1. Introduction
The ability to store lipids is basic for energy metabolism. Specifically, these high-energy molecules are stored in mammalian adipose tissue. Adipocytes are lipid-filled cells that exert many different roles, from energy storage to energy expenditure, depending on the adipocyte type [
1]. There are three types of adipose tissue were described: white, brown and beige adipose [
2,
3]. White adipose tissue (WAT) is the most abundant type of adipose in adults, and the occurrence of obesity is associated with WAT expansion. White adipocytes are non-thermogenic, and contain unilocular lipid droplets that occupy more than 95% of the adipocyte cell volume [
4]. Brown adipose tissue has a characteristic dark color due to the higher concentration of mitochondria. Mature brown adipocytes are characterized by thermogenin expression (uncoupling protein 1, UCP1). In mitochondria UCP1 provides the transfer of protons to the matrix without ATP synthesis, resulting in the release of energy in the form of heat [
5]. Brown and white adipocytes originate from different cell lineages [
6,
7]. However, it was revealed that activation of brown adipocyte differentiation program in white adipose tissue cells leads to conversion into another type of adipocytes. This process could be induced by various multiple stimuli, including chronic cold exposure [
8], and leads to conversion into beige or “brite” (brown in white) adipocytes. These beige adipocytes are inducible thermogenic cells that occur as clusters within WAT depots, exhibiting a mixed energy storage or spending function, and a mixed multilocular/unilocular/paucilocular morphology. Beige adipocytes characterized by low basal expression of UCP1, which is unducible upon stimulation by cold or other stimuli [
1,
8]. Their response includes an increase in UCP1 expression, thermogenesis, and intensification of respiratory rate and lipolysis.
Obesity is a chronic disease characterized by an excessive fat deposition, the primary cause of which is metabolic imbalance [
9]. Animal models have been used extensively as tools to elucidate human obesity genes and mechanisms of human obesity. The
agouti gene is involved in regulation of melanin synthesis in wild-type mice. When the
agouti is expressed in the hair follicle during early postnatal development it switches the pigment synthesis from black (eumelanin) to yellow (phaeomelanin), resulting in a subapical yellow coloration [
10]. Such dominant mutations of
agouti as the lethal yellow (AYIa) and viable yellow (AVYIa) are characterized not only by yellow coat color, but by a pleiotropic syndrome that includes increased body size, increased susceptibility to cancer, insulin resistance, and maturity-onset obesity [
11]. Obese animals exhibit body fat mass that are 35-50% greater than wild-type mice, and this obesity is associated with elevated hepatic lipogenesis, depressed basal lipolysis, and adipocyte hypertrophy [
12].
Nowadays, novel drugs that effectively overcome insulin resistance and counteract obesity are in development. Special attention is paid to viral and non-viral vectors targeting transcriptional pathways by overexpression of genes encoding transcription factors controlling brown adipogenesis. Studies have demonstrated that activation of specific transcriptional regulators, such as PRDM16 (the transcriptional regulator PR domain-containing protein 16), PGC-1α, and C/EBPβ, and recently discovered FoxP4 (forkhead box transcription factor 4) can drive the browning process [
3,
4,
13,
14]. Here we hypothesize that adeno-associated viral (AAV) delivery of PRDM16 or FoxP4 to agouti mice leads to weight loss and changes in lipid profile of WAT. We found the decrease in weight after AAV administration. Additionally, we discovered that gene therapy application lowers TAG content (up to 50-fold decrease for some TAG species) in lipid extracts of WAT in agouti mice after PRDM16 or FoxP4-expressing AAV injection in comparison with control agouti mice.
2. Results
2.1. Expression Cassette for AAV Production
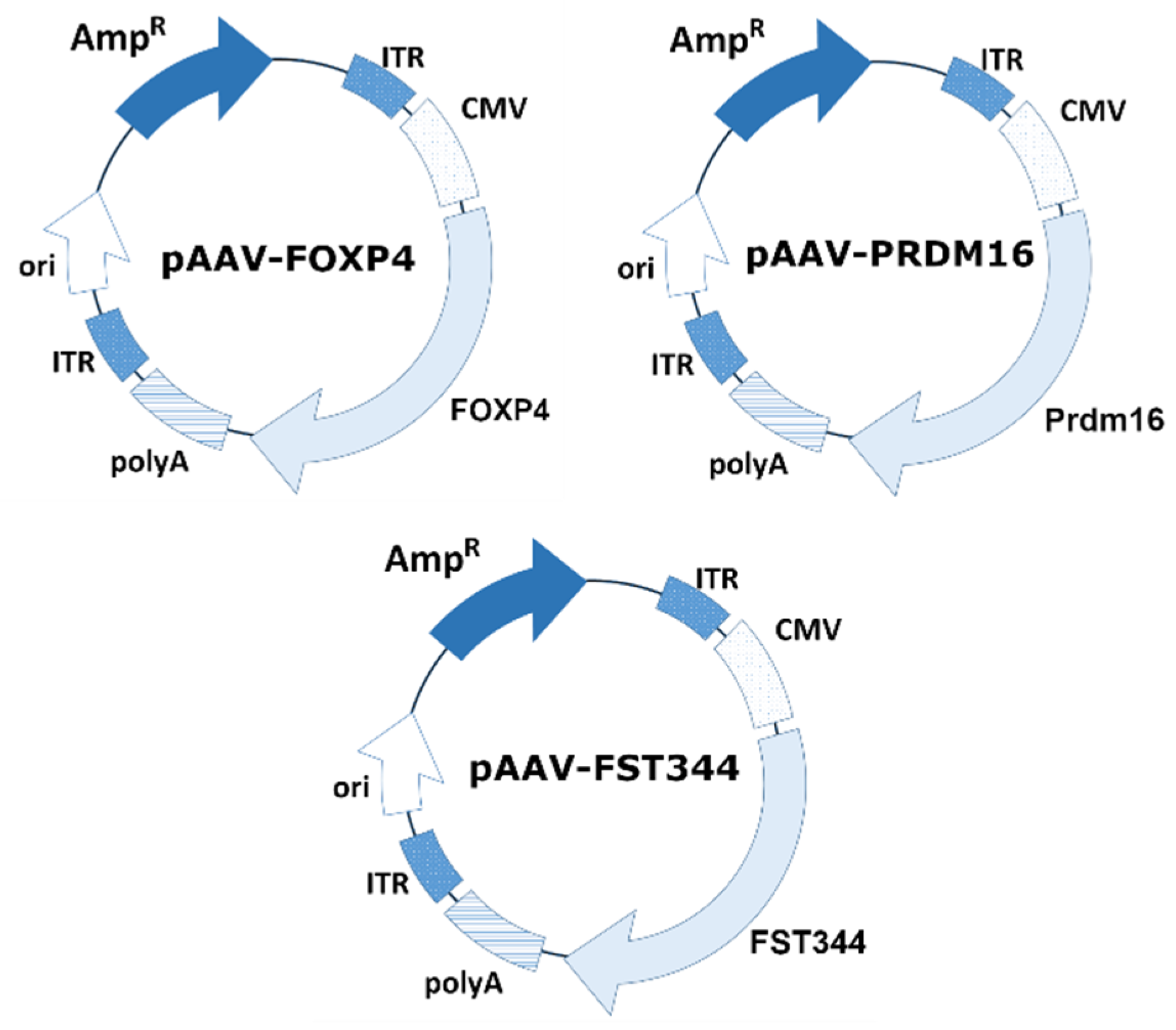
An AAV expression cassette was obtained by cloning the PRDM16, FoxP4, and FST genes between the ITRs into multiple cloning sites of the vector backbone pAAV-CMV-MSC. The schematic plasmid maps are presented in
Figure 1. The mouse PRDM16 gene was amplified from a commercially available plasmid pcDNA3.1 PRDM16 (Addgene plasmid # 15503; RRID: Addgene_15503). The human FoxP4 gene was amplified from a commercially available plasmid Flag-FoxP4 (Addgene plasmid # 153148; RRID: Addgene_153148). The human FST was amplified from a cDNA obtained from HepG2 cells.
2.2. Production of Recombinant AAV8-PRDM16, AAV8-FoxP4, AAV9-FST
The initial step in the study is the production of high-quality recombinant viruses and purification procedures (see
Figure S1 for a representation of the process of recombinant AAV production). In summary, transfection of a suspension HEK293 cell culture with three plasmids (pAAV-FoxP4/pAAV-PRDM16/pAAV-FST, pHelper, and the corresponding pRC) resulted in the production of rAAV. Five days after transfection, the cells were lysed, and the resulting cell lysates, which contained rAAV particles, were subjected to concentration by tangential flow filtration. The concentrated virus was purified by chromatography using CaptureSelect AAVX affinity resin. Subsequently, ultrafiltration was performed to exchange buffer and remove excess salt.
The produced rAAV samples were analyzed by means of dynamic light scattering (DLS). The results of the dynamic light scattering analysis demonstrated the presence of AAV8-PRDM16, AAV8-FoxP4, and AAV9-FST particles with hydrodynamic diameters of 26.92 nm, 32.08 nm, 27.49 nm, and 26.62 nm at a concentration of 1.39×1012, 3.2×1013, 2.38×1013, and 4.51×1012, respectively. The average volume fraction of particles was 99.99%.
Genomic titers of the rAAVs were determined in the range of 1×1011 – 1×1013 VG/mL (viral genomes per liter of culture), as determined by RT-qPCR. Total viral particle concentrations were 2.67×1013 (AAV8-PRDM16), 6.67×1012 (AAV8-FoxP4).
2.3. Administration of AAV with Encoded Transcriptional Regulators into Agouti Mice Results in Weight Loss within the First 3 Weeks
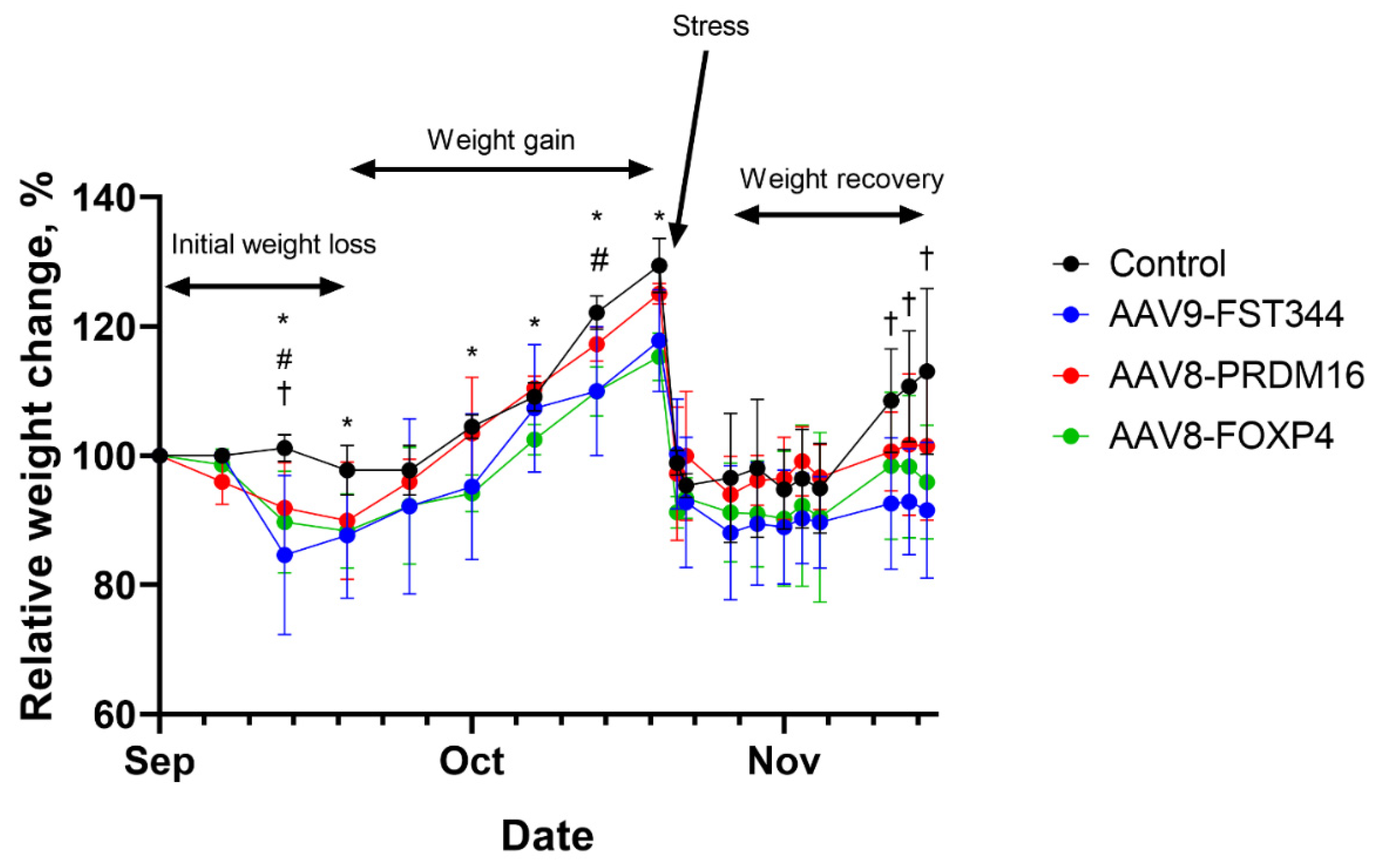
Obese agouti mice treated with AAV encoding human follistatin, FoxP4 or PRDM16 genes had significantly decreased weight gain (10-14%) within the first 3 weeks compared with levels prior to AAV administration, while there was no change in control (empty AAV) group (
Figure 2). Then mice from all groups showed a marked increase in body weight gain. After 8 weeks mice from all groups regain 25-30% of their initial weight (
Figure 2). At the end of 8 weeks, mice were transported to Sirius University to analyze the lipid composition of adipose tissue. Transport stress resulted in serious weight loss, mice from all groups showed similar weight loss of up to 40% of their pre-transport weights (
Figure 2). After transportation, mice treated with AAV-FoxP4, AAV-PRDM16 or AAV-FST vectors gained weight slowly than control animals (
Figure 2).
2.4. AAV8-PRDM16, AAV8-FoxP4, and AAV9-FST Administration Cause Significant Reduction in the Content of TAG with Relatively Low Carbon Number (40-54 Acyl Carbones) in Subcutaneous Adipose Tissue
To explore lipids in iWAT that differed between mice received FST, PRDM16 or FoxP4-expressing AAV vs. control (empty AAV), all of the significantly changed lipids were visualized using a volcano plot (
Figure S2). Lipids that were altered by more than 2.0 folds with p<0.05 were considered to be significantly altered between the two groups. Of the 168 lipids detected, 85 lipids showed significant differences in their abundance when mice received AAV9-FST and control were compared. The complete list of these lipids is shown in
Table 1. For AAV8-FoxP4, 50 lipids and for AAV8-PRDM16, 68 lipids were significantly altered compared to control (
Table 1). Photographic images of adipose tissue that was used for lipidomic analysis showed in
Figure S3.
Lipids that were similar across groups were identified and were compared based on fold-change. The comparison was made between AAV9-FST, AAV8-FoxP4, AAV8-PRDM16 groups against control (
Table 1). Based on these comparisons, we found that the levels of TAG with relatively low carbon number (40-54 acyl carbones) and containing saturated or monounsaturated fatty acids were significantly decreased (up to 50-fold) after the administration of AAV9-FST, AAV8-PRDM16, or AAV8-FoxP4.
3. Discussion
The conversion of white to beige adipose tissue occurs naturally, but also can be induced artificially in many ways. Cold exposure, dietary interventions, β3 adrenergic stimulation, exercise training are some ways that lead to WAT browning and BAT activation [
16,
17,
18,
19].
PRDM16 is a dynamic transcriptional regulator of various stem cell niches, that controls the development of brown adipocytes in BAT depots [
20]. PPARγ, which stimulates adipocyte maturation directly recruits PRDM16 to form a transcriptionally active complex that triggers a browning program in WAT. FoxP4 is expressed in adipose tissue and directly controls the levels of UCP1, a key regulator of thermogenesis that uncouples fatty acid oxidation from ATP production [
5]. Recently, overexpression of FoxP4 and PRDM16 was shown to affect thermogenic gene expression in in vitro cell-based models [
13,
20]. To our knowledge, this is the first study of use AAV-mediated delivery of FoxP4 and PRDM16 genes in obese mice. We show that AAV-PRDM16 or AAV-FoxP4 administration induces loss of body weight in obese mice within the first 3 weeks after AAV administration and decreases iWAT lipid levels. The size of iWAT of the AAV-FoxP4 and AAV-PRDM16 injection was smaller and darker than controls (
Figure S3). We also compare the efficacy of AAV-PRDM16 and AAV-FoxP4 with AAV-FST. FST have been demonstrated to play a key role in regulating white adipose browning both in in-vitro and in-vivo animal models [
21]. FST functions to bind and neutralize the activity of follicle-stimulating hormone, activin, and members of the transforming growth factor-β superfamily [
22]. Staining of the lipid droplets of the adipocytes demonstrated that that the size of subcutaneous adipocytes in FST injected mice was significantly smaller than in the controls, and multilocular lipid droplet structures appeared in the FST injection group [
22,
23].
Using targeted MRM-based lipidomics, we found that AAV-FST, AAV-PRDM16 or AAV-FoxP4 injection decreased the overall abundance of the different lipid classes in iWAT. In the AAV-FST group, 20 phosphatidylcholines (PCs), 16 free fatty acids (FFAs), 4 cholesteryl esters (CEs), 11 sphingomyelins (SMs), 1 diacylglycerol (DAG), 33 TAGs were significantly decreased compare to control, while only 2 PCs, 4 FFAs, 3 CEs, 2 DAGs, 39 TAGs were significantly decreased in the AAV-FoxP4 group, and 12 PCs, 6 FFAs, 3 CEs, 5 SMs, 3 DAGs, 39 TAGs were significantly decreased in the AAV-PRDM16 group (
Table 1). TAG species with low carbon number (40-54 acyl carbones) show the most dramatic decrease. Interestingly, it has been reported that TAG species with low carbon numbers and low double-bond content have been associated with increased cardiovascular disease [
24,
25], suggesting that AAV-FST, AAV-PRDM16 or AAV-FoxP4 injection may have beneficial effects in treating cardiovascular disease.
In this study, AAVs are directly injected into the fat pad of mice. Local AAV injections help mitigate off-target effects because of a lower dose and fewer leaks in the circulation. However, injecting fat depots is time-consuming and invasive. Systemic injection, such as intravenous and intraperitoneal injection, alternatively, could distribute AAVs in more fat depots, but it requires a higher dose than that of local injection and may provoke a greater immune response against the capsid and generate off-target effects [
26,
27]. We observed 10% body weight reduction in the first 3 weeks after AAV administration and rapid weight regain during the next 5 weeks. We suppose that local injection of AAV-FST, AAV-PRDM16 or AAV-FoxP4 vectors do not efficiently targeted subcutaneous and visceral fat depots to alleviate obesity in mice for long periods of time.
4. Materials and Methods
4.1. Animal Procedures
Female agouti mice were bred at the Center for Preclinical and Clinical Research of Belgorod State National Research University (Belgorod, Russia) and taken for study at 12 weeks of age. All the animals with the body weight exceeding 28 grams. (n = 12) were randomly divided into four groups (n = 3 mice per group) and given intro-inguinal white adipose tissue (iWAT) injections of control AAV (empty capsids), AAV-FoxP4, AAV-PRDM16 and AAV-FST [200 µL of each (2 × 1010 viral genomes in PBS)] on left side. Mice were then fed a normal chow diet. The body weight was measured once a week after injection.
Tissue sampling was performed after cervical dislocation.
4.2. Sample Analysis Using Dynamic Light Scattering (DLS) and Size-Exclusion Chromatography (SEC) Methods
Dynamic light scattering (DLS) is a non-contact method that employs the light scattering effect and is designed to measure the size of nano- and submicron particles of a dispersed phase that exhibit Brownian motion. The DLS method offers a distinct advantage over other optical methods, as it allows the sample to be measured in its native form. The samples were measured at 25 ºC on a Zetasizer Ultra analyser (Malvern Panalytical Ltd., Malvern, UK) equipped with a He-Ne laser with a wavelength of 633 nm and a maximum power of 10 mW. The multi-angle light scattering method, which is based on the sequential capture of the analytical signal from three detection angles of scattered radiation, permitted the estimation of the hydrodynamic diameter, the modality of particle distribution, and the fractional ratios. The Malvern Panalytical Ltd. (Malvern, UK) quartz cuvette was employed for the measurements. The data were processed using ZS XPLORER software, version 3.1.0 (Malvern Panalytical Ltd., Malvern, UK). The relative content of AAV monomers, high molecular weight substances (HMWS), and low molecular weight substances (LMWS) was determined by means of exclusion chromatography (SEC) using a Vanquish Flex liquid chromatograph (Thermofisher Scientific, Waltham, MA, USA). The fractions were separated in isocratic mode using an XBridge Protein BEH SEC chromatography column, 450 Å, 2.5 µm, 4.6 mm × 300 mm (Waters, Milford, MA, USA), with a mobile phase comprising: The solution was composed of 20 mM Na₂HPO₄, 150 mM KCl, and a pH of 7.0, with a flow rate of 0.5 mL/min. The fluorimetric detector was used for detection, with an excitation wavelength of 280 nm and an emission wavelength of 350 nm.
4.3. Transmission Electron Microscopy (TEM)
Transmission electron microscopy was employed for the observation of rAAV morphology. A total of 10 µL of viral suspension was applied to the freshly glow-discharged copper grids (200 mesh, formvar-carbon coated (EMCN, Beijing, China)), for 2 min, washed with distilled water, and stained with 1 droplet (10 µL) of a 1% (w/v) aqueous uranyl acetate solution (Polysciences Inc., Warrington, PA, USA, Catalogue No. 2024, 16, 138 5 of 14). The grids were observed using a transmission electron microscope, the JEM2100 Plus (JEOL, Tokyo, Japan), operating at 160 kV. A minimum of 15 grid squares were subjected to comprehensive examination, with representative micrographs captured at the same magnification.
4.4. Lipid Analysis
50 mg of adipose tissue were extracted with 500 µL of chloroform/methanol (2/1, v/v), centrifuged, the supernatant was transferred to a vial and analyzed by liquid chromatography coupled with tandem MS (LC–MS/MS).
LC-MS/MS analysis was performed on a triple quadruple mass spectrometer (EVOQ Elite, Bruker, Bremen, Germany) coupled to an Ultimate 3000 RS UHPLC system (Thermo Scientific, Germering, Germany). The total run time was 65 min (
Figure S4), and the flow rate was 0.21 mL/min. Chromatographic separation was achieved by reversed phase chromatography and gradient elution. Separation of the lipids was carried out on a Poroshell 120 EC-C18 column (100 mm×2.1 mm, particle size 1.9 μm, Agilent). The injection volume was 1 µL. Column temperature was kept at 50˚C. Mobile phase A consists of isopropanol/methanol/water (5/1/4, v/v/v), with 5 mM ammonium acetate and 0.1% acetic acid, and mobile phase B consists of isopropanol/water (95/5, v/v), with 5 mM ammonium acetate and 0.1% acetic acid. The gradient starts at 100% A, held for 3 min, increasing to 20% B in 3-5 min, increasing to 30% B in 5-25 min, increasing to 98% B in 25-42 min, and keeping 98 % B for 23 min. Subsequently, the column was re-equilibrated for 17 min at 100 % A. The detection of lipids was done in multiple reaction monitoring (MRM) mode using a triple quadruple mass spectrometer equipped with heated electrospray ionization (ESI) source. In total, 191 lipids were analyzed (
Table S1), of which 163 lipids were identified in positive mode (CE, SM, Cer, TAG, DAG, PC) and 28 identified in negative mode (FFA). All samples were measured in one run including positive and negative ion mode (
Table S1,
Figure S4). The source capillary voltage was 4400 and 3700 V in positive ion and negative ion modes, respectively. Cone temperature was set to 350˚C and cone gas to 20 psi. Heated probe temperature was set to 250˚C and probe gas flow to 30 psi. Nebulizing gas was set to 50 psi and collision gas (argon) to 1.5 mTorr.
MRM peak areas of lipids were calculated and submitted to statistical analysis. Bruker MS Workstation software (version 8.2.1; Bruker, Bremen, Germany) was used for mass spectrometry data acquisition and processing. Statistical analysis using peak areas was carried out by Metaboanalyst 5.0 (
https://www.metaboanalyst.ca/) [
15]. Fold-change analysis was used to compare the lipid peak areas between mice received control (empty AAV) or follistatin (FST), PRDM16 and FoxP4-expressing AAV.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1: schematic representation of adeno-associated virus production and purification; Figure S2: volcano plots of significantly different lipids; Figure S3: photographic images of adipose tissue extracted from mice that were injected with empty AAV (control), AAV8-FoxP4 (FoxP4), AAV8-PRDM16 (PRDM16), and AAV9-FST (Follistatin); Figure S4: total ion chromatogramms of iWAT lipids; Table S1: MS/MS parameters of analyzed lipids.
Author Contributions
Conceptualization, A.D.E.; writing—original draft preparation, M.A.Y. and S.S.B.; writing—review and editing, A.D.E.; supervision, A.D.E. AAVs production and purification was performed by S.S.B. and A.D.E. AAV administration, recording of body weight and other animal procedures were performed by P.L. and A.D.E. The lipid analysis was performed by M.A.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Russian Science Foundation and Kuban Science Foundation (project no. 22-14-20046).
Institutional Review Board Statement
Animal experiments were carried out in accordance with the international guidelines for the care and handling of experimental animals. Animal experiments were approved by the local Ethics Committee.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the
corresponding author.
Acknowledgments
We gratefully acknowledge technical support from Elena Subcheva (TEM), Elena Sahibgaraeva (DLS), Gregory Muravyev and Alina Abdullina (autopsy). We thank Anna Ryzhova and Marina Predeina for administrative support, and Alexei Deikin for donations in kind (e.g., materials used for experiments).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leiria, L.O., Tseng, Y.H. Lipidomics of brown and white adipose tissue: Implications for energy metabolism. Biochim Biophys Acta Mol Cell Biol Lipids 2020, 1865(10), 158788. [CrossRef]
- Rosen, E.D., Spiegelman, B.M. What we talk about when we talk about fat. Cell 2014, 156(1-2), 20–44. [CrossRef]
- Chouchani, E.T., Kazak, L., Spiegelman, B.M. New Advances in Adaptive Thermogenesis: UCP1 and Beyond. Cell Metab. 2019, 29(1), 27–37. [CrossRef]
- Lynes, M.D., Tseng, Y.H. (2018). Deciphering adipose tissue heterogeneity. Ann N Y Acad Sci. 2018, 1411(1), 5–20. [CrossRef]
- Boychenko, S., Egorova, V.S., Brovin, A., Egorov, A.D. White-to-Beige and Back: Adipocyte Conversion and Transcriptional Reprogramming. Pharmaceuticals (Basel) 2024, 17(6), 790. [CrossRef]
- Timmons, J.A., Wennmalm, K., Larsson, O., Walden, T.B., Lassmann, T., Petrovic, N., Hamilton, D.L., Gimeno, R.E., Wahlestedt, C., Baar, K., Nedergaard, J., Cannon, B. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci U S A. 2007, 104(11), 4401–4406. [CrossRef]
- Seale, P., Bjork, B., Yang, W., Kajimura, S., Chin, S., Kuang, S., Scimè, A., Devarakonda, S., Conroe, H.M., Erdjument-Bromage, H., Tempst, P., Rudnicki, M.A., Beier, D.R., Spiegelman, B.M. PRDM16 controls a brown fat/skeletal muscle switch. Nature 2008, 454(7207), 961–967. [CrossRef]
- Wu, J., Boström, P., Sparks, L.M., Ye, L., Choi, J.H., Giang, A.H., Khandekar, M., Virtanen, K.A., Nuutila, P., Schaart, G., Huang, K., Tu, H., van Marken Lichtenbelt, W.D., Hoeks, J., Enerbäck, S., Schrauwen, P., Spiegelman, B.M. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 2012, 150(2), 366–376. [CrossRef]
- NCD Risk Factor Collaboration (NCD-RisC) (2024). Worldwide trends in underweight and obesity from 1990 to 2022: a pooled analysis of 3663 population-representative studies with 222 million children, adolescents, and adults. Lancet 2024, 403(10431), 1027–1050. [CrossRef]
- Moussa, N.M., Claycombe, K.J. The yellow mouse obesity syndrome and mechanisms of agouti-induced obesity. Obe Res. 1999, 7(5), 506–514. [CrossRef]
- Miltenberger, R.J., Mynatt, R.L., Wilkinson, J.E., Woychik, R.P. The role of the agouti gene in the yellow obese syndrome. J Nutr. 1997, 127(9), 1902S–1907S. [CrossRef]
- Dolinoy, D.C., Huang, D., Jirtle, R.L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007, 104(32), 13056–13061. [CrossRef]
- Perie, L., Verma, N., Mueller, E. The forkhead box transcription factor FoxP4 regulates thermogenic programs in adipocytes. J Lipid Res. 2021, 62, 100102. [CrossRef]
- Ziqubu, K., Dludla, P.V., Mthembu, S.X.H., Nkambule, B.B., Mabhida, S.E., Jack, B.U., Nyambuya, T.M., Mazibuko-Mbeje, S.E. An insight into brown/beige adipose tissue whitening, a metabolic complication of obesity with the multifactorial origin. Front Endocrinol (Lausanne) 2023, 14, 1114767. [CrossRef]
- Pang, Z., Chong, J., Zhou, G., de Lima Morais, D.A., Chang, L., Barrette, M., Gauthier, C., Jacques, P.É., Li, S., Xia, J. MetaboAnalyst 5.0: narrowing the gap between raw spectra and functional insights. Nucleic Acids Res. 2021, 49(W1), W388–W396. [CrossRef]
- Scheel, A.K., Espelage, L., Chadt, A. Many Ways to Rome: Exercise, Cold Exposure and Diet-Do They All Affect BAT Activation and WAT Browning in the Same Manner?. Int J Mol Sci. 2022, 23(9), 4759. [CrossRef]
- Peres Valgas da Silva, C., Hernández-Saavedra, D., White, J.D., Stanford, K.I. Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology (Basel) 2019, 8(1), 9. [CrossRef]
- de Moura E Dias, M., Dos Reis, S.A., da Conceição, L.L., Sediyama, C.M.N.O., Pereira, S.S., de Oliveira, L.L., Gouveia Peluzio, M.D.C., Martinez, J.A., Milagro, F.I. (2021). Diet-induced obesity in animal models: points to consider and influence on metabolic markers. Diabetol Metab Syndr. 2021, 13(1), 32. [CrossRef]
- Schweizer, S., Liebisch, G., Oeckl, J., Hoering, M., Seeliger, C., Schiebel, C., Klingenspor, M., Ecker, J. The lipidome of primary murine white, brite, and brown adipocytes-Impact of beta-adrenergic stimulation. PLoS Biol. 2019, 17(8), e3000412. [CrossRef]
- Seale, P. , Conroe, H.M., Estall, J., Kajimura, S., Frontini, A., Ishibashi, J., Cohen, P., Cinti, S., Spiegelman, B.M. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011, 121(1), 96–105. [Google Scholar] [CrossRef] [PubMed]
- Pervin, S., Singh, V., Tucker, A., Collazo, J., Singh, R. Modulation of transforming growth factor-β/follistatin signaling and white adipose browning: therapeutic implications for obesity related disorders. Horm Mol Biol Clin Investig. 2017, 31(2), 20170036. [CrossRef]
- Braga, M., Reddy, S.T., Vergnes, L., Pervin, S., Grijalva, V., Stout, D., David, J., Li, X., Tomasian, V., Reid, C.B., Norris, K. C., Devaskar, S.U., Reue, K., Singh, R. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res. 2014, 55(3), 375–384. [CrossRef]
- Li, H., Zhang, C., Liu, J., Xie, W., Xu, W., Liang, F., Huang, K., He, X. Intraperitoneal administration of follistatin promotes adipocyte browning in high-fat diet-induced obese mice. PloS One 2019, 14(7), e0220310. [CrossRef]
- Xu, Z., You, W., Zhou, Y., Chen, W., Wang, Y., Shan, T. Cold-induced lipid dynamics and transcriptional programs in white adipose tissue. BMC Biol. 2019, 17(1), 74. [CrossRef]
- Stegemann, C., Pechlaner, R., Willeit, P., Langley, S.R., Mangino, M., Mayr, U., Menni, C., Moayyeri, A., Santer, P., Rungger, G., Spector, T.D., Willeit, J., Kiechl, S., Mayr, M. Lipidomics profiling and risk of cardiovascular disease in the prospective population-based Bruneck study. Circulation 2014, 129(18), 1821–1831. [CrossRef]
- O'Neill, S.M., Hinkle, C., Chen, S.J., Sandhu, A., Hovhannisyan, R., Stephan, S., Lagor, W.R., Ahima, R.S., Johnston, J.C., Reilly, M.P. Targeting adipose tissue via systemic gene therapy. Gene Ther. 2014, 21(7), 653–661. [CrossRef]
- Bates, R., Huang, W., Cao, L. Adipose Tissue: An Emerging Target for Adeno-associated Viral Vectors. Mol Ther Methods Clin Dev. 2020, 19, 236–249. [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).