1. Introduction
Copper is one of the harmful heavy metals among the various pollutants. It is discharged into wastewater streams on a daily basis from a variety of industries, including electroplating, paints and dyes, petroleum refining, fertilizers, mining and metallurgy, explosives, pesticides, and steel. Epidemiological studies have identified a correlation between copper mining activities and a variety of health conditions, including cancer, cirrhosis, kidney failure, and headaches, in individuals residing in close proximity to copper mining areas [
1]. Copper is a critical trace element that is essential for the proper functioning of numerous enzymes, including cytochrome c oxidase, superoxide dismutase, and tyrosinase. Critical processes such as energy production, antioxidant defense, and melanin synthesis are facilitated by these enzymes. Despite its necessity, maintaining copper homeostasis is crucial. An overabundance of Cu²⁺ ions can result in toxicity and contribute to the development of a variety of diseases. The toxicity of copper arises from its ability to engage in redox reactions, leading to the generation of reactive oxygen species. (ROS) [
2]. These ROS have the potential to induce oxidative stress, which can result in the destruction of cellular components, including DNA, proteins, and lipids. This oxidative damage is linked to a variety of pathological conditions, such as cardiovascular diseases, liver disorders such as Wilson's disease, and neurodegenerative diseases like Alzheimer's and Parkinson's [
3,
4,
5]. It is essential to provide a comprehensive overview of remediation techniques for various contamination scenarios, as the concentration of copper in wastewater has been reported to range from approximately 2.5 mg/L to 10000 mg/L. Consequently, it is crucial to create wastewater removal technologies that are eco-friendly, sustainable, and relatively inexpensive. Recent years have seen a significant amount of research on a variety of methods for the removal of heavy metals from wastewater. It is advisable to establish methods for the selective and sensitive detection of Cu
2+ ions in aqueous media and living cells, as the entire food chain is harmed by the excess of Cu
2+ ions, and the significance of Cu
2+ in biological activity is significant.
Numerous detection methods for the sensing of copper ions have been established to date. Inductively coupled plasma, atomic absorption spectroscopy, and various electrochemical instruments have been employed. Nonetheless, they require sample pretreatment and costly apparatus, and the samples may be compromised during the analytical procedure [
6]. Furthermore, the methods are inapplicable to living organisms for bioimaging purposes. Conversely, chemosensors have garnered significant attention due to their potential to address these deficiencies. It is user-friendly, economical, and responsive. Chemosensors utilizing fluorescence can be employed for real-time bioimaging of diverse analytes.
Fluorescence detection assays represent one of the most straightforward and convenient methodologies among various detection techniques, offering simplicity, sensitivity, and versatility for the identification of a broad spectrum of analytes. Recent advancements in nanoscale science and engineering have produced nanomaterials exhibiting distinctive optical properties. In the last ten years, various fluorescent nanoprobes have been created for the highly sensitive and selective detection and imaging of metal ions, both in vitro and in vivo [
7,
8]. Organic dyes, including rhodamine, fluorescein, and cyanine, are the predominant fluorescent probes; however, their low absorption coefficients and weak signals diminish the sensitivity and responsiveness to metal ions. Moreover, the majority of organic fluorophores experience irreversible photobleaching under extended illumination, rendering them unsuitable for continuous observation and long-term imaging in living cell studies. The aforementioned limitations have prompted the creation of alternative fluorescent probes for metal ions characterized by enhanced luminescence, superior photostability, and improved biocompatibility, facilitated by advancements in nanoscale science and engineering. The dansyl fluorophore is extensively utilized in the modification of amino acids, protein sequencing, and amino analysis due to its elevated fluorescence quantum yields and substantial Stokes shift [
9,
10].
Peptide-based fluorescent chemosensor for metal ions detection have many advantages compared with other small organic molecules [
11,
12]. The remarkable specificity and sensitivity of biodegradable peptides as ligands for detecting heavy metals in aqueous systems have garnered significant attention. Peptides contain a range of potential donor atoms and the complexes formed exist in a variety of conformations. Therefore, short sequences of amino acids can be engineered to take advantage of peptides' high affinity for binding particular target molecules, like heavy metal ions. This significantly improves their potential for the development of sensors, particularly in the fields of environmental monitoring and water quality assessment. One method that makes the ability to precisely identify unique amino acid sequences (peptides) for the detection of heavy metal ions is phage display technology. The phage display requires the use of phage libraries, which consist of diverse phages displaying distinct peptides or proteins on their surfaces. Phages with a strong affinity for a compound can be isolated via an affinity selection process. Determining the peptides presented on these specific phages can be accomplished by sequencing the gene that encodes the peptide. The efficacy of phage display is beneficial for the toxicological evaluation of chemical substances by utilizing it as a screening mechanism for identifying the most important targets. Phage display selection technology has been employed to identify specific peptides that selectively bind to cancer cells, proteins, nanoparticles, and metal ions, including Cd(II), Ni(II), Co(II), Pb(II), Cu(II), and Cr(III), since its initial development. [
13,
14,
15,
16,
17,
18]
.
It is widely known that peptides and proteins with the N-terminal motifs such as NH
2-Xxx-His (XH) and NH
2-Xxx-Zzz-His (XZH) form well-established Cu(II) complexes [
19,
20,
21,
22]. Thus, these motifs have been utilized to obtain peptides with specific functions. One of the most straightforward methods for adding a high affinity Cu(II) binding site to virtually any peptide or protein is to incorporate XH or XZH into the structure through chemical or recombinant methods [
23].
The objective of this research was to identify specific amino acid sequences through phage display technology that can be employed in the development of an ultra-selective and sensitive fluorescent chemosensor for Cu2+ detection. The results indicate that the identified peptide sequence containing the N-terminal Xxx-His motif exhibited a high selectivity toward Cu2+ ions. Moreover, when selected sequence (P-11) was modified with the dansyl fluorophore, at the N-terminus and KW amino acids at the C-terminus, the ability to detect copper(II) ions with a high sensitivity and selectivity remained intact.
2. Materials and Methods
2.1. Materials
20, 200 µM and 10 mM metal salt solutions of Cadmium chloride, Copper(II) chloride, Nickel(II) chloride, Mercury(II) chloride, Manganese(II) chloride were prepared with deionized water; 0.05% TBST buffer [50 mM Tris-HCl, 150 mM NaCl, 0.05% TWEEN 20 (v/v) pH 7.4]; glycine buffer for elution was composed of 0.2 M Glycine-HCl, pH 2.2. 10 mM Nα,Nα-Bis(carboxymethyl)-L-lysine hydrate (BCML) in deionized water (#14580, Sigma Aldrich). Maleic Anhydride Activated Plates (#15100, Thermo Scientific™).
Fmoc-Rink Amide AM Resin (0.7 mmol/g) was gained from Iris Biotech GmbH. Fmoc protected amino acids, Fmoc-Met-OH, Fmoc-Ile-OH, Fmoc-Val-OH, Fmoc-Pro-OH, Fmoc-Glu(OtBu)-OH, Fmoc-Lys(Boc)-OH and Fmoc-Trp(Boc)-OH were purchased from CSBio (Shanghai) Ltd.). Fmoc-His(Boc)-OH was from CEM Corporation. 5-Dimethylaminonaphthalene-1-sulfonyl chloride (DNS), Oxyma pure, N,N'-Diisopropylcarbodiimide (DIC), Triethylamine (TEA), Piperidine, 99%, extra pure, Triisopropylsilane (TIS), 4-(2-Hydroxyethyl)piperazine-1-ethanesulfonic acid (HEPES), Pb(NO3)2 and MnCl2 were obtained from Sigma-Aldrich. N,N-Dimethylformamide (DMF), 99.8% and Trifluoroacetic Acid (TFA) for synthesis, Chromium standard solution, Cadmium standard solution, Hg(NO3)2, CuSO4, ZnSO4, NaCl and KCl were gained from VWR International, LLC. Ethyl dieter was collected from POCH S.A.
D2O, 99.8 % was purchased from Thermo Scientific Chemicals. The Acetonitrile HPLC gradient grade, Acetonitrile hypergrade for LC-MS LiChrosolv®, water (LC-MS LiChrosolv®), trifluoracetic acid for the HPLC and formic acid (FA) (LC-MS LiChropur™) were purchased from Sigma-Aldrich. Doubly distilled water (Hydrolab-Reference purified) with conductivity not exceeding 0.05 µS/cm was used. All other chemicals used were of analytical reagent grade unless otherwise noted
2.2. Phage Library and Bacterial Strain
The Ph.D.™-7 Phage Display Peptide Library Kit was acquired from NEB (New England Biolabs GmbH, Frankfurt am Main, Germany). The host strain E. coli K12 ER 2738 (NEB) was cultivated overnight at 37°C in LB medium supplemented with 20 μg/mL tetracycline (stock solution 20 mg/mL in a 1:1 water/ethanol mixture, preserved at -20°C) and subsequently stored in 100 μL aliquots in PCR tubes at -80°C until required. The strain was preserved throughout the ongoing experiments on LB agar plates (10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl, 15 g/L agar with 20 μg/mL tetracycline).
2.3. Immobilization of Copper Ions on Plates
One hundred microliters of 10 mM Nα,Nα-Bis(carboxymethyl)-L-lysine hydrate (Sigma Aldrich Cat# 14580) in 0.1 M NaPO4, pH 8, were dispensed into each well of a maleic anhydride-activated microtiter plate (Thermo Scientific) and incubated overnight at ambient temperature. The plate was subsequently rinsed thrice with 300 µL of 0.05% TBST buffer. The plate was obstructed by incubation with 3% BSA in 0.05% TBST buffer for 2 hours at the room temperature. The plate was subsequently rinsed thrice with 300 µL of 0.05% TBST buffer. The plate was subsequently incubated with 10 mM metal salts for 20 minutes at the room temperature. Modified plates were used for biopanning.
2.4. Surface Panning Procedure
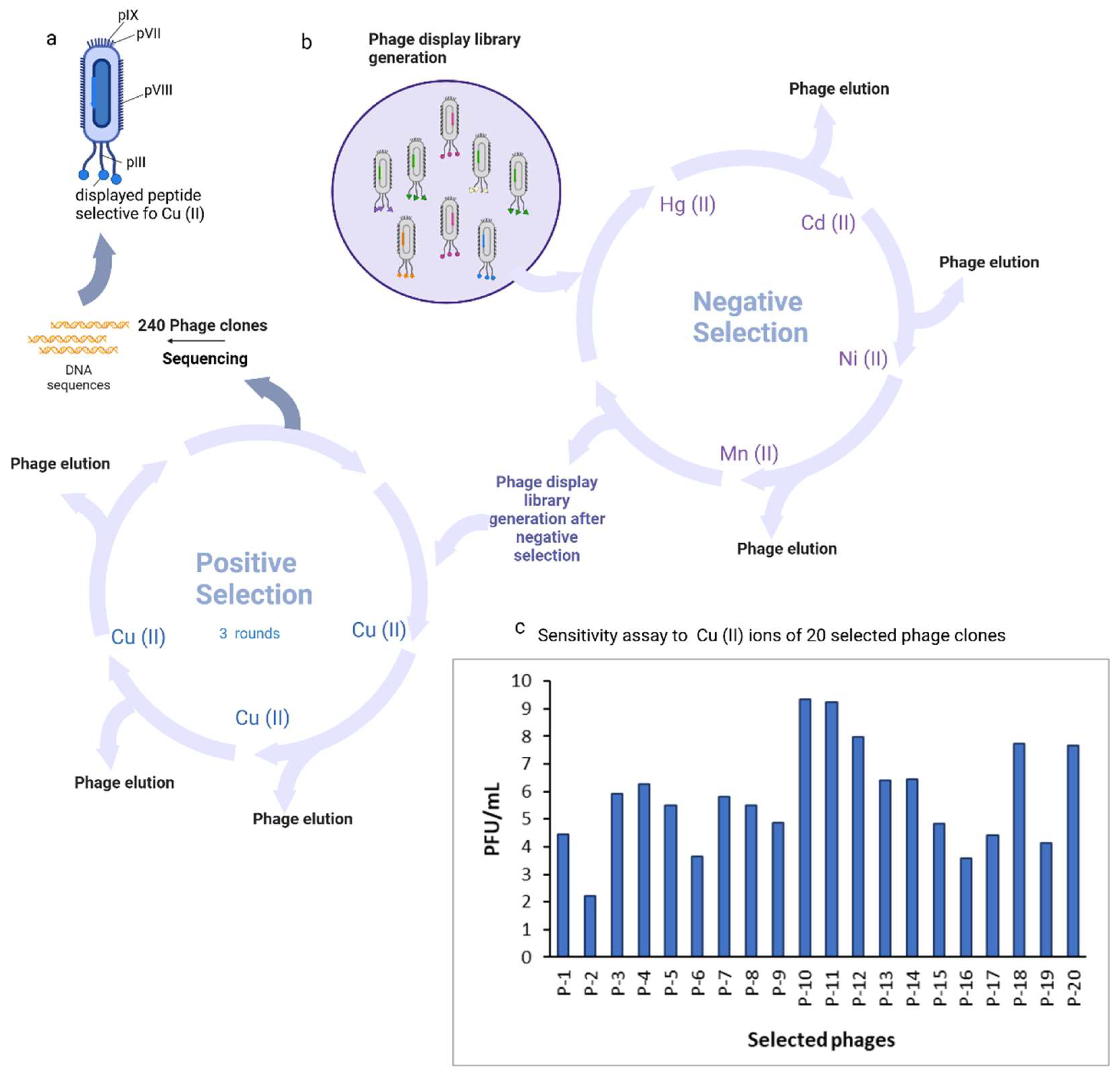
This study utilized the host bacterial strain E. coli K12 ER 2738 (NEB) and the Ph.D.™-7 Phage Display Peptide Library Kit (New England Biolabs GmbH, Frankfurt am Main, Germany). The culture was incubated overnight at 37°C in LB medium supplemented with 20 μg/L tetracycline and preserved in 100 μL aliquots in PCR tubes at -80°C for future use. The strain was preserved on LB agar plates during the experiments. The plates contained 10 g/L tryptone, 5 g/L yeast extract, 5 g/L NaCl, 15 g/L agar, and 20 μg/L tetracycline. The phage display library was screened for metallospecific peptides following the manufacturer's instructions, with minimal modifications. A negative selection step was integrated into the protocol to remove nonspecific phage clones from the library that bound to metal ions other than copper. In summary, 1 x 10^11 PFU of the library was diluted in 100 μL of 0.05% TBST buffer and subsequently introduced to a plate coated with a 10 mM nontarget metal ion. Following a 60-minute incubation at room temperature with a plate shaker set to 100 rpm, unbound phage clones were transferred into wells coated with an additional negative target and incubated for another 60 minutes at room temperature with the plate shaker at 100 rpm. All clones underwent the negative selection process. Negative selection was conducted against nickel, manganese, cadmium, and mercury. Subsequent to negative selection, the manufacturer's guidelines were adhered to in order to perform three rounds of biopanning with the designated metal ion. Two hundred forty plaques were chosen from IPTG/X-gal LB titration plates after the third round to amplify phage clones for DNA sequencing. Subsequently, the 7-amino acid copper ion-binding sequences were extracted through the sequencing of individual phages.
2.5. Binding Specificity Assay
100 µL of 10 mM Nα,Nα-Bis(carboxymethyl)-L-lysine hydrate (Sigma Aldrich Cat# 14580) in 0.1 M NaPO4, pH 8, was dispensed into each well of a maleic anhydride-activated microtiter plate (Thermo Scientific Cat#15100) and incubated overnight at room temperature. The plate was subsequently rinsed thrice with 300 µL of 0.05% TBST buffer. The plate was blocked by incubating with 3% BSA in 0.05% TBST buffer for 2 hours at the room temperature. The plate was subsequently rinsed thrice with 300 µL of 0.05% TBST buffer. The plate was subsequently incubated with 20 µM metal salts for 20 minutes at the room temperature. Plates coated with metal ions were incubated with 100 µL of 10^9 PFU of selected clones in TBS buffer per well. Plates were incubated for 60 minutes at room temperature with shaking at 100 rpm on a plate shaker. Unbound phages were eliminated, and the plate was rinsed ten times with 300 µL of 0.05% TBST buffer. For phage elution, 100 µL of 0.2 M Glycine-HCl (pH 2.2) was utilized, followed by a 15-minute incubation at room temperature, after which 15 µL of 1 M Tris-HCl (pH 9.1) was incorporated. Eluted phages were titered following the procedure outlined in The Ph.D.™-7 Phage Display Peptide Library Kit manual utilizing LB/IPTG/Xgal plates. Binding specificity is expressed as the phage titer eluted from wells coated with the chosen metal .
2.6. Peptide Synthesis, Modification and Characterization
Using microwave-assisted Fmoc solid-phase peptide synthesis (SPPS), the peptide with the sequence Dansyl-Met-His-Ile-Pro-His-Glu-Lys-Trp-NH2 was synthesized on Rink Amide resin (0,1 mmol). Peptide chain elongation was performed utilizing an Initiator+ Alstra™ (Biotage, Sweden) automated microwave peptide synthesizer. Couplings were carried out twice for 5 minutes at 75 °C utilizing Fmoc-amino acid (5 equivalents), DIC (5 equivalents), and Oxyma (5 equivalents) in DMF. The deprotection of the Fmoc group utilized a 20% piperidine solution in DMF at room temperature (1 x 3 minutes, 1 x 10 minutes). DNS was introduced at the N-terminal side of the peptide in a separate step. To this end, DNS (5 equivalents) and TEA (3 equivalents) were introduced to the peptidyl resin in darkness for 4 hours. The DNS-labeled peptide was cleaved from Rink Amide resin utilizing a cleavage solution of TFA/TIS/H2O (95:2.5:2.5) for 2 hours, precipitated with anhydrous, cold diethyl ether, and subsequently lyophilized. The resultant product was characterized utilizing a reverse-phase HPLC Shimadzu system (Prominence-i LC-2030C Plus, Shimadzu, Japan) equipped with a Jupiter 4 µm Proteo, 90Å, 4.6 x 250 mm column, employing UV detection at λ=224 nm. A linear gradient method was applied, transitioning from 5 to 95% solvent B over 60 minutes at a flow rate of 1 mL/min, where solvent A was water and solvent B was acetonitrile, both containing 0.1% TFA. Electrospray ionization mass spectrometry (ESI MS) in positive ion mode was conducted utilizing a single quadrupole mass spectrometer (LCMS 2020 Shimadzu, Japan). Isocratic elution at 60% B, with eluent A comprising water and 0.1% formic acid (LCMS grade) and eluent B consisting of acetonitrile (LCMS grade) with 0.1% formic acid, was conducted at a flow rate of 1.5 mL/min.
2.7. General Spectroscopy Measurements
DNS–P-11, a highly soluble fluorescent peptide, was dissolved in double-distilled water to create a 2 mM DNS–P-11 solution, which was stored at 4ºC. The solutions of metal ions were derived from Hg(NO3)2, Pb(NO3)2, MnCl2, ZnSO4, CuSO4, and NiCl2. Standard solutions of Cd, Cr, NaAsO2, KCl, and NaCl in double distilled water at a concentration of 10 mM. Subsequent to appropriate dilution, the resultant stock solution was utilized for all spectral measurements. The Synergy H1MG Multimode Microplate Reader (BioTek Instruments, United States) was employed to assess the fluorescence spectra on a 96-well plate featuring an F-bottom from Greiner Bio-One. The excitation wavelength for the dansyl fluorophore was established at 330 nm.
3. Results and Discussion
3.1. Phage Display Derived Unique Peptide for Cu2+ Ions Detection
After the entire biopanning procedure 240 monoclonal Cu(II)-binding M13 virus plaques containing phage monoclonal peptides were isolated for phage amplification and DNA sequencing. DNA sequences were successfully obtained for all of the clones. A total of 20 individual clones containing distinctive peptides were identified from selected 240 plaques. The biopanning procedure proved effective, demonstrated by the increased binding affinity of phage clones with Cu(II)-binding peptides to Cu(II). P-10, P-11, P-12, P-18, and P-20 demonstrated the greatest affinity (
Figure 1).
3.2. Characterization and Amio Acid Sequences Analysis of the Unique Phage Display Derive Peptides
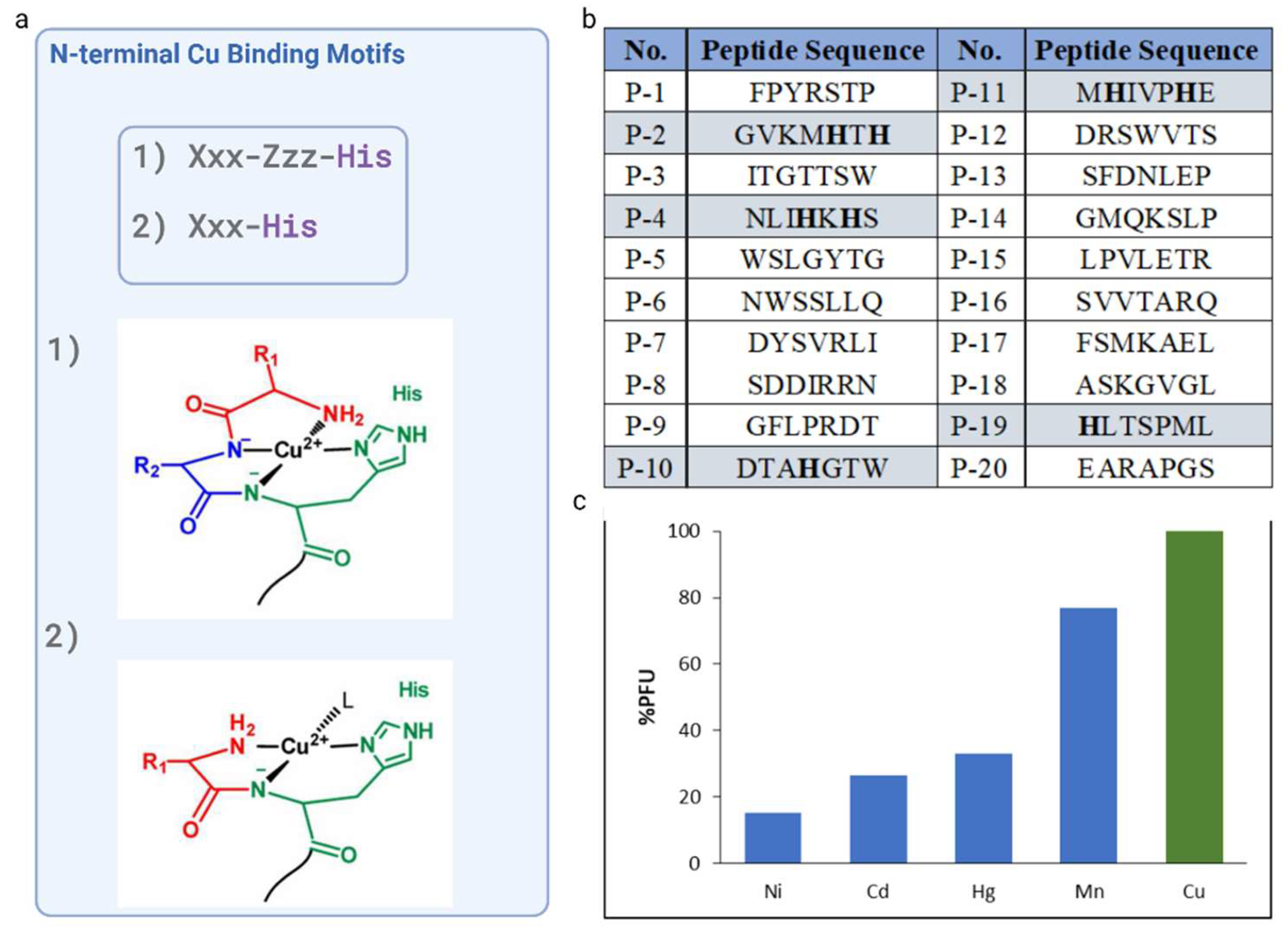
The analysis of the amino acid sequences of the twenty selected phages revealed that only four of them has a histidine residue: P-2 (GVKMHTH), P-4 (NLIHKHS), P-10 (DTAHGTW) P-11 (MHIVPHE) and P-19 (HLTSPML), with P-10 and P-11 showing the greatest selectivity towards Cu2+ ions. Thus, P-10 and P-11 peptide sequences were taken into consideration due to the well-known, potent, and short N-terminal motif, which have a His residue in the second or third position. P-10 with a sequence DTAHGTW, featured histidine at fourth position, however, the possibility of formation of chelate system is removed if there are two or more intervening amino acid residues between the N-terminal amine the imidazole donors
Since histidine residue located in position two of the peptide chain allows generation of highly desirable Cu
2+-complex, P-11 with a sequence MHIVPHE, which possessed a His residue at the second position with respect to the free N-terminal amine and high affinity toward Cu
2+ ions, was chosen as the candidate peptide for further examination. NH
2--Xxx-His motif, which generated a highly stable Cu
2+-Xxx-His complex.Peptides contained N-terminal amino acid sequence Xxx-His, where Xxx can be any amino acid, except Pro, display a high affinity for Cu(II). In this manner Cu
2+ can bind to XH in a tridentate fashion, with three nitrogens from the N-terminal amine (NH
2), the first amide (Nα) and the imidazole nitrogen at the delta ring position (Nδ) being involved (
Figure 2a). The fourth equatorial binding site is occupied by an external molecule such as water, buffer, etc.
Following a thorough investigation and the selection of the sequence, four supplementary metal ions (Ni, Cd, Hg, and Mn) were used in the cross-reactivity assays to enhance selectivity investigations. The selective Cu(II)-binding M13 phage clone that displaces the MHIVPHE peptide demonstrated a strong affinity for copper(II) ions while exhibiting minimal affinity for other metal ions, such as Ni, Cd, Hg, and Mn. This result is of great importance, as the selectivity of the chemosensor for specific ions is crucial.
3.3. Design of Modifications and Sold Phase Peptide Synthesis
The unique selective native peptide sequence Met-His-Val-Ile-Pro-His-Glu (P-11) selected from Phage Display experiments was modified to obtain specific optic functions of peptide. Metal ion-binding peptide was synthesized on Rink Amide resin by Fmoc/tBu microwave-assisted solid phase peptide synthesis [
24]. The fluorophore (dansyl chloride) and tryptophan as donor were conveniently attached during SPPS, eliminating the need for labeling reactions. Dansyl (DNS) was introduced at the N-terminal side of the peptide, tryptophan residue was inserted at the C-terminus manually, in separate step. Flexible linker like lysine residue was inserted between native sequence and donor fluorophore to increase the peptide's solubility, minimize steric hindrance and ensure proper positioning of the fluorophore (
Figure 3). Labeling peptides by use of dansyl chloride (DNS-Cl) is a versatile and highly sensitive means of fluorescent detection. DNS was chosen for the construction of the chemosensor due to its strong fluorescence, relatively long emission wavelength in the visible region and rapid reactivity with primary amines. Moreover, sequence labeling with dansyl fluprophore allows for very rapid detection at a low quantity in fluorescent spectroscopy. DNS typically exhibits strong fluorescence at 540 nm when excited at an appropriate wavelength, usually around 330 nm [
25,
26]
Obtained peptide was cleaved from the resin, isolated and liophylized. The successful synthesis was confirmed by ESI mass spectrometer. The crude peptide was puried by HPLC.Peptide DNS–P-11 chrateryzation: Yellowish solid; Synthetic yield: 89%; HPLC purity > 95%; Rt 20.708 min., ESI MS of peptide is calculated as 1408.4 (g/mol); Observed value as [M+2H]2+ m/z=706, [M+3H]3+ m/z=471.
3.4. Metal Ions Selectivity Experiment
The fluorescence spectroscopic response of DNS−P-11 peptide (10 µM) toward metal ions (Cu
2+, Hg
2+, Pb
2⁺, Cd
2⁺, Ni
2⁺, Mn
2⁺, Cr
3+, As
3+, Na
+ and K
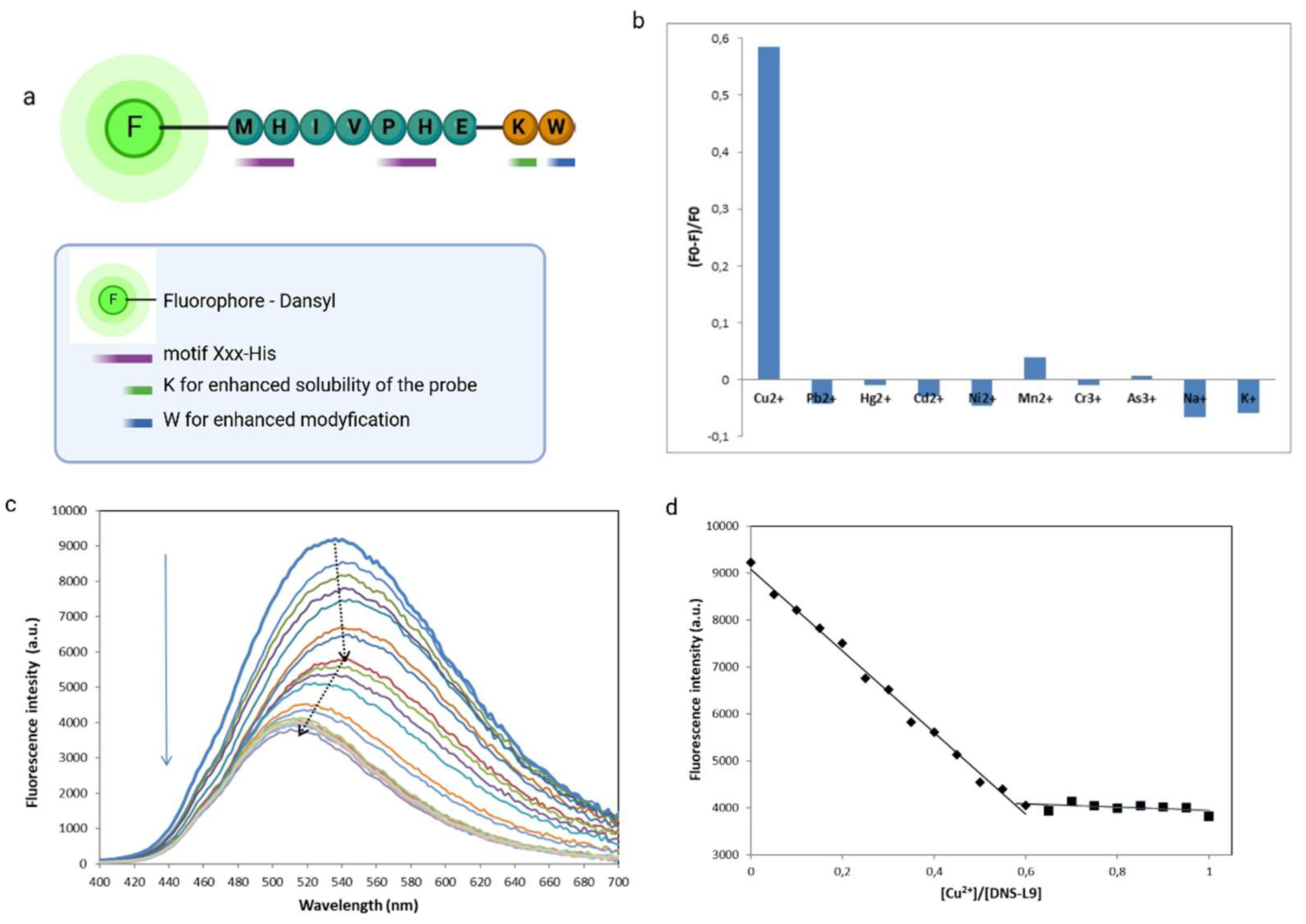
+) was evaluated in HEPES buffer solutions (50 mM, pH 7.41). Separate solutions of each metal ion prepared at concentrations of 10 µM were individually added to the DNS−P-11 solutions and fluorescence emission spectra were recoded with excitation at 330 nm. Comparing the fluorescence response of DNS−P-11 in the presence of Cu
2+ ions and other selected metal ions in the tested concentration, it was found that DNS−P-11 exhibited selectivity for Cu
2+ in aqueous solution and no fluorescent response to other metal ions. Conversely, after adding 1.0 equiv. Cu
2+ ions, the emission intensity of DNS−P-11 was significantly reduced with the fluorescence quenching rate for Cu
2+ about 58.5 % (
Figure 3).
3.5. Characterization of The Sensing Process of Fluorescent Chemosensor based on Phage Display Derived Peptide for Cu2+ Detection
In order to better understand the sensing process, the fluorescence spectra changes of fluorescent peptide, DNS−P-11 with Cu
2+ ions was demonstrated. For this purpose, the different concentrations of Cu
2+ ion solutions (0-10 µM) were added to the 10 μM DNS−P-11 in HEPES buffer solutions (50 mM, pH 7.41) and fluorescence spectra of the obtained solutions were recorded. As illustrated in
Figure 3a, the fluorescence emission peak of DNS−P-11 gradually decreased with increasing Cu
2+ concentration. The fluorescence spectroscopy of DNS−P-11 indicated that the addition of Cu
2+ (ranging from 0-1.0 equiv.) caused a fluorescent turn-off response at 540 nm.
Notably, the fluorescence intensity of DNS−P-11 at 540 nm began to gradually quench after the addition of Cu
2+.ions (0.05-0,45 equiv.), while the emission maximum was shifted to 546 nm. Following increase in Cu
2+ concentration (0,5-1.0 equiv.) resulted in a further decrease in fluorescence intensity with a concurrent blue-shifted emission peak 36 nm from 546 to 510 nm (
Figure 3a). According to the trend diagram of fluorescence titration, the fluorescence titration curve of DNS−P-11 reached a steady plateau after the addition of 0.65 equiv. of Cu
2+ (
Figure 3b), while the increasing level of Cu
2+ up to 10 equiv. maintained the saturation of DNS−P-11-Cu
2+ complex at constant level (
Figure 3c), The above results determined the binding stoichiometry of 1:2 between DNS−P-11 and Cu
2+ ions. Since the structure and stability of the formed complexes is highly dependent on pH and the metal/ligand ratio, it is possible that at neutral or basic pH two histidines (at 4 and 6 position) in peptide sequence can behave as independent co-ordination sites, and DNS−P-11 can bind up to two Cu
2+ ions.
The results of the fluorescence titration experiment clearly demonstrated that DNS−P-11 exhibits a high level of selectivity towards Cu
2+ ions by turn-off fluorescent response mechanism (
Figure 3).
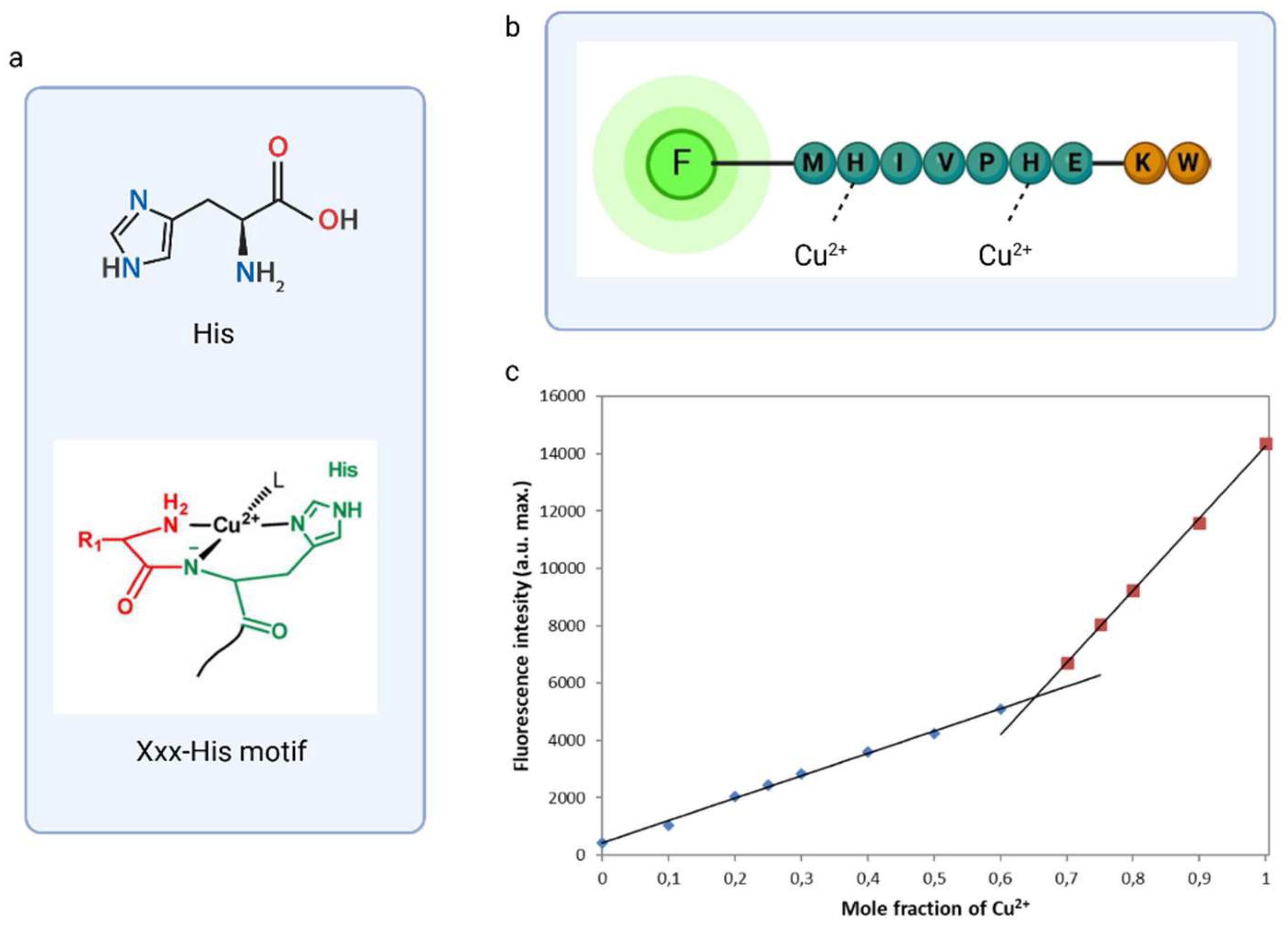
Another way to determine the binding stoichiometry of fluorescent peptide and metal ions is to determine the Job’s plot analysis curve. The typical Job's plot shows a curve that reaches a maximum at the mole fraction corresponding to the stoichiometric ratio of the complex. In some cases, the Job's plot can show a dip, indicating a minimum, instead of a maximum. This happens in situations where the measured property decreases as the complex is formed. Such a scenario occurred during the complexation of DNS−P-11 with Cu2+, during which the fluorescence intensity decreases, that is inversely related to the amount of complex formed.
The total concentration of DNS−P-11 and Cu
2+ was 10 µM. The result exhibited a minimum at 0.65 mol fraction of Cu
2+ in 50 mM HEPES buffer solution, which could be consistent with the formation of 1:2 coordination stoichiometry for DNS−P-11-Cu
2+ complex (
Figure 4).
3.6. Analysis of Selectivity and Sensitivity of Fluorescent Chemosensor (DNS−P-11) based on Phage Display Derived Peptide for Cu2+ Detection
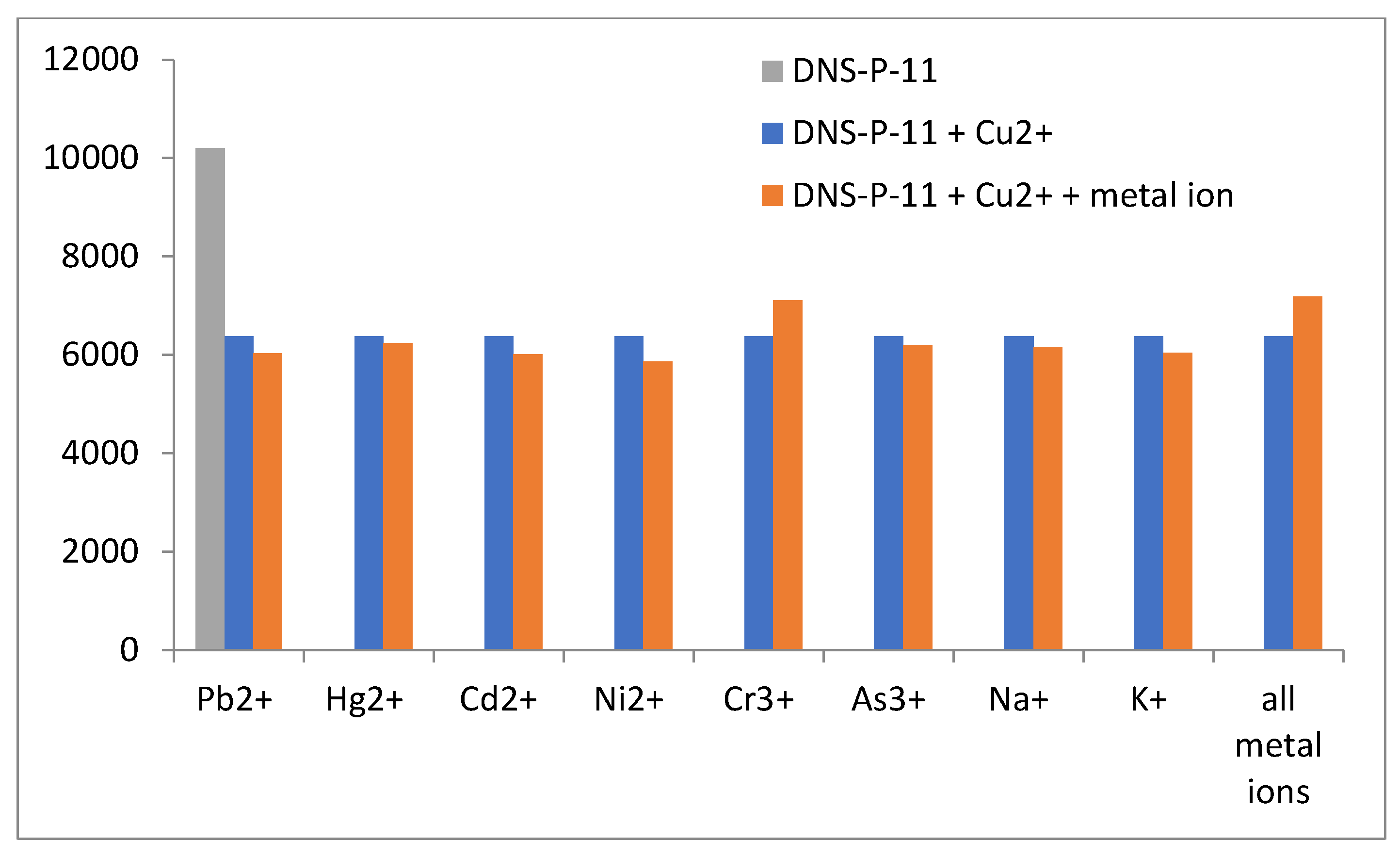
The specificity of fluorescence-based detection methods is crucial in many applications, including clinical diagnostics and environmental monitoring. To ensure this specificity, it is essential to test the response of the DNS−P-11 peptide to its target ions in the presence of other potentially interfering metal ions. The fluorescence responses of DNS−P-11 to Cu
2+ (1.0.equiv.), in the presence of other tested metal ions (5.0 equiv.) were measured in HEPES buffer solutions (50 mM, pH 7.41). Moreover, we also explored the ability of DNS−P-11 to detect Cu
2+ in the mixture of all tested ions. Cross-reactivity refers to the response of the peptide DNS−P-11 to multiple metal ions, which may affect the interpretation of the fluorescence signal. Thus, to verify the peptide biosensor's selectivity for Cu
2+ ions, the fluorescence responses of DNS−P-11 to Cu
2+ (1.0.equiv.), in the presence of other tested metal ions (5.0 equiv.) were measured in HEPES buffer solutions (50 mM, pH 7.41). The experimental results showed that the presence of other metal ions, such as Pb
2+, Hg
2+, Cd
2+, Ni
2+ and Mn
2+ or trivalent chromium and arsenic ions, had no effect on the response of DNS−P-11 to Cu
2+ and also had no impact on the interpretation of the fluorescence signal, as demonstrated in
Figure 5. This clearly indicated formation of stable complex between DNS−P-11 and Cu
2+ ions and strongly support ability of DNS−P-11 to detect Cu
2+ in the presence of other metal ions. In addition, the simultaneous introduction of all the analyzed ions (5 equiv. of each) into the sample containing the DNS−P-11 peptide probe and Cu
2+ ions (1 equiv.) did not substantially impact the reduction of the fluorescence emission of the peptide−Cu
2+ complex. This property is crucial for developing selective biosensors that accurately identify and measure Cu
2+ in complex mixtures.
The results demonstrate that the fluorescence can be controlled by an ON−OFF switch, which is in line with the powerful chelating capability of the DNS−labeled peptide towards Cu2+. It is important to highlight that the DNS−P-11 peptide remains highly responsive to Cu2+ ions even in high concentrations of other competitive ions, which emphasizes the ability of the designed DNS−P-11 peptide to selective copper(II) ions detection in aqueous systems.
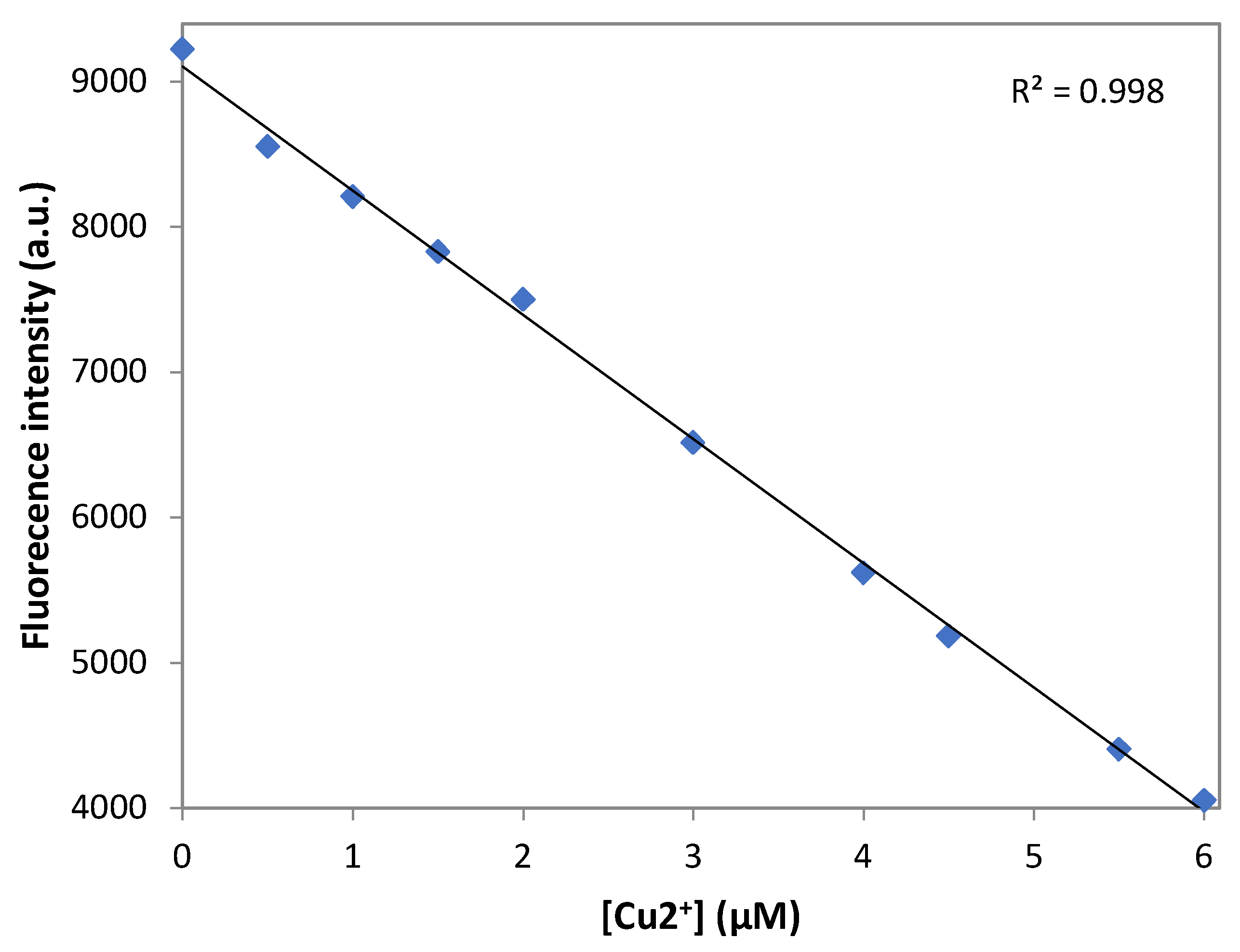
3.7. Detection Limit of DNS–P-11 for Cu2+ Ions
The limit of the detection (LOD) was calculated according to the fluorescence titration experiment. In to ascertain the S/N ratio, the fluorescence emission intensity of DNS−P-11 in the absence of Cu
2+ was measure six times and standard deviation of blank measurement was obtained. Three independent duplicate measurements of fluorescence emission intensity were evaluated in the presence of Cu
2+ ions and the mean intensity was plotted as function of Cu
2+ for determining the slope. Then LOD for Cu
2+ was obtained by the equation: LOD=3σ/a, where σ is the standard deviation of the response, a is the slope of the calibration curve of the plot. LOD for the DNS−P-11 biosensor for Cu
2+ was calculated using the linear relationships of fluorescence emission intensity at maximum wavelength with the addition of increasing concentrations of Cu
2+.
Figure 6 showed good linear relationship between fluorescence emission intensity of DNS−P-11 and Cu
2+ concentration solution in the range of 0-6 µM, in 50 mM HEPES buffer, with correlation coefficient R
2=0.998. According to the equation of LOD=3σ/a,, the detection limit of DNS−P-11 for Cu
2+ is 0.46 µM. The data indicates that the DNS−P-11 chemosensor is highly sensitive to Cu
2+ ions, and the detection limit is significantly lower than the WHO’s recommended limit value of 31 µM.
4. Conclusions
The high value of peptides in the development of chemosensors for a variety of applications, such as drug delivery systems, tissue engineering, diagnostics, and the monitoring of small analites such as emerging pollutants, is a result of their versatility. The utilisation of Phage display technology to identified novel peptides become a alternative way nowadays. The identifed peptides with proper features vey often contains specific motifs that are well known in biotechnolgy field.
The incorporation of fluorophores and the precise control over peptide sequences have been made possible by advanced techniques in peptide synthesis, such as solid-phase peptide synthesis. These developments have the potential to further improve the properties of peptide-based materials. It is possible to develop materials with specific chemical and biological properties that are specifically tailored to specific applications by customizing the sequence and structure of peptides.
In summary, we developed a highly selective and sensitive fluorescent chemosensor for Cu2+ detection based on a peptide from phage display library that carries the N-terminal Xxx-His motif.
5. Patents
The results of this work were included in the two patent applications number PL449080 and PL449085.
Author Contributions
Conceptualization, B.G. and M.S.; methodology, M.S., T.Ł., B.G.; formal analysis, B.G., M.S..; investigation, M.S., T.Ł., B.G.;. resources, B.G.; writing-original draft preparation, B.G., M.S.; writing-reviewing and editing, M.S., B.G.; visualization, M.S., B.G.; supervision, B.G.; project administration, B.G., M.O.; funding acquisition, D.N., B.G. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Warsaw University of Technology and The National Centre for Research and Development under the III program TECHMATSTRATEG—Strategic research and development program “Modern material technologies—TECHMATSTRATEG” no. TECHMATSTRATEG-III/0042/2019-00 and acronym ASTACUS, “Biopolymer materials with chemically and genetically programmed heavy metals selectivity for new generation of ultra-sensitive biosensors”.
Data Availability Statement
The datasets used and analysed in the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Taylor, A.A.; Tsuji, J.S.; Garry, M.R.; McArdle, M.E.; Goodfellow Jr, W.L; Adams, W.J.; Menzie, C.A. Critical Review of Exposure and Effects: Implications for Setting Regulatory Health Criteria for Ingested Copper. Environmental Management 2020, 65, 131–159. [Google Scholar] [CrossRef] [PubMed]
- Gaetke, L.M.; Chow, C.H. ; Copper toxicity, oxidative stress, and antioxidant nutrients, Toxicology 2003, 189, 1–2, 147-163. [CrossRef]
- Ejaz, H.W.; Wang, W.; Lang, M. Copper Toxicity Links to Pathogenesis of Alzheimer’s Disease and Therapeutics Approaches. Int. J. Mol. Sci. 2020, 21, 7660. [Google Scholar] [CrossRef] [PubMed]
- Strausak, D.; Mercer, J.F.B.; Dieter, H.H.; Stremmel, W.; Multhaup, G. Copper in disorders with neurological symptoms: Alzheimer’s, Menkes, and Wilson diseases, Brain Research Bulletin 2001, 55, 175-185. [CrossRef]
- Karpenko, M.N.; Muruzheva, Z.M.; Ilyechova, E.Y.; Babich, P.S.; Puchkova, L.V. Abnormalities in Copper Status Associated with an Elevated Risk of Parkinson’s Phenotype Development. Antioxidants 2023, 12, 1654. [Google Scholar] [CrossRef] [PubMed]
- Elkhatat, A.M.; Soliman, M.; Ismail, R.; Ahmed, S.; Abounahia, N.; Mubashir, S.; Fouladi, S.; Khraisheh, M. Recent trends of copper detection in water samples. Bull Natl Res Cent 2021, 45, 218. [Google Scholar] [CrossRef]
- Kateshiya, M.R. , Desai, M.L., Malek, N.I. et al. Advances in Ultra-small Fluorescence Nanoprobes for Detection of Metal Ions, Drugs, Pesticides and Biomarkers. J Fluoresc. 2023, 33, 775–798. [CrossRef]
- Shi, Y.; Zhang, W.; Xue, Y.; Zhang, J. Fluorescent Sensors for Detecting and Imaging Metal Ions in Biological Systems: Recent Advances and Future Perspectives. Chemosensors 2023, 11, 226. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, D.; Chen, B. A fluorescent dansyl-based peptide probe for highly selective and sensitive detect Cd2+ ions and its application in living cell imaging. Spectrochim Acta A Mol Biomol Spectrosc 2019, 207, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Xiao, L.; Gou, Y.; He, F.; Wang, P.; Yang, X. A novel peptide-based relay fluorescent probe with a large Stokes shift for detection of Hg2+ and S2− in 100 % aqueous medium and living cells: Visual detection via test strips and smartphone. Spectrochim Acta A Mol Biomol Spectrosc 2023, 285, 121836. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Wu, J.; Zhao, C. A water-soluble peptide fluorescent chemosensor for detection of cadmium (II) and copper (II) by two different response modes and its application in living LNcap cells, Spectrochim Acta A Mol Biomol Spectrosc 2020, 226, 117600. [CrossRef]
- Wang, P.; Wu, J. Highly selective and sensitive detection of Zn(II) and Cu(II) ions using a novel peptide fluorescent probe by two different mechanisms and its application in live cell imaging, Spectrochim Acta A Mol Biomol Spectrosc 2019, 208, 140-149. [CrossRef]
- Román-Azcona, M.S.; Trigüis, S.; Caballero, S.; Michajluck, J.; Sotelo, P.H. Generation of a cadmium-binding filamentous phage through cysteine-rich peptide display on PVIII. Indian J. Biotechnol. 2019, 18, 132-138.
- Li, H.; Dong, W.; Liu, Y.; Zhang, H.; Wang, G. Enhanced Biosorption of Nickel Ions on Immobilized Surface-Engineered Yeast Using Nickel-Binding Peptides. Front Microbiol. 2019, 26, 1254. [Google Scholar] [CrossRef]
- Matys, S.; Schönberger, N.; Lederer, F.L.; Pollmann, K. Characterization of specifically metal-binding phage clones for selective recovery of cobalt and nickel. J. Environ. Chem. Eng. 2020, 2, 103606. [Google Scholar] [CrossRef]
- Korkmaz, N. , Kim, M. Phage display selection of a Pb(II) specific peptide and its application as a biorecognition unit for colorimetric detection of Pb(II) ions. Biotechnol. J. 2024, 19, 2300482. [CrossRef]
- Korkmaz, N.; Hwang, C.; Kessler, K.K.; Silina, Y.E.; Müller, L.; Park, J. A novel copper (II) binding peptide for a colorimetric biosensor system design. Talanta 2021, 232, 122439. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, X.Y.; Zhang, X.X.; Chen, M.L.; Wang, J.H. Chromium(III) Binding Phage Screening for the Selective Adsorption of Cr(III) and Chromium Speciation. ACS Appl Mater Interfaces 2015, 30, 21287–21294. [Google Scholar] [CrossRef]
- . Henryk Kozłowski, H,; Bal, W.; Dyba, M.; Kowalik-Jankowska, T. Specific structure–stability relations in metallopeptide. Coord. Chem. Rev. 1999, 184,1, 319-346. [CrossRef]
- Gonzalez, P.; Vileno, B.; Bossak, K.; Khoury, Y.E.; Hellwig, P.; Bal, W.; Hureau, C.; Faller, P. Cu(II) Binding to the Peptide Ala-His-His, a Chimera of the Canonical Cu(II)-Binding Motifs Xxx-His and Xxx-Zzz-His. Inorg. Chem. 2017, 24, 14870–14879. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, P.; Bossak, K. ; Stefaniak,E. ; Hureau, C.; Raibaut, L.; Bal, W.; Falle, P. N-Terminal Cu-Binding Motifs (Xxx-Zzz-His, Xxx-His) and Their Derivatives: Chemistry, Biology and Medicinal Applications. Chem. Eur. J. 2018, 32, 8029–8041. [Google Scholar] [CrossRef]
- Noormägi, A.; Golubeva, T.; Berntsson, E.; Wärmlänr, S.K.T.S.; Tõugu, V.; Palumaa, P. Direct Competition of ATCUN Peptides with Human Serum Albumin for Copper(II) Ions Determined by LC-ICP MS. ACS Omega 2023, 8, 37, 33912–33919. [Google Scholar] [CrossRef]
- . Maiti, B.K.; Govil, N.; Kundu, T.; Moura, J.J.G. Designed Metal-ATCUN Derivatives: Redox- and Non-redox-Based Applications Relevant for Chemistry, Biology, and Medicine. iScience 2020, 23, 101792. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, S.L.; Tofteng, A.P.; Malik, L.; Jensen, K.J. Microwave heating in solid-phase peptide synthesis. Chem. Soc. Rev. 2012, 41, 1826–1844. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.; Dong, J.; Gao, L.; Wang, L. ; Yu,S. ; Kong, J.; Li, L.Dansyl-peptide dual-functional fluorescent chemosensor for Hg2+ and biothiols. Dyes Pigm. 2020, 173, 107888. [Google Scholar] [CrossRef]
- Wang, P.; Zhou, D.; Chen, B. A fluorescent dansyl-based peptide probe for highly selective and sensitive detect Cd2+ ions and its application in living cell imaging. Spectrochim Acta A Mol Biomol Spectrosc. 2019, 207, 276–283. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).