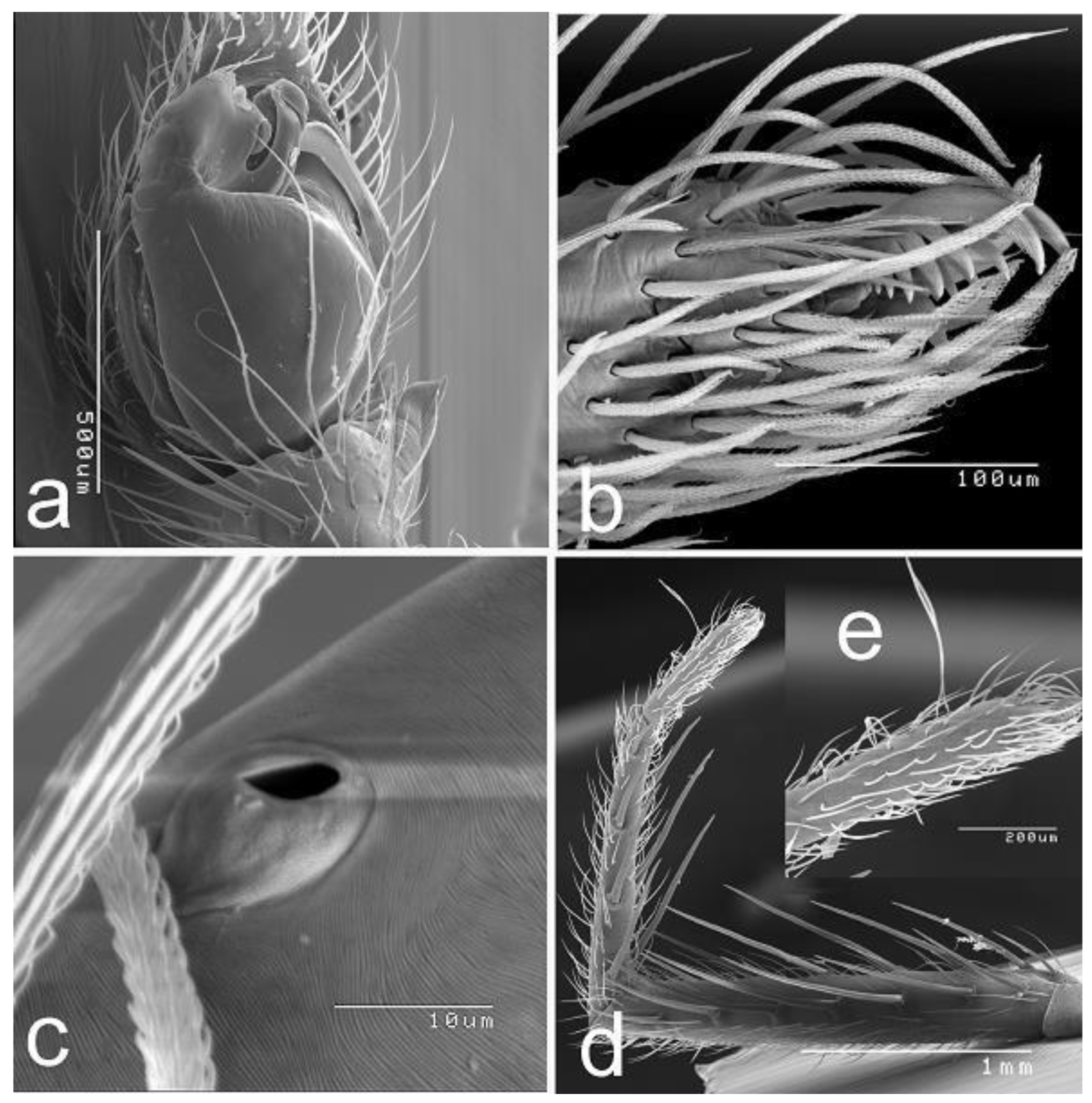
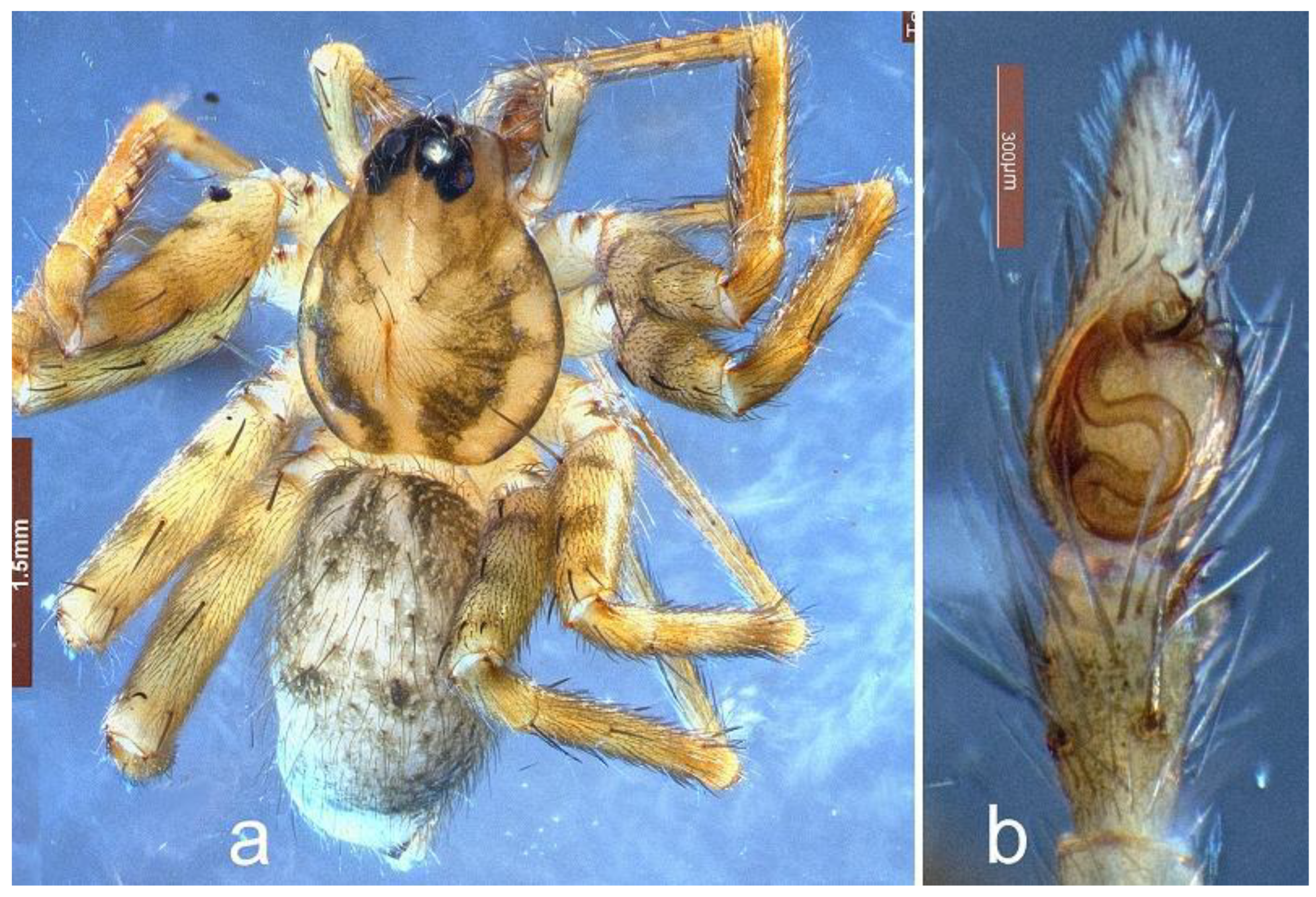
Material and Methods
Abbreviations. Morphology: Abbreviations are standard for the Araneae; in addition, RTA, retrolateral tibial apophysis; CO, copulatory orifice; FD, fertilisation duct. Leg measurements are given in the descriptions as leg 1: femur, patella, tibia, metatarsus (absent in palp), tarsus, total. Digital photographs were taken with a Leica microscope L16 with a Leica DFC 500 camera and the image stacking software program Zerene Stacker (
http://www.zerenesystems.com/). The epigyne was drawn with a camera lucida on a Zeiss Stemi SV6. Scanning electron micrographs were done on a Hitachi TM1000; material was sputter coated with gold.
Taxonomy
Toxopsoides Forster & Wilton, 1973
Toxopsoides Forster & Wilton, 1973, p. 309. Type species, Toxopsoides huttoni Forster and Wilton, 1973, by original designation.
Diagnosis. Differs from the often sympatric
Cycloctenus by the eyes being in the standard zorine (Miturgidae) configuration: AER viewed from above slightly recurved with ALE clearly closer to the AME (
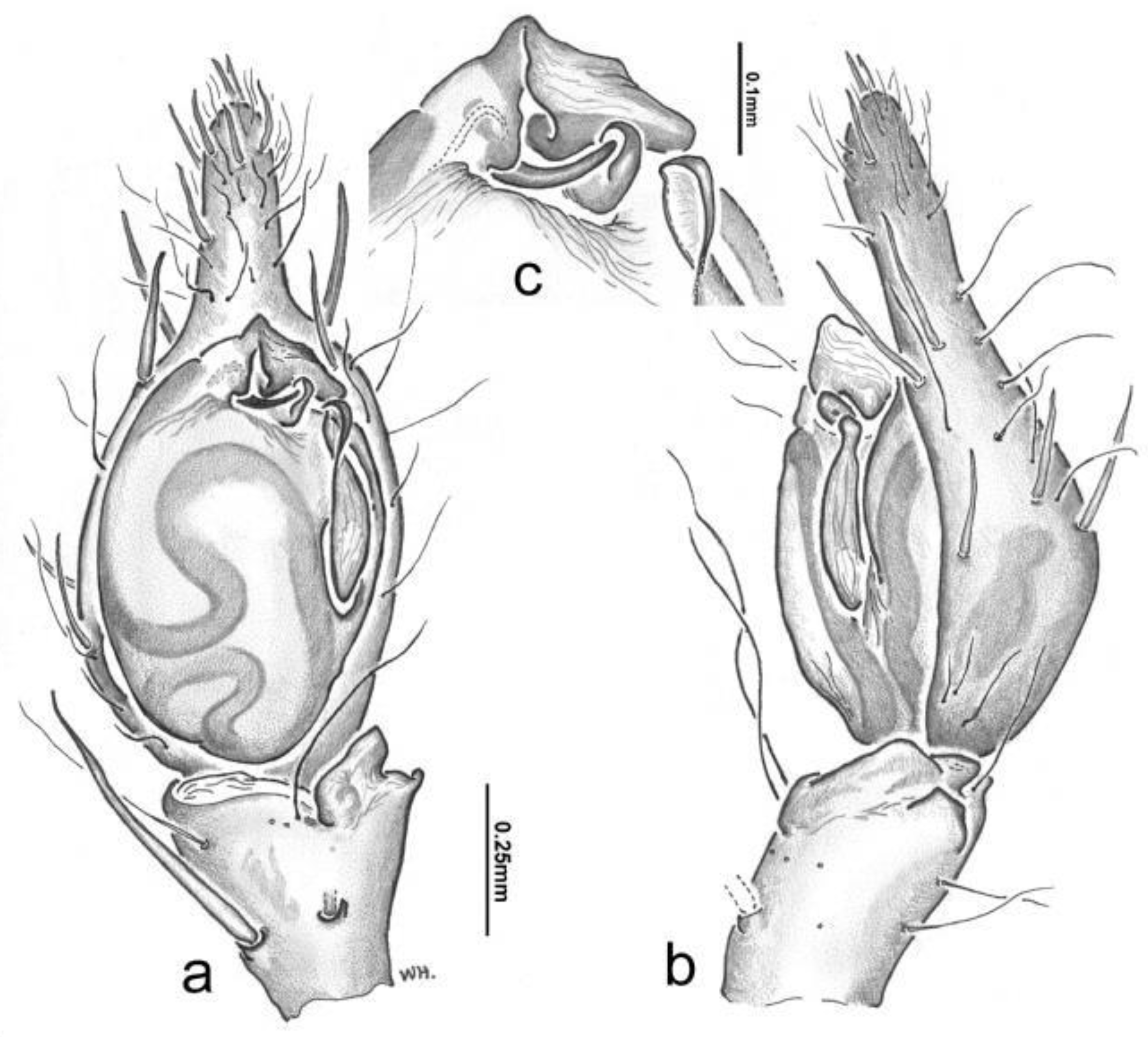
Figure 1b) than to the PME.
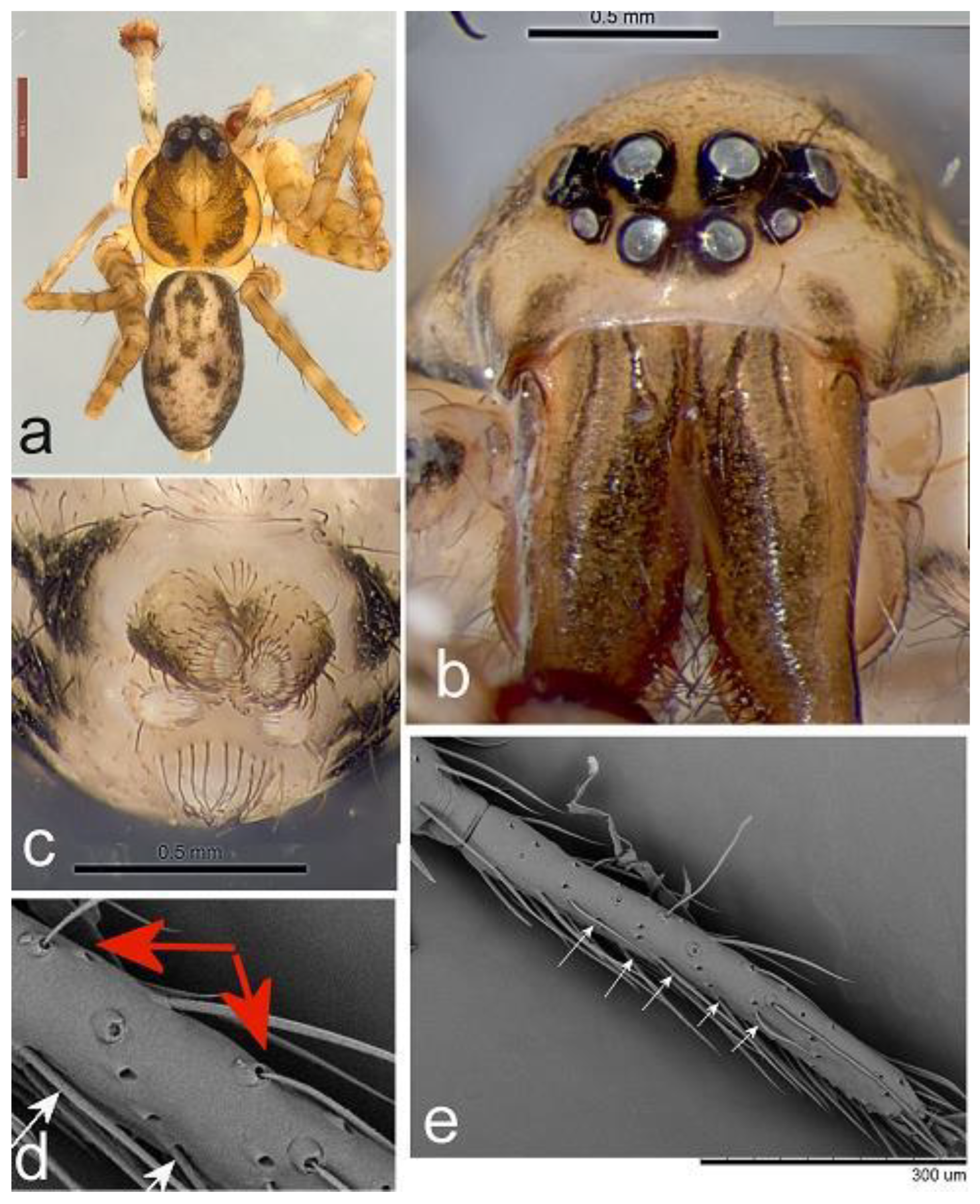
Toxopsoides also have 5-7 pairs of strong ventral spines on tibiae, I, II and 3-4 pairs of strong ventral spines in both males and females on metatarsi I and II (
Figure 1a) and a much less elaborate palpal bulb and RTA than
Cycloctenus (e.g., Figure 6a).
Relationships. The primary diagnostic character mentioned by Smith (2013), the two rows of trichobothia on the metatarsi, is a lapsus of Forster & Wilton (1973) and has not been been seen on any material herein (e.g.,
Figure 1e,f). Equally, the line of setae on the anal tubercle was used by Silva (2002) as a diagnostic character of the ctenid subfamily Calocteninae but such a line is also evident in
Toxopsoides (
Figure 1c).
As noted, although Toxopsoides is presently placed in the Toxopidae (Wheeler et al., 2017, Gorneau et al., 2023), it differs from Cycloctenus (Cycloctenidae) somatically only in the ALE being set low in a miturgid position, rather than near the PME, as in the ctenid like condition in Cycloctenus. It has not been sequenced or included in a cladogram but appears in the Toxopidae by what I call “guilty by association”. Thus, it is suggested that Toxopsoides should be transferred to the Cycloctenidae.
The male palp of Toxopsoides species is minimally divergent with a large tegulum and the small interspecific differences confined to the apical fifth of the bulb; similarly, the female epigyne is very similar across species which maximum difference shown in T. driesseni, sp. nov.
Toxopsoides
Included species: Toxopsoides huttoni Forster & Wilton, 1973; T. erici Smith, 2013, T. kathleenae Smith, 2013, T. macleayi, Smith, 2013, T. driesseni, sp. nov., T. wybalena, sp. nov., T. takayna, sp. nov., T. deux, sp. nov., T. ibisca, sp. nov.
Toxopsoides driesseni, sp. nov.
Diagnosis. Males and females have a similar abdominal pattern to those of T. erici and T. huttoni but males differ in that the long grooved median apophysis is basal on the bulb and lacks strong rugosity in the much less extensive or protruding processes on the male palpal bulb.
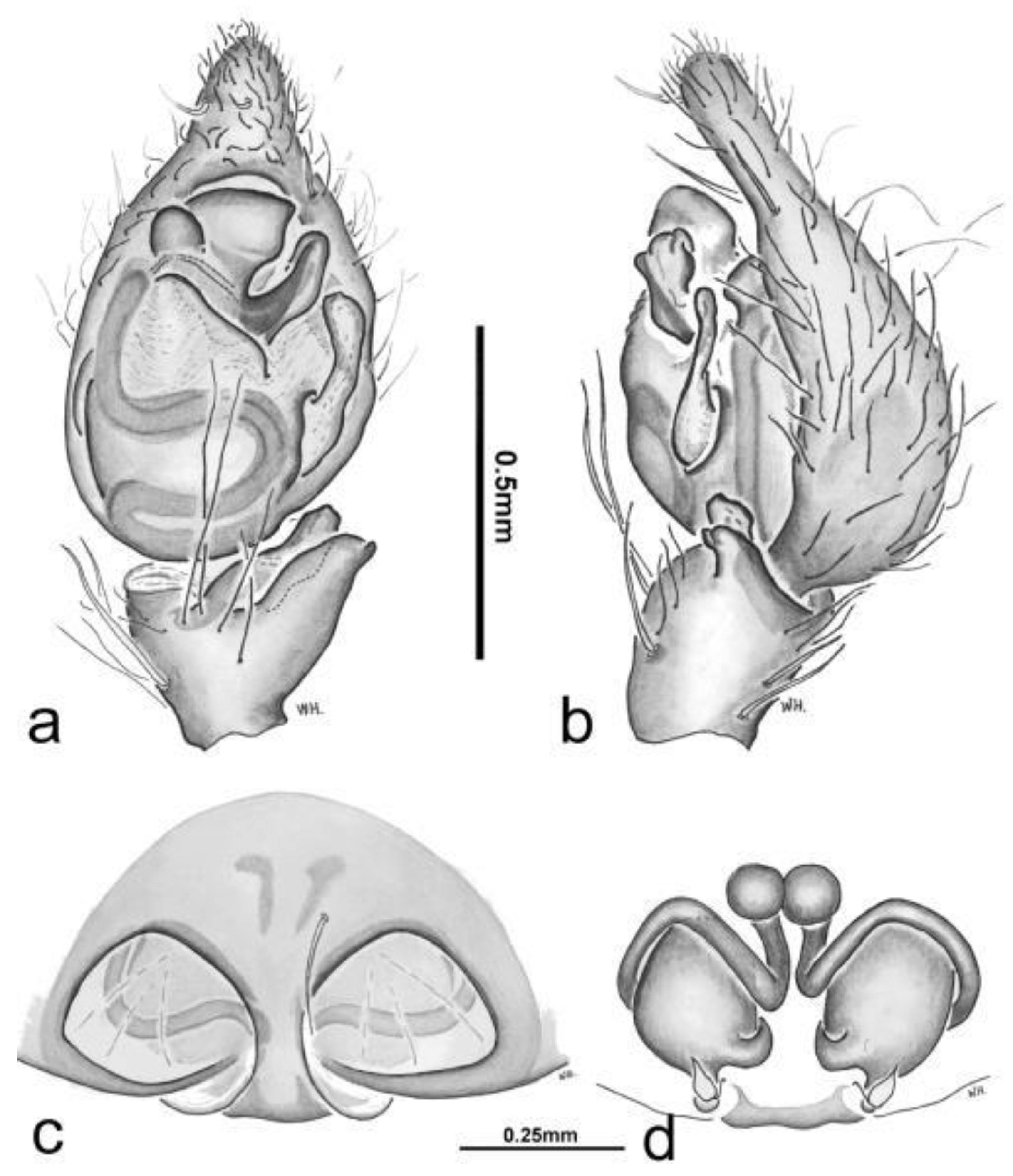
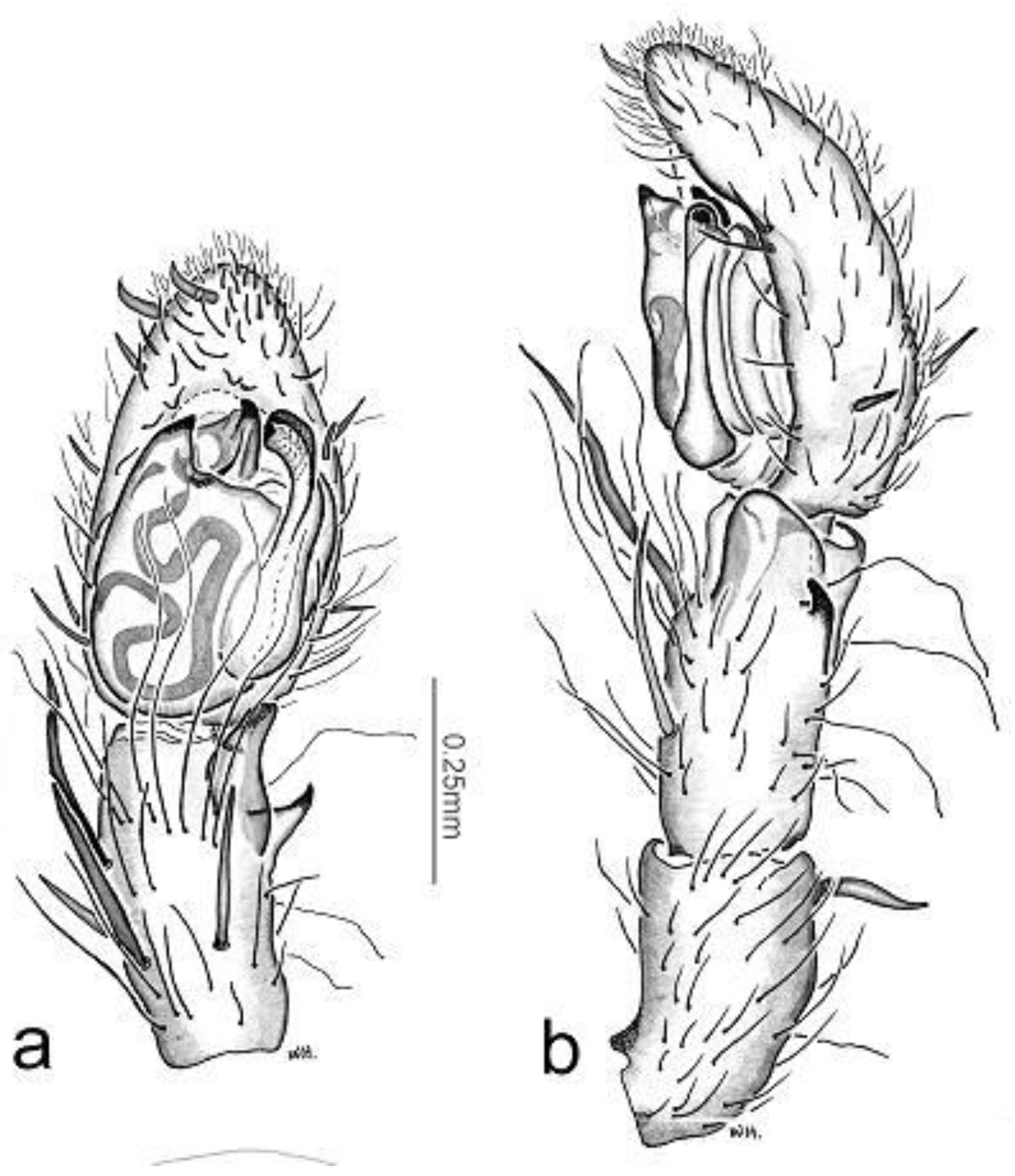
Diagnostic description. Male palpal tibia at most half length of cymbium; with RTA with two lobes; short, broad, smooth conductor, tegular envelope large, dominant with large (embolic) process (
Figure 2a); tegular base =0.65 of bulb. Female with spermathecae ca. 1.35 times longer than wide (
Figure 2d); epigyne with long median scape with widely curving lateral ridge (
Figure 2c).
Material Examined. Tasmania. Holotype male, Lake St Clair, Airstrip Rd (site 5M) [42.06S, 146.17E], 30 Oct 2001, M. Driessen, QM S65850. Paratype female, QM S86836, same data but 10 Apr 2001, QM S86036; 1 male, McPartlan Pass, 12 March 1998, M. Driessen, QM S51336;1 male, Lake St Clair (A6-2), 6 Apr 2000, M. Driessen, QM S62054.
Description. Holotype male QM S65850
Carapace 2.04 long, 1.86 wide. Abdomen 2.25 long, 1.11 wide. Total 4.30.
Leg measurements. I: 2.00; 0.57; 2.01; 2.18; 0.84; 7.59. II: 1.74; 0.51; 1.50; 0.98; 0.32; 5.04. III: 1.55; 0.65; 1.28; 1.22; 0.69; 5.37. IV: 2.00; 0.57; 2.01; 2.18; 0.84; 7.59. Palp: 0.90; 0.47; 0.95; —; 1.22; 3.53.
Colour. Carapace pallid with narrow dark edges and broad subcentral dark bands extending from PLE; abdomen pallid with dark irregular mottling laterally and a pair of long dark bands anteriorly and smaller dark spots posteriorly.
Legs pallid with a dark spot anteriorly on trochanter I—IV; femora with two dark bands; medially and indistinct dark zone apically; pedal patellae with dark zone anteriorly; two annulations on mid-tibiae I-IV; one in distal half and one apically on metatarsi I—IV. Palp entirely pallid. Sternum, maxillae and labium pallid; abdomen ventrally with large dark median arrow-shaped area, flanked with pallid zone and the incursion of the dark flanks.
Palp. Tibia clearly wider than long; RTA is two similarly long lobes, overlaid in ventral view (
Figure 2a). Cymbium with narrow lateral margins, apical cone symmetrical short conical with 2 spines on the ventral and lateral faces. Bulb ovoid, with dominant tegulum distally traversed partially by sinuous ridge; sperm ducts form tight basal fold and extend in one wide fold to embolus; large curved median apophysis and below it a long rugose conductor tip.
Female QM S86836. Similar to male except: Trichobothria, long distal on tarsi; none evident on dorsal metatarsi I. Spines: I; tibia I, II, 7 long pairs of spines; metatarsi I, I with 4 long pairs. STC with 7 teeth; ITC, small, bare.
Epigyne. Wide with large opening and narrow median plate.
Distribution. Known only buttongrass plains in the Lake St Clair area, central Tasmania.
Toxopsoides wybalena, sp. nov.
Diagnosis. Males differ from those of
T. takayna, sp. nov. in the absence of two large spines on the distal cymbium and the more slender cymbial cone (
Figure 4b).
Diagnostic description. Male palpal tibia slightly more than half length of cymbium; RTA with two lobes; short triangular conductor; tegular envelope moderately large, not dominant with small (embolic) process (
Figure 4b; tegular base =0.74 of bulb. Female unknown.
Material Examined. Tasmania. Holotype male, Flinders Island, Walkers Lookout, 40.054°S, 148.08°E, 20-30 Mar 2014, R. Raven, TMAG.
Description Holotype Male TMAG
Carapace 2.30 long, 2.15 wide. Abdomen 2.41 long, 3.07 wide. Total length, 4.7.
Leg measurements. I: 1.66; 0.62; 2.19; 1.56; 0.66; 6.69. II: 1.66; 0.63; 1.56; 1.51; 0.73; 6.09. III: 1.59; 0.33; 1.32; 1.12; 0.71; 5.06. IV: 2.07; 0.38; 1.63; 2.04; 0.89; 7.01. Palp: 0.90; 0.18; 0.38; --; 0.60; 2.07.
Colour. Carapace like T. takayna but with dark divergent lines along caput margins; abdomen like T. takayna. Legs orange brown, femora with 2-3 dark annulations and one distally on tibiae. Spines like T. takayna.
Palp. Tibia clearly longer than wide; RTA is two unequal lobes inner lobe slender spinelike, outer dorsal lobe short conical (
Figure 4b). Cymbium with narrow lateral margins, apical cone symmetrical trianguloid with 4 spines apically and 4 more basal and lateral. Bulb long ovoid, with dominant tegulum with small lateral median apophysis and small hooked embolus, sperm ducts a sinuous lobe of two folds extending to broad distal loop (
Figure 4b).
Distribution. Known only from Flinders Island, Tasmania. According to indigenous history (consistent with geology), Flinders Island is the focal point of radical drying about 4800 years ago, as much of the world’s water was bound up in polar ice and glaciations. At that time, Flinders Island connected Tasmania to Victoria as the Bass Strait closed. More significantly, it is recorded as a major catastrophe for the indigenous people. Concomitantly, in 1847, up to 130 First Nations people had died at Wybalena on Flinders Island and all were moved to Tasmania where many more died.
Toxopsoides takayna, sp. nov.
Diagnosis. Similar abdominal pattern to T. erici and T. huttoni but differs in the much less extensive or protruding processes on the male palpal bulb.
Diagnostic description. Male palpal tibia at most half length of cymbium; with RTA with two lobes; short, broad, smooth conductor, tegular envelope large, dominant with large (embolic) process (
Figure 5a); tegular base =0.79 of bulb. Females unknown.
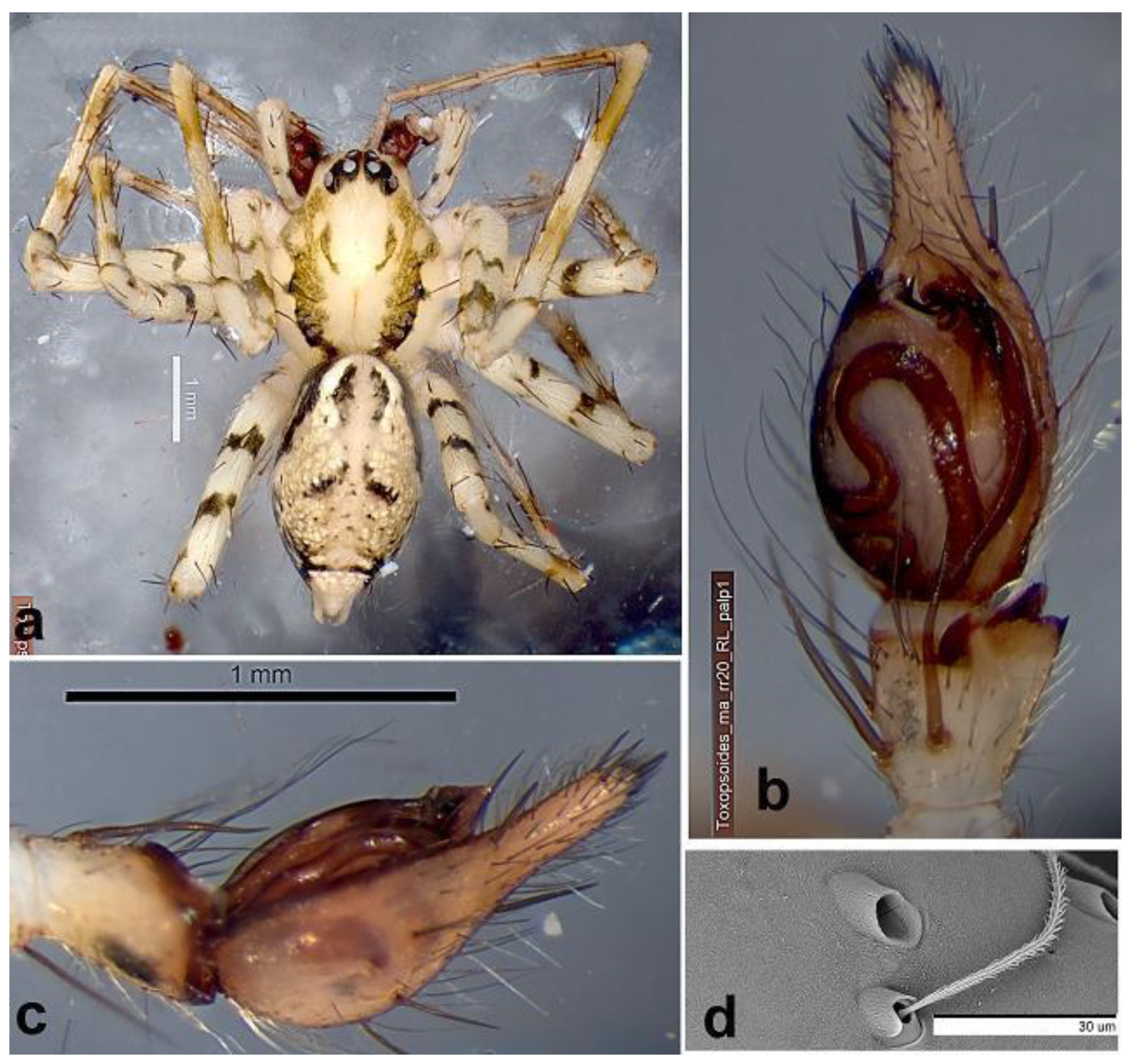
Material examined. Tasmania; Meredith Range Regional Reserve; E of Arthur River, 41.48467°S, 145.4737°E, Nothofagus with little understorey, open ground, on bark, 27 Jan-6 Feb 2015, R. Raven (RR20).
Description Holotype Male TMAG
Carapace 2.50 long, 2.00 wide. Abdomen 3.03 long, 1.75 wide. Colour in alcohol. Carapace pallid with irregular dark bands from caput margins submarginally, bands narrow posteriorly. Abdomen dorsally with black flanks for anterior half, a bright white zone with laterally dark ostiate region with posterior flares and dark irregular transverse band just anterior to spinnerets. Legs pallid: femora with two irregular dark bands medially, patellae distally dark, two brown annuli on tibiae; chelicerae fawn anteriorly darkly smudged.
Eyes. Eight in two rows, anterior row clearly narrower (0.72 of PER), eyes smaller and upcurved; AME their length from clypeal margin and two-thirds their diameter apart; all oval; AME: ALE: PME: PLE, 1.38. 1.00, 1.62. 1.90, respectively. Lower edge of ALE bisects AME. PME one AME from each other and PLE. PER so recurved as to be almost 2 rows.
Chelicerae. With a downwardly directed spine on the upper third (Fig. xx).
Spines. Tibia I, II with 7 pairs of long spines, lateroventrally; metatarsi I, II with 5 such pairs. Palp: fe: p1d1.1.1; pa d1; ti d1; cymbial cone with 10 spines. Palp: 1.18, 0.47, 0.53, --. 1.22, 3.40.
Palp. Tibia not much longer than wide; RTA is two unequal lobes overlaid in ventral view (
Figure 5a, b), ventral lobe a low broad ridge (
Figure 5b, outer dorsal lobe slender pointed. Cymbium with narrow lateral margins, apical cone symmetrical digitiform with 6 spines apically and 4 more basal and lateral 7 spines over the ventral and lateral faces. Bulb long ovoid, with dominant tegulum with small lateral median apophysis and small hooked embolus, sperm ducts a basal lobe extending to broad distal loop (
Figure 5a).
Distribution. Known only from the Meredith Range, in the Tarkine region, south-western Tasmania.
Toxopsoides deux, sp. nov.
Diagnosis. Similar abdominal pattern to T. erici and T. huttoni but differs in the much less extensive or protruding processes on the male palpal bulb.
Diagnostic description. Male palpal tibia at most half length of cymbium; with RTA with two lobes; tegulum deeply incursed distally for one-third tegulum; very small, indistinct conductor, tegular envelope almost absent with small (embolic) (
Figure 7b) process; tegular base =0.66 of bulb. Females unknown.
Material Examined. Tasmania. Holotype male, Meredith Range Regional Reserve; 41.5328°S, 145.1075°E, 27 Jan-6 Feb 2015, R. Raven, TMAG.
Description. Holotype Male TMAG
Similar to T. deux, sp. nov. but: colour in ethanol. Carapace fawn with irregular submarginal dark bands; legs fawn with dark annuli; abdomen dorsally mottled brown with two small dark chevrons posteriorly.
Abdomen. A line of long distinct setae on the anal tubercle (Fig. xx).
Palp. Tibia clearly longer than wide; RTA is two unequal lobes, juxtaposed in ventral view (Fig. xx), inner dorsal lobe slender pointer, outer lobe widely triangular. Cymbium with narrow lateral margins, apical cone symmetrical triangular with 7 spines over the ventral and lateral faces. Bulb subcircular, with dominant tegulum traversed by deeply procurved embolic ridge, sperm ducts confined to basal and prolateral; small hooked median apophysis beside large triangular lobe.
Distribution. Known only from the Tarkine region in western Tasmania.
Toxopsoides ibisca, sp. nov.
Diagnosis. Males share with
T. huttoni (
sensu Smith, 2013) the rugose conductor tip (
Figure 8a) but differ in the longer, more slender conductor, the shorter pointed tegular envelope (TEP,
Figure 8a). They differ from all other species in the long male palpal tibia (
Figure 8a).
Diagnostic description. Male palpal tibia longer than cymbium; with RTA with two lobes; narrow, fluted conductor, tegular envelope large, dominant with large (embolic) process (
Figure 8b); tegular base =0.82 of bulb. Female with spermathecae set diagonally (
Figure 2d); epigyne with broad median scape with paired lateral lobes (
Figure 8d).
Material Examined Queensland: holotype male, Lamington National Park, IBISCA survey, site 900B, 28°238”S, 153°145”E, 950m, pitfall Trap, 12-21/02/2007, K. Staunton, QM S76179; paratype female, same data but site 900C, QM S76193.
Other Material Examined. LAMINGTON NATIONAL PARK, IBSICA SURVEY: 1 fe., IQ-1100-B, SC20035, 28°259”S, 153°162”E, 1142m, Malaise Trap, 7-17/10/2006, C. Lambkin, N. Starick, QM S76454; 1 fe., IQ-500-A, SC20173, 28°216”S, 153°142”E, 560m, pitfall Trap, 8-12/10/2006, K. Staunton, QM S81032; 1 fe., IQ-300-A, SC20359, 28°148”S, 153°137”E, 267m, pitfall Trap, 9-11/10/2006, K. Staunton, QM S81047; 2 fe., IQ-900-C, SC20436, 28°24”S, 153°149”E, 944m, pitfall Trap, 11-20/10/2006, A. Cameron, QM S81079; 1 ma., 1 fe., IQ-300-B, SC20441, 28°155”S, 153°139”E, 282m, pitfall Trap, 11-21/10/2006, D. Putland, QM S81087; 1 fe., IQ-700-D, SC20443, 28°204”S, 153°129”E, 748m, pitfall Trap, 11-20/10/2006, S. Maunsell, QM S81120; 1 fe., IQ-1100-C, SC20507, 28°26”S, 153°167”E, 1106m, flight intercept, 17-27/10/2006, G. Monteith, QM S76230; 1 fe., IQ-1100-B, SC30742, 28°259”S, 153°162”E, 1142m, dung baited pitfall, 22-27/10/2006, R. Menendez, G. Monteith, QM S76425; 1 ma., IQ-1100-D, SC30714, 28°262”S, 153°17”E. 1140m, dung baited pitfall, 22-27/10/2006, R. Menendez, G. Monteith, QM S76434; 1 ma., IQ-300-A, SC30564, 28°148”S, 153°137”E, 267m, mushroom baited pitfall, 16-21/10/2006, R. Menendez, G. Monteith, QM S76361; 1 fe., IQ-300-B, SC30556, 28°155”S, 153°139”E, 282m, mushroom baited pitfall, 16-21/10/2006, R. Menendez, G. Monteith, QM S76426; 1 fe., IQ-300-D, SC30704, 28°142”S, 153°133”E, 248m, mushroom baited pitfall, 21-26/10/2006, R. Menendez, G. Monteith, QM S76372; 1 fe., IQ-500-B, SC30668, 28°212”S, 153°141”E, 514m, mushroom baited pitfall, 18-23/10/2006, R. Menendez, G. Monteith, QM S76410; 1 fe., IQ-700-A, SC30566, 28°188”S, 153°121”E, 746m, mushroom baited pitfall, 16-21/10/2006, R. Menendez, G. Monteith, QM S76365; 1 ma., IQ-700-A, SC30705, 28°188”S, 153°121”E, 746m, dung baited pitfall, 21-26/10/2006, R. Menendez, G. Monteith, QM S76337; 1 ma., IQ-700-C, SC30757, 28°193”S, 153°128”E, 748m, dung baited pitfall, 24-29/10/2006, R. Menendez, G. Monteith, QM S76335; 1 ma., IQ-700-C, SC30759, 28°193”S, 153°128”E, 748m, dung baited pitfall, 24-29/10/2006, R. Menendez, G. Monteith, QM S76382; 1 fe., IQ-900-C, SC30750, 28°24”S, 153°149”E, 944m, mushroom baited pitfall, 22-27/10/2006, R. Menendez, G. Monteith, QM S76394; 1 ma., IQ-500-B, SC22168, 28°212”S, 153°141”E, 514m, flight intercept, 15-25/01/2007, G. Monteith, QM S76302; 1 ma., IQ-1100-C, SC22177, 28°26”S, 153°167”E, 1106m, flight intercept, 16-26/01/2007, G. Monteith, QM S76279; 1 fe., IQ-1100-D, SC22178, 28°262”S, 153°17”E, 1140m, flight intercept, 16-26/01/2007, G. Monteith, QM S76291; 7 ma., fe., IQ-900-A, SC22254, 28°234”S, 153°141”E, 904m, pitfall Trap, 12-21/02/2007, K. Staunton, QM S76187; 6 ma., 6 fe., IQ-900-C, SC22256, 28°24”S, 153°149”E, 944m, pitfall Trap, 12-21/02/2007, K. Staunton, QM S76193; 7 ma., 3 fe., IQ-900-D, SC22257, 28°227”S, 153°131”E, 920m, pitfall Trap, 12-21/02/2007, K. Staunton, QM S76183; 7 ma., 3 fe., IQ-1100-A, SC22258, 28°258”S, 153°159”E, 141m, pitfall Trap, 12-21/02/2007, K. Staunton, QM S76215; 11 ma., 6 fe., IQ-1100-B, SC22259, 28°259“S, 153°162”E, 1142m, pitfall Trap, 12-21/02/2007, K. Staunton, QM S76203; 6 ma., 6 fe., IQ-1100-C, SC22260, 28°26”S, 153°167”E, 1106m, pitfall Trap, 12-21/02/2007, K. Staunton, QM S76200; 8 ma., 5 fe., IQ-1100-D, SC22261; 28°262”S, 153°17”E, 1140m, pitfall Trap, 12-21/02/2007, K. Staunton, QM S76206; 1 fe., IQ-900-D, SC20756, 28°227”S, 153°131”E, 920m, flight intercept, 15-25/10/2006, G. Monteith, R. Menendez, QM S7624.
Holotype male QM S76179
Carapace 1.12 long, 0.97 wide. Abdomen 1.08 long, 0.59 wide. Total length 2.2.
Leg measurements. I: 1.11; 0.41; 1.35; 1.19; 0.41; 4.46. II: 1.03; 0.35; 1.03; 1.00; 0.35; 3.76. III: 1.08; 0.41; 0.95; 1.03; 0.38; 3.84. IV: 1.38; 0.41; 1.30; 1.35; 0.46; 4.89. Palp: 0.49; 0.22; 0.27; --; 0.41; 1.38.
Colour. Carapace, legs and chelicerae yellow brown, carapace altar with darker central region. Abdomen dorsally pallid (other material greenish brown) with darker median band.
Carapace. Pear-shaped, caput not raised, no “saddle” anterior to fovea. Cover of light brown hairs. Eyes. Configuration: 2-4-2 from above, 2-2-4 from in front. From above, ALE beside PME. PER in two rows. Chelicerae. Small, fangs short, transverse. Pair of inwardly directed spines on upper face; other male showed spine on only one chelicera (
Figure 1b, arrow). Furrow with line of 6--8 teeth medially, dorsal of which is line of 6--8 long curved spines on ridge; 4 small spaced teeth on retromargin; boss small, distinct. Maxillae rectanguloid, lacking grooves. Labium subquadrate. Serrula long. Sternum cordate.
Legs. Femora appear dark through uniform cover of short black bristles. Trochanteral notches very shallow. Plumose hairs absent. Weak preening comb on metatarsi III, IV. Trichobothria. Metatarsi and tarsi with trichobothria in single narrow band. Erect trichobothria-like hairs on ventral tibiae and metatarsi I, II (
Figure 1d, e).
Spines. Patellae with two dorsal spines one basally and one on apex; 6 pairs of strong spines on raised bases on tibiae I, II, 4 such pairs on metatarsi I, II; ventral spines on tibiae and metatarsi III, IV weak. I: fe p1d2; pa d1.1; ti d1w v2.2.2.2.2.2; me v2.2.2.2. II: like I but fe p1d2. III: fe p1d3r1; pa d1w.1; ti p2d2r2v2.2.2. IV: fe p1d3r1; pa d1.1w; ti p2d2r2v2.2.2; me p3r4v.2+3 distal. Palp: p1d3; pa d1 basal plus strong erect spine distally; ti v2w d6w.
Palp. RTA small, distolateral, bipartite: basal a conical point, distal is broad flange that appeared acute in ventral view. Tibia as long as patella, longer than cymbium, line of setae marks invagination of distal edge of tibia ventrally. Cymbium apically rounded with 3 upwardly curved spines apically on lower face and 2 on prolateral edge; dorsal scopula distinct; no groove retrolaterally. Tegulum dominant with all sclerites in distal quarter. Spermatic duct sinuous and visible. Embolus small, distal.
Abdomen. Spiracle distinct, transverse. Colulus distinct with 6 setae. PLS conical, apical segment domed. Spinnerets. PLS 0.5 size and diameter of ALS. PMS as long as PLS but conical and less sclerotised. Fringe on ventral face of anal tubercle.
Female QM S76193
Colour in Alcohol. Carapace and abdomen dark, carapace with broad submedian lateral pallid band and median pallid band. Abdomen dorsally dark with small pallid areas, unpaired; ventrally dark with pallid genital area and booklungs, triangular area points distal from it, plus a pair of irregular pallid lines laterally, fusing posteriorly to large diamond shaped area including tracheal spiracle. Legs light brown with slighlt darker annulations on femora.
Epigyne. Spermathecae set diagonally (
Figure 8d); epigyne with broad median scape with paired lateral lobes (
Figure 8d) and median pair of small crescentic shaped insemination apertures.
Otherwise, similar to male.
Distribution. Known only from Lamington National Park, southeastern Queensland.