You are currently viewing a beta version of our website. If you spot anything unusual, kindly let us know.
Preprint
Review
Crossing Age Boundaries: The Unifying Potential of Presepsin in Sepsis Diagnosis Across Diverse Age Groups
Altmetrics
Downloads
50
Views
31
Comments
0
This version is not peer-reviewed
Abstract
Sepsis is a pervasive condition that affects individuals of all ages, with significant social and economic consequences. Early diagnosis of sepsis is fundamental for establishing appropriate treatment and is based on warning scores and clinical characteristics, with positive microbiological cultures being the gold standard. Research has yet to identify a single biomarker to meet this diagnostic demand. Presepsin is a molecule that has the potential as a biomarker for diagnosing sepsis. In this paper, we present a narrative review of the diagnostic and prognostic performance of presepsin in different age groups. Given its particularities, it is identified that presepsin is a potential biomarker for sepsis at all stages of life.
Keywords:
Subject: Medicine and Pharmacology - Clinical Medicine
1. Introduction
Sepsis is a critical clinical condition defined as life-threatening organ dysfunction secondary to a deregulated host response to infection [1]. The high prevalence of sepsis, coupled with elevated mortality rates associated with severe sepsis, imposes economic costs and exacts a heavy toll on human lives [2]. Sepsis affects individuals across all age groups, with due consideration to the specific characteristics of each group.
Cardiovascular diseases and sepsis are two of the most significant contributors to human mortality [3,4]. Regarding cardiovascular diseases, heightened awareness of early signs and improvements in health services and professional training have resulted in a commendable success rate for early diagnosis [5]. Conversely, concerning sepsis, there is a lack of public understanding regarding the risks associated with infections, and healthcare professionals face challenges in identifying clear clinical signs for early diagnosis [6]. Early detection scores such as the quick Sequential Organ Failure Assessment (qSOFA) and systemic inflammatory response syndrome (SIRS) criteria and protocol adherence can facilitate the timely diagnosis and treatment of sepsis [7]. However, these scores need more inputs in their construction to ensure sensitivity and specificity for widespread use in clinical practice [8]. Identifying new biomarkers for sepsis diagnosis and prognosis becomes an essential goal in this context.
Presepsin is a biomarker with significant diagnostic and prognostic potential, making it superior to conventional biomarkers such as C-reactive protein (CRP) or procalcitonin (PCT) [9]. This analytical review aims to identify original studies in the literature that utilize presepsin as a biomarker for sepsis across all age groups, from neonates to nonagenarians, encompassing young adults and adults.
2. Demographics of Sepsis
Statistics from the USA indicate that sepsis was associated with 201,092 deaths in 2019 [10]. It is the primary cause of in-hospital deaths, accounting for an estimated 19.7% of the overall death rate [3]. The global incidence of sepsis exhibits a bimodal distribution, with peaks occurring in childhood and older adults [3].
It is the leading cause of death among infants and children, with an estimated 1.6 million cases per year [11], despite some variability in its occurrence, depending on the diagnostic strategy [2,12]. In the neonatal period, sepsis occurs in 1 to 5 cases per 1000 live births [13], with an overall mortality rate of 24.4%. However, this rate can escalate to as high as 54% in premature infants under 24 weeks of gestation [14].
Research indicates that >60% of sepsis cases occur in patients aged ≥65 years [15]. Mortality from sepsis in older adults constitutes approximately three-quarters of all sepsis-related deaths in the USA, particularly among individuals over the age of 65 [10]. Although this index declined between 2000 and 2019, it continues to rise with age, increasing five times in those over 85 [16].
3. Pathophysiology and Immunological Aspects of Sepsis across Ages
Comprehending the pathophysiology of sepsis requires a solid understanding of the intricate interaction among various domains, precisely the convergence of the inflammation pathway with the coagulation system, leading to endothelial stimulation and microcirculatory dysfunction [17]. This framework underpins the exploration of potential biomarkers, diagnostic approaches, optimal treatment durations, and the management of antibiotic therapy [17]. As an illustration, we can consider the activation and dysfunction of endothelial cells induced by sepsis, a phenomenon that diminishes with advanced age [18]. In this section, we delve into some age-related characteristics of the sepsis response.
Sepsis in the pediatric population is a distinct entity characterized by specific features in the host response to infection and therapy. It is crucial to understand the developmental differences that set it apart from adult sepsis [19]. Neonatal sepsis, occuring within the first 28 days of life, presents its unique aspects, with maternal risk factors (chorioamnionitis, premature membrane rupture, premature pregnancy, prolonged membrane rupture, and intrapartum maternal fever) and risk factors associated with the neonate (prematurity, low birth weight, fetal distress, and low Apgar score) [20]. Neonatal sepsis can be acquired from the mother during intrauterine life or through postpartum care [20]. After the neonatal stage, the clinical signs of pediatric sepsis are nonspecific. They can be exacerbated by birth conditions or adaptation to extrauterine life [21], often resulting in a delayed diagnosis [20]. Therefore, the diagnosis is presumptive in many cases, and treatment is based on clinical findings and nonspecific laboratory tests.
Moreover, the definitions of sepsis currently used for this age group are an extrapolation of the criteria used for adults [22], needing more validation for pediatric patients, which results in a low predictive value [23]. This diagnostic challenge has recently been addressed to validate new pediatric sepsis and septic shock criteria through organic dysfunction variables called Phoenix criteria [24]. Despite its limitations, the tool made it possible to identify sepsis and septic shock, enabling improvements in clinical care and research aimed at pediatric patients [23].
The inflammatory response to sepsis in pediatric patients suggests a predominantly anti-inflammatory phenotype, which is exacerbated compared to that in adults [25]. The immaturity of the adaptive immune system causes the neonate to become more dependent on the innate immune system [26].
In children, sepsis induces an immune response characterized initially by a pro-inflammatory state, promoting classic symptomatology such as fever, tachycardia, and tachypnea [27], making it clinically indistinguishable from the inflammatory response caused by other etiologies, posing challenges for its diagnosis by pediatricians [28]. Although there is no cohesive understanding of the mechanisms involved in sepsis [29], this pro-inflammatory phase is followed by the immunoparalysis phase, characterized by anti-inflammatory activity [27]. Such information corroborates the notion that a developmental difference exists in the inflammatory response to infection or injury among children and adults, exemplified by its pattern of organ failure following sepsis [25].
The prognosis of sepsis in pediatric patients is associated with lactate clearance as well as physiological variables in the first 4 hours after admission to the intensive care unit [30]. There also appears to be a correlation between genetic profiles and endotypes for septic shock in childhood [31], suggesting the possible existence of subclasses of response in sepsis. Thus, corticotherapy may be beneficial in those subgroups [31]; however, developing clinical trials to understand immunophenotypes and their relation to immunoparalysis must improve the prognosis of childhood sepsis [27].
Scientific research on sepsis has more widely included adults. A recent study identified a relative increase in sepsis diagnoses in the 18–44 age group, possibly due to greater awareness of the syndrome in this age range [32].
The final effect on the immunological phenotype (hypo- or hyper-reactivity) is variable and individualized and depends on the molecular heterogeneity of the septic syndrome. Thus, the differentiated activation of the innate and adaptive immune systems has allowed the identification of three subgroups based on mRNA expression profiles (transcriptomics): "inflammopathic" (characterized by innate immune activation, called SRS1 and linked to higher mortality), "adaptive" (adaptive immune activation, called SRS2 and linked to lower mortality), and "coagulopathic" (with platelet degranulation and coagulation dysfunction, related to higher mortality and the older adults population).
In this age group, the inflammatory and immunosuppressive responses are simultaneous and exhibit interindividual variation in the conceptual model of immune trajectories before, during, and after sepsis [33]. Thus, chronic hyperinflammation and immunosuppression have a prolonged clinical trajectory, known as persistent inflammation/immunosuppression and catabolism syndrome [34,35]. This endotype leads to chronic critical illness, characterized by impaired functional status, rehabilitation failure, and increased mortality [35].
Despite the heterogeneity in biological ages among individuals of the same chronological age [36], sepsis in older adults holds significance due to its association with increased morbidity [37], positioning it as the quintessential disease affecting this demographic [36].
Older age can be considered an independent predictor of mortality [15,39] despite the more encouraging results in a subgroup of patients over 85 obtained in a recent study [32], which showed a reduction in mortality (< 50%) compared to previous studies [40]. As the initial signs of sepsis in this age group may be invisible, progression to septic shock can be rapid2, highlighting the particular importance of early diagnosis in this age group.
4. Biomarkers and Sepsis
Biomarkers reflect the state of health or disease at a molecular level [41]. They improve diagnosis, define subsets of diseases that may differ in response, as well as individual variability in the drug’s molecular target, and provide an early reading of the response to therapy, among other functions. The search for new molecules with this purpose has been identified as a high priority for science [42] as part of the challenge of implementing "Personalized Medicine" [41].
In the case of sepsis, the question is whether it is possible to discriminate among septic patients, which subgroups share specific biological characteristics, who are at risk of unfavorable outcomes, and who are at risk of organ failure [28].
Although no ideal single biomarker or even combination of biomarkers serves this purpose in the international consensuses on sepsis [1,20], their use in this context is commonplace because, besides being an important aid in diagnosis, they enable us to predict possible sepsis syndrome outcomes [43]. Unfortunately no single one can reliably perform as a stand-alone sepsis biomarker [44,45,46,47].
Biomarkers represent the host response and their aberrant behavior—with persistent proinflammation (CRP), maintenance of immunosuppression (IL-10, soluble programmed death ligand-1 [PDL-1]), continuation of stress metabolism (glucagon-like peptide-1), absence of anabolism, and anti-angiogenesis (insulin-like growth factor-1, insulin-like growth factor-binding protein-3) for >14 days—indicate progression to chronic critical illness [48]. These molecules cannot represent the uncontrolled inflammation and increased vascular permeability that characterizes sepsis, leading to hypotension and organ dysfunction [49]. Therefore, to develop rapid assessment and differentiation between infection and inflammation, biomarker research aims to enable point-of-care testing among many molecules [49]. However, new biomarkers may not present superior results to traditional ones, frustrating expectations of benefit, suggesting their aid after evaluation with the usual scales and biomarkers [50].
Among the various functions necessary for the ideal biomarker, it should be precise for guiding therapeutic decision-making [51]. However, its measurement is impaired due to critical disadvantages, such as the collecting timing and the insufficiency of standardization (Table 1). Although traditionally measured at single time, gathering biomarker at several time interval may show a better overview of the host response to sepsis [44].
Guiding therapeutic decisions should be one of the ideal features of sepsis biomarkers [51]. In this context, deriving diagnostic algorithms appears to be a reliable strategy for early diagnosis of sepsis, integrating the pretest probability of infection, clinical features and results of in vitro diagnostic testing [52].
Considering the pathological process, the disease stage, and individual patient characteristics, a personalized therapeutic strategy could be provided by a biomarker-guided approach, avoiding “one size fts all” sepsis therapies [53]. In other words, sepsis research must consider the individual immune status or likely response to specific treatment to avoid harmuful therapy to a patient with a particular immune response activation pattern [53].
In addition to all the issues addressed so far, we must incorporate the key concept of value-based medicine, which involves cost-effectiveness studies, comparing different interventions, and defining the viability of diagnostic means. This is fundamental in a world of limited resources [46].
In pediatrics, most researchers agree that diagnostic priority depends on clinical signs and not biomarkers, even though sepsis has a polymorphic presentation [54]. CRP and PCT have been the most widely used biomarkers in pediatric clinical practice, with the recommendation that they must be used simultaneously to increase the efficiency of the results [54]. However, low accuracy is observed, as well as variable sensitivity and specificity for detecting bacterial infection via polymerase chain reaction (PCR) (lower when a single measurement is performed) [55]. On the other hand, PCT also has some limitations, such as variable sensitivity and specificity, altered serum levels in cases of kidney dysfunction, a lack of multicenter and prognostic studies and risk stratification, and higher costs55. Lactate is used to corroborate the diagnosis of septic shock and assess the response to therapy; however, normal or slightly elevated levels do not rule out the development of sepsis and septic shock; therefore, it is of limited effectiveness in children [56].
The medical literature comprises thousands of studies evaluating the applicability of biomarkers in adult sepsis, reporting >200 potential candidate molecules for the early diagnosis of sepsis [57]. However, methodological biases in many of these articles create limitations [58]. Due to these issues and insufficient evidence, only a few are suitable for everyday clinical use, with CRP, PCT, IL-6, and presepsin among the most promising [58]. No single biomarker has sufficient diagnostic power to be used independently; instead, a panel of biomarkers is considered the best option for a point-of-care approach to sepsis [59].
The specificity and sensitivity of biomarkers can be influenced by age. Thus, a moderate to marked increase in biomarkers such as CRP, an inflammatory peptide associated with immunosenescence, can be expected with advancing age [60]. This molecule is one of the substances linked to aging-related inflammation, and its increase is described as characteristic of the aging process [61]. In adult and older adults hospitalized with sepsis, CRP can rise within 72 hours and remain elevated for extended periods in older adults, even after they are discharged from the hospital [62]. This marker has been linked to poorer clinical outcomes in these patients [63].
It seems that patients who have subclinical inflammation at the time of discharge are more likely to have a higher risk of death, as indicated by persistently elevated inflammatory biomarker levels [63]. Patients over 65 tend to have a higher baseline inflammation, as reflected in higher inflammatory biomarker levels upon admission. However, these levels converge with those found in other age groups within the first 72 hours [62].
However, contrasting perspectives exist as some research groups have yet to identify a robust association between aging and markers of systemic inflammation or cytokine release in sepsis [18]. Furthermore, older adults experiencing sepsis display a dampening of endothelial cell activation, termed endothelial tolerance. Significantly, this phenomenon is attributed to the septic event rather than age [18].
5. Presepsin as a Sepsis Biomarker across Age Groups
Presepsin is a molecule identified in many cells involved in the sepsis cascades, including macrophages, monocytes, and granulocytes, and is responsible for the intracellular transduction of endotoxin signals [64]. Granulocytes phagocytize bacteria and CD14 and secrete presepsin into the blood within 2 hours after enzymatic digestion [65]. During the induction of systemic inflammation, the increase in presepsin levels occurs earlier and more rapidly than other sepsis markers [64].
Presepsin has advantages that justify its use, such as its early elevation in infection [66], high accuracy [20], and affordability compared to the gold standard blood culture test (US$ 7 versus US$ 11-89) [67,68]. It also exhibits better prognostic validity than PCT, CRP, and erythrocyte sedimentation rate (ESR) [69,70]. Presepsin’s advantages can be explained by its correlation with the sepsis pathophysiology, unlike other biomarkers resulting from a general inflammatory reaction [71]. However, it showed inferior performance to PCT as a predictor of bacterial infection [70] (Figure 1).
The availability of laboratory assays that can measure presepsin in 17 minutes is another factor that has made it a promising marker in sepsis [64,72,73,74,75,76,77]. However, its use has disadvantages, such as non-standardized cutoff points and the fact that it is inaccessible in most clinical settings [20]. As discussed below, its use as a biomarker should be customized according to the age group, as the threshold values can vary.
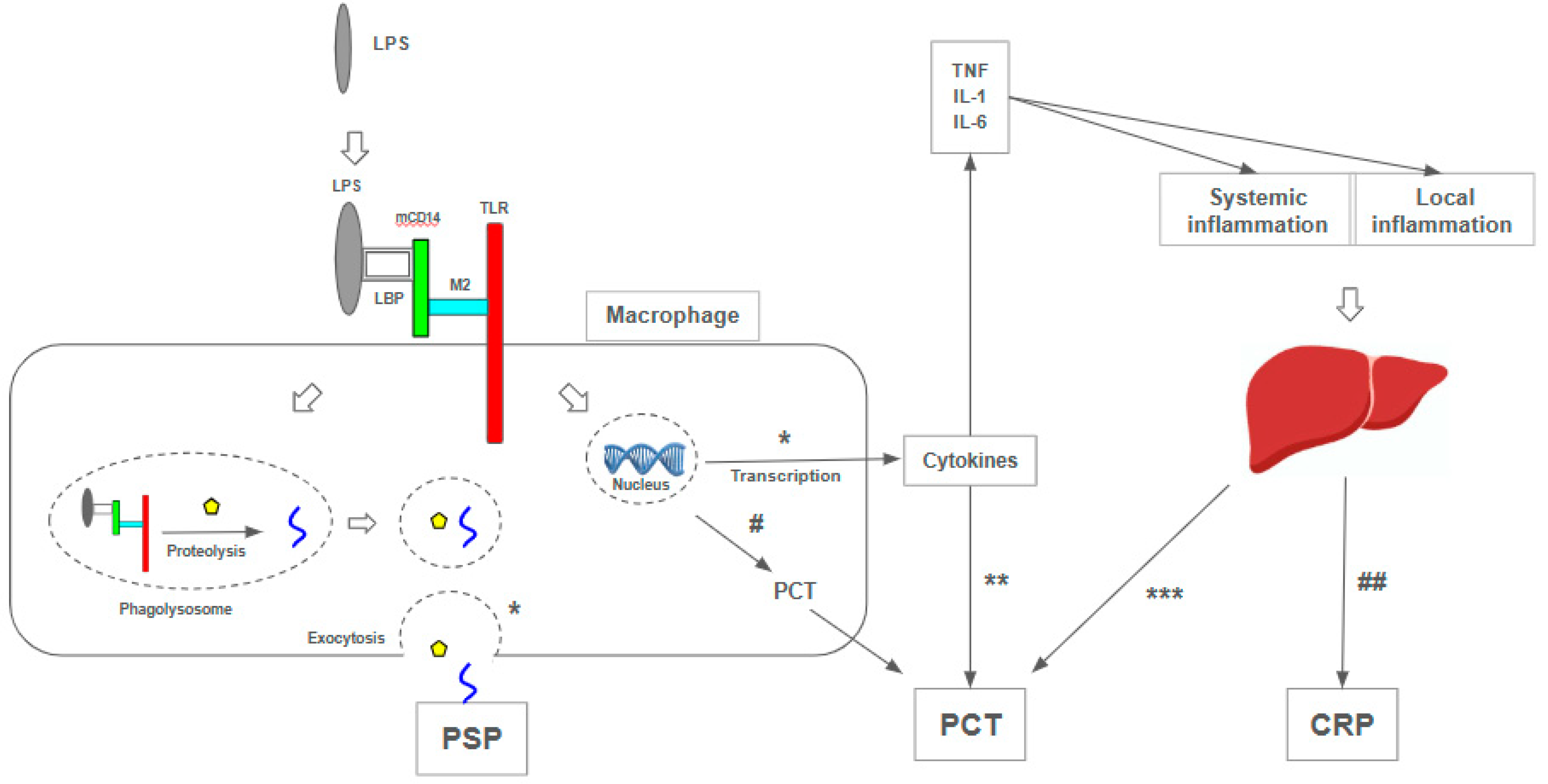
Figure 1.
Mechanisms of presepsin, procalcitonin, and C-reactive protein production [72,120,121,122,123]. (*) The molecular complex LPS-LBP-mCD14-M2-TLR is internalized into a phagolysosome; proteolysis and internalization processes release presepsin (PSP), which is released in circulation after exocytosis. CD 14 promotes the expression of genes responsible for the immune response, such as cytokine production [72]. (**) The rise of TNF, IL-1, IL-2, and IL-6 levels increases PCT [120]. (***) the liver is considered to be the most important site of production of PCT during an inflammatory response, especially those induced by bacterial infections [121]. (#) Peripheral blood mononuclear cells express PCT both on mRNA and on protein levels [122]. (##) CRP is an acute-phase protein, and its synthesis is rapidly upregulated, principally in hepatocytes, under the control of cytokines [123]. LPS: lipopolysaccharide, TLR: Toll-like receptor; LBP: Lipoprotein Binding Protein; mCD14: membrane-bound CD14; M2: co-protein of TLR; TNF: tumor necrosis factor; IL-1: interleucin-1; IL-6: interleucin-6; CPR: C-reactive protein; PCT: procalcitonin; PSP: presepsin.
Figure 1.
Mechanisms of presepsin, procalcitonin, and C-reactive protein production [72,120,121,122,123]. (*) The molecular complex LPS-LBP-mCD14-M2-TLR is internalized into a phagolysosome; proteolysis and internalization processes release presepsin (PSP), which is released in circulation after exocytosis. CD 14 promotes the expression of genes responsible for the immune response, such as cytokine production [72]. (**) The rise of TNF, IL-1, IL-2, and IL-6 levels increases PCT [120]. (***) the liver is considered to be the most important site of production of PCT during an inflammatory response, especially those induced by bacterial infections [121]. (#) Peripheral blood mononuclear cells express PCT both on mRNA and on protein levels [122]. (##) CRP is an acute-phase protein, and its synthesis is rapidly upregulated, principally in hepatocytes, under the control of cytokines [123]. LPS: lipopolysaccharide, TLR: Toll-like receptor; LBP: Lipoprotein Binding Protein; mCD14: membrane-bound CD14; M2: co-protein of TLR; TNF: tumor necrosis factor; IL-1: interleucin-1; IL-6: interleucin-6; CPR: C-reactive protein; PCT: procalcitonin; PSP: presepsin.
5.1. Presepsin as a Sepsis Biomarker in Neonates and Children
Due to its superior diagnostic performance compared to PCT and CRP [78], presepsin use has been highlighted in neonatal sepsis. Among healthy neonates, presepsin has an average plasmatic value of 649 ng/L and 720 ng/L in premature infants [79] (Table 1). A cutoff point of 788 ng/L, 93% sensitivity, and 100% specificity was obtained to diagnose early sepsis in premature infants [80] (Table 1). Its use is advocated for monitoring antibiotic therapy, as its levels decrease when treatment is effective [81]. In neonates with infection, it has the advantage that its levels are not influenced by gestational age or other perinatal factors [78]. High serum values also increase 30-day mortality [82].
Despite having demonstrated good accuracy in several studies, the use of PSP as a toll in the diagnosis and prognosis of neonatal sepsis still requires refinement. The differentiation of biomarker behavior between term and preterm neonates [79,80], between early onset (in the first 72 hours of life) and late onset [20,80,81], among others. In this age group, the diagnostic process must be remarkably rapid because, in addition to threatening life, it is a potential cause of permanent sequelae in survivors [20]. Therefore, some answers are necessary to consolidate the role of PSP as a biomarker in newborns, especially the diagnostic cutoff values, a topic that is still controversial (Table 1). Celerity, sensitivity, and specificity would reduce unnecessary treatments in symptomatic, low-risk individuals.
In children, presepsin shows similar responses; in a recent meta-analysis, presepsin showed high sensitivity and diagnostic accuracy compared to PCR and PCT but lower specificity [83]. The usefulness of presepsin extends to individuals with hematological neoplasms, where it can be a good predictor of clinical evolution with septic shock in febrile neutropenics [84]. In these patients, when there is no detectable site of infection, higher levels of presepsin can anticipate the positive result of cultures, discriminating the infectious origin of the febrile condition [85,86] (Table 2).
Biomarkers in pediatric sepsis are a valuable aid in promptly and cautiously diagnosing sepsis. Despite the emergence of promising options, such as genomic biosignature [29], older biomarkers, including CRP, PCT, ferritin, and lactate, despite their varying levels of reliability, continue to serve as useful clinical adjuncts in diagnosis [29]. Moreover, they are more readily available in most pediatric institutions [29].
Additionally, laboratory tests can determine the severity of sepsis, such as quantifying dynamic changes in levels of the antigen-presenting molecule human leukocyte antigen-DR isotype or the production of TNF-α upon stimulation (the latter representing the hyporeactivity of the innate immune system) [27].
5.2. Presepsin as a Sepsis Biomarker in Adults
As discussed previously, many current studies focus on adults, who benefit most from the results validated by scientific literature.
With average plasmatic levels of 202 pg/mL in healthy individuals [87], the increase of presepsin levels in the bloodstream correlates with the pathophysiology of sepsis rather than a general inflammatory reaction [71]. This characteristic gives it better prognostic validity than PCT, CRP, and ESR [69,70].
Presepsin levels have been shown to correlate with the severity and in-hospital mortality of patients with sepsis and septic shock [9], with mean values of 1718 and 3266 pg/ml for survivors and nonsurvivors, respectively [88] (Table 1). In a 28-day survival period, significant values of 1108 vs. 3251 pg/mL were obtained for survivors and nonsurvivors, respectively [89] (Table 1). Blood level changes, both absolute (increase above 500 pg/L) [90] and relative (reduction of >50% between admission and the seventh day) [91], correlated with unfavorable and favorable clinical outcomes, respectively. Due to its stability in various acute or chronic clinical scenarios, presepsin has helped detect sepsis in liver cirrhosis [92], rheumatoid arthritis [93], and febrile neutropenia [94], among others.
5.3. Presepsin as a Sepsis Biomarker in Older Adults
Studies suggest that presepsin could be more valuable than PCT and CRP as a predictor of bacteremia in older adult patients admitted to the emergency department. It showed significantly higher values than those without bacteremia (866.6 ± 184.6 vs. 639.9 ± 137.1 ng/L, p = 0.03) [97] (Table 1). It showed similar diagnostic and prognostic accuracy to PCT and early warning scores (qSOFA and SIRS), with the combination of the three biomarkers being superior to the use of anyone alone [98].
Aging was found to be an independent predictor of increased blood presepsin levels [99], with a significant difference comparing over 70 and under-70 age groups (470 [380–601] ng/L vs. 300 [201–457] ng/L, P < 0.001) [87]. Notably, age-related changes in renal and vascular function, such as glomerulosclerosis, vascular dysautonomia, altered tubular management of creatinine, and reduced renal reserve100, increase presepsin levels in renal dysfunction [100,101,102]. A study revealed that in older adult patients, hypercreatinemia raises the presepsin threshold value to 706 ng/L, enabling a diagnosis of sepsis [103].
However, there are caveats in the literature. Some authors postulate that for individuals over 75 years of age, a cutoff point of 380 pg/mL would be more appropriate [98]. This differs from the findings of systematic reviews focusing on predominantly younger populations, in which levels as high as 600 ng/L were found [104,105]. The rationale for this lies in the origin of presepsin; it comes from granulocytes, which are dysfunctional in this age group and hyporesponsive to infectious stimuli [98], a characteristic of immunosenescence (Table 2).
6. Published Meta-Analysis on Presepsin as Sepsis Biomarker
Published meta-analyses corroborate the promising role of presepsin as a biomarker in sepsis. In a search covering the period from 2010 to the present, several meta-analyses were found on using presepsin in neonatal sepsis. This search evaluated 28 studies and 2505 patients, recognizing the diagnostic value of presepsin in early-onset sepsis (i.e., occurring in the first 72 hours of life) [20] and late-onset sepsis [83,106,107].
The meta-analyses involving adults and older adults, evaluating the efficacy of presepsin in the context of sepsis, showed six meta-analyses in a literature review covering the period from 2010 to the present. It covered 20,544 patients in 141 selected studies, which, in general, showed good or moderate diagnostic accuracy in differentiating septic and nonseptic patients [108,109,110], indicating its suitability as a biomarker similar to PCT in the early diagnosis of sepsis [111] and showing relevant prognostic value [112,113]. None of these meta-analyses categorized older adult patients into subgroups, with mean ages ranging from 55.2 years [114] to 74 years [115], demanding efforts to conduct this type of study on the older adult population or to analyze a subgroup of this age group to support the understanding of sepsis in this population.
7. Discussion
The pathophysiological complexity of sepsis is acknowledged as the primary impediment to developing validated biomarkers, with current emphasis on the extensive study of inflammatory markers [17]. Nevertheless, distinct age groups manifest unique characteristics in their immune responses, encompassing both pro- and anti-inflammatory aspects and phenomena like immunoparalysis and immunosenescence. This variation aligns with differences in clinical and laboratory presentations, particularly concerning the levels of inflammatory biomarkers.
Prioritizing the characterization of septic syndrome behavior across different age groups affected by it is essential. Gaining insights into this aspect and recognizing the relative significance of biomarkers can aid in developing reproducible tools. These tools, in turn, facilitate the translation of clinical research findings into practical applications at the bedside.
The clinical and laboratory characteristics of different age groups present diagnostic challenges. In newborns, biomarkers show great potential for improving diagnosis, as blood cultures, considered the gold standard, have limitations. Blood cultures typically require a long turnaround time, ranging from 6 hours to 5 days for microorganisms to reach detectable levels, with an additional 24–48 hours needed for antibiotic susceptibility testing [51]. However, in newborns, the sensitivity of blood cultures is often reduced due to factors such as low blood volume during collection, low or intermittent bacteremia, and maternal antibiotic therapy [78], which can contribute to false-negative results [81]. Additionally, biomarkers provide insights into the newborn’s response to therapeutic interventions [13,107], thus potentially reducing the indiscriminate use of antibiotics.
Similarly, presepsin has shown diagnostic and prognostic value in adult studies, where we found the most significant number of publications. Consequently, its absolute plasma values and dynamic changes [115] have been described in various clinical situations, whether associated with sepsis. This predominance, combined with the intrinsic diagnostic difficulty of septic syndrome, strengthens the prominence gained by PSP as an adjunct in propaedeutics.
Despite comprising a substantial proportion of intensive care unit (ICU) patients, older adults must be more adequately represented in clinical trials, hindering the development of targeted protocols [36]. This underrepresentation can be attributed to age discrimination and significantly influences the formulation of public health policies for conditions such as sepsis.
Nevertheless, older adult survivors of intensive care frequently encounter sequelae and an accelerated age-related functional decline [116]. This scenario underscores the imperative for heightened support post-hospital discharge, particularly evident in 37.3% of patients over 85 years [32]. This population’s unique characteristics and specific needs emphasize the requirement for enhanced scientific rigor in studies encompassing this demographic. Focused clinical research on this cohort would yield invaluable insights for clinical decision-making, highlighting the importance of utilizing biomarkers to inform and streamline the process.
We observe a growing endorsement for personalized medicine, extending beyond ethnic groups to encompass individualized treatment strategies. The rationale for tailored therapies is firmly grounded in robust theoretical frameworks. Early diagnosis is pivotal in accelerating the protracted and time-intensive propaedeutic process. Hence, it becomes imperative to streamline the diagnostic trajectory of sepsis by seamlessly integrating clinical and laboratory data. This integration facilitates the anticipation of therapeutic decisions and interventions, mitigates potential complications, and optimizes overall outcomes. In pursuing a personalized, pragmatic, and efficient approach to sepsis, utilizing a multi-biomarker model propelled by genomic tools holds promise for future disease management11.
A promising trajectory for the future of sepsis management may lie in "omics" approaches, encompassing genomics, proteomics, metabolomics, and transcriptomics, alongside noteworthy strides in therapeutic interventions to optimize outcomes [117]. An illustrative instance involves the application of transcriptomic analysis panels to blood samples, enabling a precision-oriented approach in administering antimicrobials for the targeted exclusion of bacterial infections [118].
While there is a growing global awareness of sepsis, this heightened recognition has yet to translate into a substantive improvement in its management, particularly in developing or low-income countries [119]. Vulnerable populations in these regions necessitate tailored strategies, given the unavailability or unaffordability of expertise and technologies [119]. Biomarkers, emerging as promising tools, offer potential alternatives to facilitate decision-making and should be integrated into health policies.
8. Conclusions
Due to its unique characteristics, presepsin stands out as a promising biomarker for the diagnosis, therapeutic monitoring, and prognosis of sepsis across all age groups. Incorporating presepsin into quality improvement programs and consensus guidelines hinges on the foundation of rigorous research that validates its efficacy and solidifies its routine application.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Generative Intelligence Application
This article was initially written in Brazilian Portuguese and subsequently translated into English by human translators. Generative Intelligence was employed to enhance the quality of the English writing. It is important to note that no part of the manuscript contains text generated exclusively by Generative AI.
Acknowledgments
No funding was received.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. [CrossRef]
- Martín S, Pérez A, Aldecoa C. Sepsis and Immunosenescence in the Elderly Patient: A Review. Front Med. 2017;4:20. [CrossRef]
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211. [CrossRef]
- Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk A Compass for Future Health. J Am Coll Cardiol. 2022;80(25):2361-2371. [CrossRef]
- Kerrigan SW, Martin-Loeches I. Public awareness of sepsis is still poor: We need to do more. Intensiv Care Med. 2018;44(10):1771-1773. [CrossRef]
- Fiest KM, Krewulak KD, Brundin-Mather R, et al. Patient, Public, and Healthcare Professionals’ Sepsis Awareness, Knowledge, and Information Seeking Behaviors: A Scoping Review*. Crit Care Med. 2022;50(8):1187-1197. [CrossRef]
- Kim HI, Park S. Sepsis: Early Recognition and Optimized Treatment. Tuberc Respir Dis. 2018;81(1):6-14. [CrossRef]
- Luo J, Jiang W, Weng L, et al. Usefulness of qSOFA and SIRS scores for detection of incipient sepsis in general ward patients: A prospective cohort study. J Crit Care. 2019;51:13-18. [CrossRef]
- Behnes M, Bertsch T, Lepiorz D, et al. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Critical Care. 2014;18(5). [CrossRef]
- CDC C of DC and P. About Multiple Cause of Death, 1999-2020. About Multiple Cause of Death, 1999-2020. Published September 8, 2023. Accessed July 23, 2023.
- Oikonomakou MZ, Gkentzi D, Gogos C, Akinosoglou K. Biomarkers in pediatric sepsis: A review of recent literature. Biomark Med. 2020;14(10):895-917. [CrossRef]
- Balamuth F, Weiss SL, Neuman MI, et al. Pediatric Severe Sepsis in U.S. Children’s Hospitals* Pediatr Crit Care Med. 2014;15(9):798-805. [CrossRef]
- Gude SS, Peddi NC, Vuppalapati S, Gopal SV, Ramesh HM, Gude SS. Biomarkers of Neonatal Sepsis: From Being Mere Numbers to Becoming Guiding Diagnostics. Cureus. 2022;14(3):e23215. [CrossRef]
- Sharma D, Farahbakhsh N, Shastri S, Sharma P. Biomarkers for diagnosis of neonatal sepsis: A literature review. J Matern-Fetal Neonatal Med. 2018;31(12):1646-1659. [CrossRef]
- Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis* Crit Care Med. 2006;34(1):15-21. [CrossRef]
- Kramarow EA. Sepsis-related Mortality Among Adults Aged 65 and Over: United States, 2019. NCHS Data Brief. 2021 Nov;(422):1-8. [CrossRef] [PubMed]
- Arora J, Mendelson AA, Fox-Robichaud A. Sepsis: Network pathophysiology and implications for early diagnosis. Am J Physiol-Regul, Integr Comp Physiol. 2023;324(5):R613-R624. [CrossRef]
- Michels EHA, Butler JM, Reijnders TDY, et al. Association between age and the host response in critically ill patients with sepsis. Crit Care. 2022;26(1):385. [CrossRef]
- Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The Host Response to Sepsis and Developmental Impact. Pediatrics. 2010;125(5):1031-1041. [CrossRef]
- Hincu MA, Zonda GI, Stanciu GD, Nemescu D, Paduraru L. Relevance of Biomarkers Currently in Use or Research for Practical Diagnosis Approach of Neonatal Early-Onset Sepsis. Children. 2020;7(12):309. [CrossRef]
- Camargo JF de, Caldas JP de S, Marba STM. Early neonatal sepsis: Prevalence, complications and outcomes in newborns with 35 weeks of gestational age or more. Rev Paul Pediatr. 2021;40:e2020388. [CrossRef]
- Goldstein B, Giroir B, Randolph A, Sepsis ICC on P. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics* Pediatr Crit Care Med. 2005;6(1):2-8. [CrossRef]
- Schlapbach LJ, Kissoon N. Defining Pediatric Sepsis. JAMA Pediatrics. 2018;172(4):313-314. [CrossRef]
- Sanchez-Pinto LN, Bennett TD, DeWitt PE, et al. Development and Validation of the Phoenix Criteria for Pediatric Sepsis and Septic Shock. JAMA. 2024;331(8). [CrossRef]
- Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, McIntyre RC. Endotoxin induces an exaggerated interleukin-10 response in peritoneal macrophages of children compared with adults. J Pediatr Surg. 2004;39(6):912-915. [CrossRef]
- Wynn JL, Scumpia PO, Winfield RD, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112(5):1750-1758. [CrossRef]
- Hall M. Immune Modulation in Pediatric Sepsis. J Pediatr Intensiv Care. 2019;08(01):042-050. [CrossRef]
- Wong HR. Pediatric sepsis biomarkers for prognostic and predictive enrichment. Pediatr Res. 2022;91(2):283-288. [CrossRef]
- Lim PPC, Bondarev DJ, Edwards AM, Hoyen CM, Macias CG. The evolving value of older biomarkers in the clinical diagnosis of pediatric sepsis. Pediatr Res. 2023;93(4):789-796. [CrossRef]
- Kathmandu K children H, Jeevan GJ. Clinical, Demographic Profile and Outcome of Children Admitted in PICU with A Diagnosis of Severe Sepsis and Septic Shock. J. Med. Sci. Clin. Res.. 2017, 5(12). [CrossRef]
- Wong HR, Cvijanovich NZ, Anas N, et al. Developing a Clinically Feasible Personalized Medicine Approach to Pediatric Septic Shock. Am J Respir Crit Care Med. 2015;191(3):309-315. [CrossRef]
- Wardi G, Tainter CR, Ramnath VR, et al. Age-related incidence and outcomes of sepsis in California, 2008–2015. J Crit Care. 2021;62:212-217. [CrossRef]
- Poll T van der, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54(11):2450-2464. [CrossRef]
- Gentile LF, Nacionales DC, Lopez MC, et al. Protective Immunity and Defects in the Neonatal and Elderly Immune Response to Sepsis. J Immunol. 2014;192(7):3156-3165. 3156. [CrossRef]
- Darden DB, Kelly LS, Fenner BP, Moldawer LL, Mohr AM, Efron PA. Dysregulated Immunity and Immunotherapy after Sepsis. J Clin Med. 2021;10(8):1742. [CrossRef]
- Milbrandt EB, Eldadah B, Nayfield S, Hadley E, Angus DC. Toward an Integrated Research Agenda for Critical Illness in Aging. Am J Respir Crit Care Med. 2012;182(8):995-1003. [CrossRef]
- Jia L, Hao L, Li X, Jia R, Zhang HL. Comparing the predictive values of five scales for 4-year all-cause mortality in critically ill elderly patients with sepsis. Ann Palliat Med. 2021;0(0):6-6. [CrossRef]
- Wang Y, Dong C, Han Y, Gu Z, Sun C. Immunosenescence, aging and successful aging. Front Immunol. 2022;13:942796. [CrossRef]
- Martin-Loeches I, Guia MC, Vallecoccia MS, et al. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: A prospective, observational, multicenter cohort study. Ann Intensiv Care. 2019;9(1):26. [CrossRef]
- Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality Related to Severe Sepsis and Septic Shock Among Critically Ill Patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308-1316. [CrossRef]
- Woodcock J. The Prospects for “Personalized Medicine” in Drug Development and Drug Therapy. Clin Pharmacol Ther. 2007;81(2):164-169. [CrossRef]
- Woodcock J, Woosley R. The FDA Critical Path Initiative and Its Influence on New Drug Development. Annu Rev Med. 2008;59(1):1-12. [CrossRef]
- im MH, Choi JH. An Update on Sepsis Biomarkers. Infect Chemother. 2020;52(1):1-18. [CrossRef]
- Llitjos, JF., Carrol, E.D., Osuchowski, M.F. et al. Enhancing sepsis biomarker development: Key considerations from public and private perspectives. Crit Care 28, 238 (2024). [CrossRef]
- Liang, P., Wu, Y., Qu, S. et al. Exploring the biomarkers and potential therapeutic drugs for sepsis via integrated bioinformatic analysis. BMC Infect Dis 24, 32 (2024). [CrossRef]
- Deltell JMM, Boter NR. Biomarkers in emergencies: A never-ending race? Emergencias. 2024 Jan;36(1):4-6. Spanish, English. [CrossRef]
- Kadim MM, AL-Dahmoshi HOM, AL-Khikan FHO. Sepsis biomarkers: Current information and future visions. Microbes and Infectious Diseases 2024; 5(4): 201-210.
- Mankowski RT, Anton SD, Ghita GL, et al. Older adults demonstrate biomarker evidence of the persistent inflammation, immunosuppression and catabolism syndrome (PICS) after sepsis. J Gerontol: Ser A. 2021;77(1):glab080-. [CrossRef]
- Feng L, Liu S, Wang J, Gao Y et al. The performance of a combination of heparin-binding protein with other biomarkers for sepsis diagnosis: An observational cohort study BMC Infectious Diseases (2024) 24:755. [CrossRef]
- Clemente C, Fuentes ME, Ortega D, Julián A, Martín-Sánchez FJ, González del Castillo J. Utilidad de la combinación de biomarcadores de respuesta inflamatoria y escalas clínicas para la estratificación del riesgo en pacientes atendidos en urgencias por sospecha de infección. Emergencias. 2024;36:9-16.
- Lippi, Giuseppe. "Sepsis biomarkers: Past, present and future" Clinical Chemistry and Laboratory Medicine (CCLM), vol. 57, no. 9, 2019, pp. 1281-1283. [CrossRef]
- Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin Chem Lab Med 2019;57:1308–18.
- Vincent JL, van der Poll T, Marshall JC. The End of "One Size Fits All" Sepsis Therapies: Toward an Individualized Approach. Biomedicines. 2022 Sep 12;10(9):2260. [CrossRef]
- Bulatova YY, Maltabarova NA, Zhumabayev MB, Li TA, Ivanova MP. Modern Diagnostics of Sepsis and Septic Shock in Children. Electron J Gen Med. 2020;17(5):em216. [CrossRef]
- Lanziotti VS, Póvoa P, Soares M, Silva JRL e, Barbosa AP, Salluh JIF. Use of biomarkers in pediatric sepsis: Literature review. Rev Bras Ter Intensiv. 2016;28(4):472-482. [CrossRef]
- Schuh AM, Leger KJ, Summers C, Uspal NG. Lactic Acidosis in a Critically Ill Patient. Pediatr Emerg Care. 2018;34(9):e165-e167. [CrossRef]
- Kustán P, Horváth-Szalai Z, Mühl D. Nonconventional Markers of Sepsis. EJIFCC. 2017;28(2):122-133.
- Hung SK, Lan HM, Han ST, Wu CC, Chen KF. Current Evidence and Limitation of Biomarkers for Detecting Sepsis and Systemic Infection. Biomedicines. 2020;8(11):494. [CrossRef]
- Teggert A, Datta H, Ali Z. Biomarkers for Point-of-Care Diagnosis of Sepsis. Micromachines. 2020;11(3):286. [CrossRef]
- Wagner KH, Cameron-Smith D, Wessner B, Franzke B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients. 2016;8(6):338. [CrossRef]
- Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann N York Acad Sci. 2000;908(1):244-254. [CrossRef]
- Ginde AA, Blatchford PJ, Trzeciak S, et al. Age-Related Differences in Biomarkers of Acute Inflammation During Hospitalization for Sepsis. Shock. 2014;42(2):99-107. [CrossRef]
- Yende S, D’Angelo G, Kellum JA, et al. Inflammatory Markers at Hospital Discharge Predict Subsequent Mortality after Pneumonia and Sepsis. Am J Respir Crit Care Med. 2008;177(11):1242-1247.
- Velissaris D, Zareifopoulos N, Karamouzos V, et al. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus. Published online 2021. 2021. [CrossRef]
- Yaegashi Y, Shirakawa K, Sato N, et al. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. Journal of Infection and Chemotherapy. 2005;11(5):234-238. [CrossRef]
- Arai Y, Mizugishi K, Nonomura K, Naitoh K, Takaori-Kondo A, Yamashita K. Phagocytosis by human monocytes is required for the secretion of presepsin. J Infect Chemother. 2015;21(8):564-569. [CrossRef]
- MDsave. Blood culture. Published July 24, 2023. Available online: https://www.mdsave.com/procedures/blood-culture/d787ffc9.
- Truehealthlabs. 2023;(Culture). Available online: https://truehealthlabs.com/product/blood-culture/.
- Henriquez-Camacho C, Losa J. Biomarkers for sepsis. BioMed Research International. 2014;2014. [CrossRef]
- Park J, Yoon JH, Ki HK, Ko JH, Moon HW. Performance of presepsin and procalcitonin predicting culture-proven bacterial infection and 28-day mortality: A cross sectional study. Frontiers in Medicine. 2022;9. [CrossRef]
- Pierrakos C, Velissaris D, Bisdorff M, Marshall JC, Vincent JL. Biomarkers of sepsis: Time for a reappraisal. Critical Care. 2020;24(1). [CrossRef]
- Memar MY, Baghi HB. Presepsin: A promising biomarker for the detection of bacterial infections. Biomed Pharmacother. 2019;111:649-656. [CrossRef]
- Maddaloni C, Rose DUD, Santisi A, et al. The Emerging Role of Presepsin (P-SEP) in the Diagnosis of Sepsis in the Critically Ill Infant: A Literature Review. Int J Mol Sci. 2021;22(22):12154. [CrossRef]
- Piccioni A, Santoro MC, Cunzo T de, et al. Presepsin as Early Marker of Sepsis in Emergency Department: A Narrative Review. Medicina. 2021;57(8):770. [CrossRef]
- Ferreira T, Candido MA, Soriano FG. Sepsis biomarkers: A review of the diagnostic value of presepsin. Revista de Medicina. 2023;102(1). [CrossRef]
- Kyriazopoulou E, Leventogiannis K, Tavoulareas G, et al. Presepsin as a diagnostic and prognostic biomarker of severe bacterial infections and COVID-19. Scientific Reports. 2023;13(1). [CrossRef]
- Puspaningtyas NW, Karyanti MR, Paramita TN, et al. Presepsin as a promising biomarker for early detection of post-operative infection in children. Front Pediatr. 2023;11:1036993. [CrossRef]
- Tzialla C, Manzoni P, Achille C, Bollani L, Stronati M, Borghesi A. New Diagnostic Possibilities for Neonatal Sepsis. Am J Perinatol. 2018;35(06):575-577. [CrossRef]
- Pugni L, Pietrasanta C, Milani S, et al. Presepsin (Soluble CD14 Subtype): Reference Ranges of a New Sepsis Marker in Term and Preterm Neonates. PLoS ONE. 2015;10(12):e0146020. [CrossRef]
- Montaldo P, Rosso R, Santantonio A, Chello G, Giliberti P. Presepsin for the detection of early-onset sepsis in preterm newborns. Pediatr Res. 2017;81(2):329-334. [CrossRef]
- Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: A meta-analysis and systematic review. Crit Care. 2018;22(1):316. [CrossRef]
- Bellos I, Fitrou G, Pergialiotis V, Thomakos N, Perrea DN, Daskalakis G. The diagnostic accuracy of presepsin in neonatal sepsis: A meta-analysis. Eur J Pediatr. 2018;177(5):625-632. [CrossRef]
- Yoon SH, Kim EH, Kim HY, Ahn JG. Presepsin as a diagnostic marker of sepsis in children and adolescents: A systemic review and meta-analysis. BMC Infectious Diseases. 2019;19(1). [CrossRef]
- Korpelainen S, Intke C, Hämäläinen S, Jantunen E, Juutilainen A, Pulkki K. Soluble CD14 as a Diagnostic and Prognostic Biomarker in Hematological Patients with Febrile Neutropenia. Dis Markers. 2017;2017:9805609. [CrossRef]
- Olad E, Sedighi I, Mehrvar A, et al. Presepsin (scd14) as a marker of serious bacterial infections in chemotherapy induced severe neutropenia. Iran J Pediatr. 2014;24(6):715-722.
- Baraka A, Zakaria M. Presepsin as a diagnostic marker of bacterial infections in febrile neutropenic pediatric patients with hematological malignancies. Int J Hematol. 2018;108(2):184-191. [CrossRef]
- Giavarina D, Carta M. Determination of reference interval for presepsin, an early marker for sepsis. Biochem Med. 2015;25(1):64-68. [CrossRef]
- Zvyagin AA, Demidova VS, Smirnov GV. Dinamika biomarkerov sepsisa kak pokazatel’ éffektivnosti intensivnoĭ terapii. Khirurgiia. 2019;(2):53-57. [CrossRef]
- Ikeda T, Kamohara H, Suda S, et al. Comparative evaluation of endotoxin activity level and various biomarkers for infection and outcome of ICU-admitted patients. Biomedicines. 2019;7(3). [CrossRef]
- Fischer P, Grigoras C, Bugariu A, et al. Are presepsin and resistin better markers for bacterial infection in patients with decompensated liver cirrhosis? Digestive and Liver Disease. 2019;51(12):1685-1691. [CrossRef]
- Fujii E, Fujino K, Eguchi Y. An evaluation of clinical inflammatory and coagulation markers in patients with sepsis: A pilot study. Acute Medicine & Surgery. 2019;6(2):158-164. [CrossRef]
- Chen J, Huang ZB, Li H, et al. Early Diagnostic Biomarkers of Sepsis for Patients with Acute-on-Chronic Liver Failure: A Multicenter Study. Infectious Diseases and Therapy. 2021;10(1):281-290. [CrossRef]
- Tsujimoto K, Hata A, Fujita M, Hatachi S, Yagita M. Presepsin and procalcitonin as biomarkers of systemic bacterial infection in patients with rheumatoid arthritis. Int J Rheum Dis. 2018 Jul;21(7):1406-141. [CrossRef]
- Koizumi Y, Shimizu K, Shigeta M, et al. Plasma presepsin level is an early diagnostic marker of severe febrile neutropenia in hematologic malignancy patients. BMC Infectious Diseases. 2017;17(1). [CrossRef]
- Kang T, Yoo J, Choi H, Lee S, Jekarl DW, Kim Y. Performance evaluation of presepsin using a Sysmex HISCL-5000 analyzer and determination of reference interval. Journal of Clinical Laboratory Analysis. 2022;36(9). [CrossRef]
- Dragoş D, Ghenu MI, Timofte D, Balcangiu-Stroescu AE, Ionescu D, Manea MM. The cutoff value of presepsin for diagnosing sepsis increases with kidney dysfunction, a cross-sectional observational study. Medicine (United States). 2023;102(1):E32620-E32620. 3262. [CrossRef]
- Imai Y, Taniguchi K, Iida R, Nitta M, Uchiyma K, Takasu A. Diagnostic accuracy of presepsin in predicting bacteraemia in elderly patients admitted to the emergency department: Prospective study in Japan. BMJ Open. 2019;9(12):e030421. [CrossRef]
- Ruangsomboon O, Panjaikaew P, Monsomboon A, Chakorn T, Permpikul C, Limsuwat C. Diagnostic and prognostic utility of presepsin for sepsis in very elderly patients in the emergency department. Clin Chim Acta. 2020;510:723-732. [CrossRef]
- Claessens YE, Trabattoni E, Grabar S, et al. Plasmatic presepsin (sCD14-ST) concentrations in acute pyelonephritis in adult patients. Clinica Chimica Acta. 2017;464:182-188. [CrossRef]
- Musso CG, Oreopoulos DG. Aging and Physiological Changes of the Kidneys Including Changes in Glomerular Filtration Rate. Nephron Physiol. 2011;119(Suppl 1):p1-p5. [CrossRef]
- Chenevier-Gobeaux C, Trabattoni E, Roelens M, Borderie D, Claessens YE. Presepsin (sCD14-ST) in emergency department: The need for adapted threshold values? Clin Chim Acta. 2014;427:34-36. [CrossRef]
- Nagata T, Yasuda Y, Ando M, et al. Clinical impact of kidney function on presepsin levels. PLoS ONE. 2015;10(6). [CrossRef]
- Kim H, Song1 J, *, et al. Presepsin levels for discriminating sepsis and predicting mortality among organ failure patients stratified by hypercreatinemia. Signa Vitae. Published online 2023. [CrossRef]
- Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. Journal of Infection and Chemotherapy. 2011;17(6):764-769. [CrossRef]
- Endo S, Suzuki Y, Takahashi G, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. Journal of Infection and Chemotherapy. 2012;18(6):891-897. [CrossRef]
- Maldeghem I van, Nusman CM, Visser DH. Soluble CD14 subtype (sCD14-ST) as biomarker in neonatal early-onset sepsis and late-onset sepsis: A systematic review and meta-analysis. BMC Immunol. 2019;20(1):17. [CrossRef]
- Poggi C, Bianconi T, Gozzini E, Generoso M, Dani C. Presepsin for the detection of late-onset sepsis in preterm newborns. Pediatrics. 2015;135(1):68-75. [CrossRef]
- Wu CC, Lan HM, Han ST, et al. Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and C-reactive protein: A systematic review and meta-analysis. Annals of Intensive Care. 2017;7(1). [CrossRef]
- Zheng Z, Jiang L, Ye L, Gao Y, Tang L, Zhang M. The accuracy of presepsin for the diagnosis of sepsis from SIRS: A systematic review and meta-analysis. Annals of Intensive Care. 2015;5(1):1-13. [CrossRef]
- Liu Y, Hou J huan, Li Q, Chen K jun, Wang SN, Wang J min. Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome: A systematic review and meta-analysis. SpringerPlus. 2016;5(1). [CrossRef]
- Kondo Y, Umemura Y, Hayashida K, Hara Y, Aihara M, Yamakawa K. Diagnostic value of procalcitonin and presepsin for sepsis in critically ill adult patients: A systematic review and meta-analysis. J Intensiv Care. 2019;7(1):22. [CrossRef]
- Yang HS, Hur M, Yi A, Kim H, Lee S, Kim SN. Prognostic value of presepsin in adult patients with sepsis: Systematic review and meta-analysis. PLoS ONE. 2018;13(1):e0191486. [CrossRef]
- Zhu Y, Li X, Guo P, Chen Y, Li J, Tao T. The accuracy assessment of presepsin (sCD14-ST) for mortality prediction in adult patients with sepsis and a head-to-head comparison to PCT: A meta-analysis. Therapeutics and Clinical Risk Management. 2019;15:741-753. [CrossRef]
- Ali FT, Ali MAM, Elnakeeb MM, Bendary HNM. Presepsin is an early monitoring biomarker for predicting clinical outcome in patients with sepsis. Clinica Chimica Acta. 2016;460:93-101. [CrossRef]
- Yu H, Qi Z, Hang C, Fang Y, Shao R, Li C. Evaluating the value of dynamic procalcitonin and presepsin measurements for patients with severe sepsis. Am J Emerg Med. 2017;35(6):835-841. [CrossRef]
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of Activities of Daily Living in Older Adults After Hospitalization for Acute Medical Illness. J Am Geriatr Soc. 2008;56(12):2171-2179. 2171. [CrossRef]
- Wynn JL, Wong HR. Fetal and Neonatal Physiology (Fifth Edition). Sect XXVI: Pathophysiol Neonatal Dis. 2017;(J Immunol19272014):1536-1552.e10. [CrossRef]
- Sampson D, Yager TD, Fox B, et al. Blood transcriptomic discrimination of bacterial and viral infections in the emergency department: A multi-cohort observational validation study. BMC Med. 2020;18(1):185. [CrossRef]
- Kwizera A, Baelani I, Mer M, et al. The long sepsis journey in low- and middle-income countries begins with a first step...but on which road? Crit Care. 2018;22(1):64. [CrossRef]
- Maruna P, Nedelníková K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49(1):S57-61.
- Dong R, Wan B, Lin S, et al. Procalcitonin and Liver Disease: A Literature Review. J Clin Transl Hepatol. 2019;7(1):51-55. [CrossRef]
- Oberhoffer M, Stonans I, Russwurm S, et al. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Lab Clin Med. 1999;134(1):49-55. [CrossRef]
- Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Investig. 2003;111(12):1805-1812. 1805. [CrossRef]
Table 1.
Admission levels of presepsin - comparison between sepsis and non sepsis, and suvivor and non survivors. Cutoff values of presepsin in all stages of life.
Table 1.
Admission levels of presepsin - comparison between sepsis and non sepsis, and suvivor and non survivors. Cutoff values of presepsin in all stages of life.
| Age Group | Author | Admission Medium PSP Levels (ng/mL) | Cutoff Values (ng/mL) | |||
|---|---|---|---|---|---|---|
| Sepsis | Non Sepsis | Survivor | Non Survivor | |||
| Neonates & children | Poggi et al. 2015 [107] | 1295 | 562 | - | - | 885 |
| Pugni et al. 2015 [79] | - | 649 | - | - | - | |
| Montaldo et al. 2016 [80] | 598 | 328 | - | - | 788 * | |
| Korpelainen et al. 2017 [84] | 1432 | - | - | - | - | |
| Bellos et al. 2018 [82] | - | - | - | - | 650–850 ** | |
| Baraka et al. 2018 [86] | 1014 | 178 | - | - | Multiple | |
| Yoon et al. 2019 [83] | - | - | - | - | 650 ** | |
| Puspaningtyas et al. 2023 [77] | 806.5 | 717 | - | - | 761 * | |
| Adults | Shozushima et al. 2011 [104] | 817.9 | 190 | - | - | 399 |
| Endo et al. 2012 [105] | 1579 | 312 | - | - | Multiple | |
| Giavarina et al. 2015 [87] | 55-184 | - | - | - | - | |
| Ali et al. 2016 [114] | 1183 | 472 | 615,5 | 1301 | Multiple | |
| Yu et al. 2017 [115] | - | - | 1230,5 | 1269 | - | |
| Claessens et al. 2017 [99] | 476 | 200 | - | - | - | |
| Ikeda et al. 2019 [89] | - | - | 3251 | 1108 | - | |
| Zvyagyn et al. 2019 [88] | - | - | 1718 | 3266 | - | |
| Dragoş et al. 2023 [96] | 1039 | 372 | - | - | - | |
| Old adults | Imai et al. 2019 [97] | 639.93 | 866.56 | - | - | 285 |
| Ruangsomboon et al. 2020 [98] | 746 | 316 | 470 | 795 | Multiple | |
* Best of multiple values; ** Best accuracy values in the metanalysis.
Table 2.
Positive and negative aspects of presepsin in all stages of life.
| Aspects | Pediatric | Adult | Elderly |
|---|---|---|---|
| Positive | Early elevation, affordable cost, better diagnostic performance (PCT and CRP) and prognostic validity (30-day mortality), monitoring of antibiotic therapy, levels not influenced by gestational age, predictor of clinical evolution in febrile neutropenics | Better prognostic validity (PCT, CRP, ESR), correlation with hospital mortality in sepsis and septic shock, prognostic validity (28-day mortality), correlation with clinical outcomes, stable in different clinical scenarios (cirrhosis, rheumatoid arthritis, febrile neutropenia) | A better predictor of bacteremia in the Emergency Department (PCT, CRP), similar diagnostic accuracy to PCT, similar prognostic accuracy (qSOFA, SIRS) |
| Negative | Poor predictor of bacterial infection (PCT), non-standardized cutoff points, inaccessible in most scenarios | Poor predictor of bacterial infection (PCT), requires adjustments when kidney function is altered | Diagnostic and prognostic accuracy lower than combination (PCT + CRP + PSP), major renal dysfunction in older adults, specific cutoff point (immunosenescence) |
PSP: presepsin; CRP: C-reactive protein; PCT: procalcitonin; ESR: erythrocyte sedimentation rate; qSOFA: quick Sequential Organ Failure Assessment; SIRS: systemic inflammation response syndrome.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
supplementary.pdf (212.76KB )
Submitted:
25 September 2024
Posted:
25 September 2024
You are already at the latest version
Alerts
supplementary.pdf (212.76KB )
This version is not peer-reviewed
Submitted:
25 September 2024
Posted:
25 September 2024
You are already at the latest version
Alerts
Abstract
Sepsis is a pervasive condition that affects individuals of all ages, with significant social and economic consequences. Early diagnosis of sepsis is fundamental for establishing appropriate treatment and is based on warning scores and clinical characteristics, with positive microbiological cultures being the gold standard. Research has yet to identify a single biomarker to meet this diagnostic demand. Presepsin is a molecule that has the potential as a biomarker for diagnosing sepsis. In this paper, we present a narrative review of the diagnostic and prognostic performance of presepsin in different age groups. Given its particularities, it is identified that presepsin is a potential biomarker for sepsis at all stages of life.
Keywords:
Subject: Medicine and Pharmacology - Clinical Medicine
1. Introduction
Sepsis is a critical clinical condition defined as life-threatening organ dysfunction secondary to a deregulated host response to infection [1]. The high prevalence of sepsis, coupled with elevated mortality rates associated with severe sepsis, imposes economic costs and exacts a heavy toll on human lives [2]. Sepsis affects individuals across all age groups, with due consideration to the specific characteristics of each group.
Cardiovascular diseases and sepsis are two of the most significant contributors to human mortality [3,4]. Regarding cardiovascular diseases, heightened awareness of early signs and improvements in health services and professional training have resulted in a commendable success rate for early diagnosis [5]. Conversely, concerning sepsis, there is a lack of public understanding regarding the risks associated with infections, and healthcare professionals face challenges in identifying clear clinical signs for early diagnosis [6]. Early detection scores such as the quick Sequential Organ Failure Assessment (qSOFA) and systemic inflammatory response syndrome (SIRS) criteria and protocol adherence can facilitate the timely diagnosis and treatment of sepsis [7]. However, these scores need more inputs in their construction to ensure sensitivity and specificity for widespread use in clinical practice [8]. Identifying new biomarkers for sepsis diagnosis and prognosis becomes an essential goal in this context.
Presepsin is a biomarker with significant diagnostic and prognostic potential, making it superior to conventional biomarkers such as C-reactive protein (CRP) or procalcitonin (PCT) [9]. This analytical review aims to identify original studies in the literature that utilize presepsin as a biomarker for sepsis across all age groups, from neonates to nonagenarians, encompassing young adults and adults.
2. Demographics of Sepsis
Statistics from the USA indicate that sepsis was associated with 201,092 deaths in 2019 [10]. It is the primary cause of in-hospital deaths, accounting for an estimated 19.7% of the overall death rate [3]. The global incidence of sepsis exhibits a bimodal distribution, with peaks occurring in childhood and older adults [3].
It is the leading cause of death among infants and children, with an estimated 1.6 million cases per year [11], despite some variability in its occurrence, depending on the diagnostic strategy [2,12]. In the neonatal period, sepsis occurs in 1 to 5 cases per 1000 live births [13], with an overall mortality rate of 24.4%. However, this rate can escalate to as high as 54% in premature infants under 24 weeks of gestation [14].
Research indicates that >60% of sepsis cases occur in patients aged ≥65 years [15]. Mortality from sepsis in older adults constitutes approximately three-quarters of all sepsis-related deaths in the USA, particularly among individuals over the age of 65 [10]. Although this index declined between 2000 and 2019, it continues to rise with age, increasing five times in those over 85 [16].
3. Pathophysiology and Immunological Aspects of Sepsis across Ages
Comprehending the pathophysiology of sepsis requires a solid understanding of the intricate interaction among various domains, precisely the convergence of the inflammation pathway with the coagulation system, leading to endothelial stimulation and microcirculatory dysfunction [17]. This framework underpins the exploration of potential biomarkers, diagnostic approaches, optimal treatment durations, and the management of antibiotic therapy [17]. As an illustration, we can consider the activation and dysfunction of endothelial cells induced by sepsis, a phenomenon that diminishes with advanced age [18]. In this section, we delve into some age-related characteristics of the sepsis response.
Sepsis in the pediatric population is a distinct entity characterized by specific features in the host response to infection and therapy. It is crucial to understand the developmental differences that set it apart from adult sepsis [19]. Neonatal sepsis, occuring within the first 28 days of life, presents its unique aspects, with maternal risk factors (chorioamnionitis, premature membrane rupture, premature pregnancy, prolonged membrane rupture, and intrapartum maternal fever) and risk factors associated with the neonate (prematurity, low birth weight, fetal distress, and low Apgar score) [20]. Neonatal sepsis can be acquired from the mother during intrauterine life or through postpartum care [20]. After the neonatal stage, the clinical signs of pediatric sepsis are nonspecific. They can be exacerbated by birth conditions or adaptation to extrauterine life [21], often resulting in a delayed diagnosis [20]. Therefore, the diagnosis is presumptive in many cases, and treatment is based on clinical findings and nonspecific laboratory tests.
Moreover, the definitions of sepsis currently used for this age group are an extrapolation of the criteria used for adults [22], needing more validation for pediatric patients, which results in a low predictive value [23]. This diagnostic challenge has recently been addressed to validate new pediatric sepsis and septic shock criteria through organic dysfunction variables called Phoenix criteria [24]. Despite its limitations, the tool made it possible to identify sepsis and septic shock, enabling improvements in clinical care and research aimed at pediatric patients [23].
The inflammatory response to sepsis in pediatric patients suggests a predominantly anti-inflammatory phenotype, which is exacerbated compared to that in adults [25]. The immaturity of the adaptive immune system causes the neonate to become more dependent on the innate immune system [26].
In children, sepsis induces an immune response characterized initially by a pro-inflammatory state, promoting classic symptomatology such as fever, tachycardia, and tachypnea [27], making it clinically indistinguishable from the inflammatory response caused by other etiologies, posing challenges for its diagnosis by pediatricians [28]. Although there is no cohesive understanding of the mechanisms involved in sepsis [29], this pro-inflammatory phase is followed by the immunoparalysis phase, characterized by anti-inflammatory activity [27]. Such information corroborates the notion that a developmental difference exists in the inflammatory response to infection or injury among children and adults, exemplified by its pattern of organ failure following sepsis [25].
The prognosis of sepsis in pediatric patients is associated with lactate clearance as well as physiological variables in the first 4 hours after admission to the intensive care unit [30]. There also appears to be a correlation between genetic profiles and endotypes for septic shock in childhood [31], suggesting the possible existence of subclasses of response in sepsis. Thus, corticotherapy may be beneficial in those subgroups [31]; however, developing clinical trials to understand immunophenotypes and their relation to immunoparalysis must improve the prognosis of childhood sepsis [27].
Scientific research on sepsis has more widely included adults. A recent study identified a relative increase in sepsis diagnoses in the 18–44 age group, possibly due to greater awareness of the syndrome in this age range [32].
The final effect on the immunological phenotype (hypo- or hyper-reactivity) is variable and individualized and depends on the molecular heterogeneity of the septic syndrome. Thus, the differentiated activation of the innate and adaptive immune systems has allowed the identification of three subgroups based on mRNA expression profiles (transcriptomics): "inflammopathic" (characterized by innate immune activation, called SRS1 and linked to higher mortality), "adaptive" (adaptive immune activation, called SRS2 and linked to lower mortality), and "coagulopathic" (with platelet degranulation and coagulation dysfunction, related to higher mortality and the older adults population).
In this age group, the inflammatory and immunosuppressive responses are simultaneous and exhibit interindividual variation in the conceptual model of immune trajectories before, during, and after sepsis [33]. Thus, chronic hyperinflammation and immunosuppression have a prolonged clinical trajectory, known as persistent inflammation/immunosuppression and catabolism syndrome [34,35]. This endotype leads to chronic critical illness, characterized by impaired functional status, rehabilitation failure, and increased mortality [35].
Despite the heterogeneity in biological ages among individuals of the same chronological age [36], sepsis in older adults holds significance due to its association with increased morbidity [37], positioning it as the quintessential disease affecting this demographic [36].
Older age can be considered an independent predictor of mortality [15,39] despite the more encouraging results in a subgroup of patients over 85 obtained in a recent study [32], which showed a reduction in mortality (< 50%) compared to previous studies [40]. As the initial signs of sepsis in this age group may be invisible, progression to septic shock can be rapid2, highlighting the particular importance of early diagnosis in this age group.
4. Biomarkers and Sepsis
Biomarkers reflect the state of health or disease at a molecular level [41]. They improve diagnosis, define subsets of diseases that may differ in response, as well as individual variability in the drug’s molecular target, and provide an early reading of the response to therapy, among other functions. The search for new molecules with this purpose has been identified as a high priority for science [42] as part of the challenge of implementing "Personalized Medicine" [41].
In the case of sepsis, the question is whether it is possible to discriminate among septic patients, which subgroups share specific biological characteristics, who are at risk of unfavorable outcomes, and who are at risk of organ failure [28].
Although no ideal single biomarker or even combination of biomarkers serves this purpose in the international consensuses on sepsis [1,20], their use in this context is commonplace because, besides being an important aid in diagnosis, they enable us to predict possible sepsis syndrome outcomes [43]. Unfortunately no single one can reliably perform as a stand-alone sepsis biomarker [44,45,46,47].
Biomarkers represent the host response and their aberrant behavior—with persistent proinflammation (CRP), maintenance of immunosuppression (IL-10, soluble programmed death ligand-1 [PDL-1]), continuation of stress metabolism (glucagon-like peptide-1), absence of anabolism, and anti-angiogenesis (insulin-like growth factor-1, insulin-like growth factor-binding protein-3) for >14 days—indicate progression to chronic critical illness [48]. These molecules cannot represent the uncontrolled inflammation and increased vascular permeability that characterizes sepsis, leading to hypotension and organ dysfunction [49]. Therefore, to develop rapid assessment and differentiation between infection and inflammation, biomarker research aims to enable point-of-care testing among many molecules [49]. However, new biomarkers may not present superior results to traditional ones, frustrating expectations of benefit, suggesting their aid after evaluation with the usual scales and biomarkers [50].
Among the various functions necessary for the ideal biomarker, it should be precise for guiding therapeutic decision-making [51]. However, its measurement is impaired due to critical disadvantages, such as the collecting timing and the insufficiency of standardization (Table 1). Although traditionally measured at single time, gathering biomarker at several time interval may show a better overview of the host response to sepsis [44].
Guiding therapeutic decisions should be one of the ideal features of sepsis biomarkers [51]. In this context, deriving diagnostic algorithms appears to be a reliable strategy for early diagnosis of sepsis, integrating the pretest probability of infection, clinical features and results of in vitro diagnostic testing [52].
Considering the pathological process, the disease stage, and individual patient characteristics, a personalized therapeutic strategy could be provided by a biomarker-guided approach, avoiding “one size fts all” sepsis therapies [53]. In other words, sepsis research must consider the individual immune status or likely response to specific treatment to avoid harmuful therapy to a patient with a particular immune response activation pattern [53].
In addition to all the issues addressed so far, we must incorporate the key concept of value-based medicine, which involves cost-effectiveness studies, comparing different interventions, and defining the viability of diagnostic means. This is fundamental in a world of limited resources [46].
In pediatrics, most researchers agree that diagnostic priority depends on clinical signs and not biomarkers, even though sepsis has a polymorphic presentation [54]. CRP and PCT have been the most widely used biomarkers in pediatric clinical practice, with the recommendation that they must be used simultaneously to increase the efficiency of the results [54]. However, low accuracy is observed, as well as variable sensitivity and specificity for detecting bacterial infection via polymerase chain reaction (PCR) (lower when a single measurement is performed) [55]. On the other hand, PCT also has some limitations, such as variable sensitivity and specificity, altered serum levels in cases of kidney dysfunction, a lack of multicenter and prognostic studies and risk stratification, and higher costs55. Lactate is used to corroborate the diagnosis of septic shock and assess the response to therapy; however, normal or slightly elevated levels do not rule out the development of sepsis and septic shock; therefore, it is of limited effectiveness in children [56].
The medical literature comprises thousands of studies evaluating the applicability of biomarkers in adult sepsis, reporting >200 potential candidate molecules for the early diagnosis of sepsis [57]. However, methodological biases in many of these articles create limitations [58]. Due to these issues and insufficient evidence, only a few are suitable for everyday clinical use, with CRP, PCT, IL-6, and presepsin among the most promising [58]. No single biomarker has sufficient diagnostic power to be used independently; instead, a panel of biomarkers is considered the best option for a point-of-care approach to sepsis [59].
The specificity and sensitivity of biomarkers can be influenced by age. Thus, a moderate to marked increase in biomarkers such as CRP, an inflammatory peptide associated with immunosenescence, can be expected with advancing age [60]. This molecule is one of the substances linked to aging-related inflammation, and its increase is described as characteristic of the aging process [61]. In adult and older adults hospitalized with sepsis, CRP can rise within 72 hours and remain elevated for extended periods in older adults, even after they are discharged from the hospital [62]. This marker has been linked to poorer clinical outcomes in these patients [63].
It seems that patients who have subclinical inflammation at the time of discharge are more likely to have a higher risk of death, as indicated by persistently elevated inflammatory biomarker levels [63]. Patients over 65 tend to have a higher baseline inflammation, as reflected in higher inflammatory biomarker levels upon admission. However, these levels converge with those found in other age groups within the first 72 hours [62].
However, contrasting perspectives exist as some research groups have yet to identify a robust association between aging and markers of systemic inflammation or cytokine release in sepsis [18]. Furthermore, older adults experiencing sepsis display a dampening of endothelial cell activation, termed endothelial tolerance. Significantly, this phenomenon is attributed to the septic event rather than age [18].
5. Presepsin as a Sepsis Biomarker across Age Groups
Presepsin is a molecule identified in many cells involved in the sepsis cascades, including macrophages, monocytes, and granulocytes, and is responsible for the intracellular transduction of endotoxin signals [64]. Granulocytes phagocytize bacteria and CD14 and secrete presepsin into the blood within 2 hours after enzymatic digestion [65]. During the induction of systemic inflammation, the increase in presepsin levels occurs earlier and more rapidly than other sepsis markers [64].
Presepsin has advantages that justify its use, such as its early elevation in infection [66], high accuracy [20], and affordability compared to the gold standard blood culture test (US$ 7 versus US$ 11-89) [67,68]. It also exhibits better prognostic validity than PCT, CRP, and erythrocyte sedimentation rate (ESR) [69,70]. Presepsin’s advantages can be explained by its correlation with the sepsis pathophysiology, unlike other biomarkers resulting from a general inflammatory reaction [71]. However, it showed inferior performance to PCT as a predictor of bacterial infection [70] (Figure 1).
The availability of laboratory assays that can measure presepsin in 17 minutes is another factor that has made it a promising marker in sepsis [64,72,73,74,75,76,77]. However, its use has disadvantages, such as non-standardized cutoff points and the fact that it is inaccessible in most clinical settings [20]. As discussed below, its use as a biomarker should be customized according to the age group, as the threshold values can vary.
Figure 1.
Mechanisms of presepsin, procalcitonin, and C-reactive protein production [72,120,121,122,123]. (*) The molecular complex LPS-LBP-mCD14-M2-TLR is internalized into a phagolysosome; proteolysis and internalization processes release presepsin (PSP), which is released in circulation after exocytosis. CD 14 promotes the expression of genes responsible for the immune response, such as cytokine production [72]. (**) The rise of TNF, IL-1, IL-2, and IL-6 levels increases PCT [120]. (***) the liver is considered to be the most important site of production of PCT during an inflammatory response, especially those induced by bacterial infections [121]. (#) Peripheral blood mononuclear cells express PCT both on mRNA and on protein levels [122]. (##) CRP is an acute-phase protein, and its synthesis is rapidly upregulated, principally in hepatocytes, under the control of cytokines [123]. LPS: lipopolysaccharide, TLR: Toll-like receptor; LBP: Lipoprotein Binding Protein; mCD14: membrane-bound CD14; M2: co-protein of TLR; TNF: tumor necrosis factor; IL-1: interleucin-1; IL-6: interleucin-6; CPR: C-reactive protein; PCT: procalcitonin; PSP: presepsin.
Figure 1.
Mechanisms of presepsin, procalcitonin, and C-reactive protein production [72,120,121,122,123]. (*) The molecular complex LPS-LBP-mCD14-M2-TLR is internalized into a phagolysosome; proteolysis and internalization processes release presepsin (PSP), which is released in circulation after exocytosis. CD 14 promotes the expression of genes responsible for the immune response, such as cytokine production [72]. (**) The rise of TNF, IL-1, IL-2, and IL-6 levels increases PCT [120]. (***) the liver is considered to be the most important site of production of PCT during an inflammatory response, especially those induced by bacterial infections [121]. (#) Peripheral blood mononuclear cells express PCT both on mRNA and on protein levels [122]. (##) CRP is an acute-phase protein, and its synthesis is rapidly upregulated, principally in hepatocytes, under the control of cytokines [123]. LPS: lipopolysaccharide, TLR: Toll-like receptor; LBP: Lipoprotein Binding Protein; mCD14: membrane-bound CD14; M2: co-protein of TLR; TNF: tumor necrosis factor; IL-1: interleucin-1; IL-6: interleucin-6; CPR: C-reactive protein; PCT: procalcitonin; PSP: presepsin.

5.1. Presepsin as a Sepsis Biomarker in Neonates and Children
Due to its superior diagnostic performance compared to PCT and CRP [78], presepsin use has been highlighted in neonatal sepsis. Among healthy neonates, presepsin has an average plasmatic value of 649 ng/L and 720 ng/L in premature infants [79] (Table 1). A cutoff point of 788 ng/L, 93% sensitivity, and 100% specificity was obtained to diagnose early sepsis in premature infants [80] (Table 1). Its use is advocated for monitoring antibiotic therapy, as its levels decrease when treatment is effective [81]. In neonates with infection, it has the advantage that its levels are not influenced by gestational age or other perinatal factors [78]. High serum values also increase 30-day mortality [82].
Despite having demonstrated good accuracy in several studies, the use of PSP as a toll in the diagnosis and prognosis of neonatal sepsis still requires refinement. The differentiation of biomarker behavior between term and preterm neonates [79,80], between early onset (in the first 72 hours of life) and late onset [20,80,81], among others. In this age group, the diagnostic process must be remarkably rapid because, in addition to threatening life, it is a potential cause of permanent sequelae in survivors [20]. Therefore, some answers are necessary to consolidate the role of PSP as a biomarker in newborns, especially the diagnostic cutoff values, a topic that is still controversial (Table 1). Celerity, sensitivity, and specificity would reduce unnecessary treatments in symptomatic, low-risk individuals.
In children, presepsin shows similar responses; in a recent meta-analysis, presepsin showed high sensitivity and diagnostic accuracy compared to PCR and PCT but lower specificity [83]. The usefulness of presepsin extends to individuals with hematological neoplasms, where it can be a good predictor of clinical evolution with septic shock in febrile neutropenics [84]. In these patients, when there is no detectable site of infection, higher levels of presepsin can anticipate the positive result of cultures, discriminating the infectious origin of the febrile condition [85,86] (Table 2).
Biomarkers in pediatric sepsis are a valuable aid in promptly and cautiously diagnosing sepsis. Despite the emergence of promising options, such as genomic biosignature [29], older biomarkers, including CRP, PCT, ferritin, and lactate, despite their varying levels of reliability, continue to serve as useful clinical adjuncts in diagnosis [29]. Moreover, they are more readily available in most pediatric institutions [29].
Additionally, laboratory tests can determine the severity of sepsis, such as quantifying dynamic changes in levels of the antigen-presenting molecule human leukocyte antigen-DR isotype or the production of TNF-α upon stimulation (the latter representing the hyporeactivity of the innate immune system) [27].
5.2. Presepsin as a Sepsis Biomarker in Adults
As discussed previously, many current studies focus on adults, who benefit most from the results validated by scientific literature.
With average plasmatic levels of 202 pg/mL in healthy individuals [87], the increase of presepsin levels in the bloodstream correlates with the pathophysiology of sepsis rather than a general inflammatory reaction [71]. This characteristic gives it better prognostic validity than PCT, CRP, and ESR [69,70].
Presepsin levels have been shown to correlate with the severity and in-hospital mortality of patients with sepsis and septic shock [9], with mean values of 1718 and 3266 pg/ml for survivors and nonsurvivors, respectively [88] (Table 1). In a 28-day survival period, significant values of 1108 vs. 3251 pg/mL were obtained for survivors and nonsurvivors, respectively [89] (Table 1). Blood level changes, both absolute (increase above 500 pg/L) [90] and relative (reduction of >50% between admission and the seventh day) [91], correlated with unfavorable and favorable clinical outcomes, respectively. Due to its stability in various acute or chronic clinical scenarios, presepsin has helped detect sepsis in liver cirrhosis [92], rheumatoid arthritis [93], and febrile neutropenia [94], among others.
5.3. Presepsin as a Sepsis Biomarker in Older Adults
Studies suggest that presepsin could be more valuable than PCT and CRP as a predictor of bacteremia in older adult patients admitted to the emergency department. It showed significantly higher values than those without bacteremia (866.6 ± 184.6 vs. 639.9 ± 137.1 ng/L, p = 0.03) [97] (Table 1). It showed similar diagnostic and prognostic accuracy to PCT and early warning scores (qSOFA and SIRS), with the combination of the three biomarkers being superior to the use of anyone alone [98].
Aging was found to be an independent predictor of increased blood presepsin levels [99], with a significant difference comparing over 70 and under-70 age groups (470 [380–601] ng/L vs. 300 [201–457] ng/L, P < 0.001) [87]. Notably, age-related changes in renal and vascular function, such as glomerulosclerosis, vascular dysautonomia, altered tubular management of creatinine, and reduced renal reserve100, increase presepsin levels in renal dysfunction [100,101,102]. A study revealed that in older adult patients, hypercreatinemia raises the presepsin threshold value to 706 ng/L, enabling a diagnosis of sepsis [103].
However, there are caveats in the literature. Some authors postulate that for individuals over 75 years of age, a cutoff point of 380 pg/mL would be more appropriate [98]. This differs from the findings of systematic reviews focusing on predominantly younger populations, in which levels as high as 600 ng/L were found [104,105]. The rationale for this lies in the origin of presepsin; it comes from granulocytes, which are dysfunctional in this age group and hyporesponsive to infectious stimuli [98], a characteristic of immunosenescence (Table 2).
6. Published Meta-Analysis on Presepsin as Sepsis Biomarker
Published meta-analyses corroborate the promising role of presepsin as a biomarker in sepsis. In a search covering the period from 2010 to the present, several meta-analyses were found on using presepsin in neonatal sepsis. This search evaluated 28 studies and 2505 patients, recognizing the diagnostic value of presepsin in early-onset sepsis (i.e., occurring in the first 72 hours of life) [20] and late-onset sepsis [83,106,107].
The meta-analyses involving adults and older adults, evaluating the efficacy of presepsin in the context of sepsis, showed six meta-analyses in a literature review covering the period from 2010 to the present. It covered 20,544 patients in 141 selected studies, which, in general, showed good or moderate diagnostic accuracy in differentiating septic and nonseptic patients [108,109,110], indicating its suitability as a biomarker similar to PCT in the early diagnosis of sepsis [111] and showing relevant prognostic value [112,113]. None of these meta-analyses categorized older adult patients into subgroups, with mean ages ranging from 55.2 years [114] to 74 years [115], demanding efforts to conduct this type of study on the older adult population or to analyze a subgroup of this age group to support the understanding of sepsis in this population.
7. Discussion
The pathophysiological complexity of sepsis is acknowledged as the primary impediment to developing validated biomarkers, with current emphasis on the extensive study of inflammatory markers [17]. Nevertheless, distinct age groups manifest unique characteristics in their immune responses, encompassing both pro- and anti-inflammatory aspects and phenomena like immunoparalysis and immunosenescence. This variation aligns with differences in clinical and laboratory presentations, particularly concerning the levels of inflammatory biomarkers.
Prioritizing the characterization of septic syndrome behavior across different age groups affected by it is essential. Gaining insights into this aspect and recognizing the relative significance of biomarkers can aid in developing reproducible tools. These tools, in turn, facilitate the translation of clinical research findings into practical applications at the bedside.
The clinical and laboratory characteristics of different age groups present diagnostic challenges. In newborns, biomarkers show great potential for improving diagnosis, as blood cultures, considered the gold standard, have limitations. Blood cultures typically require a long turnaround time, ranging from 6 hours to 5 days for microorganisms to reach detectable levels, with an additional 24–48 hours needed for antibiotic susceptibility testing [51]. However, in newborns, the sensitivity of blood cultures is often reduced due to factors such as low blood volume during collection, low or intermittent bacteremia, and maternal antibiotic therapy [78], which can contribute to false-negative results [81]. Additionally, biomarkers provide insights into the newborn’s response to therapeutic interventions [13,107], thus potentially reducing the indiscriminate use of antibiotics.
Similarly, presepsin has shown diagnostic and prognostic value in adult studies, where we found the most significant number of publications. Consequently, its absolute plasma values and dynamic changes [115] have been described in various clinical situations, whether associated with sepsis. This predominance, combined with the intrinsic diagnostic difficulty of septic syndrome, strengthens the prominence gained by PSP as an adjunct in propaedeutics.
Despite comprising a substantial proportion of intensive care unit (ICU) patients, older adults must be more adequately represented in clinical trials, hindering the development of targeted protocols [36]. This underrepresentation can be attributed to age discrimination and significantly influences the formulation of public health policies for conditions such as sepsis.
Nevertheless, older adult survivors of intensive care frequently encounter sequelae and an accelerated age-related functional decline [116]. This scenario underscores the imperative for heightened support post-hospital discharge, particularly evident in 37.3% of patients over 85 years [32]. This population’s unique characteristics and specific needs emphasize the requirement for enhanced scientific rigor in studies encompassing this demographic. Focused clinical research on this cohort would yield invaluable insights for clinical decision-making, highlighting the importance of utilizing biomarkers to inform and streamline the process.
We observe a growing endorsement for personalized medicine, extending beyond ethnic groups to encompass individualized treatment strategies. The rationale for tailored therapies is firmly grounded in robust theoretical frameworks. Early diagnosis is pivotal in accelerating the protracted and time-intensive propaedeutic process. Hence, it becomes imperative to streamline the diagnostic trajectory of sepsis by seamlessly integrating clinical and laboratory data. This integration facilitates the anticipation of therapeutic decisions and interventions, mitigates potential complications, and optimizes overall outcomes. In pursuing a personalized, pragmatic, and efficient approach to sepsis, utilizing a multi-biomarker model propelled by genomic tools holds promise for future disease management11.
A promising trajectory for the future of sepsis management may lie in "omics" approaches, encompassing genomics, proteomics, metabolomics, and transcriptomics, alongside noteworthy strides in therapeutic interventions to optimize outcomes [117]. An illustrative instance involves the application of transcriptomic analysis panels to blood samples, enabling a precision-oriented approach in administering antimicrobials for the targeted exclusion of bacterial infections [118].
While there is a growing global awareness of sepsis, this heightened recognition has yet to translate into a substantive improvement in its management, particularly in developing or low-income countries [119]. Vulnerable populations in these regions necessitate tailored strategies, given the unavailability or unaffordability of expertise and technologies [119]. Biomarkers, emerging as promising tools, offer potential alternatives to facilitate decision-making and should be integrated into health policies.
8. Conclusions
Due to its unique characteristics, presepsin stands out as a promising biomarker for the diagnosis, therapeutic monitoring, and prognosis of sepsis across all age groups. Incorporating presepsin into quality improvement programs and consensus guidelines hinges on the foundation of rigorous research that validates its efficacy and solidifies its routine application.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Generative Intelligence Application
This article was initially written in Brazilian Portuguese and subsequently translated into English by human translators. Generative Intelligence was employed to enhance the quality of the English writing. It is important to note that no part of the manuscript contains text generated exclusively by Generative AI.
Acknowledgments
No funding was received.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. [CrossRef]
- Martín S, Pérez A, Aldecoa C. Sepsis and Immunosenescence in the Elderly Patient: A Review. Front Med. 2017;4:20. [CrossRef]
- Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet. 2020;395(10219):200-211. [CrossRef]
- Vaduganathan M, Mensah GA, Turco JV, Fuster V, Roth GA. The Global Burden of Cardiovascular Diseases and Risk A Compass for Future Health. J Am Coll Cardiol. 2022;80(25):2361-2371. [CrossRef]
- Kerrigan SW, Martin-Loeches I. Public awareness of sepsis is still poor: We need to do more. Intensiv Care Med. 2018;44(10):1771-1773. [CrossRef]
- Fiest KM, Krewulak KD, Brundin-Mather R, et al. Patient, Public, and Healthcare Professionals’ Sepsis Awareness, Knowledge, and Information Seeking Behaviors: A Scoping Review*. Crit Care Med. 2022;50(8):1187-1197. [CrossRef]
- Kim HI, Park S. Sepsis: Early Recognition and Optimized Treatment. Tuberc Respir Dis. 2018;81(1):6-14. [CrossRef]
- Luo J, Jiang W, Weng L, et al. Usefulness of qSOFA and SIRS scores for detection of incipient sepsis in general ward patients: A prospective cohort study. J Crit Care. 2019;51:13-18. [CrossRef]
- Behnes M, Bertsch T, Lepiorz D, et al. Diagnostic and prognostic utility of soluble CD 14 subtype (presepsin) for severe sepsis and septic shock during the first week of intensive care treatment. Critical Care. 2014;18(5). [CrossRef]
- CDC C of DC and P. About Multiple Cause of Death, 1999-2020. About Multiple Cause of Death, 1999-2020. Published September 8, 2023. Accessed July 23, 2023.
- Oikonomakou MZ, Gkentzi D, Gogos C, Akinosoglou K. Biomarkers in pediatric sepsis: A review of recent literature. Biomark Med. 2020;14(10):895-917. [CrossRef]
- Balamuth F, Weiss SL, Neuman MI, et al. Pediatric Severe Sepsis in U.S. Children’s Hospitals* Pediatr Crit Care Med. 2014;15(9):798-805. [CrossRef]
- Gude SS, Peddi NC, Vuppalapati S, Gopal SV, Ramesh HM, Gude SS. Biomarkers of Neonatal Sepsis: From Being Mere Numbers to Becoming Guiding Diagnostics. Cureus. 2022;14(3):e23215. [CrossRef]
- Sharma D, Farahbakhsh N, Shastri S, Sharma P. Biomarkers for diagnosis of neonatal sepsis: A literature review. J Matern-Fetal Neonatal Med. 2018;31(12):1646-1659. [CrossRef]
- Martin GS, Mannino DM, Moss M. The effect of age on the development and outcome of adult sepsis* Crit Care Med. 2006;34(1):15-21. [CrossRef]
- Kramarow EA. Sepsis-related Mortality Among Adults Aged 65 and Over: United States, 2019. NCHS Data Brief. 2021 Nov;(422):1-8. [CrossRef] [PubMed]
- Arora J, Mendelson AA, Fox-Robichaud A. Sepsis: Network pathophysiology and implications for early diagnosis. Am J Physiol-Regul, Integr Comp Physiol. 2023;324(5):R613-R624. [CrossRef]
- Michels EHA, Butler JM, Reijnders TDY, et al. Association between age and the host response in critically ill patients with sepsis. Crit Care. 2022;26(1):385. [CrossRef]
- Wynn J, Cornell TT, Wong HR, Shanley TP, Wheeler DS. The Host Response to Sepsis and Developmental Impact. Pediatrics. 2010;125(5):1031-1041. [CrossRef]
- Hincu MA, Zonda GI, Stanciu GD, Nemescu D, Paduraru L. Relevance of Biomarkers Currently in Use or Research for Practical Diagnosis Approach of Neonatal Early-Onset Sepsis. Children. 2020;7(12):309. [CrossRef]
- Camargo JF de, Caldas JP de S, Marba STM. Early neonatal sepsis: Prevalence, complications and outcomes in newborns with 35 weeks of gestational age or more. Rev Paul Pediatr. 2021;40:e2020388. [CrossRef]
- Goldstein B, Giroir B, Randolph A, Sepsis ICC on P. International pediatric sepsis consensus conference: Definitions for sepsis and organ dysfunction in pediatrics* Pediatr Crit Care Med. 2005;6(1):2-8. [CrossRef]
- Schlapbach LJ, Kissoon N. Defining Pediatric Sepsis. JAMA Pediatrics. 2018;172(4):313-314. [CrossRef]
- Sanchez-Pinto LN, Bennett TD, DeWitt PE, et al. Development and Validation of the Phoenix Criteria for Pediatric Sepsis and Septic Shock. JAMA. 2024;331(8). [CrossRef]
- Barsness KA, Bensard DD, Partrick DA, Calkins CM, Hendrickson RJ, McIntyre RC. Endotoxin induces an exaggerated interleukin-10 response in peritoneal macrophages of children compared with adults. J Pediatr Surg. 2004;39(6):912-915. [CrossRef]
- Wynn JL, Scumpia PO, Winfield RD, et al. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood. 2008;112(5):1750-1758. [CrossRef]
- Hall M. Immune Modulation in Pediatric Sepsis. J Pediatr Intensiv Care. 2019;08(01):042-050. [CrossRef]
- Wong HR. Pediatric sepsis biomarkers for prognostic and predictive enrichment. Pediatr Res. 2022;91(2):283-288. [CrossRef]
- Lim PPC, Bondarev DJ, Edwards AM, Hoyen CM, Macias CG. The evolving value of older biomarkers in the clinical diagnosis of pediatric sepsis. Pediatr Res. 2023;93(4):789-796. [CrossRef]
- Kathmandu K children H, Jeevan GJ. Clinical, Demographic Profile and Outcome of Children Admitted in PICU with A Diagnosis of Severe Sepsis and Septic Shock. J. Med. Sci. Clin. Res.. 2017, 5(12). [CrossRef]
- Wong HR, Cvijanovich NZ, Anas N, et al. Developing a Clinically Feasible Personalized Medicine Approach to Pediatric Septic Shock. Am J Respir Crit Care Med. 2015;191(3):309-315. [CrossRef]
- Wardi G, Tainter CR, Ramnath VR, et al. Age-related incidence and outcomes of sepsis in California, 2008–2015. J Crit Care. 2021;62:212-217. [CrossRef]
- Poll T van der, Shankar-Hari M, Wiersinga WJ. The immunology of sepsis. Immunity. 2021;54(11):2450-2464. [CrossRef]
- Gentile LF, Nacionales DC, Lopez MC, et al. Protective Immunity and Defects in the Neonatal and Elderly Immune Response to Sepsis. J Immunol. 2014;192(7):3156-3165. 3156. [CrossRef]
- Darden DB, Kelly LS, Fenner BP, Moldawer LL, Mohr AM, Efron PA. Dysregulated Immunity and Immunotherapy after Sepsis. J Clin Med. 2021;10(8):1742. [CrossRef]
- Milbrandt EB, Eldadah B, Nayfield S, Hadley E, Angus DC. Toward an Integrated Research Agenda for Critical Illness in Aging. Am J Respir Crit Care Med. 2012;182(8):995-1003. [CrossRef]
- Jia L, Hao L, Li X, Jia R, Zhang HL. Comparing the predictive values of five scales for 4-year all-cause mortality in critically ill elderly patients with sepsis. Ann Palliat Med. 2021;0(0):6-6. [CrossRef]
- Wang Y, Dong C, Han Y, Gu Z, Sun C. Immunosenescence, aging and successful aging. Front Immunol. 2022;13:942796. [CrossRef]
- Martin-Loeches I, Guia MC, Vallecoccia MS, et al. Risk factors for mortality in elderly and very elderly critically ill patients with sepsis: A prospective, observational, multicenter cohort study. Ann Intensiv Care. 2019;9(1):26. [CrossRef]
- Kaukonen KM, Bailey M, Suzuki S, Pilcher D, Bellomo R. Mortality Related to Severe Sepsis and Septic Shock Among Critically Ill Patients in Australia and New Zealand, 2000-2012. JAMA. 2014;311(13):1308-1316. [CrossRef]
- Woodcock J. The Prospects for “Personalized Medicine” in Drug Development and Drug Therapy. Clin Pharmacol Ther. 2007;81(2):164-169. [CrossRef]
- Woodcock J, Woosley R. The FDA Critical Path Initiative and Its Influence on New Drug Development. Annu Rev Med. 2008;59(1):1-12. [CrossRef]
- im MH, Choi JH. An Update on Sepsis Biomarkers. Infect Chemother. 2020;52(1):1-18. [CrossRef]
- Llitjos, JF., Carrol, E.D., Osuchowski, M.F. et al. Enhancing sepsis biomarker development: Key considerations from public and private perspectives. Crit Care 28, 238 (2024). [CrossRef]
- Liang, P., Wu, Y., Qu, S. et al. Exploring the biomarkers and potential therapeutic drugs for sepsis via integrated bioinformatic analysis. BMC Infect Dis 24, 32 (2024). [CrossRef]
- Deltell JMM, Boter NR. Biomarkers in emergencies: A never-ending race? Emergencias. 2024 Jan;36(1):4-6. Spanish, English. [CrossRef]
- Kadim MM, AL-Dahmoshi HOM, AL-Khikan FHO. Sepsis biomarkers: Current information and future visions. Microbes and Infectious Diseases 2024; 5(4): 201-210.
- Mankowski RT, Anton SD, Ghita GL, et al. Older adults demonstrate biomarker evidence of the persistent inflammation, immunosuppression and catabolism syndrome (PICS) after sepsis. J Gerontol: Ser A. 2021;77(1):glab080-. [CrossRef]
- Feng L, Liu S, Wang J, Gao Y et al. The performance of a combination of heparin-binding protein with other biomarkers for sepsis diagnosis: An observational cohort study BMC Infectious Diseases (2024) 24:755. [CrossRef]
- Clemente C, Fuentes ME, Ortega D, Julián A, Martín-Sánchez FJ, González del Castillo J. Utilidad de la combinación de biomarcadores de respuesta inflamatoria y escalas clínicas para la estratificación del riesgo en pacientes atendidos en urgencias por sospecha de infección. Emergencias. 2024;36:9-16.
- Lippi, Giuseppe. "Sepsis biomarkers: Past, present and future" Clinical Chemistry and Laboratory Medicine (CCLM), vol. 57, no. 9, 2019, pp. 1281-1283. [CrossRef]
- Schuetz P, Beishuizen A, Broyles M, Ferrer R, Gavazzi G, Gluck EH, et al. Procalcitonin (PCT)-guided antibiotic stewardship: An international experts consensus on optimized clinical use. Clin Chem Lab Med 2019;57:1308–18.
- Vincent JL, van der Poll T, Marshall JC. The End of "One Size Fits All" Sepsis Therapies: Toward an Individualized Approach. Biomedicines. 2022 Sep 12;10(9):2260. [CrossRef]
- Bulatova YY, Maltabarova NA, Zhumabayev MB, Li TA, Ivanova MP. Modern Diagnostics of Sepsis and Septic Shock in Children. Electron J Gen Med. 2020;17(5):em216. [CrossRef]
- Lanziotti VS, Póvoa P, Soares M, Silva JRL e, Barbosa AP, Salluh JIF. Use of biomarkers in pediatric sepsis: Literature review. Rev Bras Ter Intensiv. 2016;28(4):472-482. [CrossRef]
- Schuh AM, Leger KJ, Summers C, Uspal NG. Lactic Acidosis in a Critically Ill Patient. Pediatr Emerg Care. 2018;34(9):e165-e167. [CrossRef]
- Kustán P, Horváth-Szalai Z, Mühl D. Nonconventional Markers of Sepsis. EJIFCC. 2017;28(2):122-133.
- Hung SK, Lan HM, Han ST, Wu CC, Chen KF. Current Evidence and Limitation of Biomarkers for Detecting Sepsis and Systemic Infection. Biomedicines. 2020;8(11):494. [CrossRef]
- Teggert A, Datta H, Ali Z. Biomarkers for Point-of-Care Diagnosis of Sepsis. Micromachines. 2020;11(3):286. [CrossRef]
- Wagner KH, Cameron-Smith D, Wessner B, Franzke B. Biomarkers of Aging: From Function to Molecular Biology. Nutrients. 2016;8(6):338. [CrossRef]
- Franceschi C, Bonafè M, Valensin S, et al. Inflamm-aging: An Evolutionary Perspective on Immunosenescence. Ann N York Acad Sci. 2000;908(1):244-254. [CrossRef]
- Ginde AA, Blatchford PJ, Trzeciak S, et al. Age-Related Differences in Biomarkers of Acute Inflammation During Hospitalization for Sepsis. Shock. 2014;42(2):99-107. [CrossRef]
- Yende S, D’Angelo G, Kellum JA, et al. Inflammatory Markers at Hospital Discharge Predict Subsequent Mortality after Pneumonia and Sepsis. Am J Respir Crit Care Med. 2008;177(11):1242-1247.
- Velissaris D, Zareifopoulos N, Karamouzos V, et al. Presepsin as a Diagnostic and Prognostic Biomarker in Sepsis. Cureus. Published online 2021. 2021. [CrossRef]
- Yaegashi Y, Shirakawa K, Sato N, et al. Evaluation of a newly identified soluble CD14 subtype as a marker for sepsis. Journal of Infection and Chemotherapy. 2005;11(5):234-238. [CrossRef]
- Arai Y, Mizugishi K, Nonomura K, Naitoh K, Takaori-Kondo A, Yamashita K. Phagocytosis by human monocytes is required for the secretion of presepsin. J Infect Chemother. 2015;21(8):564-569. [CrossRef]
- MDsave. Blood culture. Published July 24, 2023. Available online: https://www.mdsave.com/procedures/blood-culture/d787ffc9.
- Truehealthlabs. 2023;(Culture). Available online: https://truehealthlabs.com/product/blood-culture/.
- Henriquez-Camacho C, Losa J. Biomarkers for sepsis. BioMed Research International. 2014;2014. [CrossRef]
- Park J, Yoon JH, Ki HK, Ko JH, Moon HW. Performance of presepsin and procalcitonin predicting culture-proven bacterial infection and 28-day mortality: A cross sectional study. Frontiers in Medicine. 2022;9. [CrossRef]
- Pierrakos C, Velissaris D, Bisdorff M, Marshall JC, Vincent JL. Biomarkers of sepsis: Time for a reappraisal. Critical Care. 2020;24(1). [CrossRef]
- Memar MY, Baghi HB. Presepsin: A promising biomarker for the detection of bacterial infections. Biomed Pharmacother. 2019;111:649-656. [CrossRef]
- Maddaloni C, Rose DUD, Santisi A, et al. The Emerging Role of Presepsin (P-SEP) in the Diagnosis of Sepsis in the Critically Ill Infant: A Literature Review. Int J Mol Sci. 2021;22(22):12154. [CrossRef]
- Piccioni A, Santoro MC, Cunzo T de, et al. Presepsin as Early Marker of Sepsis in Emergency Department: A Narrative Review. Medicina. 2021;57(8):770. [CrossRef]
- Ferreira T, Candido MA, Soriano FG. Sepsis biomarkers: A review of the diagnostic value of presepsin. Revista de Medicina. 2023;102(1). [CrossRef]
- Kyriazopoulou E, Leventogiannis K, Tavoulareas G, et al. Presepsin as a diagnostic and prognostic biomarker of severe bacterial infections and COVID-19. Scientific Reports. 2023;13(1). [CrossRef]
- Puspaningtyas NW, Karyanti MR, Paramita TN, et al. Presepsin as a promising biomarker for early detection of post-operative infection in children. Front Pediatr. 2023;11:1036993. [CrossRef]
- Tzialla C, Manzoni P, Achille C, Bollani L, Stronati M, Borghesi A. New Diagnostic Possibilities for Neonatal Sepsis. Am J Perinatol. 2018;35(06):575-577. [CrossRef]
- Pugni L, Pietrasanta C, Milani S, et al. Presepsin (Soluble CD14 Subtype): Reference Ranges of a New Sepsis Marker in Term and Preterm Neonates. PLoS ONE. 2015;10(12):e0146020. [CrossRef]
- Montaldo P, Rosso R, Santantonio A, Chello G, Giliberti P. Presepsin for the detection of early-onset sepsis in preterm newborns. Pediatr Res. 2017;81(2):329-334. [CrossRef]
- Ruan L, Chen GY, Liu Z, et al. The combination of procalcitonin and C-reactive protein or presepsin alone improves the accuracy of diagnosis of neonatal sepsis: A meta-analysis and systematic review. Crit Care. 2018;22(1):316. [CrossRef]
- Bellos I, Fitrou G, Pergialiotis V, Thomakos N, Perrea DN, Daskalakis G. The diagnostic accuracy of presepsin in neonatal sepsis: A meta-analysis. Eur J Pediatr. 2018;177(5):625-632. [CrossRef]
- Yoon SH, Kim EH, Kim HY, Ahn JG. Presepsin as a diagnostic marker of sepsis in children and adolescents: A systemic review and meta-analysis. BMC Infectious Diseases. 2019;19(1). [CrossRef]
- Korpelainen S, Intke C, Hämäläinen S, Jantunen E, Juutilainen A, Pulkki K. Soluble CD14 as a Diagnostic and Prognostic Biomarker in Hematological Patients with Febrile Neutropenia. Dis Markers. 2017;2017:9805609. [CrossRef]
- Olad E, Sedighi I, Mehrvar A, et al. Presepsin (scd14) as a marker of serious bacterial infections in chemotherapy induced severe neutropenia. Iran J Pediatr. 2014;24(6):715-722.
- Baraka A, Zakaria M. Presepsin as a diagnostic marker of bacterial infections in febrile neutropenic pediatric patients with hematological malignancies. Int J Hematol. 2018;108(2):184-191. [CrossRef]
- Giavarina D, Carta M. Determination of reference interval for presepsin, an early marker for sepsis. Biochem Med. 2015;25(1):64-68. [CrossRef]
- Zvyagin AA, Demidova VS, Smirnov GV. Dinamika biomarkerov sepsisa kak pokazatel’ éffektivnosti intensivnoĭ terapii. Khirurgiia. 2019;(2):53-57. [CrossRef]
- Ikeda T, Kamohara H, Suda S, et al. Comparative evaluation of endotoxin activity level and various biomarkers for infection and outcome of ICU-admitted patients. Biomedicines. 2019;7(3). [CrossRef]
- Fischer P, Grigoras C, Bugariu A, et al. Are presepsin and resistin better markers for bacterial infection in patients with decompensated liver cirrhosis? Digestive and Liver Disease. 2019;51(12):1685-1691. [CrossRef]
- Fujii E, Fujino K, Eguchi Y. An evaluation of clinical inflammatory and coagulation markers in patients with sepsis: A pilot study. Acute Medicine & Surgery. 2019;6(2):158-164. [CrossRef]
- Chen J, Huang ZB, Li H, et al. Early Diagnostic Biomarkers of Sepsis for Patients with Acute-on-Chronic Liver Failure: A Multicenter Study. Infectious Diseases and Therapy. 2021;10(1):281-290. [CrossRef]
- Tsujimoto K, Hata A, Fujita M, Hatachi S, Yagita M. Presepsin and procalcitonin as biomarkers of systemic bacterial infection in patients with rheumatoid arthritis. Int J Rheum Dis. 2018 Jul;21(7):1406-141. [CrossRef]
- Koizumi Y, Shimizu K, Shigeta M, et al. Plasma presepsin level is an early diagnostic marker of severe febrile neutropenia in hematologic malignancy patients. BMC Infectious Diseases. 2017;17(1). [CrossRef]
- Kang T, Yoo J, Choi H, Lee S, Jekarl DW, Kim Y. Performance evaluation of presepsin using a Sysmex HISCL-5000 analyzer and determination of reference interval. Journal of Clinical Laboratory Analysis. 2022;36(9). [CrossRef]
- Dragoş D, Ghenu MI, Timofte D, Balcangiu-Stroescu AE, Ionescu D, Manea MM. The cutoff value of presepsin for diagnosing sepsis increases with kidney dysfunction, a cross-sectional observational study. Medicine (United States). 2023;102(1):E32620-E32620. 3262. [CrossRef]
- Imai Y, Taniguchi K, Iida R, Nitta M, Uchiyma K, Takasu A. Diagnostic accuracy of presepsin in predicting bacteraemia in elderly patients admitted to the emergency department: Prospective study in Japan. BMJ Open. 2019;9(12):e030421. [CrossRef]
- Ruangsomboon O, Panjaikaew P, Monsomboon A, Chakorn T, Permpikul C, Limsuwat C. Diagnostic and prognostic utility of presepsin for sepsis in very elderly patients in the emergency department. Clin Chim Acta. 2020;510:723-732. [CrossRef]
- Claessens YE, Trabattoni E, Grabar S, et al. Plasmatic presepsin (sCD14-ST) concentrations in acute pyelonephritis in adult patients. Clinica Chimica Acta. 2017;464:182-188. [CrossRef]
- Musso CG, Oreopoulos DG. Aging and Physiological Changes of the Kidneys Including Changes in Glomerular Filtration Rate. Nephron Physiol. 2011;119(Suppl 1):p1-p5. [CrossRef]
- Chenevier-Gobeaux C, Trabattoni E, Roelens M, Borderie D, Claessens YE. Presepsin (sCD14-ST) in emergency department: The need for adapted threshold values? Clin Chim Acta. 2014;427:34-36. [CrossRef]
- Nagata T, Yasuda Y, Ando M, et al. Clinical impact of kidney function on presepsin levels. PLoS ONE. 2015;10(6). [CrossRef]
- Kim H, Song1 J, *, et al. Presepsin levels for discriminating sepsis and predicting mortality among organ failure patients stratified by hypercreatinemia. Signa Vitae. Published online 2023. [CrossRef]
- Shozushima T, Takahashi G, Matsumoto N, Kojika M, Okamura Y, Endo S. Usefulness of presepsin (sCD14-ST) measurements as a marker for the diagnosis and severity of sepsis that satisfied diagnostic criteria of systemic inflammatory response syndrome. Journal of Infection and Chemotherapy. 2011;17(6):764-769. [CrossRef]
- Endo S, Suzuki Y, Takahashi G, et al. Usefulness of presepsin in the diagnosis of sepsis in a multicenter prospective study. Journal of Infection and Chemotherapy. 2012;18(6):891-897. [CrossRef]
- Maldeghem I van, Nusman CM, Visser DH. Soluble CD14 subtype (sCD14-ST) as biomarker in neonatal early-onset sepsis and late-onset sepsis: A systematic review and meta-analysis. BMC Immunol. 2019;20(1):17. [CrossRef]
- Poggi C, Bianconi T, Gozzini E, Generoso M, Dani C. Presepsin for the detection of late-onset sepsis in preterm newborns. Pediatrics. 2015;135(1):68-75. [CrossRef]
- Wu CC, Lan HM, Han ST, et al. Comparison of diagnostic accuracy in sepsis between presepsin, procalcitonin, and C-reactive protein: A systematic review and meta-analysis. Annals of Intensive Care. 2017;7(1). [CrossRef]
- Zheng Z, Jiang L, Ye L, Gao Y, Tang L, Zhang M. The accuracy of presepsin for the diagnosis of sepsis from SIRS: A systematic review and meta-analysis. Annals of Intensive Care. 2015;5(1):1-13. [CrossRef]
- Liu Y, Hou J huan, Li Q, Chen K jun, Wang SN, Wang J min. Biomarkers for diagnosis of sepsis in patients with systemic inflammatory response syndrome: A systematic review and meta-analysis. SpringerPlus. 2016;5(1). [CrossRef]
- Kondo Y, Umemura Y, Hayashida K, Hara Y, Aihara M, Yamakawa K. Diagnostic value of procalcitonin and presepsin for sepsis in critically ill adult patients: A systematic review and meta-analysis. J Intensiv Care. 2019;7(1):22. [CrossRef]
- Yang HS, Hur M, Yi A, Kim H, Lee S, Kim SN. Prognostic value of presepsin in adult patients with sepsis: Systematic review and meta-analysis. PLoS ONE. 2018;13(1):e0191486. [CrossRef]
- Zhu Y, Li X, Guo P, Chen Y, Li J, Tao T. The accuracy assessment of presepsin (sCD14-ST) for mortality prediction in adult patients with sepsis and a head-to-head comparison to PCT: A meta-analysis. Therapeutics and Clinical Risk Management. 2019;15:741-753. [CrossRef]
- Ali FT, Ali MAM, Elnakeeb MM, Bendary HNM. Presepsin is an early monitoring biomarker for predicting clinical outcome in patients with sepsis. Clinica Chimica Acta. 2016;460:93-101. [CrossRef]
- Yu H, Qi Z, Hang C, Fang Y, Shao R, Li C. Evaluating the value of dynamic procalcitonin and presepsin measurements for patients with severe sepsis. Am J Emerg Med. 2017;35(6):835-841. [CrossRef]
- Boyd CM, Landefeld CS, Counsell SR, et al. Recovery of Activities of Daily Living in Older Adults After Hospitalization for Acute Medical Illness. J Am Geriatr Soc. 2008;56(12):2171-2179. 2171. [CrossRef]
- Wynn JL, Wong HR. Fetal and Neonatal Physiology (Fifth Edition). Sect XXVI: Pathophysiol Neonatal Dis. 2017;(J Immunol19272014):1536-1552.e10. [CrossRef]
- Sampson D, Yager TD, Fox B, et al. Blood transcriptomic discrimination of bacterial and viral infections in the emergency department: A multi-cohort observational validation study. BMC Med. 2020;18(1):185. [CrossRef]
- Kwizera A, Baelani I, Mer M, et al. The long sepsis journey in low- and middle-income countries begins with a first step...but on which road? Crit Care. 2018;22(1):64. [CrossRef]
- Maruna P, Nedelníková K, Gurlich R. Physiology and genetics of procalcitonin. Physiol Res. 2000;49(1):S57-61.
- Dong R, Wan B, Lin S, et al. Procalcitonin and Liver Disease: A Literature Review. J Clin Transl Hepatol. 2019;7(1):51-55. [CrossRef]
- Oberhoffer M, Stonans I, Russwurm S, et al. Procalcitonin expression in human peripheral blood mononuclear cells and its modulation by lipopolysaccharides and sepsis-related cytokines in vitro. J Lab Clin Med. 1999;134(1):49-55. [CrossRef]
- Pepys MB, Hirschfield GM. C-reactive protein: A critical update. J Clin Investig. 2003;111(12):1805-1812. 1805. [CrossRef]
Table 1.
Admission levels of presepsin - comparison between sepsis and non sepsis, and suvivor and non survivors. Cutoff values of presepsin in all stages of life.
Table 1.
Admission levels of presepsin - comparison between sepsis and non sepsis, and suvivor and non survivors. Cutoff values of presepsin in all stages of life.
| Age Group | Author | Admission Medium PSP Levels (ng/mL) | Cutoff Values (ng/mL) | |||
|---|---|---|---|---|---|---|
| Sepsis | Non Sepsis | Survivor | Non Survivor | |||
| Neonates & children | Poggi et al. 2015 [107] | 1295 | 562 | - | - | 885 |
| Pugni et al. 2015 [79] | - | 649 | - | - | - | |
| Montaldo et al. 2016 [80] | 598 | 328 | - | - | 788 * | |
| Korpelainen et al. 2017 [84] | 1432 | - | - | - | - | |
| Bellos et al. 2018 [82] | - | - | - | - | 650–850 ** | |
| Baraka et al. 2018 [86] | 1014 | 178 | - | - | Multiple | |
| Yoon et al. 2019 [83] | - | - | - | - | 650 ** | |
| Puspaningtyas et al. 2023 [77] | 806.5 | 717 | - | - | 761 * | |
| Adults | Shozushima et al. 2011 [104] | 817.9 | 190 | - | - | 399 |
| Endo et al. 2012 [105] | 1579 | 312 | - | - | Multiple | |
| Giavarina et al. 2015 [87] | 55-184 | - | - | - | - | |
| Ali et al. 2016 [114] | 1183 | 472 | 615,5 | 1301 | Multiple | |
| Yu et al. 2017 [115] | - | - | 1230,5 | 1269 | - | |
| Claessens et al. 2017 [99] | 476 | 200 | - | - | - | |
| Ikeda et al. 2019 [89] | - | - | 3251 | 1108 | - | |
| Zvyagyn et al. 2019 [88] | - | - | 1718 | 3266 | - | |
| Dragoş et al. 2023 [96] | 1039 | 372 | - | - | - | |
| Old adults | Imai et al. 2019 [97] | 639.93 | 866.56 | - | - | 285 |
| Ruangsomboon et al. 2020 [98] | 746 | 316 | 470 | 795 | Multiple | |
* Best of multiple values; ** Best accuracy values in the metanalysis.
Table 2.
Positive and negative aspects of presepsin in all stages of life.
| Aspects | Pediatric | Adult | Elderly |
|---|---|---|---|
| Positive | Early elevation, affordable cost, better diagnostic performance (PCT and CRP) and prognostic validity (30-day mortality), monitoring of antibiotic therapy, levels not influenced by gestational age, predictor of clinical evolution in febrile neutropenics | Better prognostic validity (PCT, CRP, ESR), correlation with hospital mortality in sepsis and septic shock, prognostic validity (28-day mortality), correlation with clinical outcomes, stable in different clinical scenarios (cirrhosis, rheumatoid arthritis, febrile neutropenia) | A better predictor of bacteremia in the Emergency Department (PCT, CRP), similar diagnostic accuracy to PCT, similar prognostic accuracy (qSOFA, SIRS) |
| Negative | Poor predictor of bacterial infection (PCT), non-standardized cutoff points, inaccessible in most scenarios | Poor predictor of bacterial infection (PCT), requires adjustments when kidney function is altered | Diagnostic and prognostic accuracy lower than combination (PCT + CRP + PSP), major renal dysfunction in older adults, specific cutoff point (immunosenescence) |
PSP: presepsin; CRP: C-reactive protein; PCT: procalcitonin; ESR: erythrocyte sedimentation rate; qSOFA: quick Sequential Organ Failure Assessment; SIRS: systemic inflammation response syndrome.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Copyright: This open access article is published under a Creative Commons CC BY 4.0 license, which permit the free download, distribution, and reuse, provided that the author and preprint are cited in any reuse.
Epidemiology and Trends of Sepsis in Young Adults Aged 20–44 Years: A Nationwide Population-Based Study
Carmen Bouza
et al.
JCM,
2019
Complementary Use of Presepsin with the Sepsis-3 Criteria Improved Identification of High-Risk Patients with Suspected Sepsis
Jong Park
et al.
Biomedicines,
2021
Sepsis Diagnostics: Intensive Care Scoring Systems Superior to MicroRNA Biomarker Testing
Fabian Link
et al.
Diagnostics,
2020
MDPI Initiatives
Important Links
© 2024 MDPI (Basel, Switzerland) unless otherwise stated






