1. Introduction
Type 1 diabetes affects 0.36% of Australian pregnancies(1). One in two babies born to mothers with type 1 diabetes have complications, most commonly preterm birth, macrosomia and admission to neonatal intensive care. The most important risk factor is maternal antenatal hyperglycaemia(2). Cohort studies and, more recently, intervention trials have unequivocally shown that pregnancy outcomes improve with improved maternal glycaemia, in modern clinical practice generally documented as the time spent within internationally agreed pregnancy specific “time in range” (TIR) using continuous subcutaneous glucose monitoring (CGM) devices for real-time contemporaneous glucose readings(2-8). Improved maternal glycaemia may be more readily achieved via continuous subcutaneous insulin infusion (9-10).
Preconception advice according to the Australasian Diabetes in Pregnancy Society (ADIPS) 2020 guideline for pre-existing diabetes (11) recommends routine use of CGM with blood glucose targets in the preconception and antenatal periods.
Our service has adopted these targets since publication of this guideline. Despite engagement in women via face-to-face clinician review at 2-4 weekly intervals, we noted significant ongoing variability in glycaemic control and patient experience, related to brittleness of diabetes, insulin resistance, access to insulin administration and sensor technologies and lifestyle factors. It was hoped that outcomes would be aided by provision of additional clinician involvement, with weekly remote review of continuous glucose monitoring and more intensive insulin adjustment, which we refer to as our Insulin Stabilisation Service (ISS).
2. Materials and Methods
This is a clinical audit study reporting on a quality improvement project in a clinic managing type 1 diabetes in pregnancy.
Pre ISS (Standard care): Medical and credential diabetes educator (CDE) review at 2-4 week intervals (gestational weeks 6-8, 10, 12, 14, 16, 18, 21, 24, 28, 30, 32, 34, 36, 37).
Post ISS (Intervention): Standard Care, plus additional weekly insulin titration from gestational week 6-8 until end of pregnancy via remote review of continuous glucose monitoring.
The ISS consisted of a weekly review of each woman’s CGM download and insulin dosing by an obstetric endocrinologist together with a diabetes nurse educator. Trends were analysed and insulin dose adjustments were suggested and conveyed to the woman by either phone call, email or text message. Our ISS commenced in mid -June 2022, and with full implementation from early-August 2022. There was a large caseload of women birthing following commencement of ISS. We thus chose to compare the CGM data of women in our service during the 12 months prior to commencement of ISS (mid-June 2021 to mid-June 2022) with those in our service during the 8 months following full implementation of ISS (early-August 2022 to early-April 2023). It was envisaged that data from some women would fall into both groups, but that each woman would contribute data only once per trimester per group (pre or post ISS). A wider time frame would have captured greater numbers, however we wished to minimise the impact of emerging technologies including hybrid closed loop insulin pump therapy on the comparison groups.
Inclusion criteria:
Type 1 diabetes (diabetes requiring intensive insulin administration for 1 year or more, with positive anti-GAD antibodies and/or history of diabetic ketoacidosis)
Pregnant in one or more trimester in the nominated time period(s) and receiving care at our tertiary care centre
Use of CGM during pregnancy
Insulin administration by any treatment regimen (either multiple daily injections (MDI) or insulin pump therapy).
Exclusion criteria:
Pregnancy-specific data not available on CGM report (time in range 3.5-7.8 mmol/L, percentage time below 3.5 mmol/L, percentage time below 3.0 mmol/L).
Primary outcome:
Percentage time in pregnancy-specific range 3.5-7.8 mmol/L per trimester
Secondary outcomes:
Glucose variability (coefficient of variation and standard deviation, per trimester)
Percentage time below 3.5 mmol/L per trimester
Percentage time below 3.0 mmol/L per trimester
Average glucose
Days CGM accessed and time CGM active
Trimester 1 was defined as 0-12 weeks’ gestation (may be shorter if late-presentation). Trimester 2 was defined as 13-26 weeks’ gestation. Trimester 3 was defined as 27-40 weeks’ gestation (may be shorter if pre-term).
This project has been carried out according to the National Statement on Ethical Conduct in Human Research (2007). All patients were treated according to best medical practice. Apart from the implementation of the quality improvement project which was being assessed, there was no other alteration in their treatment as a result of being included in this study. Consent was implied by a willingness to share CGM and insulin data with our service and a waiver of formal consent was granted after HREC review.
Statistical analysis
Results are presented as number (%) for categorical data, mean (standard deviation) for approximately normally distributed continuous data and median (interquartile range) for highly skewed continuous data. Distributions were assessed using visual inspection of histograms and quantile-quantile plots. Selected indicators of glycaemic control obtained from continuous glucose monitoring were compared between Pre ISS and Post ISS groups separately for each trimester using the two-sided two-sample t-test for continuous data and Fisher’s exact test for categorical data. Statistical analysis was completed using Stata V17.0 (Stata Corp, College Station, TX).
3. Results
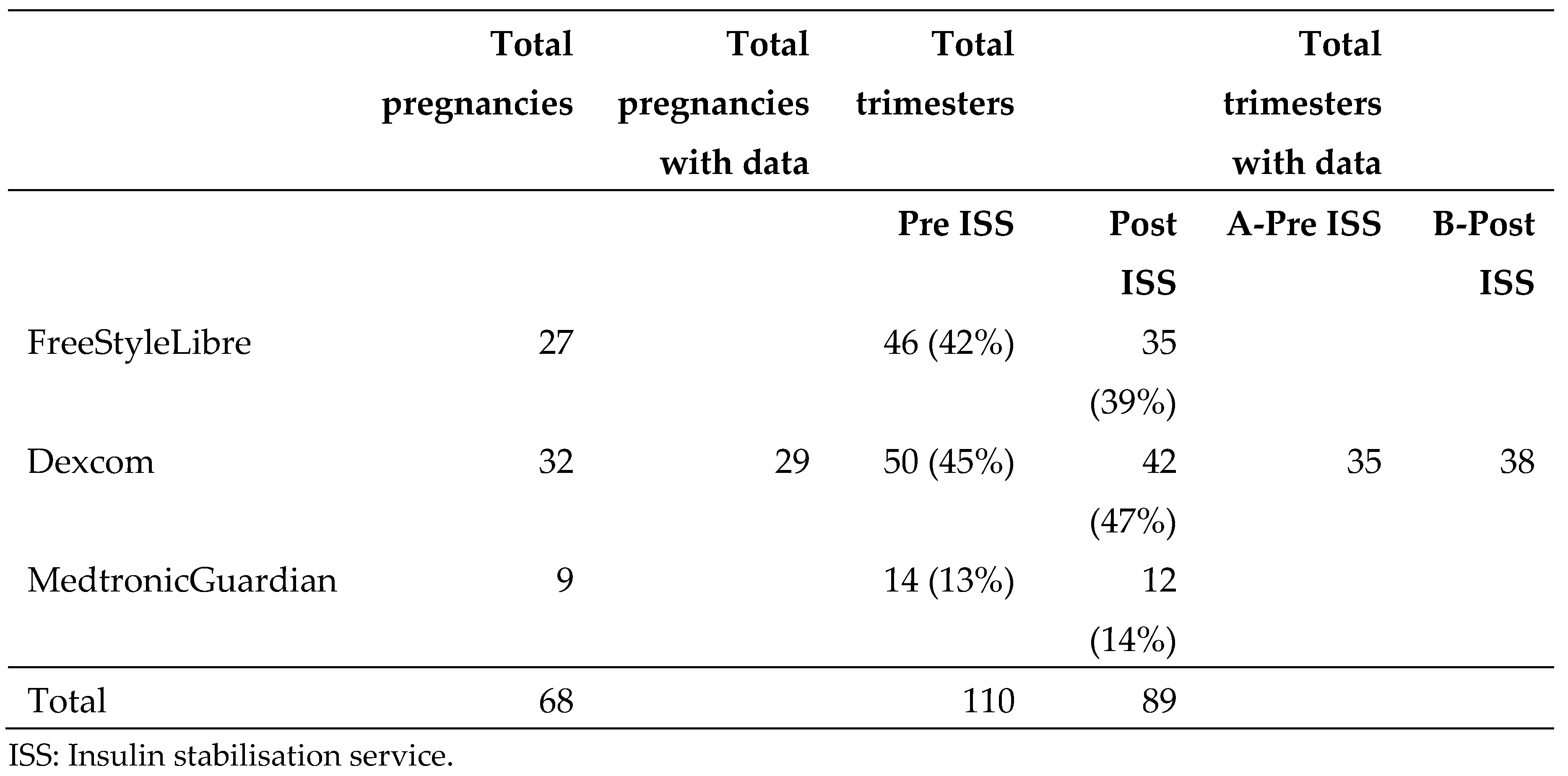
During the study period there were 70 pregnancies in women with T1D, of whom 68 utilised CGM. Of those pregnancies that used CGM, 110 trimesters occurred in the pre-ISS period, and 89 trimesters occurred in the post-ISS period. Two women contributed data for both groups (Pre ISS and Post ISS) during the same trimester. For the purposes of this report only the first data point from each trimester was included, so that each individual woman with T1D provided a maximum of one data point per trimester.
Due to lack of reporting of pregnancy-specific data available from the Libre and Medtronic systems, only data from Dexcom users was analysed in this study. The proportion of trimesters from pregnancies utilising Dexcom, FreeStyle Libre (Libre), and Medtronic Guardian Connect (Medtronic) CGM were similar pre and post ISS (
Table 1), suggesting that any factors impacting the choice of Dexcom over other CGM were similar before and after the intervention, and the impact of the intervention on the Dexcom subgroup will be representative of the impact on our entire population with T1DM.
Dexcom
32 pregnant women used Dexcom over the study period for a total of 92 trimesters (50 in Pre ISS and 42 in Post ISS). Three women (9 trimesters) provided no Dexcom data and were excluded, leaving 29 women who used Dexcom over 83 trimesters (43 in Pre ISS and 40 in Post ISS). This corresponded to 45% and 47% respectively of trimesters represented by the total cohort of women with type 1 diabetes cared for by our service. Some trimesters that fell within the study period had no Dexcom data available because the woman had not yet commenced care with our service early in their pregnancy (not yet engaged). They may have been using Dexcom prior to engagement, but their CGM wasn’t yet linked with our service. Nine women did not provide data for trimester 1 and one of these nine women also had no available data for trimester 2. Of these, 7 women were in Pre ISS and 2 were in Post ISS, leaving data for 73 trimesters (35 in Pre ISS and 38 in Post ISS). The number of women who ‘were not yet engaged’ for one or more trimesters was 7/16 (44%) pre ISS and 2/13 (15%) women Post ISS (p=0.13).
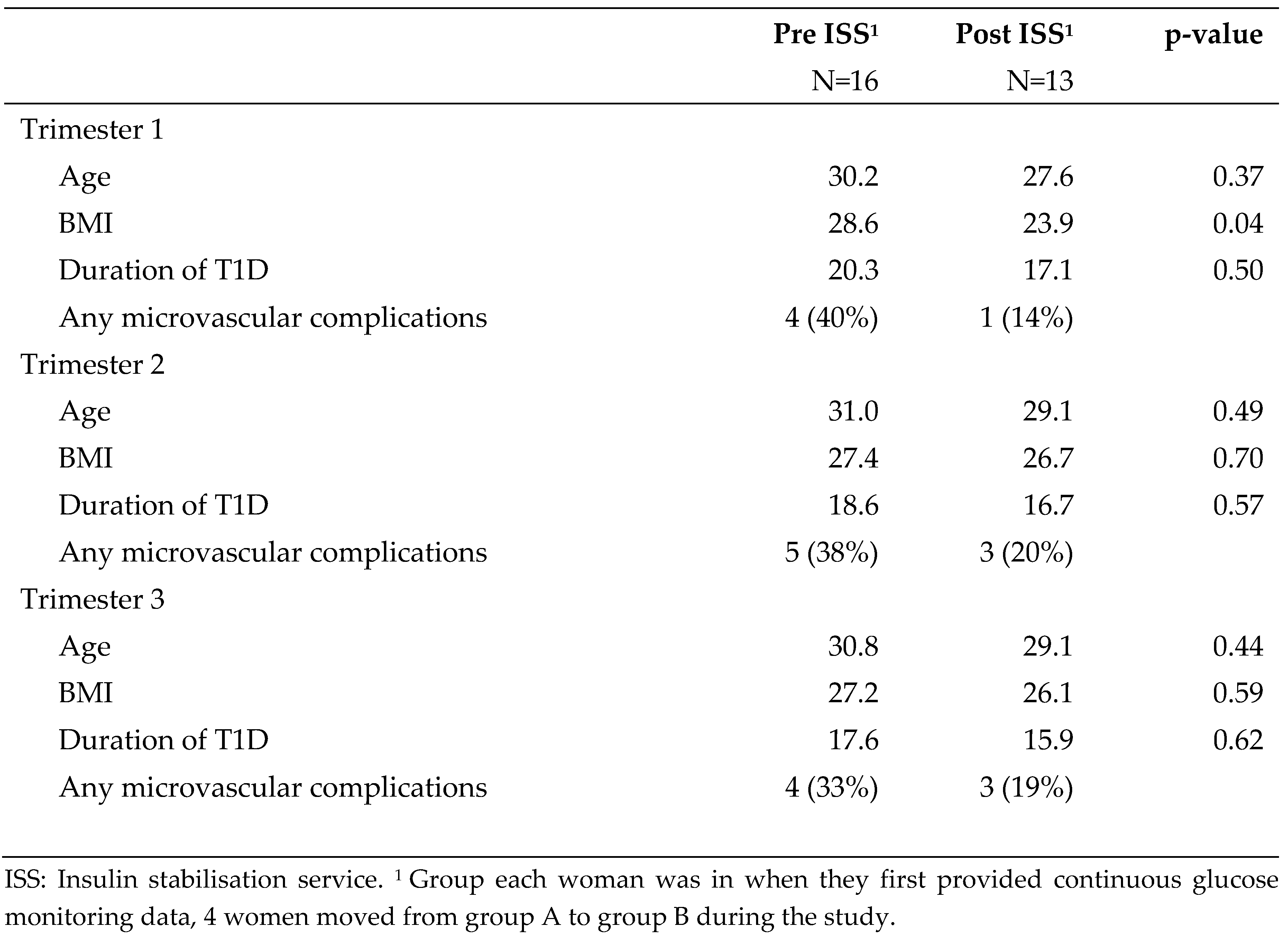
Demographic characteristics are described in
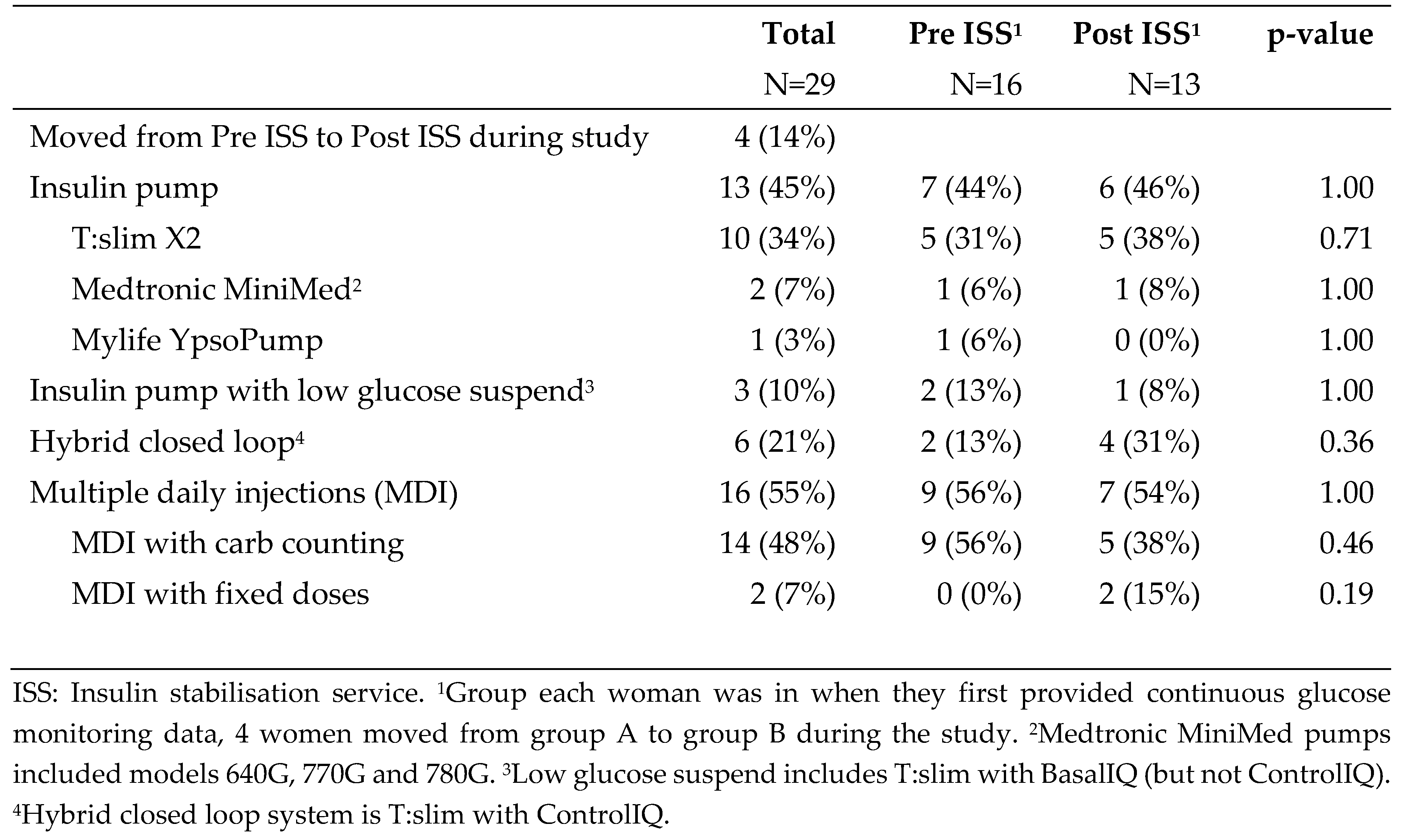
Table 2. Selected characteristics of diabetes management are described in
Table 3. Groups were similar.
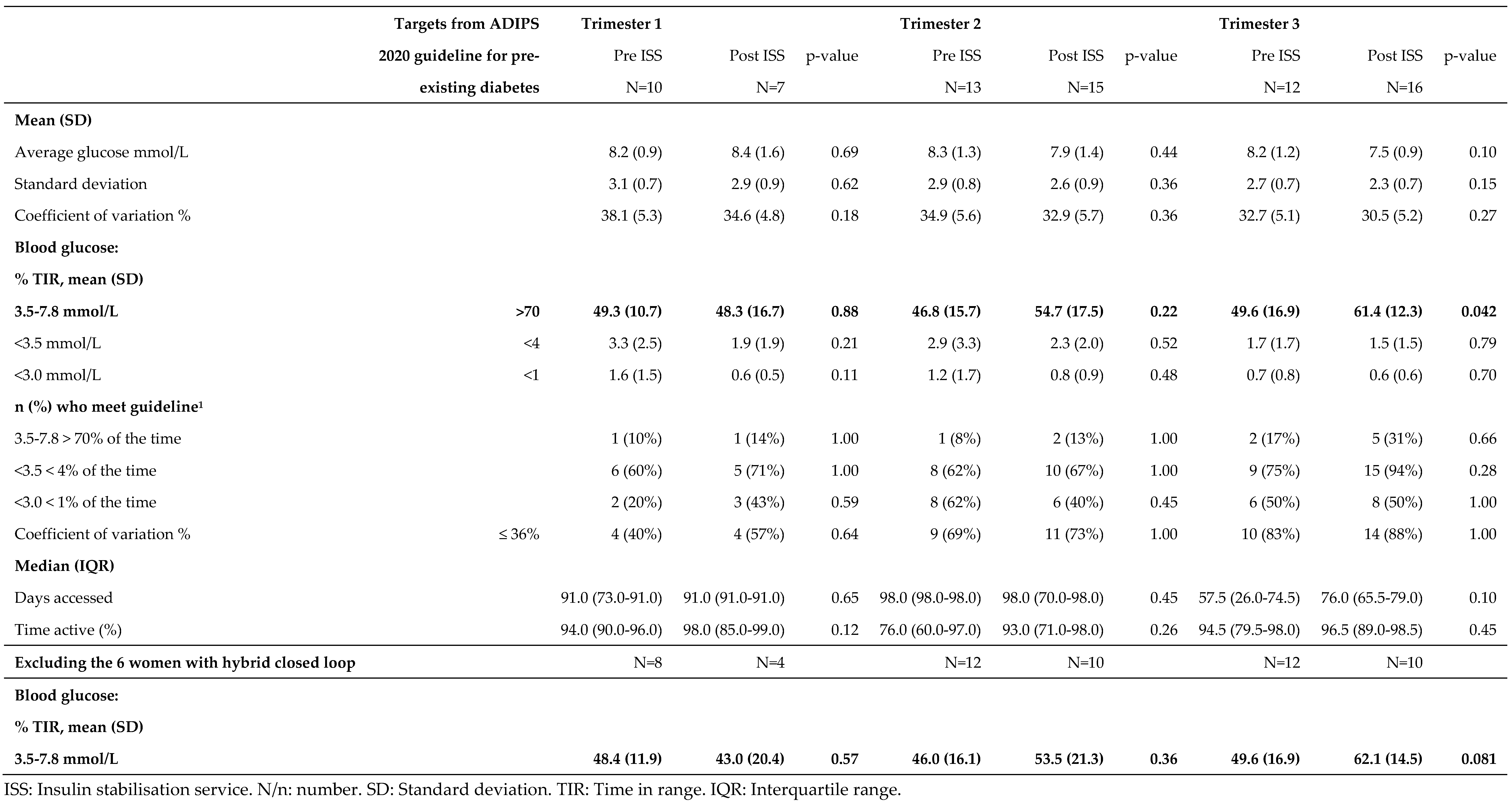
Groups Pre ISS (mean 49.3%, SD 10.7) and Post ISS (mean 38.3%, SD 16.7) had very similar % TIR during trimester 1 (p=0.88). Similarly, in trimester 2 there was no statistically significant difference between groups for % TIR (p=0.22,
Table 4). However, by trimester 3 Post ISS had a higher % TIR (mean 61.4%, SD 12.3) than Pre ISS (mean 49.6%, SD 16.9, p=0.042). When women with hybrid closed loop were excluded the difference between groups ISS and Post ISS in trimester 3 was similar in magnitude, but no longer statistically significant (
Table 4). There were no other differences between groups Pre ISS and Post ISS (
Table 4).
4. Discussion
In women using Dexcom CGM, there was an improvement in TIR following the commencement of an intensive weekly insulin stabilization service (mean TIR 49.6% increasing to 61.4%, p 0.042). This does not appear to be a function of the increased use of hybrid closed loop technology, as a similar trend was seen when hybrid closed loop technology was excluded from the analysis, albeit not then reaching statistical significance. We know from previous studies of continuous glucose monitoring metrics and pregnancy outcomes that a 5% increase in TIR corresponds to a 28% reduced risk of neonatal morbidity and 13-21% reduced risks of large for gestational age, NICU admission, neonatal hypoglycaemia, and cesarean delivery (12), highlighting the clinical importance of this improvement.
A greater proportion of women were linked with our service and using CGM early following the commencement of the ISS. Although this was not statistically significant it was suggestive of an improvement. We acknowledge there may have been factors other than our intervention impacting CGM use and engagement, such as a growing community awareness of the option of using CGM for pregnancy and pregnancy planning, or the impact of COVID-19 restrictions on access to remote technologies.
The cost of the intervention was in staff time, equating approximately to an additional 4 hours per week from a credentialled diabetes educator, and 1.5 hours per week from an endocrinologist (MO). When considering there were a total of 90 trimesters (averaging approximately 30 pregnancies) over the 8 month (35 week) post-intervention study period, the average cost per pregnancy where the intervention was applied for 8 months of the pregnancy was approximately 4.6 hours in CDE time and 1.7 hours in MO time.
A larger study incorporating formal health economic analysis would be needed to establish with accuracy the impact on time in range, however should the clinical benefits anticipated from earlier studies(12) be conferred to our population, even a modest shift in time in range would balance these costs.
5. Strengths
This paper is a clinical audit study reporting on a quality improvement project in a clinic managing type 1 diabetes in pregnancy. A clinically significant improvement in pregnancy specific time-in-range in trimester 3 occurred following the introduction of intensive weekly clinical support for pregnant women with type 1 diabetes. We consider that this study suggests benefits from more intensive support offered to women with type 1 diabetes during pregnancy and may provide an argument for more intensive support to be available through other services.
6. Weaknesses
This is a clinical audit study reporting on a quality improvement project rather than a randomized controlled clinical trial. Numbers were small due to the strict inclusion and exclusion criteria, with a narrow study period selected to minimize the impact of developing technologies on the comparison groups. This provided limited power to detect statistically significant differences between groups. There was no multivariable adjustment so confounding cannot be ruled out. There may be selection bias since analysis was limited to pregnant women using Dexcom CGM.
There is a need for prospectively, well powered studies to demonstrate the effect on pregnancy outcomes.
Author Contributions
Study concept and design ST, JN, AM; acquisition of data ST, NC; statistical analysis AG; interpretation of data ST; drafting of the manuscript ST, AG; critical revision of the manuscript for important intellectual content JN, JL, DM. All authors have read and agreed to the published version of the manuscript.
Funding
No funding was received for conducting this study. Time taken for data collection, data analysis and writing of the article was via in-kind contribution from employees of the Mater Mothers’ Hospital Brisbane and Mater Research.
Compliance with Ethical Standards
The ethical aspects of this research project have been reviewed and approved by the Mater Misericordiae Ltd HREC. This project has been carried out according to the National Statement on Ethical Conduct in Human Research (2007).
Data Availability Statement
Data available on request from the authors.
Reprint requests
Dr Stephanie Teasdale, stephanie_teasdale@hotmail.com.
Conflicts of Interest
The authors declare that they have no conflict of interest.
References
- Abell SK, Boyle JA, de Courten B, Knight M, Ranasinha S, Regan J, Soldatos G, Wallace EM, Zoungas S, Teede HJ. Contemporary type 1 diabetes pregnancy outcomes: impact of obesity and glycaemic control. Med J Aust. 2016 Aug 15;205(4):162-7. [CrossRef] [PubMed]
- Feig DS, Donovan LE, Corcoy R, Murphy KE, Amiel SA, Hunt KF, Asztalos E, Barrett JF, Sanchez JJ, de Leiva A, Hod M. Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. The Lancet. 2017 Nov 25;390(10110):2347-59.
- Wahabi HA, Alzeidan RA, Esmaeil SA. Pre-pregnancy care for women with pre-gestational diabetes mellitus: a systematic review and meta-analysis. BMC Public Health 2012; 12: 792.
- Bartal MF, Cornthwaite JAA, Ghafir D, Ward C, Ortiz G, Louis A, et al. Time in range and pregnancy outcomes in people with diabetes using continuous glucose monitoring. American Journal of Perinatology. 2023;40(05):461–6.
- Benhalima K, Jendle J, Beunen K, Ringholm L. Automated Insulin Delivery for Pregnant Women with Type 1 Diabetes: Where do we stand? Journal of diabetes science and technology. 2024;19322968231223934.
- Lawton J, Kimbell B, Closs M, Hartnell S, Lee TT, Dover AR, et al. Listening to women: experiences of using closed loop in type 1 diabetes pregnancy. Diabetes Technology & Therapeutics. 2023;25(12):845–55.
- Lee TT, Collett C, Man MS, Hammond M, Shepstone L, Hartnell S, et al. AiDAPT: automated insulin delivery amongst pregnant women with type 1 diabetes: A multicentre randomized controlled trial–study protocol. BMC pregnancy and childbirth. 2022;22(1):282.
- Sobhani NC, Goemans S, Nguyen A, Chambers ME, Richley M, Gabby LC, et al. Continuous glucose monitoring in pregnancies with type 1 diabetes: small increases in time-in-range improve maternal and perinatal outcomes. American Journal of Obstetrics and Gynecology. 2024; Available online 17 January 2024.
- Rudland VL, Price SAL, Hughes R, Barrett HL, Lagstrom J, Porter C, Britten FL, Glastras S, Fulcher I, Wein P, Simmons D, McIntyre HD, Callaway L. ADIPS 2020 guideline for pre-existing diabetes and pregnancy. Aust N Z J Obstet Gynaecol. 2020 Dec;60(6):E18-E52. [CrossRef] [PubMed]
- Jeitler K, Horvath K, Berghold A, Gratzer TW, Neeser K, Pieber TR, Siebenhofer A. Continuous subcutaneous insulin infusion versus multiple daily insulin injections in patients with diabetes mellitus: systematic review and meta-analysis. Diabetologia. 2008 Jun;51:941-51.
- Aziz KMA, Othman A. Comparison of Glycemic Control between Intensive Insulin Regimen and Continuous Subcutaneous Insulin infusion: A Meta-Analysis Report of Type-1 Diabetics from Randomized Controlled Trials. Int J Diabetol Vasc Dis Res. 2020;8(1e):1-3. [CrossRef]
- Sanusi AA, Xue Y, McIlwraith C, Howard H, Brocato BE, Casey B, Szychowski JM, Battarbee AN. Association of Continuous Glucose Monitoring Metrics With Pregnancy Outcomes in Patients With Preexisting Diabetes. Diabetes Care. 2023 Oct 2:dc230636. [CrossRef] [PubMed]
- Lee TTM, Collett C, Bergford S, Hartnell S, Scott EM, Lindsay RS, Hunt KF, McCance DR, Barnard-Kelly K, Rankin D, Lawton J, Reynolds RM, Flanagan E, Hammond M, Shepstone L, Wilinska ME, Sibayan J, Kollman C, Beck R, Hovorka R, Murphy HR; AiDAPT Collaborative Group. Automated Insulin Delivery in Women with Pregnancy Complicated by Type 1 Diabetes. N Engl J Med. 2023 Oct 26;389(17):1566-1578. [CrossRef] [PubMed]
Table 1.
Number of pregnancies and trimesters with and without continuous glucose monitoring data.
Table 1.
Number of pregnancies and trimesters with and without continuous glucose monitoring data.
Table 2.
Demographic details of n=29 pregnant women with Dexcom data, by group in which woman entered the study.
Table 2.
Demographic details of n=29 pregnant women with Dexcom data, by group in which woman entered the study.
Table 3.
Diabetes management of n=29 pregnant women with Dexcom data, overall and by group in which woman entered the study.
Table 3.
Diabetes management of n=29 pregnant women with Dexcom data, overall and by group in which woman entered the study.
Table 4.
Dexcom continuous glucose monitoring data for 29 pregnant women, comparing groups Pre ISS and Post ISS separately for each trimester.
Table 4.
Dexcom continuous glucose monitoring data for 29 pregnant women, comparing groups Pre ISS and Post ISS separately for each trimester.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).