1. Introduction
Small cell lung cancer (SCLC) is a really aggressive and poorly differentiated malignancy and approximately only one third of patients are diagnosed with localized or locoregional disease. SCLC accounts for about 13%–15% of new diagnosis of lung cancers and it represents the second most common thoracic malignancy [
1]. Its incidence is strongly related to smoking habit and only 2% of SCLC cases arises in never smokers. Within this subgroup of patients some cases derive from histological transformation of oncogene addicted (EGFR or ALK driven) lung adenocarcinoma to SCLC [
2].
SCLC is usually staged according to the Veterans Administration Lung Study Group (VALG) staging system which defines the limited-stage (LS-SCLC) as the disease confined to one hemithorax potentially being treated with radical radiotherapy (corresponding to stage I-III of the 8th edition of the American Joint Committee on Cancer—AJCC) and the extensive-stage (ES-SCLC) corresponding to stage IVA/B of the 8th edition AJCC [
3].
LS-SCLC is potentially curable and actually the standard of care is concurrent chemoradiotherapy, with a median overall survival (OS) of about 25-30 months and a 5-year OS rate of 20-30%, followed by prophylactic cranial irradiation (PCI) [
1,
4]. Furthermore the ADRIATIC trial recently showed that immunotherapy with the immune checkpoint inhibitor (ICI) durvalumab, targeting programmed death-ligand 1 (PD-L1), led to significantly longer OS and progression-free survival (PFS) than placebo, building a potential new approach to care in patients with LS-SCLC [
5].
On the other hand prognosis for extensive stage (ES-SCLC) has remained poor over the past 20 years, with a median OS of about 10 months and a 5-years OS rate < 5%. The treatment was historically based on six cycles chemotherapy with cisplatin or carboplatin plus etoposide; PCI and consolidative thoracic RT (ctRT) were offered to patients with a tumor response after initial systemic treatment to control local disease and improve OS [
6,
7].
Recently, two phase III randomized clinical trials (RCT) have changed the treatment paradigm of the ES-SCLC. These trials showed the benefit of adding durvalumab or atezolizumab, another ICI targeting PD-L1, to platinum-based chemotherapy followed by maintenance with ICIs alone, leading to an OS and PFS benefit [
8,
9,
10].
However, both trials did not include ctRT for which robust evidence on safety, efficacy and optimal timing in the chemo-immunotherapy era is not yet available. The results from several early-phase and retrospective analyses suggest improved clinical outcome and good toxicity profiles of ctRT as such that American Society for Radiation Oncology (ASTRO) guidelines and a more recent Canadian consensus recommended ctRT in patients responding to first line chemo-immunotherapy and having good performance status (PS). Even if these findings seem to be very promising, still actually we need more robust data, so that randomized study are currently ongoing [
11,
12,
13,
14].
PCI is another highly-debated topic in SCLC management considering that among patients who undergo chemo-radiotherapy, approximately 59% to 69% tough develop brain metastases. PCI is recommended in patients responding to systemic therapy and having good PS, however Takayasu et al. recently highlighted the value of MRI surveillance showing no survival benefit of PCI compared with observation in patients with ES-SCLC [
10]. These results are likely to have influenced clinical practice, given the potential impact of PCI on quality of life even if more data are needed to confirm these insights [
1,
15]. Additionally, CASPIAN trial [
8,
9] and IMPOWER133 trial [
10] resulted in a low adoption of PCI, making more challenging the role of PCI in extensive disease in this era of immunotherapy.
Many questions still remain open, such as the optimal timing and dose of ctRT in the era of chemo-immunotherapy in ES-SCLC, the best management of patients with poor performance status or the use of PCI and its optimal timing. So, on behalf of Italian Association of Radiotherapy and Clinical Oncology (AIRO), this survey was designed to evaluate the current management of ES-SCLC in Italy. The focus was the above-mentioned issues in order to identify differences in SCLC care practices and consequently to optimize patients’ treatment.
2. Materials and Methods
As well as some years ago with a first survey on the management of SCLC [
16], the thoracic oncology AIRO study group planned a 21-question web-based survey about the ES-SCLC management.
The survey was planned in November 2022 in light of a national event that involved many different specialists (pneumologists, medical and radiation oncologists, radiologists). After obtaining the endorsement of the AIRO scientific committee, the final version was finalized and sent out by e-mail for the first time in June 2023, with the link to the online questionnaire, to the heads of allitalian radiation oncology departements or to the radiation oncologists deputies for lung cancer disease (205) and to the cancer care professionals involved in the national event (5 pneumologists, 12 medical oncologists, 3 other cancer care professionals). A second call was sent out two months later to maximize the response rate. A newsletter from the Thoracic Oncology AIRO study group and the list of Italian radiation departments’ chief was used to get in touch with as much as possible specialists.
The survey was conducted anonymously and it was structured in two parts: the first one regarded demographic questions about physicians’ experience in lung cancer management, number of patients with SCLC treated annually in each oncological center and role of multidisciplinary discussion in the clinical decision-making process. Conversely, the second part of the survey was dedicated to the specific management of patients affected by ES-SCLC.
Physicians were asked to select the answers that most closely matched their standard of care about:—the use of immunotherapy in association to standard chemotherapy regimen in both fit and frail patients,—the combination of ctRT with chemo-immunotherapy focusing on its timing and fractionation,—the factors influencing the use of PCI and—the type of RT planning techniques and delivery. It was developed using survey-monkey web link and all answers were deemed eligible for statistical analysis.
The study was approved by the scientific committee of AIRO (Nr. 6/2022).
3. Results
3.1. Demographics and Expertise in ES-SCLC Treatment
A total of 90 out of 225 Italian Cancer Care professionals answered the survey (40% response rate); characteristics are listed in
Table 1.
The majority of respondents were radiation oncologists (89%), then medical oncologists (6%), pneumologists (94%), and other specialties (1%); however, 69% of them spent more than 50% of time working within the scope of lung cancer disease.
Fifty-one per cent of respondents had more than 10 years of experience, the 46% worked in non-academic hospitals; almost the other half worked in academic hospitals or cancer care centers (48%).
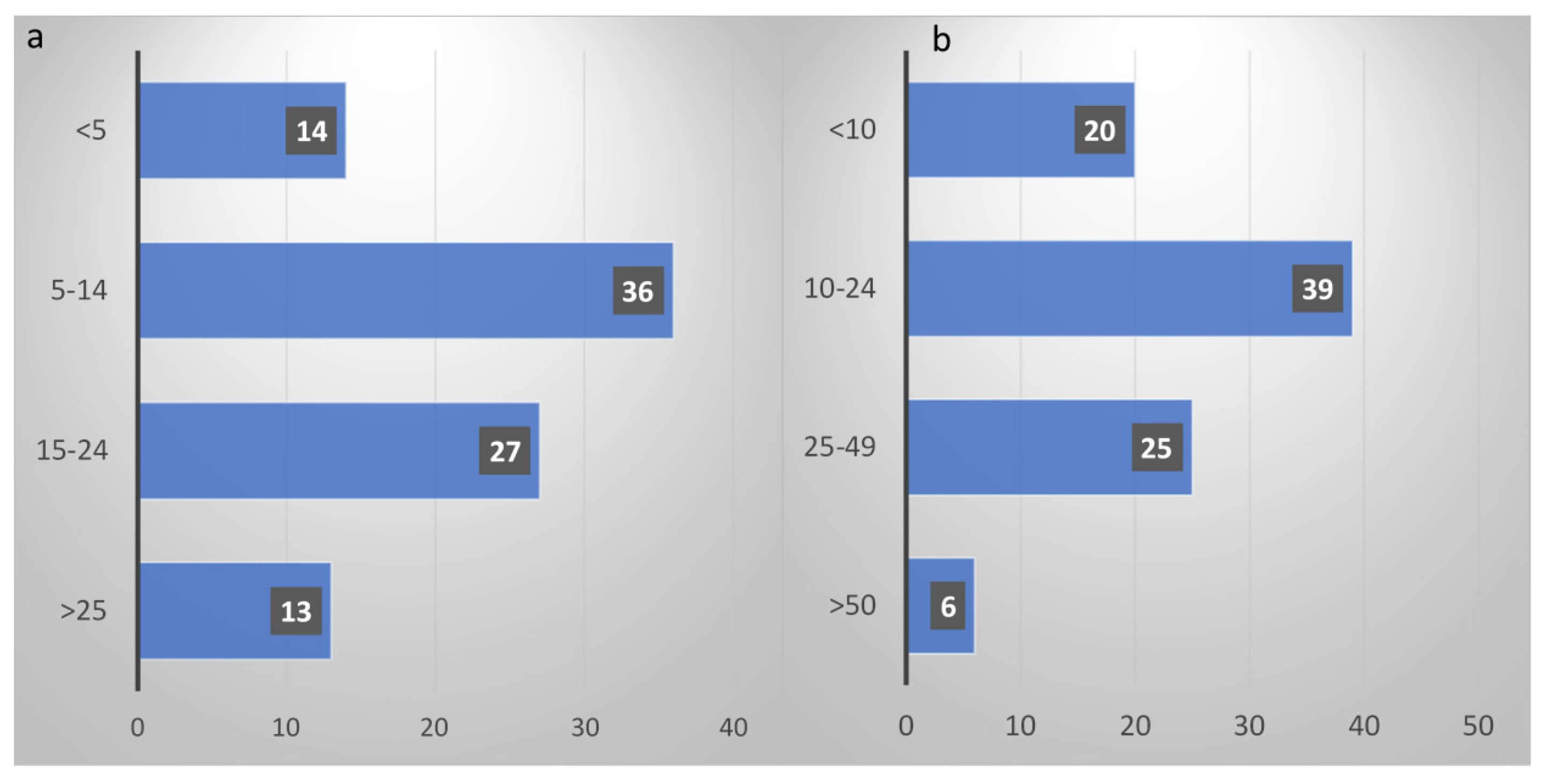
Weekly MDT discussions for SCLC cases were reported by 79% of respondents; specialists more commonly involved in diagnosis and staging were clinical oncologists (46%) and pneumologists (39%). The number of newly diagnosed SCLC patients seen annually was >50 only in 7% of cases, less than 10 in 22% and between 10 and 49 for over a half (71%), as shown in
Figure 1a. Specifically for newly diagnosed ES-SCLC, the majority of the respondents (70%) treated between 5 and 24 patients/year (
Figure 1b).
3.2. Management of ES-SCLC
3.2.1. Role of Systemic Treatment
Regarding patients with ES-SCLC and PS=0-1, 84% of respondents declared that their first therapeutic approach was concomitant chemo-immunotherapy; specifically, 66% of them preferred to start both concomitantly from the 1st cycle, while 18% of them preferred it from the 2nd or 3rd cycle of chemotherapy.
When choosing a chemotherapy regimen for ES-SCLC, the majority of the respondents preferred carboplatin+etoposide+atezolizumab both in PS 0-1 (36%) and PS 2 patients (32%); as second option cisplatin+etoposide+/- durvalumab was administered in patients with PS=0-1, but usually it was not chosen in PS 2 ones.
Interestingly, no responders declared to preclude an immunotherapy-based treatment in patients with PS 0-1 and only 6% did not offer immunotherapy in patients with PS 2; four cycles of chemotherapy were the preferred option for 68% of clinicians if a combination of chemotherapy and immunotherapy was offered.
3.2.2. Role of PCI
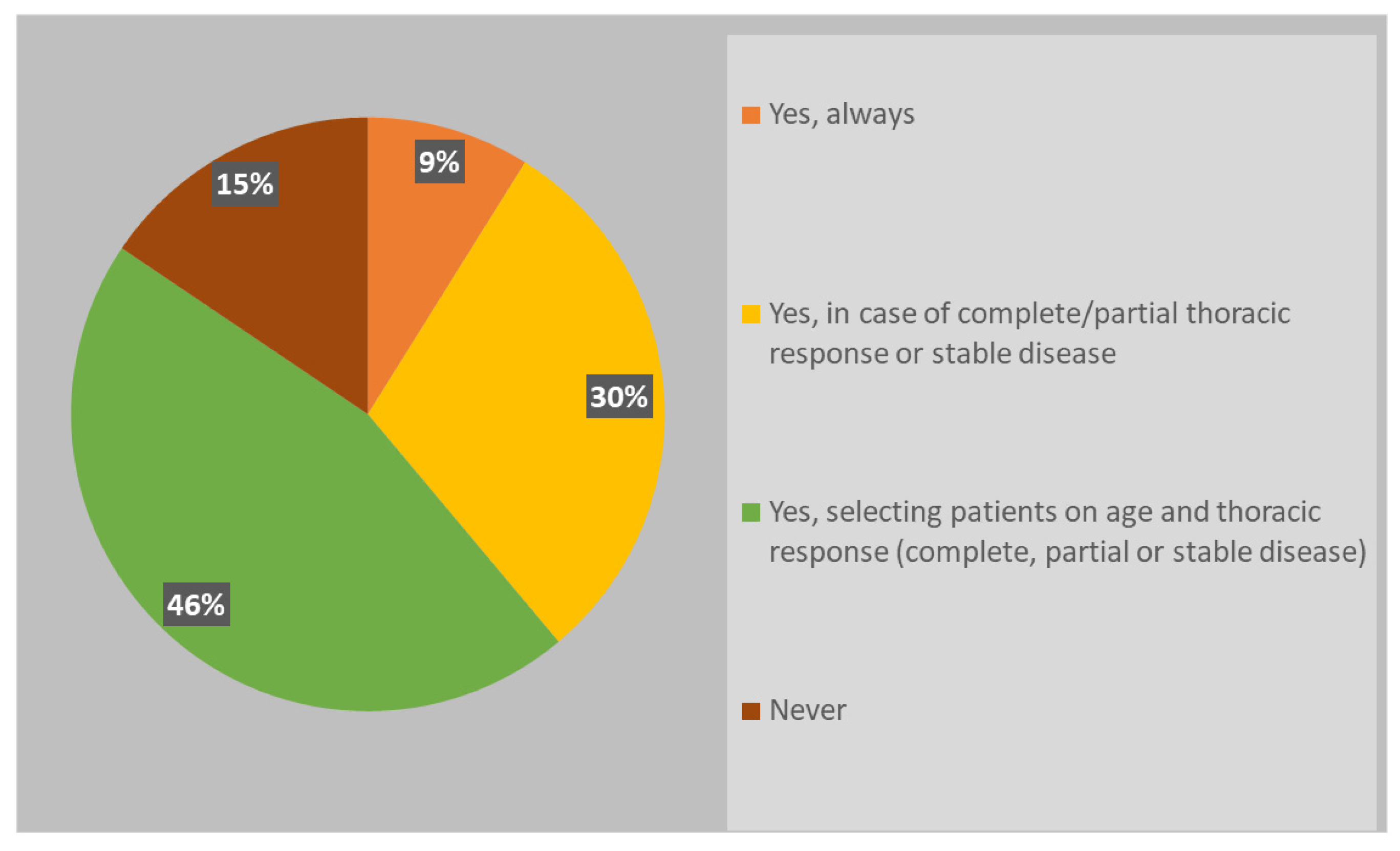
Although the new scenario related to the widely adoption of immunotherapy in ES-SCLC, almost all radiation oncologists (85%) usually recommend PCI (
Figure 2).
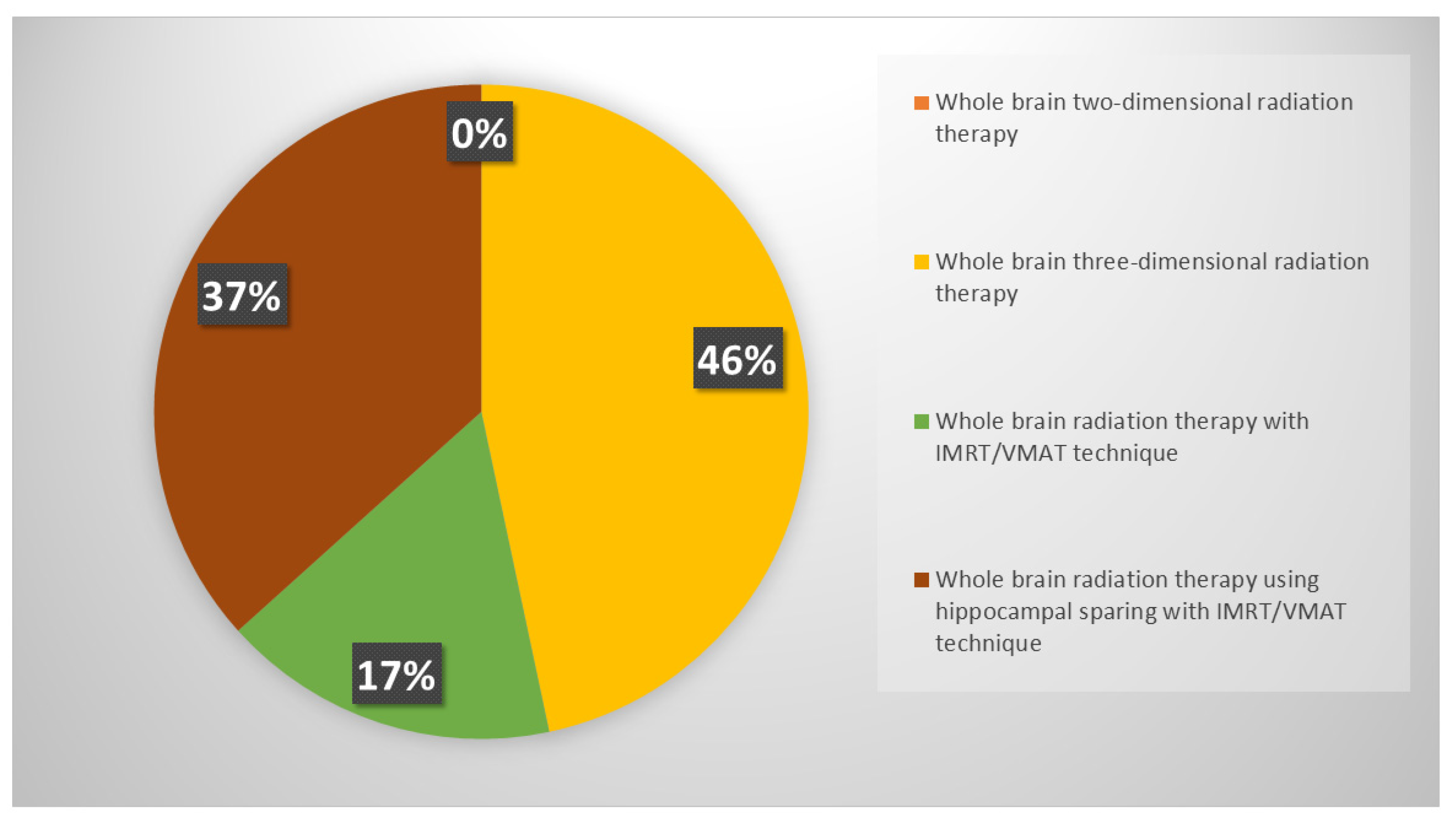
Respondents were keener to perform PCI in patients select on age and thoracic response (complete, partial or stable disease) (46%), while 30% of respondents declared to consider only thoracic response. PCI was never offered in ES-SCLC setting by 15% of clinicians. If prescribed, PCI is mostly performed after the end of chemo-immunotherapy, before starting the first cycle of maintenance immunotherapy (37%) or in concomitance with it (22%); several respondents declared to wait until the completion of first cycle of immunotherapy, both discontinuing immunotherapy during PCI (24%) and not (17%). Whole brain three-dimensional conformal radiation therapy (3DCRT) was used in case of PCI by 46% of respondents, while intensity modulated RT (IMRT)/volumetric modulated arc therapy (VMAT) with or without hippocampal avoidance was chosen by 37% and 17% of responders, respectively (
Figure 3).
3.2.3. Role of Thoracic Consolidative RT
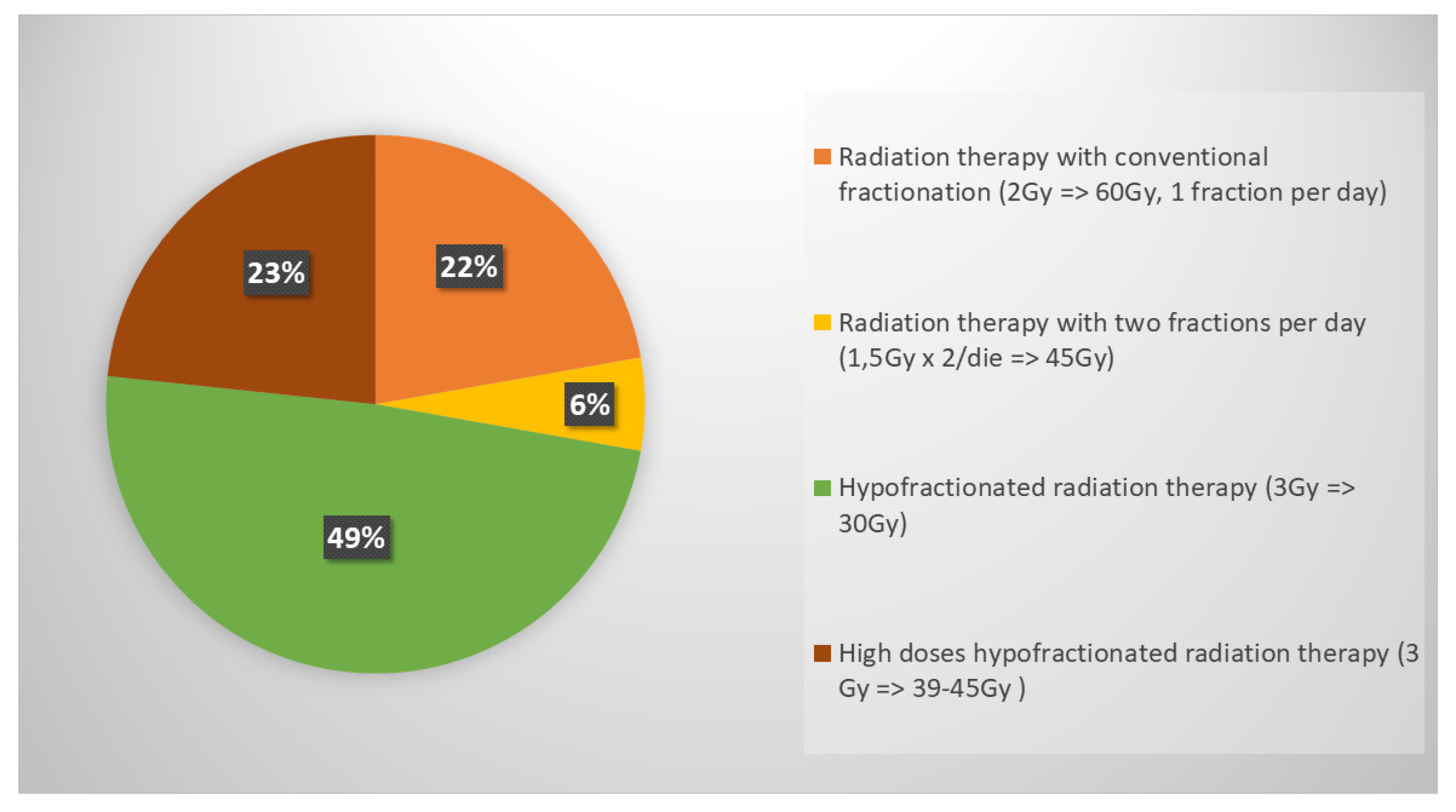
In ES-SCLC, the role of ctRT in ES-SCLC was investigated in this survey resulting that 83% of respondents choose to deliver RT in case of both intrathoracic and extrathoracic response (complete, partial or stable disease); a minority opted for RT considering only extrathoracic response (10%) and 7% declared to consider it regardless of response. The RT schedule was 30 Gy in 10 daily fractions for the majority of respondents (49%) (
Figure 4).
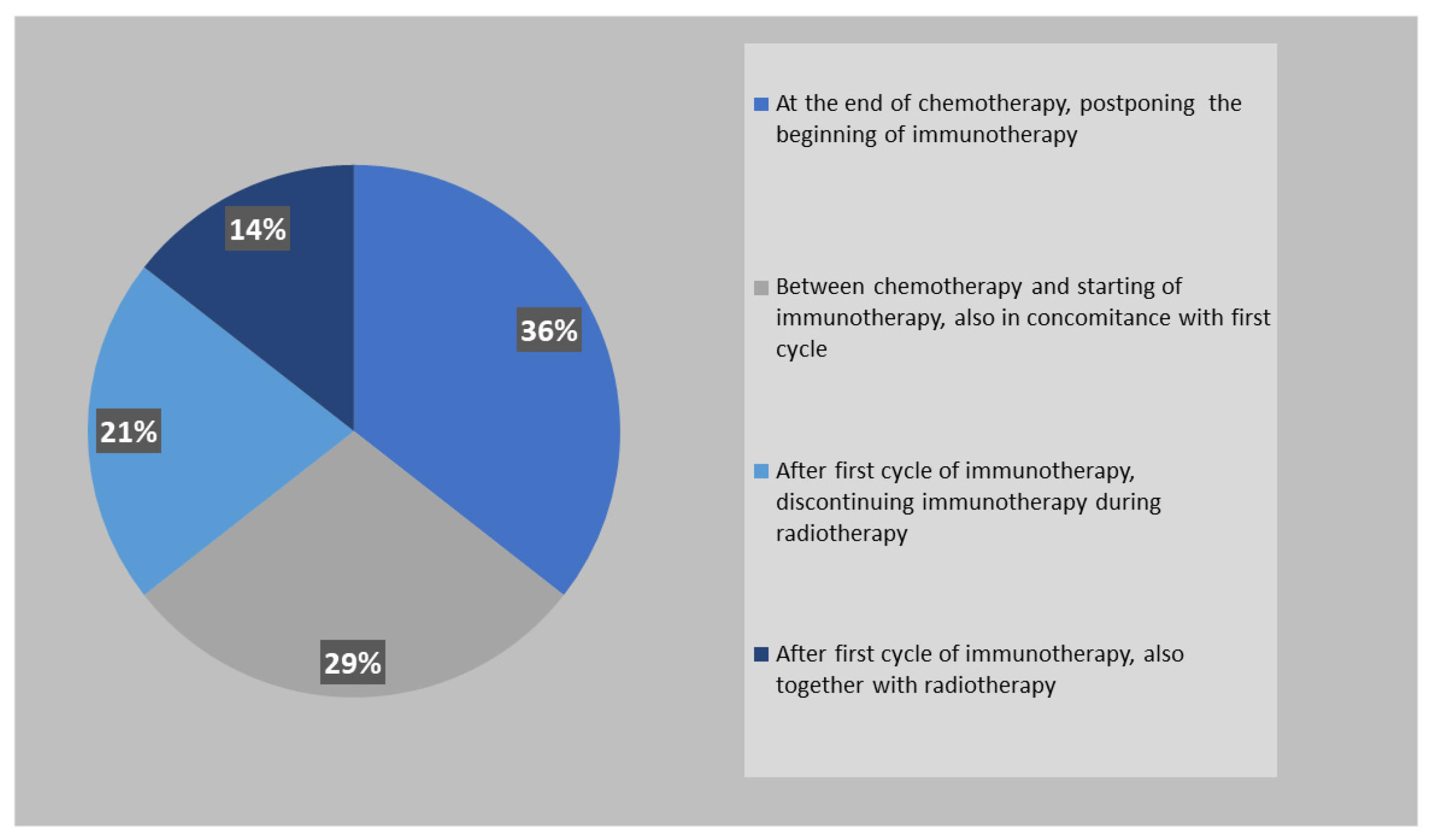
Similarly to PCI, also ctRT is mostly performed between the end of chemotherapy and the beginning of immunotherapy (65%) (
Figure 5).
3.2.4. Management of oligoprogression
If an intracranial oligoprogression occurred during maintenance immunotherapy, the treatment of choice was stereotactic RT (SRT) for 43% of clinicians, regardless of previous PCI. Conversely, if patients were RT-naive, 39% opted for WBRT. On the other hand, if extracranial oligoprogression occurred during maintenance immunotherapy, 67% of responders choose continuing the current systemic treatment adding local treatment (SRT or palliative radiotherapy).
4. Discussion
The results of this survey, driven by the radiation oncology’ community, showed the variability of the real-world management of ES-SCLC after the introduction of first-line immunotherapy. The CASPIAN and the IMpower 133 randomized clinical trials demonstrated that the addition of Durvalumab or Atezolizumab to first-line chemotherapy, improved OS HR: 0.73 (95% CI 0.59–0.91; p=0.0047) and 0.70 (95% CI 0.54–0.91; p=0.007), respectively [
8,
9,
10], if compared to chemotherapy alone. In both trials, patients received four cycles of chemotherapy in association with immunotherapy followed by maintenance immunotherapy without a significant increase in toxicities. Our survey demonstrated that only 67% of clinicians strictly follow the CASPIAN or IMpower 133 schedules. Indeed, 18% of respondents prescribes immunotherapy from the second or third cycle of chemotherapy, 8% only after chemotherapy and 8% do not prescribe immunotherapy at all (commonly due to the frailty of these patients). The majority of clinicians usually perform four cycles of chemotherapy concomitant to immunotherapy; these results could be due to the recent introduction in clinical practice of these new regimens that are counterintuitive if compared to the previous standard of care in which 6 cycles of chemotherapy were provided to obtain the most of chemotherapy efficacy. Furthermore, another reason of this discrepancy from guidelines may be a consequence of not being fully dedicated to pulmonary pathology as reported by almost 30% of respondents. According to our survey, 36% of clinicians preferred atezolizumab instead of durvalumab (25%), while for 24% of the participants both treatments were comparable. This is probably due to the fact that in Italy Atezolizumab was refundable from 2020 while durvalumab from 2022 and clinicians feel more confident in the use of one regimen rather than another. In addition, the fact that Atezolizumab administration was made with carboplatin, that is commonly considered more manageable and less toxic particularly in frail patients, should be considered. On the other hand, even if the use of cisplatin was allowed in the CASPIAN trial for fit patients, only a quarter of the population in both durvalumab and placebo arm received it. Our findings revealed that the majority of clinicians still prefer to use carboplatin instead of cisplatin, probably because most patients were considered cisplatin unfit. Both CASPIAN and IMpower 133 trial enrolled patients with an ECOG PS 0-1, so no data are available for ECOG PS 2 patients. Agarwal et al. recently compared the use of immunotherapy regimens in patients affected by ES-SCLC with ECOG PS 0-1 vs ECOG PS 2-3 patients without finding any difference in terms of both PFS and OS [
17]. Many studies showed that in real-world practice, ES-SCLC patients with ECOG PS 2 treated as per CASPIAN or IMpower 133 regimens did not present increased toxicities without any differences in terms of efficacy [
18,
19]. Similarly, our results showed that only 9% of clinicians proposed chemotherapy alone to these patients, while the vast majority use a combination of chemo and immunotherapy, and 6% even use a cisplatin combination.
Most Italian radiation oncologists (about 46%) would offer PCI only to patients with complete or partial remission after the first chemo-immunotherapy phase of treatment, considering the patient’s age and discouraging PCI in older patients with many comorbidities. On the contrary, 16% of respondents would never offer PCI in ES-SCLC. As we know, PCI was allowed during maintenance treatment only in the IMpower 133 trial and it was offered to just 10% of patients in both the immunotherapy and placebo groups. In the pre-immunotherapy era, the phase III EORTC trial compared PCI to observation in ES-SCLC with any response to first-line chemotherapy demonstrating to increase DFS HR: 0.76 (95% CI 0.59–0.96; p=0.02), to improve OS HR: 0.68 (95% CI 0.52–0.88; p=0.003) and 1-year survival rate 27.1% (95% CI 19.4 to 35.5) vs. 13.3% (95% CI 8.1 to 19.9) of the irradiation group compared to the observation group [
20]. This trial was heavily criticized as pre-PCI brain imaging was not required, and patients in the PCI group presented a lower risk of brain metastases development (14% compared to 40.4% of the control group) and long-term neurocognitive deterioration issues arose [
21]. Opposite results were obtained from the phase III Japanese trial where the authors demonstrated a detrimental effect of PCI compared to observation, which consisted of brain MRI every three months for the first 12 months and every six months thereafter [
15].
Few evidences are available in the immunotherapy era. At ASTRO 2020 Higgings et al. reported the results of an exploratory analysis about the pattern of disease progression in patients enrolled in the Impower 133 trial. They observed an improved intra-cranial PFS in those patients who underwent PCI both in the atezolizumab and the placebo arm, even though only 22 (10%) of them underwent PCI in the two groups. The median time to intra cranial progression was 20.2 months for atezolizumab and 10.5 months for placebo in the PCI group, and 16.5 months for atezolizumab and 9.8 months for placebo in the group without PCI, confirming a positive role of PCI in delaying the appearance of brain metastases [
22].
Similarly, Chen et al. reported median time to RECIST-defined disease progression in the brain or brain radiotherapy (excluding PCI), whichever occurred first was 19.2 months and 10.2 months for Durvalumab plus chemotherapy and chemotherapy alone, respectively. In the CASPIAN trial , PCI was allowed only for placebo group and applied in the 8 % of patients [
23].
Gross et al. performed a study to evaluate the impact of PCI and thoracic radiotherapy after the introduction of immunotherapy. Considering all the limits of a monocentric non-randomized study, they observed in 54 patients who underwent PCI an improved OS from 10 to 15 months and an improved median PFS from 5 to 8.5 months [
24].
The respondents in favor of PCI prefer to perform it before the first cycle of maintenance immunotherapy (37%) or even in association (39%); only 24% prefer to discontinue immunotherapy during PCI. Forty-seven percent of radiation oncologists use a 3D technique, 17% use IMRT, and 37% are in favor of hippocampal sparing brain RT as it demonstrated a significant reduction in the risk of neurocognitive impairments [
25]. Unfortunately, the role of PCI in ES-SCLC is still unclear, and the results of ongoing trials such as the MAVERIK and PRIMAlung studies are still awaited in order to find an answer to this important issue [
26,
27].
Before the CASPIAN and IMpower 133 publications, ctRT for ES-SCLC achieving clinical and radiological response to chemotherapy was part of the management of these patients and it is still suggested by American and European guidelines [
28,
29]. The RTOG 0937 trial randomized patients with ES-SCLC with any response to chemotherapy to receive PCI vs. PCI plus cRT (45 Gy in 15 fractions). The study did not meet the primary endpoint (1-year OS) even if it was able to delay thoracic progression in the RT group [
30]. The phase III CREST trial randomized ES-SCLC with any response to 4-6 cycles of chemotherapy into a ctRT group (30 Gy in 10 fractions) compared to the observation one. The results showed a 2-year OS in favor of ctRT 13% vs. 3% (p=0.004), and also the PFS was improved in the ctRT group HR: 0.73 (95% CI 0.61–0.87; p=0.001) without determining excess of toxicities [
31]. More recently, the Italian TRENDS trial confirmed that ctRT reduces the risk of intrathoracic progression being also well tolerated [
32]. Even the role of ctRT is still debated as no data can be clearly drawn from the two immunotherapy trials where ctRT was not allowed. As a matter of fact, thoracic ctRT after any response to chemo-immunotherapy is not recommended in the American and European guidelines [
1,
29]. However, retrospective data, including patients with ES-SCLC treated with immunotherapy combined with chemotherapy with/without ctRT, demonstrated that the addition of thoracic ctRT improved PFS and local control, even if it did not improve OS [
33]. On the other hand, the TREASURE study, recently presented at ESMO 2023, showed that the addition of thoracic ctRT after first-line chemo-immunotherapy can be associated with an increased rate of severe toxicities, (especially pneumonitis, sepsis and multiorgan failure), so that the recruitment was permanently discontinued [
34]. The results of our survey showed that the majority of respondents (83%) propose ctRT when patients present an intra- and extra thoracic response to systemic chemo-immunotherapy. The most used schedule is 30 Gy in 10 fractions (49%), followed by the use of higher doses such as 39-45 Gy in 13-15 fractions (23%) or 60 Gy in 30 fractions (22%). Only 6% of radiation oncologists use the twice-daily regimen (45 Gy in 30 fractions BID) in this setting. The use of modern radiotherapy techniques are preferable in order to achieve a reduction in treatment-related toxicity and improve treatment tolerance. Half of respondents perform ctRT in association with immunotherapy, 36% before starting immunotherapy, and only 14% suspend immunotherapy during thoracic cRT.
Finally, the treatment of brain metastases is very challenging, as patients are often excluded from clinical trials evaluating the use of radiosurgery (RS) and SRT. The FIRE study concluded that the use of RS or SRT for the first-line treatment of brain metastases from SCLC presents similar outcomes to those shown by other hystologies [
35]. After the publication of these results, an American survey showed an improved use of SRT for the treatment of brain metastases from SCLC among American radiation oncologists [
36]. In our survey, 39% prefer WBRT in case of intracranial failure if PCI was not previously performed, while 43% always propose SRT independently from the previous PCI. The introduction of immunotherapy revolutionized the treatment in patients with progressive disease. Recently, in the SAMOS study, data about the use of SRT for extracranial and intracranial metastases from oligoprogressive SCLC were retrospectively collected [
37]. The results suggested that the use of SRT could delay further systemic therapies prolonging the use of the same treatment. In our survey, only 30% of respondents propose a second-line systemic treatment in case of oligoprogressive disease, while the majority of respondents (about 70%) continue immunotherapy, adding SRT to the site of oligoprogression.
To our knowledge our paper represents the first survey in the immunotherapy era regarding ES-SCLC open questions in the Italian radiation oncology community, and its results may help to optimize and personalized the treatment of this aggressive disease.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Table S1: Questions posed to respondents.
Author Contributions
Conceptualization, A.B. and P.B.; methodology, A.B., P.B., F.B., J.I., C.B.; validation, F.C., M.M., M.G., A.P., S.B., F.B., J.I., C.B, M.T., R.S., E.A., M.A.Z., E.O.; formal analysis, S.B., M.T., A.B., P.B.; data curation, A.B., P.C., V.S., N.G.L., S.V.; writing—original draft preparation, M.A.Z, R.S., E.A., E.O.; writing—review and editing, P.B., A.B., M.A.Z, E.O., M.T., N.J.L, P.C.; supervision, A.B., P.B.;. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding
Institutional Review Board Statement
Not applicable
Informed Consent Statement
Not applicable
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
The Authors thank the Scientific Committee and Board of the AIRO for the critical revision and final approval of the manuscript (Nr. 6/2022).
Conflicts of Interest
N.GL. received speakers’ and consultants’ fee from Astra-Zeneca, Amgen. M.T. received speakers’ and consultants’ fee from Astra-Zeneca, Pfizer, Eli-Lilly, BMS, Novartis, Roche, MSD, Boehringer Ingelheim, Takeda, Amgen, Merck, Sanofi, Janssen, Daiichi Sankyo; M.T. received institutional research grants from Astra-Zeneca, Boehringer Ingelheim and Roche; M.T. received travel support from Amgen and Takeda. S.B. received speakers’ fees from Elekta , Accuray, Astra-Zeneca.
References
- Daly ME, Ismaila N, Decker RH, Higgins K, Owen D, Saxena A, Franklin GE, Donaldson D, Schneider BJ. Radiation Therapy for Small-Cell Lung Cancer: ASCO Guideline Endorsement of an ASTRO Guideline. J Clin Oncol. 2021 Mar 10;39(8):931-939. [CrossRef]
- Rudin CM, Brambilla E, Faivre-Finn C, Sage J. Small-cell lung cancer. Nat Rev Dis Primers. 2021 Jan 14;7(1):3. [CrossRef]
- Nicholson AG, Chansky K, Crowley J, Beyruti R, Kubota K, Turrisi A, Eberhardt WE, van Meerbeeck J, Rami-Porta R; Staging and Prognostic Factors Committee, Advisory Boards, and Participating Institutions; Staging and Prognostic Factors Committee Advisory Boards and Participating Institutions. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the Clinical and Pathologic Staging of Small Cell Lung Cancer in the Forthcoming Eighth Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016 Mar;11(3):300-11. [CrossRef]
- Khurshid H, Ismaila N, Bian J, Dabney R, Das M, Ellis P, Feldman J, Hann C, Kulkarni S, Laskin J, Manochakian R, Mishra DR, Preeshagul I, Reddy P, Saxena A, Weinberg F, Kalemkerian GP. Systemic Therapy for Small-Cell Lung Cancer: ASCO-Ontario Health (Cancer Care Ontario) Guideline. J Clin Oncol. 2023 Dec 10;41(35):5448-5472. [CrossRef]
- Cheng Y, Spigel DR, Cho BC, Laktionov KK, Fang J, Chen Y, Zenke Y, Lee KH, Wang Q, Navarro A, Bernabe R, Buchmeier EL, Chang JW, Shiraishi Y, Goksu SS, Badzio A, Shi A, Daniel DB, Hoa NTT, Zemanova M, Mann H, Gowda H, Jiang H, Senan S; ADRIATIC Investigators. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N Engl J Med. 2024 Sep 13. [CrossRef]
- Lally BE, Urbanic JJ, Blackstock AW, Miller AA, Perry MC. Small cell lung cancer: have we made any progress over the last 25 years? Oncologist (2007) 12:1096-104. [CrossRef]
- Levy A, Botticella A, Le Péchoux C, Faivre-Finn C. Thoracic radiotherapy in small cell lung cancer-a narrative review. Transl Lung Cancer Res (2021) 10:2059-70. [CrossRef]
- Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Garassino MC, Voitko O, Poltoratskiy A, Ponce S, Verderame F, Havel L, Bondarenko I, Każarnowicz A, Losonczy G, Conev NV, Armstrong J, Byrne N, Thiyagarajah P, Jiang H, Paz-Ares L; CASPIAN investigators. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021 Jan;22(1):51-65. [CrossRef]
- Paz-Ares L, Chen Y, Reinmuth N, Hotta K, Trukhin D, Statsenko G, Hochmair MJ, Özgüroğlu M, Ji JH, Garassino MC, Voitko O, Poltoratskiy A, Musso E, Havel L, Bondarenko I, Losonczy G, Conev N, Mann H, Dalvi TB, Jiang H, Goldman JW. Durvalumab, with or without tremelimumab, plus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer: 3-year overall survival update from CASPIAN. ESMO Open. 2022 Apr;7(2):100408. [CrossRef]
- Liu SV, Reck M, Mansfield AS, Mok T, Scherpereel A, Reinmuth N, Garassino MC, De Castro Carpeno J, Califano R, Nishio M, Orlandi F, Alatorre-Alexander J, Leal T, Cheng Y, Lee JS, Lam S, McCleland M, Deng Y, Phan S, Horn L. Updated Overall Survival and PD-L1 Subgroup Analysis of Patients With Extensive-Stage Small-Cell Lung Cancer Treated With Atezolizumab, Carboplatin, and Etoposide (IMpower133). J Clin Oncol. 2021 Feb 20;39(6):619-630. [CrossRef]
- Sun A, Abdulkarim B, Blais N, Greenland J, Louie AV, Melosky B, Schellenberg D, Snow S, Liu G. Use of radiation therapy among patients with Extensive-stage Small-cell lung cancer receiving Immunotherapy: Canadian consensus recommendations. Lung Cancer. 2023 May;179:107166. [CrossRef]
- Chemo-immunotherapy Plus Thoracic Radiotherapy in Extensive Stage Small-cell Lung Cancer (TRIPLEX). Available online: https://classic.clinicaltrials.gov/ct2/show/NCT05223647 (accessed on 1 September 2024).
- Bozorgmehr F, Christopoulos P, Chung I, Cvetkovic J, Feißt M, Krisam J, Schneider MA, Heußel CP, Kreuter M, Müller DW, Thomas M, Rieken S. Protocol of the TREASURE study: Thoracic RadiothErapy with Atezolizumab in Small cell lUng canceR Extensive disease—a randomized, open-label, multicenter phase II trial. BMC Cancer. 2022 Sep 24;22(1):1011. [CrossRef]
- NIH, Testing the Addition of Radiation Therapy to the Usual Immune Therapy Treatment (Atezolizumab) for Extensive Stage Small Cell Lung Cancer, The RAPTOR Trial. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04402788 (accessed on 1 September 2024).
- Takahashi T, Yamanaka T, Seto T, Harada H, Nokihara H, Saka H, Nishio M, Kaneda H, Takayama K, Ishimoto O, Takeda K, Yoshioka H, Tachihara M, Sakai H, Goto K, Yamamoto N. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2017 May;18(5):663-671. [CrossRef]
- Ciammella P, Timon G, Bruni A, Franceschini D, Borghetti P, Giaj-Levra N, Greco C, Scotti V, Trovo M; On the behalf of Associazione Italiana Radioterapia Oncologica (AIRO). Radiation therapy in small cell lung cancer: a national Italian survey. Radiol Med. 2018 Jul;123(7):554-560. [CrossRef]
- Agarwal M, Liu A, Almquist D, Langlais BT, Leventakos K, Yu NY, Manochakian R, Ernani V. Chemoimmunotherapy in patients with extensive-stage small cell lung cancer and a poor performance status. Cancer. 2023 Nov 15;129(22):3546-3553. [CrossRef]
- Agarwal M, Liu A, Langlais BT, Leventakos K, Yu NY, Almquist D, Manochakian R, Ernani V. Chemoimmunotherapy as the First-Line Treatment for Patients With Extensive-Stage Small-Cell Lung Cancer and an ECOG Performance Status 2 or 3. Clin Lung Cancer. 2023 Nov;24(7):591-597. [CrossRef]
- Longo V, Pizzutilo P, Catino A, Montrone M, Pesola F, Marerch I, Galetta D. Prognostic factors for survival in extensive-stage small cell lung cancer: An Italian real-world retrospective analysis of 244 patients treated over the last decade. Thorac Cancer. 2022 Dec;13(24):3486-3495. [CrossRef]
- Slotman B, Faivre-Finn C, Kramer G, Rankin E, Snee M, Hatton M, Postmus P, Collette L, Musat E, Senan S; EORTC Radiation Oncology Group and Lung Cancer Group. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Engl J Med. 2007 Aug 16;357(7):664-72. [CrossRef]
- Gondi V, Paulus R, Bruner DW, Meyers CA, Gore EM, Wolfson A, Werner-Wasik M, Sun AY, Choy H, Movsas B. Decline in tested and self-reported cognitive functioning after prophylactic cranial irradiation for lung cancer: pooled secondary analysis of Radiation Therapy Oncology Group randomized trials 0212 and 0214. Int J Radiat Oncol Biol Phys. 2013 Jul 15;86(4):656-64. [CrossRef]
- Higgins KA, Curran WJ Jr, Liu SV, Yu W, Brockman M, Johnson A, Bara I, Bradley JD. Patterns of Disease Progression after Carboplatin/Etoposide + Atezolizumab in Extensive-Stage Small-Cell Lung Cancer (ES-SCLC). Int J Radiat Oncol Biol Phys. 2020 Dec 1;108(5):1398. [CrossRef]
- Chen Y, Paz-Ares L, Reinmuth N, Garassino MC, Statsenko G, Hochmair MJ, Özgüroğlu M, Verderame F, Havel L, Losonczy G, Conev NV, Hotta K, Ji JH, Spencer S, Dalvi T, Jiang H, Goldman JW. Impact of Brain Metastases on Treatment Patterns and Outcomes With First-Line Durvalumab Plus Platinum-Etoposide in Extensive-Stage SCLC (CASPIAN): A Brief Report. JTO Clin Res Rep. 2022 Apr 26;3(6):100330. [CrossRef]
- Gross AJ, Sheikh S, Kharouta M, Chaung K, Choi S, Margevicius S, Fu P, Machtay M, Bruno DbS, Dowlati A, Biswas T. The Impact of Prophylactic Cranial Irradiation and Consolidative Thoracic Radiation Therapy for Extensive Stage Small-Cell Lung Cancer in the Transition to the Chemo-Immunotherapy Era: A Single Institution Series. Clin Lung Cancer. 2023 Dec;24(8):696-705. [CrossRef]
- Zhao R, Kong W, Shang J, Zhe H, Wang YY. Hippocampal-Sparing Whole-Brain Radiotherapy for Lung Cancer. Clin Lung Cancer. 2017 Mar;18(2):127-131. [CrossRef]
- S1827 (MAVERICK) Testing Whether the Use of Brain Scans Alone Instead of Brain Scans Plus Preventive Brain Radiation Affects Lifespan in Patients With Small Cell Lung Cancer. Available online: https://clinicaltrials.gov/study/NCT04155034 (accessed on 1 September 2024).
- PRophylactic Cerebral Irradiation or Active MAgnetic Resonance Imaging Surveillance in Small-cell Lung Cancer Patients (PRIMALung Study) (PRIMALung). Available online: https://clinicaltrials.gov/study/NCT04790253 (accessed on 1 September 2024).
- Expert Panel Thoracic Malignancies:; Higgins KA, Simone CB 2nd, Amini A, Chetty IJ, Donington J, Edelman MJ, Chun SG, Kestin LL, Movsas B, Rodrigues GB, Rosenzweig KE, Slotman BJ, Rybkin II, Wolf A, Chang JY. American Radium Society Appropriate Use Criteria on Radiation Therapy for Extensive-Stage SCLC. J Thorac Oncol. 2021 Jan;16(1):54-65. [CrossRef]
- Dingemans AC, Früh M, Ardizzoni A, Besse B, Faivre-Finn C, Hendriks LE, Lantuejoul S, Peters S, Reguart N, Rudin CM, De Ruysscher D, Van Schil PE, Vansteenkiste J, Reck M; ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up☆. Ann Oncol. 2021 Jul;32(7):839-853. [CrossRef]
- Gore EM, Hu C, Sun AY, Grimm DF, Ramalingam SS, Dunlap NE, Higgins KA, Werner-Wasik M, Allen AM, Iyengar P, Videtic GMM, Hales RK, McGarry RC, Urbanic JJ, Pu AT, Johnstone CA, Stieber VW, Paulus R, Bradley JD. Randomized Phase II Study Comparing Prophylactic Cranial Irradiation Alone to Prophylactic Cranial Irradiation and Consolidative Extracranial Irradiation for Extensive-Disease Small Cell Lung Cancer (ED SCLC): NRG Oncology RTOG 0937. J Thorac Oncol. 2017 Oct;12(10):1561-1570. [CrossRef]
- Slotman BJ, van Tinteren H, Praag JO, Knegjens JL, El Sharouni SY, Hatton M, Keijser A, Faivre-Finn C, Senan S. Use of thoracic radiotherapy for extensive stage small-cell lung cancer: a phase 3 randomised controlled trial. Lancet. 2015 Jan 3;385(9962):36-42. [CrossRef]
- Cozzi S, Bruni A, Ruggieri MP, Borghetti P, Scotti V, Franceschini D, Fiore M, Taraborrelli M, Salvi F, Galaverni M, Savoldi L, Braglia L, Botti A, Finocchi Ghersi S, Niccolò GL, Lohr F, Iotti C, Ciammella P. Thoracic Radiotherapy in Extensive Disease Small Cell Lung Cancer: Multicenter Prospective Observational TRENDS Study. Cancers (Basel). 2023 Jan 10;15(2):434. [CrossRef]
- Wu, J. & Zhang, J. & Sun, H. & Sun, Y. & Ge, Y. & Cheng, Q. & Wang, D. & Wang, X. & Fu, X. & Li, Jhon & Gao, A.. (2023). 2000P Efficacy and safety of thoracic radiotherapy after first-line immunotherapy in extensive stage small cell lung cancer: A multi-center retrospective study. Annals of Oncology, Volume 34, 2023, Page S1067. [CrossRef]
- Bozorgmehr F., Weykamp F., Overbeck T.R., Maguire N., Buchmeier E.L., Hammer-Hellmig M.,. Gauler T.C, Wermke M., Troost E.G.C., Ulmer M, Mueller A-C., Kokowski K, Röper B., Wehler T., Hey-Koch S., Consdorf N-S., Behnisch R., Christopoulos P., Thomas M., Rieken S. 1988MO Recruitment discontinuation in TREASURE trial (thoracic radiotherapy with atezolizumab in small cell lung cancer extensive disease) due to unexpected safety data, Annals of Oncology, Volume 34, Supplement 2, 2023, Page S1060. [CrossRef]
- Rusthoven CG, Yamamoto M, Bernhardt D, Smith DE, Gao D, Serizawa T, Yomo S, Aiyama H, Higuchi Y, Shuto T, Akabane A, Sato Y, Niranjan A, Faramand AM, Lunsford LD, McInerney J, Tuanquin LC, Zacharia BE, Chiang V, Singh C, Yu JB, Braunstein S, Mathieu D, Touchette CJ, Lee CC, Yang HC, Aizer AA, Cagney DN, Chan MD, Kondziolka D, Bernstein K, Silverman JS, Grills IS, Siddiqui ZA, Yuan JC, Sheehan JP, Cordeiro D, Nosaki K, Seto T, Deibert CP, Verma V, Day S, Halasz LM, Warnick RE, Trifiletti DM, Palmer JD, Attia A, Li B, Cifarelli CP, Brown PD, Vargo JA, Combs SE, Kessel KA, Rieken S, Patel S, Guckenberger M, Andratschke N, Kavanagh BD, Robin TP. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol. 2020 Jul 1;6(7):1028-1037. Erratum in: JAMA Oncol. 2020 Sep 1;6(9):1473. 10.1001/jamaoncol.2020.3404. [CrossRef]
- Gjyshi O, Lin SH, Pezzi TA, Ning MS, Ma J, Liu S, Rusthoven CG. Care Patterns for Stereotactic Radiosurgery in Small Cell Lung Cancer Brain Metastases. Clin Lung Cancer. 2022 Mar;23(2):185-190. [CrossRef]
- Borghetti P, Facheris G, Ciammella P, Galaverni M, Granello L, Scotti V, Franceschini D, Romei A, Giaj Levra N, Federico M, La Vecchia M, Merlotti A, Sepulcri M, Piperno G, Marvaso G, Simoni N, Alì E, Pontoriero A, Cappelli A, Dionisi V, Menis J, Martino A, Vagge S, Canova S, Montesi G, Cuccia F, Boldrini L, Franzese C, Grisanti S, Bruni A, Scorsetti M. Sterotactic Ablative Radiotherapy in a Multicentric Series of Oligometastatic SCLC: The SAMOS Cohort. Clin Lung Cancer. 2024 Mar;25(2):151-158. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).