1. Introduction
Post-transplant lymphoproliferative disorders (PTLD) represent a severe and often insidious complication that can occur after kidney transplantation, characterized by its potential for aggressive dissemination [
1]. PTLD predominantly arises in the context of Epstein-Barr virus (EBV) infection and is most frequently observed within the first year following transplantation. In planning for and treatment of transplant recipients, it is essential to make balance between preventing certain complications such as transplant rejection via utilization of immunosuppressive agents and the potential risk of infectious complications and the risk of PTLD [
2,
3]. High-dose immunosuppression has been linked to an increased risk of PTLD, and the clinical manifestations of this condition can vary significantly, ranging from localized lesions to widespread disease, and may mimic benign disorders [
4].
The incidence of PTLD varies depending on factors such as the type of organ transplanted and the immunosuppressive regimen employed. A study utilizing data from the French kidney transplant registry, which followed adult patients prospectively from 1998 to 2007, reported a 1% incidence of PTLD at 5 years, increasing to 2.1% at 10 years post-transplant [
5]. The likelihood of PTLD decreased with more recent transplants. Similarly, a Canadian single-center cohort study involving 1,642 kidney transplant recipients from 2000 to 2012 documented a PTLD incidence rate of 0.18 cases per 100 person-years, with EBV mismatch between donor and recipient identified as a significant risk factor [
6]. Key risk factors for PTLD include the EBV serostatus of the donor and recipient (with PTLD more common in EBV-seropositive donors and EBV-seronegative recipients), the intensity and duration of immunosuppressive therapy, and the status of EBV infection.
Given the relatively low incidence of PTLD, there is a notable gap in the literature regarding management strategies for affected patients. Consequently, this case report aims to present a patient with PTLD who developed B-cell large cell lymphoma (DLBCL) following kidney and pancreas transplantation.
2. Case Presentation
A 44-year-old female patient was admitted to the Clinic of Nephrology, University Clinical Center of Serbia with elevated body temperature (40.2°C), accompanied by night sweats and general weakness. In the laboratory analyses were registered worsening transplant kidney function with an increase in serum creatinine (sCr) values 180umol/l (59-104umol/l) and inflammation parameters, C-reactive protein (CRP) 275mg/l (0-8mg/l). The patient had a history of type 1 diabetes mellitus since the age of six, which led to diabetic nephropathy. Consequently, she underwent simultaneous cadaver kidney and pancreas transplantation in January 2014 in NewYork-Presbyterian Hospital in the USA. Since then, she has been treated with triple immunosuppressive therapy (Tacrolimus, Azathioprine, Prednisone). She was treated with double antibiotic therapy (cefixime, ciprofloxacin), with the withdrawal of complaints, normalization of inflammation parameters and kidney allograft function (sCr 99 umol/l) with negative blood and urine cultures and also negative results PCR EBV and CMV.
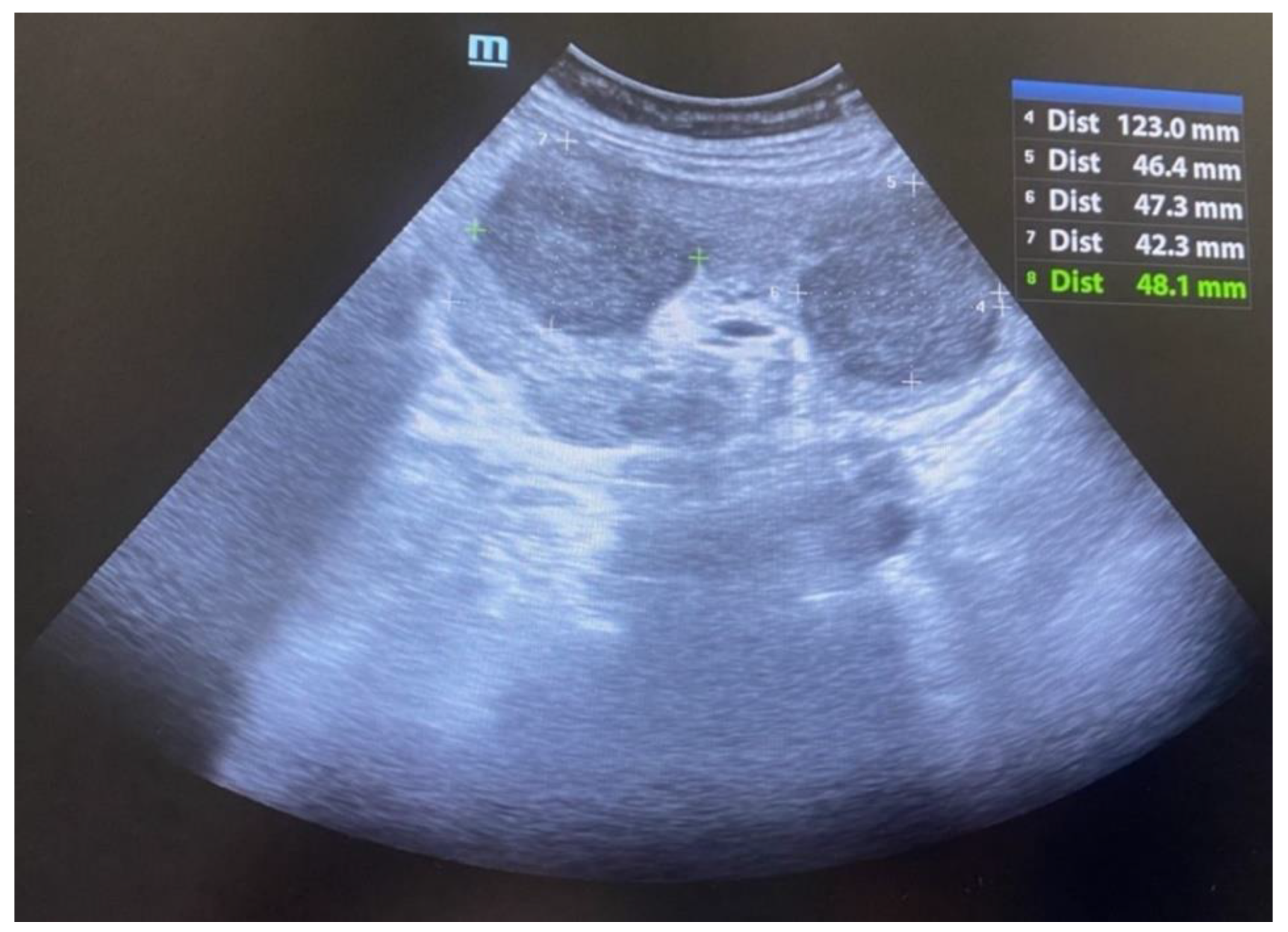
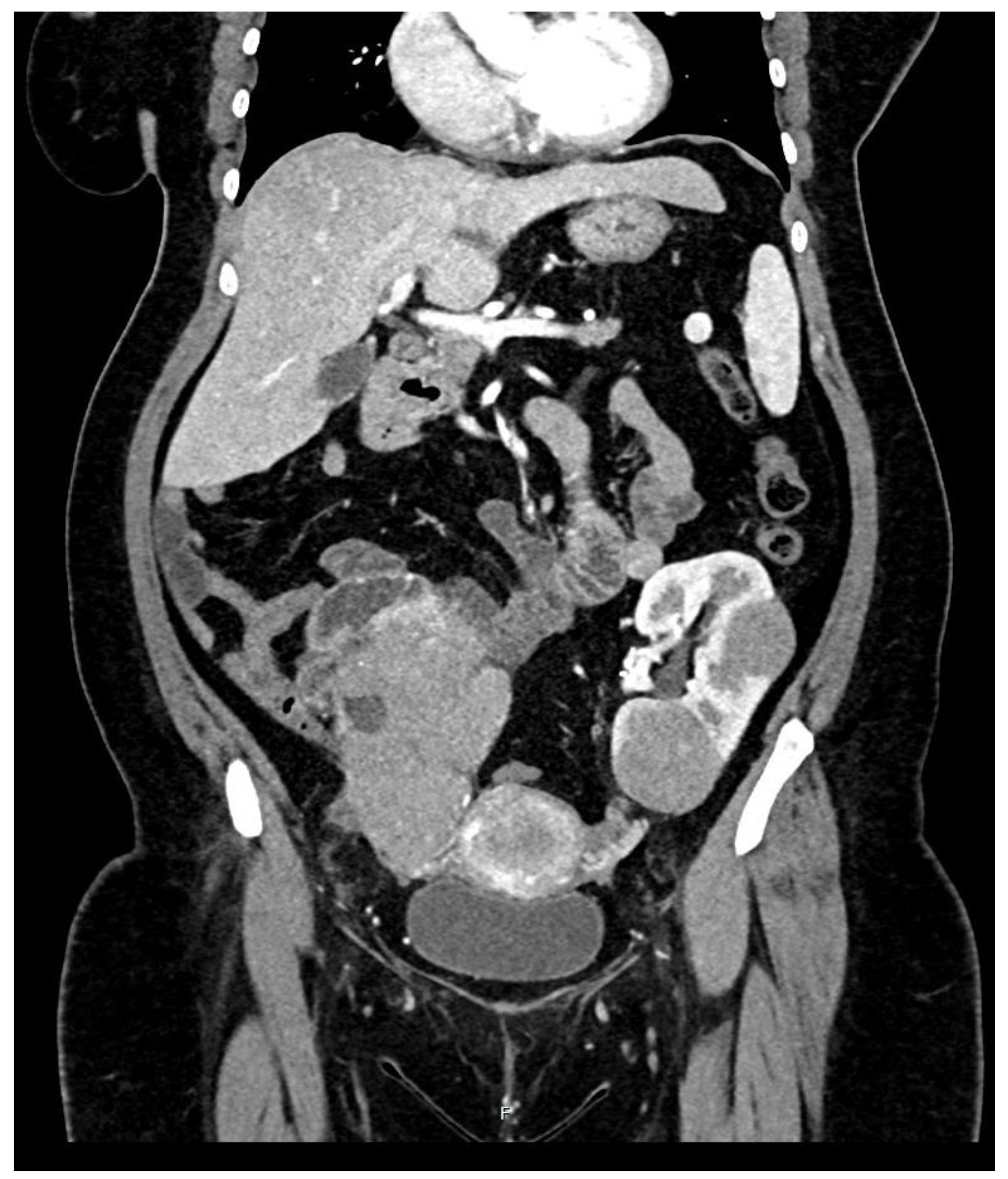
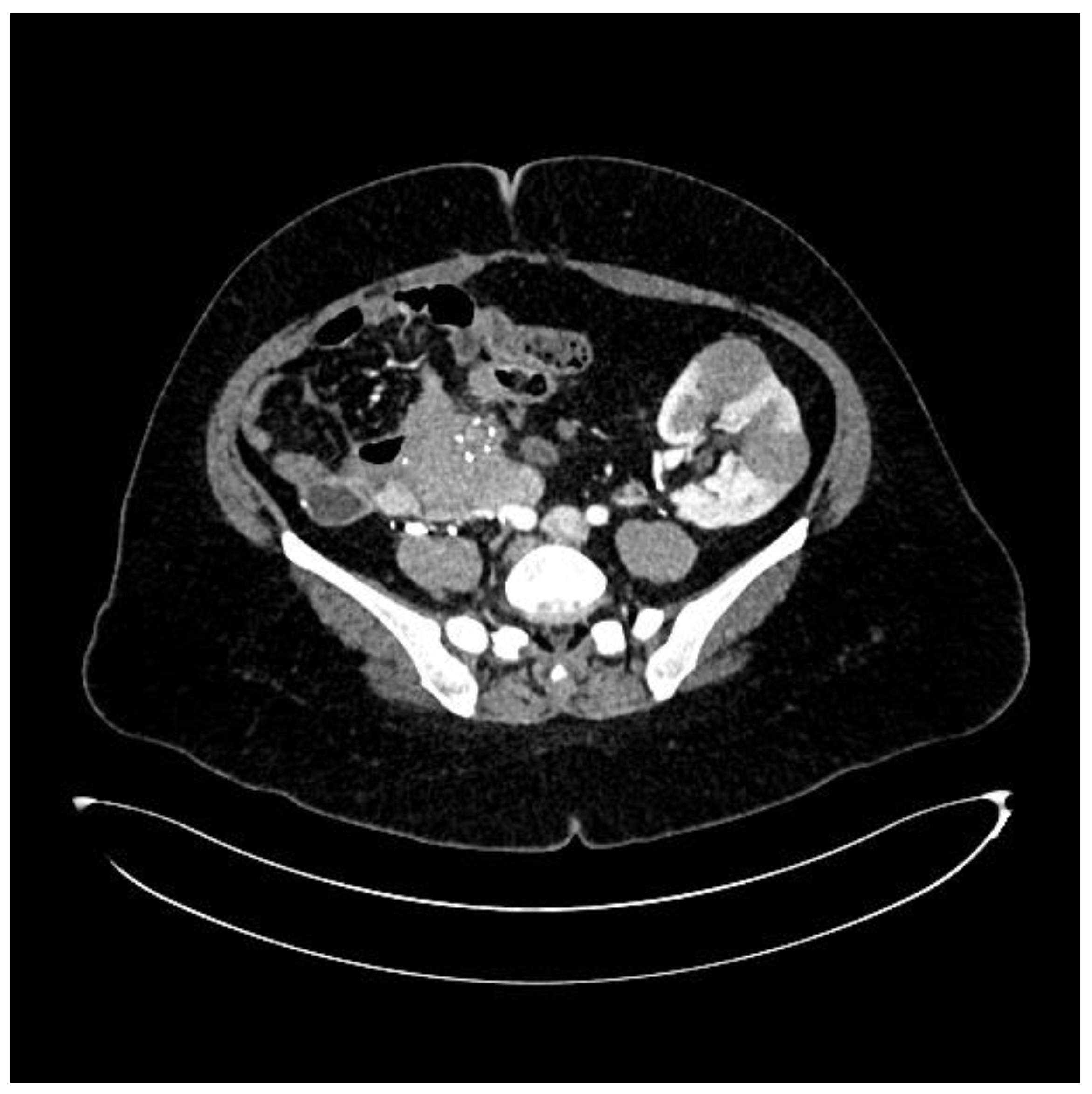
During hospitalization, an ultrasound of the transplant kidney was preformed and after the registered three infiltrative circular lesions isoechoic with parenchyma, a CT scan was also performed using CT-GE Medical Systems, LCC, Revolution HD, Belgrade, Serbia, revealing three lesions in the transplanted kidney, a larger lesion in the body of the pancreas, and additional lesions in the native kidneys (
Figure 1,
Figure 2 and
Figure 3). Additionally, a lesion was identified in the medial lobe of the right lung adjacent to the sixth rib cartilage.
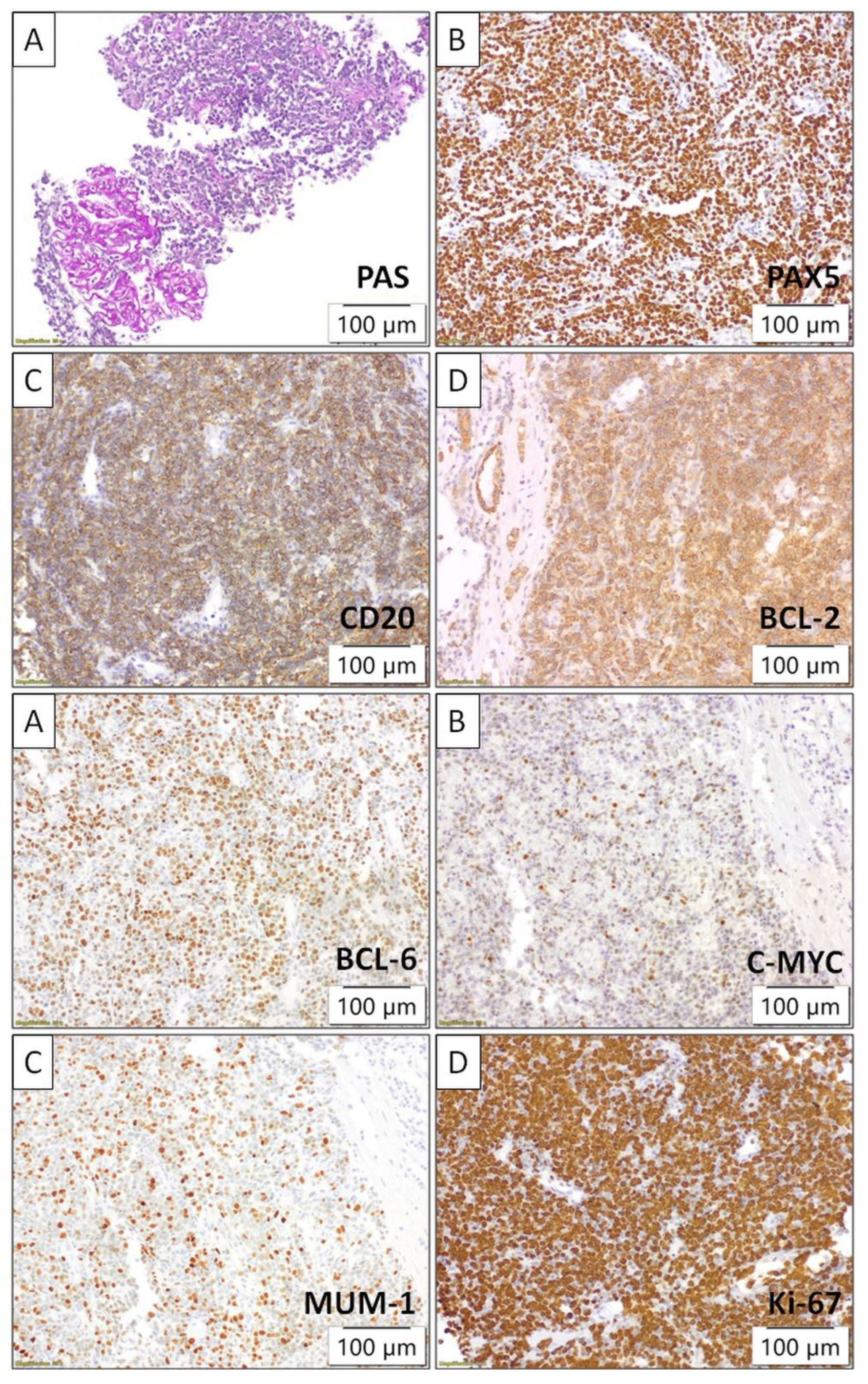
Following the identification of lesions, the patient underwent a biopsy of the lower pole of the transplanted kidney as the most accessible organ. Pathological and immunohistochemical analyses were conducted, which showed positive staining for CD20, Pax5, CD10, BCL6, BCL2, MUM1, c-myc, and lambda, while kappa, CD138, CD3, CD5, CD23, CyclinD1, Sox11, CD30, CD15, CD35, TdT, CKAE1/AE3, INSM1, and CD99 were negative. The Ki67 proliferation index was >90%. A diagnosis from two independent pathologists confirmed PTLD, specifically diffuse large B-cell lymphoma (DLBCL), GCS subtype, with double-positive expression of BCL2 and c-myc (
Figure 4).
Upon arrival of the PH findings of PTLD, a conversion was made from TAC to mTORi (everolimus) with dose correction according to target values (6-8ng/ml), antimetabolic agent was excluded and corticosteroid was continued in a low dose.
A multidisciplinary team recommended chemotherapy according to the R-CHOP protocol (Rituximab, Cyclophosphamide, Doxorubicin, Vincristine, Prednisone). During the first cycle, the patient experienced an infusion-related reaction (IRR) of grade II, which was managed symptomatically. Following the third cycle, the patient developed a complication of small bowel perforation, which was surgically addressed with the creation of a terminal stoma on the anterior abdominal wall. After a recovery period of one month and ten days, the patient resumed the fourth cycle of chemotherapy, followed by the fifth cycle. After the fifth cycle of chemotherapy, the clinical course was complicated by the appearance of pyelonephritis caused by Proteus mirabilis, leading to hospitalization at Kidney Transplantation Center for treatment. After the sixth cycle of chemotherapy, the patient developed grade IV neutropenia with a neutrophil count of (0,5 x109/l), WBC 1,2x109/l), which was treated with the application of Granulocyte colony stimulating factor (G-CSF).
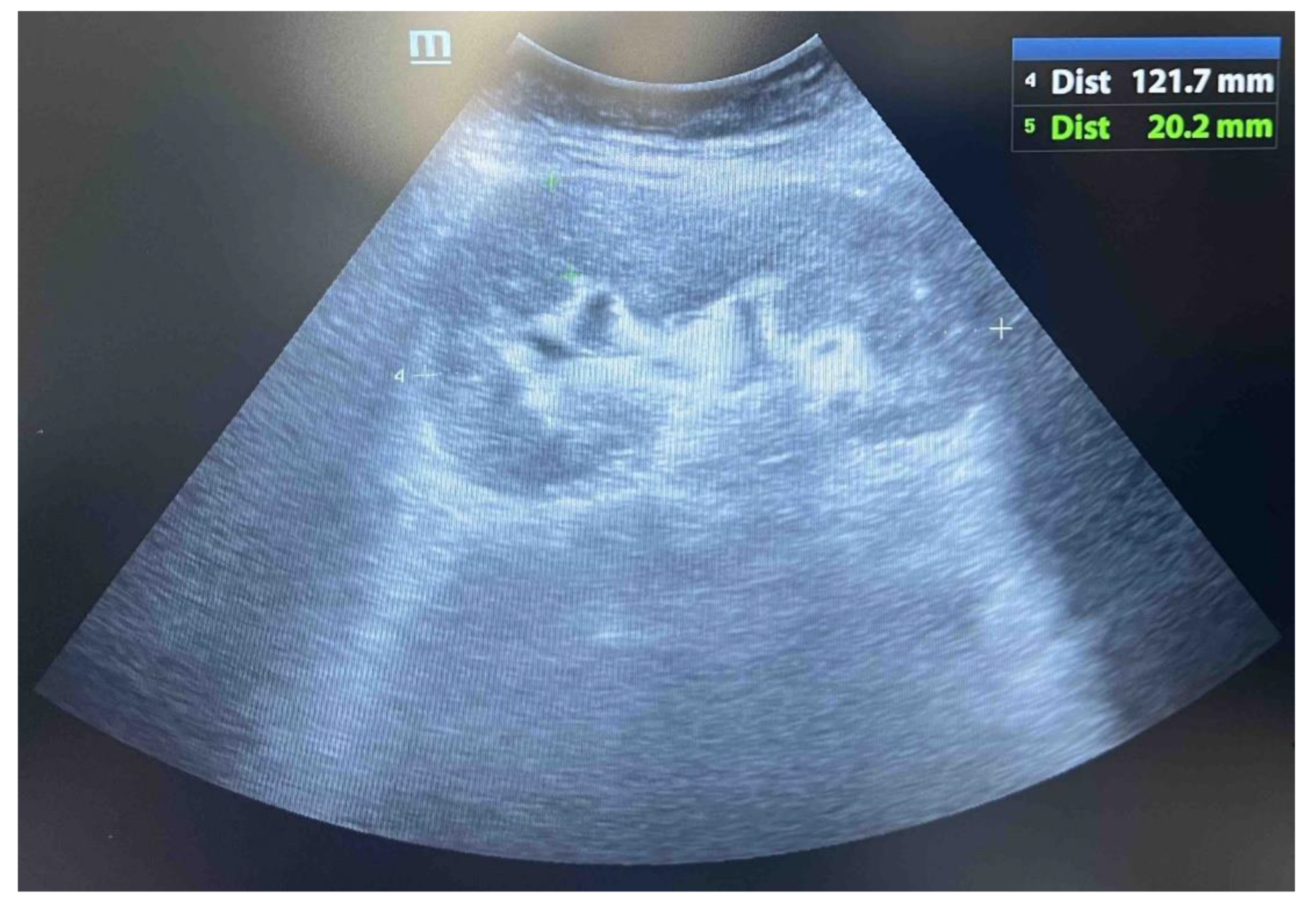
After six months of chemotherapy, the patient was in good general condition, afebrile, with an ultrasound noted the retreat of the infiltrative changes on the transplanted kidney and maintenance of the normal function of the allograft (
Figure 5). However, due to the persistence of tumor changes on the transplanted pancreas and active hematological disease, specific hematological treatment was continued.
The approval for publishing this case was granted by the Ethics Committee of the University Clinical Center of Serbia (No. 1264/20, from July 04, 2024).
3. Discussion
A rare case of PTLD, specifically DLBCL, is presented in a patient who underwent simultaneous pancreas and kidney transplantation due to longstanding type 1 diabetes mellitus and diabetic nephropathy. The patient presented with elevated body temperature, a recognized sign of PTLD. According to Samant et al. [
7], PTLD manifestations are heterogeneous, non-specific, and highly variable, presenting either as localized or disseminated disease. Common symptoms include malaise, fatigue, fever, and a mononucleosis-like syndrome. B-symptoms such as night sweats, weight loss, and lymphadenopathy are also prevalent [
7]. PTLD can progress rapidly and may lead to compressive symptoms at the tumor site. High-risk patients, such as those with EBV IgG-positive donors and EBV-negative recipients, are particularly susceptible to PTLD, which can impair graft function [
8]. Due to the diverse clinical presentation, a high index of suspicion for PTLD is essential, especially with increasing EBV PCR levels in post-transplant recipients, which heightens the likelihood of PTLD [
9].
In addition to radiological diagnostics, pathological analysis is essential for a definitive diagnosis of PTLDs. According to the latest World Health Organization (WHO) guidelines from 2022, PTLD are classified into four distinct categories based on their morphological, immunophenotypic, genetic, and clinical characteristics [
10]. Accurate classification is crucial for directing appropriate management strategies [
11]. The first category includes non-neoplastic lesions such as plasmacytic hyperplasia, infectious mononucleosis-like reactions, and florid follicular hyperplasia, which are considered early and benign proliferations. The remaining categories are neoplastic and are defined by the presence of a lymphoid tumor combined with at least two of the following features: disruption of tissue architecture by lymphoid proliferation, presence of monoclonal or oligoclonal lymphoid cell populations, and widespread EBV infection [
10]. Monomorphic PTLD is characterized by lymphomas of B or T/NK cell origin, with diffuse large B cell lymphoma being the most prevalent, though Burkitt lymphoma, plasma cell neoplasms, and other T/NK cell lymphomas may also occur. Polymorphic PTLD involves a heterogeneous lymphoid infiltrate that does not meet the criteria for specific B or T/NK cell lymphomas. Lastly, Hodgkin lymphoma-like PTLD matches the diagnostic criteria for classical Hodgkin lymphoma and is the rarest form of PTLD [
10].
The management of PTLD involves several strategies distinct from those used for lymphoproliferative disorders in immunocompetent patients [
12]. Initial treatment typically includes reducing immunosuppression by lowering calcineurin inhibitors and discontinuing antimetabolic agents [
13]. Rituximab, an anti-CD20 monoclonal antibody, is used for patients who do not respond adequately to reduced immunosuppression, either alone or in combination with chemotherapy. For those unresponsive to these measures, chemotherapy, particularly the R-CHOP regimen, is employed [
13,
14]. Radiation therapy is used for localized disease or central nervous system involvement [
15]. Additionally, adoptive immunotherapy utilizing EBV-specific cytotoxic T lymphocytes or donor lymphocyte infusion may be considered, though it carries a risk of graft-versus-host disease [
16].
The differential diagnosis of PTLD should account for its variable presentation, considering allograft rejection, opportunistic infections, and common infectious causes depending on the clinical context. The prognosis for PTLD has notably improved with the advent of rituximab and lymphoma-specific treatment regimens. An international trial demonstrated that sequential treatment with rituximab followed by CHOP chemotherapy resulted in a complete or partial response in 53 of 59 patients, with 40 achieving a complete response [
17]. Retransplantation is feasible following PTLD treatment but is recommended only after a minimum of one year [
18].
4. Conclusions
This case report details a rare instance of DLBCL as a PTLD in a 44-year-old female with a history of type 1 diabetes and simultaneous kidney and pancreas transplantation. Considering the manifestation of lymphoproliferative disease in the form of infiltrative changes on the transplanted kidney, an earlier diagnosis of DLBCL was possible. Due to the normal function of the transplanted kidney, it was possible to carry out necessary chemotherapy in full doses of drugs, which will certainly affect the prognosis and survival of both the patient and the transplanted organs. This case highlights the importance of both radiological and pathological evaluation for accurate PTLD diagnosis and effective management. Despite complications such as infusion reactions and bowel perforation, the patient's health improved with ongoing follow-up and treatment.
Author Contributions
Conceptualization, V.K.R., J.P. and M.B.; methodology, V.B., A.B. and M.B.; validation, V.K.R., V.B., M.M., D.V., D.A. and V.V.; formal analysis, V.K.R., J.P. and M.Ž.; investigation, V.K.R., A.B. and M.K.; resources, D.V., M.Ž., D.A., V.V. and M.R.; data curation, V.K.R., M.M., M.K. and M.Ž.; writing—original draft preparation, V.K.R. and J.P.; writing—review and editing, V.K.R., J.P. and M.R.; visualization, V.K.R. and M.Ž.; supervision, J.P., M.R.; project administration, V.K.R. and M.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the University Clinical Center of Serbia (No. 1264/20, from July 04, 2024).
Informed Consent Statement
Written informed consent has been obtained from the patient to publish this paper.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Atallah-Yunes, S.A.; Salman, O.; Robertson, M.J. Post-transplant Lymphoproliferative Disorder: Update on Treatment and Novel Therapies. Br J Haematol 2023, 201, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Dharnidharka, V.R.; Ruzinova, M.B.; Marks, L.J. Post-Transplant Lymphoproliferative Disorders. Semin Nephrol 2024, 44, 151503. [Google Scholar] [CrossRef] [PubMed]
- Lückemeier, P.; Radujkovic, A.; Holtick, U.; Kurch, L.; Monecke, A.; Platzbecker, U.; Herling, M.; Kayser, S. Characterization and Outcome of Post-Transplant Lymphoproliferative Disorders within a Collaborative Study. Front Oncol 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Veltmaat, N.; Zhong, Y.; de Jesus, F.M.; Tan, G.W.; Bult, J.A.A.; Terpstra, M.M.; Mutsaers, P.G.N.J.; Stevens, W.B.C.; Mous, R.; Vermaat, J.S.P.; et al. Genomic Profiling of Post-Transplant Lymphoproliferative Disorders Using Cell-Free DNA. J Hematol Oncol 2023, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Caillard, S.; Lamy, F.X.; Quelen, C.; Dantal, J.; Lebranchu, Y.; Lang, P.; Velten, M.; Moulin, B. Epidemiology of Posttransplant Lymphoproliferative Disorders in Adult Kidney and Kidney Pancreas Recipients: Report of the French Registry and Analysis of Subgroups of Lymphomas. American Journal of Transplantation 2012, 12, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Husain, S.; Famure, O.; Li, Y.; Kim, S.J. Incidence, Risk Factors, Clinical Management, and Outcomes of Posttransplant Lymphoproliferative Disorder in Kidney Transplant Recipients. Progress in Transplantation 2019, 29, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Samant, H.; Vaitla, P.; Kothadia, J.P. Posttransplant Lymphoproliferative Disorders; 2024.
- Shahid, S.; Prockop, S.E. Epstein-Barr Virus-Associated Post-Transplant Lymphoproliferative Disorders: Beyond Chemotherapy Treatment. Cancer Drug Resistance 2021. [Google Scholar] [CrossRef] [PubMed]
- Nijland, M.L.; Kersten, M.J.; Pals, S.T.; Bemelman, F.J.; ten Berge, I.J.M. Epstein-Barr Virus–Positive Posttransplant Lymphoproliferative Disease After Solid Organ Transplantation. Transplant Direct 2016, 2, e48. [Google Scholar] [CrossRef] [PubMed]
- Markouli, M.; Ullah, F.; Omar, N.; Apostolopoulou, A.; Dhillon, P.; Diamantopoulos, P.; Dower, J.; Gurnari, C.; Ahmed, S.; Dima, D. Recent Advances in Adult Post-Transplant Lymphoproliferative Disorder. Cancers (Basel) 2022, 14, 5949. [Google Scholar] [CrossRef] [PubMed]
- Dierickx, D.; Pociupany, M.; Natkunam, Y. Epstein-Barr Virus-Associated Posttransplant Lymphoproliferative Disorders: New Insights in Pathogenesis, Classification and Treatment. Curr Opin Oncol 2022, 34, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Vergote, V.K.J.; Deroose, C.M.; Fieuws, S.; Laleman, W.; Sprangers, B.; Uyttebroeck, A.; Van Cleemput, J.; Verhoef, G.; Vos, R.; Tousseyn, T.; et al. Characteristics and Outcome of Post-Transplant Lymphoproliferative Disorders After Solid Organ Transplantation: A Single Center Experience of 196 Patients Over 30 Years. Transplant International 2022, 35. [Google Scholar] [CrossRef] [PubMed]
- Zaffiri, L.; Chambers, E.T. Screening and Management of PTLD. Transplantation 2023, 107, 2316–2328. [Google Scholar] [CrossRef] [PubMed]
- DeStefano, C.B.; Desai, S.H.; Shenoy, A.G.; Catlett, J.P. Management of Post-transplant Lymphoproliferative Disorders. Br J Haematol 2018, 182, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Kirkpatrick, J.P.; Halperin, E.C. Low-Dose Radiation for Posttransplant Lymphoproliferative Disorder. Am J Clin Oncol 2003, 26, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Bollard, C.M.; Rooney, C.M.; Heslop, H.E. T-Cell Therapy in the Treatment of Post-Transplant Lymphoproliferative Disease. Nat Rev Clin Oncol 2012, 9, 510–519. [Google Scholar] [CrossRef] [PubMed]
- Trappe, R.; Oertel, S.; Leblond, V.; Mollee, P.; Sender, M.; Reinke, P.; Neuhaus, R.; Lehmkuhl, H.; Horst, H.A.; Salles, G.; et al. Sequential Treatment with Rituximab Followed by CHOP Chemotherapy in Adult B-Cell Post-Transplant Lymphoproliferative Disorder (PTLD): The Prospective International Multicentre Phase 2 PTLD-1 Trial. Lancet Oncol 2012, 13, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Prasad, P.; Khullar, D.; Gupta, N.; Grover, R.; Chhabra, G.; Gandhi, K.R.; Gupta, S.; Bagai, S. Retransplantation after Post Transplant Lymphoproliferative Disorder: Overcoming the Obstacles! CEN Case Rep 2020, 9, 200–203. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).