Submitted:
24 September 2024
Posted:
25 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Questionnaire Items
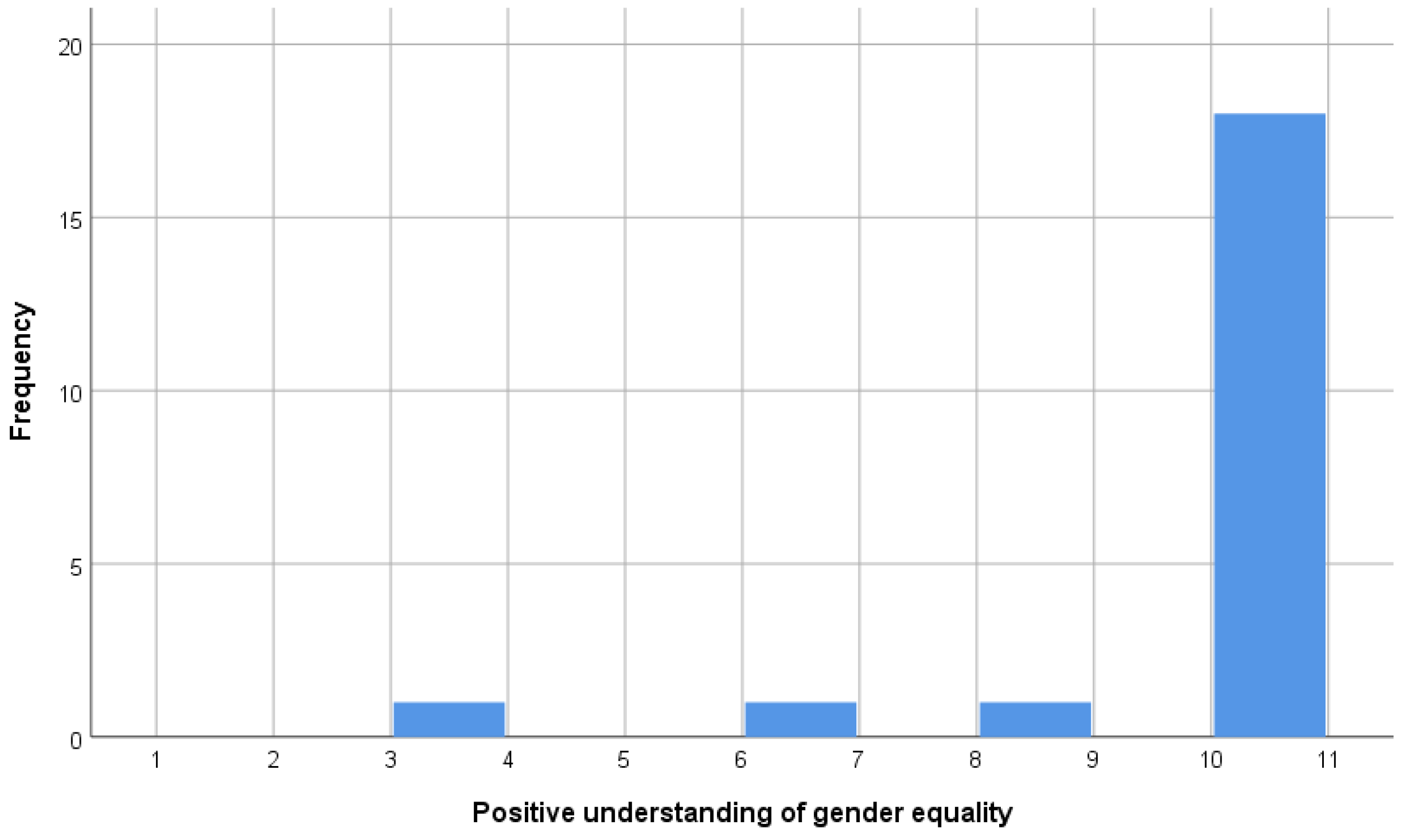
- (i) Gender: Please tell me how essential you think it is as a characteristic of democracy.
- “Women have the same rights as men.” (Q249)
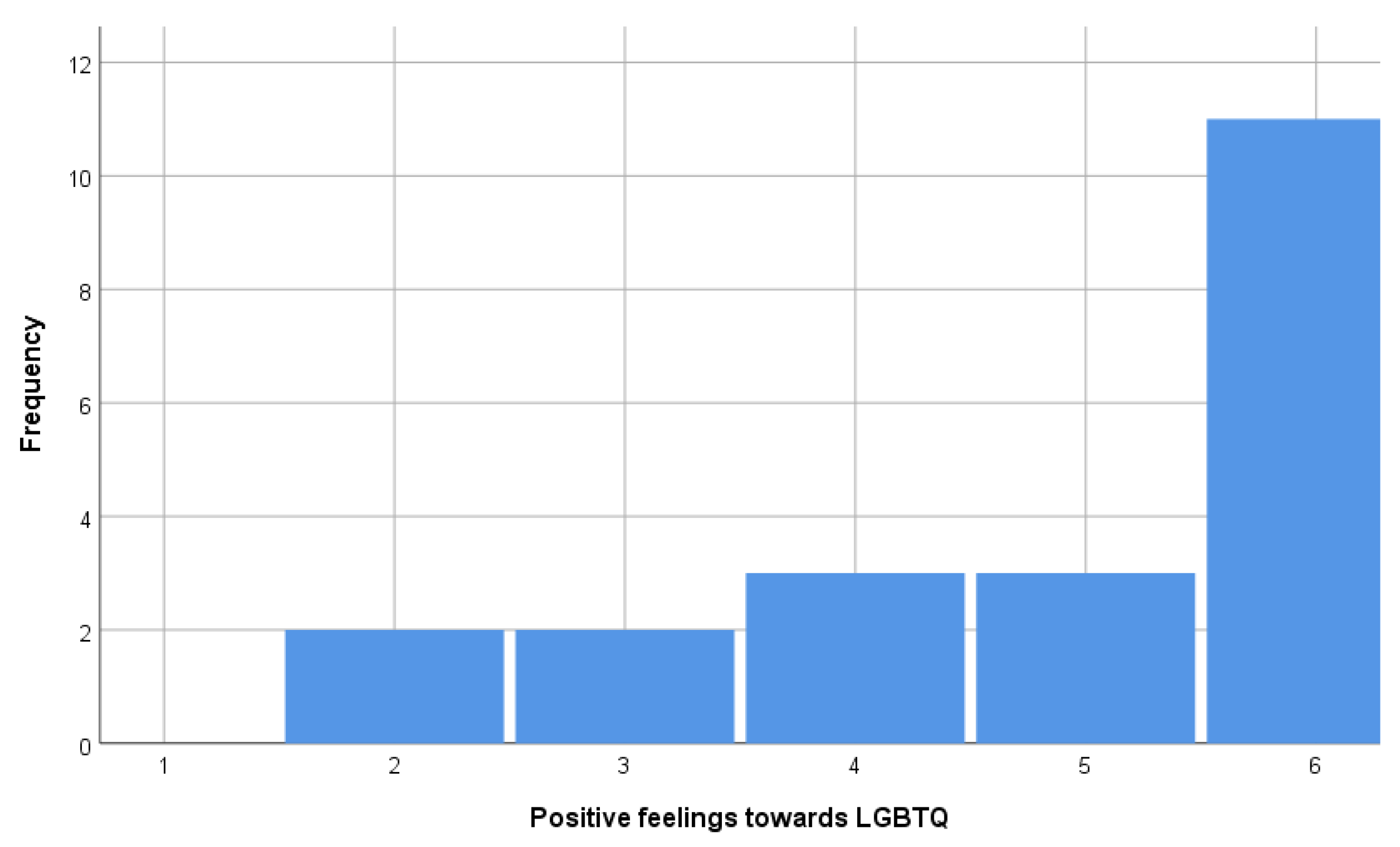
- (ii) LGBTQ: Could you please mention any that you would not like to have as neighbors?
- “Homosexual” (Q22)
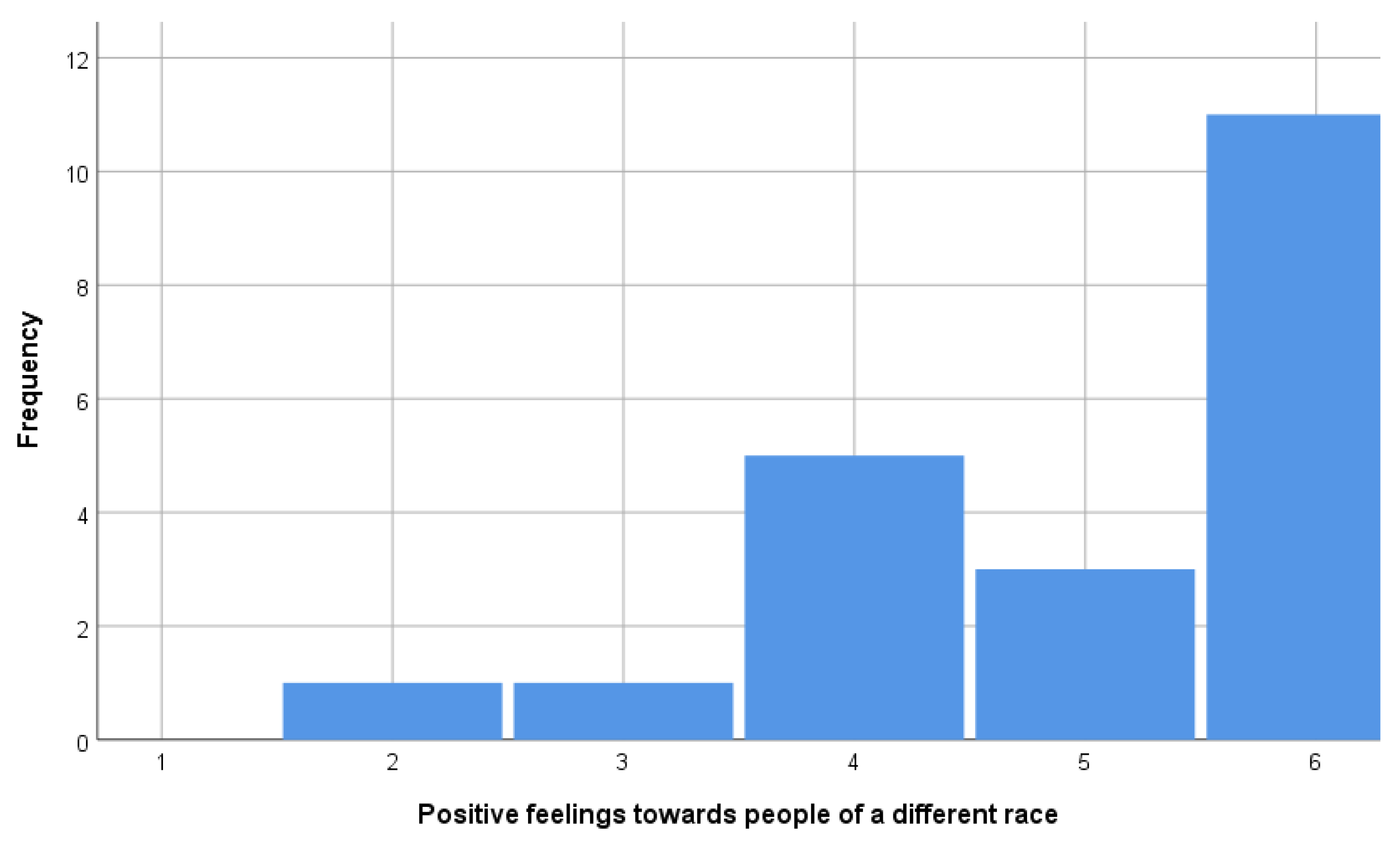
- (iii) Different race: Could you please mention any that you would not like to have as neighbors? “People of a different race” (Q19)
2.3. Calculation of ΔGMV
2.4. Analytical Method
3. Results
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moonen, J.E.; Nasrallah, I.M.; Detre, J.A.; Dolui, S.; Erus, G.; Davatzikos, C.; Meirelles, O.; Bryan, R.N.; Launer, L.J. Race, sex, and mid-life changes in brain health: Cardia MRI substudy. Alzheimers Dement. 2022, 18, 2428–2437. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, Y.; Wang, Z.; Yin, H.; Zhang, D.; Yang, J. Evaluating age-and gender-related changes in brain volumes in normal adult using synthetic magnetic resonance imaging. Brain Behav. 2024, 14, e3619. [Google Scholar] [CrossRef] [PubMed]
- García, J.J. Heart, brain and mental health disparities for LGBTQ people of color; Palgrave Macmillan: London, United Kingdom, 2021. [Google Scholar]
- Lanz, K.; Brown, P. All the brains in the business: the engendered brain in the 21st century organisation (The neuroscience of business); Palgrave Macmillan: London, United Kingdom, 2019. [Google Scholar] [CrossRef]
- Rodríguez-Nieto, G.; Mercadillo, R.E.; Pasaye, E.H.; Barrios, F.A. Affective and cognitive brain-networks are differently integrated in women and men while experiencing compassion. Front. Psychol. 2022, 5693. [Google Scholar] [CrossRef] [PubMed]
- Menon, B. Towards a new model of understanding–The triple network, psychopathology and the structure of the mind. Med. Hypotheses 2019, 133, 109385. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.D.; Corbetta, M.; Snyder, A.Z.; Vincent, J.L.; Raichle, M.E. Spontaneous neuronal activity distinguishes human dorsal and ventral attention systems. Proc. Natl. Acad. Sci. U. S. A. 2006, 103, 10046–10051. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, I.; Harel, M.; Malach, R. When the brain loses its self: prefrontal inactivation during sensorimotor processing. Neuron 2006, 50, 329–339. [Google Scholar] [CrossRef]
- Koechlin, E.; Summerfield, C. An information theoretical approach to prefrontal executive function. Trends Cogn. Sci. 2007, 11, 229–235. [Google Scholar] [CrossRef]
- Miller, E.K.; Cohen, J.D. An integrative theory of prefrontal cortex function. Annu. Rev. Neurosci. 2001, 24, 167–202. [Google Scholar] [CrossRef]
- Owen, A.M.; McMillan, K.M.; Laird, A.R.; Bullmore, E. N-back working memory paradigm: A meta-analysis of normative functional neuroimaging studies. Hum. Brain Mapp. 2005, 25, 46–59. [Google Scholar] [CrossRef]
- Smith, E.E.; Jonides, J. Neuroimaging analyses of human working memory. Proc. Natl. Acad. Sci. U. S. A. 1998, 95, 12061–12068. [Google Scholar] [CrossRef]
- Wager, T.D.; Smith, E.E. Neuroimaging studies of working memory: a meta-analysis. Cogn. Affect. Behav. Neurosci. 2003, 3, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef] [PubMed]
- Goulden, N.; Khusnulina, A.; Davis, N.J.; Bracewell, R.M.; Bokde, A.L.; McNulty, J.P.; Mullins, P.G. The salience network is responsible for switching between the default mode network and the central executive network: replication from DCM. Neuroimage. 2014, 99, 180–190. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Chen, T.; Ryali, S.; Kochalka, J.; Li, C.S.R.; Menon, V. Causal interactions within a frontal-cingulate-parietal network during cognitive control: Convergent evidence from a multisite–multitask investigation. Cereb. Cortex. 2016, 26, 2140–2153. [Google Scholar] [CrossRef]
- Chen, T.; Michels, L.; Supekar, K.; Kochalka, J.; Ryali, S.; Menon, V. Role of the anterior insular cortex in integrative causal signaling during multisensory auditory–visual attention. Eur J Neurosci. 2015, 41, 264–274. [Google Scholar] [CrossRef]
- Ham, T.; Leff, A.; de Boissezon, X.; Joffe, A.; Sharp, D.J. Cognitive control and the salience network: an investigation of error processing and effective connectivity. J. Neurosci. 2013, 33, 7091–7098. [Google Scholar] [CrossRef]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: a network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef]
- Sridharan, D.; Levitin, D.J.; Menon, V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc. Natl. Acad. Sci. U. S. A. 2008, 105, 12569–12574. [Google Scholar] [CrossRef]
- Uddin, L.Q. Salience processing and insular cortical function and dysfunction. Nat. Rev. Neurosci. 2015, 16, 55–61. [Google Scholar] [CrossRef]
- Andrews-Hanna, J.R.; Reidler, J.S.; Sepulcre, J.; Poulin, R.; Buckner, R.L. Functional-anatomic fractionation of the brain’s default network. Neuron 2010, 65, 550–562. [Google Scholar] [CrossRef]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The brain’s default network - anatomy, function, and relevance to disease. Ann. N.Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Shulman, G.L.; Fiez, J.A.; Corbetta, M.; Buckner, R.L.; Miezin, F.M.; Raichle, M.E.; Petersen, S.E. Common blood flow changes across visual tasks.2. Decreases in cerebral cortex. J. Cogn. Neurosci. 1997, 9, 648–663. [Google Scholar] [CrossRef]
- Li, W.; Mai, X.; Liu, C. The default mode network and social understanding of others: what do brain connectivity studies tell us. Front. Hum. Neurosci. 2014, 8, 74. [Google Scholar] [CrossRef] [PubMed]
- Krönke, K.M.; Wolff, M.; Shi, Y.; Kräplin, A.; Smolka, M.N.; Bühringer, G.; Goschke, T. Functional connectivity in a triple-network saliency model is associated with real-life self-control. Neuropsychologia 2020, 149, 107667. [Google Scholar] [CrossRef]
- Acevedo, B.P.; Aron, E.N.; Aron, A.; Sangster, M.D.; Collins, N.; Brown, L.L. The highly sensitive brain: an fMRI study of sensory processing sensitivity and response to others’ emotions. Brain Behav. 2014, 4, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Bilevicius, E.; Kolesar, T.A.; Smith, S.D.; Trapnell, P.D.; Kornelsen, J. Trait emotional empathy and resting state functional connectivity in default mode, salience, and central executive networks. Brain Sci. 2018, 8, 128. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, S.E.; Kim, H.E.; Han, K.; Jeong, B.; Kim, J.J.; Namkoong, K.; Kim, J.W. Altered functional connectivity of the default mode network in low-empathy subjects. Yonsei Med J. 2017, 58, 1061–1065. [Google Scholar] [CrossRef]
- Nummenmaa, L.; Hirvonen, J.; Parkkola, R.; Hietanen, J.K. Is emotional contagion special? An fMRI study on neural systems for affective and cognitive empathy. NeuroImage 2008, 43, 571–580. [Google Scholar] [CrossRef]
- Shamay-Tsoory, S.G.; Aharon-Peretz, J.; Perry, D. Two systems for empathy: a double dissociation between emotional and cognitive empathy in inferior frontal gyrus versus ventromedial prefrontal lesions. Brain 2009, 132, 617–627. [Google Scholar] [CrossRef]
- Hansen, T.M.; Muthulingam, J.A.; Brock, B.; Drewes, A.M.; Juhl, A.; Vorum, H.; Jakobsen, P.E.; Karmisholt, J.; Brock, C.; Frøkjær, J.B. Reduced gray matter brain volume and cortical thickness in adults with type 1 diabetes and neuropathy. Neurosci. Res. 2022, 176, 66–72. [Google Scholar] [CrossRef]
- Ferguson, S.C.; Blane, A.; Wardlaw, J.; Frier, B.M.; Perros, P.; McCrimmon, R.J.; Deary, I.J. Influence of an early-onset age of type 1 diabetes on cerebral structure and cognitive function. Diabetes Care 2005, 28, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- World values survey: round seven - country-pooled datafile version 5.0. Madrid; Haerpfer, C., Inglehart, R., Moreno, A., Welzel, C., Kizilova, K., Diez-Medrano, J., Lagos, M., Norris, P., Ponarin, E., Puranen, B., Eds.; JD Systems Institute & WVSA Secretariat: Spain & Vienna, Austria, 2022. [Google Scholar] [CrossRef]
- Kokubun, K.; Yamakawa, Y.; Hiraki, K. Association between behavioral ambidexterity and brain health. Brain Sci. 2020, 10, 137. [Google Scholar] [CrossRef]
- Kokubun, K.; Yamakawa, Y.; Nemoto, K. The link between the brain volume derived index and the determinants of social performance. Curr. Psychol. 2023, 42, 12309–12321. [Google Scholar] [CrossRef]
- Watanabe, K.; Kakeda, S.; Nemoto, K.; Onoda, K.; Yamaguchi, S.; Kobayashi, S.; Yamakawa, Y. Grey-matter brain healthcare quotient and cognitive function: A large cohort study of an MRI brain screening system in Japan. Cortex 2021, 145, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, K.; Nemoto, K.; Oka, H.; Fukuda, H.; Yamakawa, Y.; Watanabe, Y. Association of fatigue and stress with gray matter volume. Front. Behav. Neurosci. 2018, 12, 154. [Google Scholar] [CrossRef] [PubMed]
- Kokubun, K.; Yamakawa, Y. Association between food patterns and gray matter volume. Front. Hum. Neurosci. 2019, 13, 384. [Google Scholar] [CrossRef]
- Kokubun, K.; Pineda, J.C.D.; Yamakawa, Y. Unhealthy lifestyles and brain condition: Examining the relations of BMI, living alone, alcohol intake, short sleep, smoking, and lack of exercise with gray matter volume. Plos one 2021, 16, e0255285. [Google Scholar] [CrossRef]
- Nemoto, K.; Oka, H.; Fukuda, H.; Yamakawa, Y. MRI-based Brain Healthcare Quotients: A bridge between neural and behavioral analyses for keeping the brain healthy. PLoS One. 2017, 12, e0187137. [Google Scholar] [CrossRef]
- Farah, M.J. The neuroscience of socioeconomic status: Correlates, causes, and consequences. Neuron 2017, 96, 56–71. [Google Scholar] [CrossRef]
- Gallese, V. The roots of empathy: the shared manifold hypothesis and the neural basis of intersubjectivity. Psychopathology 2003, 36, 171–180. [Google Scholar] [CrossRef]
- Baron-Cohen, S.; Wheelwright, S.; Hill, J.; Raste, Y.; Plumb, I. The “Reading the Mind in the Eyes” Test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psychiatry. 2001, 42, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Baron-Cohen, S.; Wheelwright, S. The empathy quotient: an investigation of adults with Asperger syndrome or high functioning autism, and normal sex differences. J. Autism. Dev. Disord. 2004, 34, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Maurage, P.; Grynberg, D.; Noël, X.; Joassin, F.; Philippot, P.; Hanak, C.; Verbanck, P.; Luminet, O.; de Timary, P.; Campanella, S. Dissociation between affective and cognitive empathy in alcoholism: a specific deficit for the emotional dimension. Alcohol. Clin. Exp. Res. 2011, 35, 1662–1668. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.S.; Mateen, M.A.; Brozovich, F.A.; Zaki, J.; Goldin, P.R.; Heimberg, R.G.; Gross, J.J. Empathy for positive and negative emotions in social anxiety disorder. Behav. Res. Ther. 2016, 87, 232–242. [Google Scholar] [CrossRef]
- Farrow, T.F.; Zheng, Y.; Wilkinson, I.D.; Spence, S.A.; Deakin, J.F.W.; Tarrier, N.; Griffiths, P.D.; Woodruff, P.W.R. Investigating the functional anatomy of empathy and forgiveness. Neuroreport 2001, 12, 2433–2438. [Google Scholar] [CrossRef]
- Alagapan, S.; Lustenberger, C.; Hadar, E.; Shin, H.W.; Frӧhlich, F. Low-frequency direct cortical stimulation of left superior frontal gyrus enhances working memory performance. Neuroimage 2019, 184, 697–706. [Google Scholar] [CrossRef]
- Boisgueheneuc, F.D.; Levy, R.; Volle, E.; Seassau, M.; Duffau, H.; Kinkingnehun, S.; Samson, Y.; Zhang, S.; Dubois, B. Functions of the left superior frontal gyrus in humans: a lesion study. Brain 2006, 129, 3315–3328. [Google Scholar] [CrossRef]
- Banaji, M.R.; Baron, A.S.; Dunham, Y.; Olson, K. The development of intergroup social cognition. In Intergroup attitudes and relations in childhood through adulthood; Levy, S.R., Killen, M., Eds.; Oxford University Press: New York, NY, USA, 2008; pp. 87–102. [Google Scholar]
- Jankowiak-Siuda, K.; Zajkowski, W. A neural model of mechanisms of empathy deficits in narcissism. Med. Sci. Monit. 2013, 19, 934–941. [Google Scholar] [CrossRef]
- Fallon, N.; Roberts, C.; Stancak, A. Shared and distinct functional networks for empathy and pain processing: a systematic review and meta-analysis of fMRI studies. Soc. Cogn. Affect. Neurosci. 2020, 15, 709–723. [Google Scholar] [CrossRef]
- Gu, X.; Liu, X.; Guise, K.G.; Naidich, T.P.; Hof, P.R.; Fan, J. Functional dissociation of the frontoinsular and anterior cingulate cortices in empathy for pain. J. Neurosci. 2010, 30, 3739–3744. [Google Scholar] [CrossRef]
- Timmers, I.; Park, A.L.; Fischer, M.D.; Kronman, C.A.; Heathcote, L.C.; Hernandez, J.M.; Simons, L.E. Is empathy for pain unique in its neural correlates? A meta-analysis of neuroimaging studies of empathy. Front Behav Neurosci. 2018, 12, 289. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Pei, Z.; Zhu, Z.; Wu, X.; Feng, C. Intrinsic brain activity patterns across large-scale networks predict reciprocity propensity. Hum. Brain Mapp. 2022, 43, 5616–5629. [Google Scholar] [CrossRef] [PubMed]
- Mars, R.B.; Neubert, F.X.; Noonan, M.A.P.; Sallet, J.; Toni, I.; Rushworth, M.F.S. On the relationship between the “default mode network” and the “social brain”. Front. Hum. Neurosci. 2012, 6, 189. [Google Scholar] [CrossRef]
- Sampaio, A.; Soares, J.M.; Coutinho, J.; Sousa, N.; Gonçalves, Ó.F. The Big Five default brain: functional evidence. Brain. Struct. Funct. 2014, 219, 1913–1922. [Google Scholar] [CrossRef]
- Coutinho, J.F.; Sampaio, A.; Ferreira, M.; Soares, J.M.; Gonçalves, O.F. Brain correlates of pro-social personality traits: a voxel-based morphometry study. Brain Imaging Behav. 2013, 7, 293–299. [Google Scholar] [CrossRef]
- Silva, P.O.; Maia, L.; Coutinho, J.; Frank, B.; Soares, J.M.; Sampaio, A.; Gonçalves, Ó. Empathy by default: Correlates in the brain at rest. Psicothema 2018, 30, 97–103. [Google Scholar] [CrossRef]
- Esménio, S.; Soares, J.M.; Oliveira-Silva, P.; Zeidman, P.; Razi, A.; Gonçalves, O.F.; Friston, K.; Coutinho, J. Using resting-state DMN effective connectivity to characterize the neurofunctional architecture of empathy. Sci Rep. 2019, 9, 2603. [Google Scholar] [CrossRef]
- Novak, L.; Malinakova, K.; Mikoska, P.; van Dijk, J.P.; Tavel, P. Neural correlates of compassion–An integrative systematic review. Int. J. Psychophysiol. 2022, 172, 46–59. [Google Scholar] [CrossRef]
- Kheloui, S.; Jacmin-Park, S.; Larocque, O.; Kerr, P.; Rossi, M.; Cartier, L.; Juster, R.P. Sex/gender differences in cognitive abilities. Neurosci. Biobehav. Rev. 2023, 105333. [Google Scholar] [CrossRef]
- Mirziyoyeva, Z.; Salahodjaev, R. Does representation of women in parliament promote economic growth? Considering evidence from Europe and Central Asia. Front. Polit. Sci. 2023, 5, 1120287. [Google Scholar] [CrossRef]
- Ministry of Economy, Trade and Industry. Diversity 2.0: One step ahead of competitive strategy. Economic and Social Policy Office; Economic and Industrial Policy Bureau: Tokyo, Japan, September 2020. [Google Scholar]
- Cross, N. Design thinking: Understanding how designers think and work. Bloomsbury Publishing: London, United Kingdom, 2023. [Google Scholar]
- Stickdorn, M.; Schneider, J. This is service design thinking: Basics, tools, cases; John Wiley & Sons: New York, United States, 2012. [Google Scholar]
- Sinha, S.; Sinha, D. Neurodiversity at the workplace: The new paradigm of talent acquisition and retention. In Global Practices on Effective Talent Acquisition and Retention; Christiansen, B., Aziz, M.A., O’Keeffe, E.L., Eds.; IGI Global: Pennsylvania, United States, 2024; pp. 333–348. [Google Scholar] [CrossRef]
- Kirby, A.; Smith, T. Neurodiversity at work: Drive innovation, performance and productivity with a neurodiverse workforce; Kogan Page Publishers: London, United Kingdom, 2021. [Google Scholar]
- LeFevre-Levy, R.; Melson-Silimon, A.; Harmata, R.; Hulett, A.L.; Carter, N.T. Neurodiversity in the workplace: Considering neuroatypicality as a form of diversity. Ind. Organ. Psych. 2023, 16, 1–19. [Google Scholar] [CrossRef]
- Schimmelpfennig, R.; Razek, L.; Schnell, E.; Muthukrishna, M. Paradox of diversity in the collective brain. Philos. Trans. R. Soc. B 2022, 377, 20200316. [Google Scholar] [CrossRef] [PubMed]
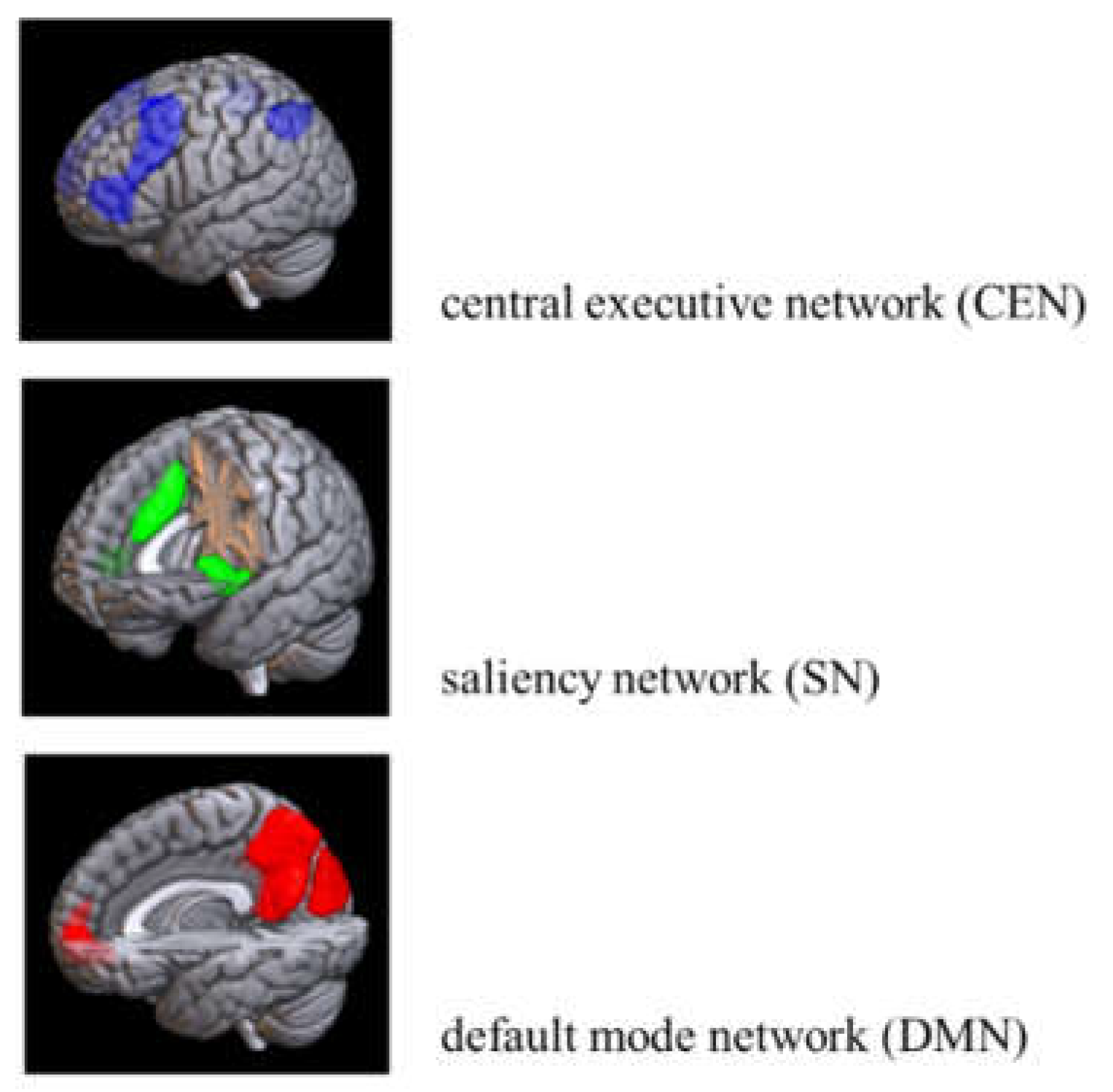
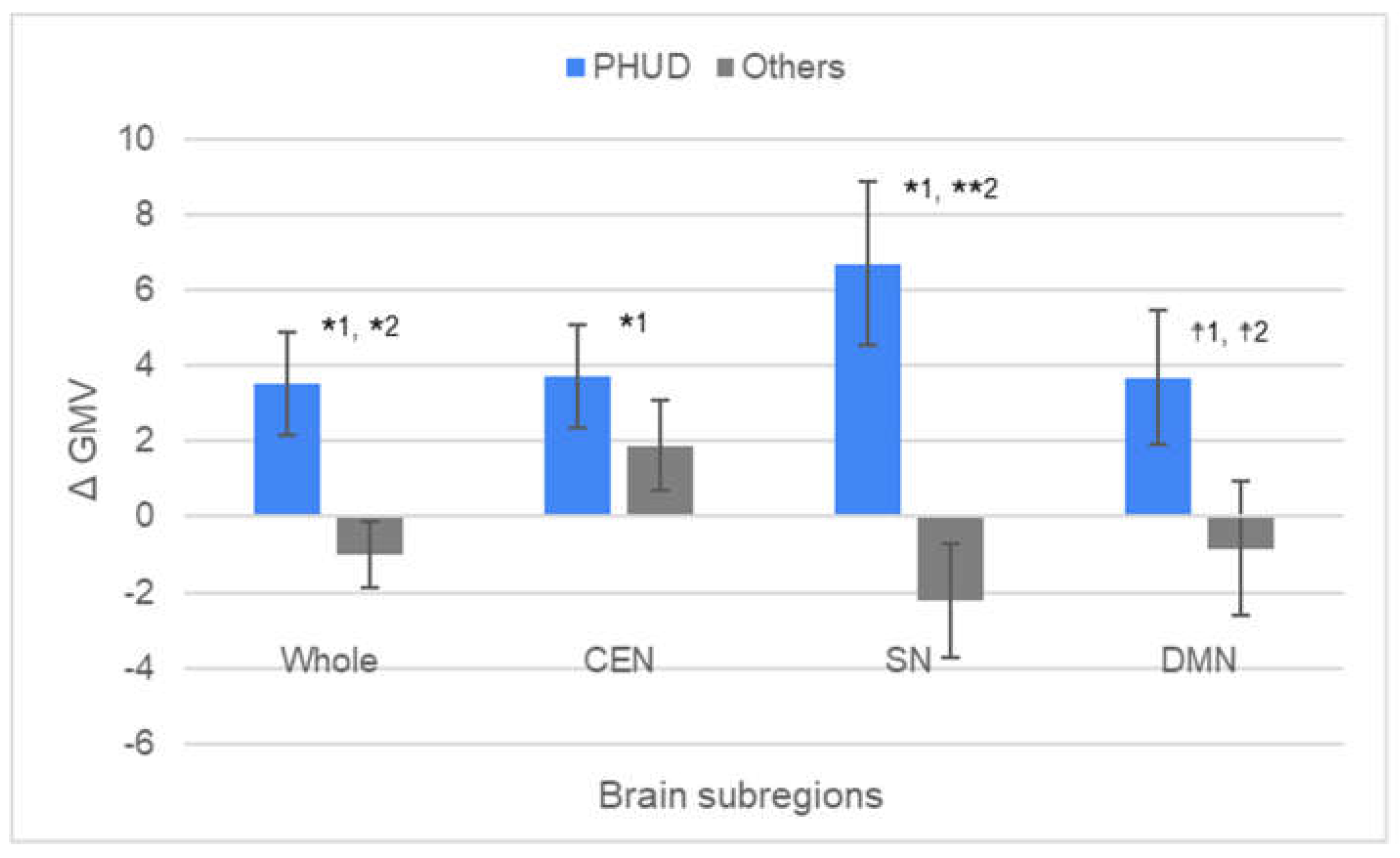
| PHUD | Others | |||||
| Mean | SD | Mean | SD | t | p | |
| GMV | ||||||
| Whole | 102.569 | 7.316 | 98.853 | 8.062 | 1.108 | 0.282 |
| DMN | 102.479 | 8.292 | 99.427 | 10.044 | 0.762 | 0.455 |
| CEN | 104.527 | 6.147 | 104.036 | 7.734 | 0.162 | 0.873 |
| SN | 103.576 | 10.605 | 95.884 | 10.319 | 1.681 | 0.109 |
| Years of schooling | 17.550 | 2.296 | 17.500 | 1.080 | 0.057 | 0.955 |
| BMI | 22.342 | 2.331 | 23.606 | 3.080 | 1.066 | 0.300 |
| Age | 50.000 | 12.116 | 44.100 | 13.486 | 1.056 | 0.304 |
| PHUD | Others | |
| Drinking frequency | ||
| Every day | 3 | 2 |
| 5-6 days a week | 1 | 0 |
| 3-4 days a week | 3 | 1 |
| 1-2 days a week | 1 | 2 |
| 1-3 days a month | 1 | 2 |
| Seldom drink | 1 | 1 |
| Quitted | 1 | 0 |
| Don’t drink (can’t drink) | 0 | 2 |
| χ2 | 5.832 | |
| p | 0.559 | |
| Smoking frequency | ||
| Every day | 0 | 3 |
| Haven’t smoked for over a month | 2 | 2 |
| Don’t smoke | 9 | 5 |
| χ2 | 4.105 | |
| p | 0.128 | |
| Marriage | ||
| Married | 10 | 8 |
| Single | 1 | 2 |
| χ2 | 0.509 | |
| p | 0.476 | |
| Occupation | ||
| Professional/technical | 5 | 7 |
| Management | 4 | 3 |
| Sales | 1 | 0 |
| Service/security | 1 | 0 |
| χ2 | 2.434 | |
| p | 0.487 |
| N | Mean | SD | SE | t1 | p1 | d1 | t2 | p2 | d2 | |
| PHUD | ||||||||||
| Whole | 11 | 3.544 | 4.542 | 1.369 | 2.587 | 0.027* | 0.780 | 2.728 | 0.013* | 1.206 |
| CEN | 11 | 3.732 | 4.584 | 1.382 | 2.700 | 0.022* | 0.814 | 0.994 | 0.333 | 0.436 |
| SN | 11 | 6.712 | 7.181 | 2.165 | 3.100 | 0.011* | 0.935 | 3.308 | 0.004** | 1.460 |
| DMN | 11 | 3.696 | 5.943 | 1.792 | 2.063 | 0.066☨ | 0.622 | 1.795 | 0.089☨ | 0.786 |
| Others | ||||||||||
| Whole | 10 | -1.011 | 2.811 | 0.889 | 1.137 | 0.285 | 0.360 | |||
| CEN | 10 | 1.896 | 3.791 | 1.199 | 1.582 | 0.148 | 0.500 | |||
| SN | 10 | -2.202 | 4.797 | 1.517 | 1.452 | 0.181 | 0.459 | |||
| DMN | 10 | -0.831 | 5.575 | 1.763 | -0.472 | 0.648 | 0.149 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





