Submitted:
27 September 2024
Posted:
29 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
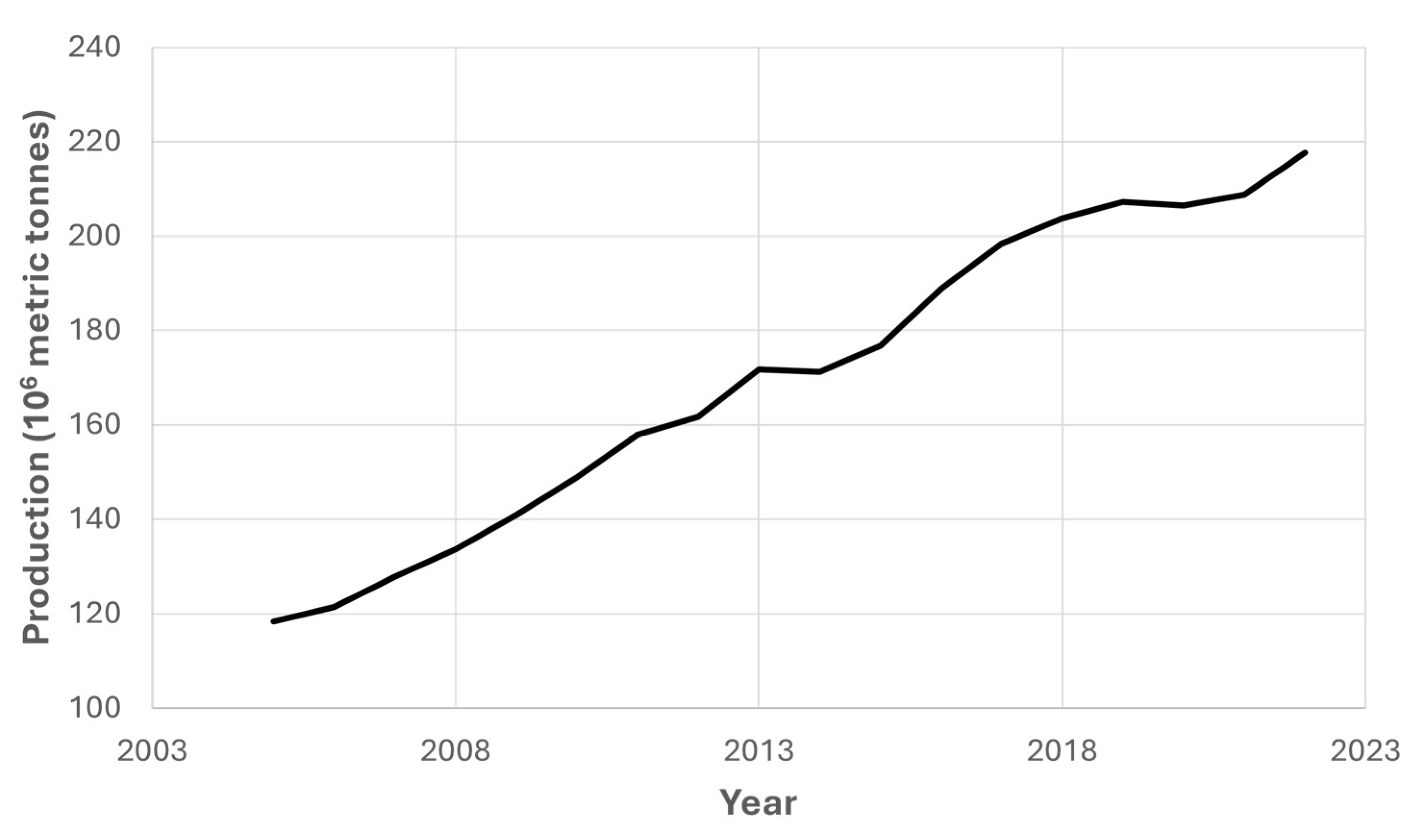
1.1. Global Environmental Scenario
1.2. Biolubricant Production: A Biorefinery Approach
- It is a process that can be easily implemented at industrial scale (first and second transesterification are similar to typical biodiesel production in industries).
- A wide range of products can be obtained, like biodiesel, glycerol or biolubricants.
- Some intermediate products can be reused, like methanol or biodiesel (as the starting point for biolubricant production).
- As a consequence, low amounts of pollutants are evolved to the environment, implying a high atom economy.
1.3. Catalytic Biolubricant Production through Double Transesterification
| Patent number | Details | Ref. |
|---|---|---|
| KR102564510B1 | Method for producing neopentyl glycol diester as a biolubricant using enzymatic reaction. The use of lipase derived from Thermomyces lanuginosus, along with the correct temperature, time (8-10 h) and vacuum conditions, obtained high conversions (>95%) | [17] |
| JP2015059176A | There is provided a method for manufacturing a bio lubricant by stirring castor oil methyl biodiesel and/or jatropha oil methyl biodiesel (a mixture of methyl oleate and methyl linoleate), trimethylol propane (TMP) as poly hydroxylated alcohol, neopentyl glycol or pentaerythritol, water and an enzymatic catalyst, under specific stirring and vacuum conditions | [18] |
| Authors | Details | Ref. |
|---|---|---|
| Greco-Duarte et al. | The authors performed two-step enzymatic biolubricant production, through castor oil hydrolysis and esterification with NPG (using lipase), among others, with interesting physico-chemical properties. | [19] |
| Cavalcanti et al. | Different commercial lipases (including Lipomod 34MDP) were used to obtain biolubricants through sterification with soybean fatty acids, with conversions above 97%. | [20] |
| Papadaki et al. | Biolubricants from extracted microbial oils were obtained through hydrolysis and esterification combining Lipomod 34-MDP with NPG. The highest conversion yields were above 80%. | [21] |
| Fernandes et al. | Different biocatalysts from Candida rugosa lipase were used to produce biolubricants from soybean oil byproducts, through esterification with NPG, obtaining up to 90% conversion. | [22] |
| Pucko et al. | Lubricants based on lauric acid and different polyols (such as NPG) were produced through esterification (using p-toluenesulfonic acid monohydrate as a catalyst), with purities above 97% | [23] |
| Kim et al. | NPG diester as a biodegradable biolubricant was synthetized by esterification of fatty acids and NPG using immobilized lipase, with a maximum conversion of 97% after 10 h of reaction. | [24] |
1.3. Aim of This Work
2. Materials and Methods
2.1. Raw Material
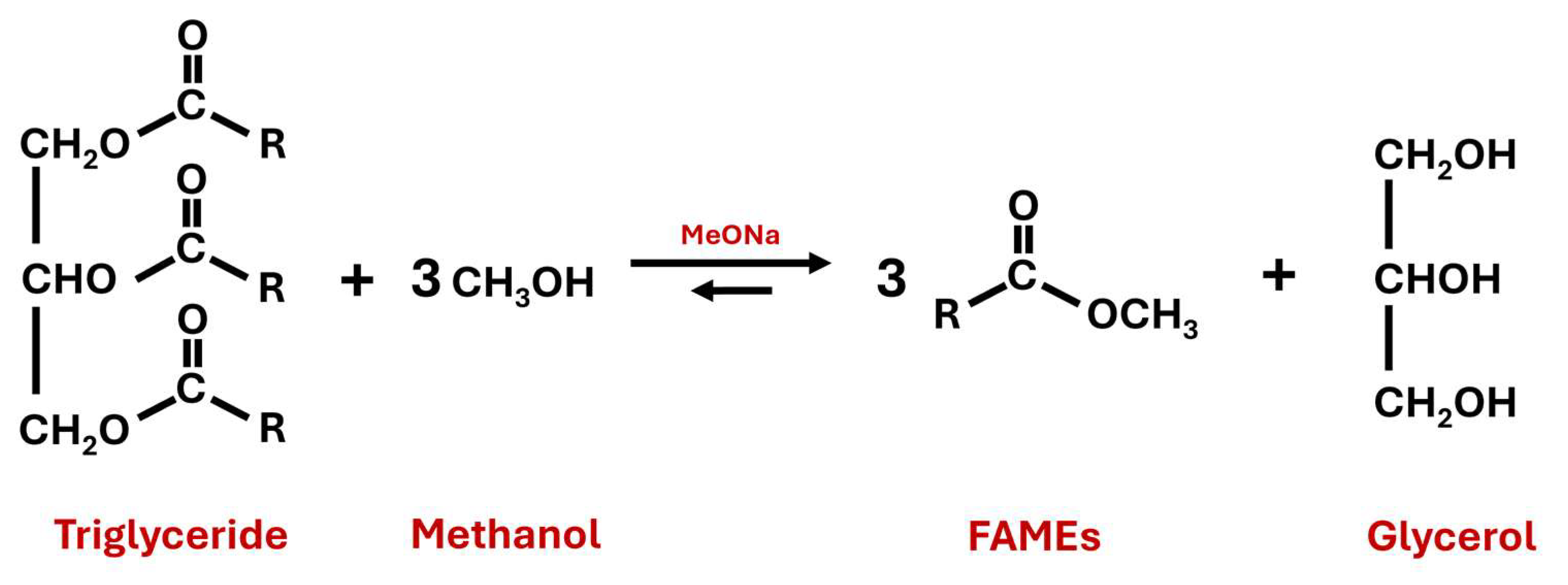
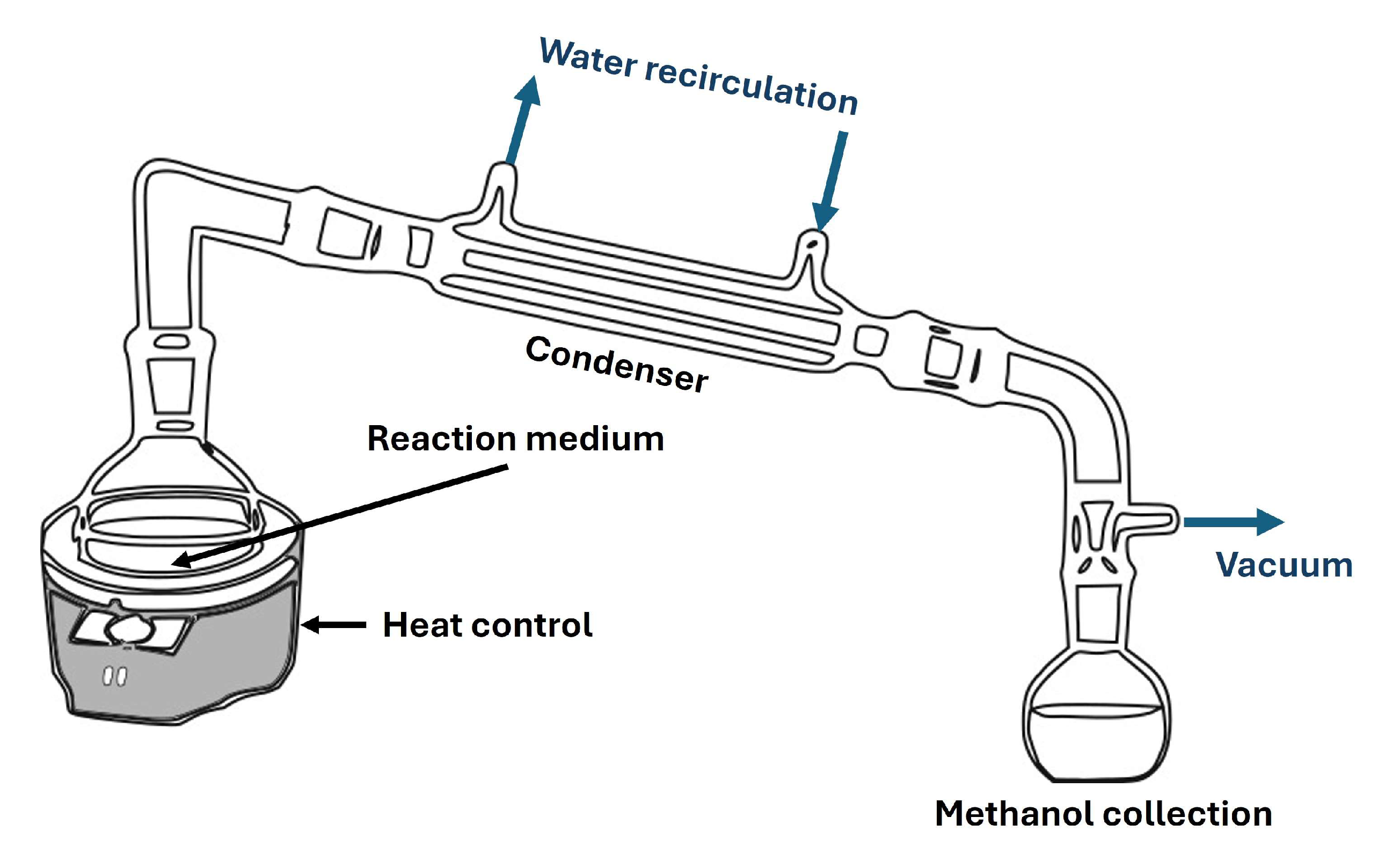
2.2. First Transesterification
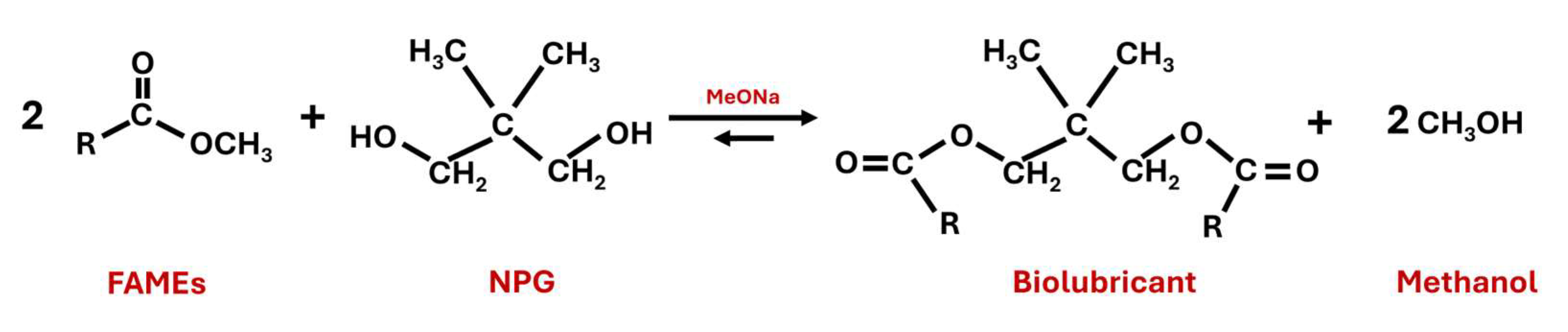
2.3. Second Transesterification
2.4. Biolubricant Characterization
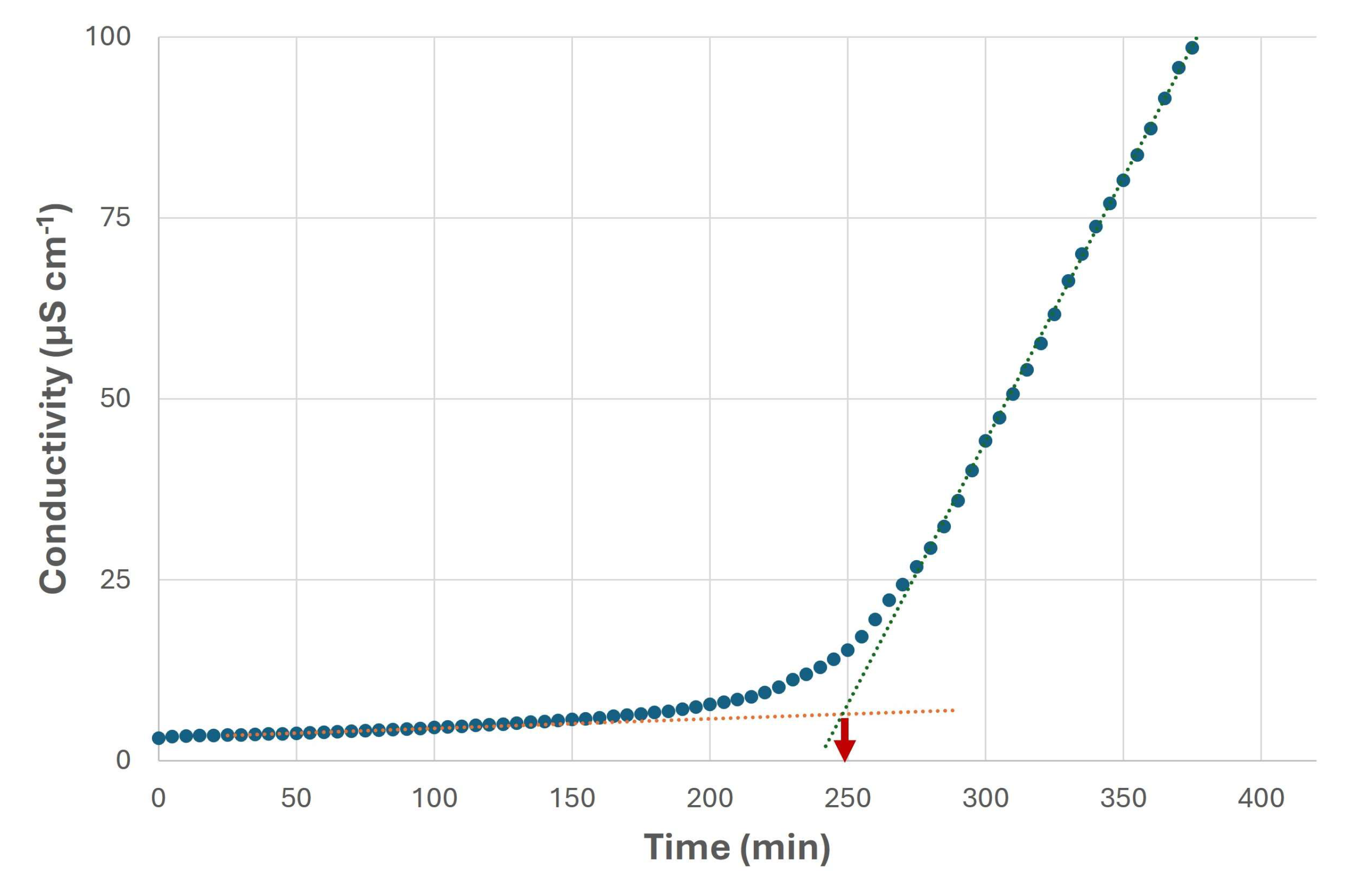
3. Results and Discussion
4. Conclusions
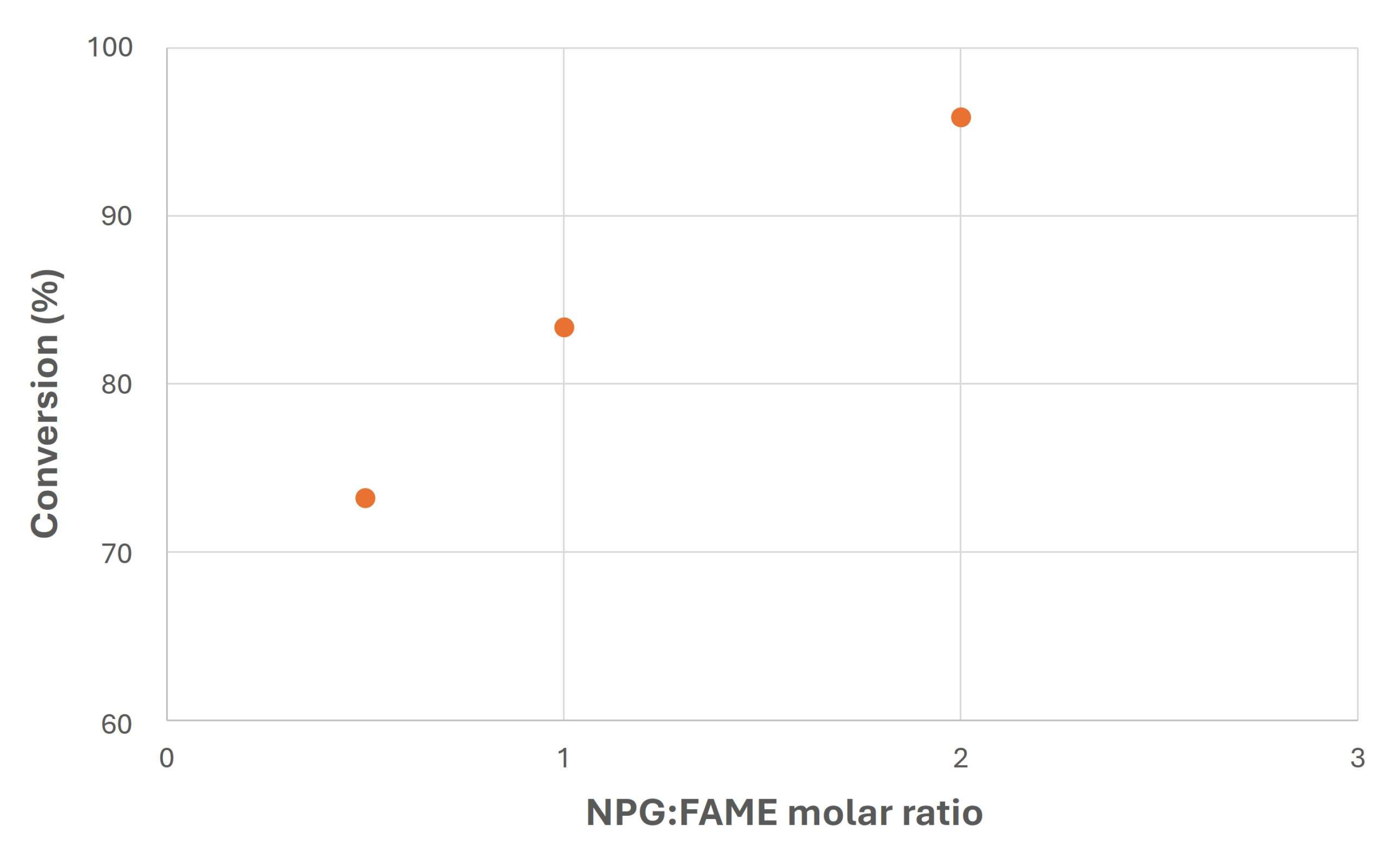
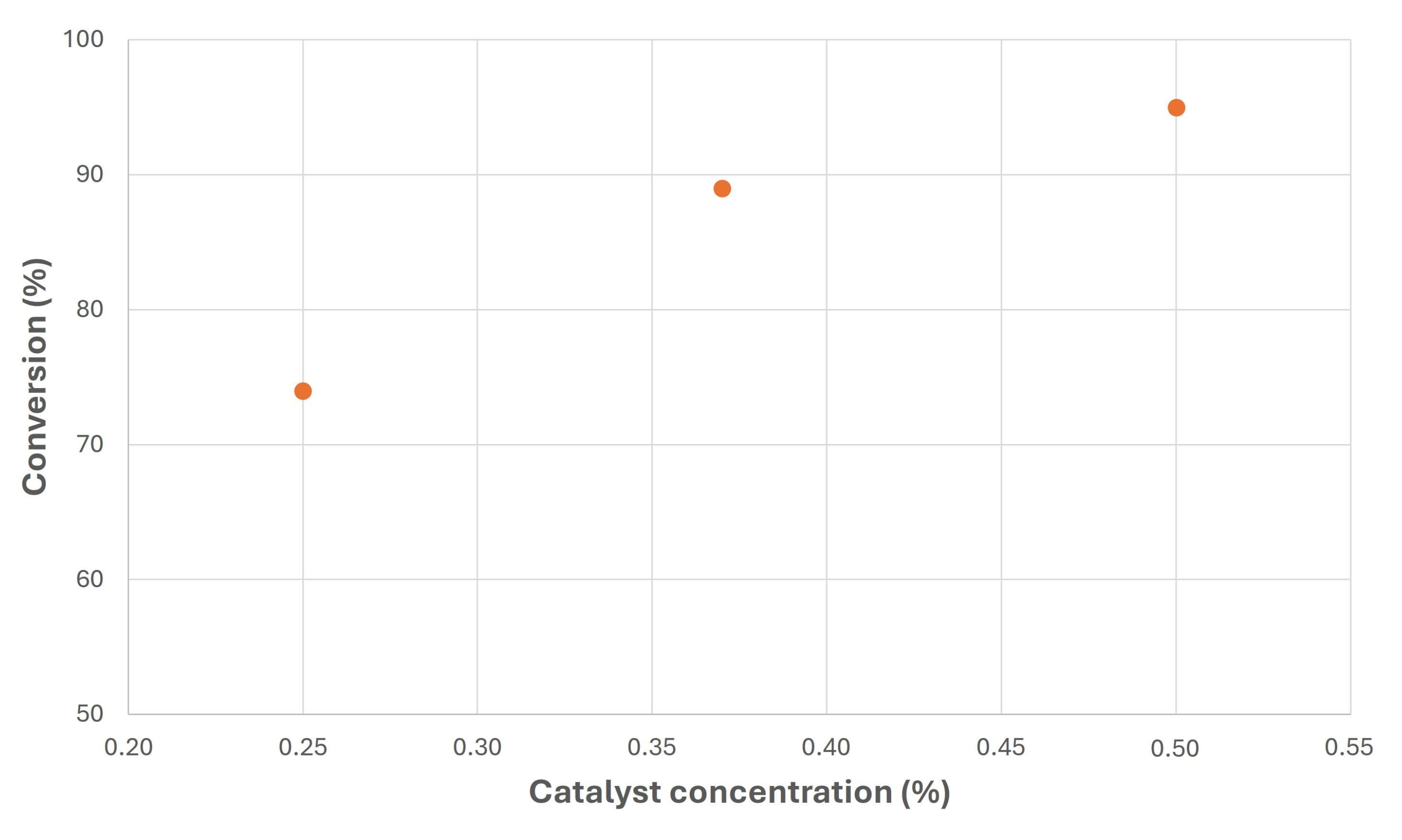
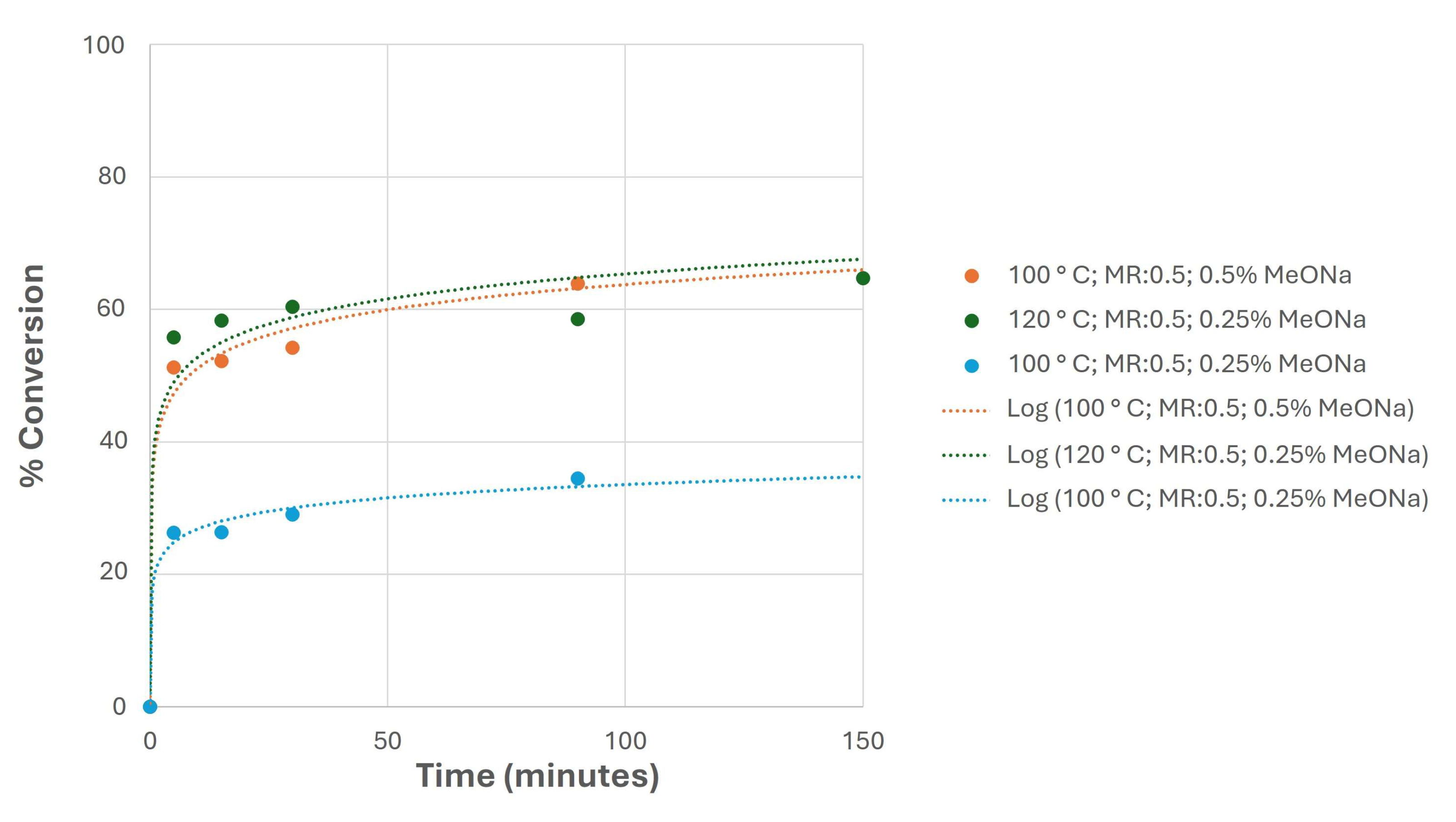
- Biodiesel and biolubricant production from canola oil through double transesterification with methanol and neopentyl glycol was carried out. For this purpose, homogeneous catalysts were used, with high conversion levels in these two stages.
- Specifically, relatively small amounts of catalysts were necessary to exceed 90% conversions to produce biolubricants, proving the suitability of these catalysts for this purpose.
- In this sense, canola oil, along with other vegetable oils, could be an interesting starting point in a biorefinery context, where different products (in this case, glycerol, biodiesel and biolubricant) can be obtained with a high atom economy.
- The characteristics of NPG-based biolubricant were analyzed, considering the use of this product as engine oil (SAE 5W).
- Further studies, like the use of different kinds of catalysts (including heterogeneous ones, which should present high reusability to make the process sustainable) and tribological studies, are suggested.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bazilian, M.; Bradshaw, M.; Gabriel, J.; Goldthau, A.; Westphal, K. Four Scenarios of the Energy Transition: Drivers, Consequences, and Implications for Geopolitics. Wiley Interdiscip Rev Clim Change 2020, 11. [CrossRef]
- Vakulchuk, R.; Overland, I.; Scholten, D. Renewable Energy and Geopolitics: A Review. Renewable and Sustainable Energy Reviews 2020, 122.
- Granjo, J.F.O.; Duarte, B.P.M.; Oliveira, N.M.C. Integrated Production of Biodiesel in a Soybean Biorefinery: Modeling, Simulation and Economical Assessment. Energy 2017. [CrossRef]
- Gottumukkala, L.D.; Haigh, K.; Collard, F.X.; van Rensburg, E.; Görgens, J. Opportunities and Prospects of Biorefinery-Based Valorisation of Pulp and Paper Sludge. Bioresour Technol 2016, 215, 37–49.
- Caldeira, C.; Vlysidis, A.; Fiore, G.; De Laurentiis, V.; Vignali, G.; Sala, S. Sustainability of Food Waste Biorefinery: A Review on Valorisation Pathways, Techno-Economic Constraints, and Environmental Assessment. Bioresour Technol 2020, 312. [CrossRef]
- Ubando, A.T.; Anderson S. Ng, E.; Chen, W.H.; Culaba, A.B.; Kwon, E.E. Life Cycle Assessment of Microalgal Biorefinery: A State-of-the-Art Review. Bioresour Technol 2022, 360.
- Okeke, E.S.; Ejeromedoghene, O.; Okoye, C.O.; Ezeorba, T.P.C.; Nyaruaba, R.; Ikechukwu, C.K.; Oladipo, A.; Orege, J.I. Microalgae Biorefinery: An Integrated Route for the Sustainable Production of High-Value-Added Products. Energy Conversion and Management: X 2022, 16.
- Moncada B, J.; Aristizábal M, V.; Cardona A, C.A. Design Strategies for Sustainable Biorefineries. Biochem Eng J 2016, 116, 122–134. [CrossRef]
- Bauer, F.; Coenen, L.; Hansen, T.; McCormick, K.; Palgan, Y.V. Technological Innovation Systems for Biorefineries: A Review of the Literature. Biofuels, Bioproducts and Biorefining 2017, 11, 534–548.
- Ubando, A.T.; Felix, C.B.; Chen, W.H. Biorefineries in Circular Bioeconomy: A Comprehensive Review. Bioresour Technol 2020, 299.
- Beyzi, E.; Gunes, A.; Buyukkilic Beyzi, S.; Konca, Y. Changes in Fatty Acid and Mineral Composition of Rapeseed (Brassica Napus Ssp. Oleifera L.) Oil with Seed Sizes. Ind Crops Prod 2019, 129, 10–14. [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Pinilla, A. Biolubricant Production through Double Transesterification: Reactor Design for the Implementation of a Biorefinery Based on Rapeseed. Processes 2021, 9, 1224. [CrossRef]
- Hanif, M.A.; Nisar, S.; Akhtar, M.N.; Nisar, N.; Rashid, N. Optimized Production and Advanced Assessment of Biodiesel: A Review. Int J Energy Res 2018, 42, 2070–2083. [CrossRef]
- Checa, M.; Nogales-Delgado, S.; Montes, V.; Encinar, J.M. Recent Advances in Glycerol Catalytic Valorization: A Review. Catalysts 2020, 10, 1–41.
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. A Review on Biolubricants Based on Vegetable Oils through Transesterification and the Role of Catalysts: Current Status and Future Trends. Catalysts 2023, 13, 1299. [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M.; Sánchez Ocaña, M. Use of Mild Reaction Conditions to Improve Quality Parameters and Sustainability during Biolubricant Production. Biomass Bioenergy 2022, 161, 106456. [CrossRef]
- Kim, I.; Won, K.J. Method for Producing Neopentyl Glycol Diester as a Biolubricant Using Enzymatic Reaction 2023.
- Cavalcanti da Silva, J.A.; Guimaraes Freire, D.M.; Habert, A.C.; Ferreira Soares, V. Method for Manufacturing a Bio Lubricant from Methyl Biodiesel and the Bio Lubricant Obtained by the Method 2015.
- Greco-Duarte, J.; Cavalcanti-Oliveira, E.D.; Da Silva, J.A.C.; Fernandez-Lafuente, R.; Freire, D.M.G. Two-Step Enzymatic Production of Environmentally Friendly Biolubricants Using Castor Oil: Enzyme Selection and Product Characterization. Fuel 2017, 202, 196–205. [CrossRef]
- Cavalcanti, E.D.C.; Aguieiras, É.C.G.; da Silva, P.R.; Duarte, J.G.; Cipolatti, E.P.; Fernandez-Lafuente, R.; da Silva, J.A.C.; Freire, D.M.G. Improved Production of Biolubricants from Soybean Oil and Different Polyols via Esterification Reaction Catalyzed by Immobilized Lipase from Candida Rugosa. Fuel 2018, 215, 705–713. [CrossRef]
- Papadaki, A.; Fernandes, K.V.; Chatzifragkou, A.; Aguieiras, E.C.G.; da Silva, J.A.C.; Fernandez-Lafuente, R.; Papanikolaou, S.; Koutinas, A.; Freire, D.M.G. Bioprocess Development for Biolubricant Production Using Microbial Oil Derived via Fermentation from Confectionery Industry Wastes. Bioresour Technol 2018, 267, 311–318. [CrossRef]
- Fernandes, K. V.; Cavalcanti, E.D.C.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, M.C.C.; Tavares, F.A.; da Silva, P.R.; Fernandez-Lafuente, R.; Arana-Peña, S.; Pinto, J.C.; et al. Enzymatic Synthesis of Biolubricants from By-Product of Soybean Oil Processing Catalyzed by Different Biocatalysts of Candida Rugosa Lipase. Catal Today 2021, 362, 122–129. [CrossRef]
- Pucko, I.; Crnjac, K.; Faraguna, F. Lauric Acid-Based Polyol Esters as Potential Bio-Based Lubricants for Diesel Fuel. Chem Biochem Eng Q 2023, 37, 143–151. [CrossRef]
- Kim, J.W.; Kim, B.H.; Kim, Y.; Lee, M.W.; Im, D.J.; Kim, I.H. Lipase-Mediated Synthesis of Neopentyl Glycol Diester Using a Combination of Reduced and Standard Pressure. JAOCS, Journal of the American Oil Chemists’ Society 2021, 98, 1001–1007. [CrossRef]
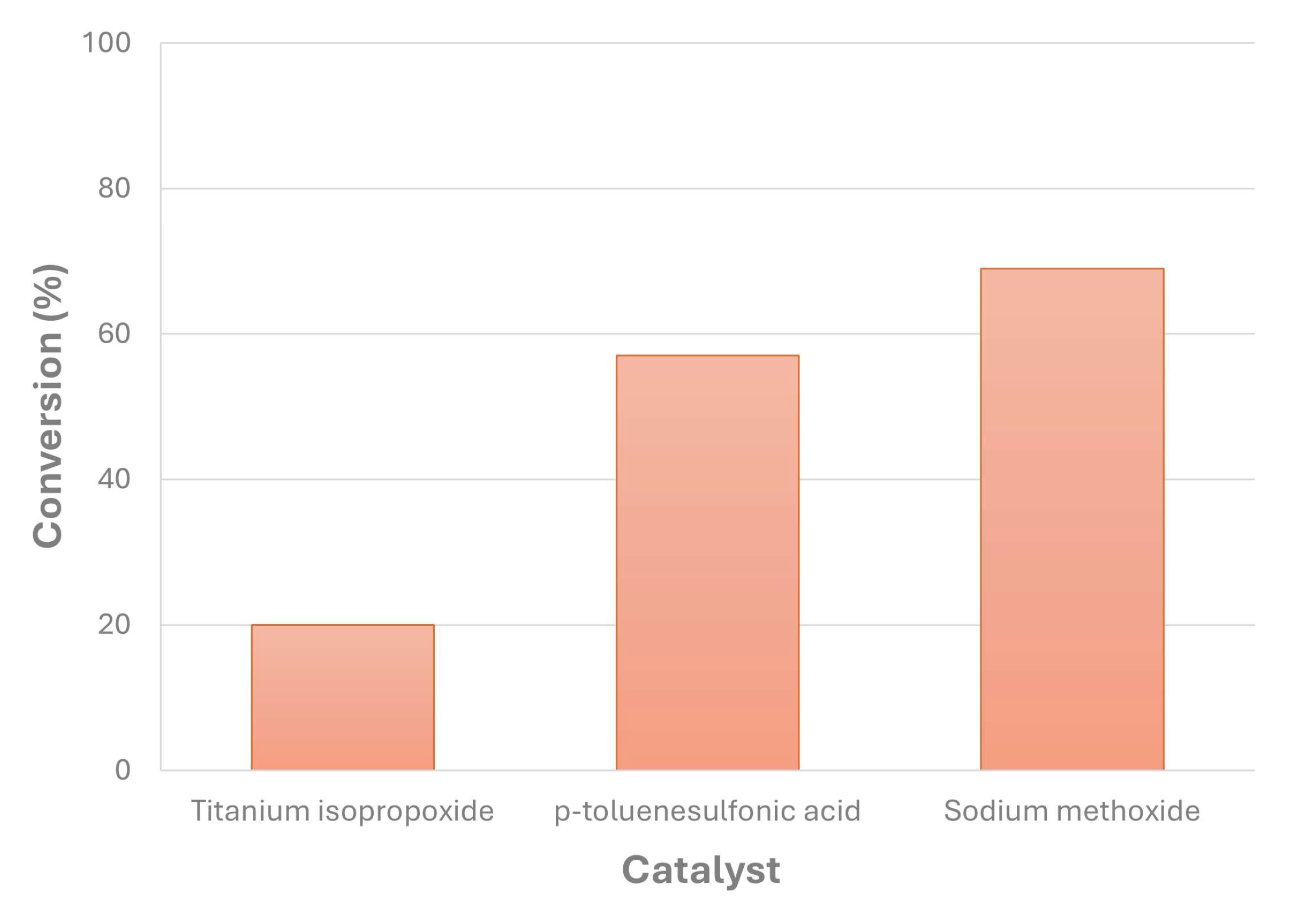
- Encinar, J.M.; Nogales-Delgado, S.; Sánchez, N.; González, J.F. Biolubricants from Rapeseed and Castor Oil Transesterification by Using Titanium Isopropoxide as a Catalyst: Production and Characterization. Catalysts 2020, 10. [CrossRef]
- Nogales-Delgado, S.; Encinar Martín, J.M. Cardoon Biolubricant through Double Transesterification: Assessment of Its Oxidative, Thermal and Storage Stability. Mater Lett 2021, 302. [CrossRef]
- Encinar, J.M.; Nogales, S.; González, J.F. Biodiesel and Biolubricant Production from Different Vegetable Oils through Transesterification. Engineering Reports 2020, 1–10. [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González, J.F. Safflower Biodiesel: Improvement of Its Oxidative Stability by Using BHA and TBHQ. Energies (Basel) 2019, 12. [CrossRef]
- UNE-EN 14214:2013 V2+A1:2018 Liquid Petroleum Products - Fatty Acid Methyl Esters (FAME) for Use in Diesel Engines and Heating Applications - Requirements and Test Methods. 2018.
- UNE-EN ISO 3104/AC:1999 Petroleum Products. Transparent and Opaque Liquids. Determination of Kinematic Viscosity and Calculation of Dynamic Viscosity (ISO 3104:1994). 1999.
- UNE-EN-ISO 3675 Crude Petroleum and Liquid Petroleum Products. Laboratory Determination of Density. Hydrometer Method 1999.
- UNE-EN ISO 12966-2:2011 Animal and Vegetable Fats and Oils – Gas Chromatography of Fatty Acid Methyl Esters – Part 2: Preparation of Methyl Esters of Fatty Acids. 2011.
- UNE-EN 14104:2003 Oil and Fat Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Acid Value. 2003.
- UNE-EN 14111:2003 Fat and Oil Derivatives. Fatty Acid Methyl Esters (FAME). Determination of Iodine Value. 2003.
- UNE-EN 14112 Fat and Oil Derivatives - Fatty Acid Methyl Esters (FAME) - Determination of Oxidation Stability (Accelerated Oxidation Test) 2017.
- UNE-EN 51023:1990 Petroleum Products. Determination of Flash and Fire Points. Cleveland Open Cup Method. 1990.
- Issariyakul, T.; Kulkarni, M.G.; Meher, L.C.; Dalai, A.K.; Bakhshi, N.N. Biodiesel Production from Mixtures of Canola Oil and Used Cooking Oil. Chemical Engineering Journal 2008. [CrossRef]
- Kania, D.; Yunus, R.; Omar, R.; Abdul Rashid, S.; Mohamad Jan, B. A Review of Biolubricants in Drilling Fluids: Recent Research, Performance, and Applications. J Pet Sci Eng 2015, 135, 177–184.
- Ajala, O.E.; Aberuagba, F.; Odetoye, T.E.; Ajala, A.M. Biodiesel: Sustainable Energy Replacement to Petroleum-Based Diesel Fuel - A Review. ChemBioEng Reviews 2015, 2, 145–156. [CrossRef]
- Kumar, N. Oxidative Stability of Biodiesel: Causes, Effects and Prevention. Fuel 2017.
- Jemima Romola, C.V.; Meganaharshini, M.; Rigby, S.P.; Ganesh Moorthy, I.; Shyam Kumar, R.; Karthikumar, S. A Comprehensive Review of the Selection of Natural and Synthetic Antioxidants to Enhance the Oxidative Stability of Biodiesel. Renewable and Sustainable Energy Reviews 2021, 145, 111109. [CrossRef]
- Caldeira, C.; Freire, F.; Olivetti, E.A.; Kirchain, R. Fatty Acid Based Prediction Models for Biodiesel Properties Incorporating Compositional Uncertainty. Fuel 2017, 196, 13–20. [CrossRef]
- Ge, J.C.; Yoon, S.K.; Choi, N.J. Using Canola Oil Biodiesel as an Alternative Fuel in Diesel Engines: A Review. Applied Sciences (Switzerland) 2017, 7. [CrossRef]
- Encinar, J.M.; Nogales-Delgado, S.; Álvez-Medina, C.M. High Oleic Safflower Biolubricant through Double Transesterification with Methanol and Pentaerythritol: Production, Characterization, and Antioxidant Addition. Arabian Journal of Chemistry 2022, 15. [CrossRef]
- Nogales-Delgado, S.; Encinar, J.M.; González Cortés, Á. High Oleic Safflower Oil as a Feedstock for Stable Biodiesel and Biolubricant Production. Ind Crops Prod 2021, 170. [CrossRef]
- Sanjurjo, C.; Rodríguez, E.; Viesca, J.L.; Battez, A.H. Influence of Molecular Structure on the Physicochemical and Tribological Properties of Biolubricants: A Review. Lubricants 2023, 11.
- Aguieiras, É.C.G.; Cavalcanti, E.D.C.; da Silva, P.R.; Soares, V.F.; Fernandez-Lafuente, R.; Bessa Assunção, C.L.; da Silva, J.A.C.; Freire, D.M.G. Enzymatic Synthesis of Neopentyl Glycol-Bases Biolubricants Using Biodiesel from Soybean and Castor Bean as Raw Materials. Renew Energy 2020, 148, 689–696. [CrossRef]
- Nor, N.M.; Salih, N.; Salimon, J. Optimization and Lubrication Properties of Malaysian Crude Palm Oil Fatty Acids Based Neopentyl Glycol Diester Green Biolubricant. Renew Energy 2022, 200, 942–956. [CrossRef]
- Ng, B.Y.S.; Ong, H.C.; Lau, H.L.N.; Ishak, N.S.; Elfasakhany, A.; Lee, H.V. Production of Sustainable Two-Stroke Engine Biolubricant Ester Base Oil from Palm Fatty Acid Distillate. Ind Crops Prod 2022, 175. [CrossRef]
- Nogales-Delgado, S.; Cabanillas, A.G.; Romero, Á.G.; Encinar Martín, J.M. Monitoring Tert-Butylhydroquinone Content and Its Effect on a Biolubricant during Oxidation. Molecules 2022, 27. [CrossRef]
- Nogales-Delgado, S.; Sánchez, N.; Encinar, J.M. Valorization of Cynara Cardunculus L. Oil as the Basis of a Biorefinery for Biodiesel and Biolubricant Production. Energies (Basel) 2020, 13. [CrossRef]


| Property | Details | Ref. |
|---|---|---|
| Viscosity | A Cannon-Fenske viscometer was used, controlling temperature at 40 °C | [30] |
| Density | A densimeter (for densities between 900 to 1000 mg/kg) was selected | [31] |
| FAME content | FAMEs were analyzed with a gas chromatograph (VARIAN 3900) coupled to a flame ionization detector (FID). A silica capillary column was used (Zebron ZB Waxplus, 30 m long, 0.32 mm inner diameter, and 0.5 μm film thickness). Helium was the carrier gas (0.7 mL·min−1), and the injector and FID temperatures were 270 and 300 °C, respectively. Oven temperature was 200 °C for 21 min, and afterward, it increased to 220 °C. Standards of the corresponding FAME and an internal standard (methyl heptadecanoate) were used. All the standards were analytical grade (Merck, Darmstadt, Germany). | [32] |
| Acid value | Methods previously described in the literature were used | [33] |
| Iodine value | Methods previously described in the literature were used | [34] |
| Oxidation stability | Through the Rancimat method | [35] |
| Flash and fire points | These parameters were determined by using the Cleveland open cup method | [36] |
| Property | Result |
|---|---|
| Viscosity, cSt | 4.86 |
| Density, kg·m-3 | 871 |
| FAME content, % | 97.0 |
| Acid value, mgKOH·g-1 | 0.39 |
| Iodine value, gI2·100g-1 | 122 |
| Oxidation stability, min | 273 |
| Flash and fire points, °C | 175-182 |
| Property | Result |
|---|---|
| Conversion, % | 94.9 |
| Viscosity, cSt | 20.7 |
| Density, kg·m-3 | 855 |
| Acid value, mgKOH·g-1 | 0.51 |
| Oxidation stability, min | 248 |
| Flash and fire points, °C | 184-193 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





