1. Introduction
Soil serves a critical function within the hydrological cycle as a physical element of a watershed [
1]. It regulates surface, hypodermic, and groundwater runoff, while also contributing to atmospheric water vapor through various processes. These include direct physical evaporation, biological transpiration, or evapotranspiration when the soil surface is covered by vegetation.
Energy forces such as gravity, osmosis, and capillarity drive water movement within the soil. Capillary action occurs at suctions exceeding 33 kPa [
1], from wetter to drier regions. As soil desiccation occurs more rapidly at the surface compared to deeper layers, a non-saturated saline capillary flow is generated from deeper horizons toward the surface. However, in a drained medium, the capillary rise is significantly slow. In contrast, when a shallow layer of free water is present, capillary movement is much more effective due to the sustained flow of capillary water that is not retained by the soil. In a well-balanced soil texture, capillary rise exhibits significant impact, supplying approximately 2 to 3 mm of water per day up to a height of 0.8 to 1 m above the water table. This rate corresponds to the potential evapotranspiration value of a meadow in June [
2].
When these vapor currents encounter calcareous rocks with increased porosity due to weathering, they induce a hygroscopic effect through the crystallization of dissolved salts as the water evaporates. This process reduces the mechanical strength of the rocks, depending on pore size and the degree of saturation [
3,
4]. Additionally, if the salt present can exist in multiple hydration states, such as sodium sulfate, transitions between these states—caused by dissolution and subsequent recrystallization—can lead to volumetric expansion. This expansion generates overpressures within the rock cavities, resulting in structural damage [
5]. The most significant salts involved in this deterioration process are those with exchangeable ions between the soil's solid and liquid phases, including sulfates, sodium, magnesium, and chloride [
3].
The cycles of dissolution, migration, and crystallization of salts are recognized as primary contributors to the weathering of calcareous rocks, both on the surface and within the substrate, which constitute historical monuments [
6]. This review aims to elucidate the mechanisms driving water movement in the soil, the role of soluble salts, and the impact of saline capillary rise on the mechanical integrity of calcareous rocks that form the structures of historical buildings. This study addresses a significant gap in the literature, where the relationship between soil water dynamics, upward saline flow, and the degradation of porous construction materials in cultural heritage has been insufficiently explored.
2. Energy State and Hydraulic Conductivity
Water movement within the soil occurs through transmission macropores, which are interconnected channels with diameters greater than 30 µm [
2]. This flow brings water into contact with the surfaces of the mineral and organic particles that comprise the soil's solid matrix, subjecting it to a complex array of forces primarily resulting from the interaction with the solid matrix, the gravitational field, and the presence of dissolved ions in the water.
Due to the challenges in quantitatively determining the magnitude and direction of these forces, the concept of the energy state of water in the soil is employed [
2]. The energy state and movement of water are governed by its potential energy at each point within the soil, expressed relative to an arbitrarily defined reference state. The various independent components contributing to the total potential (Ψ
t) are represented in equation (1).
Ψg: gravitational potential, related to the vertical position of water within the soil. This potential is critical in the removal of excess water from the surface horizons following rainfall or irrigation.
Ψo: osmotic potential, which arises from the presence of dissolved ions in the soil solution. This potential is particularly significant in saline soils, where the presence of salts reduces the total potential.
Ψ
p: pressure potential, which encompasses all other combined effects influencing soil water, including air pressure, hydrostatic pressure, and the configuration of the soil matrix. The various components of the pressure potential (Ψ
p) are detailed in equation (2).
Ψpa: pneumatic or air pressure potential reflects the effect of excess gas pressure on a soil sample at a specific moisture content.
Ψpu: hydrostatic potential represents the pressure exerted by a column of water above a particular point in saturated soil.
Ψpm: matric or capillary potential arises primarily from capillary phenomena, including water retention due to capillary and adsorption forces. This potential becomes especially pronounced in smaller pores, meaning that as soil dries, the remaining water is increasingly held within these small pores. The matric potential is higher in soils with finer granulometry, such as clayey soils.
The matric potential (Ψ
pm), commonly utilized in soil studies, reflects the influence of the soil's solid matrix. It is negative in unsaturated soils, reaching values as low as -2000 kPa, but becomes zero below the water table [
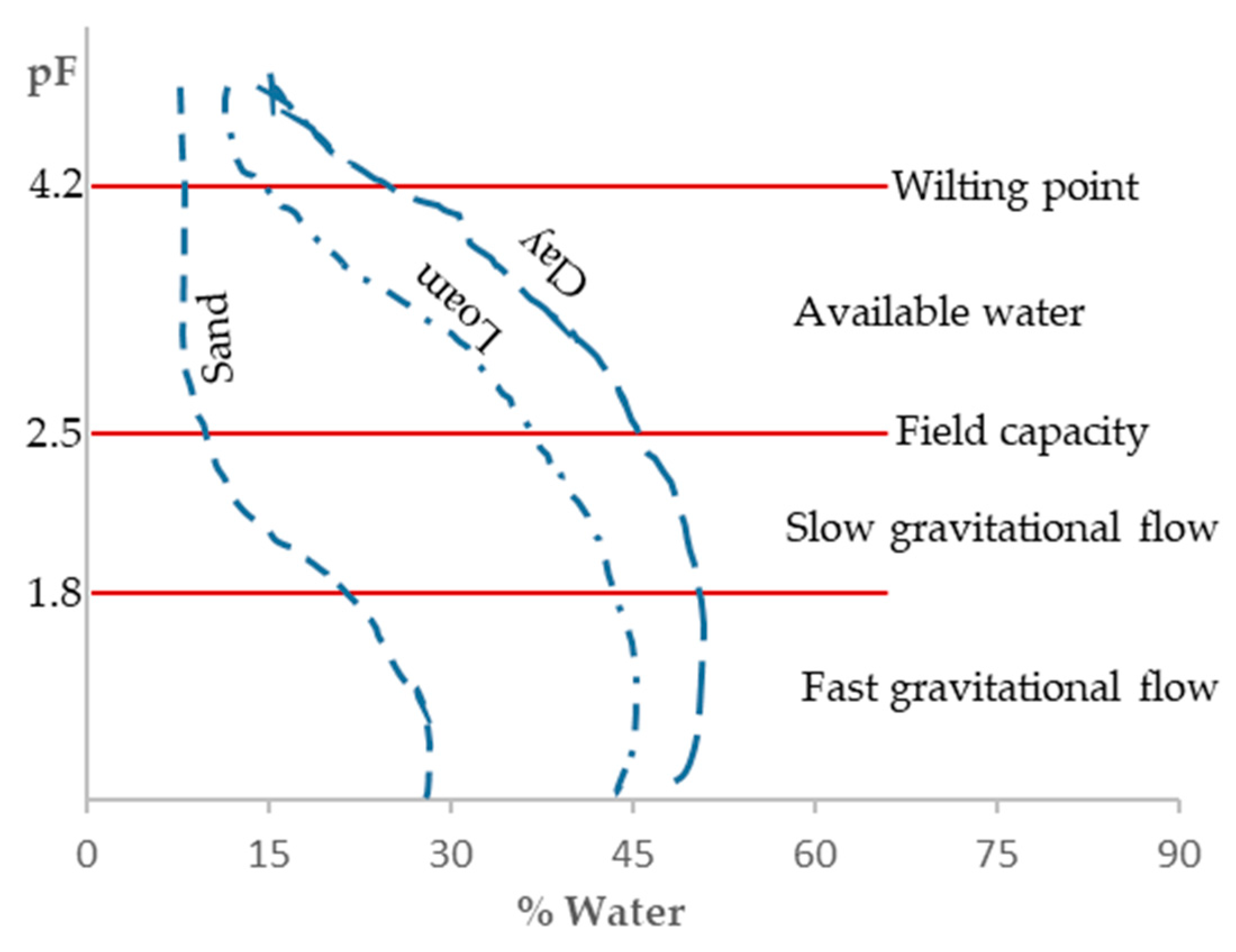
7]. The logarithm of this soil water retention force is referred to as pF. The pF is a practical term because the values of capillary potential can reach very high negative numbers, making it more convenient to express them as logarithms. The pF is defined as the logarithm of the height in cm of a water column that is equivalent to the suction or pressure with which the weakest water is retained in the soil. To better understand this concept, pF reflects the pressure required to extract water from the soil, as the force with which water is retained in the soil is measured by the force needed to remove it. Therefore, the different constants that characterize soil-water relationships can be expressed in terms of a capillary potential, whose range of variation is smaller. For instance, the permanent wilting point corresponds, regardless of the soil type, to a capillary potential of 16 atm, which translates to a pF = 4.2 (
Figure 1). Conversely, the pF of water holding capacity varies with soil texture, being 2 for sandy soils, 2.5 for loamy soils, and 3 for clay soils [
8].
Hydraulic conductivity, the proportionality factor in Darcy’s law when applied to the viscous flow of water through soil, quantifies a porous medium's (soil's) ability to transmit water [
9]. It depends among other factors, on the water content and is thus influenced by the matric potential. Soil resistance to water flow arises from cohesive forces between liquid molecules and adhesive forces between the liquid and pore walls. Under saturated flow conditions, hydraulic conductivity remains constant over time if soil structure is unchanged.
Measuring hydraulic conductivity at different soil depths allows for the differentiation of water transmission capacity across soil horizons, highlighting soil heterogeneity and vertical anisotropy [
9]. To address environmental or engineering challenges related to water movement, calculations often rely on saturated hydraulic conductivity (Ks) values. Indicative Ks values for different soil textures and structures include approximately 10 mm day⁻¹ for fine-textured soils, 10 to 1000 mm day⁻¹ for well-structured loamy soils, and values exceeding 1000 mm day⁻¹ for coarse-textured soils, such as sandy soils [
9].
3. Water Movement in Soil
When water movement is primarily driven by the gravitational field, at pressures lower than -33 kPa, the movement is described as saturated flow, governed by Darcy’s law. In contrast, unsaturated flow occurs when water experiences suctions greater than the gravitational force, with other forces, such as osmotic and capillary forces, becoming predominant, described by the Buckingham-Darcy law [
2].
Saturated flow refers to the movement of water in soils where the pore space is completely filled with water, typically in the presence of a surrounding water table. If the flow rate is very slow and oxygen renewal is insufficient, anaerobic conditions may develop due to inadequate drainage. Saturated flow typically begins with the infiltration of water following precipitation or irrigation. Infiltration is the process by which water moves from the soil surface into the subsurface. A portion of the infiltrated water is retained in the soil against gravitational forces due to interactions with the soil's solid matrix, and this water may subsequently evaporate or be lost through evapotranspiration. Another fraction of the infiltrated water escapes evaporation and transpiration processes, moving to deeper soil layers until it eventually reaches the water table [
10]. The continued movement of water through soil that is already saturated is referred to as percolation. Percolation water contributes to groundwater recharge, transporting soluble salts from the upper soil layers as it moves downward.
Unsaturated flow is most common in soils that undergo cycles of wetting and drying. When the soil is relatively dry, vapor water transfer driven by thermal gradients becomes significant, whereas capillary flow in any direction becomes more important when the soil is wet. Under unsaturated conditions, the pore volume is only partially filled with water, resulting in hydraulic conductivity that is no longer constant. Instead, it varies with the matric potential and is lower than in saturated conditions [
9].
Water infiltration through soil occurs rapidly via macropores and is diminished by any factor that reduces pore size or number. Several factors influence the rate of infiltration, including [
11]: (1) Soil texture and structure, where coarse-textured soils, such as sandy soils, facilitate rapid infiltration, and soils with stable aggregates and a granular structure similarly tend to exhibit higher overall infiltration rates; (2) Organic matter content, which helps to stabilize aggregates and reduce the erosive effect of raindrop impact; (3) Depth to bedrock or impermeable layers, as shallow soils limit the volume of water infiltration compared to deeper soils; (4) Initial soil moisture content, with lower infiltration rates observed in soils that are already saturated; and (5) Soil temperature, as warmer soils generally exhibit higher water uptake rates compared to colder soils.
Infiltration rates are categorized as follows [
12]: very low, typically associated with soils containing a high percentage of clay, with rates less than 0.25 cm hour⁻¹; low, common in shallow soils with high clay content or low organic matter, with rates between 0.25 and 1.25 cm hour⁻¹; medium, observed in loam soils, with rates ranging from 1.25 to 2.5 cm hour⁻¹; and high, characteristic of deep sands and silty loams, with rates exceeding 2.5 cm hour⁻¹.
During hot, dry periods characterized by high rates of evaporation (or evapotranspiration in the presence of vegetation), soil water content decreases below field capacity. However, even under prolonged drought conditions, the soil retains a minimal amount of water, known as hygroscopic water, which is held tightly at a potential of pF 4.7, or approximately 50 atmospheres of pressure.
4. Soluble Salts and Capillary Rise
The accumulation of soluble salts in soils primarily originates from the weathering of rocks, which release their constituent elements. Two principal salinization cycles can be distinguished [
13]: (1) Continental cycles, where chlorides, sulfates, sodium carbonate, and bicarbonates are mobilized, redistributed, and accumulated in depressed areas with poor drainage, resulting from rock weathering, and (2) Marine cycles, in which soils in coastal plains and marshes accumulate marine salts, primarily sodium chloride, due to shallow saline water tables, tidal flooding, or the deposition of salts from maritime air masses. These salts may be deposited either as aerosols containing suspended salt crystals or as saline droplets.
In Spain, the continental salinization cycle is the most prevalent, particularly associated with calcilutites (marl) from the Keuper facies, Oligocene, or Miocene periods. These formations serve as sources for the redistribution of salts across multiple drainage basins [
2].
The use of electrical conductivity (EC) and the exchangeable sodium percentage (ESP) has become widely adopted for evaluating soil behavior in relation to salinity and sodicity. These two parameters allow for the classification of soils based on their soluble salt content and exchangeable sodium percentage [
14] (
Table 1).
The mineralogy of salts is highly complex and exhibits significant spatial and temporal variability, depending on the temperature and humidity of the environment in which they crystallize. As a general reference, the most representative types of salts are listed in
Table 2 [
2].
Rainfall causes a downward movement of water in the soil, resulting in the leaching of salts. During dry months, when precipitation (P) is lower than potential evapotranspiration (PET) (P - PET < 0), evapotranspiration will cause the flow to reverse, leading to the upward movement of salts. These processes occur cyclically in a non-percolating moisture regime. The temporal variability of soil salinity must be studied across different periods. For example, it has been observed that the top 10 centimeters of soil can reach a salinity approximately 10 times higher in May compared to late October, after the autumn rains [
15].
5. Efflorescence and Cryptoflorescence
The UNE 41805-5: 2009 standard, "Building Diagnosis. Part 5: Pathological Diagnosis of Building Structures." [
16], defines mechanical, physical, and chemical pathological processes that can affect buildings constructed with load-bearing walls. Among these, the most complex are those caused by rising capillary water, whose effects range from simple stains (efflorescence) to more significant deterioration that may even compromise the stability of the structure.
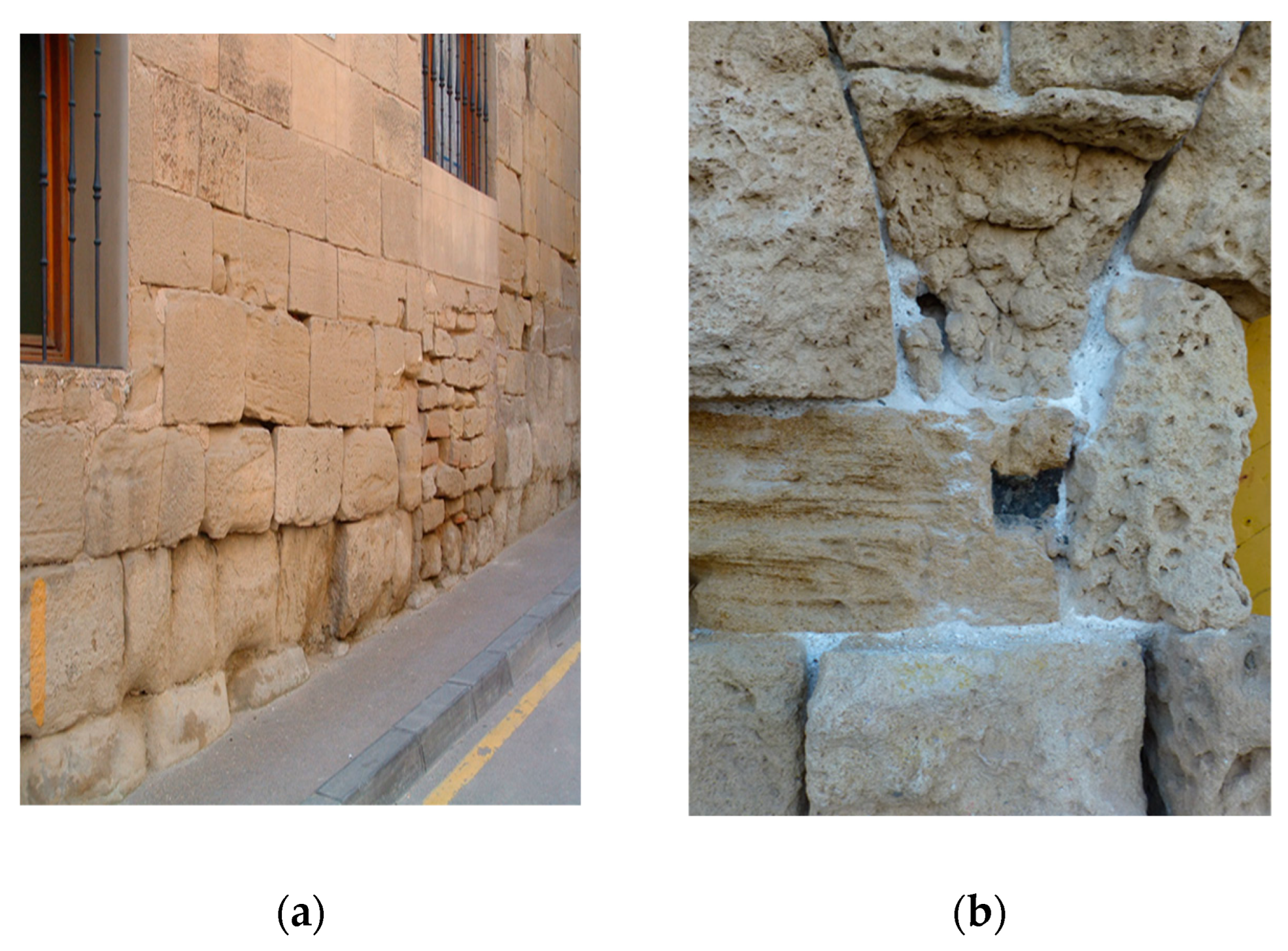
Efflorescence refers to the whitish, powdery, and loosely coherent outer layer formed by the evaporation of water contained in the masonry materials, resulting in the surface precipitation of dissolved salts (
Figure 2). These salts can originate from the building materials themselves, the masonry mortars, the water that comes into contact with the foundation, or even from improperly applied treatments aimed at preserving the masonry [
17].
Sometimes, these crystalline deposits are loose and powdery, making them easily removable by manual means, while at other times, they form hard and compact deposits that are more difficult to remove.
In this type of defect, the level of damage caused to vertical surfaces is usually not severe, as it typically consists of light-colored stains visible on the exterior of the wall, which generally do not affect the materials that make up the structure. In the worst-case scenario, only some types of pictures or thin-layer coatings may be degraded.
The detachments and mass losses associated with exfoliation, flaking or disaggregation of masonry structures are much more severe phenomena. The origin of many of these pathological processes is also rising capillary water. However, in this case, there is segregation of the constituent materials of the wall due to thermos-hydric variations, frost, and especially the crystallization of soluble salts within the pores of the material [
18].
This latter phenomenon, in which alveolar fractures occur, resulting in a reduction in the cross-section of the structure, as well as a decline in its mechanical and elastic properties, is known as cryptoflorescence (
Figure 3). These subflorescences or interstitial crystals form below the surface or within the body of the stone or mortar. The most severe manifestation of this process can cause creep, which can affect the building's integrity and stability [
19].
6. Conclusions
Capillary moisture is a common phenomenon in virtually all buildings constructed with load-bearing walls in contact with water. Its effects are wide-ranging, from creating conditions of insalubrity to long-term durability issues, which can eventually lead to mechanical problems that directly affect the stability of the structure.
In practice, the process of capillary rise is highly complex, as it involves several factors. These include the diameter of the capillary channels, electrical charges, the influence of migratory gases, the interaction between soil, masonry, water, air, temperature, and the types of salts present. Regarding the latter, in Spain, the most common and representative salts found in soils are chlorides and sulfates, with the latter being more harmful due to the larger size of their crystals.
Determining the type and origin of the salts is crucial for understanding the extent of degradation in load-bearing wall structures, identifying the causes of this deterioration, and, most importantly, defining the most appropriate intervention procedures for the maintenance and preservation of monuments.
Author Contributions
Conceptualization, A.L.M. and E.A.K.; methodology, E.A.K. and A.L.M.; validation, R.F.C., J.I.L. and B.L.G.; formal analysis, E.A.K.; investigation, E.A.K. and A.L.M.; resources, A.L.M., J.I.L., B.L.G. and R.F.C.; data curation, E.A.K. and A.L.M.; writing—original draft preparation, E.A.K. and A.L.M.; writing—review and editing, E.A.K., A.L.M., J.I.L., B.L.G. and R.F.C.; visualization, A.L.M.; supervision, E.A.K.; project administration, A.L.M. and E.A.K.; funding acquisition, A.L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
All the data related to this study can be provided upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Shaw, E.M.; Beven, K.J.; Chappell, N.A.; Lamb, R. Hydrology in practice, 4th ed.; CRC Press: London, United Kingdom, 2017. [Google Scholar] [CrossRef]
- Porta, J.; Reguerín, M.; Roquero, C. Edafología para la agricultura y el medio ambiente, 3rd ed.; Mundi-Prensa: Madrid, Spain, 2003. [Google Scholar]
- Maurício, A.M.; Pacheco, A.M.; Brito, P.S.; Castro, B.; Figueiredo, C.; Aires-Barros, L. An ionic conductivity-based methodology for monitoring salt systems in monuments stones. Journal of cultural heritage (JCH) 2005, 6, 287–293. [Google Scholar] [CrossRef]
- Marzal, R.M.E.; Franke, L.; Deckelmann, G. Predicting Efflorescence and Subflorescences of Salts. MRS Online Proceedings Library 2007, 1047, article number 403. [CrossRef]
- Doehne, E.; Price, C.A. Stone conservation: An overview of current research, 2nd ed.; Getty Conservation Institute: Los Angeles, USA, 2011. [Google Scholar]
- Zedef, V.; Kocak, K.; Doyen, A.; Ozsen, H.; Kekec, B. Effect of salt crystallization on stones of historical buildings and monuments, Konya, Central Turkey. Building and environment 2007, 42, 1453–1457. [Google Scholar] [CrossRef]
- Gallage, C.P.K.; Uchimura, T. Effects of dry density and grain size distribution on soil-water characteristic curves of sandy soils. Soils and foundations 2010, 50, 161–172. [Google Scholar] [CrossRef]
- Carter, M.R.; Gregorich, E. G. Soil sampling and methods of analysis, 2nd ed.; CRC Press: Boca Raton, USA, 2007. [Google Scholar]
- Youngs, E.G. Hydraulic conductivity of saturated soils. In Soil and Environmental Analysis, 2nd ed.; Smith, K.A., Ed.; CRC Press: Boca Raton, USA, 2000; pp. 153–194. [Google Scholar] [CrossRef]
- Rawls, W.J.; Ahuja, L.R.; Brakensiek, D.L.; Shirmohammadi, A. Infiltration and soil water movement. In Handbook of Hydrology, 1st ed.; Maidment, D.R., Ed.; McGraw-Hill Inc.: New York, USA, 1992; 5.1-5.51 ref. 123. [Google Scholar]
- Morbidelli, R.; Saltalippi, C.; Flammini, A.; Govindaraju, R.S. Role of slope on infiltration: A review. Journal of Hydrology 2018, 557, 878–886. [Google Scholar] [CrossRef]
- Patle, G.T.; Sikar, T.T.; Rawat, K.S.; Singh, S.K. Estimation of infiltration rate from soil properties using regression model for cultivated land. Geology, Ecology, and Landscapes 2019, 3, 1–13. [CrossRef]
- Ondrasek, G.; Rengel, Z. Environmental salinization processes: Detection, implications & solutions. Science of the Total Environment 2021, 754, 142432. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Shahid, S.A.; Heng, L. Guideline for salinity assessment, mitigation and adaptation using nuclear and related techniques, 1st ed.; Springer Nature: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Aragüés, R.; Cerdá, A. Salinidad de aguas y suelos en la agricultura de regadío. In Agricultura Sostenible, 1st ed.; Jiménez, R., Lamo de Espinosa, J., Eds.; Mundi-Prensa: Madrid, Spain, 1998; pp. 249–274. [Google Scholar]
- UNE 41805-5: 2009, IN. Building Diagnosis - Part 5 - Pathological study of the structure of the building - Masonry units. Available online: https://www.normadoc.com/english/une-41805-5-2009-in.html (accessed on 02 September 2024).
- Siedel, H. Salt efflorescence as indicator for sources of damaging salts on historic buildings and monuments: a statistical approach. Environ Earth Sci. 2018, 77, 572. [Google Scholar] [CrossRef]
- Koca, A.; Uğural, M.N.; Yaman, E. Rising Damp Treatment in Historical Buildings by Electro Osmosis: A Case Study. Buildings 2024, 14, 1460. [Google Scholar] [CrossRef]
- Granneman, S.J.; Lubelli, B.; van Hees, R.P. Mitigating salt damage in building materials by the use of crystallization modifiers–a review and outlook. Journal of Cultural Heritage 2019, 40, 183–194. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).