1. Introduction
Plastic waste is a severe problem for the global economy. Municipal solid waste contains more than 10% synthetic polymers [
1,
2,
3]. Over time, this will continue to increase as an environmental problem as societal demand continues to increase for polymers. One approach to alleviating this oncoming environmental concern is to develop polymers for multiple uses, i.e., recover and repurpose. Therefore, polymers synthesized with end-of-life considerations that allow for the recovery and reprocessing of the material are of significant interest. Polymers designed for recovery and reprocessing should be easy and cost-effective to synthesize. Diels-Alder (DA) chemistry is a powerful tool for studying the recovery and reprocessing of materials. DA reaction is a [4 + 2] cycloaddition reaction between an electron-rich diene and an electron-poor dienophile, which forms a DA adduct without any catalyst. It is a thermally reversible reaction that can be applied to various polymers [
4,
5,
6,
7,
8]. It has been extensively studied due to the relatively fast kinetics and mild reaction conditions [
8,
9,
10,
11]. The crosslinking of polymers can be achieved by reacting polymers functionalized with furan (diene) and maleimide (dienophile) moieties. Moreover, its retro-DA reaction takes place at relatively low temperatures, which is the decrosslinking process. This, therefore, opens up exciting applications, such as recyclable networks and self-healing materials using functional polymers containing furans and maleimide. Polymer networks, polymer gels, and dendrimers have been prepared by reacting furan and maleimide functionalized polymers. The first report on this approach to preparing polymeric materials was by Stevens and Jenkins [
12]. They used the Friedel-Crafts alkylation to functionalize polystyrene with a maleimide functional group and crosslinked the polystyrene using a bifunctional furan structure to create self-healing materials. A decade later, Canary and Stevens used this idea by mixing polystyrene functionalized by maleimide with di-furfuryl adipate [
13]. Several groups have used furan as a diene in Diels-Alder reactions [
14,
15,
16,
17]. Bapat et al. and Barthel et al. have reported using a thermo-reversible Diels-Alder reaction to regulate the crosslinking of micelles [
14,
15]. Das et al. recently reported the formation of novel thermo-resettable thermosetting polymeric materials of furyl-modified copolymers (FMPs) and BMI using DA reactions [
18]. Furthermore, Kang et al. used DA reactions for block copolymers that contain pendant triphenylamine [
17]. The cross-linked network formed at 50 °C, which was confirmed by the DSC studies, which showed an increase in Tg, and for the decrosslinking reaction, a retro-Diels-Alder peak was observed at 149 °C in the DSC thermogram. Buonerba et al. reported the formation of the thermo-reversible crosslinked network via the Diels-Alder (DA) reaction of copolymers of 2-vinylfuran and styrene (S-co-2VFs) with BMI [
19]. The temperature range for crosslinking and decrosslinking of S-co-2VFs with BMI via DA and r-DA reaction was between 50 °–150 °C. Recently, Liang et al. studied the kinetics of the forward Diels–Alder reaction between furfuryl-terminated polybutadiene (FTPB) and N, N0-1,3-phenylenedimaleimide (PDMI) by UV-Vis spectroscopy [
20]. The reaction kinetics of FTPB-PDMI was measured by the change in the peak that appears at 310 nm because of the conjugated C=C–C=O moiety. The kinetics were studied at different temperatures, and it was found that the reaction rate increased with temperature.
Thermoplastic elastomers (TPEs) are an important class of materials and can be found in various high-value applications, such as luxurious footwear, catheters, tires, etc. They show rubber and plastic-like properties at room temperature due to their two glass transition temperatures, one higher than room temperature and another below room temperature. Hairy nanoparticles (HNPs) can be described as a material with an inner core and an outer hairy layer, sometimes called the shell. The combination of characteristics and properties of core components and shell components in HNPs can show excellent properties that make them attractive [
21,
22]. Furthermore, because of their small size (10 nm to 100 nm), multiple functionalities on the shells can be included, and further, because HNPs have a high surface area to volume ratio, they have unique physical, chemical, and sometimes even biological properties. Therefore, they can be found as active materials for many applications in various areas of technology [
23], such as catalysis [
24], resistant coatings [
25,
26], pollution control, tires, the rubber industry [
27], sensing nanocarriers for drug and gene delivery [
28,
29]. Moreover, polymeric nanoparticles that have self-assembling properties have been used as building blocks for self-assembled nano-micro structures [
30,
31].
“Living anionic polymerization” (LAP) is a powerful synthetic method to synthesize well-defined polymers, block copolymers, and functional polymers [
32]. Living anionic polymerization has been successfully used to synthesize well-defined thermoplastic elastomers (TPE). Zheng et al. synthesized TPE by grafting polybutadiene brushes to polystyrene core particles prepared by LAP (living anionic polymerization) [
33]. Zhou et al. synthesized all-polymeric HNP with PDMS brushes and crosslinked polystyrene core by living anionic polymerization via a one-pot approach [
34]. The synthesized HNPs were microphase-separated and acted like nanoscale TPE materials because two glass transition temperatures were observed.
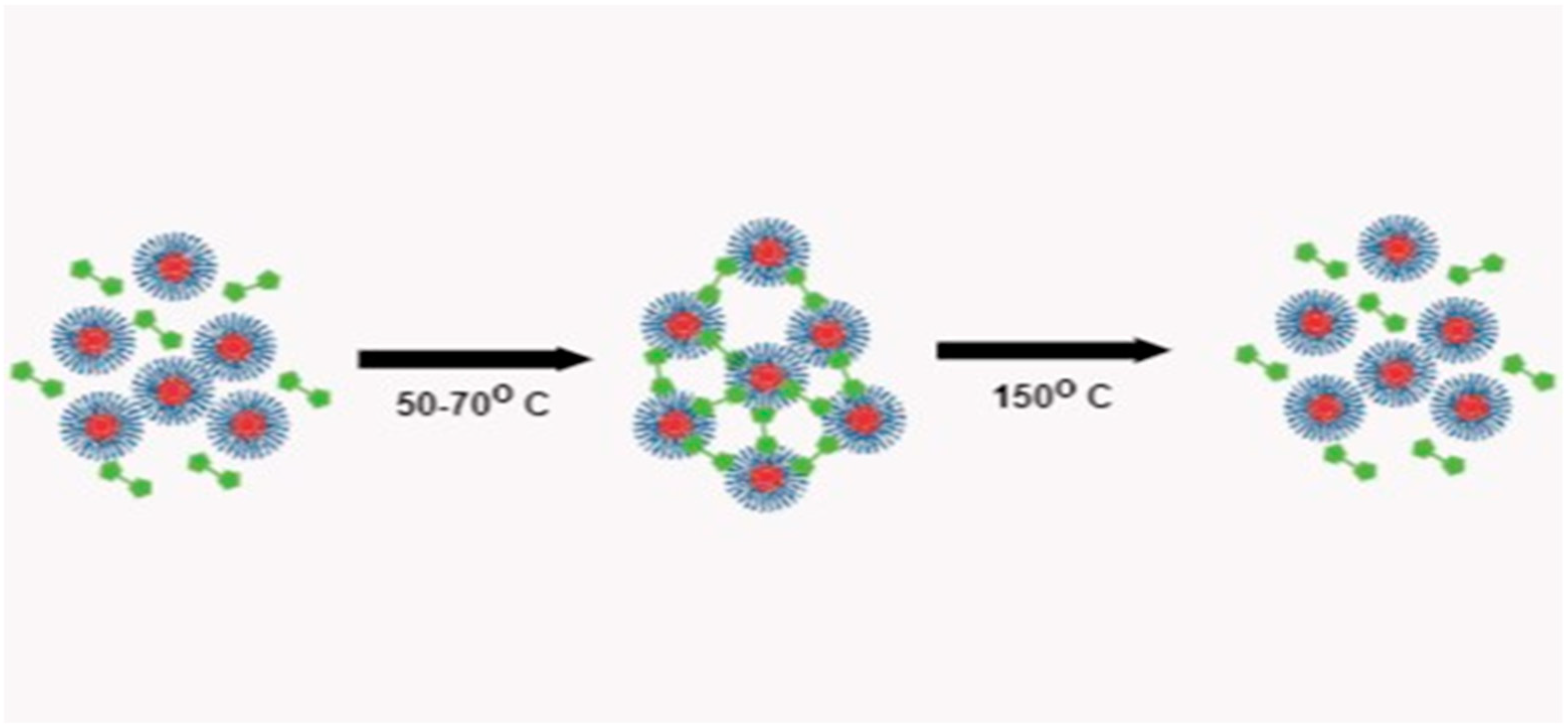
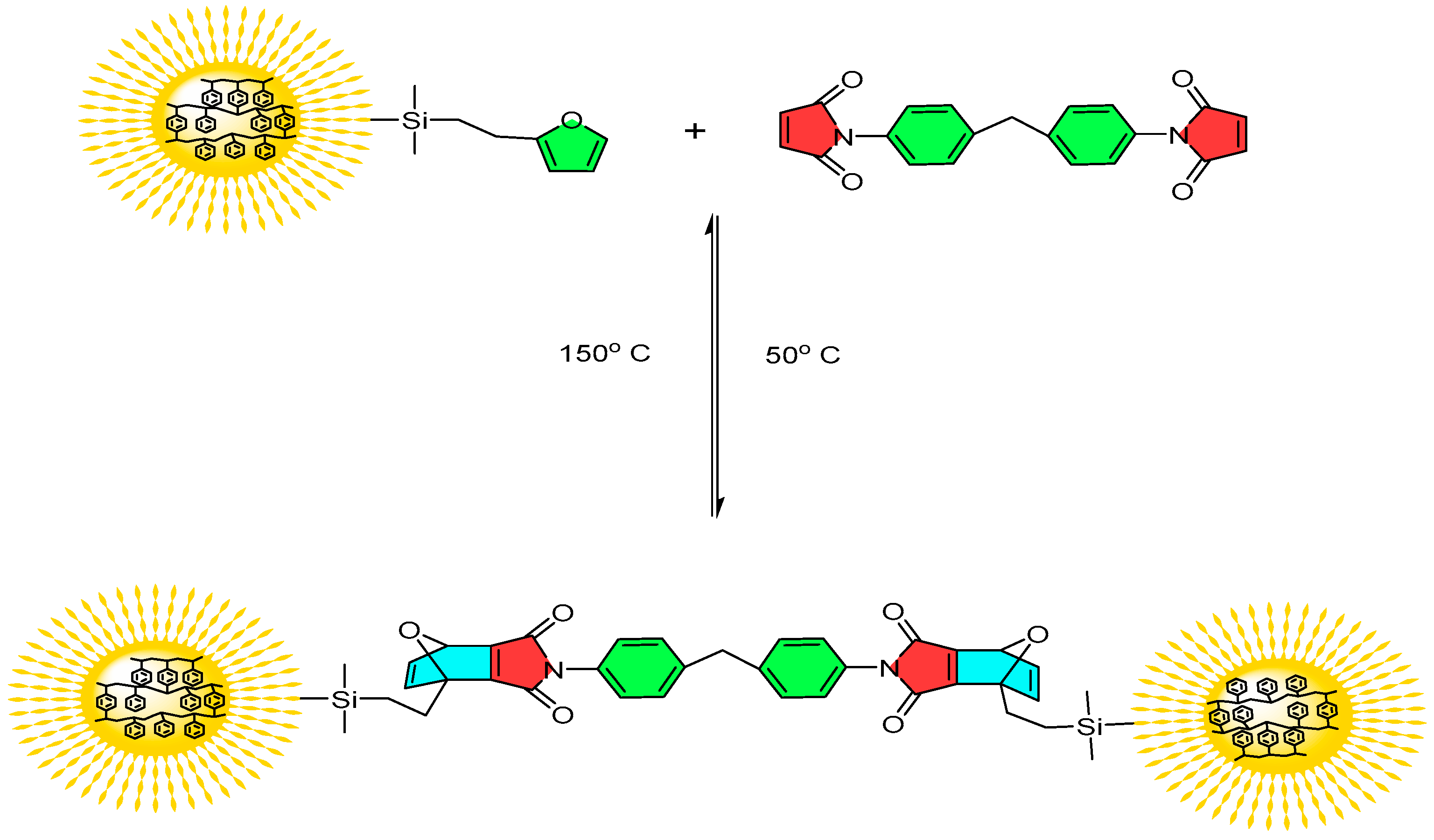
In this communication, we report the synthesis of HNPs functionalized with the furan group by combining living anionic polymerization and hydrosilylation reactions. The furan group at the hair end makes the HNPs interesting as it can undergo crosslinking reaction with bismaleimide at low temperature to form thermoplastic elastomeric networks, which can easily be de-crosslinked at relatively low temperature ~150 °C as shown in
Figure 1.
2. Materials and Methods
Calcium hydride (CaH
2), sec-butyllithium (sec-BuLi, 1.4M in cyclohexane), sodium metal, benzophenone, platinum (0), deuterated chloroform (CDCl
3), 1,1′-(Methylenedi-4,1-phenylene) bismaleimide (BMI), nitrobenzene, anhydrous potassium carbonate, methyl triphenyl phosphonium bromide were purchased from Millipore Sigma chemical company. Furfural, Styrene (S), divinylbenzene (DVB), hexamethylcyclotrisiloxane (D3), chlorodimethylsilane, and tetrahydrofuran (THF) were also purchased from Millipore Sigma and were purified before use. The procedures of purification are described below in detail. Methanol, 2-Propanol, and TEMPO were purchased from the Fisher Chemical Company. 2-Vinylfuran was synthesized in our lab, and a
1H NMR spectrum is provided to demonstrate purity (
Figure S1). Speier’s catalyst (H
2PtCl
6) was purchased from Millipore Sigma. The hydrosilylation reactions were conducted under an inert nitrogen atmosphere. Tetrahydrofuran (THF) was purified by refluxing over sodium metal in the presence of benzophenone. A styrene and divinylbenzene mixture (90:10 S/DVB) was stirred overnight over finely powdered calcium hydride. On the vacuum line, the mixture was frozen-degassed-thawed three times before being distilled into pre-calibrated high vacuum storage tubes. Styrene, hexamethylcyclotrisiloxane (D3), 2-vinylfuran, and the terminating agent chlorodimethylsilane were purified similarly.
Synthesis of Furan End-Functionalized Hairy Nanoparticles (HNP-F)
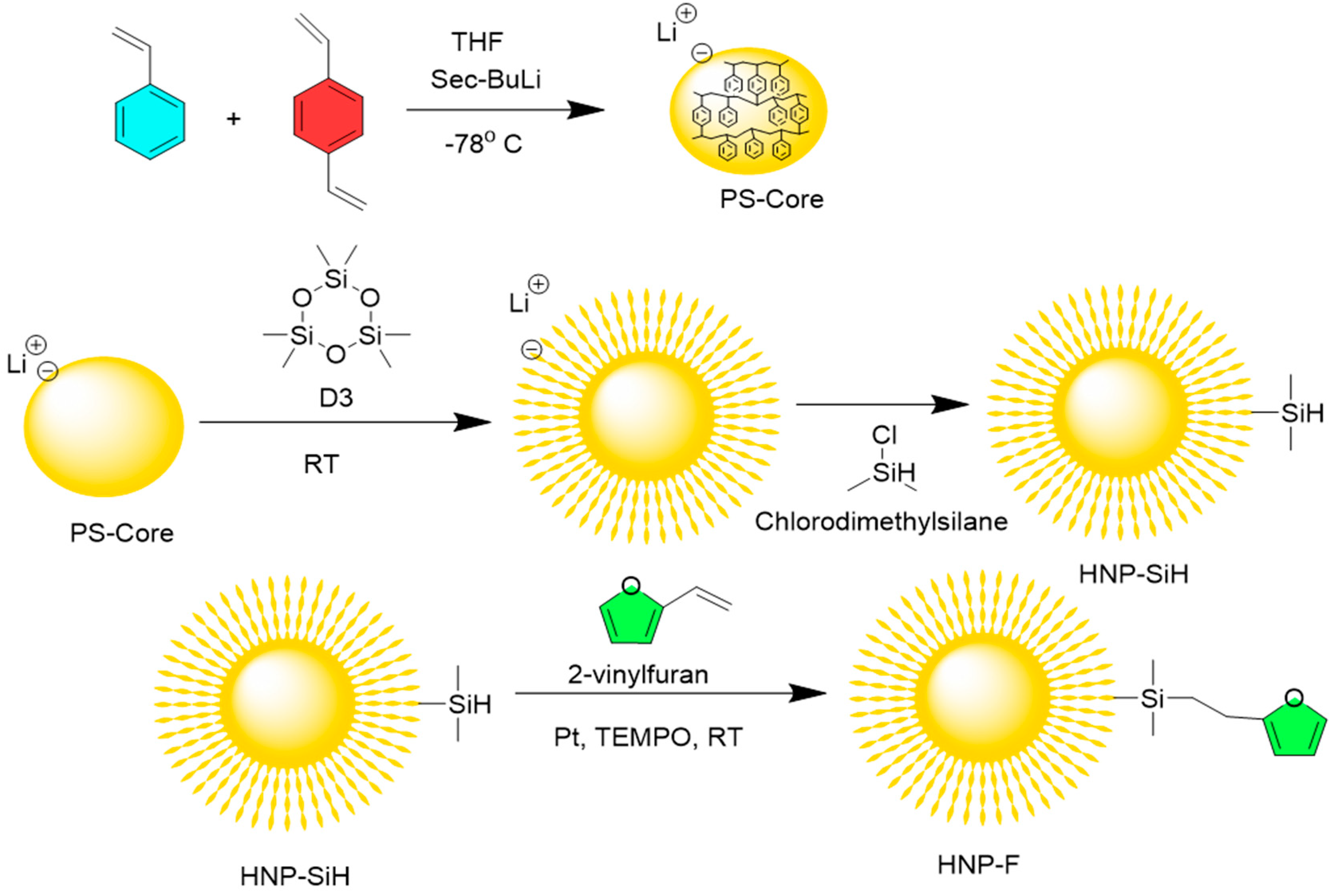
The HNPs functionalized with furan were synthesized by the high vacuum living anionic polymerization technique. The high vacuum storage tubes containing the mixture of monomer and cross-linker (S and DVB), hexamethylcyclotrisiloxane (D3), chloro-dimethylsilane, 2-vinylfuran and tetrahydrofuran (THF) were attached in advance to the reactor. The reactor was placed under high vacuum and flushed several times with dry nitrogen. At first 25 mL of dry distilled THF was transferred to the reactor from high vacu-um storage tube. After that, the mixture of styrene (1.50 g) and divinylbenzene (0.22 g) was transferred to the reactor. The temperature of the monomer solution was reduced to -78 °C. Under vigorous stirring, 1 mL of sec-BuLi (1.4 M) in cyclohexane was rapidly added to the reactor by septum. The color of the reaction mixture immediately turned red, which is characteristic of the living styryllithium anion indicating the occurrence of anionic polymerization. The first-step polymerization was carried out at -78 °C for 1.5 hr. The second-step polymerization was initiated by living styryllithium anion by adding 1.80 g of hexamethylcyclotrisiloxane (D3) dissolved in THF to the reaction mixture and gradually warmed the reactor temperature to room temperature. The red color initially became or-ange and lightened over time until finally it disappeared completely. The second polymer-ization was then carried out at room temperature for 15 h. Finally, purified chlorodime-thylsilane was added to the reactor from high vacuum storage tube to the reactor to terminate the polymerization. After 30 min 2 mL of reaction mixture was pulled by syringe for 1HNMR and FTIR characterization before adding 0.3gm 2-vinylfuran monomer to the reactor. Under continuous dry nitrogen flow to the reactor, 3–5 drops of Speier’s catalyst (H2PtCl6) and 5 mol% of TEMPO dissolved in dry THF were added, and the reaction mixture and the reaction was conducted for another 24 h at room temperature to complete the hydrosilylation reaction. Then the resulted polymer was precipitated into a large excess of methanol. It was dried in a vacuum oven at room temperature to produce 3.5 g furan functionalized HNPs.
Thermo-Reversible Crosslinking via Diels–Alder Reaction
The cross-linked TPE network was prepared by Diels–Alder reaction between bismaleimide (BMI) and the furan functionalized HNP. The polymers were dissolved in N,N-dimethylformamide, and THF (1:1) into 50 mL round bottom flasks. The necessary amount of the BMI was then added to the polymer solution. The experimental details are given in
Table 1. The mixture was stirred and placed into an oil bath set to 50 °C for 24 h. After that, the mixtures were precipitated into methanol to obtain the crosslinked yellow polymer. Then, the cross-linked polymer was dried under a vacuum at 50 °C and analyzed by DSC and ATR-IR. The DA reaction kinetics between all polymers and BMI were also studied at 50 °, 60 °, and 70 °C.
3. Results and Discussion
The hairy nanoparticles (HNP) were synthesized from the sequential addition of the monomers (
Scheme 1). In the first step, the mixture of styrene (S) and divinylbenzene (DVB) (90 S: 10 DVB molar ratio) was polymerized in dry THF using sec-BuLi (1.4 M) at -78 °C. In the second step, the cyclic hexamethylcyclotrisiloxane, D3, monomer was added to the core and polymerized at room temperature. The living polymers were terminated by dimethylchlorosilane to obtain the HNP-SiH. The spectral data is consistent with published data [
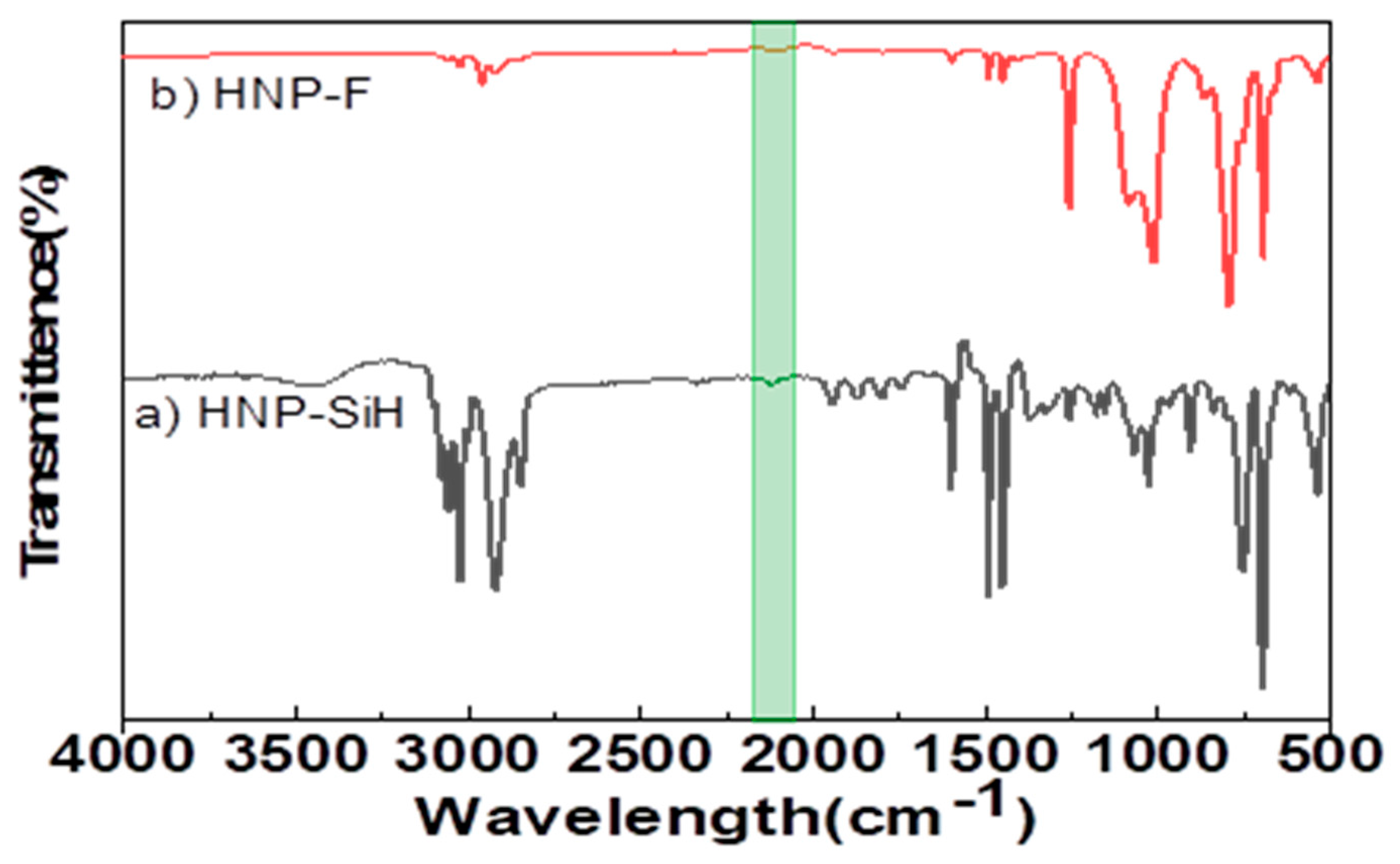
34]. ATR-FTIR spectroscopy analysis of HNP-SiH in
Figure 2 shows absorption bands of PDMS hairs and a characteristic absorption band of silyl hydride (Si-H) functionality at the end. A characteristic absorption band at 2120 cm
−1 corresponds to a Si-H bond stretching vibration. The absorption band at 1261 cm
−1 corresponds to the Si–CH
3 group whereas Si–O–Si anti-symmetrical stretching peaks are observed at 1097–1032 cm
−1. The strong peak at 805 cm
−1 corresponds to Si-C stretching. For the PS-core in HNP-SiH, the 2900–3000 cm
−1 peaks correspond to the aliphatic C-H stretching, and the C-H aromatic peaks can be seen in the range of 3000–3090 cm
−1. The peaks for the C=C aromatic are observed at 1453 cm
−1, 1494 cm
−1, and 1602 cm
−1.
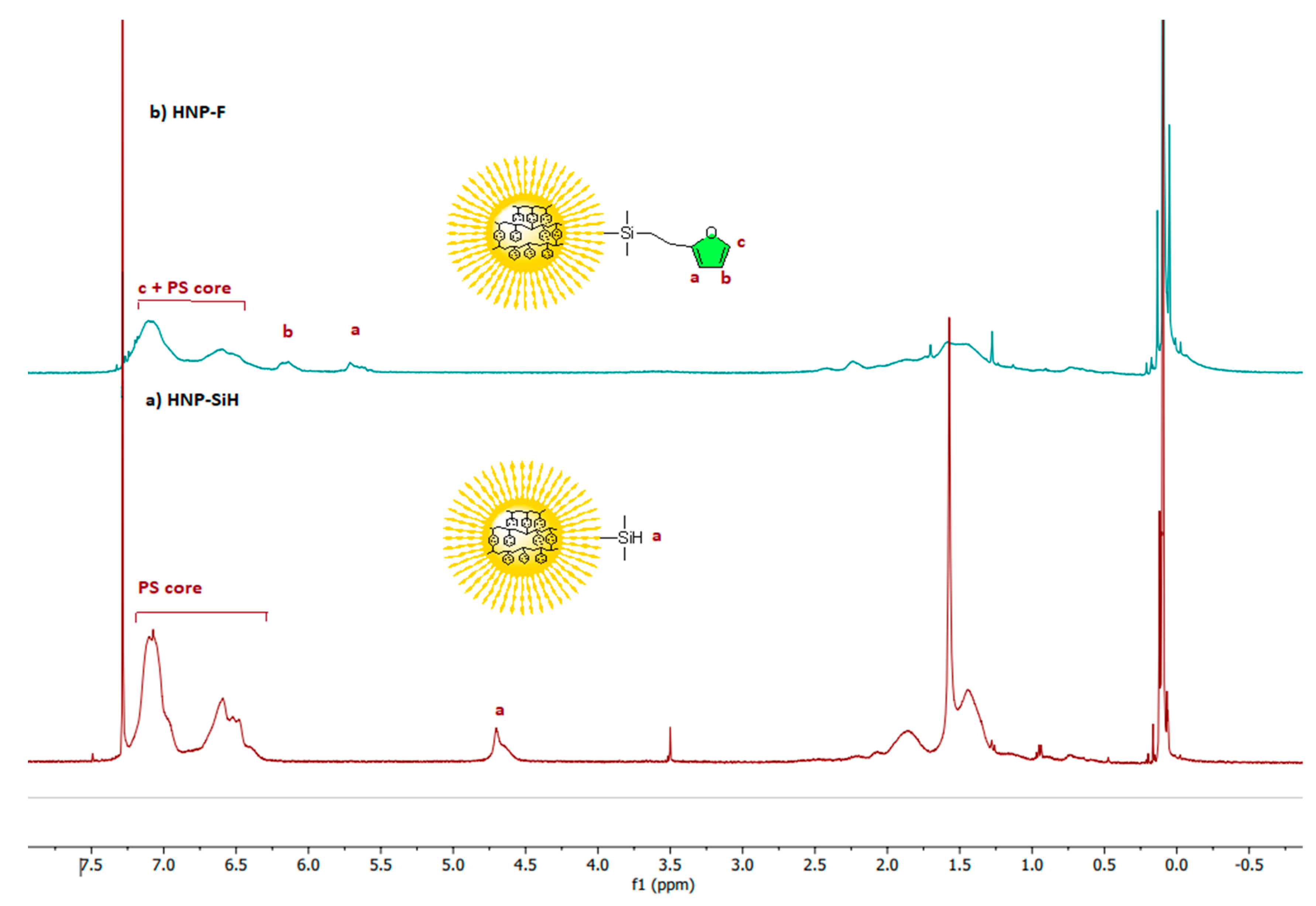
1H NMR spectrum shows the characteristic peak for the silane proton at 4.72 ppm. The characteristic signals at 0.25 to −0.25 ppm correspond to the PDMS brushes in HNP-SiH (
Figure 3a). Alkyl protons of the backbone of the polystyrene (-CH
2-CH-) cores appear in the range from 0.9 to 2.0 ppm, and the aryl protons from the phenyl ring show the characteristic peak ranging from 6.4 to 7.25 ppm. The methyl protons introduced from the sec-butyl group at the chain ends are observed at 0.74 ppm (
Figure 3a). The range between 5.1 to 5.6 ppm shows no signals attributed to methylene protons (CH
2=CH
2) of unreacted vinyl groups stemming from the crosslinker DVB, indicating that all vinyl groups of the crosslinkers were copolymerized to form the crosslinked polystyrene core. The styrene and the hexamethylcyclotrisiloxane (D3) monomers were polymerized by the living anionic polymerization (LAP) technique using Sec-BuLi as initiator and terminated by dimethylchlorosilane, according to
Scheme S1, to get silyl hydride terminated polystyrene and poly(dimethylsiloxane) linear polymers. The functionalization of the materials was confirmed by the disappearance of the Si-H peaks and the appearance of new peaks that are characteristics of the furan in both ATR-FTIR and
1H NMR spectra (
Figure 2 and
Figure 3;
Figure S2 and S3). The
1H NMR spectrum of the HNP-F shown in
Figure 3b confirmed the disappearance of the Si-H peak at 4.54 ppm. The characteristic furan peaks appeared at 6.1–6.4 ppm, which corresponded to protons of the furan ring attached to the polymer—the methylene protons of furan overlap with the alkyl protons of the polystyrene backbone. No residual allyl protons between 5.1 to 5.6 ppm were detected in unreacted vinyl groups in the furan.
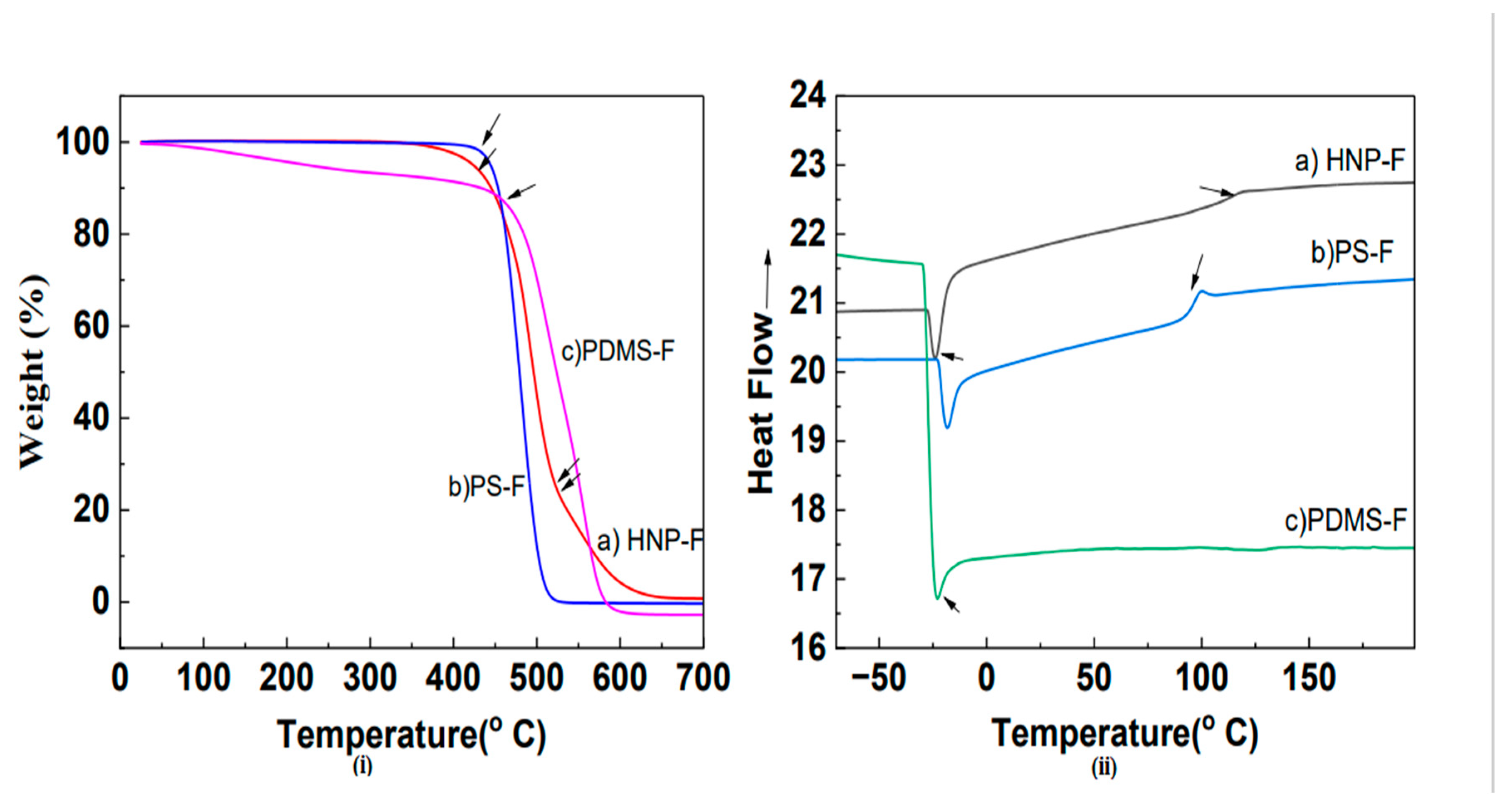
Thermogravimetric analysis (TGA) was used to study the thermal stability of all synthesized materials. The analyses were performed using nitrogen flow and scanned from room temperature to 700 °C.
Figure 4i(b) shows the decomposition of PS-F, which began at ~ 413 °C. The thermal decomposition of HNP-F shows a two-step mass loss process between 25 ° and 700 °C, which indicates a two-phase system of hairy nanoparticles. The first degradation step, at approximately 382 °C,
Figure 4i (a), is attributed to the decomposition of the PS Core. The second degradation step, at around 515 °C, corresponds to the decomposition of the PDMS hairs of nanoparticles. When compared with the decomposition temperature for PS-core, the lowering of the decomposition temperature of PS in the HNP is attributed to the interplay between the PS and PDMS segments.
Furthermore, DSC was also used to determine the thermal properties and the glass transition temperature (Tg) for all polymers, and the polymers’ thermograms are shown in
Figure 4(ii). As seen in the DSC thermogram, a glass transition temperature of the PS was observed at 103 °C. The DSC thermogram of the HNP showed two distinct transition temperatures. The polystyrene core’s glass transition temperature (Tg) was observed at 110 °C. The higher glass transition temperature than the pure homopolymers is because the polystyrene core of the HNPs is cross-linked. Because of the operational limitations of the DSC instrument, a glass transition temperature (Tg) of the PDMS segment was not determined. However, a melting transition temperature (Tm) of the PDMS phase was observed at -41 °C. Therefore, the HNPs are microphase-separated materials and should function as thermoplastic elastomers [
34].
Crosslinking via a Diels–Alder Reaction
The cross-linking of HNP-F was carried out by the reaction with a bismaleimide (BMI), i.e., a Diels–Alder reaction (
Scheme 2). The reaction between PDMS-F and PS-F and BMI was also studied. The FT-IR and UV-Vis spectrums were used to study the Diels-Alder reactions.
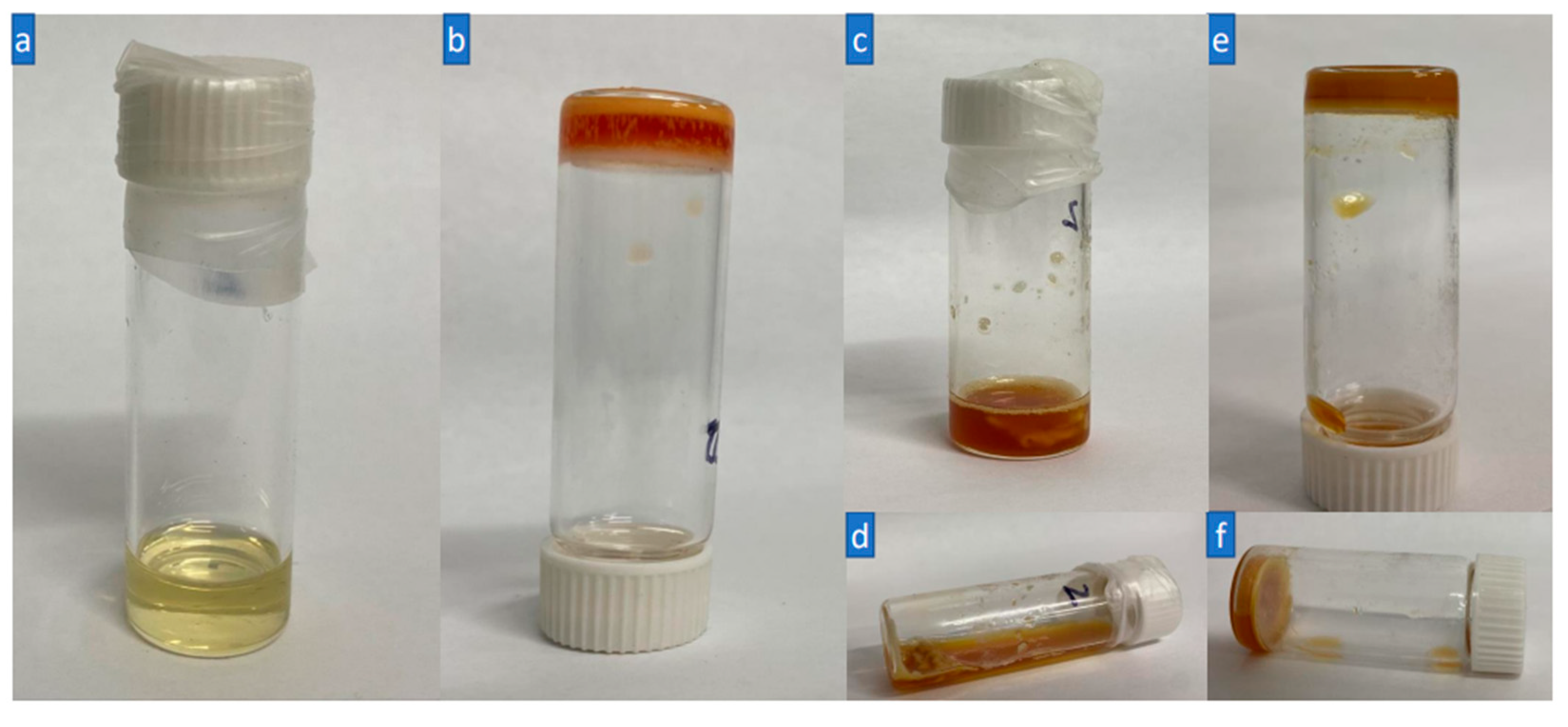
Figure 5 shows the visual observation of the crosslinking and decrosslinking of the HNP-F with BMI.
Figure 5a is a solution of the HNP-F and BMI, and
Figure 5b is the crosslinked gel prepared by heating to 50 °C for two (2) hours. The gel can be quickly decrosslinked (
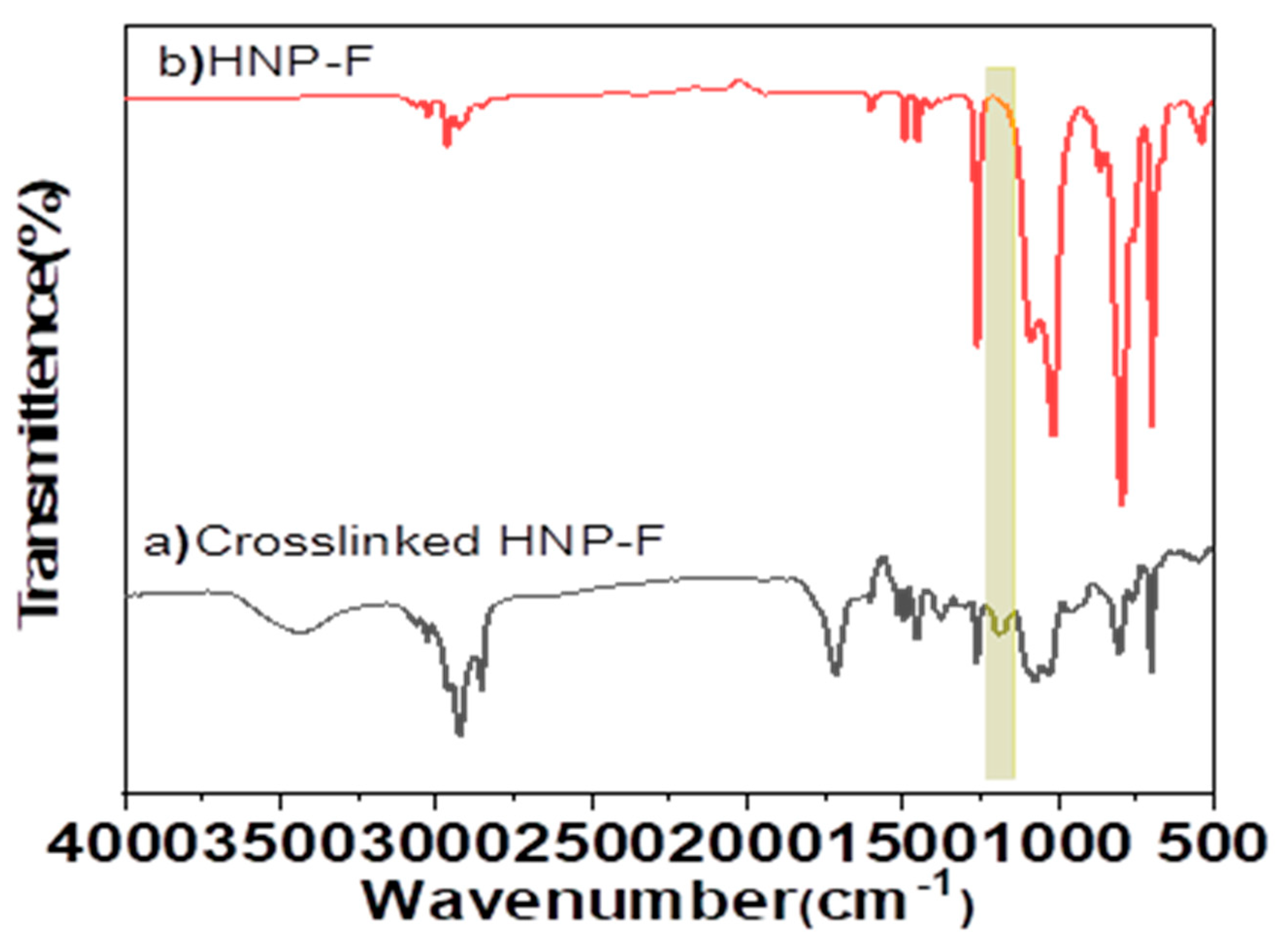
Figure 5c,d) by heating to 150 °C for one minute to recover the uncrosslinked HNP-F. The recovered HNP-F can be crosslinked again by heating it to 50 °C for two hours. The most interesting conclusion is how quickly and easily the decrosslinking reaction takes place and strongly suggests the utilization of HNPs and building blocks for recoverable and reusable networks. The chemical reaction of the crosslinking and decrosslinking can be followed by FT-IR spectroscopy. The absorption peaks in the range 1190 cm
−1 correspond to the characteristic succinimide bands that result from the formation of the DA adduct, which indicates the crosslinking reaction (
Figure 6) [
35,
36]. The peaks for C-O-C and Si-O-Si decrease upon crosslinking, as do the absorption bands at 599 cm
−1, which relate to the furan ring. New absorption peaks are observed at 1770 and 1720 cm
−1, corresponding to the BMI carbonyl groups [
37,
38].
Kinetics Study of the Diels–Alder Reaction by UV-Vis spectroscopy
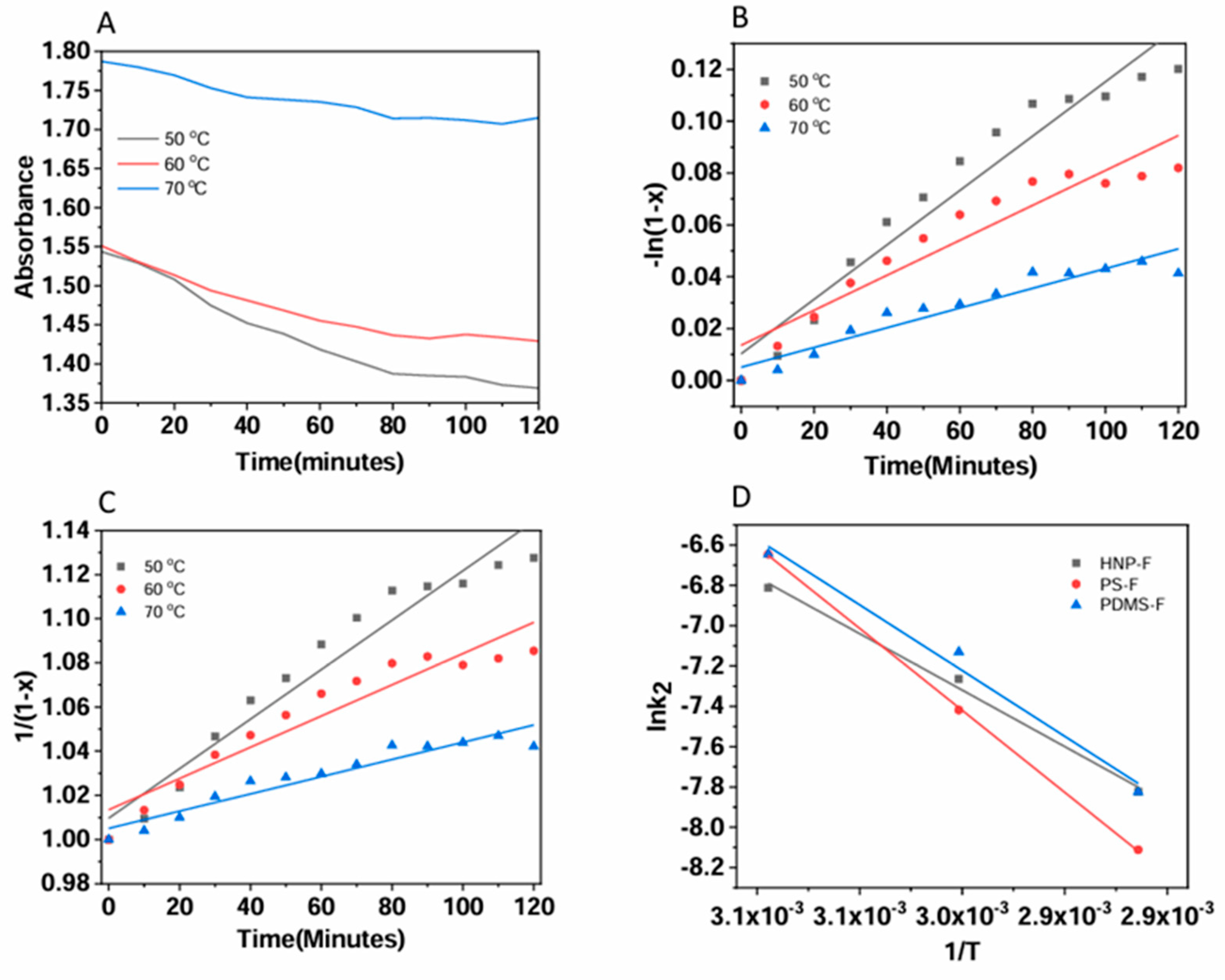
The kinetics of the forward Diels–Alder reaction of all HNP-F, PSF, and PDMS-F with BMI were studied by UV-Vis spectroscopy at different temperatures (50 °C, 60 °C, and 70 °C. As seen in the UV-Vis spectra of the HNP-F, the maleimide absorption decreases with time at 310 nm. For HNP-F with BMI, the absorption at 310 nm dropped rapidly initially, indicating that maleimide groups reacted quickly (
Figure 7a). After that, the absorption changed slightly only. On the other hand, the maleimide absorption of PS-MBI and PDMS-BMI decreased considerably slower than the HNP-F (
Figure S4).
The degree of the reaction with the reaction time was calculated using the following expressions:
where x is the conversion rate, which can be expressed as:
By rearranging equation (2), we get
where A
0 is the initial absorbance at (t=0), and
At is the absorbance at 310 nm at time t.
When the reaction is first-order (n=1) equation (1) can be written as for pseudo-first-order reaction:
When the reaction is second order (n=2) equation (1) can be written as:
The reaction rate constants for first and second order (
k1, k2) for the DA reaction were calculated for each temperature and are listed in
Table 2. The rate constants were derived from the slope of the graphs shown in (
Figure 7) The reaction rate constants (
k1, k2) increased when the temperature rises from 50 °C to 70 °C. This would significantly accelerate the cross-linking reaction rate because the rate constants’ value significantly changes with temperature. When comparing all the results of order kinetics, the DA reaction between the polymers and BMI followed second-order kinetics [
39,
40,
41].
The DA reactions’ activation energy was calculated using the Arrhenius plot.
E
a is the activation energy, R is the universal gas constant (R = 8.314 J.mol-1.K-1), T is the absolute temperature (K), and A is the pre-exponential factor. The DA reaction activation energy was calculated from the rate constants of the second-order reaction kinetics at 50 °C, 60 °C, and 70 °C, which was determined by the slope of the line from graphing ln k versus 1/T, shown in
Figure 7d. The activation energy of the cross-linking reaction for the HNP, PS, and PDMS with BMI are 36.91, 45.31, and 41.34 kJ mol−1, respectively, and are listed in
Table 3. The values are similar to those found in previous literature for the DA reaction in polymeric systems [
36,
39,
40]. Compared to the three cross-linking reactions of HNP, PS, and PDMS, the Ea of Diels-Alder reaction of HNP with BMI is smaller than furan functionalized homopolymers. This result may be because of the HNP-F spherical particle shape, which may be an approach to control assembly and disassembly processes. Furthermore, the chain flexibility of PDMS-F resulted in a lower Ea compared to the more rigid of PS-F chain in the cross-linking reaction of homopolymers.
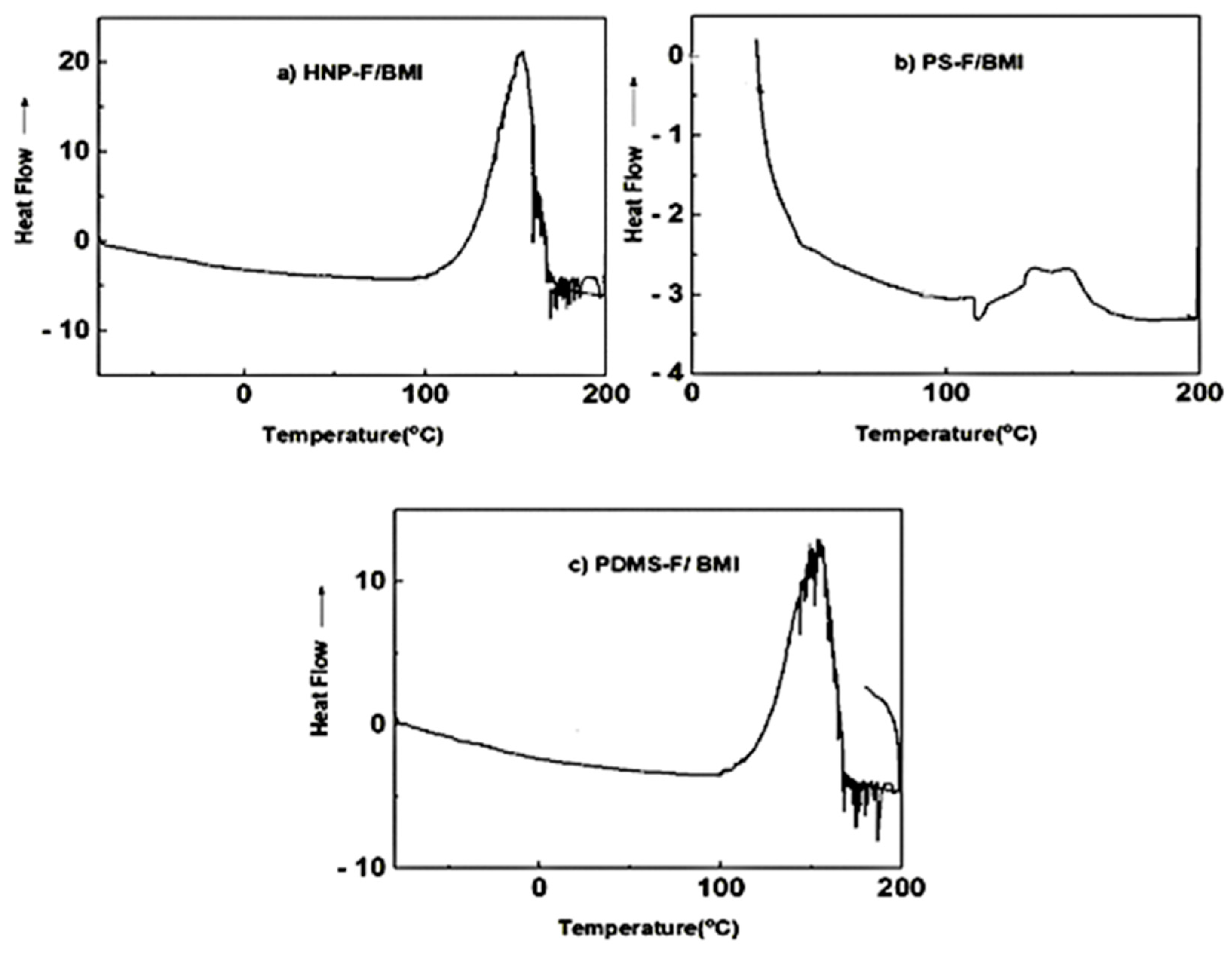
Differential scanning calorimetry (DSC) was used to study the retro Diels Alder reaction of the crosslinked polymers with BMI.
Figure 8a–c shows the heating curves of the DSC analysis of HNP-F, PS-F, and PDMS-F after reaction with the BMI. As seen in the thermogram, a broad peak at 154 °C was observed, indicating the cleavage of HNP-BMI via a retro-DA (rDA) reaction in the first heating curve. No peak was observed in the second heating curve since the cooling process time was not long enough to form the DA adduct [
37].
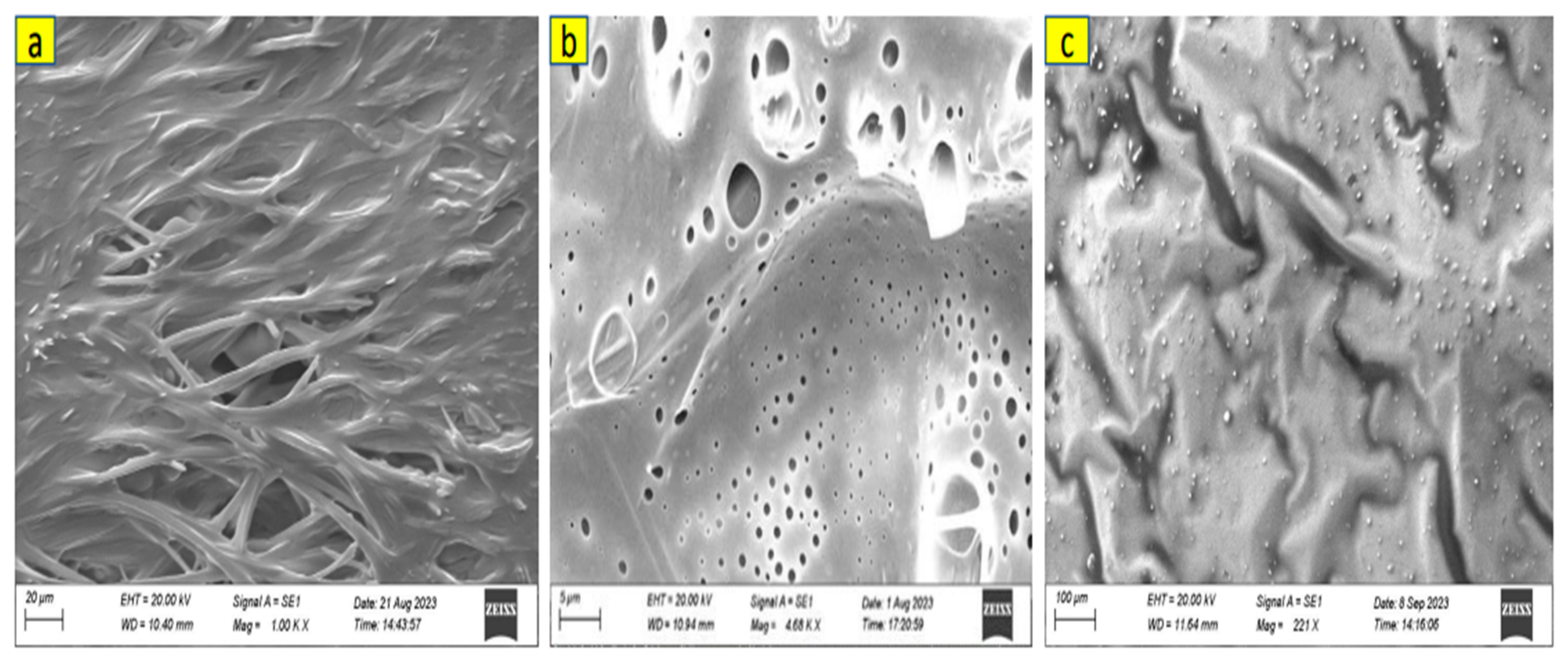
Scanning electron microscopy was used to investigate the morphology of the HNP-F, PS-F, and PDMS-F before and after reaction with BMI. All samples were prepared by drop-casting on silicon wafers using chloroform as solvent. The SEM images reveal the surface continuity of the polymer samples. The crosslinked polymers showed a continuous surface with porosity. Crosslinked HNP-F TPEs show the most uniform surface with some anisotropic pores (
Figure 9b), which is expected. The polystyrene and PDMS are in crosslinked state, they are not continuous but rather broken up or blistering surface (
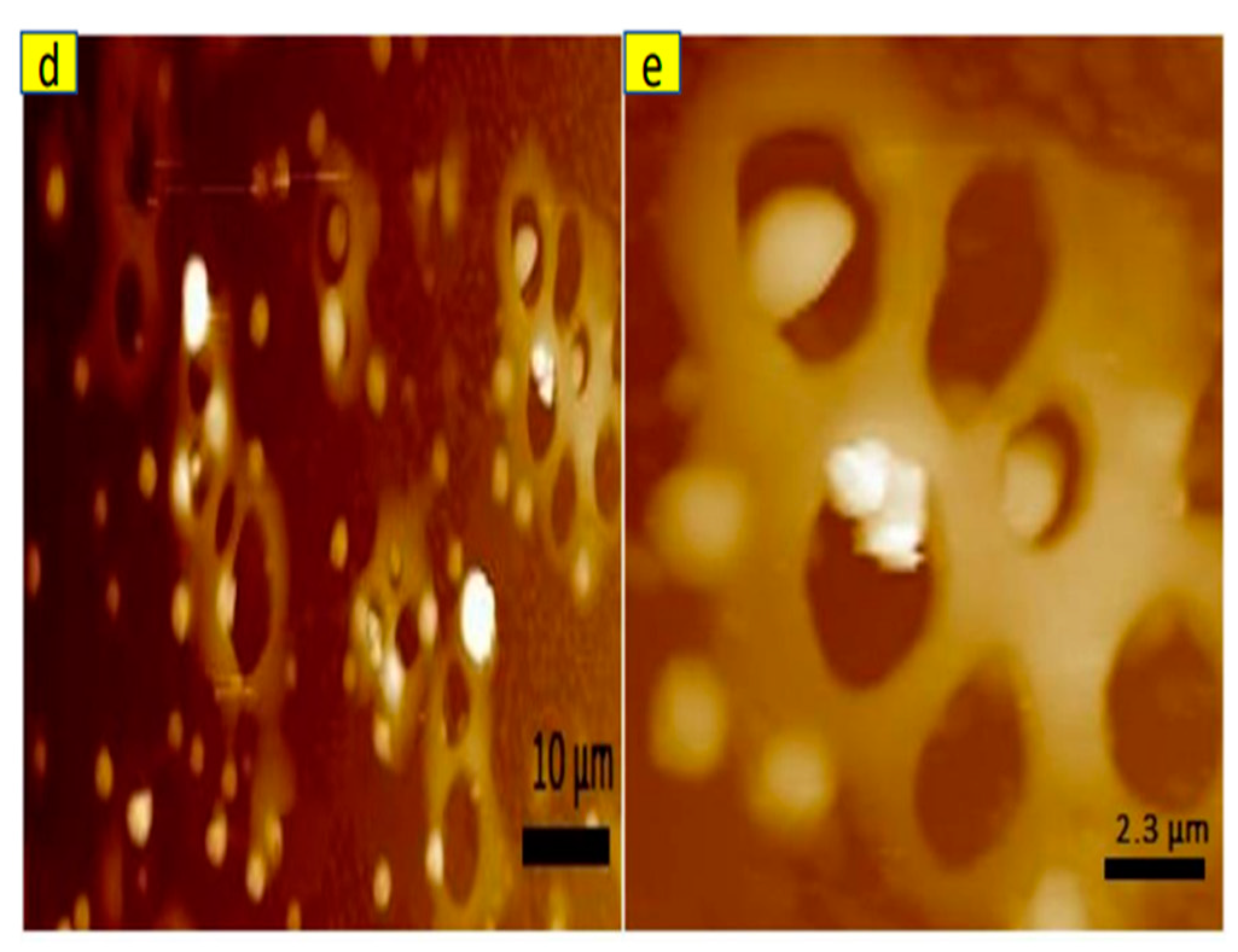
Figure S5b,c). Furthermore, AMF images of crosslinked HNP-F also reveals porous surfaces (
Figure 9d,e).
4. Conclusions
In summary, we have synthesized chain-end furan functionalized hairy nanoparticles (HNP-F) with hard polystyrene (PS) core and soft polydimethylsiloxane (PDMS) shells via a one-pot approach. The furan functionalized HNPs were synthesized, combining sequential living anionic polymerization and hydrosilylation reaction. Differential scanning calorimetry shows the presence of two thermal transitions, indicative of the presence of a poly (dimethylsiloxane) soft phase and a poly(styrene) hard phase, suggesting that the HNPs are a thermoplastic elastomer. Additionally, furan-functionalized PS and PDMS were successfully synthesized and reacted with bis-maleimide (BMI). The activation energy of the forward Diels-Alder reaction of BMI with HNP-F, PS-F, and PDMS-F was determined to be 46.57, 67.61, and 54.22 kJ mol−1, respectively. These results indicated that the activation energy (Ea) was highly dependent on the polymer shape and flexibility. HNP-F, due to its shape, flexibility of hairs, and uniformity, needs less energy to react with BMI compared to linear polymers. Moreover, SEM and AFM images show uniform crosslinked surfaces originating from HNP-F. BMI can crosslink the HNP-F by heating it to 50 eating it to 150 C for one minute to recover the un-crosslinked HNP-F. The recovered HNP-F can be crosslinked again by heating it to 50 o C for two hours. The most interesting conclusion is how quickly and easily the decrosslinking reaction takes place and strongly suggests the utilization of HNPs and building blocks for recoverable and reusable networks. The study indicates furan functionalized HNPs can be promising TPE building blocks for tailored recoverable and reprocess-able and have the potential to minimize thermoplastic elastomeric waste in the environment.
Supplementary Materials
The following supporting information can be downloaded at:
www.mdpi.com/xxx/s1, Procedures for synthesis of 2-vinylfuran, PDMS-F and PS-F (Scheme S1). 1H NMR spectra of 2-vinylfuran (Figure S1), FTIR and 1H NMR spectra of PS-F, PS-SiH, PDMS-F and PDMS-SiH (Figures S2 and S3). UV-Vis spectra and reaction kinetic plots (Figure S4). SEM images of before and after Diels-Alder reaction of PS-F and PDMS-F with BMI.
Author Contributions
Supervisor and Conceptualization, I.K. and M.U.; Writing—original draft preparation, I.K., Sultan A. and M.U.; Methodology, I.K., E.A., M.U., and Sultan A.; Investigation, M.U., E.A., Sultan A., N.W. and Saber A., Validation, M.U., E.A., Sultan A., N.W., I.K. and Saber A.: Data curation, Sultan A., E.A. and M.U. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the US ARMY RESEARCH OFFICE WN911NF2010272; NSF DMR-2122147 and NSF PREC-2216807.
Institutional Review Board Statement
Not Applicable.
Data Availability Statement
Data supporting this study is included within the article.
Acknowledgments
Not Applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Foundation, E.M. The New Plastics Economy: Rethinking the Future of Plastics. Ellen MacArthur Found No. 2016, 120.
- Miller, L.; Soulliere, K.; Sawyer-Beaulieu, S.; Tseng, S.; Tam, E.C. and Alternatives to Plastics Recycling in the Automotive Sector. Materials 2014, 7, 5883–5902. [Google Scholar] [CrossRef] [PubMed]
- Duflou, J.R.; de Moor, J.; Verpoest, I.; Dewulf, W. Environmental Impact Analysis of Composite Use in Car Manufacturing. CIRP Ann 2009, 58, 9–12. [Google Scholar] [CrossRef]
- Yuan, C.; Zhang, M.Q.; Rong, M.Z. Application of Alkoxyamine in Self-Healing of Epoxy. J Mater 2014, 2, 6558–6566. [Google Scholar] [CrossRef]
- Toncelli, C.; de Reus, D.C.; Picchioni, F.; Broekhuis, A.A. Properties of Reversible Diels-Alder Furan/Maleimide Polymer Networks as Function of Crosslink Density. Macromol Chem 2012, 213, 157–165. [Google Scholar] [CrossRef]
- Goiti, E.; Huglin, M.B.; Rego, J.M. Thermal Breakdown by the Retro Diels-Alder Reaction of Crosslinking in Poly [Styrene-Co-(Furfuryl Methacrylate)]. Macromol Rapid Commun 2003, 24, 692–696. [Google Scholar] [CrossRef]
- Gheneim, R.; Perez-Berumen, C.; Gandini, A. Diels-Alder Reactions with Novel Polymeric Dienes and Dienophiles: Synthesis of Reversibly Cross-Linked Elastomers. Macromolecules 2002, 35, 7246–7253. [Google Scholar] [CrossRef]
- Franc, G.; Kakkar, A.K. Diels-Alder ``Click’’ Chemistry in Designing Dendritic Macromolecules. Chem 2009, 15, 5630–5639. [Google Scholar] [CrossRef]
- Gandini, A.; Silvestre, A.; Coelho, D. Reversible Click Chemistry at the Service of Macromolecular Materials. Polym. Chem. 2013, 4, 1364–1371. [Google Scholar] [CrossRef]
- Nandivada, H.; Jiang, X.; Lahann, J.C.C.V. and Control in the Hands of Materials Scientists. Adv Mater 2007, 19, 2197–2208. [Google Scholar] [CrossRef]
- Chen, X.; Dam, M.A.; Ono, K.; et al. A Thermally Re-Mendable Cross-Linked Polymeric Material. Science (1979) 2002, 295, 1698–1702. [Google Scholar] [CrossRef]
- Stevens, M.P.; Jenkins, A.D. Crosslinking of polystyrene via pendant maleimide groups. J Polym Sci Polym Chem Ed. 1979, 17, 3675–3685. [Google Scholar] [CrossRef]
- Canary, S.A.; Stevens, M.P. Thermally Reversible Crosslinking of Polystyrene via the Furan-Maleimide Diels--Alder Reaction. J Polym Sci 1992, 30, 1755–1760. [Google Scholar] [CrossRef]
- Bapat, A.P.; Ray, J.G.; Savin, D.A.; Hoff, E.A.; Patton, D.L.; Sumerlin, B.S. Dynamic-Covalent Nanostructures Prepared by Diels-Alder Reactions of Styrene-Maleic Anhydride-Derived Copolymers Obtained by One-Step Cascade Block Copolymerization. Polym Chem 2012, 3, 3112–3120. [Google Scholar] [CrossRef]
- Barthel, M.J.; Rudolph, T.; Crotty, S.; Schacher, F.H.; Schubert, U.S.H.; Copolymers, D. of Poly(Furfuryl Glycidyl Ether) by Living Anionic Polymerization: Toward Reversibly Core-Crosslinked Micelles. J Polym Sci 2012, 50, 4958–4965. [Google Scholar] [CrossRef]
- Kuang, X.; Liu, G.; Dong, X.; Liu, X.; Xu, J.; Wang, D. Facile Fabrication of Fast Recyclable and Multiple Self-Healing Epoxy Materials through Diels-Alder Adduct Cross-Linker. J Polym Sci 2015, 53, 2094–2103. [Google Scholar] [CrossRef]
- Kang, B.G.; Pramanik, N.B.; Singha, N.K.; Lee, J.S.; Mays, J. Precise Synthesis of Thermoreversible Block Copolymers Containing Reactive Furfuryl Groups via Living Anionic Polymerization: The Countercation Effect on Block Copolymerization Behavior. Polym Chem 2015, 6, 6732–6738. [Google Scholar] [CrossRef]
- Das, S.; Samitsu, S.; Nakamura, Y.; et al. Thermo-Resettable Cross-Linked Polymers for Reusable/Removable Adhesives. Polym Chem 2018, 9, 5559–5565. [Google Scholar] [CrossRef]
- Buonerba, A.; Speranza, V.; Capacchione, C.; Milione, S.; Grassi, A. Improvement of Tensile Properties, Self-Healing and Recycle of Thermoset Styrene/2-Vinylfuran Copolymers via Thermal Triggered Rearrangement of Covalent Crosslink. Eur Polym J 2018, 99, 368–377. [Google Scholar] [CrossRef]
- Liang, C.; Li, J.; Xia, M.; Li, G.; Luo, Y.P.; Study, K. of Self-Repairing Hydroxyl-Terminated Polybutadiene Binders Based on the Diels-Alder Reaction. Polymers 2017, 9, 6. [Google Scholar] [CrossRef]
- Jiang, X.; Zhao, B.; Zhong, G; et al. Microphase Separation of High Grafting Density Asymmetric Mixed Homopolymer Brushes on Silica Particles. Macromolecules 2010, 43, 8209–8217. [Google Scholar] [CrossRef]
- Mohan, S.J.; Mohan, E.C.; Yamsani, M.R. One-Pot Synthesis of Hairy Nanoparticles by Emulsion ATRP. Macromolecules 2009, 42, 1597–1603. [Google Scholar]
- Wang, X.; Hall, E.; Warren, J.; et al. Characterization, and Application of Novel Polymeric Nanoparticles. Macromolecules 2007, 40, 499–508. [Google Scholar] [CrossRef]
- Helms, B.; et al. One-Pot Reaction Cascades Using Star Polymers with Core-Confined Catalysts. Angew. Chemie Int. Ed. 44, 6384–6387 (2005).
- Klos, J.; Pakula, T. Interaction of a Spherical Particle with Linear Chains. II. Chains End-Grafted at the Particle Surface. J Chem 2003, 118, 16. [Google Scholar] [CrossRef]
- Yezek, L.; Sch”artl, W.; Chen, Y.; Gohr, K.; Schmidt, M. Influence of Hair Density and Hair Length on Interparticle Interactions of Spherical Polymer Brushes in a Homopolymer Matrix. Macromolecules 2003, 36, 4226–4235. [Google Scholar] [CrossRef]
- Wang, X.; Foltz, V.J.; Rackaitis, M.; Bohm, G.G.A. Dispersing Hairy Nanoparticles in Polymer Melts. Polymer 2008, 49, 5683–5691. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Whitehead, K.A.; Nuhn, L.; et al. Combinatorial Synthesis of Chemically Diverse Core-Shell Nanoparticles for Intracellular Delivery. Proc Natl Acad Sci U.S. A 2011, 108, 12996–13001. [Google Scholar] [CrossRef]
- Werne, V.; Patten, T.E.T. Preparation of Structurally Well-Defined Polymer-Nanoparticle Hybrids with Controlled/Living Radical Polymerizations. J Am 1999, 121, 7409–7410. [Google Scholar] [CrossRef]
- Ueda, K.; Hirao, A.; Nakahama, S. Synthesis of Polymers with Amino End Groups. 3. Reactions of Anionic Living Polymers with α-Halo-ι-Aminoalkanes with a Protected Amino Functionality 2002, 23, 939–945. [Google Scholar]
- Takenaka, K.; Hirao, A.; Nakahama, S. Synthesis of End functionalized Polymer by Means of Living Anionic Polymerization. 4. Synthesis of Polyisoprene and Polystyrene with Epoxy Termini by Reaction of Their Anionic Living Polymers with 2-bromoethyloxirane. Polym. Int. 1995, 37, 291–295. [Google Scholar] [CrossRef]
- Quirk, R.P.; Zhuo, Q.; Jang, S.H.; Lee, Y.; Lizarraga, G. Principles of Anionic Polymerization: An Introduction. Published online 1998:2–27. [CrossRef]
- Zheng, L.; Xie, A.F.; Lean, J.T. Polystyrene Nanoparticles with Anionically Polymerized Polybutadiene Brushes. Macromolecules 2004, 37, 9954–9962. [Google Scholar] [CrossRef]
- Zhou, G.; Person, V.; Khan, I.M. Hairy Nanoparticles with Hard Polystyrene Cores and Soft Polydimethylsiloxane Shells: One-Pot Synthesis by Living Anionic Polymerization and Characterization. Macromol. Chem 2016, 217, 2601–2610. [Google Scholar] [CrossRef]
- Polgar, L.M.; Duin, M.; van Broekhuis, A.A.; Picchioni, F. Use of Diels − Alder Chemistry for Thermoreversible Cross-Linking of Rubbers: The Next Step toward Recycling of Rubber Products? Macromolecules 2015, 48, 7096–7105. [Google Scholar] [CrossRef]
- Liu, X.; Du, P.; Liu, L.; Zheng, Z. Kinetic Study of Diels -- Alder Reaction Involving in Maleimide -- Furan Compounds and Linear Polyurethane. Polym Bull. 2013, 70, 2319–2335. [Google Scholar] [CrossRef]
- Xu, Z.; Zhao, Y.WX. ATL Thermally Healable Polyhedral Oligomeric Silsesquioxane (POSS) Nanocomposite Based on Diels--Alder Chemistry. Chem. 2013, 49, 6755–6757. [Google Scholar]
- Gandini, A.; Hodge, P. Application of the Diels - Alder Reaction to Polymers Bearing Furan Moieties. 2. Diels - Alder and Retro-Diels - Alder Reactions Involving Furan Rings in Some Styrene Copolymers. Macromolecules 1998, 1, 314–321. [Google Scholar]
- Liu, S.; Liu, X.; He, Z.; Liu, L.; Niu, H. Thermoreversible Cross-Linking of Ethylene/Propylene Copolymers Based on Diels-Alder Chemistry: The Cross-Linking Reaction Kinetics. Polym. Chem. 2020, 11, 5851–5860. [Google Scholar] [CrossRef]
- Tian, Q.; Rong, M.Z.; Zhang, M.Q.; Yuan, Y.C.S. and Characterization of Epoxy with Improved Thermal Remendability Based on Diels-Alder Reaction. Polym Int 2010, 59, 1339–1345. [Google Scholar] [CrossRef]
- Polgar, L.M.; Kingma, A.; Roelfs, M.; van Essen, M.; van Duin, M.; Picchioni, F. Kinetics of Cross-Linking and de-Cross-Linking of EPM Rubber with Thermoreversible Diels-Alder Chemistry. Eur Polym J 2017, 90, 150–161. [Google Scholar] [CrossRef]
Figure 1.
Schematic illustration of crosslinking and decrosslinking of furan functionalized HNPs with bismaleimide.
Figure 1.
Schematic illustration of crosslinking and decrosslinking of furan functionalized HNPs with bismaleimide.
Scheme 1.
Synthesis of furan functionalized HNP-F.
Scheme 1.
Synthesis of furan functionalized HNP-F.
Figure 2.
ATR-FTIR spectrum of a) Silylhyride functionalized HNP, b) Furan functionalized HNP.
Figure 2.
ATR-FTIR spectrum of a) Silylhyride functionalized HNP, b) Furan functionalized HNP.
Figure 3.
500 MHz 1H NMR spectra of a) Silylhydride functionalized HNP(HNP-SiH) and b) Furan functionalized HNP(HNP-F).
Figure 3.
500 MHz 1H NMR spectra of a) Silylhydride functionalized HNP(HNP-SiH) and b) Furan functionalized HNP(HNP-F).
Figure 4.
(i) TGA and (ii) DSC thermograms of HNP-F, PS-F, PDMS-F.
Figure 4.
(i) TGA and (ii) DSC thermograms of HNP-F, PS-F, PDMS-F.
Scheme 2.
The Crosslinking of HNP-F with bismaleimide (BMI).
Scheme 2.
The Crosslinking of HNP-F with bismaleimide (BMI).
Figure 5.
(a) 10% (w/w) HNP-F and BMI dissolved in DMF and THF (1:1) before crosslinking, (b) after Crosslinked gel after heating for 2 h at 50 °C, (c,d) Decrosslinking after heating at 150 °C for 1 min and quench cooling; (e,f) crosslinking again at 50 °C for 2 h.
Figure 5.
(a) 10% (w/w) HNP-F and BMI dissolved in DMF and THF (1:1) before crosslinking, (b) after Crosslinked gel after heating for 2 h at 50 °C, (c,d) Decrosslinking after heating at 150 °C for 1 min and quench cooling; (e,f) crosslinking again at 50 °C for 2 h.
Figure 6.
ATR-FTIR spectra of a) crosslinked HNP-F with BMI and b) un-crosslinked HNP-F.
Figure 6.
ATR-FTIR spectra of a) crosslinked HNP-F with BMI and b) un-crosslinked HNP-F.
Figure 7.
(A)UV absorbance of BMI reaction with HNP-F with time (B) 1st order kinetics of HNP-F with BMI (C) 2nd order reaction kinetics of HNP-F and (D) Activation energies of HNP-F, PS-F and PDMS-F reaction with BMI.
Figure 7.
(A)UV absorbance of BMI reaction with HNP-F with time (B) 1st order kinetics of HNP-F with BMI (C) 2nd order reaction kinetics of HNP-F and (D) Activation energies of HNP-F, PS-F and PDMS-F reaction with BMI.
Figure 8.
a) Heating curve of DSC analysis of crosslinked HNP-F with BMI (b) shows the heating curve of the DSC analysis of PS with BMI. The retro-DA peak, which indicates the cleavage of PS-BMI, was observed at 143 °C. PDMS shows cleavage of PDMS-BMI at around 150 °C.
Figure 8.
a) Heating curve of DSC analysis of crosslinked HNP-F with BMI (b) shows the heating curve of the DSC analysis of PS with BMI. The retro-DA peak, which indicates the cleavage of PS-BMI, was observed at 143 °C. PDMS shows cleavage of PDMS-BMI at around 150 °C.
Figure 9.
SEM image of (a) Uncrosslinked HNP-F with BMI (b) crosslinked HNP-F with BMI, and (c) decrosslinked HNP-F after heated at 150 °C. AFM images of crosslinked HNP-F with BMI at different magnifications (d,e).
Figure 9.
SEM image of (a) Uncrosslinked HNP-F with BMI (b) crosslinked HNP-F with BMI, and (c) decrosslinked HNP-F after heated at 150 °C. AFM images of crosslinked HNP-F with BMI at different magnifications (d,e).
Table 1.
Composition for Diels -Alder Reactions.
Table 1.
Composition for Diels -Alder Reactions.
| Furan Functionalized Samples |
Weight of Polymers (mg) |
Weight of BMI (mg) |
DMF + THF (1:1 v/v) |
| HNP-F |
50 |
30 |
10 mL |
| PS-F |
25 |
10 |
7 mL |
| PDMS-F |
50 |
30 |
10 mL |
Table 2.
The Reaction Rate Constants (k1, k2) of HNP-F, PS-F and PDMS-F.
Table 2.
The Reaction Rate Constants (k1, k2) of HNP-F, PS-F and PDMS-F.
| Polymer |
Kinetic Order |
T/oC |
50 |
60 |
70 |
HNP-F |
n = 1 |
k1/min-1 |
0.001 |
0.0007 |
0.0004 |
| n = 2 |
k2/(min-1 mol-1 L) |
0.0011 |
0.0007 |
0.0004 |
PS-F |
n = 1 |
k1/min-1 |
0.0012 |
0.0006 |
0.0003 |
| n = 2 |
k2/(min-1 mol-1 |
0.0013 |
0.0006 |
0.0003 |
PDMS-F |
n = 1 |
k1/min-1 |
0.0012 |
0.0007 |
0.0004 |
| n = 2 |
k2/(min-1 mol-1 L) |
0.0013 |
0.0008 |
0.0004 |
Table 3.
Activation Energy for the Diels-Alder reactions of the furan functionalized polymers with BMI.
Table 3.
Activation Energy for the Diels-Alder reactions of the furan functionalized polymers with BMI.
Furan Functionalized End-Group
Samples |
The Activation Energy of the Crosslinking Reaction with BMI |
| HNP-F |
46.57 KJmol-1
|
| PS-F |
67.61 KJmol-1
|
| PDMS-F |
54.22 KJmol-1
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).