Submitted:
27 September 2024
Posted:
27 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. PRISMA Guidelines
2.2. Search Strategy
2.3. Study Selection
2.4. Data Extraction
2.5. Quality Assessment
2.6. Statistical Analysis
3. Results
3.1. Search Results
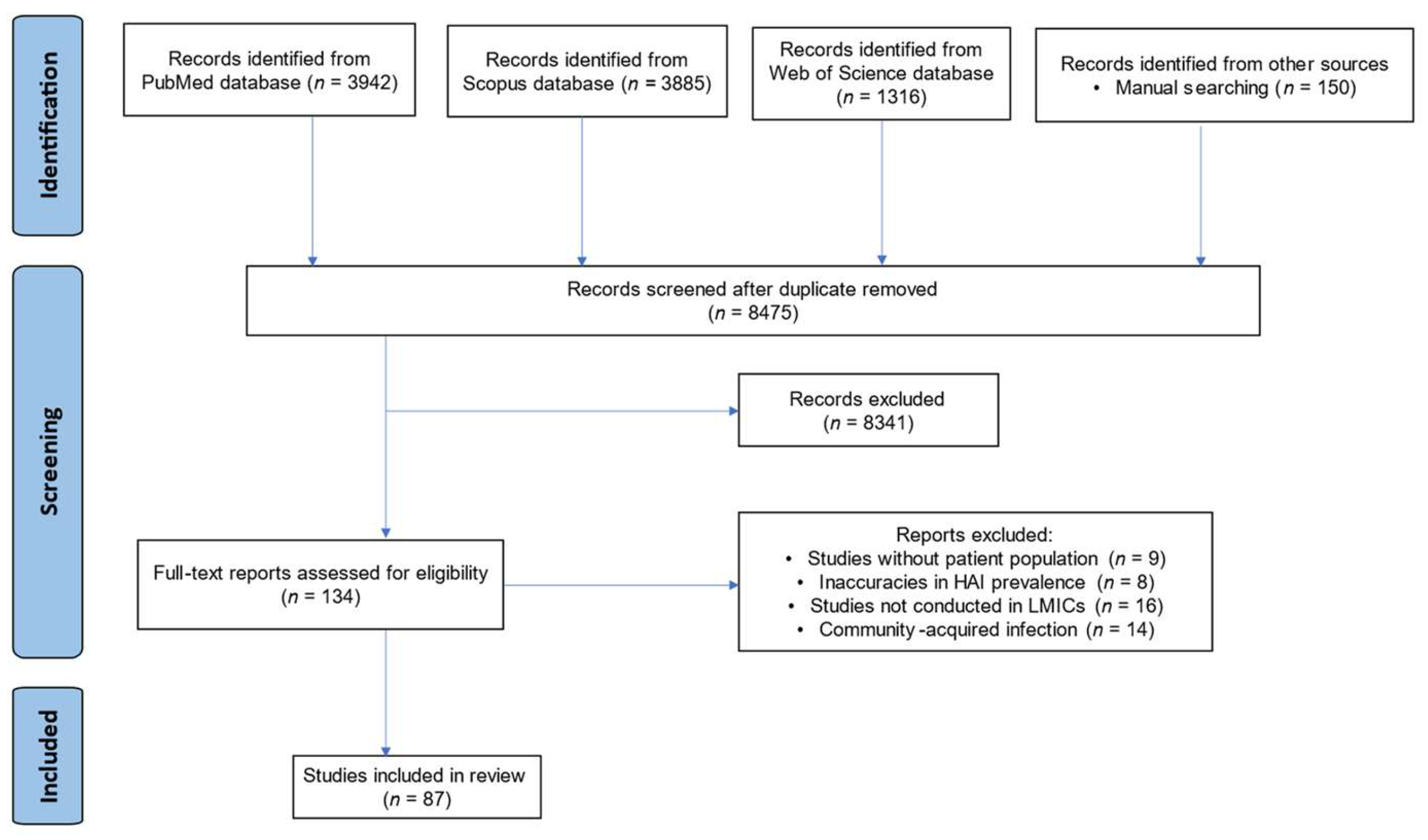
3.2. Study Distribution
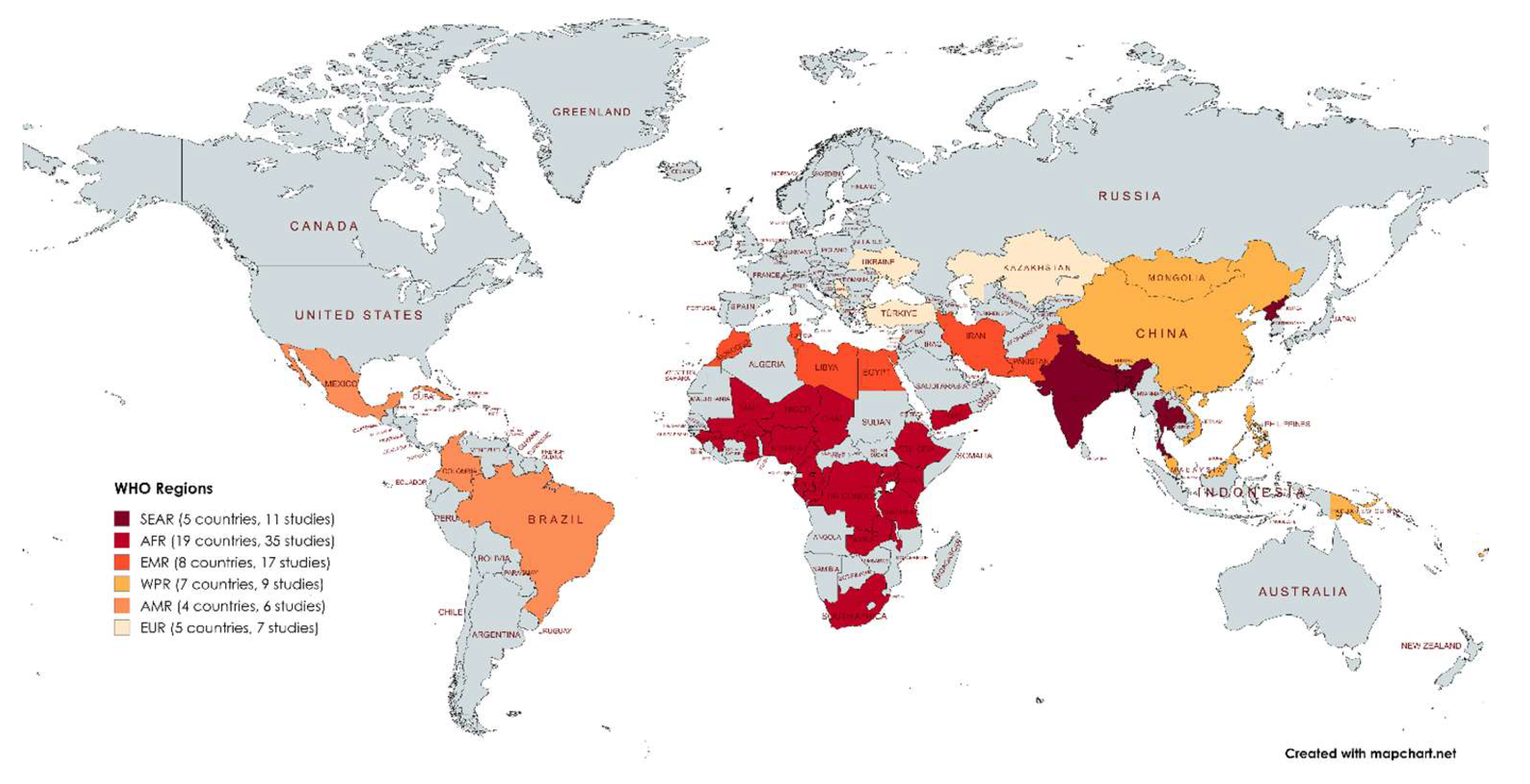
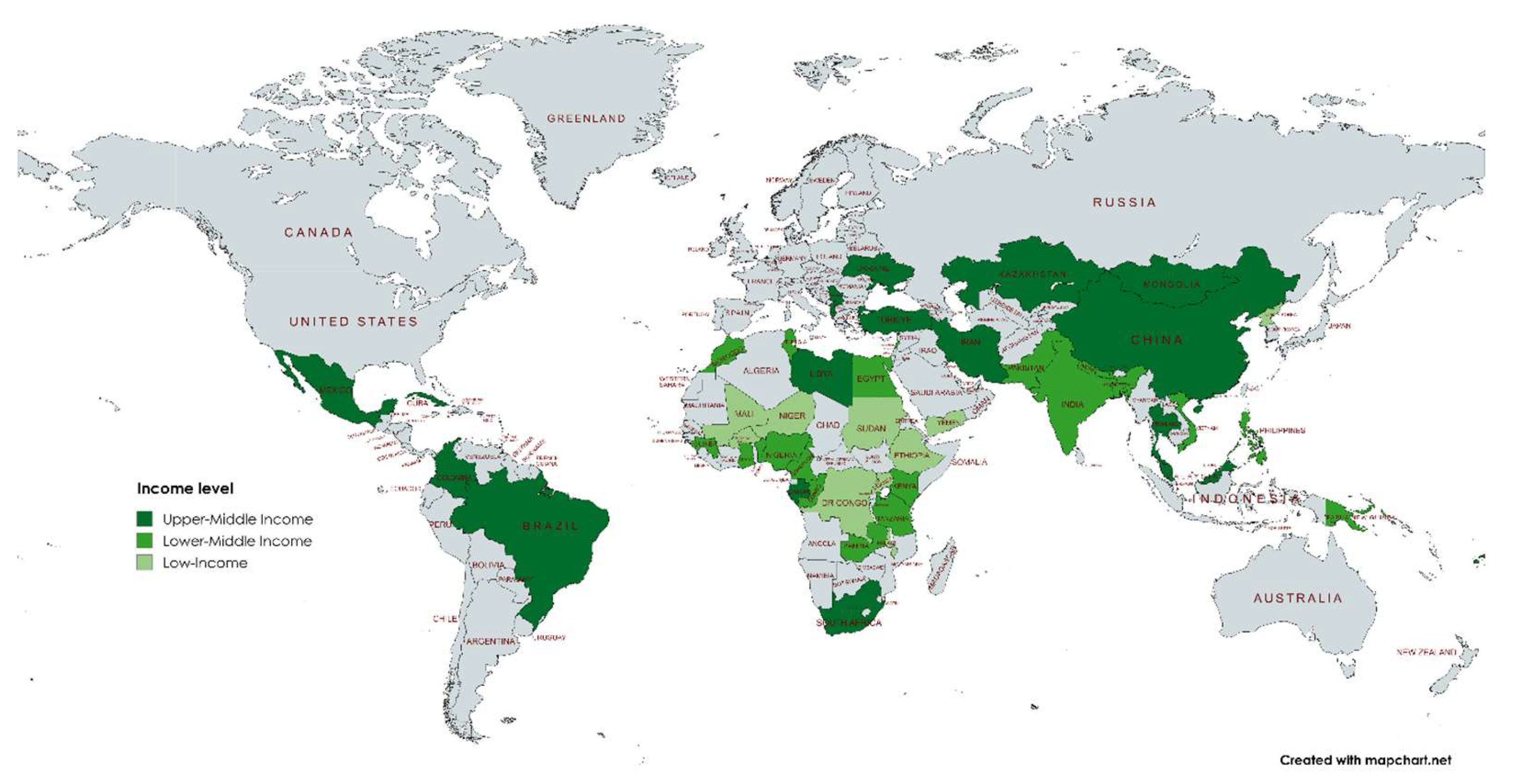
3.2. Pool Prevalence of HAIs in LMICs
3.3. HAI Type
3.4. Quality of the Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Haque M, Sartelli M, McKimm J, Abu Bakar M. Health care-associated infections - an overview. Infect Drug Resist 2018, 11, 2321–2333. [Google Scholar] [CrossRef] [PubMed]
- Alamer A, Alharbi F, Aldhilan A, Almushayti Z, Alghofaily K, Elbehiry A, et al. Healthcare-Associated Infections (HAIs): Challenges and Measures Taken by the Radiology Department to Control Infection Transmission. Vaccines (Basel) 2022, 10, 2060. [Google Scholar] [CrossRef] [PubMed]
- Maki G, Zervos M. Health Care-Acquired Infections in Low- and Middle-Income Countries and the Role of Infection Prevention and Control. Infect Dis Clin North Am 2021, 35, 827–839. [Google Scholar] [CrossRef] [PubMed]
- WHO launches first ever global report on infection prevention and control. Available online: https://www.who.int/news/item/06-05-2022-who-launches-first-ever-global-report-on-infection-prevention-and-control (accessed on 7 August 2024).
- Scott RD, Culler SD, Rask KJ. Understanding the Economic Impact of Health Care-Associated Infections: A Cost Perspective Analysis. Journal of Infusion Nursing. 2019, 42, 61–69. [Google Scholar] [CrossRef]
- Stone, PW. Economic burden of healthcare-associated infections: an American perspective. Expert Rev Pharmacoecon Outcomes Res. 2009, 9, 417–422. [Google Scholar] [CrossRef]
- Report on the Burden of Endemic Health Care-Associated Infection Worldwide Clean Care is Safer Care. 2011. Available online: www.who.int (accessed on 6 August 2024).
- Magill SS, O’Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, et al. Changes in Prevalence of Health Care–Associated Infections in U.S. Hospitals. New England Journal of Medicine. 2018, 379, 1732–1744.
- Suetens C, Latour K, Kärki T, Ricchizzi E, Kinross P, Moro ML, et al. Prevalence of healthcare-associated infections, estimated incidence and composite antimicrobial resistance index in acute care hospitals and long-term care facilities: results from two European point prevalence surveys, 2016 to 2017. Euro Surveill 2018, 23, 1800516. [Google Scholar]
- Ling ML, Apisarnthanarak A, Madriaga G. The Burden of Healthcare-Associated Infections in Southeast Asia: A Systematic Literature Review and Meta-analysis. Clinical Infectious Diseases 2015, 60, 1690–1699. [Google Scholar] [CrossRef]
- Abubakar U, Amir O, Rodríguez-Baño J. Healthcare-associated infections in Africa: a systematic review and meta-analysis of point prevalence studies. J Pharm Policy Pract 2022, 15, 99. [Google Scholar] [CrossRef]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, n71. [CrossRef]
- Munn Z, Moola S, Lisy K, Riitano D, Tufanaru C. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc. 2015, 13, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Newman, MJ. Nosocomial and Community Acquired Infections in Korle Bu Teaching Hospital, Accra. West Afr J Med 2010, 28. [Google Scholar] [CrossRef] [PubMed]
- Iwuafor AA, Ogunsola FT, Oladele RO, Oduyebo OO, Desalu I, Egwuatu CC, et al. Incidence, Clinical Outcome and Risk Factors of Intensive Care Unit Infections in the Lagos University Teaching Hospital (LUTH), Lagos, Nigeria. PLoS One 2016, 11, e0165242–e0165242. [Google Scholar] [CrossRef] [PubMed]
- Dayyab FM, Iliyasu G, Aminu A, Habib ZG, Tiamiyu AB, Tambuwal SH, et al. A prospective study of hospital-acquired infections among adults in a tertiary hospital in north-western Nigeria. Trans R Soc Trop Med Hyg 2018, 112, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Labi AK, Obeng-Nkrumah N, Owusu E, Bjerrum S, Bediako-Bowan A, Sunkwa-Mills G, et al. Multi-centre point-prevalence survey of hospital-acquired infections in Ghana. Journal of Hospital Infection 2019, 101, 60–68. [Google Scholar] [CrossRef]
- Abubakar, U. Point-prevalence survey of hospital acquired infections in three acute care hospitals in Northern Nigeria. Antimicrob Resist Infect Control 2020, 9, 63. [Google Scholar] [CrossRef]
- Andersen CT, Langendorf C, Garba S, Sayinzonga-Makombe N, Mambula C, Mouniaman I, et al. Risk of community- and hospital-acquired bacteremia and profile of antibiotic resistance in children hospitalized with severe acute malnutrition in Niger. International Journal of Infectious Diseases 2022, 119, 163–171. [Google Scholar] [CrossRef]
- Ige OK, Adesanmi AA, Asuzu MC. Hospital-acquired infections in a Nigerian tertiary health facility: An audit of surveillance reports. Niger Med J 2011, 52, 239–243. [Google Scholar] [CrossRef]
- Onuzo CN, Sefogah PE, Nuamah MA, Ntumy M, Osei MM, Nkyekyer K. Surgical site infections following caesarean sections in the largest teaching hospital in Ghana. Infection prevention in practice 2022, 4, 100203. [Google Scholar] [CrossRef]
- Iliyasu G, Dayyab FM, Abubakar S, Inuwa S, Tambuwal SH, Tiamiyu AB, et al. Laboratory-confirmed hospital-acquired infections: An analysis of a hospital’s surveillance data in Nigeria. Heliyon. 2018, 4, e00720. [Google Scholar] [CrossRef]
- Olawale KO, Fadiora SO, Taiwo SS. Prevalence of hospital-acquired enterococci infections in two primary-care hospitals in osogbo, southwestern Nigeria. Afr J Infect Dis 2011, 5, 40–46. [Google Scholar]
- Ahoyo TA, Bankolé HS, Adéoti FM, Gbohoun AA, Assavèdo S, Amoussou-Guénou M, et al. Prevalence of nosocomial infections and anti-infective therapy in Benin: results of the first nationwide survey in 2012. Antimicrob Resist Infect Control 2014, 3, 17. [Google Scholar] [CrossRef] [PubMed]
- Hien H, Drabo KM, Ouédraogo L, Konfé S, Zeba S, Sangaré L, et al. Healthcare-associated infection in Burkina Faso: an assessment in a district hospital. J Public Health Afr 2012, 3, e29–e29. [Google Scholar] [CrossRef]
- Ngolet LO, Fortuné A, Liboko B, Roland B, Ibara O, Dokekias AE. Hospital acquired infection in a department of hematology-oncology care in the Congo. Am J Blood Res 2021, 11, 191–198. Available online: www.AJBlood.us/ (accessed on 14 April 2024).
- Hassan R, El-Gilany AH, Abd Elaal AM, El-Mashad N, Azim DA. An overview of healthcare-associated infections in a tertiary care hospital in Egypt. Infection prevention in practice 2020, 2, 100059. [Google Scholar] [CrossRef]
- See I, Lessa FC, ElAta OA, Hafez S, Samy K, El-Kholy A, et al. Incidence and pathogen distribution of healthcare-associated infections in pilot hospitals in Egypt. Infect Control Hosp Epidemiol 2013, 34, 1281–1288. [Google Scholar] [CrossRef]
- Hamam S, Sakr A, Zahran W, Kholy R, Kasemy Z, Ibrahem R, et al. Health care-associated infections at an Egyptian tertiary care hospital: a 2-year prospective study. Menoufia Medical Journal. 2021, 34, 514. [Google Scholar] [CrossRef]
- Scherbaum M, Kösters K, Mürbeth RE, Ngoa UA, Kremsner PG, Lell B, et al. Incidence, pathogens and resistance patterns of nosocomial infections at a rural hospital in Gabon. BMC Infect Dis 2014, 14, 124. [Google Scholar]
- Keita AK, Doumbouya N, Sow MS, Konaté B, Dabo Y, Panzo DA, et al. Prévalence des infections nosocomiales dans deux hôpitaux de Conakry (Guinée). Sante Publique (Paris) 2016, 28, 251–255. [Google Scholar] [CrossRef]
- Kalagouda R, Kabera B, Muia CK, Ale BM. Hospital acquired infections in a private paediatric hospital in Kenya: a retrospective cross-sectional study. Pan African Medical Journal. 2022, 41.
- Bunduki GK, Feasey N, Henrion MYR, Noah P, Musaya J. Healthcare-associated infections and antimicrobial use in surgical wards of a large urban central hospital in Blantyre, Malawi: a point prevalence survey. Infection prevention in practice 2021, 3, 100163. [Google Scholar] [CrossRef] [PubMed]
- Jroundi I, Khoudri I, Azzouzi A, Zeggwagh AA, Benbrahim NF, Hassouni F, et al. Prevalence of hospital-acquired infection in a Moroccan university hospital. Am J Infect Control 2007, 35, 412–416. Available online: https://pubmed.ncbi.nlm.nih.gov/17660013/ (accessed on 14 April 2024). [CrossRef] [PubMed]
- Kallel H, Bahoul M, Ksibi H, Dammak H, Chelly H, Hamida CB, et al. Prevalence of hospital-acquired infection in a Tunisian hospital. Journal of Hospital Infection 2005, 59, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Ginawi I, Saleem M, Sigh M, Vaish AK, Ahmad I, Srivastava VK, et al. Hospital acquired infections among patients admitted in the medical and surgical wards of a non-teaching secondary care hospital in northern India. J Clin Diagn Res 2014/02/03. 2014, 8, 81–83. [Google Scholar]
- Nair V, Sahni AK, Sharma D, Grover N, Shankar S, Chakravarty A, et al. Point prevalence & risk factor assessment for hospital-acquired infections in a tertiary care hospital in Pune, India. Indian J Med Res 2017, 145, 824–832. [Google Scholar]
- Vergeire-Dalmacion GR, Itable JR, Baja ES. Hospital-acquired infection in public hospital buildings in the Philippines: Is the type of ventilation increasing the risk? The Journal of Infection in Developing Countries 2016, 10, 1236–1242. [Google Scholar] [CrossRef]
- Viderman D, Khamzina Y, Kaligozhin Z, Khudaibergenova M, Zhumadilov A, Crape B, et al. An observational case study of hospital associated infections in a critical care unit in Astana, Kazakhstan. Antimicrob Resist Infect Control 2018, 7, 57. [Google Scholar] [CrossRef]
- Askarian M, Yadollahi M, Assadian O. Point prevalence and risk factors of hospital acquired infections in a cluster of university-affiliated hospitals in Shiraz, Iran. J Infect Public Health 2012, 5, 169–176. [Google Scholar] [CrossRef]
- Le NK, HF W, Vu PD, Khu DTK, Le HT, Hoang BTN, et al. High prevalence of hospital-acquired infections caused by gram-negative carbapenem resistant strains in Vietnamese pediatric ICUs. Medicine. 2016, 95, e4099. [Google Scholar] [CrossRef]
- Phu VD, Wertheim HFL, Larsson M, Nadjm B, Dinh QD, Nilsson LE, et al. Burden of Hospital Acquired Infections and Antimicrobial Use in Vietnamese Adult Intensive Care Units. PLoS One. 2016, 11, e0147544. [Google Scholar]
- Arif S, Sadeeqa S, Saleem Z, Latif S, Sharif M. The burden of healthcare-associated infections among pediatrics: a repeated point prevalence survey from Pakistan. Hosp Pract 2020, 49, 34–40. [Google Scholar] [CrossRef]
- Saleem Z, Hassali MA, Godman B, Hashmi FK, Saleem F. A multicenter point prevalence survey of healthcare–associated infections in Pakistan: Findings and implications. Am J Infect Control 2019, 47, 421–424. [Google Scholar] [CrossRef] [PubMed]
- Shrestha SK, Shrestha S, Ingnam S. Point prevalence of healthcare-associated infections and antibiotic use in a tertiary care teaching hospital in Nepal: A cross-sectional study. J Infect Prev 2022, 23, 29–32. Available online: https://pubmed.ncbi.nlm.nih.gov/35126679 (accessed on 29 August 2021). [CrossRef] [PubMed]
- Shrestha SK, Trotter A, Shrestha PK. Epidemiology and Risk Factors of Healthcare-Associated Infections in Critically Ill Patients in a Tertiary Care Teaching Hospital in Nepal: A Prospective Cohort Study. Infect Dis 2022, 15, 11786337211071120–11786337211071120. [Google Scholar]
- Loftus MJ, Curtis SJ, Naidu R, Cheng AC, Jenney AWJ, Mitchell BG, et al. Prevalence of healthcare-associated infections and antimicrobial use among inpatients in a tertiary hospital in Fiji: a point prevalence survey. Antimicrob Resist Infect Control 2020, 9, 146. [Google Scholar] [CrossRef]
- Braga IA, Campos PA, Gontijo-Filho PP, Ribas RM. Multi-hospital point prevalence study of healthcare-associated infections in 28 adult intensive care units in Brazil. Journal of Hospital Infection 2018, 99, 318–324. [Google Scholar] [CrossRef]
- Oliveira AC de, Kovner CT, Silva RS da. Nosocomial Infection in an Intensive Care Unit in a Brazilian University Hospital. Rev Lat Am Enfermagem 2010, 18, 233–239. [Google Scholar] [CrossRef]
- Ferreira GB, Donadello JCS, Mulinari LA. Healthcare-Associated Infections in a Cardiac Surgery Service in Brazil. Braz J Cardiovasc Surg 2020, 35, 614–618. [Google Scholar]
- Salmanov A, Vozianov S, Kryzhevsky V, Litus O, Drozdova A, Vlasenko I. Prevalence of healthcare-associated infections and antimicrobial resistance in acute care hospitals in Kyiv, Ukraine. Journal of Hospital Infection. 2019, 102, 431–437. [Google Scholar] [CrossRef]
- Hema A, Arsène Some S, Kaboré O, Sanou S, Poda A, Meda C, et al. Risk and outcomes of healthcare-associated infections in three hospitals in Bobo Dioulasso, 2 2022 (Burkina Faso): a longitudinal study Authors’ contributions. medRxiv 2024. [CrossRef]
- Yallew WW, Kumie A, Yehuala FM. Point prevalence of hospital-acquired infections in two teaching hospitals of Amhara region in Ethiopia. Drug Healthc Patient Saf 2016, 8, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Tassew SG, Alebachew Woldu M, Amogne Degu W, Shibeshi W. Management of hospital-acquired infections among patients hospitalized at Zewditu memorial hospital, Addis Ababa, Ethiopia: A prospective cross-sectional study. PLoS One 2020, 15, e0231949–e0231949. [Google Scholar] [CrossRef] [PubMed]
- Sahiledengle B, Seyoum F, Abebe D, Geleta EN, Negash G, Kalu A, et al. Incidence and risk factors for hospital-acquired infection among paediatric patients in a teaching hospital: a prospective study in southeast Ethiopia. BMJ Open 2020, 10, e037997–e037997. [Google Scholar] [CrossRef] [PubMed]
- Ali S, Birhane M, Bekele S, Kibru G, Teshager L, Yilma Y, et al. Healthcare associated infection and its risk factors among patients admitted to a tertiary hospital in Ethiopia: longitudinal study. Antimicrob Resist Infect Control 2018, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Endalafer N, Gebre-Selassie S, Kotiso B. Nosocomial bacterial infections in a tertiary hospital in Ethiopia. J Infect Prev 2010, 12, 38–43. [Google Scholar] [CrossRef]
- Atalay YA, Gebeyehu NA, Gelaw KA. The prevalence of hospital acquired infection and associated factors among patients admitted at Wolaita Sodo University Comprehensive Specialized Hospital, in Ethiopia. IP Journal of Nutrition, Metabolism and Health Science 2024, 7, 43–50. [CrossRef]
- Coulibaly Y, Kone O, Amadou I, Diop THM, Coulibaly O, Doumbia A, et al. Hospital Acquired Infections at the Service of Pediatric Surgery in Gabriel Touré Academic Hospital, Bamako, Mali. Open J Pediatr 2020, 10, 185–193. [Google Scholar] [CrossRef]
- Aminata M, Alioune BS, Yacouba C, Agaly DO, Bassirou D, Abdoulaye T, et al. Multidrug resistant bacteria isolated from nosocomial infections at University Teaching Hospital of Point-G, Bamako, Mali. African Journal of Bacteriology Research 2023, 15, 1–7. Available online: https://academicjournals.org/journal/JBR/article-abstract/39116BE70184 (accessed on 7 August 2024).
- Bocoum A, Fané S, Traoré Y, Sanogo SA, Kanté I, Kouma A, et al. Bacteriology of Healthcare-Associated Infections in the Gynecology and Obstetrics Department of CHU Gabriel Touré. Open J Obstet Gynecol 2019, 09, 1336–1346. [Google Scholar] [CrossRef]
- Kim EJ, Kwak YG, Kwak SH, Ko SH, Kweon OM, Kim ES, et al. Korean National Healthcare-associated Infections Surveillance System, Intensive Care Unit Module Report: Summary of Data from July 2019 through June 2020. Korean Journal of Healthcare-Associated Infection Control and Prevention 2021, 26, 115–128. [Google Scholar] [CrossRef]
- Kim EJ, Kwak YG, Kwak SH, Ko SH, Kweon OM, Kim ES, et al. Korean National Healthcare-associated Infections Surveillance System, Intensive Care Unit Module Report: Summary of Data from July 2020 through June 2021. Korean Journal of Healthcare-Associated Infection Control and Prevention 2023, 28, 64–77. [Google Scholar] [CrossRef]
- Kim EJ, Kwak YG, Kwak SH, Ko SH, Kim JH, Kim ES, et al. Korean National Healthcare-associated Infections Surveillance System, Intensive Care Unit Module Report: Summary of Data from July 2018 to June 2019. Korean J healthc assoc Infect Control Prev 2020, 25, 115–127. [Google Scholar] [CrossRef]
- Osman, T. Prevalence of Intensive Care Unit-Acquired Infections in different Hospitals in Khartoum State-Sudan. American Journal of PharmTech Research 2019, 9, 176–186. [Google Scholar] [CrossRef]
- Greco D, Magombe I. Hospital acquired infections in a large north Ugandan hospital. J Prev Med Hyg 2011, 52, 55–58. [Google Scholar]
- Al-Shami HZ, Al-Haimi MA. Nosocomial Infections in Six Major Hospitals in Sana’a Capital City and in Some Governorates in Yemen. Applied Microbiology: Open Access 2018, 4. [CrossRef]
- Mukomena PN, Munsaka S, Simunza M, Kwenda G, Yamba K, Kabwe J, et al. Nosocomial infections and associated risk factors at two tertiary healthcare facilities in Lusaka and Copperbelt Provinces, Zambia. Sci Afr 2023, 20, e01644. [Google Scholar] [CrossRef]
- Afroz H, Fakruddin M, Masud MR, Islam K. Incidence of and risk factors for Hospital Acquired Infection in a Tertiary Care Hospital of Dhaka, Bangladesh. Bangladesh Journal of Medical Science 2017, 16, 358–369. [Google Scholar] [CrossRef]
- Amin ZA, Nahar N. Hospital Acquired Infection in a Tertiary Military Hospital in Dhaka, Bangladesh. International Journal of Infectious Diseases and Therapy 2017, 2, 35–39. [Google Scholar]
- Nouetchognou JS, Ateudjieu J, Jemea B, Mesumbe EN, Mbanya D. Surveillance of nosocomial infections in the Yaounde University Teaching Hospital, Cameroon. BMC Res Notes 2016, 9, 505. [Google Scholar]
- Kakupa DK, Muenze PK, Byl B, Wilmet MD. Study of the prevalence of nosocomial infections and associated factors in the two university hospitals of Lubumbashi, Democratic Republic of Congo. Pan Afr Med J 2016, 24, 275. [Google Scholar]
- Razine R, Azzouzi A, Barkat A, Khoudri I, Hassouni F, Chefchaouni AC, et al. Prevalence of hospital-acquired infections in the university medical center of Rabat, Morocco. Int Arch Med 2012, 5, 26. [Google Scholar] [CrossRef] [PubMed]
- Curtis SJ, Barnabas R, Cairns KA, Cameron D, Coghlan B, Jones R, et al. Healthcare-associated infections and antimicrobial use at a major referral hospital in Papua New Guinea: a point prevalence survey. Lancet Reg Health West Pac. 2024, 48, 101120. [Google Scholar]
- Gosling R, Mbatia R, Savage A, Mulligan JA, Reyburn H. Prevalence of hospital-acquired infections in a tertiary referral hospital in northern Tanzania. Annals of Tropical Medicine & Parasitology 2003, 97, 69–73. [Google Scholar] [CrossRef]
- Ider BE, Clements A, Adams J, Whitby M, Muugolog T. Prevalence of hospital-acquired infections and antibiotic use in two tertiary Mongolian hospitals. Journal of Hospital Infection 2010, 75, 214–219. [Google Scholar] [CrossRef]
- Aiesh BM, Qashou R, Shemmessian G, Swaileh MW, Abutaha SA, Sabateen A, et al. Nosocomial infections in the surgical intensive care unit: an observational retrospective study from a large tertiary hospital in Palestine. BMC Infect Dis 2023, 23, 686. [Google Scholar]
- Faria S, Sodano L, Gjata A, Dauri M, Sabato AF, Bilaj A, et al. The first prevalence survey of nosocomial infections in the University Hospital Centre ‘Mother Teresa’ of Tirana, Albania. Journal of Hospital Infection 2007, 65, 244–250. [Google Scholar] [CrossRef]
- Tao XB, Qian LH, Li Y, Wu Q, Ruan JJ, Cai DZ, et al. Hospital-acquired infection rate in a tertiary care teaching hospital in China: a cross-sectional survey involving 2434 inpatients. International Journal of Infectious Diseases 2014, 27, 7–9. [Google Scholar] [CrossRef]
- Huang G, Huang Q, Zhang G, Jiang H, Lin Z. Point-prevalence surveys of hospital-acquired infections in a Chinese cancer hospital: From 2014 to 2018. J Infect Public Health 2020, 13, 1981–1987. [Google Scholar] [CrossRef]
- Wintaco LM, Quintero-Lesmes DC, Vargas-Soler JA, Barrera DM, Palacio LN, Granados U, et al. Analysis of Healthcare-associated Infections before and during the COVID-19 pandemic in a Colombian hospital. Revista Cuidarte 2024. [CrossRef]
- Izquierdo-Cubas F, Zambrano A, Frómeta I, Gutiérrez A, Bastanzuri M, Guanche H, et al. National Prevalence of Nosocomial Infections. Cuba 2004. Journal of Hospital Infection 2008, 68, 234–240. [Google Scholar] [CrossRef]
- Tabatabaei SM, Behmanesh Pour F, Osmani S. Epidemiology of Hospital-Acquired Infections and Related Anti-Microbial Resistance Patterns in a Tertiary-Care Teaching Hospital in Zahedan, Southeast Iran. International Journal of Infection 2015, 2. [Google Scholar] [CrossRef]
- Eshrati B, Masoumi Asl H, Afhami S, Pezeshki Z, Seifi A. Health care-associated infections in Iran: A national update for the year 2015. Am J Infect Control 2018, 46, 663–667. [Google Scholar] [CrossRef] [PubMed]
- Masoudifar M, Gouya M, Pezeshki Z, Eshrati B, Afhami S, Farzami M, et al. Health care-associated infections, including device-associated infections, and antimicrobial resistance in Iran: The national update for 2018. J Prev Med Hyg. 2022, 62, E943–9.
- Rajabi M, Abdar ME, Rafiei H, Aflatoonia MR, Abdar ZE. Nosocomial Infections and Epidemiology of Antibiotic Resistance in Teaching Hospitals in South East of Iran. Glob J Health Sci 2015, 8, 190–197. [Google Scholar]
- Choobdar F, Vahedi Z, Khosravi N, Khalesi N, Javid A, Shojaee S. Nosocomial Infection in an Iranian Neonatal Intensive Care Unit: Hospital Epidemiology and Risk Factors. Arch Pediatr Infect Dis 2020, 8. [Google Scholar] [CrossRef]
- Raka L, Spahija G, Gashi-Gecaj A, Hamza A, Haxhiu E, Rashiti A, et al. Point prevalence survey of healthcare-associated infections and antimicrobial use in Kosovo hospitals. Infect Dis Rep 2019, 11, 7975. [Google Scholar] [CrossRef]
- Azzam R, Dramaix M. A one-day prevalence survey of hospital-acquired infections in Lebanon. Journal of Hospital Infection 2001, 49, 74–78. [Google Scholar] [CrossRef]
- Daw MA, Mahamat MH, Wareg SE, El-Bouzedi AH, Ahmed MO. Epidemiological manifestations and impact of healthcare-associated infections in Libyan national hospitals. Antimicrob Resist Infect Control 2023, 12, 122. [Google Scholar] [CrossRef]
- Hughes AJ, Ariffin N, Huat TL, Molok HA, Hashim S, Sarijo J, et al. Prevalence of Nosocomial Infection and Antibiotic Use at a University Medical Center in Malaysia. Infection Control & Hospital Epidemiology 2005, 26, 100–104. [Google Scholar] [CrossRef]
- Hernández Orozco H, Lucas Resendiz E, Luis Castañeda J, De Colsa A, Ramirez Mayans J, Johnson KM, et al. Surveillance of Healthcare Associated Infections in Pediatric Cancer Patients Between 2004 and 2009 in a Public Pediatric Hospital in Mexico City, Mexico. J Pediatr Hematol Oncol 2014, 36, 96–98. [Google Scholar] [CrossRef]
- Ćirković I, Marković-Denić L, Bajčetić M, Dragovac G, Đorđević Z, Mioljević V, et al. Microbiology of Healthcare-Associated Infections: Results of a Fourth National Point Prevalence Survey in Serbia. Antibiotics (Basel) 2022, 11, 1161. [Google Scholar]
- Šuljagić V, Bajčetić M, Mioljević V, Dragovac G, Mijović B, Janićijević I, et al. A nationwide assessment of the burden of healthcare-associated infections and antimicrobial use among surgical patients: results from Serbian point prevalence survey, 2017. Antimicrob Resist Infect Control 2021, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Ilic M, Markovic-Denic L. Nosocomial infections prevalence study in a Serbian university hospital. Vojnosanit Pregl 2009, 66, 868–875. [Google Scholar] [CrossRef] [PubMed]
- Olivier C, Kunneke H, O’Connell N, Von Delft E, Wates M, Dramowski A. Healthcare-associated infections in paediatric and neonatal wards: A point prevalence survey at four South African hospitals. South African Medical Journal 2018, 108, 418. [Google Scholar] [CrossRef]
- Nair A, Steinberg WJ, Habib T, Saeed H, Raubenheimer JE. Prevalence of healthcare-associated infection at a tertiary hospital in the Northern Cape Province, South Africa. South African Family Practice 2018, 60, 162–167. [Google Scholar] [CrossRef]
- Manosuthi W, Thientong V, Moolasart V, Rongrungrueng Y, Sangsajja C, Danchaivijitr S. HEALTHCARE-ASSOCIATED INFECTIONS AT SELECTED HOSPITALS IN THAILAND. Southeast Asian J Trop Med Public Health 2017, 48, 204–212. Available online: https://pubmed.ncbi.nlm.nih.gov/29644841/ (accessed on 14 April 2024).
- Moolasart V, Srijareonvijit C, Charoenpong L, Kongdejsakda W, Anugulruengkitt S, Kulthanmanusorn A, et al. Prevalence and Risk Factors of Healthcare-Associated Infections among Hospitalized Pediatric Patients: Point Prevalence Survey in Thailand 2021. Children (Basel) 2024, 11, 738. [Google Scholar]
- Kepenekli E, Soysal A, Yalindag-Ozturk N, Ozgur O, Ozcan I, Devrim I, et al. Healthcare-Associated Infections in Pediatric Intensive Care Units in Turkey: a National Point-Prevalence Survey. Jpn J Infect Dis 2015, 68, 381–386. [Google Scholar] [CrossRef]
- World Bank country classifications by income level for 2024-2025. Available online: https://blogs.worldbank.org/en/opendata/world-bank-country-classifications-by-income-level-for-2024-2025 (accessed on 8 August 2024).
- Kärki T, Plachouras D, Cassini A, Suetens C. Burden of healthcare-associated infections in European acute care hospitals. Wien Med Wochenschr 2019, 169 (Suppl. 1), 3–5. Available online: https://pubmed.ncbi.nlm.nih.gov/30680486/. [CrossRef]
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 2014, 370, 1198–1208. [Google Scholar] [CrossRef]
- Raoofi S, Pashazadeh Kan F, Rafiei S, Hosseinipalangi Z, Noorani Mejareh Z, Khani S, et al. Global prevalence of nosocomial infection: A systematic review and meta-analysis. PLoS One. 2023, 18, e0274248. [Google Scholar]
- Goh LPW, Marbawi H, Goh SM, Bin Abdul Asis AK, Gansau JA. The prevalence of hospital-acquired infections in Southeast Asia (1990-2022). The Journal of Infection in Developing Countries 2023, 17, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Bunduki GK, Masoamphambe E, Fox T, Musaya J, Musicha P, Feasey N. Prevalence, risk factors, and antimicrobial resistance of endemic healthcare-associated infections in Africa: a systematic review and meta-analysis. BMC Infect Dis. 2024, 24, 158. [Google Scholar]
- Gomaa K, Abdelraheim AR, El Gelany S, Khalifa EM, Yousef AM, Hassan H. Incidence, risk factors and management of post cesarean section surgical site infection (SSI) in a tertiary hospital in Egypt: a five year retrospective study. BMC Pregnancy Childbirth 2021, 21. Available online: /pmc/articles/PMC8449867/. (accessed on 15 April 2024). [Google Scholar]
- Lansing SS, Moley JP, McGrath MS, Stoodley P, Chaudhari AMW, Quatman CE. High Number of Door Openings Increases the Bacterial Load of the Operating Room. Surg Infect (Larchmt) 2020/12/23. 2021, 22, 684–689. [Google Scholar] [CrossRef]
- Romano F, Milani S, Gustén J, Joppolo CM. Surgical Smoke and Airborne Microbial Contamination in Operating Theatres: Influence of Ventilation and Surgical Phases. Int J Environ Res Public Health 2020, 17, 5395. [Google Scholar] [CrossRef]
- Healthcare-associated infections. Available online: https://www.ecdc.europa.eu/en/healthcare-associated-infections (accessed on 6 August 2024).
- Leistner R, Kohlmorgen B, Brodzinski A, Schwab F, Lemke E, Zakonsky G, et al. Environmental cleaning to prevent hospital-acquired infections on non-intensive care units: a pragmatic, single-centre, cluster randomized controlled, crossover trial comparing soap-based, disinfection and probiotic cleaning. EClinicalMedicine. 2023, 59, 101958. [Google Scholar] [CrossRef]
- Dancer, SJ. Cleaning and decontamination of the healthcare environment. In Decontamination in Hospitals and Healthcare; Elsevier: Amsterdam, The Netherlands, 2014; pp. 370–97. [Google Scholar]
- Umscheid CA, Mitchell MD, Doshi JA, Agarwal R, Williams K, Brennan PJ. Estimating the Proportion of Healthcare-Associated Infections That Are Reasonably Preventable and the Related Mortality and Costs. Infect Control Hosp Epidemiol. 2011, 32, 101–114. [Google Scholar] [CrossRef]
- Boyce, JM. Modern technologies for improving cleaning and disinfection of environmental surfaces in hospitals. Antimicrob Resist Infect Control. 2016, 5, 10. [Google Scholar] [CrossRef]
- Kubde D, Badge AK, Ugemuge S, Shahu S. Importance of Hospital Infection Control. Cureus 2023. [Google Scholar]
- NHSN | CDC. Available online: https://www.cdc.gov/nhsn/index.html (accessed on 6 August 2024).
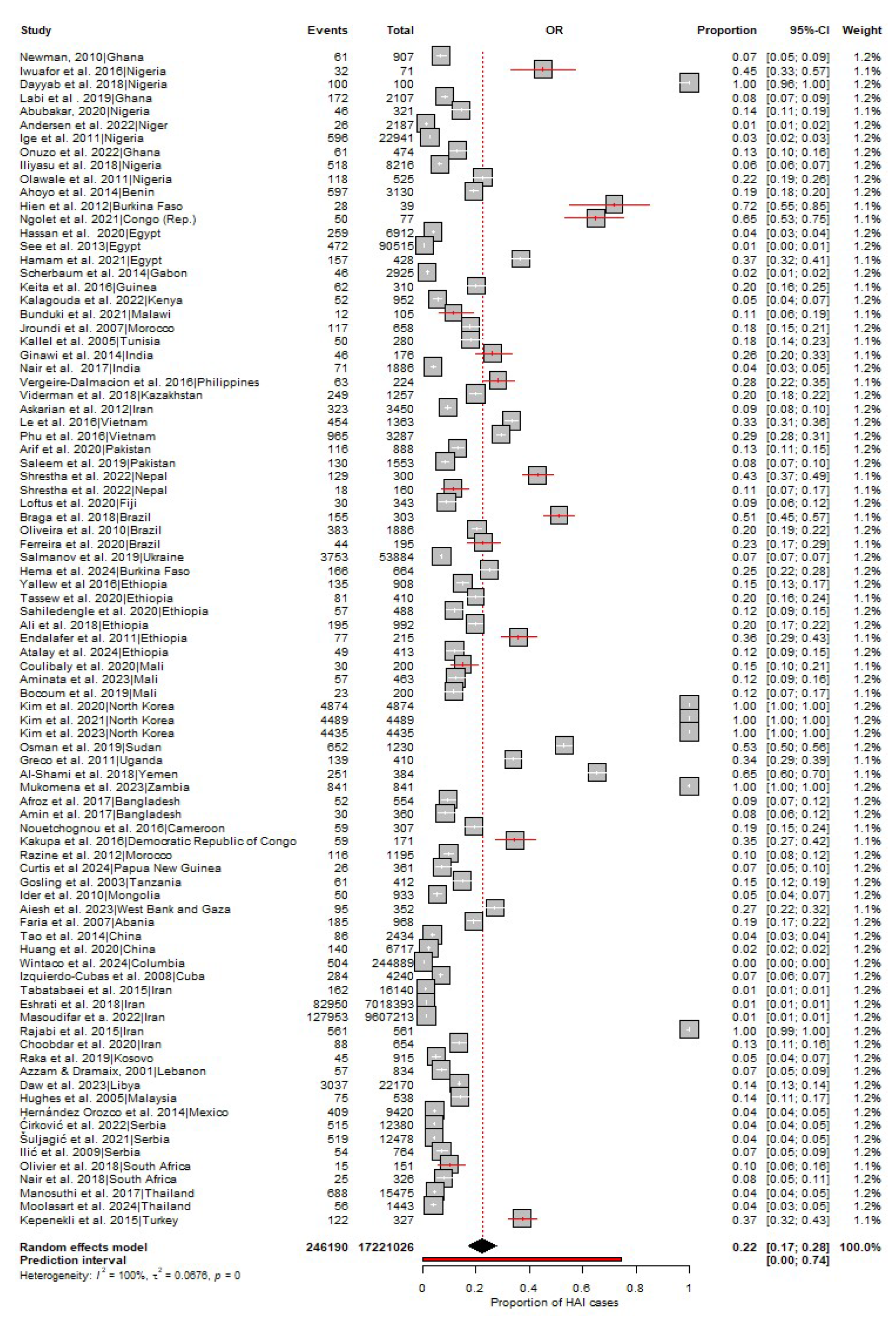
| Variable | Prevalence (95% CI) | I2 | p value |
|---|---|---|---|
| WHO region | |||
| SEAR | 37% (13-62) | 100% | 0 |
| AFR | 25% (16-33) | 100% | 0 |
| EMR | 19% (6-31) | 100% | 0 |
| AMR | 17% (3-32) | 100% | 0 |
| WPR | 15% (7-23) | 100% | 0 |
| EUR | 14 % (5-23) | 99% | <0.01 |
| World Bank income level (2024/2025) | |||
| Low-Income | 37% (23-52) | 100% | 0 |
| Lower-Middle Income | 22% (15-30) | 100% | 0 |
| Upper-Middle Income | 13% (6-20) | 100% | 0 |
| HAI | No. of countries | No. of studies | No. of cases | Prevalence (95% CI) | I2 | p- value |
|---|---|---|---|---|---|---|
| SSIs | 43 | 70 | 44312 | 27% (23-31) | 99% | 0 |
| Pneumonia | 27 | 41 | 62633 | 23% (18-28) | 99% | 0 |
| UTIs | 46 | 77 | 65505 | 22% (19-26) | 99% | 0 |
| BSIs | 40 | 65 | 33731 | 21% (16-25) | 99% | 0 |
| LRTIs | 20 | 26 | 1211 | 19% (13-25) | 98% | <0.01 |
| SSTIs | 24 | 33 | 812 | 10% (6-13) | 95% | <0.01 |
| GIs | 16 | 19 | 174 | 6% (3-9) | 88% | <0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





