Submitted:
24 September 2024
Posted:
25 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Site, Plant Material and Experimental Design
2.2. Photosynthetically Active Radiation and Daily Light Integral
2.3. Leaf Area
2.4. Number and Dimensions of Stomata, Stomatal Pore and Guard Cell
2.5. Parameters of Photosynthetic Activity and Canopy Architecture
2.6. Fruit Quality
2.5. Statistical Analyses
3. Results
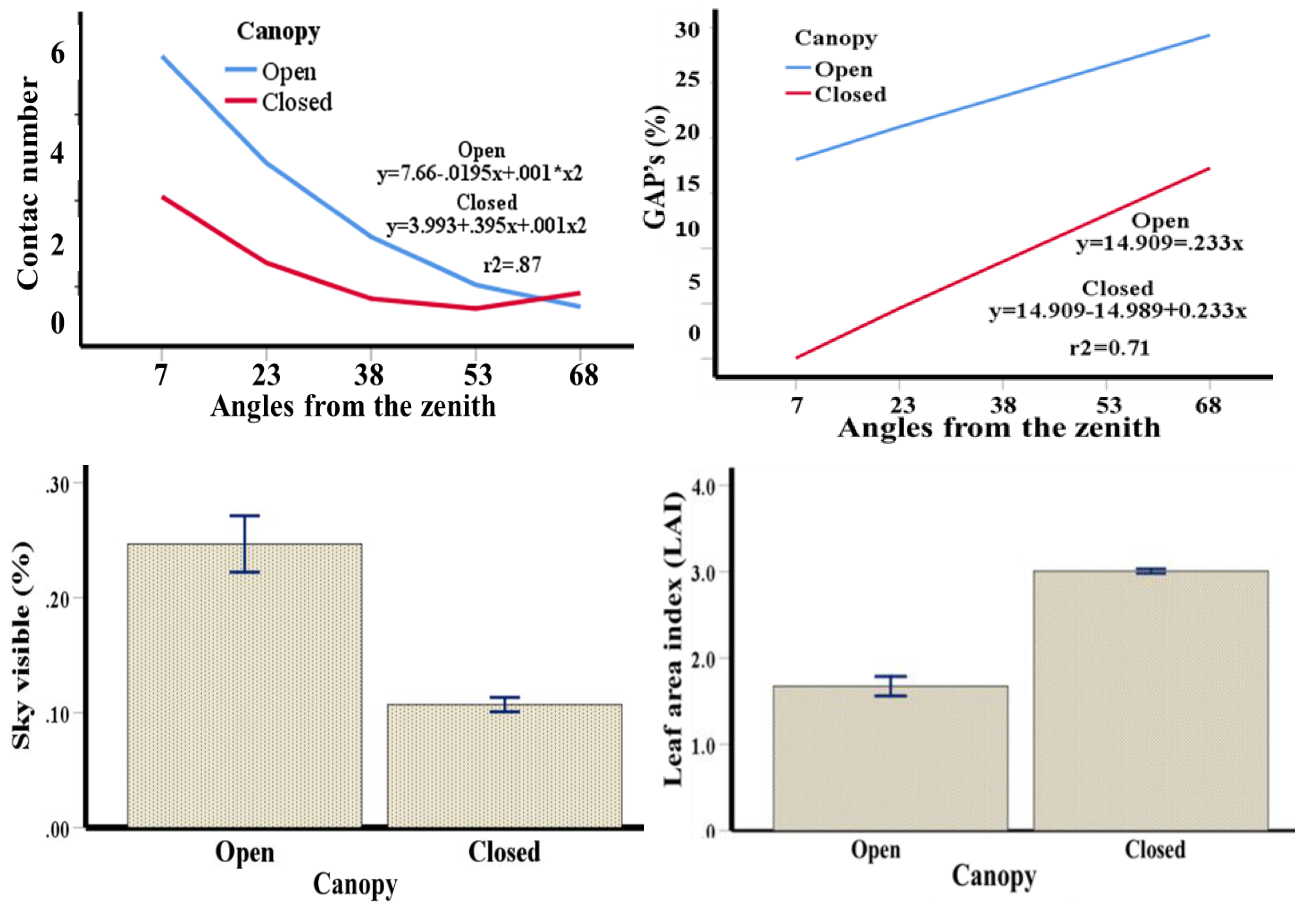
3.1. Photosynthetically Active Radiation and Photosynthetic Activity Parameters
| Treatment | NS mm2 | LS | WS | LSP | SPO | WGC |
|---|---|---|---|---|---|---|
| Open canopy | 47.5 a | 31.62 a | 24.10 a | 13.62 a | 1.88 a | 8.42 a |
| Closed canopy | 47.5 a | 31.35 a | 23.15 a | 15.2 a | 1.55 a | 7.40 a |
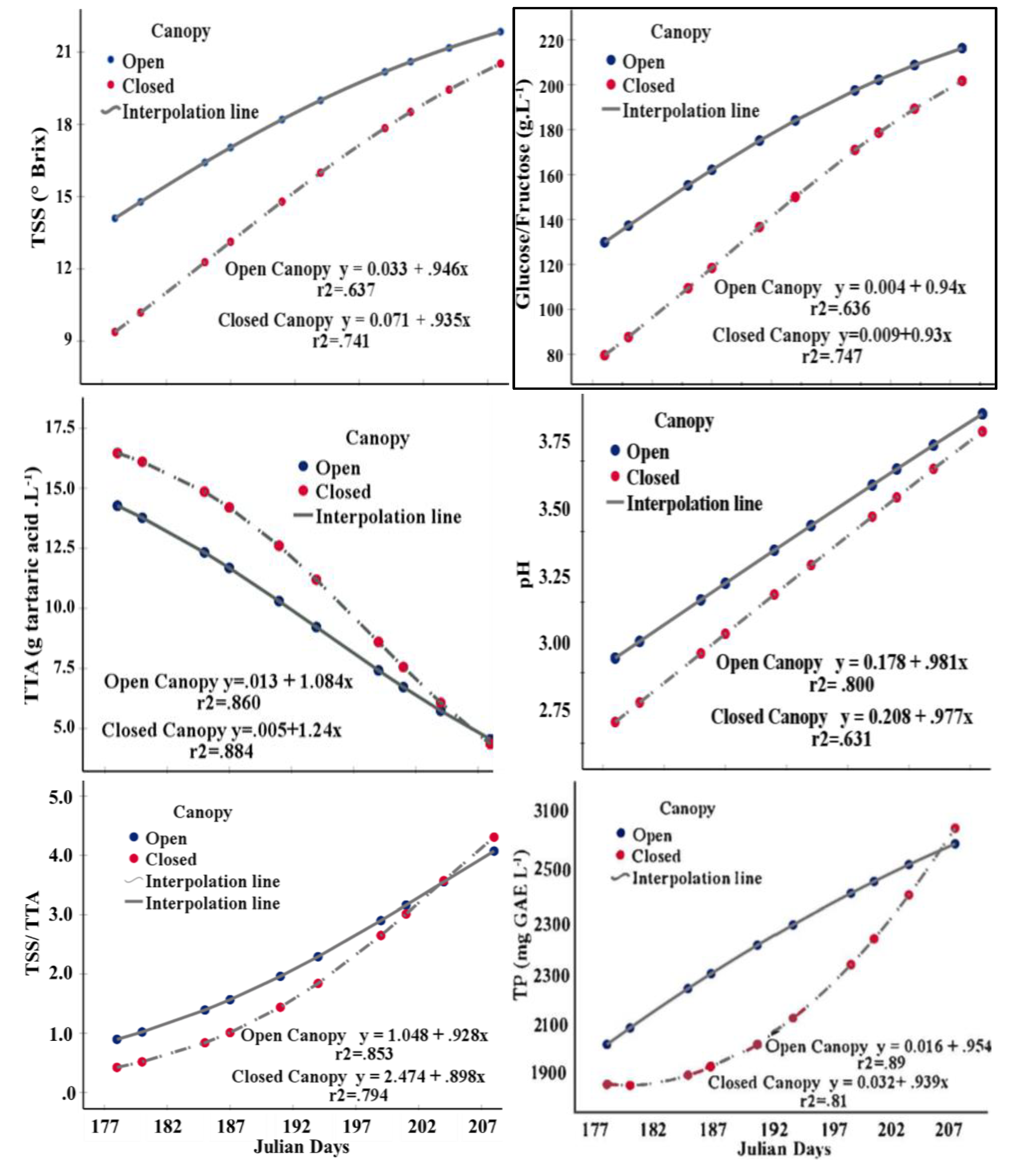
3.2. Fruit Quality
4. Discussion
4.1. Photosynthetically Active Radiation and Photosynthetic Activity Parameters
4.2. Quality of Fruit
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Yu R, Torres N, Tanner JD, Kacur SM, Marigliano LE, et al. Adapting wine grape production to climate change through canopy architecture manipulation and irrigation in warm climates. Frontiers in Plant Science. 2022;13:1015574. [CrossRef]
- Ontiveros-Capurata RE, Juárez-López P, Mendoza-Tafolla RO, Alia-Tejacal I, Villegas-Torres OG, et al. Relationship between chlorophyll and nitrogen concentration, and fresh matter production in basil ‘Nufar’ (Ocimum basilicum) with three handheld chlorophyll meter readings: SPAD, atLEAF and MC-100. Revista Chapingo. Serie Horticultura. 2022;28(3):189-202. [CrossRef]
- Gavhane KP, Hasan M, Singh DK, Kumar SN, Sahoo RN, et al. Determination of optimal daily light integral (DLI) for indoor cultivation of iceberg lettuce in an indigenous vertical hydroponic system. Scientific Reports. 2023;13(1):10923. [CrossRef]
- Furini G, de Oliveira Anese R, Reinehr J, Silva FN, Casa RT, et al. Ethephon effect on defoliation of cluster zone, Botrytis bunch rot, and viticultural performance of ‘Cabernet Sauvignon’ grapevine in highland region of southern Brazil. Ciencia Rural. 2024;54(6):e20230207. [CrossRef]
- Durand M, Murchie EH, Lindfors AV, Urban O, Aphalo PJ, et al. Diffuse solar radiation and canopy photosynthesis in a changing environment. Agricultural and Forest Meteorology. 2021;311:108684. [CrossRef]
- Dechant B, Ryu Y, Badgley G, Zeng Y, Berry JA, et al. Canopy structure explains the relationship between photosynthesis and sun-induced chlorophyll fluorescence in crops. Remote Sensing of Environment. 2020;241:111733. [CrossRef]
- Li Z, Zhao T, Liu J, Li H, Liu B. Shade-induced leaf senescence in plants. Plants. 2023;12(7):1550. [CrossRef]
- Liu X, Xu Y, Wang Y, Yang Q, Li Q. Rerouting artificial light for efficient crops production: A review of lighting strategy in PFALs. Agronomy. 2022;12(5):1021. [CrossRef]
- Jediyi H, Naamani K, Elkoch AA, Dihazi A, El Fels AE, et al. First study on technological maturity and phenols composition during the ripeness of five Vitis vinifera L grape varieties in Morocco. Scientia Horticulturae. 2019;246:390-7. [CrossRef]
- Emmel C, D'Odorico P, Revill A, Hörtnagl L, Ammann C, et al. Canopy photosynthesis of six major arable crops is enhanced under diffuse light due to canopy architecture. Global Change Biology. 2020;26(9):5164-77. [CrossRef]
- Neo DCJ, Ong MMX, Lee YY, Teo EJ, Ong Q, et al. Shaping and tuning lighting conditions in controlled environment agriculture: A review. ACS Agricultural Science & Technology. 2022;2(1):3-16. [CrossRef]
- Torregrosa L, Carbonneau A, Kelner JJ. The shoot system architecture of Vitis vinifera ssp. sativa. Scientia Horticulturae. 2021;288:110404. [CrossRef]
- Jogaiah S, Striegler KR, Bergmeier E, Harris J. Influence of canopy management practices on canopy characteristics, yield, and fruit composition of ‘Norton’ grapes (Vitis aestivalis Michx). International Journal of Fruit Science. 2013;13(4):441-58. [CrossRef]
- Prieto JA, Louarn G, Pérez-Peña J, Ojeda H, Simonneau T, et al. A functional–structural plant model that simulates whole-canopy gas exchange of grapevine plants (Vitis vinifera L.) under different training systems. Anales de Botánica. 2020;126(4):647-60. [CrossRef]
- Gao XT, Sun D, Wu MH, Li HQ, Liu FQ, et al. Influence of cluster positions in the canopy and row orientation on the flavonoid and volatile compound profiles in Vitis vinifera L. Cabernet franc and Chardonnay berries. Food Research International. 2021;143:110306. [CrossRef]
- Nistor E, Dobrei A, Dobrei A, Ciorica G. Climate variability and canopy management influence on grape berries quality in Merlot and Pinot Noir varieties. Scientific Papers. Series B. Horticulture. 2020;64(1):299-306.
- Greer DH, Ghannoum O. Changes in photosynthesis and chlorophyll a fluorescence in relation to leaf temperature from just before to after harvest of Vitis vinifera cv. Shiraz vines grown in outdoor conditions. Functional Plant Biology. 2021;49(2):170-85. [CrossRef]
- Wu YS, Gong WZ, Wang YM, Yang WY. Shading of mature leaves systemically regulates photosynthesis and leaf area of new developing leaves via hormones. Photosynthetica. 2019;57(1):303-10. [CrossRef]
- Espinoza S, Ortega-Farías S, Ahumada-Orellana L. Characterization of stomatal density and size of different Vitis vinifera L. cultivars growing in Mediterranean climate conditions. Ciência e Técnica Vitivinícola. 2024;39(1):196-208. [CrossRef]
- Pereira GE, Padhi EM, Girardello RC, Medina-Plaza C, Tseng D, et al. Trunk girdling increased stomatal conductance in Cabernet Sauvignon grapevines, reduced glutamine, and increased malvidin-3-glucoside and quercetin-3-glucoside concentrations in skins and pulp at harvest. Frontiers in Plant Science. 2020;11:707. [CrossRef]
- Wang X, De Bei R, Fuentes S, Collins C. Influence of canopy management practices on canopy architecture and reproductive performance of Semillon and Shiraz grapevines in a hot climate. American Journal of Enology and Viticulture. 2019;70(4):360-72. [CrossRef]
- Korczynski PC, Logan J, Faust JE. Mapping monthly distribution of daily light integrals across the contiguous United States. HortTechnology. 2002;12(1):12-6. [CrossRef]
- Sánchez C, Fischer G, Sanjuanelo DW. Stomatal behavior in fruits and leaves of the purple passion fruit (Passiflora edulis Sims) and fruits and cladodes of the yellow pitaya [Hylocereus megalanthus (K. Schum. ex Vaupel) Ralf Bauer]. Agronomía Colombiana. 2013;31(1):38-47.
- Olmedo L, Henning MF, Pappalardo B, García SM, Pellon-Maison M. Validation of an enzymatic colorimetric assay for fructose content determination in soft drinks. Revista Española de Nutrición Humana y Dietética. 2021;25(1):69-77. [CrossRef]
- Waterman PG, Mole S. Analysis of Phenolic Plant Metabolites. Oxford, UK: Blackwell Scientific Publications; 1994.
- Rescic J, Mikulic-Petkovsek M, Rusjan D. The impact of canopy managements on grape and wine composition of cv. ‘Istrian Malvasia’ (Vitis vinifera L.). Journal of the Science of Food and Agriculture. 2016;96(14):4724-35. [CrossRef]
- Bubola M, Sivilotti P, Janjanin D, Poni S. Early leaf removal has a larger effect than cluster thinning on grape phenolic composition in cv. Teran. American Journal of Enology and Viticulture. 2017;68(2):234-42. [CrossRef]
- Sanchez-Rodriguez LA, Spósito MB. Influence of the trellis/training system on the physiology and production of Vitis labrusca cv. Niagara Rosada in Brazil. Scientia Horticulturae. 2020;261:109043. [CrossRef]
- Reshef N, Walbaum N, Agam N, Fait A. Sunlight modulates fruit metabolic profile and shapes the spatial pattern of compound accumulation within the grape cluster. Frontiers in Plant Science. 2017;8:70. [CrossRef]
- Sebastian V, Nicolas O, Alvaro G, Samuel OF. Effect of irrigation management on the relationship between stomatal conductance and stem water potential on cv. Cabernet Sauvignon. BIO Web of Conferences. 2023;56:01012. [CrossRef]
- Anić M, Osrečak M, Andabaka Ž, Tomaz I, Večenaj Ž, et al. The effect of leaf removal on canopy microclimate, vine performance and grape phenolic composition of Merlot (Vitis vinifera L.) grapes in the continental part of Croatia. Scientia Horticulturae. 2021;285:110161. [CrossRef]
- Zhang J, Serra S, Leisso RS, Musacchi S. Effect of light microclimate on the quality of ‘d’Anjou’ pears in mature open-centre tree architecture. Biosystems Engineering. 2016;141:1-11. [CrossRef]
- Munitz S, Schwartz A, Netzer Y. Effect of timing of irrigation initiation on vegetative growth, physiology and yield parameters in Cabernet Sauvignon grapevines. Aust J Grape Wine Res. 2020;26(3):220-32. [CrossRef]
- Munitz S, Schwartz A, Netzer Y. Water consumption, crop coefficient and leaf area relations of a Vitis vinifera cv. 'Cabernet Sauvignon' vineyard. Agric Water Manag. 2019;219:86-94. [CrossRef]
- Hunter JJ, Volschenk CG, Zorer R. Vineyard row orientation of Vitis vinifera L. cv. Shiraz/101-14 Mgt: climatic profiles and vine physiological status. Agricultural and Forest Meteorology. 2016;228:104-19. [CrossRef]
- Boso S, Gago P, Alonso-Villaverde V, Santiago JL, Martinez MC. Density and size of stomata in the leaves of different hybrids (Vitis sp.) and Vitis vinifera varieties. Vitis. 2016;55(1):17-22. [CrossRef]
- Speirs J, Binney A, Collins M, Edwards E, Loveys B. Expression of ABA synthesis and metabolism genes under different irrigation strategies and atmospheric VPDs is associated with stomatal conductance in grapevine (Vitis vinifera L. cv Cabernet Sauvignon). Journal of Experimental Botany. 2013;64(7):1907-16. [CrossRef]
- Hunter JK, Tarricone L, Volschenk C, Giacalone C, Melo MS, et al. Grapevine physiological response to row orientation-induced spatial radiation and microclimate changes. Oeno One. 2020;54(2):411-33. [CrossRef]
- Römermann C, Bucher SF, Hahn M, Bernhardt-Römermann M. Plant functional traits–fixed facts or variable depending on the season? Folia Geobotanica. 2016;51:143-59. [CrossRef]
- Niimi J, Tomic O, Næs T, Bastian SE, Jeffery DW, et al. Objective measures of grape quality: From Cabernet Sauvignon grape composition to wine sensory characteristics. LWT - Food Science and Technology. 2020;123:109105. [CrossRef]
- Sams B, Bramley RG, Sanchez L, Dokoozlian N, Ford C, et al. Remote sensing, yield, physical characteristics, and fruit composition variability in Cabernet Sauvignon vineyards. American Journal of Enology and Viticulture. 2022;73(2):93-105. [CrossRef]
- González CV, Prieto JA, Mazza C, Jeréz DN, Biruk LN, et al. Grapevine morphological shade acclimation is mediated by light quality whereas hydraulic shade acclimation is mediated by light intensity. Plant Science. 2021;307:110893. [CrossRef]
- Torres N, Martínez-Lüscher J, Porte E, Kurtural SK. Optimal ranges and thresholds of grape berry solar radiation for flavonoid biosynthesis in warm climates. Frontiers in Plant Science. 2020;11:931. [CrossRef]
- Güler S, Kunter B, Şehit A. Stomatal density, type and their relationships with leaf morphological traits in Vitis vinifera L. varieties. International Journal of Agriculture Environment and Food Sciences. 2023;8(1):78-87. [CrossRef]
- Sivilotti P, Herrera JC, Lisjak K, Česnik HB, Sabbatini P, et al. Impact of leaf removal applied before and after flowering on anthocyanin, tannin, and methoxypyrazine concentrations in ‘Merlot' (Vitis vinifera L.) grapes and wines. Journal of Agricultural and Food Chemistry. 2016;64:4487-96. [CrossRef]
- Alonso R, Muñoz F, Bottini R, Piccoli P, Berli FJ. Effects of wind exposure and deficit irrigation on vegetative growth, yield components and berry composition of Malbec and Cabernet Sauvignon. Plants. 2024;13(10):1292. [CrossRef]
| Treatment | 9 June 2022 (vegetative growth) | |||||
|---|---|---|---|---|---|---|
| PAR | DLI | PR | SC | CI | LA | |
| Open canopy | 415.9 a | 5.9 a | 8.9 a | 150.1 a | 291.4 a | 97.92 a |
| Closed canopy | 266.0 b | 3.8 b | 7.2 b | 140.2 a | 272.1 b | 95.20 b |
| 22 September 2022 (after harvest ) | ||||||
| Open canopy | 320.7 a | 4.6 a | 4.2 a | 90.34 a | 304.5 a | 91.39 a |
| Closed canopy | 298.6 a | 4.3 a | 3.0 b | 90.82 a | 289.6 b | 90.67 a |
| Treatment | 9 June 2022 (vegetative growth) | ||||
|---|---|---|---|---|---|
| Transpiration | CO2 /H2O | H2O/CO2 | LT | AT | |
| Open canopy | 6.4 a | 3.5 a | 1180.4 b | 35.9 a | 36.9 a |
| Closed canopy | 6.0 a | 3.4 a | 1760.4 a | 36.2 a | 37.3 a |
| 22 September 2022 (after harvest) | |||||
| Open canopy | 2.9 a | 4.0 a | 359.5 a | 32.9 a | 33.2 a |
| Closed canopy | 2.8 a | 2.5 b | 355.2 b | 32.17 b | 32.4 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





