1. Introduction
Informed consent is fundamental to perioperative patient management in general. With regard to obtaining such consent for anesthesia in particular, major challenges in daily clinical practice arise from legal requirements and from the need to explain procedures in a way that patients can actually understand. Inadequate information, either by too short an interval between consenting and the procedure or by content that is not readily comprehended, may engender uncertainty in patients and can even result in lawsuits [
1]. Informed consent also needs to involve correct and reproducible documentation [
2].
This challenging ethical and legal background prompted the publication, in 2017, of guidelines for consent by the Association of Anaesthetists of Great Britain and Ireland [
3]. The American Society of Anesthesiologists, in turn, clearly states in its ethical guidelines that the "patient-physician relationship involves special obligations for the physician that include personal interaction with the patient, placing the patient’s interests foremost, faithfully caring for the patient and being truthful" [
4]. The same source highlights the importance of adequately interacting with patients, requiring that anesthesiologists "respect the right of every patient to self-determination".
There is an ever-present need to apply the highest of ethical standards in daily clinical practice. Given reports from around the world that patients do not fully grasp the messages communicated for informed consent [
5,
6,
7], our quest to maintain these standards must include an effort to convey information as lucidly as possible to raise as many patients as possible to adequate levels of understanding. Various obstacles come into play here, including physicians' everyday use of jargon that is not readily intelligible to patients, but factors such as age, cultural background, or level of education may also influence a given patient's ability to adequately comprehend the information provided.
It is a fact that, despite being a time- and staff-consuming medium, paper continues to be the gold standard of informed-consent media in anesthesia [
8]. Recent studies have explored alternatives like videos to illustrate urological procedures or 'digital media' for bariatric surgery [
9,
10], and preliminary reports are also available on virtual reality (VR) supporting the informed-consent process for surgical procedure [
11,
12]. The present report follows suit by comparing 'VR-assisted informed consent' for anesthesia with the conventional 'dialog-only' method in terms of time requirements, patient satisfaction, and cost implications.
2. Materials and Methods
2.1. Trial Authorization
The protocol for this trial had been approved by the Vinzenz Gruppe institutional ethics committee (ref. 17/2022) on July 7, 2022, and the study had been registered with the German Clinical Trials Register (DRKS00029225) on August 17, 2022.
2.2. Study Design and Patients
Fifty adults who had been scheduled for elective knee or hip arthroplasty in a tertiary center (Orthopedic Hospital Speising, Vienna, Austria) were prospectively enrolled and randomized. A study group and control group were thus formed, where informed consent for anesthesia was obtained using either a VR-assisted approach or by relying entirely on the usual patient-specialist dialogs. An adequate command of German was the only inclusion criterion, and any patients with known epilepsy, vestibular disease, or vision disorder were excluded. All participants were duly informed of the research objectives, and written informed consent for the study itself was obtained before the actual informed-consent steps under investigation. The 'VR-assisted' procedures in the study group, including the dialogs to address open questions, were then followed by a regular process of informed consent to meet all legal requirements for anesthesia.
2.3. Randomization and Control Group
A person not otherwise involved in the study used a web-based tool (
www.randomizer.org) for random assignment to a control or a study group. The randomization numbers thus returned were then used as identifiers for sealed envelopes containing the allocation. Two sets (one being a spare set for backup) of sealed envelopes that contained the allocation details were kept at a safe place throughout the study. One envelope was opened for each consecutive patient. Patients allocated to the control group were asked to complete a paper-based form for informed consent, approved by the Austrian Society of Anesthesiology, Resuscitation, and Intensive Care Medicine. This was followed by a one-on-one conversation with an anesthesiologist. All specialists involved in patient dialogs during the study were blinded to the group assignment.
2.4. Study Group ('VR-Assisted Informed Consent')
The VR hardware consisted of a standalone head-mounted display (Pico Neo3 Pro/Pro Eye; Pico Interactive Europe, Barcelona, Spain) with preinstalled software that included a VR movie (XRSynergies; Vienna, Austria). This film, close to 8 minutes long, featured 3D rooms (clinician's office, operating room) with doctors' avatars giving routine information about general anesthesia and its risks. This content (individual frames are shown for illustration in
Figure 1) had been developed from evidence-based guidelines issued by the German Network of Evidence-Based Medicine and the Austrian Society of Anaesthesiology, Resuscitation, and Intensive Care Medicine. A translation of the German-language voice track is provided as
Supplementary Text S1.
Note was taken of any adverse events related to patients wearing the head-mounted display. Having watched the VR movie, each patient was asked to rate on a Likert scale, with applicable scores ranging from 1 (strongly agree) to 5 (strongly disagree), whether he or she felt that the audiovisual explanations had been a satisfying experience:
Item 1: I experienced this VR-assisted way of giving my informed consent as useful.
Item 2: I would use this VR-assisted way of giving my informed consent again.
Item 3: I would recommend this VR-assisted method to my family and close friends.
2.5. Primary and Secondary Endpoints
The procedures in the study group just outlined were followed by one-on-one sessions with anesthesiologists for the patients to ask any open questions. The durations of the two different modes of patient-specialist dialog in both groups were recorded and analyzed as primary outcome variable. As secondary variables, we analyzed the Likert-scale items above and calculated, in US dollars, cost differences between both groups in anesthesiologists' time, based on 1700 man-hours per year and average incomes earned by US [
13] and UK [
14] anesthesiologists in 2022.
2.6. Sample Size Calculation
We searched PubMed and Google Scholar for effect sizes of VR interventions in studies dealing with informed consent. This returned one study analyzing comprehension and anxiety levels after informed-consent procedures carried out either conventionally or using 3D VR. 12 Based on a remarkably high effect size for comprehension levels between both groups, we calculated post hoc a Cohen's d of 2.48 and an effect size of r = 0.77 for the present study.
Our in-house records indicate a mean time requirement of ≈10 min for these patient-specialist dialogs. From the above literature search and considerations, we assumed a moderate-to-high effect size of Cohen's d (> 0.5) for this endpoint. A power analysis yielded a sample size of 50 patients for an 80% chance of detecting, at the 5% significance level, a decrease from ≈600 ± 200 s (i.e. the 10 min just mentioned) in the control group to 435 s in the study group, allowing for a dropout rate of 8%. The intention-to-treat principle was applied to account for non-compliance, protocol deviations, withdrawal, or, indeed, any unforeseen events after randomization [
15].
2.7. Statistical Analysis
A Kolmogorov-Smirnov test was used to check for normal distribution, followed by a non-parametric Mann-Whitney U-test for intergroup comparisons of metric and not normally distributed data. For intergroup comparisons of proportions, we used cross-tabulation and Pearson’s chi-square test. Results are expressed as medians with interquartile ranges (IQRs) and/or absolute values with percentages and differences considered significant at P<0.05. All operations were performed with IBM® SPSS® statistical software (v. 26.0.0.0; IBM, Armonk, NY, USA) and other dedicated tools for data analysis (Prism v. 10.1.1; GraphPad Software, Boston, MA, USA).
3. Results
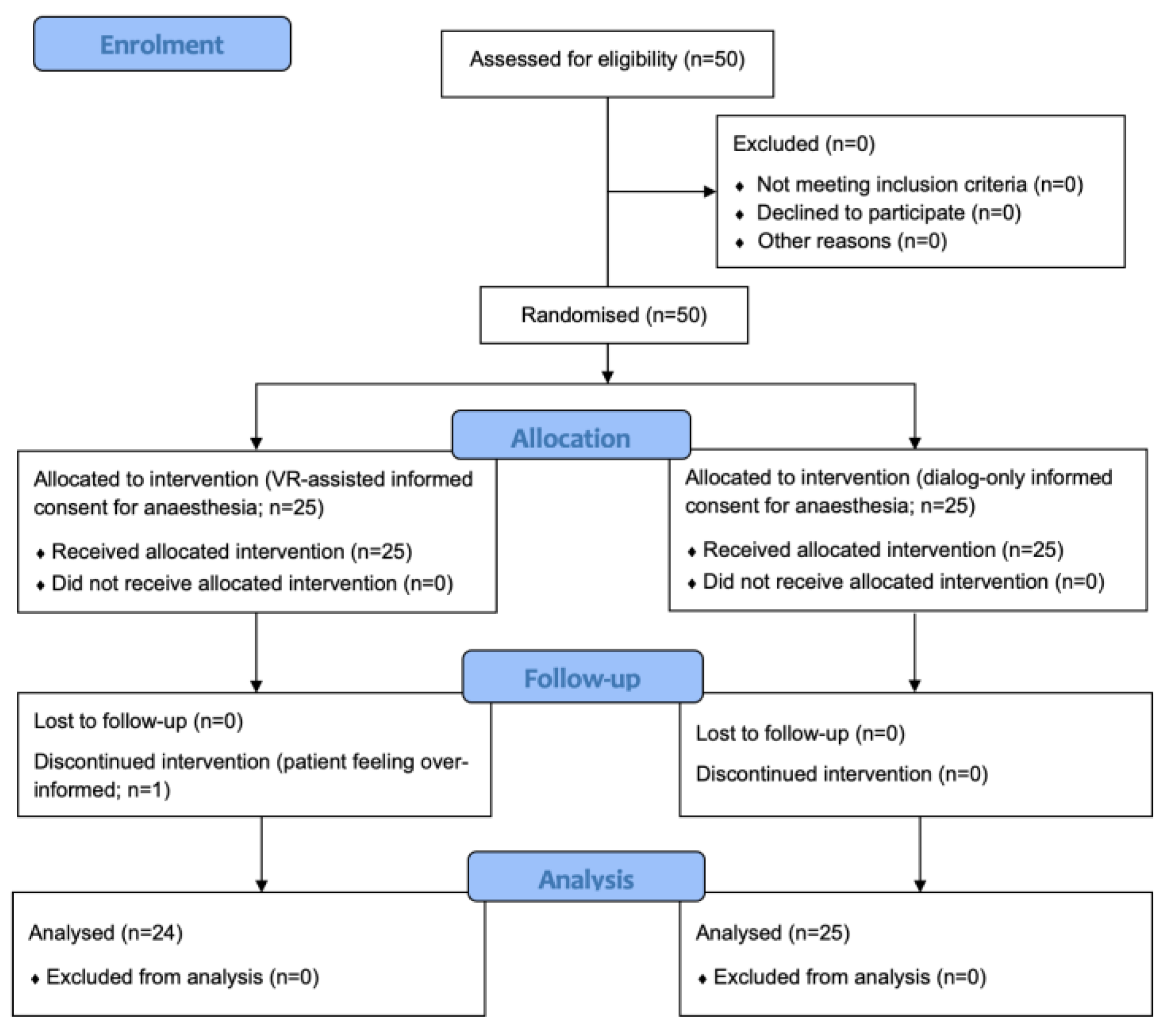
Patient recruitment took place from August 23, 2022, to April 6, 2023. As required by the result of sample-size calculation, 50 patients were initially enrolled, 49 of whom completed the study.
Figure 2 displays a flow chart of the study in accordance with the CONSORT guidelines. Pertinent patient data are summarized in
Table 1. No adverse events were noted in connection with wearing the head-mounted display.
In the study group, the patient-specialist dialogs preceded by exposure to the VR movie, which was itself 472 seconds (7:52 min) long, required a median of 93 seconds (IQR: 20−182 s). In the control group, the median duration of the conventional dialogs not preceded by exposure to a VR movie was 665 seconds (IQR: 260.5−829 s). This difference in median time requirements was statistically significant (P≤0.001).
Table 2 lists the patient ratings in the study group for the three-item Likert scale. Note that all patients exposed to the VR movie rated this experience as favorable (87.5%) or as neutral at worst (12.5%), while none of them indicated a negative impression. In addition, a vast majority (87.5%) affirmed both that they would use this VR-assisted method again and that they would recommend it to their family and close friends.
Table 3 illustrates the potential of cost savings from the shorter dialogs in the study group. Based on average anesthesiologists' incomes in the United States and United Kingdom, our method of 'VR-assisted informed consent' would reduce the cost of each informed-consent procedure by around 40 or 10.7 US dollars, respectively (P≤0.001).
Cost differences in anesthesiologists' time for each informed consent handled in the VR-assisted versus conventional way, based on 1700 man-hours per year (6,120.000 seconds) and average incomes earned by US [
13] and UK [
14] anesthesiologists in 2022. Values are expressed as US dollars.
4. Discussion
In this study, we compared a VR-assisted procedure of informed consent for anesthesia with the customary approach of placing the entire burden of information on the patient-specialist dialogs. These were found to be significantly shorter with those patients who had previously watched the VR movie, thus reducing expenditures for anesthesiologists' time. Also, the VR experience was found to be well accepted by the patients.
Informed consent is a mandatory requirement with both medical and legal implications. Despite an abundance of man-hours going into conventional procedures of informed consent on a daily basis [
8], there are limitations to how well these explanations of medical treatment are accepted and understood by the patients [
5,
7,
16]. In neurosurgery, to name but one high-risk discipline, deficiencies in informed consent are central to about 10 percent of malpractice claims [
1].
Intelligibility to patients and the cost factor are two major concerns in connection with obtaining informed consent before medical treatment. As shown by Bai and colleagues [
17], patients were scarcely able to recall the risks of interscalene blockade right after having been informed of them during the conversations. This is just one example for the dilemma existing between medico-legal eventualities by too little, and nocebo-style eventualities by too much, information. Effects of the latter type may arise from negatively biased expectations in patients comprehensively informed of all potential risks and complications [
18]. For truly informed consent, therefore, an adequate balance has to be struck that avoids both extremes.
Regarding the cost factor of informed-consent procedures, Kieninger and colleagues [
8] found 33 ± 16 minutes to be the mean time spent on patient-specialist dialogs for anesthesia. Also, experienced anesthesiologists were found to require significantly less time for patient interaction and documentation tasks. This may appear to suggest greater involvement of experienced anesthesiologists in preoperative dialogs, but this goal could only be achieved at the expense of availability in operating rooms when the waiting lists for any kind of surgery are constantly growing.
As an alternative, it may reasonably be expected that the current generation of 'smart' technologies may increase the time and cost efficiency of informed-consent procedures for anesthesia. 'Digital media' for bariatric surgery have been found to improve patients' understanding of procedure-specific risks/benefits and to bring the time requirements from the conversation-only approach down by 50 percent [
10].
VR, being the latest advancement in this area, offers 3D experiences much more emotionally stimulating than 2D media. Tian and colleagues [
19] used multichannel electroencephalography and skin conductance responses, showing that more brain activity was evoked by 3D VR than by conventional 2D media. We are only beginning to understand the neuronal mechanisms underlying this experience. Further studies will be needed to corroborate the idea of 3D VR content performing significantly better than regular 2D visual media for informed consent.
Our study reveals that 'VR-assisted informed consent' for anesthesia can meet with high acceptance by patients. Even though our specific evaluation of patient satisfaction was confined to the study group, previous studies have demonstrated low degrees of overall satisfaction with the conventional patient-physician interactions used for preoperative informed consent [
20]. Likewise, the degree to which anesthesia-related content thus communicated is actually understood by the patients has been questioned [
21]. An instrument to assess the 'understandability' of audiovisual materials, a category broadly applying to our study group, is available in the form of PEMAT-A/V [
22]. This is a tool, however, whose items were written to be answered by experts rather than by patients.
Also, for patients to watch an immersive VR movie covering all relevant aspects of general anesthesia does not impose any additional requirements on medical staff. The approach we used for study reduced the patient-specialist dialogs by two-thirds of the time spent on the conventional conversations. This data holds a promise, based on documented incomes earned by anesthesiologists in the US and UK, of cost savings on the order of 40.0 or 10.7 US dollars, respectively, for each case handled in this way.
The documentation tasks related to informed consent, too, are time-consuming activities with medico-legal implications. Negash and colleagues [
7] found documentation of informed consent to be inadequate in clinical practice. Even though speculative at this point, one consideration would be to explore the possibilities of eye tracking offered by head-mounted displays (like the device used in the present study) as a means to improve documentation. On an even more speculative note, and legal requirements allowing, one might toy with the idea of eye tracking one day superseding the need for handwritten signatures [
23].
Language barriers and related problems of accessibility pose yet another challenge to informed consent. While the present study was confined to German-language material for 'VR-assisted informed consent,' obviously its content can be personalized in various ways. Not only could the material be produced in any useful languages (reducing the need and outlays for interpreters to any questions the patients may still want to ask the anesthesiologist after having watched the movie), but its content could also be fine-tuned to reflect sociocultural differences, thereby improving comprehension of consent-relevant information by patients belonging to specific population groups.
As a related thought, readily understandable formats of preoperative information also gain importance as increasingly older patients are undergoing surgery. Giampieri and colleagues 5 identified three important considerations in this regard: (i) individual cognitive function; (ii) degrees of cognitive impairment; and (iii) legal guardianship. Theoretically, it should be possible for artificial intelligence [
24] and VR to adapt their conveying of information to different degrees of cognitive impairment. That being said, while more studies are needed to shed light on VR-assisted informed consent, it seems clear enough that older patients as such are perfectly able to use and understand VR [
25].
Limitations of the present study include, first of all, that patient satisfaction with the VR experience was tested in the study group ('VR-assisted informed consent') but not in the control group (conventional dialogs only) and that a tool similar to PEMAT-A/V (designed for use by experts) to assess the 'understandability' of audiovisual materials from the patients' perspective was not available. We do, however, know from previous studies that many patients are dissatisfied with the informed-consent process and that their grasp of the information thus provided is limited [
20]. Second, our VR movie did not cover regional anesthesia, but note that this study was only a first step in evaluating VR for informed consent in anesthesia and that subsequent versions of the movie will cover both general and techniques of regional anesthesia.
In summary, a sample of patients scheduled for orthopedic surgery was prospectively randomized to compare a VR-assisted method with the traditional dialog-only method of obtaining informed consent for anesthesia. This study revealed that 'VR-assisted informed consent' was well accepted by the patients without involving any observable side effects or complications. We demonstrated a significant potential for cost savings with the VR-assisted method due to reduced time requirements for anesthesiologists engaging in patient dialogs. Well-designed clinical studies are needed to confirm these results of 'VR-assisted informed consent' for anesthesia in other fields of surgery.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org, Figure S1: Translation of the VR movie's German-language voice track. (©XRSynergies LLC 2022; Lagergasse 6/10, 1030 Vienna, Austria;
www.xrs-medical.com).
Author Contributions
Conceptualization, S.S., J.H. and P.M.; methodology, S.S. and P.M.; validation, P.O.; formal analysis, P.O.; investigation, S.S.; resources, J.H.; data curation, S.S. writing—original draft preparation, S.S. and P.M.; writing—review and editing, S.S. and P.M.; supervision, J.H.; project administration, S.S. and J.H.; funding acquisition, S.S. and J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by institutional/departmental resources. Support for this study (equipment) was also received from XRSynergies LLC (Vienna, Austria).
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Ethics Committee of the Vinzenz Gruppe (ref. 17/2022, date of approval: July 7, 2022 and registered with the German Clinical Trials Register (DRKS00029225) on August 17, 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.” OR “Patient consent was waived due to REASON (please provide a detailed justification).” OR “Not applicable.” for studies not involving humans.
Data Availability Statement
Anonymized data will be made available upon reasonable request. Please contact the corresponding author.
Acknowledgments
We wish to thank Wilfried Preinfalk, Mag. phil., for language editing.
Conflicts of Interest
J.H. is shareholder of XRSynergies LLC (Vienna, Austria). All other authors declare no competing interests. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript and in the decision to publish the results.
References
- Iqbal, J.; Shafique, M.A.; Mustafa, M.S.; et al. Neurosurgical Malpractice Litigation: A Systematic Review and Meta-Analysis. World Neurosurg 2024, 188, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Bloomstone, J.A.; Houseman, B.T.; Sande, E.V.; et al. Documentation of individualized preoperative risk assessment: a multi-center study. Perioper Med (Lond) 2020, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Yentis, S.M.; Hartle, A.J.; Barker, I.R.; et al. AAGBI: Consent for anaesthesia 2017: Association of Anaesthetists of Great Britain and Ireland. Anaesthesia 2017, 72, 93–105. [Google Scholar] [CrossRef]
- American Society of Anesthesiologists C.O.E. Guidelines for the ethical practice of anesthesiology. Available online: https://www.asahq.org/standards-and-practice-parameters/guidelines-for-the-ethical-practice-of-anesthesiology (accessed on 24 September 2024).
- Giampieri, M. Communication and informed consent in elderly people. Minerva Anestesiol 2012, 78, 236–242. [Google Scholar] [PubMed]
- Mogili, A.R.; Mukisa, D.; Campbell, P.; et al. Do patients actually understand? An evaluation of the informed consent process for endoscopic procedures in rural Uganda. Surg Endosc 2024, 38, 4024–4030. [Google Scholar] [CrossRef]
- Negash, T.; Teshome, D.; Fenta, E.; et al. Patients’ and Healthcare Professionals’ Perspectives on Preoperative Informed Consent Procedure Obstacles and Potential Solutions, 2021: A Qualitative Study. Patient Prefer Adherence 2023, 17, 2343–2351. [Google Scholar] [CrossRef]
- Kieninger, M.; Eissnert, C.; Seitz, M.; et al. Analysis and options for optimization of preoperative assessment for anesthesia at a university hospital. Anaesthesist 2018, 67, 93–108. [Google Scholar] [CrossRef]
- Winter, M.; Kam, J.; Nalavenkata, S.; et al. The use of portable video media vs standard verbal communication in the urological consent process: a multicentre, randomised controlled, crossover trial. BJU Int 2016, 118, 823–828. [Google Scholar] [CrossRef]
- Zevin, B.; Almakky, M.; Mancini, U.; Robertson, D.I. Digital approach to informed consent in bariatric surgery: a randomized controlled trial. Surg Endosc 2022, 36, 809–816. [Google Scholar] [CrossRef]
- Graf, S.; Feldmann, H.; Hunold, L.S.; et al. Use of virtual reality in port implantation to reduce perioperative anxiety and pain: protocol for a randomised controlled pilot trial at a single German university hospital. BMJ Open 2023, 13, e074738. [Google Scholar] [CrossRef]
- Perin, A.; Galbiati, T.F.; Ayadi, R.; et al. Informed consent through 3D virtual reality: a randomized clinical trial. Acta Neurochir 2021, 163, 301–308. [Google Scholar] [CrossRef]
- Salary.com. Anesthesiologists salary in the United States. Available online: https://www.salary.com/research/salary/alternate/anesthesiologist-salary (accessed on 30 June 2024).
- Payscale. Avarage Anesthesiologists Salary in United Kingdom. Available online: https://www.payscale.com/research/UK/Job=Anesthesiologist/Salary (accessed on 30 June 2024).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Routledge: New York; Oxfordshire, 1988; 567. [Google Scholar]
- Tait, A.R.; Teig, M.K.; Voepel-Lewis, T. Informed consent for anesthesia: a review of practice and strategies for optimizing the consent process. Can J Anaesth 2014, 61, 832–842. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.W.; Abdallah, F.W.W.; Cohn, M.; Ladowski, S.; Madhusudan, P.; Brull, R. Say what? Patients have poor immediate memory of major risks of interscalene block disclosed during the informed consent discussion. Reg Anesth Pain Med 2019, 44, 981–985. [Google Scholar] [CrossRef] [PubMed]
- Fortunato, J.T.; Wasserman, J.A.; Menkes, D.L. When Respecting Autonomy Is Harmful: A Clinically Useful Approach to the Nocebo Effect. Am J Bioeth 2017, 17, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Hua, M.; Zhang, W.; Li, Y.; Yang, X. Emotional arousal in 2D versus 3D virtual reality environments. PLoS One 2021, 16, e0256211. [Google Scholar] [CrossRef]
- Ayele, T.T.; Negash, T.T.; Oumer, K.E.; et al. Patients’ satisfaction and associated factors towards preoperative informed consent process: A cross-sectional study. Ann Med Surg (Lond) 2022, 79, 104104. [Google Scholar] [CrossRef]
- Chrimes, N.; Marshall, S.D. The illusion of informed consent. Anaesthesia 2018, 73, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, S.J.; Wolf, M.S.; Brach, C. Development of the Patient Education Materials Assessment Tool (PEMAT): a new measure of understandability and actionability for print and audiovisual patient information. Patient Educ Couns 2014, 96, 395–403. [Google Scholar] [CrossRef]
- Adhanom, I.B.; MacNeilage, P.; Folmer, E. Eye Tracking in Virtual Reality: a Broad Review of Applications and Challenges. Virtual Real 2023, 27, 1481–1505. [Google Scholar] [CrossRef]
- Pruski, M. AI-Enhanced Healthcare: Not a new Paradigm for Informed Consent. J Bioeth Inq 2024. Online ahead of print. [Google Scholar] [CrossRef]
- Song, S.; Liu, Z.; Zhang, Q. Application of virtual reality technology in postoperative rehabilitation following total knee arthroplasty: A scoping review. Int J Orthop Trauma Nurs 2024, 54, 101124. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).