1. Introduction
Africa’s economic and social development depends largely on clean energy transition, thus, influencing the drive for energy development goals. Essentially, there is the need for affordable and clean energy for all Africans [
1]. The continent potentially holds the gate for the world to achieve net zero energy transition. In 2021, biofuel and waste accounted for 45.4% of the total energy supply in Africa. It is projected that by 2025, the world will generate 6 million tons of waste∙[
2].Presently,∙64% of Africans use agricultural and animal waste and wood for cooking, with possibilities of resulting in deforestation [
1]. Biogas generation is one option to ensure a sustainable energy supply and to offer an alternative for clean energy transition; especially through simple cooking applications; deriving energy from animal and agricultural waste [
2,
3,
4,
5]. Biogas, a renewable energy resource is produced from the microbial breakdown of organic materials (biomass: manure, food waste, agricultural waste, waste water, green waste, sewage, municipal waste etc.) under anaerobic conditions [
3,
6,
7,
8]. Bejor (2020) defines anaerobic as the absence of free oxygen [
9]. This process involves relevant microorganisms of four separate generation processes: hydrolytic bacteria for hydrolysis, acidogenic bacteria for acidogenesis, acetogenic bacteria for acetogenesis and methanogens for methanogenesis [
10]. At the hydrolysis and acidogenic stages of biogas production, lipids, proteins and carbohydrates are broken into complex long-chain fatty acids, glycerol, amino acids and sugars. These are eventually converted into short-chain fatty acids, alcohols, hydrogen, carbon dioxide and acetate. Through methanogenic actions, biogas is then produced from the acetates, carbon dioxide and hydrogen [
11,
12], thus, producing methane (CH
4:55-65%), Carbon dioxide (CO
2: 35-40%), Hydrogen sulphide (H
2S), moisture and siloxanes in small amounts [
13]. Biogas composition can generally summarised as 50-75% of CH
4, 25-45% of CO
2, 2-7% of H
2 according to Wukovits and Schnitzhofer (2009) [
14]. A generation equation for biogas generation is cited by Bejor (2020) as organic matter + Combined Oxygen +∙Anaerobic microbes' → CH
4 + CO
2+Other end-products [
9].The energy content of biogas primarily depends on the fraction of methane [
15].The semi-solid or solid effluent produced is a source or useful raw material for biofertilizer applications [
16]. While biogas, technically, the CH
4 component is useful for cooking, electricity generation, vehicle fuelling and bio-methanation, its production and use cycle is continuous, with no net carbon dioxide emissions being produced. That is, carbon absorbed during biomass growth offsets the carbon associated with its energy conversion, if transport and processing emission are not taken into account [
17]. Despite the positives of anaerobic digestion for biogas generation, there are still technological and microbiological considerations necessary to ensure economic feasibility [
11].
Náthia-Neves (2018) classifies biogas production processes as wet digestion, dry digestion and semi-dry digestion, based on the dry matter or total solid content of the initial substrate. A typical wet digestion should have an initial dry matter content that is below 10% and more than 20% for a dry digestion. Wet digestion has been used and suited for sewage sludge treatment and liquid waste with high moisture contents. Dry digestion on the other hand is suited for substrates with high dry matter contents [
16]. A wet digestion, semi-dry or dry digestion process could be transformed to another by transforming the substrate in order to affect the hydrolysis process. Hydrolysis in biogas production has two challenges: the most-time intensive of all four generation stages and the limitation of speed in the use of substrates which are in the forms of particles [
18,
19,
20,
21,
22,
23,
24,
25,
26]. Intensifying the hydrolysis process therefore results in an increased performance [
18]. Mechanical pre-treatment is one way of doing this (with biological, chemical or a combination of any of the three as the others) [
19]. Pressing out fluid from biomass which is difficult to process by anaerobic digestion, e.g. due to its texture and high fibre content, is an emerging mechanical pre-treatment approach [
20,
21,
22,
23,
24,
25] and one-way by which the hydrolysis stage can be modified.
Mechanical pre-treatment by pressing has been driven by several forces [
26,
30,
31,
32,
33,
34,
35]. For instance, Corton et al. (2014) cites the use of∙mechanical pressing to obtain press water for biogas while densifying the resulting solid cake,∙which is fibrous in nature for the purposes of solid fuelling [
20]. The approach was seen as an innovation to maximise the conversion of energy from low input high diversity biomass. Other researches related to this integrated approach have been conducted by other authors [
27,
28]. Nayono et al. (2010) co-digested food waste with press water from organic municipal solid waste with the view to improve production of biogas [
23]. The authors observed that adding more of either food waste or press water to biowaste co-digestion resulted in a stronger buffer medium. The Biomethane potential obtained over a 11 day period was 500 ml/g
oTS [
23]. Nayono et al. (2010) concludes that using press water from the organic part of municipal solid waste is a good resource for biogas generation [
22]. In a mesophilic digestion at 37°C, a BMP of 540 ml/g
oTS was obtained in a batch test for press water from organic fraction of municipal solid waste. This feedstock was obtained from a composting plant, thus allowing the remaining cake to continue in the composting chain. This positive result is the basis for their conclusion. The focus of the study was to characterise and assess press water’s suitability for anaerobic digestion: to evaluate the potential of its energy recovery and as a mitigation for problems with its handling [
22].
Hensgen et al. (2014) used a screw press to obtain press waters from twelve (12) material types sourced from semi-natural habitat in Germany, Estonia and Wales [
27]. Biogas production determinations were done for press waters from the silages using VDI standards, at a mesophilic temperature of 37°C. A fermentation time of 14 days was used in this trial. In Hensgen et al. (2014), the different sources of the materials used and their different compositions did not influence the Biomethane potentials of their press waters significantly. Biomethane potential ranged from 312.1 to 405 ml/g
oTS. In the long run, the press cake ended up with a high fibre content [
27].
Richter et al. (2011) studied biomass materials with the aim of using press water from the material (herbage from a low land hay meadow) to generate biogas while using the resulting cake for combustion [
28]. The authors work is underscored by the challenges associated with the combustion of low-input-high diversity materials. Hydrothermal carbonisation treatments were conducted for three (3) out of six (6) herbage biomass materials obtained on six different dates. Levels of elements that are detrimental to combustion were reduced in the press cake but higher in the press waters [
28]. The Biomethane potential for the press waters without hydrothermal treatment ranged from 6.35 to 13.15 %
TS. Between 0.09 and 0.23 % of total solids (TS) contained in the silage were transferred into the press waters.
Sailer et al. (2022) highlights dewatering biogenic wood fuels in the form of wood chips through mechanical pressing to reduce moisture content: press waters are generated as by-products. As its oxygen demand is very high, biogas generation was examined as an option to utilise these waters [
24]. They researched into the anaerobic digestion potential of spruce-based and poplar-based press waters. Obtaining a Biomethane potential (BMP) of 160±12 ml/g
oTS for both inoculum and press water, as against 95±26 ml/g
oTS for only inoculum, the authors recommend that a further research could explore the potential of press water as a co-substrate [
24]. However, the challenge with press waters is that its characteristics and utilisation potentials are not significantly known [
24].
Like most biological wastes, managing slaughter waste presents a challenge in Africa, especially due to increasing urbanisation and population growth. It is critical to transition the waste management landscape to a sustainable circular economy. There is a potential in generating valuable energy from slaughter waste, and additionally remedy huge costs and associated environmental challenges with disposal systems [
29,
30,
31,
32,
33]. Wang et al. (2018) stress, that AD has been used as a viable technology for slaughter house waste: to generate biogas (energy) and reduce negative environmental impacts [
34]. This is evident in several studies highlighting biogas’ potential from slaughter waste: basic research, performance improvement, optimisation, application, process techniques advancement and a blend of any or all of these [
34,
35,
36,
37,
38,
39,
40,
41,
42] [
32,
43,
44,
45,
46,
47,
48,
49,
50,
51]. With a focus on anaerobic digestion, energy potential of a cattle-slaughter house was done in Ireland. The study found that there is a methane potential of 49.5 to 650.9 ml∙CH
4/g
oTS [
52]. Omoni et al. (2023) co-digested water hyacinth with ruminant waste from a slaughterhouse. They observed that the slaughter house waste enhanced the production, and yield of biogas from water hyacinth by 113% [
53].
Salehin et al. (2021) studied the potential of biogas generation from slaughter wastes in Dhaka. It finds that 7,915 tons of slaughter waste is generated per year in the city, with a biogas potential of 2.15 million m
3 [
35]. Samadi et al. (2021) studied the potential of biogas generation from slaughter waste of poultry, co-digested with vegetables and fruits, in order to produce optimal conditions for a biogas generation. In this study, the highest biogas yield occurred for a C/N ratio of 30. The study stresses that generating biogas from slaughter waste (with co-digestion) is more advantageous than the use of depositing or burning as a disposal method [
36]. Ware et al. (2016) obtained a BMP of 465-650 ml/g
oTS for offal of cattle, pig and poultry from slaughter houses, presenting an empirical pointer to a good biogas potential from slaughter house waste [
54]. Aklaku et al. (2006) assessed the performance of a small-scale biogas digester for a slaughterhouse in Ghana [
55]. This slaughterhouse produces waste from cattle, sheep and goat. The study found that the digester is able to deliver energy in a form of biogas to replace wood as fuels, while delivering by-products useful for fertilising land [
55].
These studies establish that slaughter waste has a huge biogas potential for the energy landscape in Africa, with different studies targeting improvement in yield. The high moisture content of such feedstock (slaughterhouse waste) could therefore be subjected to mechanical pressing as a pre-treatment method that aims at an overall performance improvement of a biogas digestion system, including protecting digester-pumping piping and systems.
This study aimed at assessing the potential of generating biogas from waste deposits generated at a typical abattoir (slaughterhouse) in Africa and evaluating the technical comparative performance of using absolute waste mixtures at slaughterhouses and pressing out liquid (water with solved and dissolved organics). The main objective of the study was to assess the technical potential (biogas yield) of rumen waste mixtures from cow, sheep and goat produced at the Sunyani Abattoir in Ghana. Additionally, the research sought to find out if it is technically prudent to press out water from these mixtures and use digester in the biogas generation process, in mind that the remnant (cake) will be considered in practical scenarios for other conversion processes or uses, such as composting.
2. Materials and Methods
The substrate for anaerobic digestion (AD) trials was collected from the Sunyani Abattoir (Asuakwaa slaughterhouse) in Ghana, located on latitude 7.34376° and longitude -2.29457° [
56]. The substrate was obtained as the key waste generated at the facility, as all types of offal are utilised, except partially digested rumen contents. They were obtained as cow rumen content in “pasty” form and a mixture of goat and sheep rumen in medium-liquid form. The current form of disposal is an irregular, but mostly weekly collection cycle with storage in a waste dump. This not only entails the challenge of prolonged and exposed storage in a trough container, along with associated costs and irregular pickups by the waste disposal company but also involves the release of emissions from methanogenic bacteria present in the waste.
After collection in Sunyani, the samples were weighed and dried at 105°C until constant weight. They were further sealed and kept airtight for further transportation to Germany for analysis. The samples were kept in an oven at 105°C for a day before the experimental trial started in Germany.
Figure 1 shows the current form of disposal in an open container, while in the background heaps of dried rumen content can be seen, that are dumped there, when the container limits are reached.
Figure 2 shows the weighing of rumen content at the Sunyani abattoir.
A subsequent resource assessment was carried out to evaluate the specific waste generation and composition at the facility. This involved counting each cow, goat and sheep slaughtered per day. This was repeated for three (3) days. For all three days, the masses of rumen content per cow, goat and sheep slaughtered were measured, each in duplicate. This was used for estimating cow, goat and sheep’s average rumen content production. The quantities of each animal slaughtered and the resulting rumen masses were used to obtain a relationship between the mixtures produced by the slaughterhouse. The obtained ratios were subsequently used to mix the received samples.
Table 2 summarises the masses obtained for the rumen contents, the quantities of cow, goat and sheep slaughtered at the facility and the expected annual outputs.
The Hohenheim University Biogas Laboratory has a 400 L laboratory reactor, designed and designated for standardised inoculum production. This facility cultivates bacteria continuously, under controlled and favourable conditions. This reactor is supplied with rapeseed oil, soybean meal, shredded wheat, maize sludge, and digestate from different biogas plants in Baden-Württemberg, southwest Germany. The inoculum production plant had an organic loading rate (OLR) of 0.3 kg
oTS/m
3d on dry organic matter basis and a temperature of 37°C. It had a hydraulic retention time (HRT) of 200 + 25 d. The inoculum was collected and transported in sealed and airtight containers, over a period of one (1) hour. There was constant degassing of the inoculum while transporting it. Its use was preceded with sieving using a mesh with a size of 0.5 mm, same as in the case of Hülsemann et al. (2020) [
57]. Characteristics of the inoculum are described by previous authors [
24,
57,
58,
59].
Analytical methods in this research were undertaken at the Central Laboratory of the Rottenburg University of Applied Forest Sciences. These methods followed the VDI 4630 [
59] and associated standards.
In determining the total solids, approximately 50 g of each sample was fetched into evaporating dishes and oven-dried at 105°C for >12 hours. The masses were checked after the drying period and further dried until consistent masses were achieved. The determination was carried out in four (4) replicates to ensure accuracy.
After transportation to Germany and repeated drying to ensure an anhydrous state, the oTS was determined according to ISO 21656 and VDI guidelines [
59]. The samples were milled with a sieve size of 1 mm, uniform particle size was achieved according to ISO 3310-1 [
59]. Empty crucibles were weighed and 1g of each sample was measured into the empty crucibles. The masses of the empty and filled crucibles were determined and recorded. They were kept in a muffle furnace and set to operate with defined heating ramps (defined by standard DIN EN ISO 18122:2016-03) [
60] up to a temperature of 550°C for about 24 hours. For each sample, measurements were done in four (4) replicates. The crucibles were weighed after cooling to room temperature in a desiccator. The ash contents were determined and the oTS calculated as the difference in masses between pre-ashing and post-ashing. The ash contents were expressed as a percentage of the total mass of dried samples used. These processes were repeated for inoculum obtained from HHU biogas laboratory, see
Table 3.
5 g of TS of each sample (cow solid, cow liquid, goat-sheep mixture and press cake) was measured and milled using a 0.25 mm mesh. Approximately 80 mg of each sample was measured for elemental determination in an elemental analyser (LECO CHN 828). The CHN proportions of these samples were determined according to VDI 4630. The oxygen fractions were determined as the difference between the total composition of all elements (100%) and the sum of the measured CHN organic fractions, as well as the ash content, neglecting the fraction of sulphur.
Table 3 shows the different fractions of C, H, N and O present in each type of substrate.
Inductively coupled plasma-optical emission spectroscopy (ICP-OES) was used to evaluate the study samples’ concentration of trace and minor elements. The Aqua Regia treatment in accordance with ISO 11885 and DIN 22022-2 were followed in this procedure [
24,
61]. Each sample was analysed with n=4 or n=3 repetitions; except for press cake, which was done with n=2 due to limitation of sample quantity. In the case of press water, the sample was diluted in a ratio of 1:10 using double distilled water. 1 mL of nitric acid (HNO
3) was added to acidify each sample. A SPECTROBLUE system (SPECTRO Analytical Instruments GmbH) was used to measure the trace element concentration of these samples.
Sample preparation for calorimetry was done in accordance with ISO 14780 [
62]. About 1 g of each dry sample was pressed to a tablet size (ISO 1834:03) [
62]. Those tablets were burned in a bomb calorimeter (IKA C6000 ISOPERIBOL) to measure the higher heating values (HHVs) of the samples: in accordance with DIN EN14918 [
60]. The set-up had a bath, ensuring a fixed heat transfer between the bomb and the water. Double distilled water was used to collect the liquid resulting from the calorimetry and rinsing the bomb. Chloride and sulphate anions were determined using ion chromatography (with Metrohm 883 Basic IC plus).
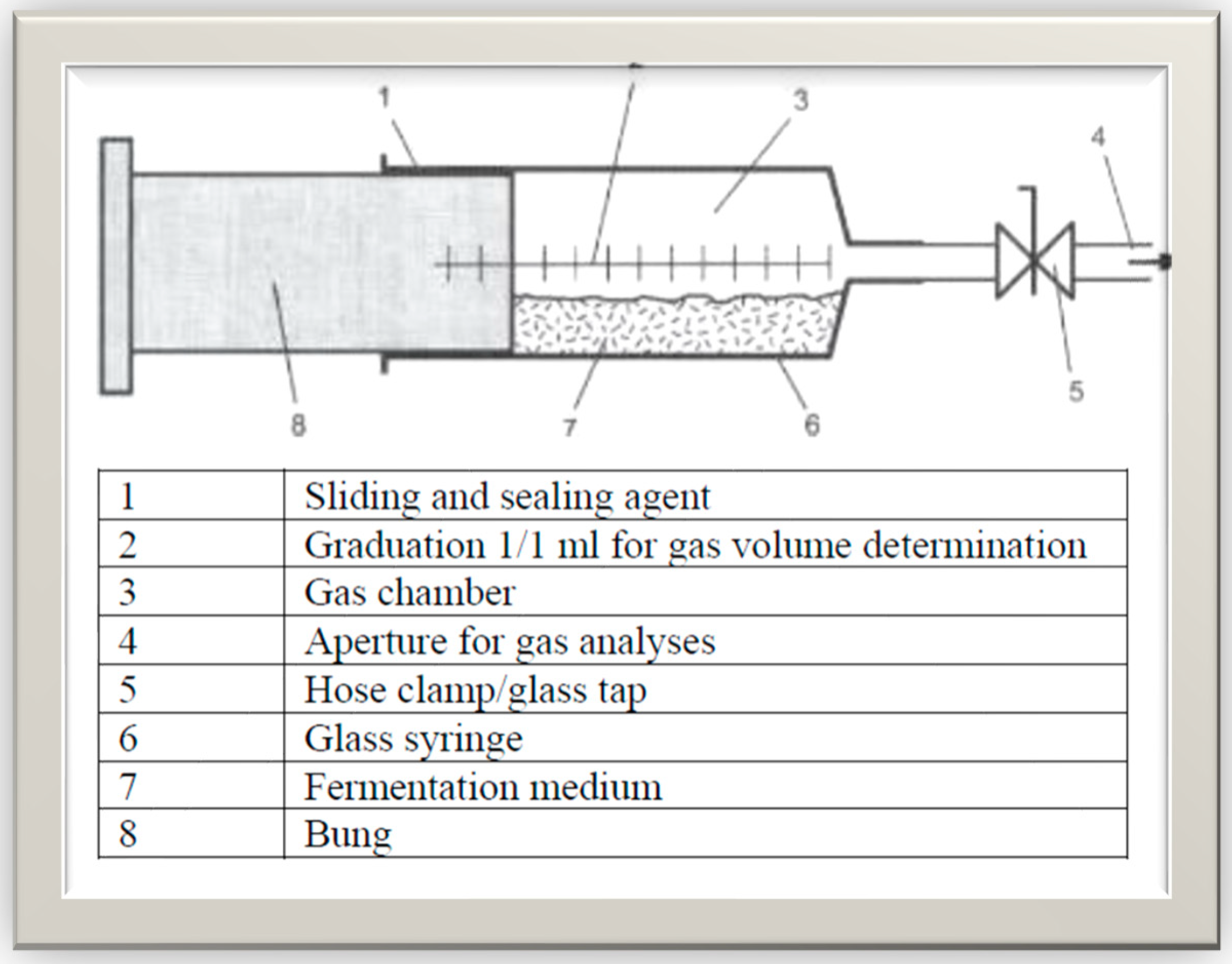
The Hohenheim Biogas Yield Test (D-HBT) was used for the Biomethane potential trials. This set-∙up consisted of sixteen (16) 100 mL syringes. In the D-HBT set-up, gas volume measurements were done manually. Due to the absence of a rotating drum, the substrates were regularly stirred by turning syringes in clockwise and anti-clockwise directions. The tests were performed as according guidelines and procedures specified by VDI 4630 [
59].
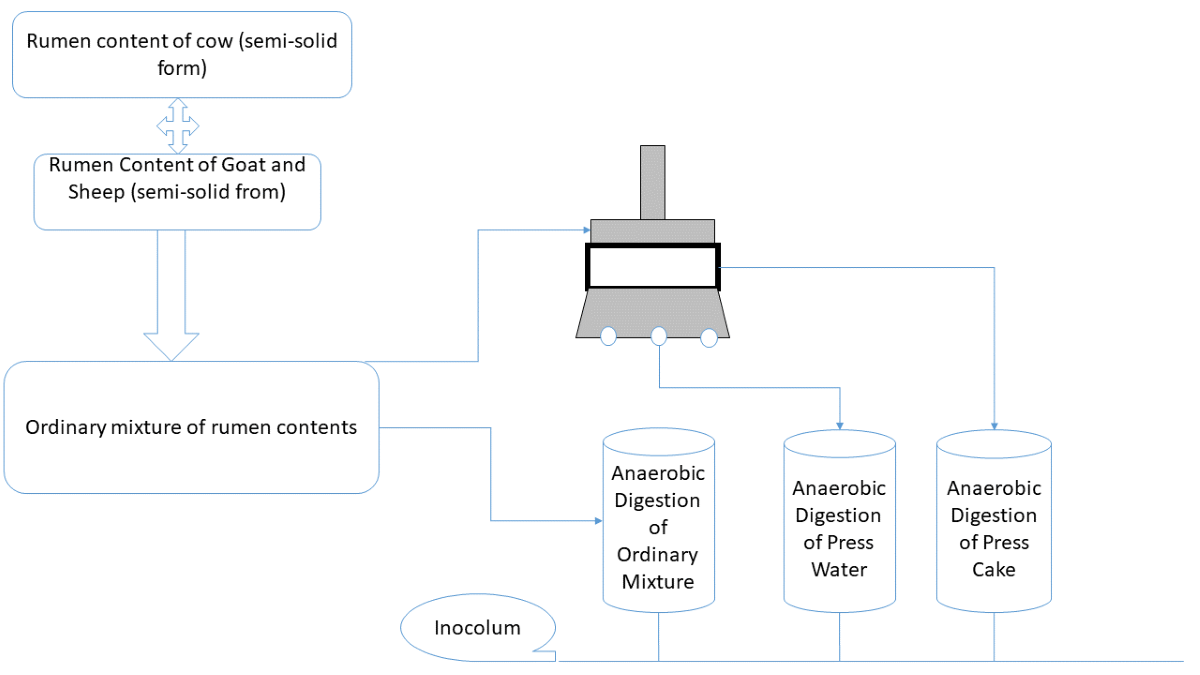
The inoculum obtained from Hohenheim University Biogas Laboratory was used together with two different forms of waste portions prepared from effluent/feedstock obtained from the Sunyani Abattoir. These resulting forms are a dried mixture of cow rumen content, goat rumen content and sheep rumen content (CGS). This mixture was based on a scientifically determined ratio, see chapter 2.1.1.1 and Press Water (PW) obtained from a solution of the aforementioned mixture.
RC-CGS-M (mixture of rumen contents of cow, goat and sheep from the Sunyani Abattoir) on a dry basis was formed using the deduced production ratio. on dry basis to two different dried samples: Rumen Content of Cow-Liquid form (RCCL) and Rumen Content of Goat, Sheep-Mixture-Liquid form
| Variant |
Sample ID |
Description |
| 1 |
RCCL |
Rumen Content of Cow – Liquid form |
| 2 |
RC-GSL |
Rumen Content of Goat, Sheep – Mixture-Liquid form |
| 3 |
RCGL |
Rumen content of goat – Liquid form |
| 4 |
RCSL |
Rumen content of sheep – Liquid form |
| Variant I |
RC-CGS-M |
A dried mixture of cow rumen content, goat-sheep rumen content |
| Variant II |
PC-CGS-M |
The solid residue obtained from Variant I after mechanical pressing |
| Variant III |
PW-CGS-M |
The liquid extract obtained from Variant I after mechanical pressing |
| Variant description 1: Description of different samples and their identities. |
(RC-GSL). The wet equivalences of dry masses of RCCL and RC-GSL (RCGL + RCSL) available for the experimental trial were measured and mixed in the established ratio. The mixing was effectively done with the use of a magnetic stirrer.
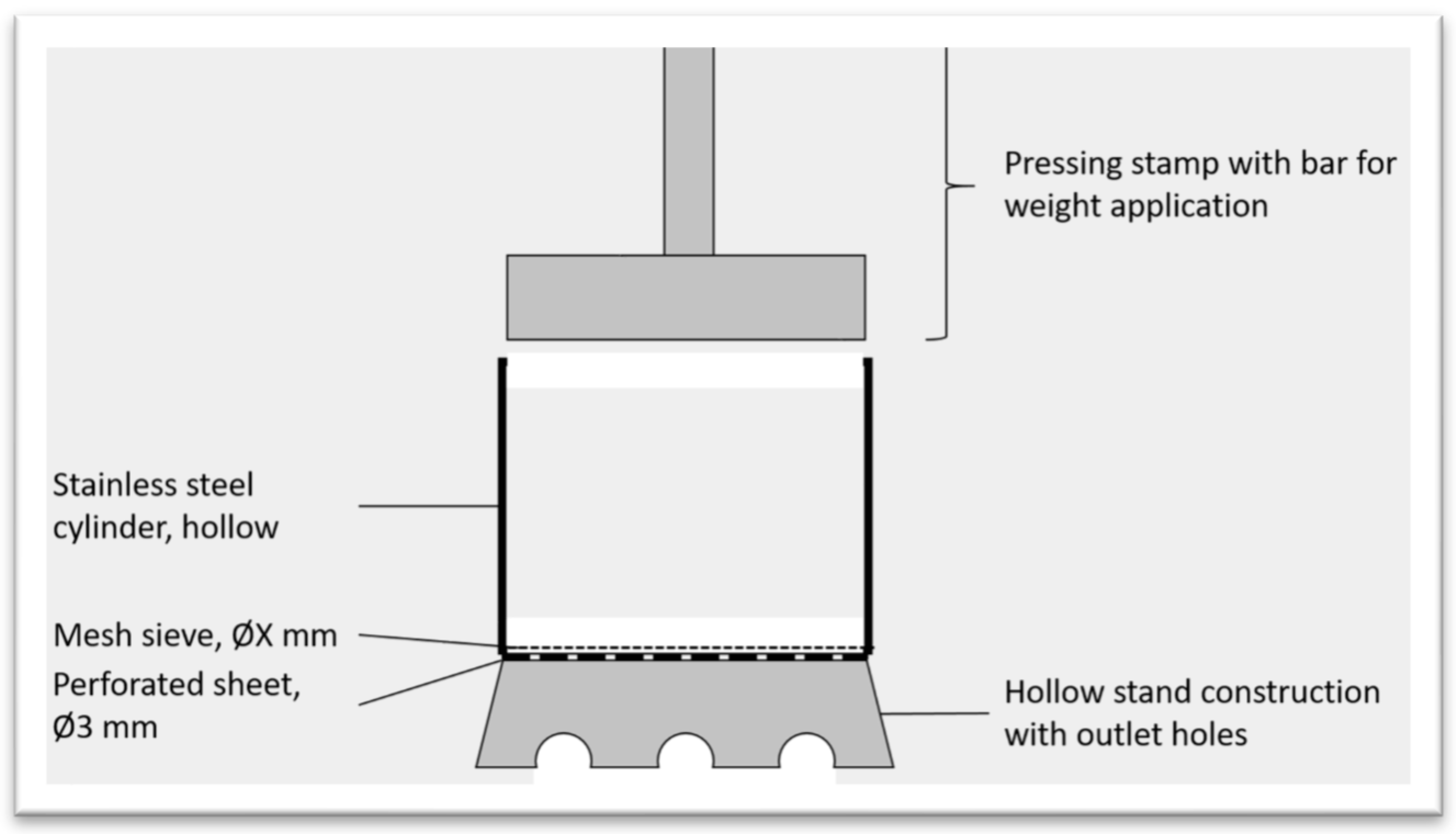
The preparation of PW-CGS involved two key intermediate steps: Step I and Step II. The activities of Step I were repeated; except for relevant masses, which were used on dry matter basis. The equivalent relevant water was calculated and added to the mixture. With the help of a magnetic stirrer, it was stirred to mimic the initial forms. The rehumidified samples were then stored overnight. The Step II involved the design of a mechanical press to squeeze out the liquid available in the substrate. An appropriate mechanical press was designed and fabricated at the HFR’s mechanical laboratory. An appropriate weight was derived from an easily commercially available press. Thus, a pressure of 24.1 kg/cm
2 should act on the press surface, reinforced with a fine sieve, of 52.81 cm², induced by a pressing weight of 200 kg.
Figure 3 is the design of the mechanical press while.
Figure 4 is a picture of the fabricated mechanical press.
After preparing the wet mixture; sample RC-CGS-M was pressed. The pressed out water (liquid) was collected, weighed and labelled as press water (PW-CGS-M).
After pressing in step II, the remnant cake was weighed and labelled as press cake (PC-CGS-M). The structure and set-up of the HBT is available in literature [
24,
57,
59]. The experimental procedure was carried out according to standards in VDI 4630 [
2,
3].
Figure 5 is a schematic of the HBT digester design used. All determinations were done in four replicates. The four (4) replications for each substrate was in accordance with VDI 4630 [
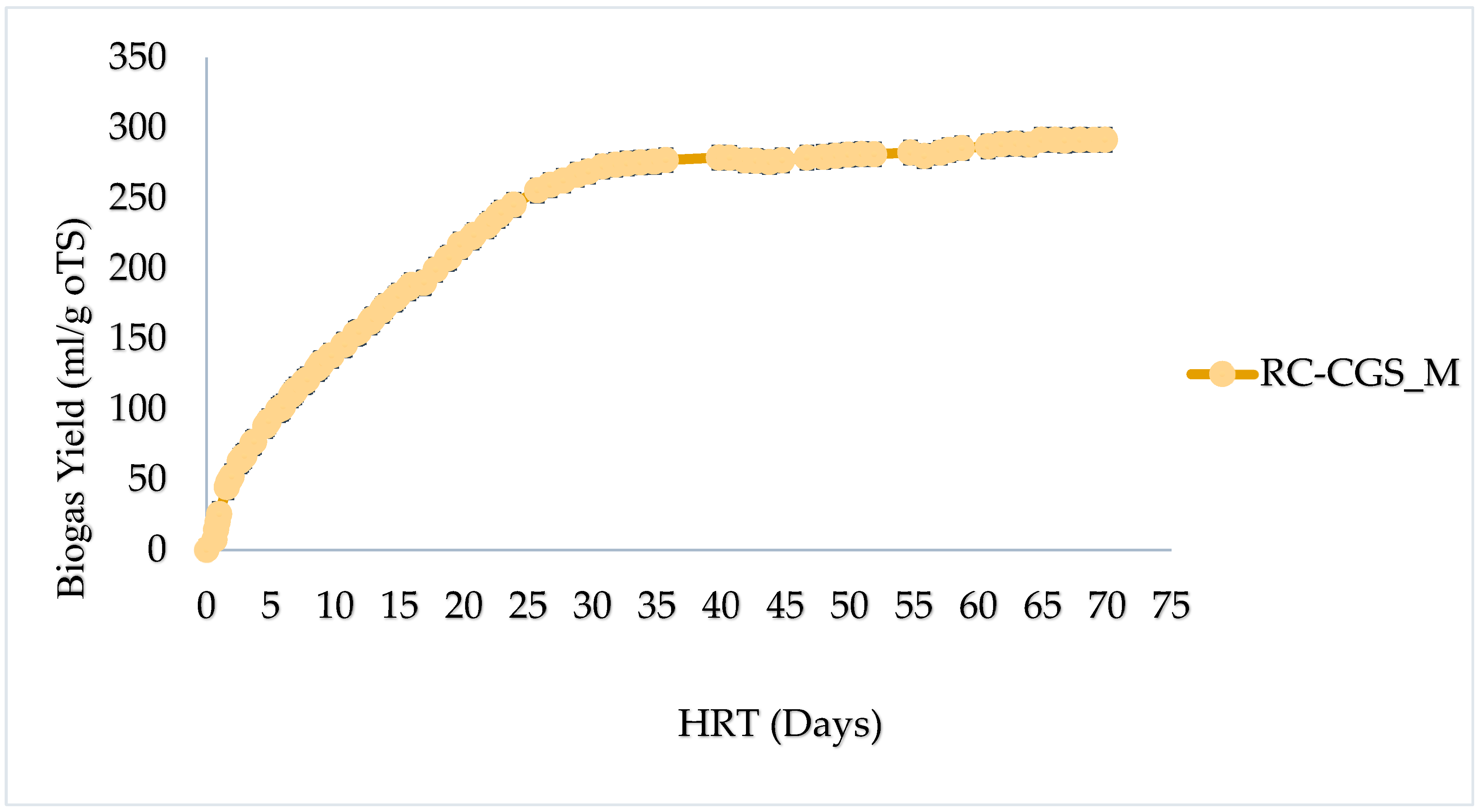
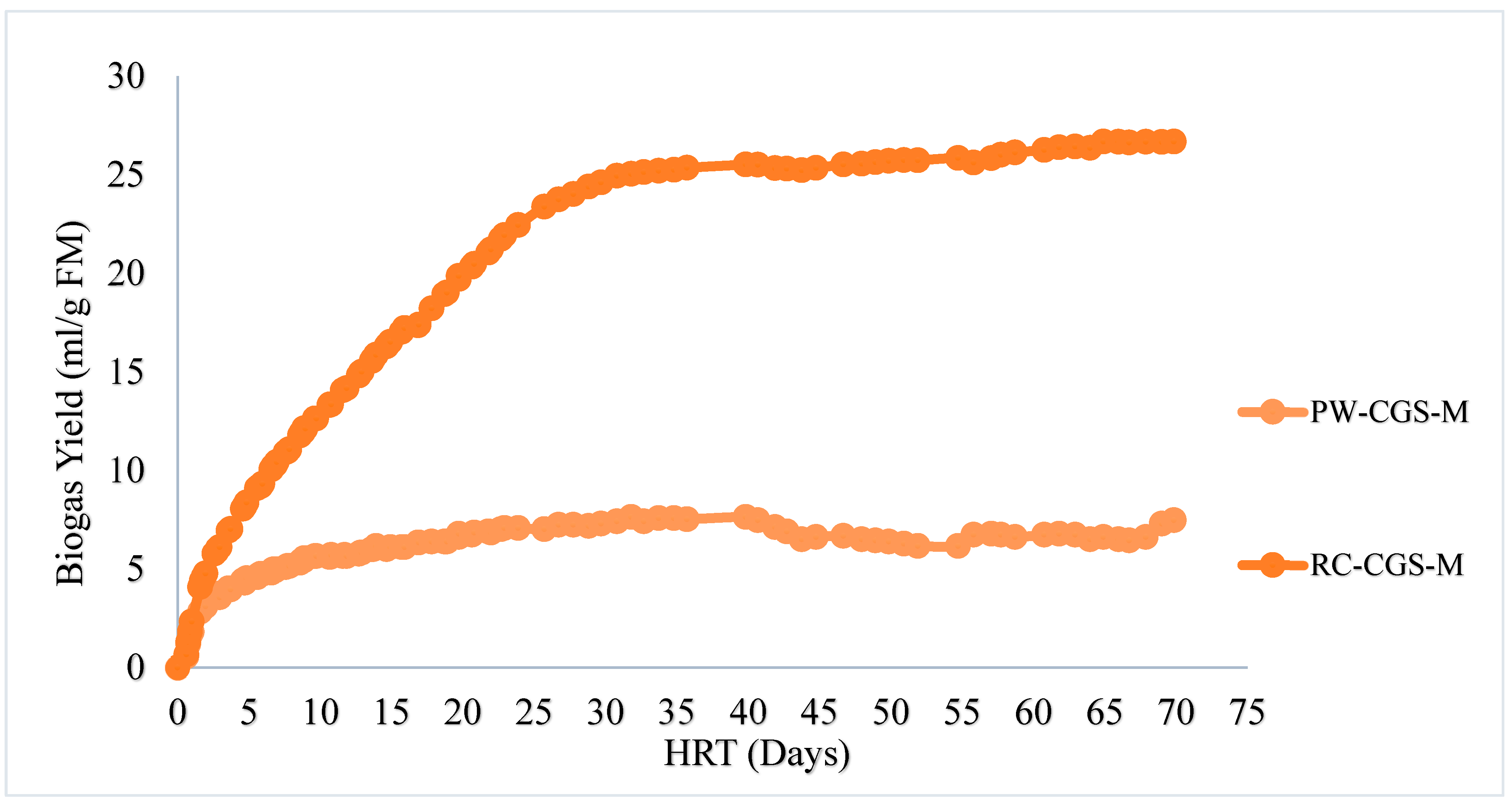
2], ensuring measuring accuracy. Each digester contained 30 mL of inoculum and X g of TS of sample, in a manner that it will achieve an oTS ratio of less than 0.5. For PW-CGS-M, X g of 2.21 g FM was used and for RC-CGS-M, X g of 0.49 g TS was used. One set-up, however, contained only inoculum to serve as control. For all three set-ups (Inoculum, RC-CGS-M and PW-CGS-M), their starting volumes were manually read and recorded. The set-up was left for a 70-day period at a mesophilic temperature of 37±0.5℃. Volume levels of each substrate and/or inoculum was read over the period. The differences in volume between two measurements was estimated as the gas generation in ml over that period (under the specified operating conditions). The experimental systems were degassed in turns at appropriate times (when volumes of content of syringes were reasonably high, to prevent uncontrolled leakage). In the initial stages of the trial (about 8 days), readings were taken averagely for three (3) to four (4) times a day. Measurements were restricted to once a day in the last weeks of the trial. Considering the 0.5% criteria (with an increase in gas production of less than 0.5% per day for three days), the digestion process was terminated.
The biogas and specific methane yields were evaluated for both biogas yield and the estimated methane fractions (determined according to VDI 4630). Using first principle, the biogas (methane) potentials of the different variants were determined from the mass of waste generation and the biomethane potentials determined in this study[
64] [
65]. The total waste generation per week was first transformed to the effective mass usable in biogas generation for each variant. That is, the mass of PW-CGS-M obtained after pressing and that obtained without pressing for RC-CGS-M variant.
Table 1 presents the factors used to transform the waste generation for each variant. The potentials in terms of cubic meters of methane were converted to kWh of energy using equations 1 and 2.
Theoretical models were adopted to determine the theoretical maximum biomethane potentials (TMBMP) of the studied samples, in quest to validate the obtained results. The Boyle’s model, dependent on elemental composition was used in the case of RC-CGS-M and dependent on chemical oxygen demand (COD) measurement in the case of PW-CGS-M [
66]. The equations used in the estimation is available in Rodrigues et al. (2016) [
66].
A dilute solution of the press water was obtained by applying a ratio of 1:30 using distilled water. 2 mL of the dilute solution was fetched into a test tube containing Test 0-29 Nanocolor CSB 1500 (COD/DCO/DQ0) (ISO 15705). The mixture was shaken thoroughly and placed in a Nanocolor Vario C2 device to heat. for two (2) hours. Afterwards, the COD was measured in a photometrically. The measured COD is presented in
Table 3.
Table 1.
Fraction of waste generation extractible for biogas generation using different variants.
Table 1.
Fraction of waste generation extractible for biogas generation using different variants.
| Variant |
Fraction of waste extractible for biogas generation (%) |
| RC-CGS-M |
100 |
| PW-CGS-M |
53 |
Equation 1: Conversion factor for cubic meters to MJ
X 1 m
3 of methane = 34 MJ of energy [
67]
Equation 2: Conversion factor for MJ to kWh
4. Conclusions
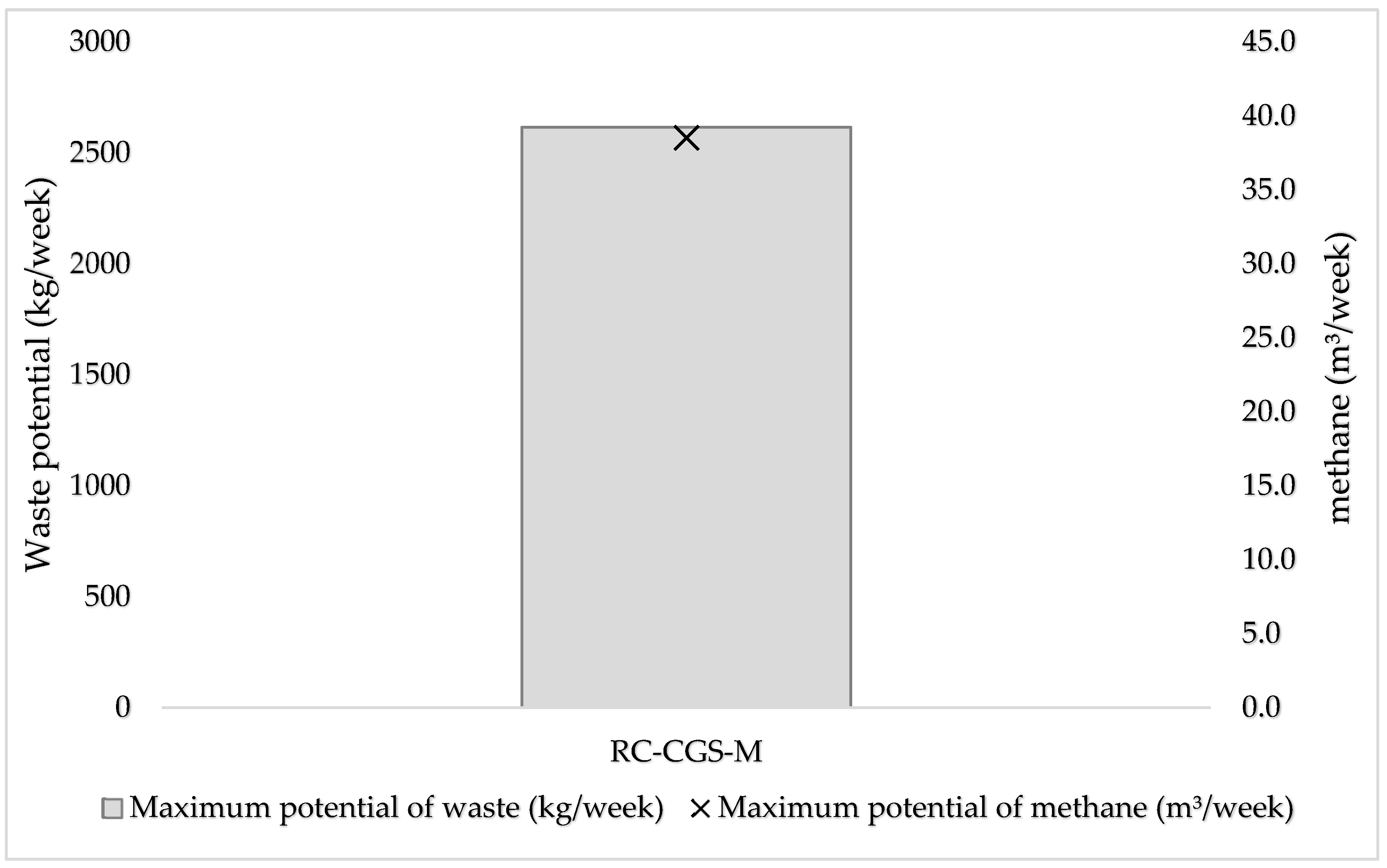
The study evaluates the comparative performance of biogas generation from the raw mixture of slaughter waste and the use of its mechanically separated press water that could potentially enable additional use cases in the sense of a bioeconomy. It finds that the facility has a waste generation potential of 2,615 kg per week, which is sourced from rumen contents of cattle, goats and sheep, slaughtered at the facility for 6 out of 7 days in a week. The biogas yield from this slaughter waste in ordinarily existing mixture is 291.60± 28.78 ml/g oTS or 26.70± 28.78 ml/g FM. The Biomethane potential (BMP) of this material is: 160.4±28.78 ml/goTS for its ordinary mixture. For the press water, the biogas yield is 7.47±1.12∙ml/g FM. It has a TMBMP of 20.39 ml/g oTS. In terms of nominal biogas volumes, the potential of the waste generated per week is 38.7 m3 , which is equivalent to 364 kWh: in the event of using the resource in its semi-solid form without pressing. The study concludes that although the use of pressing is technically viable, it is comparatively not as resourceful as the use of its ordinary mixture.
In any case, it is necessary to assess for each process chain why only press water is available for biogas generation. The high oxygen demand necessitates post-treatment of the PW, wherein anaerobic conversion can play a significant role. Furthermore, co-digestion with other materials, which are richer in surface area and carbon, can deliver substantially better results and thus energy output. However, the general energy output loss due to the separation of the two phases should always be compared with the desired additional benefits of this additional process chain. For instance, alternative use of the fibres in the context of bioeconomy or facilitated pumpability of the biogas substrate through addition of liquid/press water, as it is considerable in the presented setting without co-digestion.
Notwithstanding, with additional benefits such a reduced deterioration of pump and hydraulic systems, the approach is technically viable. It is recommended that the feedstock (slaughterhouse waste) is co-digested with more fibrous materials (in West-African context e.g. cassava, yam, or plantain waste) as that could enhance the C/N ratio and effectively increase the biogas generation output. HHVs of 17.1-17.5 MJ/kg were obtained for the studied samples. These are higher than 14.7 MJ/kg reported in literature for cow dung, probably since rumen contents might have lost methane (energy carrier) in the event of digestion before being released as faecal waste (cow dung). It can be inferred that the use of rumen content for biogas generation will be more valuable per TS amount that for animal faeces.