Submitted:
27 September 2024
Posted:
30 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Non-intensive therapy for newly diagnosed patients
3. Definition of fitness
4. Determination of ongoing criteria to evaluate fitness
4.1 Age and comorbidities
4.2 Performance status
4.3 Multi-parameter assessment tools
5. Fitness criteria
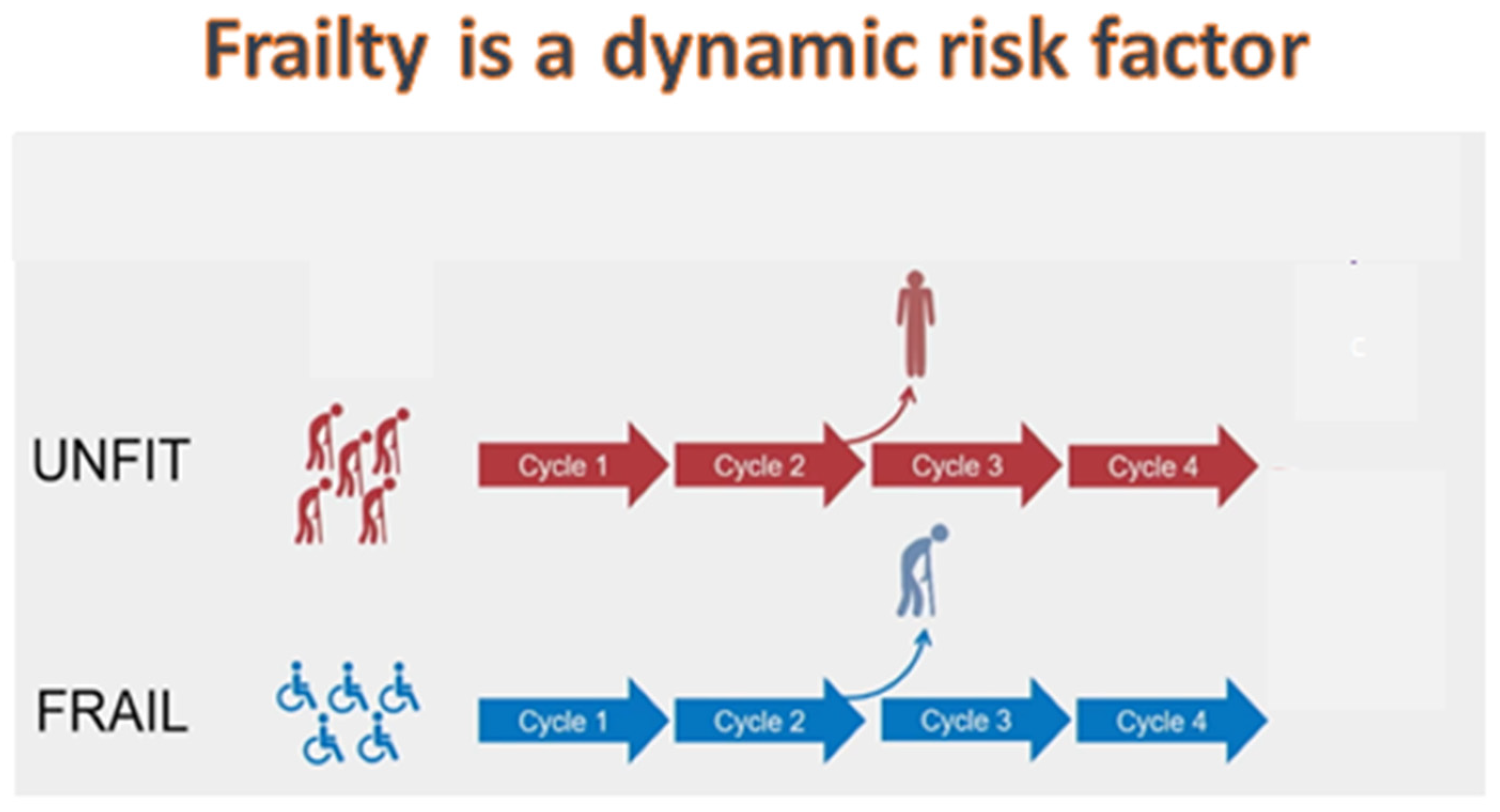
6. The concept of clinical dynamic fitness
7. The concept of biological dynamic fitness
Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dohner H, Weisdorf DJ, Bloomfield CD. Acute myeloid leukemia. N Engl J Med. 2015;373(12):1136-1152.
- Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik V, Paschka P, Roberts, N, Potter N, Campbell P. Genomic classification and prognosis in acute myeloid leukemia. N Engl J Med. 2016; 374(23):2209-2221.
- Juliusson G, Antunovic P, Derolf A, Lehmann S, Möllgård L, Stockelberg D, Tidefelt U, Wahlin A, Höglund M. Age and acute myeloid leukemia: real world data on decision to treat and outcomes from the Swedish Acute Leukemia Registry. Blood. 2009;113(18):4179-4187.
- Thein MS, Ershler WB, Jemal A, Yates JW, Baer MR. Outcome of older patients with acute myeloid leukemia: An Analysis of SEER Data Over 3 Decades. Cancer 2013; 119:2720-7.
- Ferrara F, Barosi G, Venditti A, Angelucci E, Gobbi M, Pane F, Tosi P, Zinzani P, Tura S. Consensus-based definition of unfitness to intensive and non-intensive chemotherapy in acute myeloid leukemia: a project of SIE, SIES and GITMO group on a new tool for therapy decision making. Leukemia 2013; Leukemia. 2013;27:997-9.
- Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh A, Candoni A, et al. International phase 3 study of azacitidine vs conventional care regimens in older patients with newly diagnosed AML with >30% blasts. Blood. 2015;126(3):291-299.
- Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, Gau JP, ChouWC, Buckstein R, Cermak J, et al. Multicenter, randomized,open-label, phase III trial of Decitabine versus patient choice, with physician advice, of either supportive care or low-dose cytarabine for the treatment of older patients with newly diagnosed acute myeloid leukemia. J Clin Oncol. 2012;30(21):2670-2677.
- Pollyea DA, Pratz K, Letai A, Jonas B, Wei A, Pullarkat V, Konopleva M, Thirman MJ, Arellano M, Becke P, et al. Venetoclax with azacitidine or decitabine in patients with newly diagnosed acute myeloid leukemia: long term follow-up from a phase 1b study. Am J Hematol. 2021;96:208–217.
- DiNardo CD, Jonas BA, Pullarkat V,, Thirman MJ, Garcia J, Wei A, Konopleva M, Döhner H, Letai A, Fenaux P, et al. Azacitidine and venetoclax in previously untreated acute myeloid leukemia. N Engl J Med. 2020; 383(7):617-629.
- Wei AH, Montesinos P, Ivanov V, DiNardo C, Novak J, Laribi K, Kim I, Stevens D, Fiedler W, Pagoni M, et al. Venetoclax plus LDAC for newly diagnosed AML ineligible for intensive chemotherapy: a phase 3 randomized placebo-controlled trial. Blood. 2020;135(24):2137-2145.
- Montesinos P, Recher C, Vives S, Zarzycka E, Wang J, Bertani G, Heuser M, Calado R, Schuh A, Yeh SP, et al. Ivosidenib and azacitidine in IDH1-mutated acute myeloid leukemia. N Engl J Med. 2022;386(16): 1519-1531.
- DiNardo CD, Schuh AC, Stein EM, Montesinos P, Wei A, de Botton S, Zeidan A, Fathi A, Kantarjian H, Bennett J, et al. Enasidenib plus azacitidine versus azacitidine alone in patients with newly diagnosed, mutant- IDH2 acute myeloid leukaemia (AG221-AML-005): a single-arm, phase 1b and randomised, phase 2 trial. Lancet Oncol. 2021;22(11): 1597-1608.
- Heuser M, Smith BD, Fiedler W, Sekeres M, Montesinos P, Leber B, Merchant A, Papayannidis C, Pérez-Simón J, et al. Clinical benefit of glasdegib plus low-dose cytarabine in patients with de novo and secondary acute myeloid leukemia: long-term analysis of a phase II randomized trial. Ann Hematol. 2021;100:1181–1194.
- Appelbaum FR, Gundacker H, Head DR, Willman C, Godwin J, Anderson J, Petersdorf S. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485.
- Mangaonkar AA, Patnaik MM. Patterns of care and survival for elderly acute myeloid leukemia-challenges and opportunities. Curr Hematol Malig Rep. 2017;12:290–299.
- Medeiros BC, Satram-Hoang S, Hurst D, Hoang KQ, Momin F, Reyes C. Big data analysis of treatment patterns and outcomes among elderly acute myeloid leukemia patients in the United States. Ann Hematol. 2015;94:1127-1138.
- Talati C, Dhulipala VC, Extermann MT, Ali N, Kim J, Komrokji R, Sweet K, Kuykendall A, Sehovic M, Reljic T, et al. Comparisons of commonly used front-line regimens on survival outcomes in patients aged 70 years and older with acute myeloid leukemia. Haematologica. 2020;105:398-406.
- Juliusson G, Hoglund M, Lehmann S. Hypo, hyper, or combo: new paradigm for treatment of acute myeloid leukemia in older people. Haematologica. 2020;105:249-251.
- Lazarevic VL, Bredberg A, Lorenz F, Öhlander E, Antunovic P, Cammenga J, Wennström L, Möllgård L, Deneberg S, Derolf A, et al. Acute myeloid leukemia in very old patients. Haematologica. 2018;103:e578-e580.
- Klepin, H.D. Geriatric perspective: how to assess fitness for chemotherapy in acute myeloid leukemia. Hematology Am Soc Hematol Educ Program. 2014;2014:8–13. 41.
- Etienne A, Esterni B, Charbonnier A, Mozziconacci M, Arnoulet C, Coso D, Puig B, Gastaut J, Maraninchi D, Vey N. Comorbidity is an independent predictor of complete remission in elderly patients receiving induction chemotherapy for acute myeloid leukemia. Cancer. 2007;109:1376–1383.
- Giles FJ, Borthakur G, Ravandi F, Faderl S, Verstovsek S, Thomas D, Wierda W, Ferrajoli A, Kornblau S, Pierce S, et al. The haematopoietic cell transplantation comorbidity index score is predictive of early death and survival in patients over 60 years of age receiving induction therapy for acute myeloid leukaemia. Br J Haematol. 2007;136:624-627.
- Sorror ML, Maris MB, Storb R, Baron F, Sandmaier B, Maloney D, Storer B. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912-2919.
- Kadia TM, Cortes J, Ravandi F, Jabbour E, Konopleva M, Benton C, Burger J, Sasaki K, Borthakur G, DiNardo C, et al. Cladribine and low-dose cytarabine alternating with decitabine as front-line therapy for elderly patients with acute myeloid leukaemia: a phase 2 single-arm trial. Lancet Haematol. 2018;5:e411–e421.
- Bonanad S, De la Rubia J, Gironella M, Persona E, González E, Lago C, Arnan M, Zudaire M, Hernández Rivas J, Soler A, et al. Development and psychometric validation of a brief comprehensive health status assessment scale in older patients with hematological malignancies: the GAH scale. J Geriatr Oncol. 2015;6:353-361.
- Klepin HD, Geiger AM, Tooze JA, Kritchevsky S, Williamson J, Pardee T, Ellis L, Powell B. Geriatric assessment predicts survival for older adults receiving induction chemotherapy for acute myelogenous leukemia. Blood. 2013;121:4287-4294.
- Deschler B, Ihorst G, Platzbecker U, Germing U, März E, de Figuerido M, Fritzsche K, Haas P, Salih H, Giagounidis A, et al. Parameters detected by geriatric and quality of life assessment in 195 older patients with myelodysplastic syndromes and acute myeloid leukemia are highly predictive for outcome. Haematologica. 2013;98:208-216.
- Kantarjian H, O'Brien S, Cortes J, Giles F, Faderl S, Jabbour E, Garcia-Manero G, Wierda W, Pierce S, Shan J, Estey E. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: predictive prognostic models for outcome. Cancer. 2006;106:1090-1098.
- Röllig C, Thiede C, Gramatzki M, Aulitzky W, Bodenstein H, Bornhäuser M, Platzbecker U, Stuhlmann R, Schuler U, Soucek S, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: results of 909 patients entered into the prospective AML96 trial. Blood. 2010;116:971-978.
- Krug U, Röllig C, Koschmieder A, Heinecke A, Sauerland MC, Schaich M, Thiede C, Kramer M, Braess J, Spiekermann K, et al. Complete remission and early death after intensive chemotherapy in patients aged 60 years or older with acute myeloid leukaemia: a web-based application for prediction of outcomes. Lancet. 2010;376:2000-2008.
- Borlenghi E, Pagani C, Zappasodi P, Bernardi M, Basilico C, Cairoli 6, Fracchiolla N, Todisco E, Turrini M, Cattaneo C, et al. Validation of the “fitness criteria” for the treatment of older patients with acute myeloid leukemia: a multicenter study on a series of 699 patients by the Network Rete Ematologica Lombarda (REL). J Geriatr Oncol. 2021;12:550–556.
- Borlenghi E, Pagani C, Zappasodi P, Bernardi M, Basilico C, Todisco E, Fracchiolla N, Mancini V, Turrini M, Da Vià M, Sala E, et al. Secondary acute myeloid leukaemia in elderly patients: patient’s fitness criteria and ELN prognostic stratification can be applied to guide treatment decisions. An analysis of 280 patients by the network rete ematologica lombarda (REL). Am J Hematol. 2018;93:E54–E57.
- Palmieri R, Paterno G, De Bellis E, Buzzatti E, Rossi V, Di Veroli A, Esposito F, Mercante L, Gurnari C, et al. Validation of SIE, Sies, GITMO operational criteria for the definition of fitness in elderly patients affected with acute myeloid leukemia: a six-years retrospective real-life experience. Blood. 2019;134:2150.
- Palmieri R, Othus M, Halpern AB, Percival ME, Godwin C, Becker P, Walter R. Accuracy of SIE/SIES/GITMO consensus criteria for unfitness to predict early mortality after intensive chemotherapy in adults with AML or other high-grade myeloid neoplasm. J Clin Oncol. 2020;38:4163–4174.
- Gratwohl, A. The EBMT risk score. Bone Marrow Transplant. 2012;47:749–756.
- Yanada M, Konuma T, Mizuno S, Saburi M, Shinohara A, Tanaka M, Marumo A, Sawa M, Uchida N, Ozawa Y, et al. Predicting non-relapse mortality following allogeneic hematopoietic cell transplantation during first remission of acute myeloid leukemia. Bone Marrow Transplant. 2021;56:387–394.
- Wei AH, Döhner H, Pocock C, Montesinos P, Afanasyev B, Dombret H, Ravandi F, Sayar H, Jang JH, Porkka K, et al. Oral Azacitidine Maintenance Therapy for Acute Myeloid Leukemia in First Remission. N. Engl. J. Med. 2020;383:2526–2537.
- Pratz KW, Dinardo C, Arellano ML, Letai AG, Thirman M, Pullarkat VA, Roboz GJ, Becker PS, Hong WJ, Jiang Q, et al. Outcomes after Stem Cell Transplant in Older Patients with Acute Myeloid Leukemia Treated with Venetoclax-Based Therapies. Blood. 2019;134:264. [CrossRef]
- Vosberg S, Greif PA. Clonal evolution of acute myeloid leukemia from diagnosis to relapse. Genes Chromosom. Cancer. 2019;58:839–849.
- Chen S, Xie J, Yang X, Shen H, Cen J, Yao L, Hu X, Wu Q, Zhang J, Qiu H, et al. Venetoclax Plus Decitabine for Young Adults with Newly Diagnosed ELN Adverse-Risk Acute Myeloid Leukemia: Interim Analysis of a Prospective, Multicenter, Single-Arm, Phase 2 Trial. Blood. 2021;138:35. [CrossRef]
| Criteria | Tools | Findings |
|---|---|---|
| Age and comorbidities | CCI HCT-CI |
WHO: considered the chronological age of 65 years for the definition of elderly Menderios et al. patients under 75 years had similar mortality risk reduction to those over 75 years. Comordities, low PS, previous MDS were releted to early mortality Talti et al; Juliusson et al.: WBC, platelet count, hemoglobin level, poor-risk cytogenetics, PS, secondary AML. Lazarevic et al.: underlines the importance of a complete molecular study also in patients over 80 years for the therapeutic implications with targeted drugs. |
| Performance status | ECOG KPS | Kadia et al: PS does not correlated with age. Juliunsonn et al: older patients with better PS had reduced early death rates however age and PS do not determine the fitness of the patients |
| Multi -parameter assessment tools | GAH SSBP MMS ADLs |
Bonadad et al demonstrated that higher GAH score predictive of survival Klepin et al: showed that cytogenetic risk group, previous MDS, and baseline hemoglobin level, SPPB score <9 and MMS<77 were releted to poor OS. Kantarjian et al: developped a score system integrating patients’ and disease features demonstrated a good survival for favorable and intermediate risk group. Rollig et al: ideated a score integrating age, karyotype, NPM1 mutation status, WBC count, LDH level, and CD34 expression. Four prognostic profiles have been identify and associate dwith different prognosis. |
| Criteria | Methodology | Findings |
|---|---|---|
| Ferrara et al, 2013 | Delphi consensus-based process involving a panel of Italian hematologists | Definition of patients not fit for intensive and non-intensive chemotherapy. The panel provide conceptual and operational criteria to evaluate the fitness of AML patients. These criteria resulted easily applicable in clinical practice determining three fitness groups: fit, unfit and frail. |
| Palmieri et al, 2019, 2020 | Retrospective and real-life studies | - In a cohort of 180 patients resulted a high concordance between fitness classes identified by the Ferrara criteria and overall survival. - In a retrospective study of 622 AML patients, Ferrara criteria showed a good accuracy in predicting 28-day and 100-day mortality. The authors conclude that the validity of the Ferrara criteria must be integrated with the molecular cytogenetic risk class of AML. |
| Borlenghi et al, 2018, 2021 | Retrospective and real-life studies | - In a retrospective analysis of 208 patients with secondry AML>64 years the authors integrated the Ferrara criteria with ELN risk classes. The Ferrara criteria correlated with survival of fit, unfit and frail subgroups. - The Ferrara criteria applied on 699 patients demonstrated to predict survival. However, these criteria should be integrated with biological risk classes. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





