Submitted:
26 September 2024
Posted:
29 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Peptide Synthesis and Characterization
2.3. General Fluorescence Measurements
2.4. Fluorescence Response of FY7 Peptide for Hg2+ Ions. Metal selectivity and Cross-Reactivity Studies
2.5. Characterization of the Peptide-Metal Ion Complexation
2.6. Low-Volume Fluorescence Measurements
3. Results and Discussion
3.1. FY7 Peptide Synthesis and Characterization
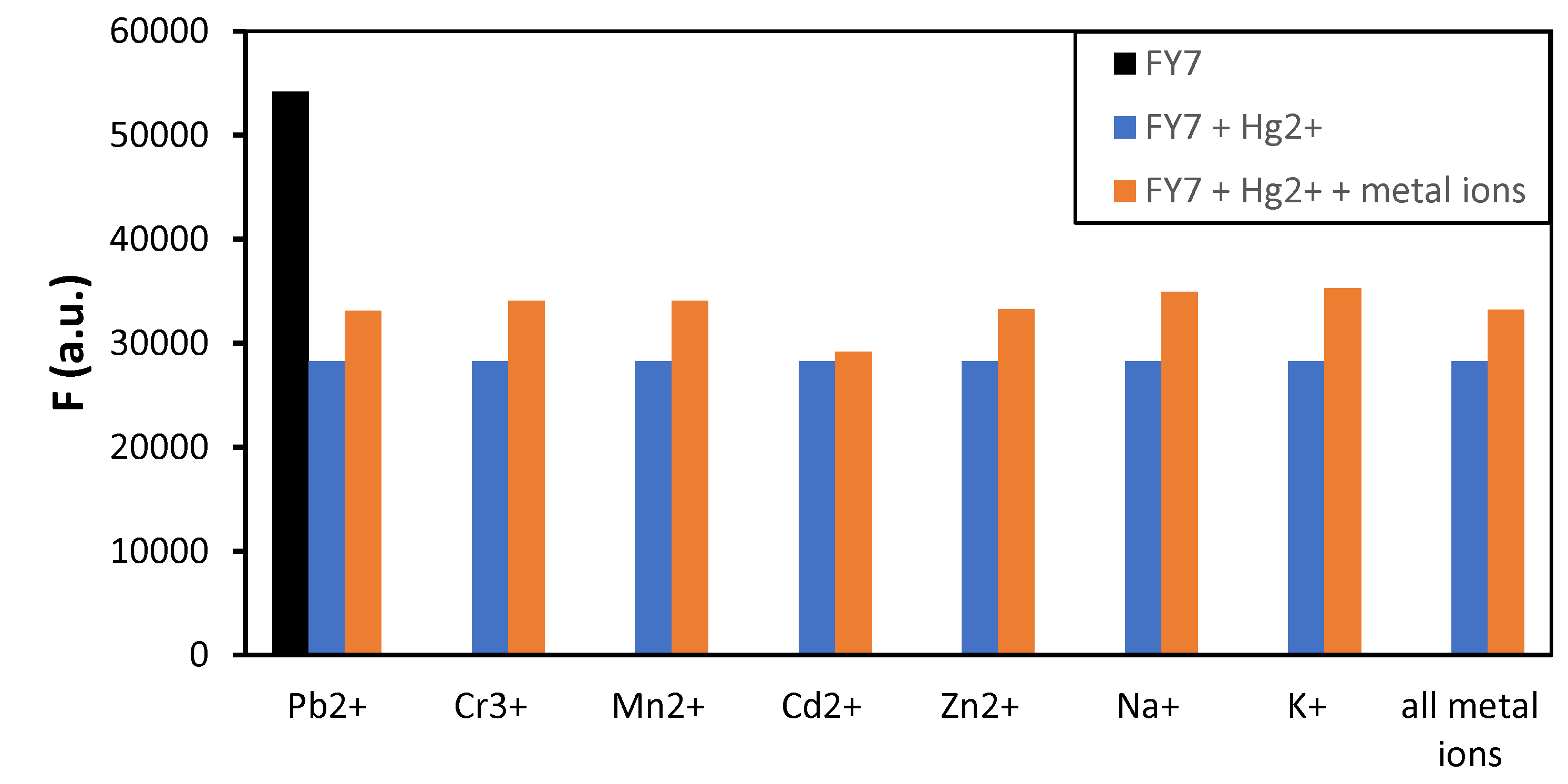
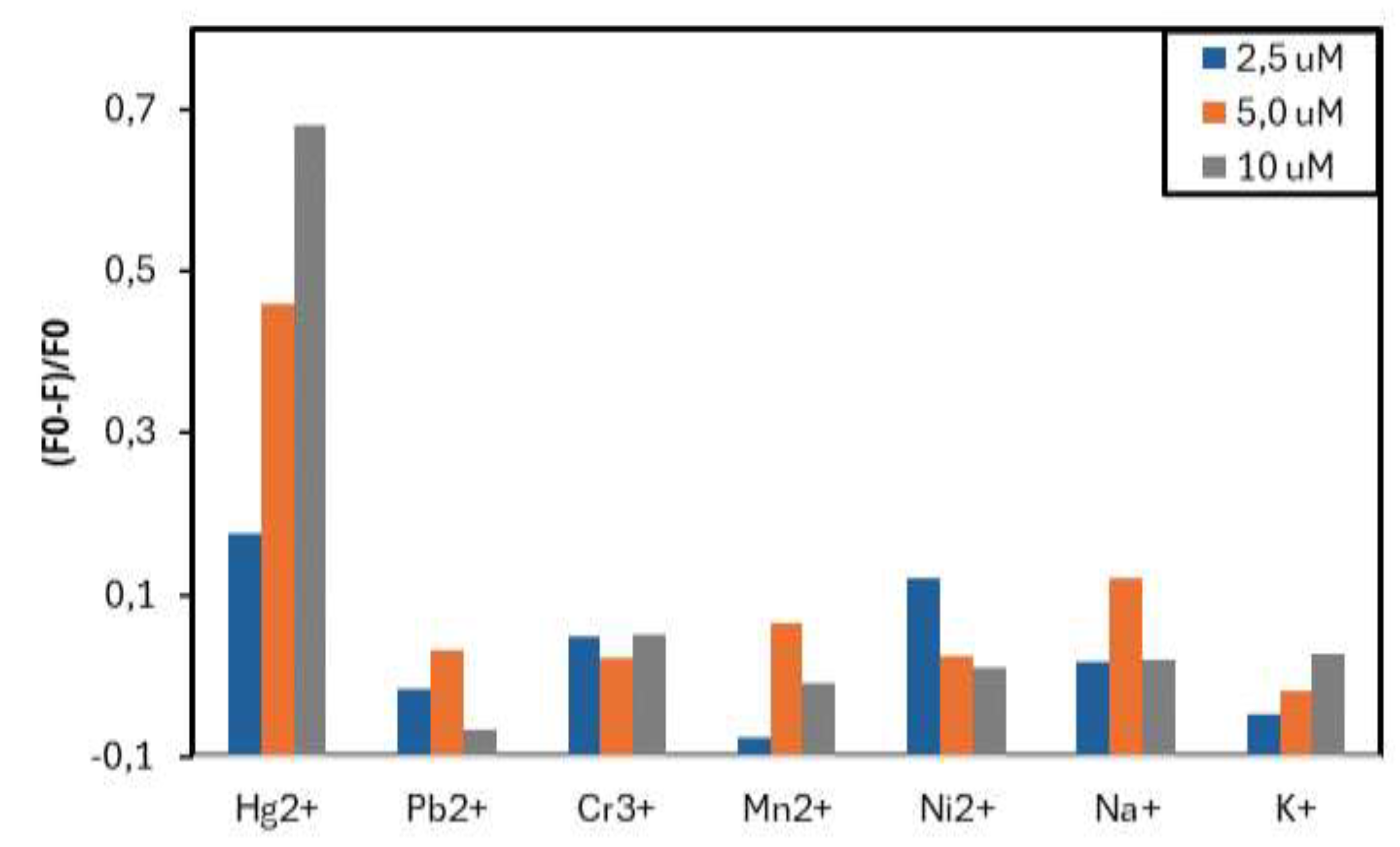
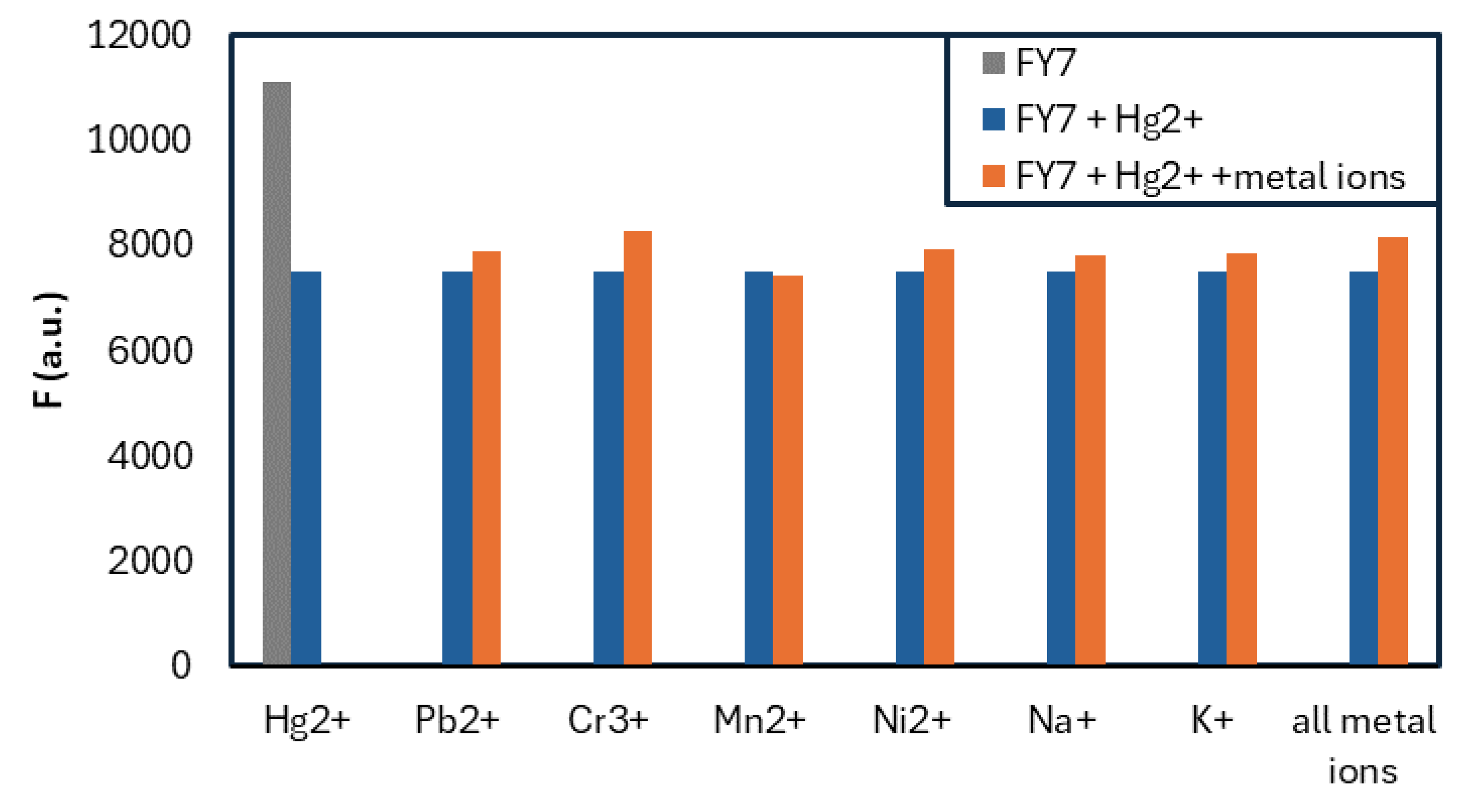
3.2. FY7 Peptide Metal Selectivity and Cross-Reactivity Studies
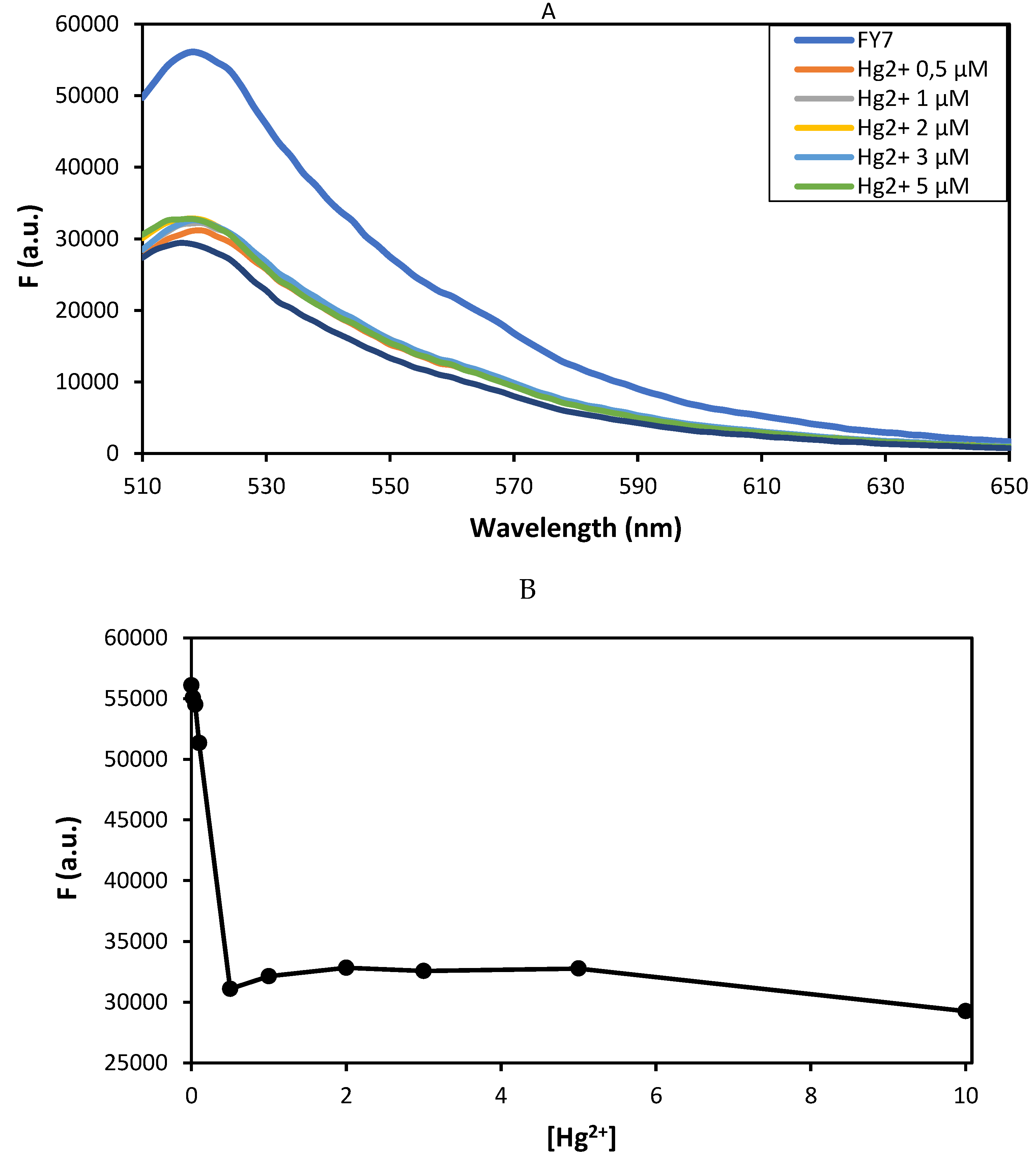
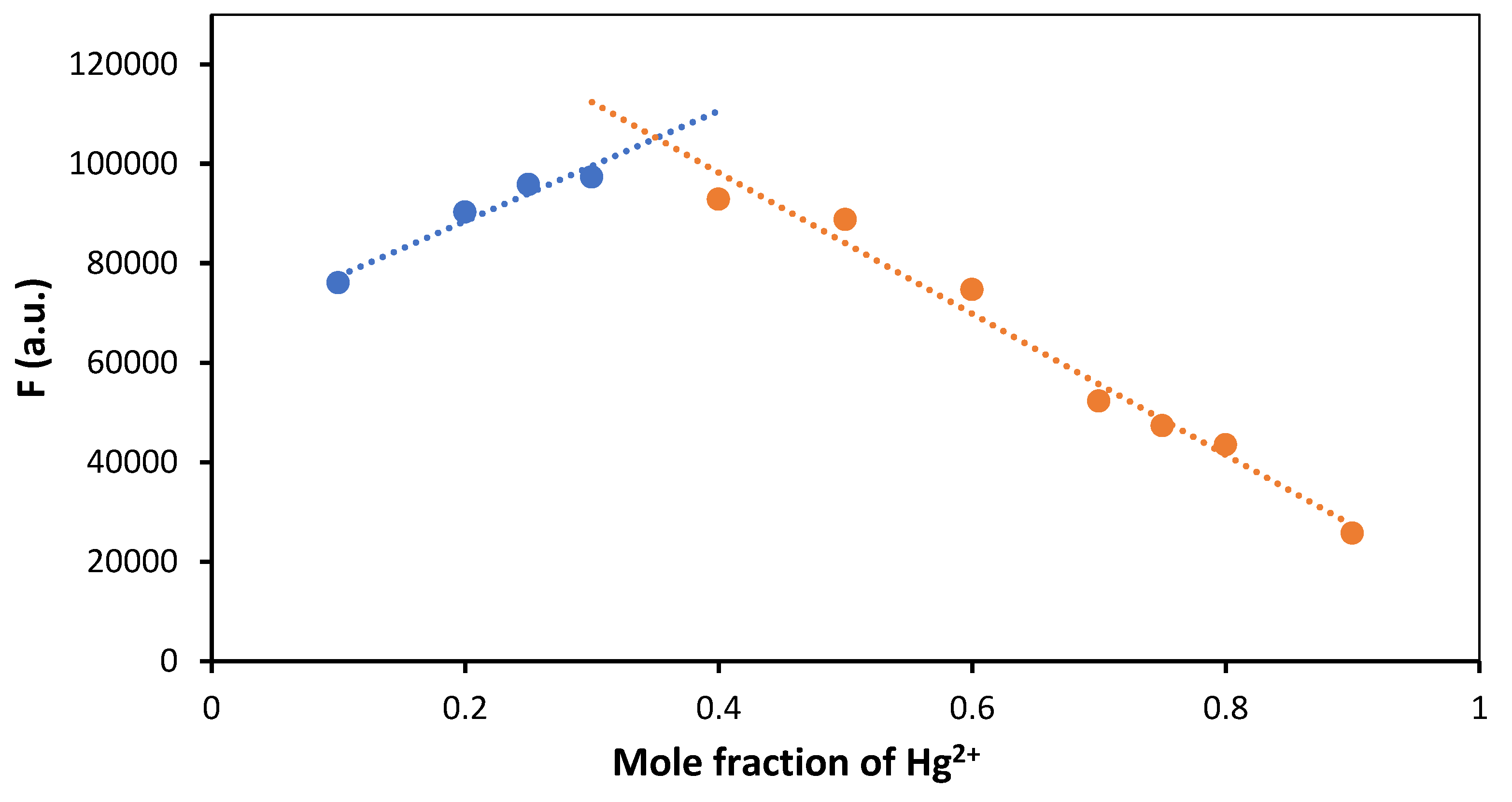
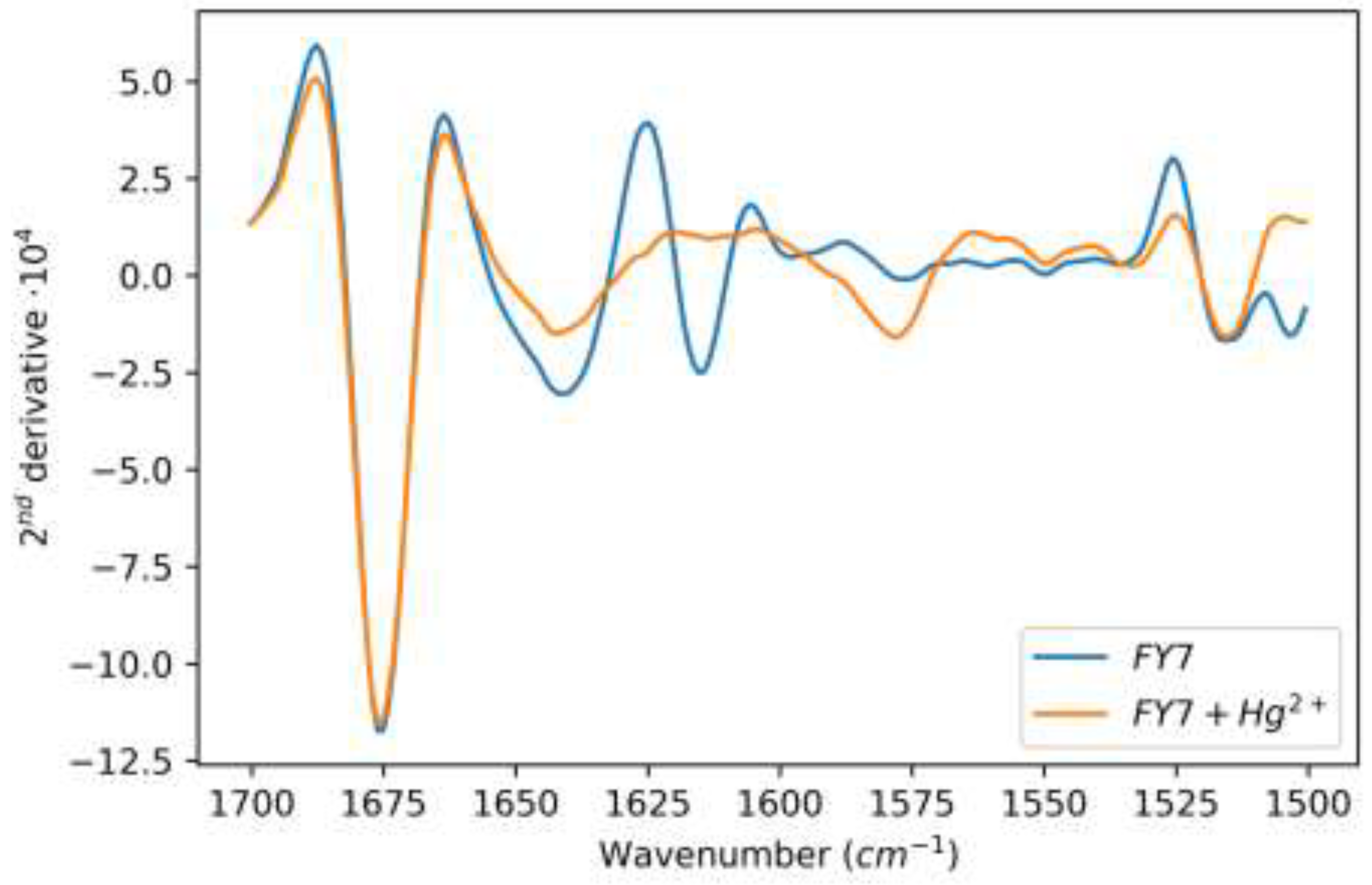
3.3. Molecular Characterisation of FY7 and Hg2+ Ions Interation
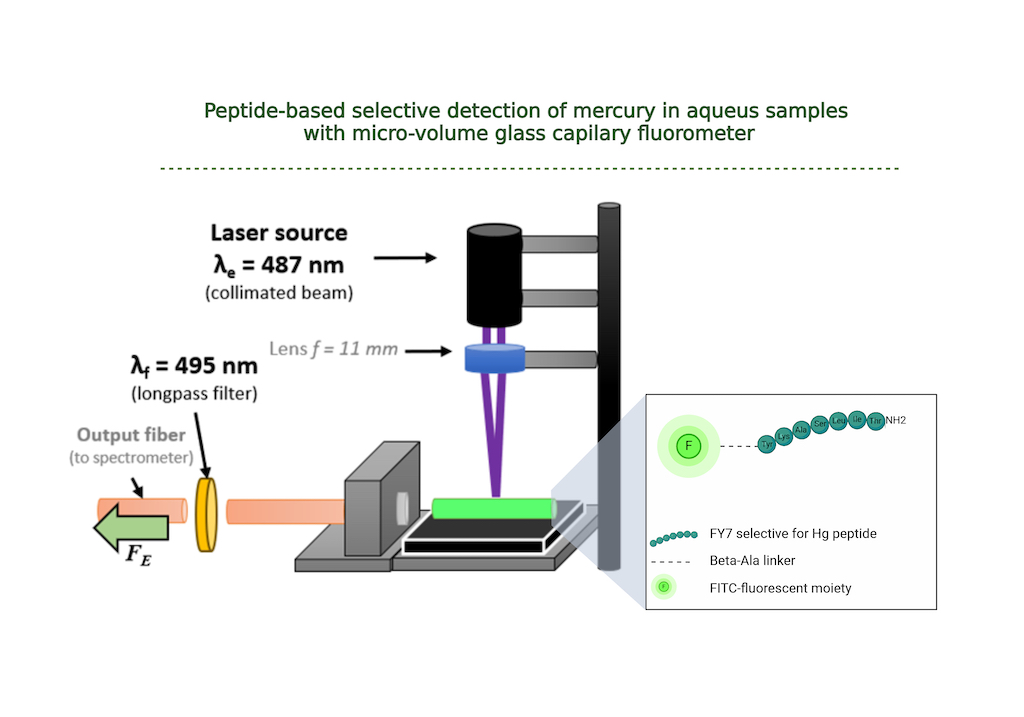
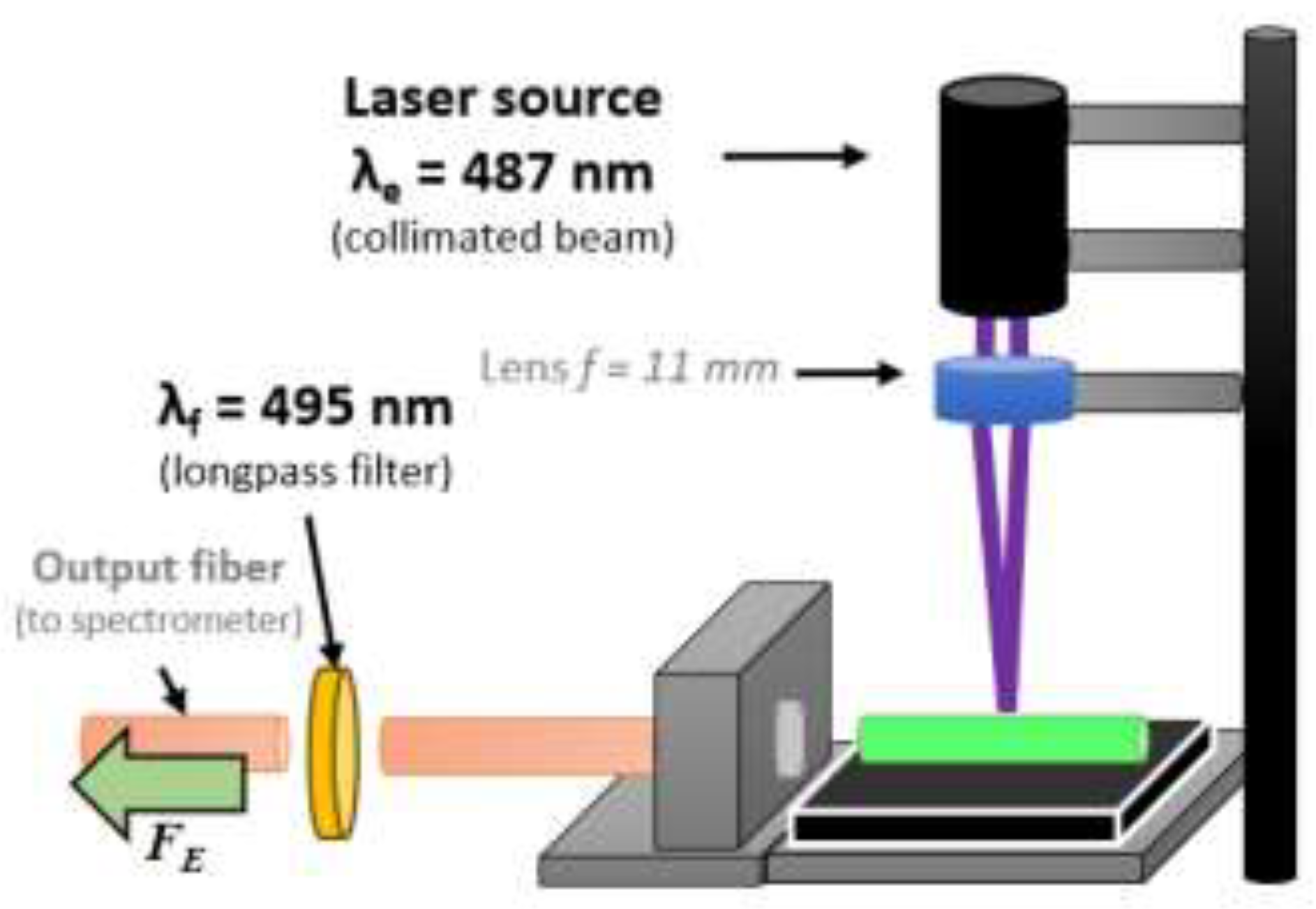
3.4. Low-Volume Miniaturized Optical Nano-Biosensor for the Rapid, Sensitive, and Selective Detection of Toxic Mercury in Aqueous Samples based on FY7 Peptide
4. Conclusions
5. Patents
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Song, S.; Li, Y.; Liu, Q.S.; Wang, H.; Li, P.; Shi, J.; Hu, L.; Zhang, H.; Liu, Y.; Li, K.; Zhao, X.; Cai, Z. Interaction of mercury ion (Hg2+) with blood and cytotoxicity attenuation by serum albumin binding. J. Hazard. Mater. 2021, 412, 125158. [Google Scholar] [CrossRef] [PubMed]
- Park, J.M.; Smith, J.C. Modeling mercury in proteins. Methods Enzymol. 2016, 578, 103–122. [Google Scholar] [CrossRef]
- Bjørklund, C.; Dadar, M.; Mutter, J.; Aaseth, J. The toxicology of mercury: Current research and emerging trends. Environ. Res. 2017, 159, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Streets, D.G.; Horowitz, H.M.; . Jacob, D.J.; Lu, Z.; Levin, L.; Schure, A.F.; Sunderland, E.M. Total Mercury Released to the Environment by Human Activities. Environ. Sci. Technol. 2017, 51, 5969–5977. [Google Scholar] [CrossRef]
- Ajsuvakova, O.P; Tinkov, A.A.; Aschner, M.; Rocha, J.B.T.; Michalke, B.; Skalnaya, M.G.; Skalny, A.V.; Butnariu, M.; Dadar, M.; Sarac, I.; Aaseth, J.; Bjørklund, G. Sulfhydryl groups as targets of mercury toxicity. Coord. Chem. Rev. 2020, 417, 213343. [Google Scholar] [CrossRef] [PubMed]
- Rupa, S.A.; Patwary, M.A.M.; Matin, M.M.; Ghann, W.E.; Uddin, J.; Kazi, M. Interaction of mercury species with proteins: towards possible mechanism of mercurial toxicology. Toxicol. Res. (Camb) 2023, 12, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, T.W.; Magos, L.; Myers, G.J. The toxicology of mercury-current exposures and clinical manifestations. N. Engl. J. Med. 2003, 349, 1731–1737. [Google Scholar] [CrossRef]
- Khan, F.; Momtaz, S.; Abdollahi, M. The relationship between mercury exposure and epigenetic alterations regarding human health, risk assessment and diagnostic strategies. J. Trace Elem. Med. Biol. 2019, 52, 37–47. [Google Scholar] [CrossRef]
- Flores-Cáceres, M.L.; Ortega-Villasante, C.; Carril, P.; Sobrino-Plata, J.; Hernández, L.E. The Early Oxidative Stress Induced by Mercury and Cadmium Is Modulated by Ethylene in Medicago sativa Seedlings. Antioxidants 2023, 12, 551. [Google Scholar] [CrossRef]
- Ciacci, C.; Betti, M.; Abramovich, S.; Cavaliere, M.; Frontalini, F. Mercury-Induced Oxidative Stress Response in Benthic Foraminifera: An In Vivo Experiment on Amphistegina lessonii. Biology (Basel) 2022, 11, 960. [Google Scholar] [CrossRef]
- Pyatha, S.; Kim, H.; Lee, D.; Kim, K. Association between Heavy Metal Exposure and Parkinson’s Disease: A Review of the Mechanisms Related to Oxidative Stress. Antioxidants (Basel) 2022, 11, 2467. [Google Scholar] [CrossRef]
- Doroszkiewicz, J.; Farhan, J.A.; Mroczko, J.; Winkel, I.; Perkowski, M.; Mroczko, B. Common and Trace Metals in Alzheimer’s and Parkinson’s Diseases. Int. J. Mol. Sci. 2023, 24, 15721. [Google Scholar] [CrossRef] [PubMed]
- Wallin, C.; Friedemann, M.; Sholts, S.B.; Noormägi, A.; Svantesson, T.; Jarvet, J.; Roos, P.M.; Palumaa, P.; Gräslund, A.; Wärmländer, S.K.T.S. ; Mercury and Alzheimer’s Disease: Hg(II) Ions Display Specific Binding to the Amyloid-β Peptide and Hinder Its Fibrillization. Biomolecules 2019, 44. [Google Scholar] [CrossRef] [PubMed]
- Kaur, B.; Gupta, A.; Kaur, N. A novel, anthracene-based naked eye probe for detecting Hg2+ ions in aqueous as well as solid state media. Microchem. J. 2020, 153, 104508. [Google Scholar] [CrossRef]
- Naija,A. ; Yalcin, H.C.; Evaluation of cadmium and mercury on cardiovascular and neurological systems: Effects on humans and fish. Toxicol. Rep. 2023, 10, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Chirumbolo, S.; Dadar, M.; Pivina, L.; Lindh, U.; Butnariu, M. J. Aaseth. Mercury exposure and its effects on fertility and pregnancy outcome. Basic Clin. Pharmacol. Toxicol. 2019, 125, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sharma, A.; Sedha, S. Occupational and environmental mercury exposure and human reproductive health - a review. J. Turk. Ger. Gynecol. Assoc. 2022, 23, 199–210. [Google Scholar] [CrossRef]
- Erxleben, H.; Ruzicka, J. Atomic Absorption Spectroscopy for Mercury, Automated by Sequential Injection and Miniaturized in Lab-on-Valve System. Anal. Chem. 2005, 77, 5124–5128. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Lessmann, F.; Harth, V. A method for reliable quantification of mercury in occupational and environmental medical urine samples by inductively coupled plasma mass spectrometry. Anal. Methods 2023, 15, 2030–2038. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhu, Z.; Zhen, H.; Jin, L.; Hu, S. Significant signal enhancement of dielectric barrier discharge plasma induced vapor generation by using non-ionic surfactants for determination of mercury and cadmium by atomic fluorescence spectrometry. J. Anal. At. Spectrom. 2016, 31, 383–389. [Google Scholar] [CrossRef]
- Komatsu, T.; Johnsson, K.; Okuno, H.; Bito, H.; Inoue, T.; Nagano, T.; Urano, Y. Real-Time Measurements of Protein Dynamics Using Fluorescence Activation-Coupled Protein Labeling Method. J. Am. Chem. Soc. 2011, 133, 17–6745. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Shi, Q.; Song,N. ;, Xiao, Y.;, Wang, L.; Chen, Z.; James, T.D. Current trends in the detection and removal of heavy metal ions using functional materials. Chem. Soc. Rev. 2023, 52, 5827–5860. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.K.; Jeon, J.; Ryu, K.; Park, S-H. ; Lee, K-H. Ratiometric fluorescent detection of lead ions in aquatic environment and living cells using a fluorescent peptide-based probe. J. Hazard. Mater. 2022, 427, 128161. [Google Scholar] [CrossRef]
- Gou, Y.; Hou, P.; Wang, O.; He, F.; Wang, P.; Yang, X. A novel AIE peptide-based fluorescent probe for highly selective detection of mercury(II) ions and its application in food samples and cell imaging. Microchem. J. 2023, 195, 109400. [Google Scholar] [CrossRef]
- Pang, X.; Gao, L.; Feng, H.; Li, X.; Kong, J.; Li, L. A peptide-based multifunctional fluorescent probe for Cu2+, Hg2+ and biothiols. New J. Chem. 2018, 42, 15770–15777. [Google Scholar] [CrossRef]
- Yu, H.; Ryu, K.; Park, J.; Subedi, S.; Lee, K-H. Design and synthesis of fluorescent peptide-based probes with aggregation-induced emission characteristic for detecting CH3Hg+ and Hg2+ in aqueous environment: Tuning fluorescent detection for CH3Hg+ by replacing peptide receptors. Dyes Pigm. 2022, 204, 110461. [Google Scholar] [CrossRef]
- Merrifield, R.B. Solid Phase Peptide Synthesis. I. The Synthesis of a Tetrapeptide. J. Am. Chem. Soc. 1963, 85(14), 2149–2154. [Google Scholar] [CrossRef]
- Fields, G. B.; Noble, R. L. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Intern. J Pep. and Prot. Res. 1990, 35, 161–214. [Google Scholar] [CrossRef] [PubMed]
- Brelje, T.C.; Wessendorf, M.W.; Sorenson, R. L. Multicolor Laser Scanning Confocal Immunofluorescence Microscopy: Practical Application and Limitations Methods. Cell Biol. 2002, 70, 70,165–249. [Google Scholar] [CrossRef]
- Hermanson, G.T. Fluorescent Probes. In: Bioconjugate Techniques, Third Edition, Academic Press, New York, 2013, 395-463. [CrossRef]
- Zhou, H.; Gao, T.; Liu, Y.; Wu, Y.; Fang, Y.; Wang, B.; Xu, B. Targeted fluorescent imaging of a novel FITC-labeled PSMA ligand in prostate cancer. Amino Acids 2022, 54, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Li, Y-S. ; Ju, X.; Gao, X-F.; Zhao, Y-Y.; Wu, Y-F. Immobilization enzyme fluorescence capillary analysis for determination of lactic acid. Anal. Chim. Acta 2008, 610, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y-S. ; Li, O-J.; Gao, X-F. A novel immobilization fluorescence capillary analysis method and its applications. Analyst 2020, 145, 1980–1996. [Google Scholar] [CrossRef] [PubMed]
- Pituła, E.; Janik, M.; Sikora, J.; Kasztelanic, R.; Stępniewski, G.; Gong, Y.; Olszewski, M.; Buczyński, B.; Koba, M.; Śmietana, M. Glass capillary systems for micro-volume fluorometry. Measurement 2025, 240, 15569. [Google Scholar] [CrossRef]
- Bruździak, P. Vapor correction of FTIR spectra – A simple automatic least squares approach. Acta A Mol. Biomol. Spectrosc. 2019, 223, 117373. [Google Scholar] [CrossRef]
- Yang, T.; . Zhang, X-Y. ; Zhang, X-X.; Chen, M-L.; Wang, J-H. Chromium(III) Binding Phage Screening for the Selective Adsorption of Cr(III) and Chromium Speciation. ACS Appl. Mater. Interfaces. 2015, 7, 21287–21294. [Google Scholar] [CrossRef]
- Jullian, M.; Hernandez, A.; Maurras, A.; Puget, K.; Amblard, M.; Martinez, J.; Subra, G. N-terminus FITC labeling of peptides on solid support: the truth behind the spacer. Tetrahedron Lett. 2009, 50, 260–263. [Google Scholar] [CrossRef]
- Kuroda, D.G.; Vorobyev, D.Y.; Hochstrasser, R.M. Ultrafast relaxation and 2D IR of the aqueous trifluorocarboxylate ion. J. Chem. Phys. 2010, 132, 044501. [Google Scholar] [CrossRef]
- Lotze, S.; Bakker, H. J. Structure and dynamics of a salt-bridge model system in water and DMSO. J. Chem. Phys. 2015, 142, 212436. [Google Scholar] [CrossRef]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef]
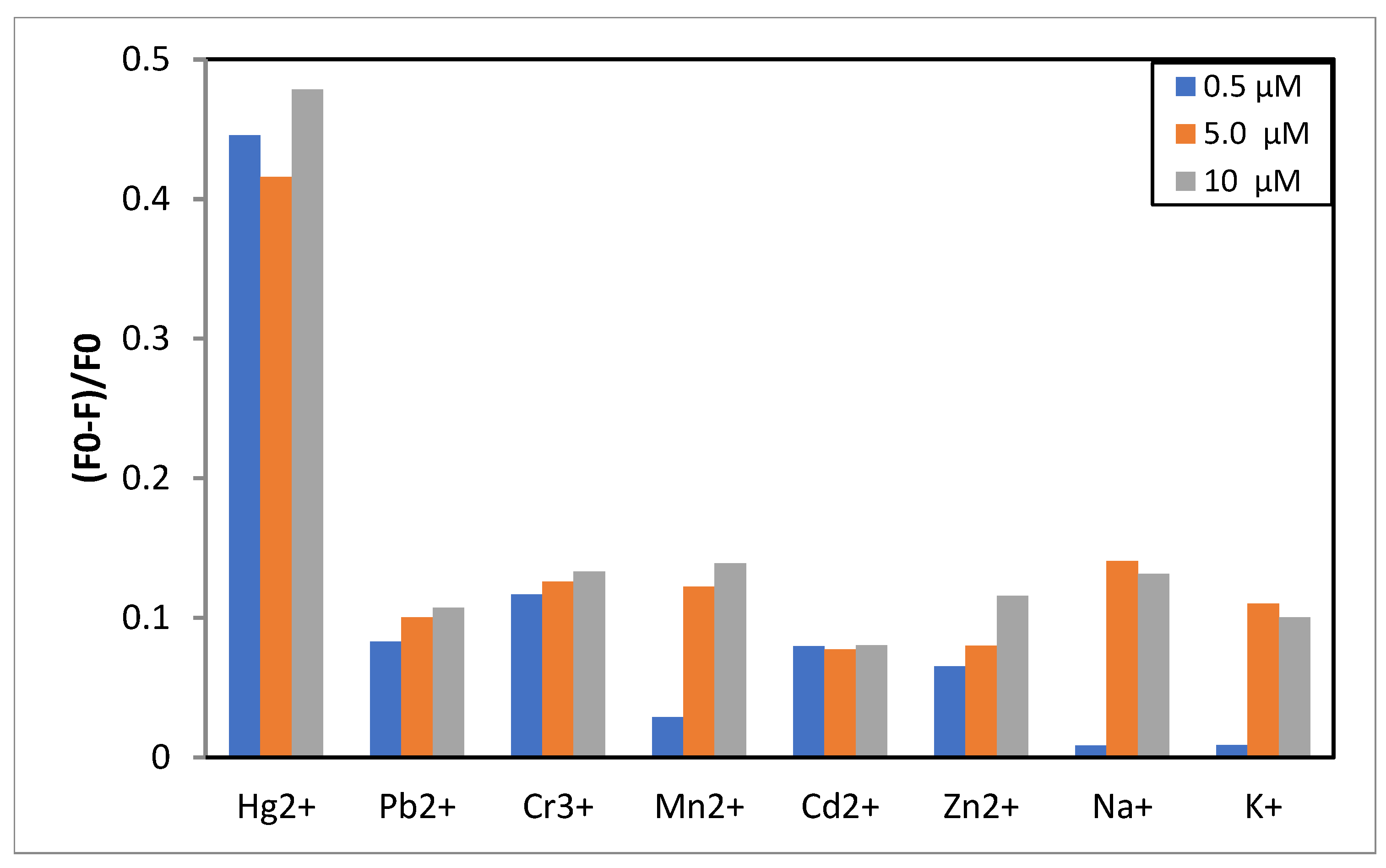
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




