Submitted:
29 September 2024
Posted:
29 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Current Poultry Vaccine Technology
2.1 Inactivated Virus Vaccines:
2.2 Live Attenuated Vaccines:
2.3 Subunit Vaccines:
2.4 Virus-like Particles (VLPs) Vaccines:
2.5 DNA Vaccines:
2.6 mRNA Vaccines:
2.7 Recombinant Viral Vectored Vaccines:
2.8. Nanotechnology:
2.9 Conclusions:
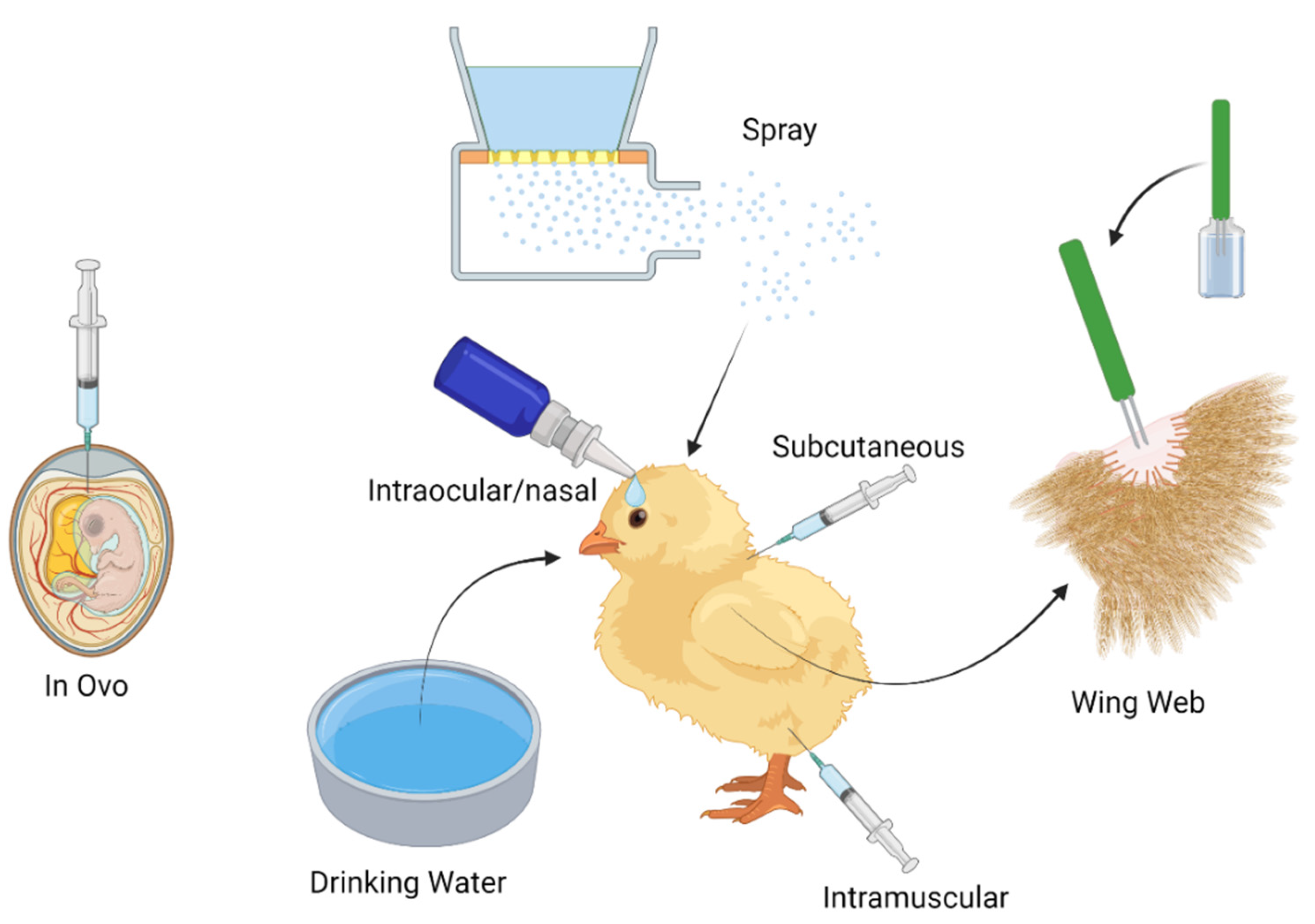
3. Delivery Mechanisms
3.1 In ovo:
3.2 Spray:
3.3 Drinking Water:
3.4 Subcutaneous/Intramuscular:
3.5 Intraocular/Nasal Drop:
3.6 Wing Web:
4. The Use of Adjuvants in Poultry Vaccines:
4.1 Emulsions (Delivery Agent and Immunostimulant):
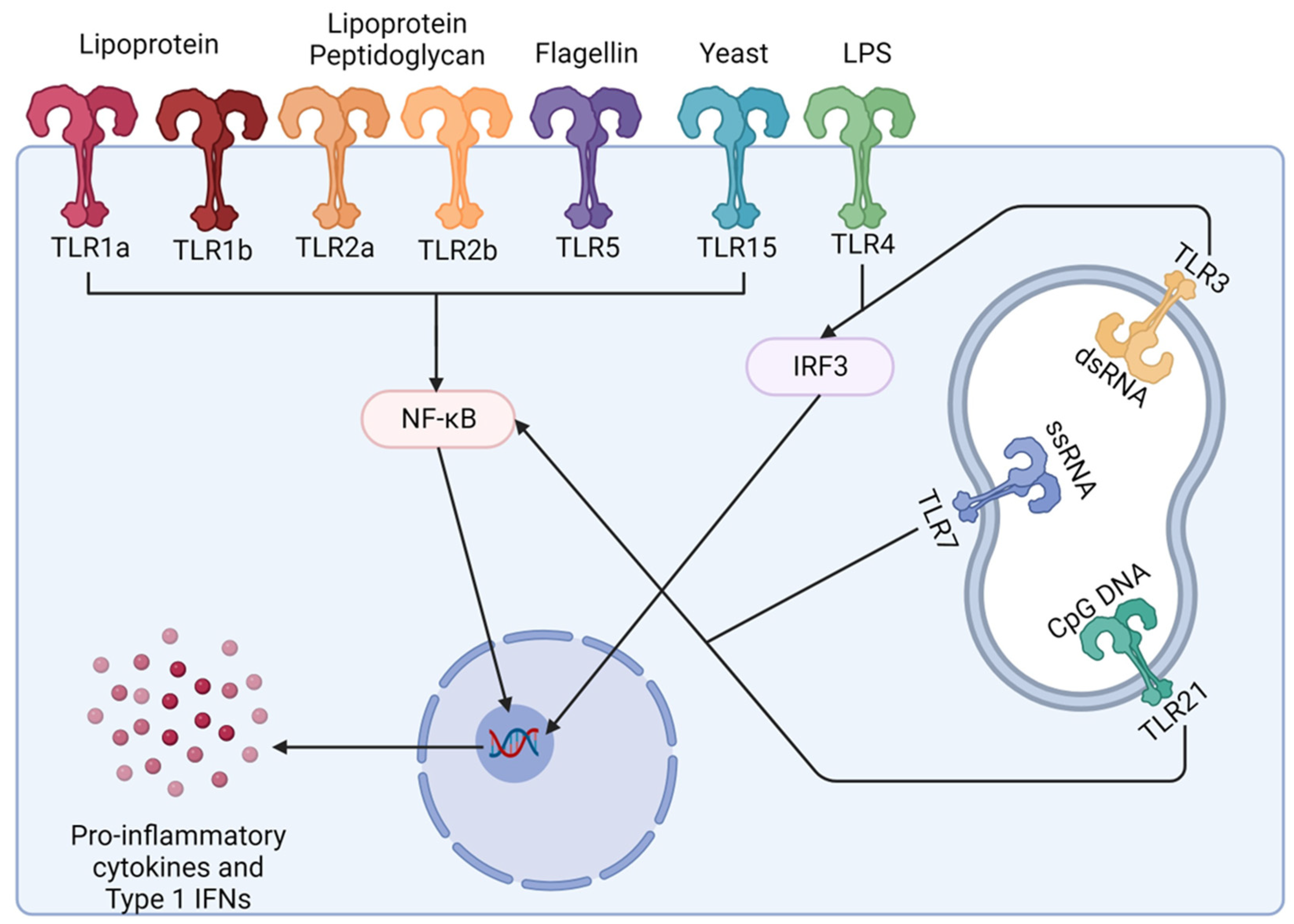
4.2 Toll Like Receptors (TLRs) (Immunostimulatory):
4.3 Cytokines as Adjuvants (Immunostimulatory):
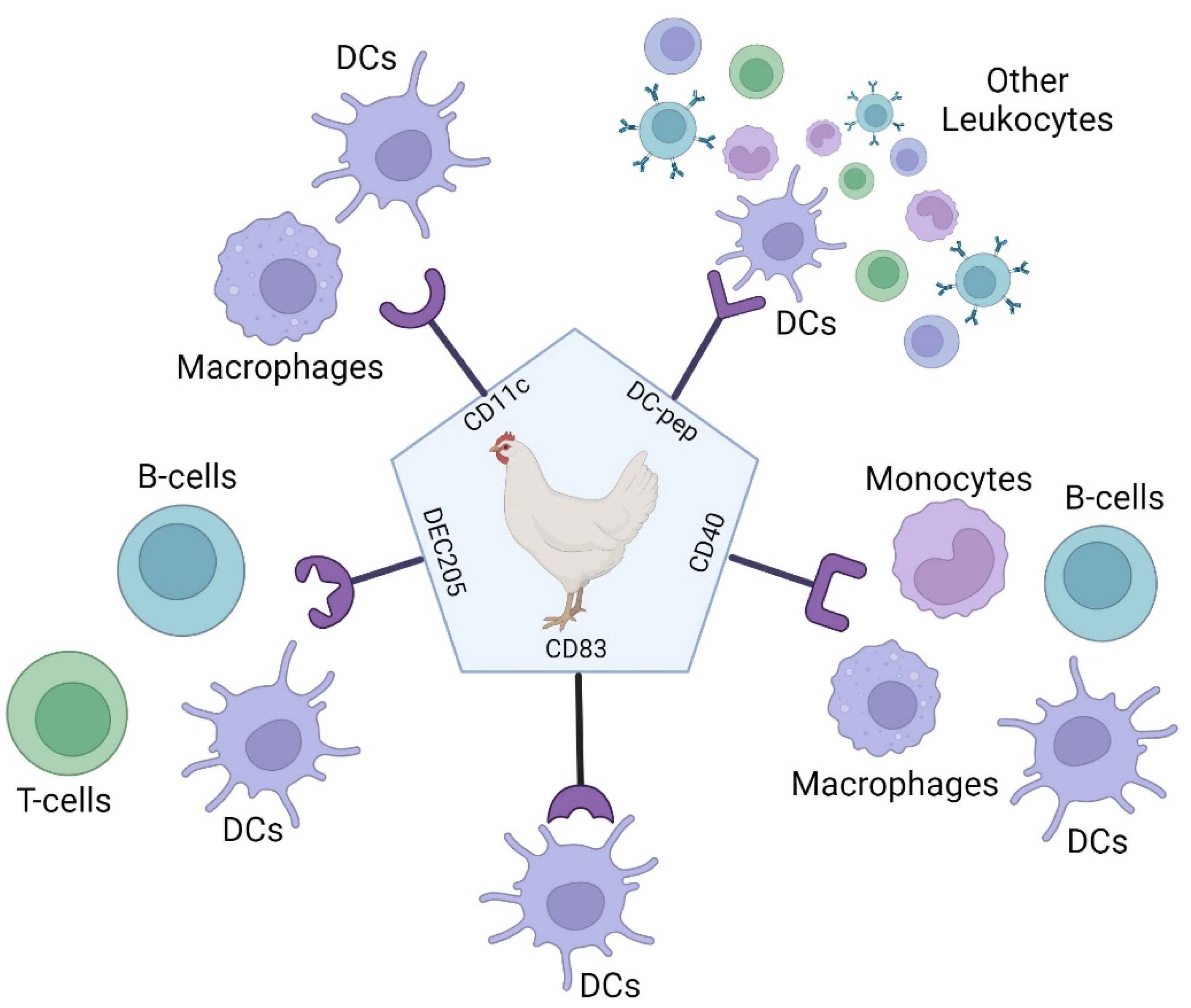
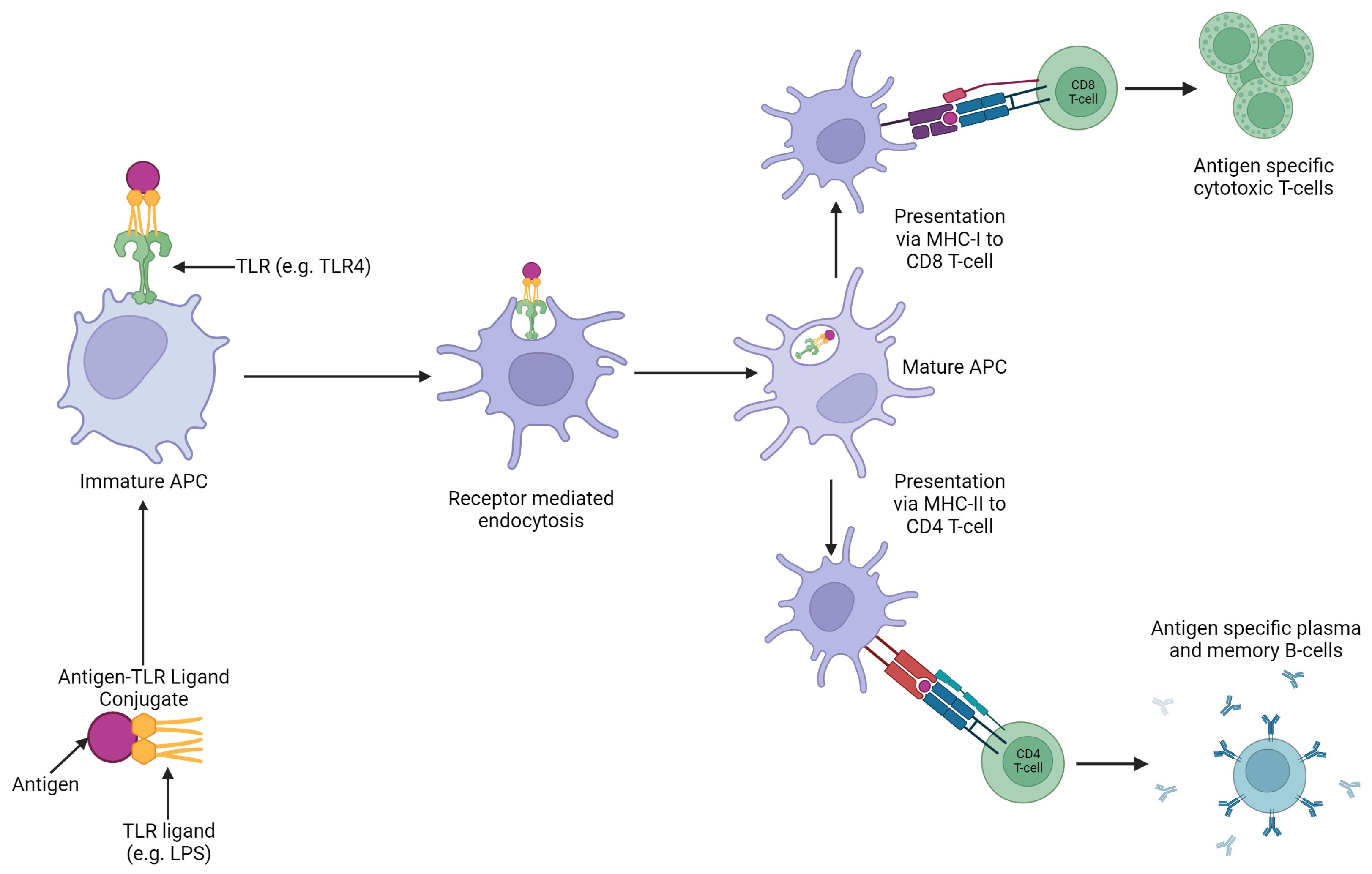
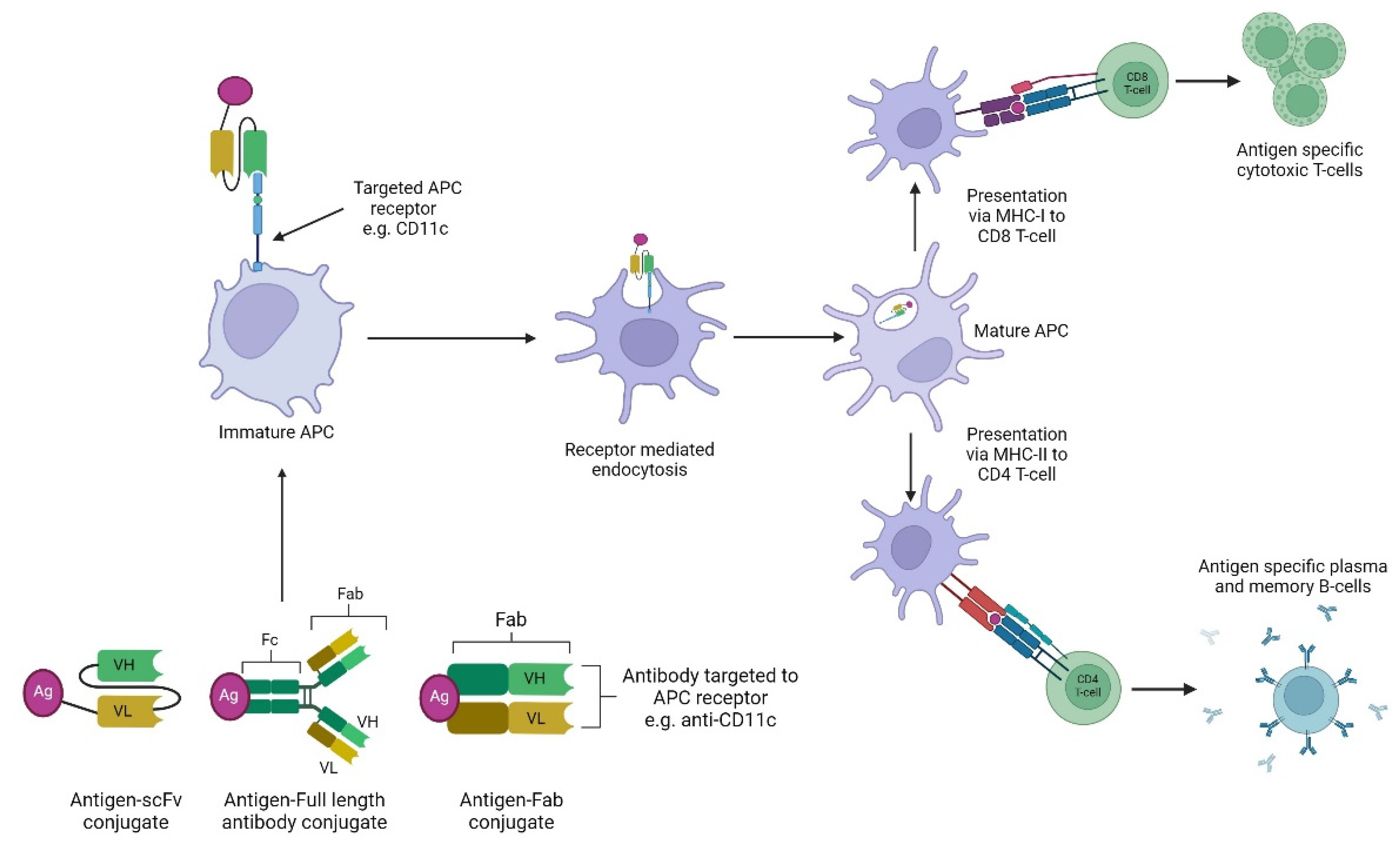
5. Targeting the Antigen to Antigen Presenting Cells:
5.1 Ligand Based Targeting of APCs:
5.2 Antibody Based Targeting of APCs:
6. Probiotics:
7. Enhancement of Vaccine Efficacy by the Incorporation of Multiple Antigens:
8. Conclusions:
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAO. MEAT MARKET REVIEW: Emerging Trends and Outlook 2022. Available online: https://www.fao.org/markets-and-trade/publications/detail/en/c/1620239/ (accessed on 19 December 2023).
- OECD-FAO Agricultural Outlook 2020-2029; OECD-FAO Agricultural Outlook; OECD, 2020; ISBN 9789264317673.
- Ravikumar, R.; Chan, J.; Prabakaran, M. Vaccines against Major Poultry Viral Diseases: Strategies to Improve the Breadth and Protective Efficacy. Viruses 2022, 14, 1195. [Google Scholar] [CrossRef] [PubMed]
- Charkhkar, S.; Bashizade, M.; Sotoudehnejad, M.; Ghodrati, M.; Bulbuli, F.; Akbarein, H. The Evaluation and Importance of Newcastle Disease’s Economic Loss in Commercial Layer Poultry. Journal of Poultry Sciences and Avian Diseases 2024, 2, 1–4. [Google Scholar] [CrossRef]
- Scharff, R.L. Food Attribution and Economic Cost Estimates for Meat- And Poultry-Related Illnesses. J Food Prot 2020, 83, 959–967. [Google Scholar] [CrossRef] [PubMed]
- Dejyong, T.; Chanachai, K.; Prarakamawongsa, T.; Kongkaew, W.; Thiptara, A.; Songserm, T.; Rukkwamsuk, T.; TagoPacheco, D.; Phimpraphai, W. Economic and Value Chain Analysis to Support an Investigation and Risk Mitigation Efforts on Marek’s Disease in Layers in the Southern Part of Thailand. Vet World 2023, 16, 35–45. [Google Scholar] [CrossRef]
- Gashaw, M. A Review on Avian Influenza and Its Economic and Public Health Impact. Int J Vet Sci Technol 2020, 4, 15–027. [Google Scholar]
- Shrestha, A.; Sadeyen, J.-R.; Iqbal, M. Enhancing Protective Efficacy of Poultry Vaccines through Targeted Delivery of Antigens to Antigen-Presenting Cells. Vaccines (Basel) 2018, 6, 75. [Google Scholar] [CrossRef]
- Abdul-Cader, M.S.; Palomino-Tapia, V.; Amarasinghe, A.; Ahmed-Hassan, H.; De Silva Senapathi, U.; Abdul-Careem, M.F. Hatchery Vaccination Against Poultry Viral Diseases: Potential Mechanisms and Limitations. Viral Immunol 2018, 31, 23–33. [Google Scholar] [CrossRef]
- Hofacre, C.L.; Singer, R.S.; Johnson, T.J. Antimicrobial Therapy (Including Resistance). In Diseases of Poultry; 2013; pp. 40–43.
- Swayne, D.E. Impact of Vaccines and Vaccination on Global Control of Avian Influenza. Avian Dis 2012, 56, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Romanutti, C.; Keller, L.; Zanetti, F.A. Current Status of Virus-Vectored Vaccines against Pathogens That Affect Poultry. Vaccine 2020, 38, 6990–7001. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E.; Kapczynski, D.R. Vaccines and Vaccination for Avian Influenza in Poultry. In Avian Influenza; 2017; pp. 378–434.
- Veterinary Medicines Directorate. Available online: https://www.vmd.defra.gov.uk/productinformationdatabase (accessed on 17 June 2024).
- Swayne, D.E. Avian Influenza Vaccines and Therapies for Poultry. Comp Immunol Microbiol Infect Dis 2009, 32, 351–363. [Google Scholar] [CrossRef]
- Jang, H.; Elaish, M.; Mahesh, K.C.; Abundo, M.C.; Ghorbani, A.; Ngunjiri, J.M.; Lee, C.W. Efficacy and Synergy of Live-Attenuated and Inactivated Influenza Vaccines in Young Chickens. PLoS One 2018, 13. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, S.; Brokstad, K.; Cox, R. Influenza Vaccination Strategies: Comparing Inactivated and Live Attenuated Influenza Vaccines. Vaccines (Basel) 2015, 3, 373–389. [Google Scholar] [CrossRef]
- Peek, H.W.; Landman, W.J.M. Coccidiosis in Poultry: Anticoccidial Products, Vaccines and Other Prevention Strategies. Veterinary Quarterly 2011, 31, 143–161. [Google Scholar] [CrossRef]
- Steel, J.; Lowen, A.C.; Pena, L.; Angel, M.; Solórzano, A.; Albrecht, R.; Perez, D.R.; García-Sastre, A.; Palese, P. Live Attenuated Influenza Viruses Containing NS1 Truncations as Vaccine Candidates against H5N1 Highly Pathogenic Avian Influenza. J Virol 2009, 83, 1742–1753. [Google Scholar] [CrossRef]
- Grødeland, G.; Fossum, E.; Bogen, B. Polarizing T and B Cell Responses by APC-Targeted Subunit Vaccines. Front Immunol 2015, 6. [Google Scholar] [CrossRef] [PubMed]
- Meunier, M.; Chemaly, M.; Dory, D. DNA Vaccination of Poultry: The Current Status in 2015. Vaccine 2016, 34, 202–211. [Google Scholar] [CrossRef]
- Lauer, K.B.; Borrow, R.; Blanchard, T.J. Multivalent and Multipathogen Viral Vector Vaccines. Clinical and Vaccine Immunology 2017, 24. [Google Scholar] [CrossRef]
- Aida, V.; Pliasas, V.C.; Neasham, P.J.; North, J.F.; McWhorter, K.L.; Glover, S.R.; Kyriakis, C.S. Novel Vaccine Technologies in Veterinary Medicine: A Herald to Human Medicine Vaccines. Front Vet Sci 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Harper, D.M.; Franco, E.L.; Wheeler, C.; Ferris, D.G.; Jenkins, D.; Schuind, A.; Zahaf, T.; Innis, B.; Naud, P.; De Carvalho, N.S.; et al. Efficacy of a Bivalent L1 Virus-like Particle Vaccine in Prevention of Infection with Human Papillomavirus Types 16 and 18 in Young Women: A Randomised Controlled Trial. Lancet 2004, 364, 1757–1765. [Google Scholar] [CrossRef]
- Keating, G.M.; Noble, S. Recombinant Hepatitis B Vaccine (Engerix-B): A Review of Its Immunogenicity and Protective Efficacy against Hepatitis B. Drugs 2003, 63, 1021–1051. [Google Scholar] [CrossRef]
- Zepeda-Cervantes, J.; Ramírez-Jarquín, J.O.; Vaca, L. Interaction Between Virus-Like Particles (VLPs) and Pattern Recognition Receptors (PRRs) From Dendritic Cells (DCs): Toward Better Engineering of VLPs. Front Immunol 2020, 11, 1100. [Google Scholar] [CrossRef] [PubMed]
- Mejía-Méndez, J.L.; Vazquez-Duhalt, R.; Hernández, L.R.; Sánchez-Arreola, E.; Bach, H. Virus-like Particles: Fundamentals and Biomedical Applications. Int J Mol Sci 2022, 23, 8579. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Qian, J.; Qin, L.; Li, J.; Xue, C.; Ding, J.; Wang, W.; Ding, W.; Yin, R.; Jin, N.; et al. Chimeric Newcastle Disease Virus-like Particles Containing DC-Binding Peptide-Fused Haemagglutinin Protect Chickens from Virulent Newcastle Disease Virus and H9N2 Avian Influenza Virus Challenge. Virol Sin 2020, 35, 455–467. [Google Scholar] [CrossRef] [PubMed]
- Firouzamandi, M.; Helan, J.A.; Moeini, H.; Soleimanian, A.; Khatemeh, S.; Hosseini, S.D. Developing a Vaccine against Velogenic Sub-Genotype Seven of Newcastle Disease Virus Based on Virus-like Particles. AMB Express 2023, 13, 114. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.Y.; Park, J.K.; Lee, D.H.; Yuk, S.S.; Kwon, J.H.; Lee, S.W.; Lee, J.B.; Park, S.Y.; Choi, I.S.; Song, C.S. Chimeric Bivalent Virus-like Particle Vaccine for H5N1 HPAI and ND Confers Protection Against a Lethal Challenge in Chickens and Allows a Strategy of Differentiating Infected from Vaccinated Animals (DIVA). PLoS One 2016, 11. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, Y.; Hu, Y.; Qiu, J.; Lei, W.; Ji, W.; Li, X.; Wu, Q.; Shi, X.; Li, Z. Protection of Chickens against Infectious Bronchitis Virus with a Multivalent DNA Vaccine and Boosting with an Inactivated Vaccine. J Vet Sci 2013, 14, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Faurez, F.; Dory, D.; Le Moigne, V.; Gravier, R.; Jestin, A. Biosafety of DNA Vaccines: New Generation of DNA Vectors and Current Knowledge on the Fate of Plasmids after Injection. Vaccine 2010, 28, 3888–3895. [Google Scholar] [CrossRef]
- Robinson, H.L.; Hunt, L.A.; Webster, R.G. Protection against a Lethal Influenza Virus Challenge by Immunization with a Haemagglutinin-Expressing Plasmid DNA. Vaccine 1993, 11. [Google Scholar] [CrossRef] [PubMed]
- Bublot, M. Poultry Vaccine Technology Platforms. Avian Dis 2023, 67. [Google Scholar] [CrossRef]
- Jazayeri, S.D.; Poh, C.L. Recent Advances in Delivery of Veterinary DNA Vaccines against Avian Pathogens. Vet Res 2019, 50, 78. [Google Scholar] [CrossRef]
- Martinon, F.; Krishnan, S.; Lenzen, G.; Magné, R.; Gomard, E.; Guillet, J. -G; Lévy, J. -P; Meulien, P. Induction of Virus-specific Cytotoxic T Lymphocytes in Vivo by Liposome-entrapped MRNA. Eur J Immunol 1993, 23. [Google Scholar] [CrossRef] [PubMed]
- Fang, E.; Liu, X.; Li, M.; Zhang, Z.; Song, L.; Zhu, B.; Wu, X.; Liu, J.; Zhao, D.; Li, Y. Advances in COVID-19 MRNA Vaccine Development. Signal Transduct Target Ther 2022, 7, 94. [Google Scholar] [CrossRef] [PubMed]
- Karikó, K.; Muramatsu, H.; Welsh, F.A.; Ludwig, J.; Kato, H.; Akira, S.; Weissman, D. Incorporation of Pseudouridine into MRNA Yields Superior Nonimmunogenic Vector with Increased Translational Capacity and Biological Stability. Molecular Therapy 2008, 16, 1833–1840. [Google Scholar] [CrossRef] [PubMed]
- Kauffman, K.J.; Webber, M.J.; Anderson, D.G. Materials for Non-Viral Intracellular Delivery of Messenger RNA Therapeutics. Journal of Controlled Release 2016, 240, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. MRNA Vaccines — a New Era in Vaccinology. Nat Rev Drug Discov 2018, 17, 261–279. [Google Scholar] [CrossRef]
- Chen, N.; Xia, P.; Li, S.; Zhang, T.; Wang, T.T.; Zhu, J. RNA Sensors of the Innate Immune System and Their Detection of Pathogens. IUBMB Life 2017, 69, 297–304. [Google Scholar] [CrossRef]
- Snoeck, J.; Chiers, K.; Tam, Y.; Sanders, N.N.; Garmyn, A. Evaluation of a Self-Amplifying MRNA Reporter Vaccine in Explant Models of Broiler Chickens. Poult Sci 2023, 102, 103078. [Google Scholar] [CrossRef]
- Xu, S.; Zhang, B.; Yao, J.; Ruan, W. A New H9 Influenza Virus MRNA Vaccine Elicits Robust Protective Immunity against Infection. Vaccine 2023, 41, 2905–2913. [Google Scholar] [CrossRef]
- Hein, R.; Koopman, R.; García, M.; Armour, N.; Dunn, J.R.; Barbosa, T.; Martinez, A. Review of Poultry Recombinant Vector Vaccines. Avian Dis 2021, 65. [Google Scholar] [CrossRef]
- Swayne, D.E.; Beck, J.R.; Kinney, N. Failure of a Recombinant Fowl Poxvirus Vaccine Containing an Avian Influenza Hemagglutinin Gene to Provide Consistent Protection against Influenza in Chickens Preimmunized with a Fowl Pox Vaccine. Avian Dis 2000, 44, 132. [Google Scholar] [CrossRef]
- Vagnozzi, A.; Zavala, G.; Riblet, S.M.; Mundt, A.; García, M. Protection Induced by Commercially Available Live-Attenuated and Recombinant Viral Vector Vaccines against Infectious Laryngotracheitis Virus in Broiler Chickens. Avian Pathology 2012, 41, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hamid, H.S.; Ellakany, H.F.; Elbestawy, A.R.; Setta, A. The Combined Use of RHVT-H5 and RHVT-F Vector Vaccines in the Hatchery Enhances Immunity against Highly Pathogenic Avian Influenza H5N1 and Velogenic Newcastle Disease Viral Infections in Commercial Chickens. Poultry Science Journal 2018, 6, 165–171. [Google Scholar] [CrossRef]
- Williams, C.J.; Hopkins, B.A. Field Evaluation of the Accuracy of Vaccine Deposition by Two Different Commercially Available in Ovo Injection Systems. Poult Sci 2011, 90, 223–226. [Google Scholar] [CrossRef] [PubMed]
- Gergen, L.; Cook, S.; Ledesma, B.; Cress, W.; Higuchi, D.; Counts, D.; Cruz-Coy, J.; Crouch, C.; Davis, P.; Tarpey, I.; et al. A Double Recombinant Herpes Virus of Turkeys for the Protection of Chickens against Newcastle, Infectious Laryngotracheitis and Marek’s Diseases. Avian Pathology 2019, 48, 45–56. [Google Scholar] [CrossRef]
- Huang, Z.; Elankumaran, S.; Panda, A.; Samal, S. Recombinant Newcastle Disease Virus as a Vaccine Vector. Poult Sci 2003, 82, 899–906. [Google Scholar] [CrossRef]
- Zhao, H.; Peeters, B.P.H. Recombinant Newcastle Disease Virus as a Viral Vector: Effect of Genomic Location of Foreign Gene on Gene Expression and Virus Replication. J Gen Virol 2003, 84, 781–788. [Google Scholar] [CrossRef]
- Lozano, D.; Larraga, V.; Vallet-Regí, M.; Manzano, M. An Overview of the Use of Nanoparticles in Vaccine Development. Nanomaterials 2023, 13, 1828. [Google Scholar] [CrossRef]
- Gregory, A.E.; Titball, R.; Williamson, D. Vaccine Delivery Using Nanoparticles. Front Cell Infect Microbiol 2013, 4. [Google Scholar] [CrossRef]
- Calderon-Nieva, D.; Goonewardene, K.B.; Gomis, S.; Foldvari, M. Veterinary Vaccine Nanotechnology: Pulmonary and Nasal Delivery in Livestock Animals. Drug Deliv Transl Res 2017, 7, 558–570. [Google Scholar] [CrossRef]
- Zhao, K.; Rong, G.; Hao, Y.; Yu, L.; Kang, H.; Wang, X.; Wang, X.; Jin, Z.; Ren, Z.; Li, Z. IgA Response and Protection Following Nasal Vaccination of Chickens with Newcastle Disease Virus DNA Vaccine Nanoencapsulated with Ag@SiO2 Hollow Nanoparticles. Sci Rep 2016, 6, 25720. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Rogers, C.J.; Hance, K.W.; Schlom, J.; Greiner, J.W. Chitosan Solution Enhances Both Humoral and Cell-Mediated Immune Responses to Subcutaneous Vaccination. Vaccine 2007, 25, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Volkova, M.A.; Irza, A. V.; Chvala, I.A.; Frolov, S.F.; Drygin, V. V.; Kapczynski, D.R. Adjuvant Effects of Chitosan and Calcium Phosphate Particles in an Inactivated Newcastle Disease Vaccine. Avian Dis 2014, 58, 46–52. [Google Scholar] [CrossRef]
- Renu, S.; Han, Y.; Dhakal, S.; Lakshmanappa, Y.S.; Ghimire, S.; Feliciano-Ruiz, N.; Senapati, S.; Narasimhan, B.; Selvaraj, R.; Renukaradhya, G.J. Chitosan-Adjuvanted Salmonella Subunit Nanoparticle Vaccine for Poultry Delivered through Drinking Water and Feed. Carbohydr Polym 2020, 243, 116434. [Google Scholar] [CrossRef] [PubMed]
- Schat, K.A.; Kaspers, B.; Kaiser, P. Avian Immunology; Elsevier, 2014; ISBN 9780123969651.
- Peebles, E.D. In Ovo Applications in Poultry: A Review. Poult Sci 2018, 97, 2322–2338. [Google Scholar] [CrossRef]
- Toro, H.; Tang, D.C.; Suarez, D.L.; Sylte, M.J.; Pfeiffer, J.; Van Kampen, K.R. Protective Avian Influenza in Ovo Vaccination with Non-Replicating Human Adenovirus Vector. Vaccine 2007, 25, 2886–2891. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.E.C.; Branton, S.L.; Evans, J.D.; Gerard, P.D.; Peebles, E.D. Layer Chicken Embryo Survival to Hatch When Administered an in Ovo Vaccination of Strain F Mycoplasma Gallisepticum and Locations of Bacteria Prevalence in the Newly Hatched Chick. Poult Sci 2017, 96, 3879–3884. [Google Scholar] [CrossRef] [PubMed]
- Wakenell, P.S.; Bryan, T.; Schaeffer, J.; Avakian, A.; Williams, C.; Whitfill, C. Effect of in Ovo Vaccine Delivery Route on Herpesvirus of Turkeys/SB-1 Efficacy and Viremia. Avian Dis 2002, 46, 274–280. [Google Scholar] [CrossRef]
- de Wit, J.J.; Montiel, E. Practical Aspects of Poultry Vaccination. In Avian Immunology; Elsevier, 2021; pp. 469–488 ISBN 9780128190715.
- Gentry, R.F.; Braune, M.O. Prevention of Virus Inactivation During Drinking Water Vaccination of Poultry. Poult Sci 1972, 51, 1450–1456. [Google Scholar] [CrossRef]
- Gharaibeh, S.; Mahmoud, K.; Al-Natour, M. Field Evaluation of Maternal Antibody Transfer to a Group of Pathogens in Meat-Type Chickens. Poult Sci 2008, 87, 1550–1555. [Google Scholar] [CrossRef]
- Burakova, Y.; Madera, R.; McVey, S.; Schlup, J.R.; Shi, J. Adjuvants for Animal Vaccines. Viral Immunol 2018, 31, 11–22. [Google Scholar] [CrossRef]
- Cox, J.C.; Coulter, A.R. Adjuvants - A Classification and Review of Their Modes of Action. Vaccine 1997, 15, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Herbert, W.J. The Mode of Action of Mineral-Oil Emulsion Adjuvants on Antibody Production in Mice. Immunology 1968, 14, 301–318. [Google Scholar] [PubMed]
- Freund, J.; Casals, J.; Hosmer, E.P. Sensitization and Antibody Formation after Injection of Tubercle Bacilli and Paraffin Oil. Proceedings of the Society for Experimental Biology and Medicine 1937, 37. [Google Scholar] [CrossRef]
- Leenaars, P.P.A.M.; Hendriksen, C.F.M.; Angulo, A.F.; Koedam, M.A.; Claassen, E. Evaluation of Several Adjuvants as Alternatives to the Use of Freund’s Adjuvant in Rabbits. Vet Immunol Immunopathol 1994, 40. [Google Scholar] [CrossRef]
- Arous, J. Ben; Deville, S.; Pal, J.K.; Baksi, S.; Bertrand, F.; Dupuis, L. Reduction of Newcastle Disease Vaccine Dose Using a Novel Adjuvant for Cellular Immune Response in Poultry. Procedia Vaccinol 2013, 7, 28–33. [Google Scholar] [CrossRef]
- Jang, S.I.; Lillehoj, H.S.; Lee, S.H.; Lee, K.W.; Park, M.S.; Bauchan, G.R.; Lillehoj, E.P.; Bertrand, F.; Dupuis, L.; Deville, S. Immunoenhancing Effects of MontanideTM ISA Oil-Based Adjuvants on Recombinant Coccidia Antigen Vaccination against Eimeria Acervulina Infection. Vet Parasitol 2010, 172, 221–228. [Google Scholar] [CrossRef]
- Chada, K.E.; Forshee, R.; Golding, H.; Anderson, S.; Yang, H. A Systematic Review and Meta-Analysis of Cross-Reactivity of Antibodies Induced by Oil-in-Water Emulsion Adjuvanted Influenza H5N1 Virus Monovalent Vaccines. Vaccine 2017, 35, 3162–3170. [Google Scholar] [CrossRef] [PubMed]
- Banzhoff, A.; Gasparini, R.; Laghi-Pasini, F.; Staniscia, T.; Durando, P.; Montomoli, E.; Capecchi, P.; di Giovanni, P.; Sticchi, L.; Gentile, C.; et al. MF59®-Adjuvanted H5N1 Vaccine Induces Immunologic Memory and Heterotypic Antibody Responses in Non-Elderly and Elderly Adults. PLoS One 2009, 4, e4384. [Google Scholar] [CrossRef]
- Barnett, P.V.; Pullen, L.; Williams, L.; Doel, T.R. International Bank for Foot-and-Mouth Disease Vaccine: Assessment of Montanide ISA 25 and ISA 206, Two Commercially Available Oil Adjuvants. Vaccine 1996, 14, 1187–1198. [Google Scholar] [CrossRef]
- Aslam, B.; Hussain, I.; Mahmood, M.S.; Khan, A. Preparation and Evaluation of Montanide ISA 206 Adjuvanted Bacterin of Borrelia Anserina in Laying Chickens. Journal of Applied Poultry Research 2013, 22, 196–203. [Google Scholar] [CrossRef]
- Gupta, S.K.; Deb, R.; Dey, S.; Chellappa, M.M. Toll-like Receptor-Based Adjuvants: Enhancing the Immune Response to Vaccines against Infectious Diseases of Chicken. Expert Rev Vaccines 2014, 13, 909–925. [Google Scholar] [CrossRef]
- Temperley, N.D.; Berlin, S.; Paton, I.R.; Griffin, D.K.; Burt, D.W. Evolution of the Chicken Toll-like Receptor Gene Family: A Story of Gene Gain and Gene Loss. BMC Genomics 2008, 9, 62. [Google Scholar] [CrossRef]
- Chaung, H.C.; Cheng, L.T.; Hung, L.H.; Tsai, P.C.; Skountzou, I.; Wang, B.; Compans, R.W.; Lien, Y.Y. Salmonella Flagellin Enhances Mucosal Immunity of Avian Influenza Vaccine in Chickens. Vet Microbiol 2012, 157, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Fu, J.; Kang, H.; Lin, J.; Yu, Q.; Yang, Q. Comparison of 3 Kinds of Toll-like Receptor Ligands for Inactivated Avian H5N1 Influenza Virus Intranasal Immunization in Chicken. Poult Sci 2013, 92, 2651–2660. [Google Scholar] [CrossRef] [PubMed]
- Bhadouriya, S.; Sharma, B.K.; Kakker, N.K.; Chhabra, R. Toll like Receptors and Cytokines as Immunostimulatory Adjuvants in Poultry Vaccines: Current Status and Future Trends. Worlds Poult Sci J 2019, 75, 417–427. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, M.; Li, J.; Cao, T.; Tian, X.; Zhou, F. Enhancement of Mucosal Immune Responses by Intranasal Co-Delivery of Newcastle Disease Vaccine plus CpG Oligonucleotide in SPF Chickens in Vivo. Res Vet Sci 2008, 85, 495–502. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Lowry, V.K.; Swaggerty, C.L.; Ferro, P.J.; Kogut, M.H. In Vitro Activation of Chicken Leukocytes and in Vivo Protection against Salmonella Enteritidis Organ Invasion and Peritoneal S. Enteritidis Infection-Induced Mortality in Neonatal Chickens by Immunostimulatory CpG Oligodeoxynucleotide. FEMS Immunol Med Microbiol 2005, 43, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Barjesteh, N.; Shojadoost, B.; Brisbin, J.T.; Emam, M.; Hodgins, D.C.; Nagy, É.; Sharif, S. Reduction of Avian Influenza Virus Shedding by Administration of Toll-like Receptor Ligands to Chickens. Vaccine 2015, 33, 4843–4849. [Google Scholar] [CrossRef]
- Hung, L.-H.; Tsai, P.-C.; Wang, C.-H.; Li, S.-L.; Huang, C.-C.; Lien, Y.-Y.; Chaung, H.-C. Immunoadjuvant Efficacy of Plasmids with Multiple Copies of a CpG Motif Coadministrated with Avian Influenza Vaccine in Chickens. Vaccine 2011, 29, 4668–4675. [Google Scholar] [CrossRef]
- Alqazlan, N.; Astill, J.; Raj, S.; Sharif, S. Strategies for Enhancing Immunity against Avian Influenza Virus in Chickens: A Review. Avian Pathology 2022, 51, 211–235. [Google Scholar] [CrossRef]
- Schijns, V.E.C.J.; Weining, K.C.; Nuijten, P.; Rijke, E.O.; Staeheli, P. Immunoadjuvant Activities of E. Coli- and Plasmid-Expressed Recombinant Chicken IFN-α/β, IFN-γ and IL-1β in 1-Day- and 3-Week-Old Chickens. Vaccine 2000, 18, 2147–2154. [Google Scholar] [CrossRef]
- Gan, L.; Tian, Y.; Zhao, Y.; Shan, X.; Zhou, W.; Xia, B.-B.; Chen, J.; Wang, M.-L.; Zhao, J. Enhancing Immunogenicity and Protective Efficacy of Inactivated Avian Influenza H9N2vaccine with Recombinant Chicken IFN-α in Chicken. Vet Microbiol 2019, 234, 77–82. [Google Scholar] [CrossRef]
- Karaca, K.; Sharma, J.M.; Winslow, B.J.; Junker, D.E.; Reddy, S.; Cochran, M.; McMillen, J. Recombinant Fowlpox Viruses Coexpressing Chicken Type I IFN and Newcastle Disease Virus HN and F Genes: Influence of IFN on Protective Efficacy and Humoral Responses of Chickens Following in Ovo or Post-Hatch Administration of Recombinant Viruses. Vaccine 1998, 16, 1496–1503. [Google Scholar] [CrossRef]
- Xiaowen, Z.; Qinghua, Y.; Xiaofei, Z.; Qian, Y. Co-Administration of Inactivated Avian Influenza Virus with CpG or RIL-2 Strongly Enhances the Local Immune Response after Intranasal Immunization in Chicken. Vaccine 2009, 27, 5628–5632. [Google Scholar] [CrossRef]
- Yang, Y.; Leggat, D.; Herbert, A.; Roberts, P.C.; Sundick, R.S. A Novel Method to Incorporate Bioactive Cytokines as Adjuvants on the Surface of Virus Particles. Journal of Interferon and Cytokine Research 2009, 29, 9–22. [Google Scholar] [CrossRef]
- Lim, K.-L.; Jazayeri, S.D.; Yeap, S.K.; Alitheen, N.B.M.; Bejo, M.H.; Ideris, A.; Omar, A.R. Co-Administration of Avian Influenza Virus H5 Plasmid DNA with Chicken IL-15 and IL-18 Enhanced Chickens Immune Responses. BMC Vet Res 2012, 8. [Google Scholar] [CrossRef]
- Melgoza-González, E.A.; Bustamante-Córdova, L.; Hernández, J. Recent Advances in Antigen Targeting to Antigen-Presenting Cells in Veterinary Medicine. Front Immunol 2023, 14. [Google Scholar] [CrossRef]
- Chen, P.; Liu, X.; Sun, Y.; Zhou, P.; Wang, Y.; Zhang, Y. Dendritic Cell Targeted Vaccines: Recent Progresses and Challenges. Hum Vaccin Immunother 2016, 12, 612–622. [Google Scholar] [CrossRef]
- Longet, S.; Lundahl, M.L.E.; Lavelle, E.C. Targeted Strategies for Mucosal Vaccination. Bioconjug Chem 2018, 29, 613–623. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Ju, X.; Silveira, P.A.; Abadir, E.; Hsu, W.-H.; Hart, D.N.J.; Clark, G.J. CD83: Activation Marker for Antigen Presenting Cells and Its Therapeutic Potential. Front Immunol 2019, 10. [Google Scholar] [CrossRef]
- Sun, P. Structural Recognition of Immunoglobulins by Fcγ Receptors. In Antibody Fc: Linking adaptive and innate immunity; 2014; pp. 131–144.
- Shrestha, A.; Sadeyen, J.-R.; Lukosaityte, D.; Chang, P.; Van Hulten, M.; Iqbal, M. Targeting Haemagglutinin Antigen of Avian Influenza Virus to Chicken Immune Cell Receptors Dec205 and CD11c Induces Differential Immune-Potentiating Responses. Vaccines (Basel) 2021, 9, 784. [Google Scholar] [CrossRef]
- Shrestha, A.; Meeuws, R.; Sadeyen, J.-R.; Chang, P.; Van Hulten, M.; Iqbal, M. Haemagglutinin Antigen Selectively Targeted to Chicken CD83 Overcomes Interference from Maternally Derived Antibodies in Chickens. NPJ Vaccines 2022, 7, 33. [Google Scholar] [CrossRef]
- Shrestha, A.; Sadeyen, J.-R.; Lukosaityte, D.; Chang, P.; Smith, A.; Van Hulten, M.; Iqbal, M. Selectively Targeting Haemagglutinin Antigen to Chicken CD83 Receptor Induces Faster and Stronger Immunity against Avian Influenza. NPJ Vaccines 2021, 6, 90. [Google Scholar] [CrossRef]
- Chou, W.K.; Chen, C.H.; Vuong, C.N.; Abi-Ghanem, D.; Waghela, S.D.; Mwangi, W.; Bielke, L.R.; Hargis, B.M.; Berghman, L.R. Significant Mucosal SIgA Production after a Single Oral or Parenteral Administration Using in Vivo CD40 Targeting in the Chicken. Res Vet Sci 2016, 108, 112–115. [Google Scholar] [CrossRef]
- Yin, G.; Lin, Q.; Qiu, J.; Qin, M.; Tang, X.; Suo, X.; Huang, Z.; Liu, X. Immunogenicity and Protective Efficacy of an Eimeria Vaccine Candidate Based on Eimeria Tenella Immune Mapped Protein 1 and Chicken CD40 Ligand. Vet Parasitol 2015, 210, 19–24. [Google Scholar] [CrossRef]
- Pecora, A.; Malacari, D.A.; Perez Aguirreburualde, M.S.; Bellido, D.; Nuñez, M.C.; Dus Santos, M.J.; Escribano, J.M.; Wigdorovitz, A. Development of an APC-Targeted Multivalent E2-Based Vaccine against Bovine Viral Diarrhea Virus Types 1 and 2. Vaccine 2015, 33, 5163–5171. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Orouji, S.; Esufali, J.; Mallick, A.I.; Parvizi, P.; Shewen, P.E.; Sharif, S. Oral Treatment of Chickens with Lactobacilli Influences Elicitation of Immune Responses. Clinical and Vaccine Immunology 2011, 18, 1447–1455. [Google Scholar] [CrossRef]
- Callaway, T.R.; Edrington, T.S.; Anderson, R.C.; Harvey, R.B.; Genovese, K.J.; Kennedy, C.N.; Venn, D.W.; Nisbet, D.J. Probiotics, Prebiotics and Competitive Exclusion for Prophylaxis against Bacterial Disease. Anim Health Res Rev 2008, 9, 217–225. [Google Scholar] [CrossRef]
- Brisbin, J.T.; Gong, J.; Parvizi, P.; Sharif, S. Effects of Lactobacilli on Cytokine Expression by Chicken Spleen and Cecal Tonsil Cells. Clinical and Vaccine Immunology 2010, 17, 1337–1343. [Google Scholar] [CrossRef]
- Schaupp, L.; Muth, S.; Rogell, L.; Kofoed-Branzk, M.; Melchior, F.; Lienenklaus, S.; Ganal-Vonarburg, S.C.; Klein, M.; Guendel, F.; Hain, T.; et al. Microbiota-Induced Type I Interferons Instruct a Poised Basal State of Dendritic Cells. Cell 2020, 181, 1080–1096. [Google Scholar] [CrossRef] [PubMed]
- Casteleyn, C.; Doom, M.; Lambrechts, E.; Van den Broeck, W.; Simoens, P.; Cornillie, P. Locations of Gut-Associated Lymphoid Tissue in the 3-Month-Old Chicken: A Review. Avian Pathol 2010, 39, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, D.; Kolożyn-Krajewska, D. Food-Origin Lactic Acid Bacteria May Exhibit Probiotic Properties: Review. Biomed Res Int 2018, 2018, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shojadoost, B.; Kulkarni, R.R.; Brisbin, J.T.; Quinteiro-Filho, W.; Alkie, T.N.; Sharif, S. Interactions between Lactobacilli and Chicken Macrophages Induce Antiviral Responses against Avian Influenza Virus. Res Vet Sci 2019, 125, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Alqazlan, N.; Astill, J.; Taha-Abdelaziz, K.; Nagy, É.; Bridle, B.; Sharif, S. Probiotic Lactobacilli Enhance Immunogenicity of an Inactivated H9N2 Influenza Virus Vaccine in Chickens. Viral Immunol 2021, 34, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Kye, Y.-C.; Park, S.-M.; Shim, B.-S.; Yoo, S.; Hwang, E.; Kim, H.; Kim, S.-J.; Han, S.H.; Park, T.S.; et al. Bacillus Subtilis Spores as Adjuvants against Avian Influenza H9N2 Induce Antigen-Specific Antibody and T Cell Responses in White Leghorn Chickens. Vet Res 2020, 51, 68. [Google Scholar] [CrossRef]
- Bavananthasivam, J.; Alizadeh, M.; Astill, J.; Alqazlan, N.; Matsuyama-Kato, A.; Shojadoost, B.; Taha-Abdelaziz, K.; Sharif, S. Effects of Administration of Probiotic Lactobacilli on Immunity Conferred by the Herpesvirus of Turkeys Vaccine against Challenge with a Very Virulent Marek’s Disease Virus in Chickens. Vaccine 2021, 39, 2424–2433. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Awad, A.M.; El-Hack, M.E.A.; Naiel, M.A.E.; Othman, S.I.; Allam, A.A.; Sedeik, M.E. The Simultaneous Administration of a Probiotic or Prebiotic with Live Salmonella Vaccine Improves Growth Performance and Reduces Fecal Shedding of the Bacterium in Salmonella-Challenged Broilers. Animals 2020, 10. [Google Scholar] [CrossRef]
- Yousaf, A.; Anwar, H. Effect of Gutcare TM and Enterogermina ® on Humoral Response of Avian Influenza Immunization in Broilers. International Journal of Veterinary Sciences and Animal Husbandry 2019, 4, 19–23. [Google Scholar]
- Wu, P.; Lu, J.; Feng, L.; Wu, H.; Zhang, X.; Mei, M.; Hou, J.; Liu, X.; Tang, Y. Antigen-Sparing and Enhanced Efficacy of Multivalent Vaccines Adjuvanted with Immunopotentiators in Chickens. Front Microbiol 2017, 8. [Google Scholar] [CrossRef]
- Lauer, K.B.; Borrow, R.; Blanchard, T.J. Multivalent and Multipathogen Viral Vector Vaccines. Clinical and Vaccine Immunology 2017, 24. [Google Scholar] [CrossRef]
- Arevalo, C.P.; Bolton, M.J.; Le Sage, V.; Ye, N.; Furey, C.; Muramatsu, H.; Alameh, M.G.; Pardi, N.; Drapeau, E.M.; Parkhouse, K.; et al. A Multivalent Nucleoside-Modified MRNA Vaccine against All Known Influenza Virus Subtypes. Science (1979) 2022, 378, 899–904. [Google Scholar] [CrossRef] [PubMed]
| Disease | Biological Agent | Type of Vaccine | Method of Vaccine Production | Delivery Method | Adjuvant Used | Veterinary Medicine (VM) Number |
| Avian encephalomyelitis virus | Virus | Live | Apathogenic strain (for adult birds) | Drinking water | None specified | 00879/5005 01708/4281 42058/5134 |
| Avian influenza Virus (H5N2) | Virus | Inactivated | Inactivation method unspecified | Subcutaneous Intramuscular |
Paraffin oil | 01708/5044 |
| Avian metapneumovirus | Virus | Live strain Live attenuated |
Apathogenic strain; Attneuated (exact method unspecified) |
Drinking water Spray Intraocular/nasal |
None specified | 15052/4086 08327/5008 42058/4112 |
| Avian reovirus | Virus | Inactivated | Formaldehyde inactivated | Intramuscular Subcutaneous |
None specified | 01708/4329 |
| Avian rhinotracheitis virus |
Virus | Live attenuated; Inactivated |
Continuous passage in tissue culture; Formaldehyde or β-propiolactone inactivated |
Intraocular/nasal Spray Intramuscular |
Paraffin oil | 08327/5022 08327/5025 01708/4505 01708/5093 01708/5083 01708/5105 08327/4134 |
| Chicken Anaemia Virus | Virus | Live; Live attenuated |
Apathogenic strain (for adult birds) | Drinking water; Intramuscular Subcutaneous |
dl-α-tocopherol acetate (emulsion) | 00879/5042 01708/4322 |
| Coccidia (Eimeria) | Parasite | Live attenuated | Attneuated (exact method unspecified) | Spray Drinking water In ovo |
None specified | 17533/5014 30282/5018 30282/4032 01708/5101 01708/4572 |
| E. coli | Bacteria | Inactivated; Live |
Genetically modified | Intramuscular Subcutaneous Spray Drinking water |
Paraffin oil | 42058/5046 01708/5085 |
| Egg drop syndrome (EDS) | Virus | Inactivated | Inactivation method unspecified | Intramuscular | Paraffin oil | 08327/5012 08327/5025 01708/4275 01708/5083 |
| Erysipelothrix rhusiopathiae | Bacteria | Inactivated | Inactivation method unspecified | Subcutaneous |
None specified | 01708/4546 |
| Herpes Virus of Turkeys (HVT) | Virus | Live recombinant; Live |
Genetic recombination |
Subcutaneous In ovo |
None specified | 15052/4089 01708/5082 01708/5040 01708/5041 01708/4289 04491/5043 15052/5001 04491/5060 |
| Infectious Bronchitis Virus (IBV) | Virus | Inactivated; Live; Live attenuated; |
Attneuated (exact method unspecified); Formaldehyde or β-propiolactone inactivated |
Spray Intraocular/nasal Drinking water Intramuscular |
Paraffin oil | 43676/4005 43676/4002 15052/5043 15052/4088 08327/5012 08327/5025 08327/4265 08327/5005 01708/4275 01708/5043 01708/4283 01708/5042 01708/4315 01708/5093 01708/5083 42058/5121 42058/4103 42058/5103 42058/5140 |
| Infectious Bursal disease (IBD) | Virus | HVT Vector expressing IBD VP2 protein; Live; Live attenuated; |
Viral Vector Attenuation in eggs; Attenuation in tissue culture |
Subcutaneous In ovo Drinking water Spray Intraocular/nasal |
Paraffin oil | 00879/3032 00879/4187 43676/5000 15052/4059 15052/4030 08327/4192 08327/4323 17533/5005 17533/5017 17533/4002 01708/5082 01708/5040 15052/4154 01708/4333 01708/4237 01708/5093 15052/5032 42058/5138 42058/5137 04491/5043 15052/5001 04491/5060 |
| Infectious Laryngotracheitis (ILT) | Virus | HVT Vector expressing ILT gD and gl glycoproteins | Viral Vector | Subcutaneous; In ovo Intraocular |
None specified | 01708/5039 01708/5082 01708/5041 42058/4106 |
| Mareks Disease Virus (MDV) | Virus | Live apathogenic; HVT Vector, Live recombinant Live attenuated |
Live, Viral vector, Genetic recombination; Attneuated (exact method unspecified) |
Subcutaneous Intramuscular In ovo |
None specified | 15052/4089 15052/4151 08327/4104 01708/4294 01708/4354 42058/4107 42058/4108 42508/4109 04491/5042 04491/5043 |
|
Mycoplasma gallisepticum; Mycoplasma synovia |
Bacteria | Live attenuated; Inactivated |
Attneuated (exact method unspecified) | Spray Subcutaneous Intraocular Intraocular/nasal |
Paraffin Oil | 43877/5001 43877/5000 42983/5000 01708/5103 01708/4607 |
| Newcastle disease (NDV) | Virus | HVT Vector expressing NDV F protein; Inactivated; Live ; Live attenuated ; Live lentogenic; |
Viral Vector, Attneuated (exact method unspecified) Formaldehyde or β-propiolactone inactivated, Lentogenic strain |
Subcutaneous Intramuscular In ovo Spray Intraocular/nasal Drinking water |
Paraffin Oil | 08327/4266 00879/5035 43676/4003 43676/4000 08327/5012 08327/5025 01708/5040 01708/5041 01708/4275 01708/4315 01708/5088 01708/4276 01708/5093 01708/5083 |
| Paramyxovirus Type 3 | Virus | Inactivated | Inactivation method unspecified | Intramuscular | Paraffin oil | 08327/4134 |
| Salmonella (enteritidis, typhimurium and infantis) | Bacteria | Live attenuated; Inactivated |
Attenuation method unspecified; Attenuation via genetic mutation, Inactivation method unspecified |
Intramuscular Drinking water |
Paraffin oil, aluminium hydroxide | 00879/4188 00879/4189 00879/5037 15052/5045 08327/4222 01708/5073 01708/5114 20634/5002 20634/5000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





