Introduction
Pregnancy is a period during which more than 90% of women experience changes in their skin, which can lead to negative emotions and impact a woman's self-confidencePregnancy-related skin conditions can be categorized into three groups: normal skin changes during pregnancy, pre-existing skin conditions affected by pregnancy, and pregnancy-specific skin diseases [
1]. Although most of these conditions are benign and subside after childbirth, some may pose a risk to the fetus and require prenatal monitoring. The prenatal period is a critical phase for the fetal development and is divided into two stages. The first stge lasts 56 days from fertilization, during which many organ systems grow rapidly. At this time, the sexual glands also differentiate into either testes or ovaries. During this stage, the developing fetus is particularly vulnerable, and malformations can occur during this "window of vulnerability." The second stage, known as the fetal period, begins at the 9th week and continues until birth. During this period, the development and differentiation of organs formed in the earlier stage take place [
2].
Pregnancy and Skin Changes
During pregnancy, a woman's body undergoes several modifications, including an increase in uterine volume, which shifts the center of gravity forward. The chest doubles in size, stretch marks may appear, and the venous network becomes more prominent. While stretch marks are common on the chest, they can also develop on other parts of the body and are influenced by various risk factors, such as:
A pre-conception BMI greater than 26,
Young age, especially in teenage pregnancies,
Weight gain during pregnancy exceeding 15 kg,
Baby's birth weight greater than 3.5 kg.
Other factors, such as carbohydrate intolerance and the pregnant woman's medical history, may also affect the development of stretch marks. Additionally, the breasts may undergo hyperpigmentation around the nipple, affecting 40 to 100% of pregnant women, which typically resolves completely after childbirth [
3]. Apart from hormonal changes, pregnancy, childbirth, and lactation can cause alterations in the skin, nails, and hair, which are briefly summarized in the table below:
Table 1.
Various changes during the pregrancy, childbirth, and lactation. (modified by Dr. F.Biskanaki, 2024).
Table 1.
Various changes during the pregrancy, childbirth, and lactation. (modified by Dr. F.Biskanaki, 2024).
| Melasma |
3rd month of pregnancy, in women with dark skin. It is located on the forehead, cheeks and chin. Melasma or the mask of pregnancy, is the second most common complaint after striae and may resolve within 1 year of delivery in 70% women without taking any treatment) [1] |
| Scars |
During pregnancy, the level of active melanocytes is higher, leading to a higher concentration of melanin in the pregnant woman's body. The scars look darker. The scars formed due to the extension of the skin. |
|
Vascular system
|
Telangiectatic arteriole, Telangiectasia, Palmar Εrythema, Varicose veins. [4] |
| Linea Nigra |
Hyperpigmentation of the white abdomen line (1st trimester) affects 90% of pregnant women. After the childbirth, it recedes enough or completely. [5] |
| Stretch marks (striae) |
>6th month of pregnancy in the percentage of 50 to 90%. They are characterized by thinner skin, but mainly a reduction in the density of the elastin fiber network. The collagen fibers break. At the level of striations, skin appendages are absent. The appearance of striae is mainly due to: hormonal changes, hereditary factors, and significant weight gain or loss. [5,6] |
| Acne |
The acne development is random. Indeed, it can worsen, improve, or remain unchanged [7] |
| Nails |
White milk spots on nails (Leukonychia). Nails can change during pregnancy. Extra hormones can make them grow faster and become stronger. Some women, though, find that their nails split and break more easily during pregnancy, but nail changes aren't permanent. [8] |
| Cellulite |
Due to high levels of estrogen and progesterone. [9] |
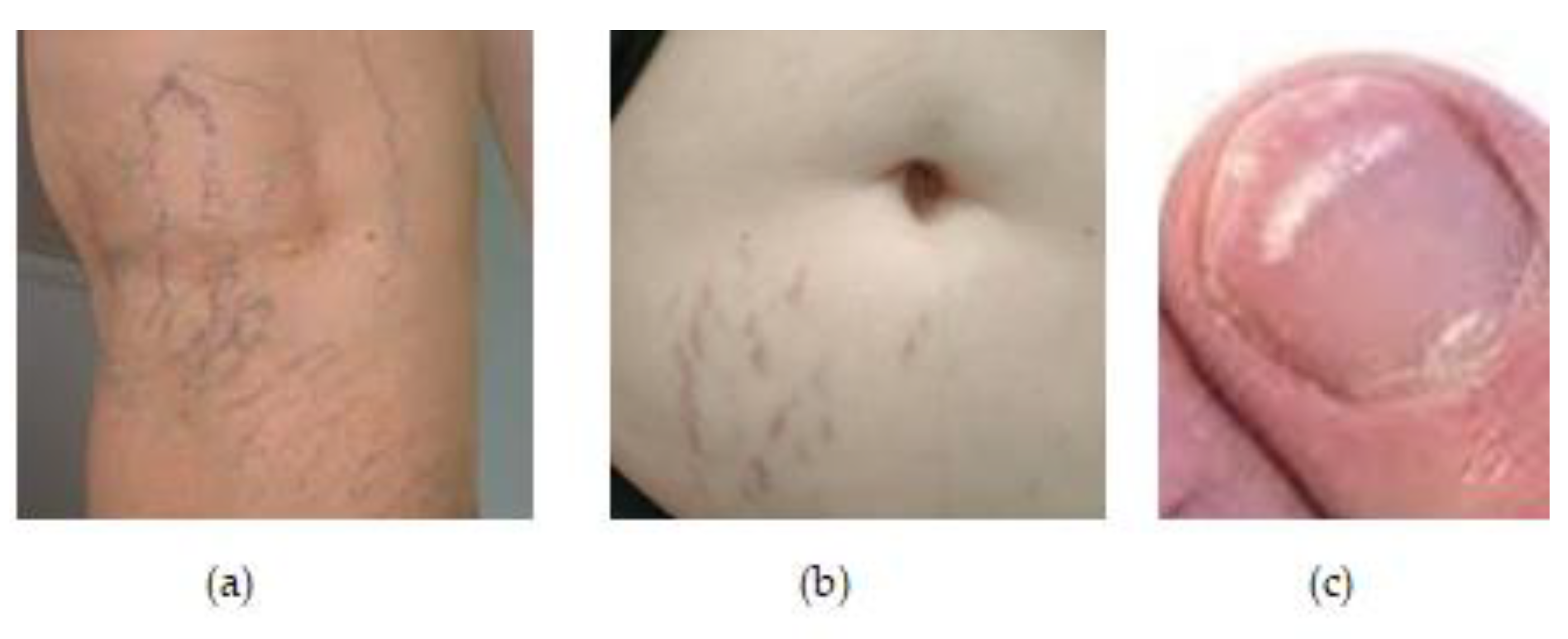
Figure 1.
(a) Changes in the vascular system during the pregrancy in a women 38 years old. (b) Red streaks one month after the childbirth in a women 32 y.o. (c) White milk spots on nails during pregrancy in a women 30 y.o.
Figure 1.
(a) Changes in the vascular system during the pregrancy in a women 38 years old. (b) Red streaks one month after the childbirth in a women 32 y.o. (c) White milk spots on nails during pregrancy in a women 30 y.o.
Cosmetics and Pregrancy
The term "cosmetic" originates from the ancient Greek word "cosmetikos," derived from "kosmos," which refers to beauty, ornament, and appearance. According to the Public Health Code (CSP), a cosmetic is defined as "any substance or mixture intended to come into contact with the various surface parts of the human body, particularly the skin, hair, nails, lips, and external genitalia, or the teeth and mucous membranes of the mouth, for the sole or principal purpose of cleaning, perfuming, modifying appearance, protecting, keeping in good condition, or correcting body odor" [
10].
Cosmetic products do not require prior marketing authorization. They do not possess therapeutic or preventive properties concerning human diseases and are not intended to be ingested, inhaled, or implanted in the body, clearly distinguishing them from drugs.
However, after the Mohrange talc scandal [
11] — in which several talc products, contaminated with high concentrations of hexachlorophene, resulted in 36 deaths in 1972 cosmetics became subject to regulations encoded in the CSP. Among these regulations is a fundamental principle: "The cosmetic product must not harm human health under normal or reasonably foreseeable conditions of use." Despite this, many cosmetic products still contain chemicals that are potentially harmful or dangerous to the body.
Given the widespread use of these substances, special attention must be paid to pregnant women and their fetuses. During pregnancy, consumers should be vigilant, as certain changes they notice can indicate various risks. Preserving maternal immunity is crucial, and beauty products known to be harmful remain prohibited. The use of cosmetic products during pregnancy requires careful recommendations due to substances that may be harmful to the fetus. Intrauterine exposure to certain chemicals can have irreversible effects on the developing child, particularly if exposure occurs during the first 8 weeks of development.
Pregnant women may be inclined to overuse cosmetic products due to the extensive physical changes associated with pregnancy (e.g., stretch marks) [
10,
12]. The concerns of aestheticians and dermatologists have drawn the attention of legislators to the real dangers posed by cosmetology. The perception of risks associated with cosmetic use during pregnancy, both for the health of the woman and her future child, is closely linked to the crucial contributions of cosmetovigilance [
13].
Cosmetovigilance
Cosmetovigilance is "a system for monitoring and recording adverse reactions associated with yhe use of cosmetics in humans". It applies to all cosmetic products once they are available on the market and is governed by Regulation (EC) No. -8 and Articles R.5131-6 to R.5131-15 of the CSP (ANSM, 2021). The ANSM (French National Agency for Medicines and Health Products Safety) ensures, through its assessments, expertise, and surveillance policies, that health products available in the EU are safe, effective, accessible, and used correctly. Cosmetovigilance is based on the following principles:
a) Reporting adverse effects and collecting all relevant information concerning them.
b) Recording, evaluating, and using information related to these effects for prevention purposes.
c) Conducting studies and research concerning the safety of cosmetic use.
d) Implementing and monitoring corrective actions if necessary [
14].
Cosmetovigilance covers any adverse reaction, serious or not, that occurs during the use of a cosmetic product under normal or reasonably foreseeable conditions or as a result of misuse. A side effect is an unwanted reaction caused by a cosmetic, though it is not necessarily harmful. In contrast, an adverse reaction is characterized as harmful to human health and is attributable to the normal or reasonably foreseeable use of a cosmetic product. A serious adverse event is defined as a reaction that leads to temporary or permanent functional incapacity, disability, hospitalization, congenital anomalies, an immediate threat to life, or death [
15].
The Risks of Cosmetics and Cosmetic Procedures
Humans are exposed daily to pollution from the ground, air, and water, and anything that enters the body can trigger inflammation. Chronic inflammation can affect the immune system and the body's defenses, potentially leading to allergies and neoplasms. Fetal life is particularly vulnerable to external pollutants, including cosmetics [
16]. However, confirming this observation is challenging, as research on the effects of cosmetics during the prenatal period is limited.
There is significant concern about the ingredients (raw materials) in cosmetic products and the treatments women undergo during this critical period. Safety guidelines prioritize the well-being of the mother and her unborn child, resulting in heightened attention to the potential negative effects of these products. The safety of cosmetics and aesthetic applications for women during pregnancy or breastfeeding remains a complex clinical issue surrounded by uncertainty [
17].
Cosmetic Procedures
The most common minor surgeries include the removal of benign skin lesions, such as seborrheic keratosis, nevi, and hemangiomas, through methods like shave excision, cryotherapy, electrocautery, or radiation. These procedures have been used safely for many years in pregnant women. Electrocautery is considered harmless to the fetus when used on the mother. However, precautions should be taken to reduce exposure to the smoke plume, which can be mutagenic and carcinogenic. This risk applies to both pregnant and non-pregnant patients, and, by extension, to the fetus. Masks are considered inadequate barriers against the plume; therefore, using a smoke evacuator is preferred to minimize smoke exposure [
18,
19].
Sclerotherapy may be safe during pregnancy but should be avoided in the first trimester and after the 36th week of pregnancy. Sclerotherapy and laser-assisted liposuction are not recommended for breastfeeding women. CO2 lasers have shown good efficacy and a low complication rate in the treatment of human papillomavirus (HPV) genital warts and laryngeal verrucous carcinoma in pregnant women due to their coagulating properties, with no reported adverse effects on the fetus [
19]. However, there are concerns regarding maternal exposure to potentially mutagenic airborne particles generated during laser vaporization. This risk can be mitigated using aspirators, fume extraction devices, and masks for both the patient and physician. Several studies support the safety of carbon dioxide lasers in treating genital warts in pregnant patients [
20,
21]. Additionally, Nd: YAG lasers have also been used safely to treat genital warts during pregnancy [
22].
Laser lithotripsy during pregnancy has been shown to be safe. Another study reported the successful and safe use of a Pulse Dye Laser to treat symptomatic urolithiasis in a woman who was 20 weeks pregnant [
23].
Botulinum toxin type A (BTX) has both cosmetic and medical applications [
24]. Current studies suggest, though do not fully confirm, that the toxin does not reach significant systemic concentrations when properly administered intramuscularly or intradermally. Additionally, the size of the BTX molecule makes it unlikely to cross the placental barrier [
25]. There are no clinical studies on the cosmetic use of Botulinum toxin type A during pregnancy. However, two cases were reported by De Oliveira Monteiro, in which BTX was used cosmetically during the 6th and 5th weeks of pregnancy without adverse effects on the fetus. However, two cases were reported by De Oliveira Monteiro (2006), in which BTX was used cosmetically during the 6th and 5th weeks of pregnancy without adverse effects on the fetus [
26]. Two studies have shown the safety of botulinum toxin A for the treatment of achalasia in women who were pregnant. Hooft et al. reported the administration of an intradose injection of BTX during the 14th week of pregnancy without negative effects on fetal health and a successful childbirth outcome [
27]. Wataganara et al. described a similar case in which BTX was administered during the 33rd week of pregnancy to treat persistent esophageal spasm and subsequent malnutrition, with no adverse effects observed five days postpartum [
28]. Robinson and Grogan also reported the safe use of BTX at the 18th week of pregnancy to treat migraines, with no issues noted in the infant for 6.5 years following birth.
A study of 900 doctors revealed that only 12 had performed accidental BTX injections in 16 patients during pregnancy. Only one of these patients experienced a miscarriage after the injection [
29]. Concerns have been raised that high doses (>600 U) of BTX are associated with cases of systemic weakness. However, the doses used in cosmetic procedures for pregnant women are typically much lower. If pregnant women choose to undergo BTX treatment, they should provide informed consent before the procedure. Procedures that are absolutely contraindicated during pregnancy include fillers, sclerotherapy, and liposuction.
For topical anesthesia, various forms are available, such as EMLA cream (lidocaine 2.5% + prilocaine 2.5%), EMLA anesthetic disc (lidocaine 2.5% + prilocaine 2.5%), ELA-Max topical anesthetic cream (lidocaine 4%), and Lidoderm (lidocaine 5% patch). Unlike injectable forms, topical creams are generally considered much safer. However, anecdotal reports advise caution with some topical agents. Prilocaine, in high doses, carries a risk of fetal methemoglobinemia; therefore, lidocaine-prilocaine (both Category B) cream should be used in moderation. Occlusion and use near periocular and mucosal surfaces should be avoided, as these can increase systemic absorption and irritation. Tetracaine and benzocaine (both Category C) are associated with fetal methemoglobinemia. Consequently, a lidocaine/prilocaine mixture (in moderation) is the preferred choice for topical anesthesia, especially for those concerned about safety [
30].
Biotechnology, and Aesthetic Applications
Endermology was developed in France by Louis Paul Guitay in the 1970s after he sustained soft tissue injuries in a car accident. The resulting scars were treated using hand massage, which inspired Guitay to develop a device that mimics this technique. The device was initially used for scars caused by trauma and burns. However, researchers observed that it also improved the appearance of cellulite and the distribution of fat. The most recent versions of this device are the Integral and Endermolab (LPG Systems). The mechanism of action involves negative pressure suction combined with a roller that passes over areas with fat deposits. Medical LPG is also suitable for pregnant women, providing relief to the legs from swelling caused by increased weight. Additionally, it can alleviate lower back pain by facilitating lymphatic system mobility. The treatment is painless and non-invasive, and for pregnant women, it is conducted with great care, with the patient lying on their side rather than on the abdomen [
31].
Massage is the oldest method of pain management and is integral to many facial and body aesthetic treatments. It involves a variety of techniques with numerous benefits at the organic level. Massage requires special attention during pregnancy, and the therapist must be knowledgeable about the anatomy and physiology of the female body. Stimulating massage is contraindicated during this sensitive period as it can cause contractions and potentially lead to premature birth. The massage should be gentle and is not recommended if there are pregnancy complications. The primary goal is to relax and relieve the muscles bearing the extra weight, improve blood and lymph circulation, and reduce stress. It also helps alleviate fatigue, muscle cramps, headaches, back pain, and leg swelling [
32]. Studies conducted over the past decade on pregnant women have shown a significant reduction in stress hormones such as cortisol, while dopamine and serotonin levels increased in women who received massage twice a week for five consecutive weeks. Low levels of dopamine and serotonin are associated with depression, so these hormonal changes can contribute to a reduction in pregnancy and childbirth complications. During the first trimester, when fetal organ formation occurs and the risk of miscarriage is high, it is recommended to avoid massage to prevent any association between massage and potential pregnancy failure. Almond oil is considered an ideal product for massage application, as strong-smelling oils may cause discomfort and nausea [
33].
Radiofrequency (RF) applications have been used since 2002 to improve cellulite, skin laxity, and wrinkles, and more recently to reduce fat. The mechanism of action is based on volumetric heating of the skin and subcutaneous tissues. Unipolar RF devices can reduce fat circumference in about 60%–80% of individuals. However, there are no studies examining the use of RF during pregnancy [
34,
35].
During pregnancy and breastfeeding, laser hair removal, IPL, or electrolysis is generally not recommended due to a lack of data and clinical studies on their safety and efficacy [
36]. There is a theoretical concern about electrolysis, as amniotic fluid is a conductor of galvanic current. For hair removal during pregnancy, shaving and depilatory creams are recommended. The use of lasers for aesthetic treatments has not been studied in pregnant women.
Repeated and prolonged exposure to ultraviolet radiation (UVA, UVB, and UVC) increases the risk of skin cancer due to DNA damage and impaired repair mechanisms. While the lamps used in IPL hair removal devices are capable of emitting UV radiation, these devices are designed to include cut-off filters that allow only wavelengths above 400 nm to pass through, preventing UV exposure. However, if these filters become damaged, UV emission could occur. The potential effects of IPL devices, based on their operating principles, have not been conclusively reported in recent studies. Only long-term epidemiological studies that analyze IPL exposure as a specific variable could confirm or refute the associated risks [
37]. There is a potential risk of developing basal cell and squamous cell carcinomas in keratinocytes and melanomas in melanocytes. However, this risk is primarily linked to severe damage to the cut-off filter within the IPL device. No publications have yet identified cancers caused by the thermal effects associated with IPL. The limited follow-up of IPL use, the short duration of follow-up in existing studies, and the small number of subjects included do not provide sufficient information on these potential long-term effects (which may be observable 5 to 15 years later).
The safety of using laser and IPL for hair removal depends on the energy absorbed by the epidermis, hair follicle, and adjacent tissues, which varies with the emission characteristics of the devices and the individual characteristics of the users. Key factors for safe exposure include:
- a)
The nominal operating characteristics of the device defined during its design.
- b)
The stability of the device's operating characteristics, which depend on design and maintenance.
- c)
Correct device usage, which is linked to the training and skill of operators, proper user information for household devices, and adherence to best practices.
- d)
The characteristics of the pregnant woman at the time of hair removal, including phototype, skin color, hair color, and individual response.
- e)
Possible contraindications, such as pre-existing diseases, photosensitive treatments, etc. [
38].
Despite concerns, lasers have been used safely to treat medical conditions in pregnant women. Laser and IPL are generally considered safe for patients with granulomatous conditions and warts. Limited studies indicate that ND: YAG lasers can be safely used to treat skin lesions during pregnancy. Genital warts have been successfully treated with the ND:YAG laser without complications for the mother or fetus [
39]. Other studies report the safe use of the ND: YAG for severe inflammatory acne and pyogenic granulomas during pregnancy, with no observed side effects [
40]. In a recent study, the Pulse Dye Laser (PDL) (585 nm) was used to treat pyogenic granulomas and warts during pregnancy, demonstrating it to be a safe treatment [
41]. Available studies indicate a good safety profile for PDL use in pregnancy, with minimal side effects such as mild erythema and pain. In conclusion, laser treatments appear relatively safe for medical conditions in pregnant women. However, laser and IPL are not recommended for cosmetic procedures during pregnancy due to a lack of safety studies [
42].
Risks of Ingredients of Ccosmetics
Cosmetics are a significant tool for many professional aestheticians. Previous studies indicate that aestheticians who are constantly in contact with cosmetics have 2-4 times more exposure to toxins compared to those who use cosmetics intermittently. Over 10,000 chemicals are used in cosmetics, but many are not completely harmless [
43].
Numerous synthetic chemical ingredients should be avoided due to their toxicity. Toxic substances are those capable of destroying life. When toxic substances enter the body, they are metabolized in the liver, where they are inactivated and then excreted by the kidneys or bioaccumulated [
44]. Some of the most commonly dangerous chemicals used in cosmetics include:
Acetone: This solvent, used in nail polish removers, can cause dry mouth, dizziness, nausea, difficulty speaking, and, in extreme cases, coma. It acts as a central nervous system (CNS) depressant [
45]
Aluminum Compounds: Commonly found in deodorants and antiperspirants, aluminum compounds can block sweat glands and pores, preventing the body's natural sweating process. There are concerns that these compounds may be linked to breast cancer, breast cysts, and Alzheimer's disease. However, dermal absorption of aluminum is not well understood, and it is not yet known whether aluminum can travel from the skin to the brain to cause Alzheimer's disease. While aluminum may cause gene instability, alter gene expression, or enhance oxidative stress, its carcinogenicity has not been proven. Epidemiological research on aluminum exposure has been inconsistent and conflicting [
45,
46].
Ammonia: Found in many cosmetics, ammonia can cause allergic reactions and, with long-term exposure, may lead to coughing, respiratory arrest, and pulmonary edema, potentially resulting in death [
47].
Titanium Dioxide: Commonly used in sunscreens and toothpastes, titanium dioxide can be absorbed through the skin or inhaled during manufacturing and use. Despite its extensive use, the biological effects and cellular response mechanisms of titanium dioxide nanoparticles (TiO2-NPs) are not fully understood. The toxicity of TiO2-NPs appears to involve reactive oxygen species (ROS) production, leading to oxidative stress, inflammation, genotoxicity, metabolic changes, and potential carcinogenesis. The extent and type of cell damage depend on the chemical and physical characteristics of TiO2-NPs, including size, crystal structure, and photo-activation [
48].
Diethanolamines (DEA): Used mainly as surfactant foam boosters or viscosity-increasing agents, DEA can cause allergic reactions, eye and skin irritation, and is a potential carcinogen if used long-term. Recent reports have raised concerns about an increase in allergic dermatitis associated with DEA [
49].
Mineral Oil: Found in many creams for eyes, lips, and hydration, mineral oil forms a barrier on the skin that can prevent it from breathing, expelling toxins, hydrating, and producing healthy cells. Common reactions include premature skin aging, cardiac disorders, kidney and liver damage, and breathing difficulties.
Formaldehyde: Used as a preservative in cosmetics, formaldehyde is a toxic substance. Other preservatives such as parabens and Imidazolidinyl Urea can cause dermatitis and are potential carcinogens. Parabens are used to inhibit microbial growth and extend the shelf life of products, but they have been associated with dermatitis and allergies. Many cosmetics also contain synthetic fragrances, which may include up to 600 different ingredients. The exact components are often not disclosed, only labeled as "Perfume" or "Fragrance," leading to potential issues such as rashes, itching, cough, asthma, and vomiting, with some fragrances having a narcotic effect [
50].
A study involving 9,710 pregnant women in Zhuzhou City and Xiangtan City in Hunan province during 2016-2017 found no significant association between daily personal cosmetics use and the risk of preterm birth, low birth weight, macrosomia, or being large for gestational age. However, the study suggested that personal cosmetics use might increase the risk of small for gestational age (SGA), warranting further research to identify specific cosmetic products contributing to this risk.
Melasma, a common skin condition during pregnancy, is associated with increased melanocyte hormone secretion, elevated estrogen-progestin levels, and sun exposure. It can also be exacerbated by cosmetics, phototoxic drugs, or anticonvulsants. Melasma often resolves partially or completely within 6 to 18 months postpartum [
50]. Ingredients that can improve melasma include glycolic acid, lactic acid, TCA, and salicylic acid. Glycolic and lactic acid peels are considered safe during pregnancy, while TCA and salicylic acid peels should be avoided.
Acne, a common skin condition affecting adolescents and adults, may change unpredictably during pregnancy. High-quality guidelines for acne treatment during pregnancy are scarce, making management challenging. Topical azelaic acid or benzoyl peroxide is recommended as baseline therapy. For inflammatory acne, a combination of topical erythromycin or clindamycin with benzoyl peroxide is advised. Oral erythromycin or cephalexin is generally safe for moderate to severe inflammatory acne when used short-term. A brief course of oral prednisolone may be useful for treating severe nodular cystic acne after the first trimester. Topical and oral antibiotics should be used in combination with benzoyl peroxide to reduce bacterial resistance.[
51]. Oral retinoids are teratogenic and contraindicated during pregnancy. While some complementary therapies, including micronutrients and non-pharmacologic treatments, appear to be well tolerated, there is limited data on their safety and efficacy, and they are not currently recommended during pregnancy [
7].
Breastfeeding Women
There are very few studies in the public literature on the safety of cosmetics for breastfeeding women. The main concern with using any cosmetic procedures during lactation is the potential systemic absorption of agents and their subsequent incorporation into breast milk, which could affect neonatal growth and development. [
52] Most cosmetic procedures, such as Botox and chemical peels, are generally considered safe during lactation, as there is little concern about significant systemic absorption of the agents involved. However, procedures or surgeries that involve fat redistribution or removal, such as liposuction, are not recommended. Sclerotherapy should also be avoided during breastfeeding. Although hypertonic saline solutions used in sclerotherapy are generally considered safe, there are no studies on whether other sclerosing agents are excreted in breast milk. Therefore, it is recommended to avoid this treatment while breastfeeding.
Some women who have undergone sclerotherapy have continued breastfeeding by pumping and discarding breast milk for the first 48 hours after treatment. However, there are no reported results, complications, or success rates associated with this approach.
Risks for Aestheticians and Dermatologists During Pregnancy
The combination of work and pregnancy in environments conducive to smooth functioning is a concern for many pregnant women According to the National Perinatal Survey (ENP, 2016), 70.2% of women in the EU worked during pregnancy in 2010. Additionally, the percentage of aestheticians workers exposed to certain occupational hazards is significantly higher than that of other professional groups. Specifically, 88.3% of aestheticians are exposed to chemical pollutants, compared to 33.2% of workers in other professions [
53]. Numerous studies have examined the effects of exposure to occupational hazards, such as chemical agents (especially solvents), musculoskeletal disorders (MSDs), and noise, on pregnant women across various occupations. Some researchers have specifically focused on the cosmetics sector due to its unique occupational hazards and demographic characteristics, including the predominance of young women [
54].
Discussion
Skin changes during pregnancy, such as melasma, striae, varicose veins, hirsutism, and increased skin growth, can negatively impact psychological well-being. Women may seek cosmetic procedures during pregnancy, necessitating a careful risk-benefit assessment by the aesthetician or dermatologist. However, only those cosmetics and procedures that are deemed safe and necessary should be performed on pregnant women. [
3,
50]. Procedures such as electrocautery, cryotherapy, and lasers for warts (particularly genital warts), as well as surgical interventions for skin malignancies and other small growths, can be safely performed [
27]. Caution is advised with chemical peels, botulinum toxin treatments, and biopsies for questionable lesions. Procedures that are contraindicated include fillers, sclerotherapy, and liposuction [
17]. Aesthetic procedures should be conducted ethically, with specialized personnel adhering to current legislation.
The lack of clinical studies prevents a clear determination of the effects of aesthetic applications during pregnancy. Future research should focus on real-life scenarios involving long-term exposure to cosmetics ingredients and personal care products during pregnancy [
54]. Further studies and new data on cosmetics should be added to better understand the safety and effects of cosmetics during pregnancy.
Aesthetic treatments and cosmetic choices for pregnant women must be managed by qualified professionals to ensure safety. Furthermore, pregnant aestheticians or dermatologists should be treated with respect, considering the unique aspects of their pregnancy [
53].
Author Contributions
Author Contributions: Conceptualization: F.B. and E.R.; methodology: F.B. and V.K.; software: E.S. and E.A.; validation: F.B and E.R.; formal analysis: F.B. and E.S..; investigation, F.B. and E.R.; resources, E.A..; data curation F.B.; writing—original draft preparation, F.B.; writing—review and editing, E.R. and V.K.; visualization: N.T. and V.K.; supervision: E.R.; and project administration; F.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest
References
- Snarskaya E.S.; OlisovaY.O.; Makatsariya D.; Kochergin N.G.; Radetskaya L.; Bitsadze V.; Khizroeva J. Skin pathologies in pregnancy. J Perinat Med. 2019, 27, 47(4):371-380.
- Amiard-Triquet, C. Pollution Tolerance in Aquatic Animals: From Fundamental Biological Mechanisms to Ecological Consequences. Ecotoxicology. 2019, 33–91. [Google Scholar]
- Putra, Ι.Β.; Jusuf, Ν.Κ.; Dewi Ν., Κ. Skin Changes and Safety Profile of Topical Products during Pregnancy. J Clin Aesthet Dermatol. 2022, 15(2):49-57.
- Kalra, A.; Lowe, A. Mechanical Behaviour of Skin, J Material Sci Eng, 2016, 5:4.
- Koumantaki-Mathioudaki, E.; Rallis, E. ; Dermatology and Pregnancy, Constantaras Publications, 2014, 95-98.
- Borrelli MR.; Griffin M.; Ngaage LM.; Longaker MT.; Lorenz HP. Striae Distensae: Scars without Wounds. Plast Reconstr Surg. 2021, 148(1):77-87.
- Chien A.L., Qi J., Rainer B., Sachs D.L., Helfrich Y.R. Treatment of Acne in Pregnancy. J Am Board Fam. Med. 2016, 29(2):254-62.
- Liji T. That Causes White Milk Spots on Nails? News-Medical., 2024. https://www.news-medical.net/health/What-Causes-White-Milk-Spots-on-Nails.aspx.
- Biskanaki, F.; Kefala, V.; Kalofiri, P. The Latest in Non-Invasive Local Fat Treatment Method with Diode Laser (1060nm), Review of Clinical Pharmacology and Pharmacokinetics, International Edition, 2019, 33 (2):35-38.
- Kalofiri, P.; Biskanaki, F.; Kefala, V.; Tertipi, N.; Sfyri, E.; Rallis, E. Endocrine Disruptors in Cosmetic Products and the Regulatory Framework: Public Health Implications, Cosmetics 2023, 10(6), 160.
- Gutierrez P. M.; Osman A.; Barrios, F. X.; Kopper B. A.; Baker M. T.; Haraburda C. M. Development of the Reasons for Living Inventory for Young Adults. Journal of Clinical Psychology, 2002, 58(4), 339–357.
- Tonelli, C.; Chio, I.I.J.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid Redox Signal. 2018, 29(17):1727-1745.
- Jones, J.; Toklu, HZ. Cosmetovigilance: A New Concept for the Residency Curriculum. HCA, Healthc J Med. 2020, 1(4):181-183.
- Toklu HZ.; Antigua A.; Lewis V.; Reynolds M.; Jones J. Cosmetovigilance: A review of the current literature. J Family Med Prim Care. 2019, 8(5):1540-1545.
- Altıokka İ.; Üner M. Safety in Cosmetics and Cosmetovigilance, Current Regulations in Türkiye. Turk J Pharm Sci. 2022, 19(5):610-617.
- Biskanaki F.; Kefala V.; Lazaris A.C.; Rallis E. Aging and the Impact of Solar Ultraviolet Radiation on the Expression of Type I and Type VI Collagen. Cosmetics 2023, 10(2), 48.
- Kaličanin B.; Velimirović D. A Study of the Possible Harmful Effects of Cosmetic Beauty Products on Human Health. Biol Trace Elem Res. 2016, 170(2):476-84.
- Garg A.M.; Mysore V. Dermatologic and Cosmetic Procedures in Pregnancy. Indian J Plast Surg. 2021, 54(4):507-513.
- Katoch, S.; Mysore, V. Surgical Smoke in Dermatology: Its Hazards and Management. Journal of Cutaneous and Aesthetic Surgery, 2019, 12(1), 1-7.
- Fulgham P.F..; Assimos D.J.; Pearle M.S.; Preminger G.M. Clinical effectiveness protocols for imaging in the management of ureteral calculous disease: AUA technology assessment. J Urol. 2013, 189(4):1203-13.
- Laing K.; Lam T.B. L; McClinton. S.; Cohen N.P; Traxer O.; Somani B.K. Outcomes of ureteroscopy for stone disease in pregnancy: results from a systematic review of the literature, Urol Int. 2012, 89(4):380-6.
- Rashid AO.; Attar A.; Mohammed KS.; Fakhralddin SS.; Abdulla LN.; Buchholz N. Direct Comparison of Pneumatic and Ho:YAG Laser Lithotripsy in the Management of Lower Ureteric Stones. Urol Int. 2020, 104(9-10):765-768.
- Hendawy AF.; Aly DG.; Shokeir HA.; Samy NA. Comparative Study Between the efficacy of Long-Pulsed Neodymium- YAG Laser and Fractional Co2 Laser in the Treatment of Striae Distensae. J Lasers Med Sci. 2021, 12: e57.
- Tan, M.; Kim, E.; Koren, G.; Bozzo, P. Botulinum toxin type A in pregnancy, 2013, 59(11): 1183-1184.
- Kroumpouzos G.; Bercovitch L. Ethics of esthetic procedures in pregnancy. Int J Womens Dermatol. 2018, 4(4): 194–197.
- Oliveira Monteiro E. Botulinum toxin and pregnancy. Skinmed. 2006, 5(6):308.
- Abdelkader, A.; Arslan, E.K.; Erol, H. Pregnancy outcomes following botulinum toxin type A exposure: A systematic review, International Journal of Scientific Research and Management, 2018, 6(05).
- Robinson A.Y.; Grogan P.M; Onabotulinumtoxin A successfully used as migraine prophylaxis during pregnancy: a case report, Mil Med. 2014, 179(6):e703-4.
- Jakharia-Shah N.; Chadha P.; Watson L. A review of the safety of common aesthetic procedures during pregnancy. J Plast Reconstr Aesthet Surg. 2018, 71:1362–80.
- Moortgat P.; Anthonissen M.; Meirte J.; Daele U.V.; Maertens K. The physical and physiological effects of vacuum massage on the different skin layers: a current status of the literature. Burns Trauma. 2016, 19:4:34. 19:4.
- Andreou E.; Biskanaki F.; Sfyri E.; Tertipi N.; Kefala V.; Rallis E. The Lymphatic Massage and its results on the healthy body. Epith. Clin.Pharm. 2023, 41 (1): 87-91.
- Pachtman Shetty, SL.; Fogarty, S. Massage During Pregnancy and Postpartum. Clin Obstet Gynecol. 2021, 64(3):648-660.
- Biskanaki F.; Kefala V.; Skouras G. Radiofrequency Innovation-New Non-Contact Skin Liposuction Method. Review of Clinical Pharmacology and Pharmacokinetics. 2019, 32 (1):17-21.
- Kim H.S.; Choi H.D.; Pack J.K.; Kim N.; Ahn Y.H. Biological Effects of Exposure to a Radiofrequency Electromagnetic Field on the Placental Barrier in Pregnant Rats. Bioelectromagnetics. 2021, 42(3):191-199.
- Biskanaki, F.; Rallis, E.; Andreou, E.; Sfyri, E.; Tertipi, N.; Ninos, G.; Kefala, V. Laser Innovations in Aesthetics. Review of Clinical Pharmacology and Pharmacokinetics. 2024, 38 (1):17-21.
- Kralik J. Relaxing School Suncreen Restrictions. NCSL Legisbrief. 2017, 25(28):1-2.
- Kefala, V.; Biskanaki, F.; Andreou, Ε.; Sfyri, Ε.; Tertipi, Ν.; Rallis, E. Laser for hair removal. Challenges-considerations., Review of Clinical Pharmacology and Pharmacokinetics. 2020, 38 (1): 17-22.
- Abedi, A.R.; Razzaghi, M.R. .; Allameh F.; Aliakbari F.; Karkan M.F.; Ranjbar A. Pneumatic Lithotripsy Versus Laser Lithotripsy for Ureteral Stones. J Lasers Med Sci. 2018, 9(4):233-236.
- Abedi A.; Razzaghi M.; Allameh F.; Fallahkarkan A.R. Pneumatic Lithotripsy Versus Laser Lithotripsy for Ureteral Stones. Journal of Lasers in Medical Sciences. 2018, 9(4):233-236.
- Wang, Z. On-line time pressure manipulations: L2 speaking performance under five types of planning and repetition conditions. In P. Skehan (Ed.), Processing Perspectives on Task Performance. Investigating a processing perspective on task performance. Amsterdam, the Netherlands: John Benjamins. 2014, 27–62.
- Kwiatek M.; Kojak A.; Kwaśniewska A.; OX40 (CD134) Expression on T Regulatory Cells Is Related to Serious Hypertensive Disorders in Pregnancy. J Cardiovasc Dev Dis. 2023, 10(10): 431.
- Sharmeen J.B.; Mahomoodally F.M.; Zengin G.; Maggi F. Essential Oils as Natural Sources of Fragrance Compounds for Cosmetics and Cosmeceuticals. Molecules. 2021, 26(3):666.
- Kaličanin, B.; Velimirović, D. ; A Study of the Possible Harmful Effects of Cosmetic Beauty Products on Human Health, Biol Trace Elem Res. 2016, 170(2):476-84.
- Scheers C.; Andre J.; Richert B.; Nail cosmetology. Hand Surg Rehabil. 2024, 43S:101657.
- Sanajou S.; Şahin G.; Baydar T. Aluminium in cosmetics and personal care products. J Appl Toxicol. 2021, 41(11):1704-1718.
- Luo L.; Yan T.; Yang L.; Zhao M. Aluminum chloride and D-galactose induced a zebrafish model of Alzheimer's disease with cognitive deficits and aging. Comput Struct Biotechnol J. 2024, 23:2230-2239.
- Symanzik C.; Weinert P.; Babić Z.; Hallmann S.; Havmose M.S.; Johansen G.D.; Kezic S.; Macan M.; Macan J.; Strahwald J.; Turk R.; Molen H.F.; John S.M.; Uter W. Skin Toxicity of Selected Hair Cosmetic Ingredients: A Review Focusing on Hairdressers. Int J Environ Res Public Health. 2022, 19(13):7588.
- Tucci, G.P. Tucci G.P. Titanium Dioxide Nanoparticles: a Risk for Human Health? Mini Rev Med Chem. 2016, 16(9):762-9.
- Kaličanin, B.; Velimirović, D. A Study of the Possible Harmful Effects of Cosmetic Beauty Products on Human Health. Biol Trace Elem Res. 2016, 170(2):476-84.
- Vitale M.; Gómez-Sánchez M.J.; Vicente M.H.; Colombo F.; Milani M. Efficacy, Tolerability, and Face Lipidomic Modification of New Regimen with Cleanser and Corrective Serum in Women with Acne-Prone. Skin Appl. Sci. 2024, 14(17): 7799.
- Lee, K.; Bull, R.; Ho, R.M.H. Developmental Changes in Executive Functioning, Child Dev. 2013, 84(6):1933-53.
- Viotti S.; Guidetti G.; Loera B.; Martini M.; Sottimano I.; Converso D.; Stress, work ability, and an aging workforce: A study among women aged 50 and over. International Journal of Stress Management, 24(1), 98–121. International Journal of Stress Management.
- Sanajou S.; Şahin G.; Baydar T. Aluminium in cosmetics and personal care products. J Appl Toxicol. 2021, 41(11):1704-1718.
- Cuny J.F.; Porphyries cutanées. The cutaneous porphyrias. Annales de Dermatologie et de Vénéréologie. 2019, (2) 146:143-159.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).