Materials and Methods
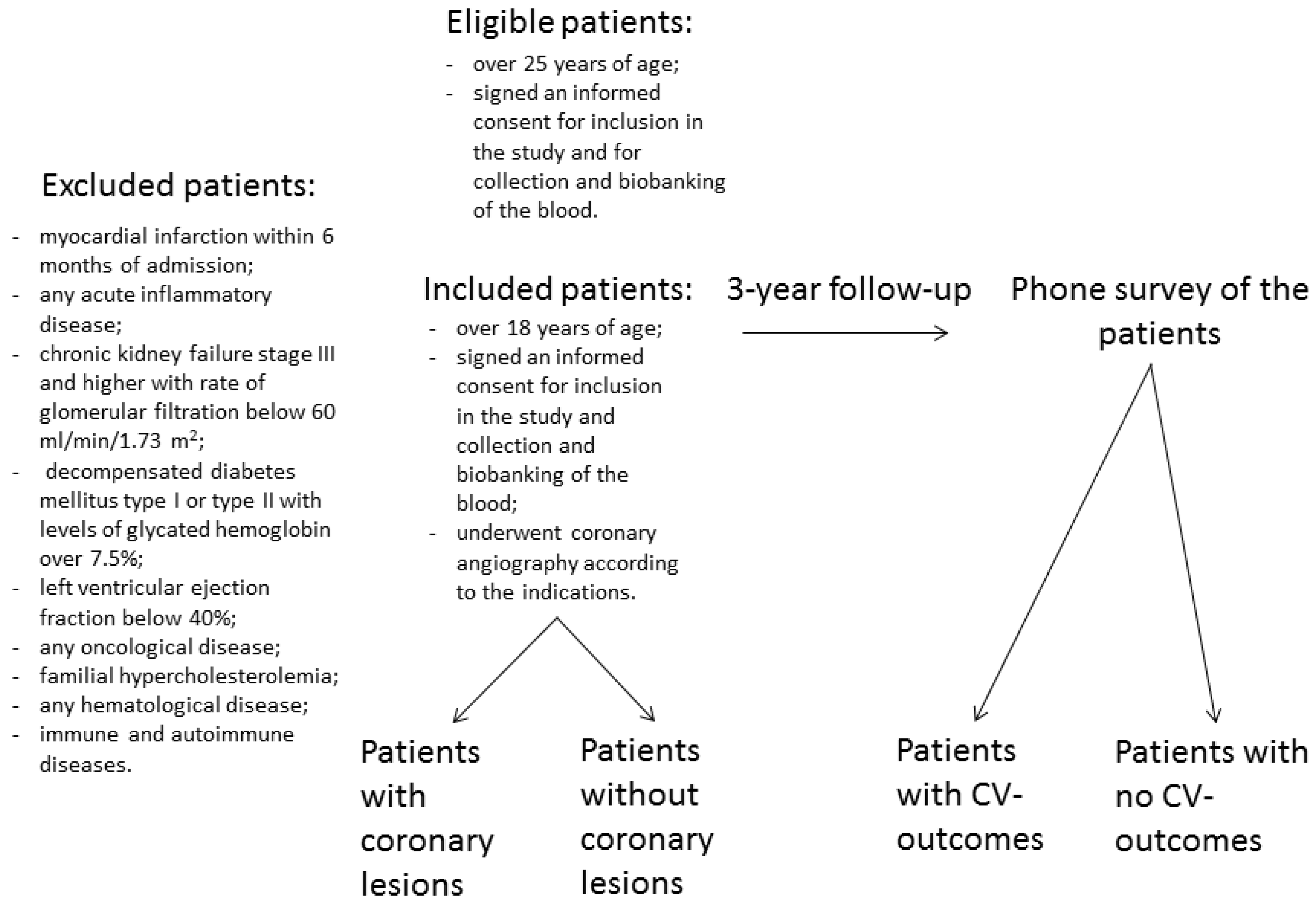
The study cohort included patients (men and women aged ≥25 years) hospitalized at the National Research Center for Preventive Medicine (NRCPM) in 2018-2020. Eligible patients were over 25 years of age and signed an informed consent for inclusion in the study and the collection and biobanking of the blood. Inclusion criteria were as follows: over 18 years of age, signed an informed consent for inclusion in the study and the collection and biobanking of the blood, and underwent coronary angiography according to the indications. Indications for angiography included positive exercise test, positive stress echocardiography, symptoms of advanced angina pectoris, arrhythmia, pathological changes in electrocardiogram with physical inability to perform exercise or stress tests, or high Duke score. All included patients underwent the procedure of coronary angiography according to guidelines of European Society of Cardiology [
6]. After 3 years, all participants were followed up by phone survey. The survey recorded the occurrence of cardiovascular (CV) events: CV death; ischemic stroke; acute myocardial infarction; unplanned revascularization or procedure of coronary angiography at least 90 days after discharge due to deterioration of the symptoms, including coronary percutaneous coronary angioplasty or artery bypass grafting; hospitalization related to deterioration due to coronary heart disease for noninvasive treatment in a cardiology department. Family history of coronary artery disease was assessed as follows: 0, no pathogenic heredity burden; 1, CV-diseases were documented in the immediate family members (parents and siblings). Flowchart of the study is presented in
Figure 1.
Exclusion criteria were as follows: myocardial infarction within 6 months of admission; any acute inflammatory disease; chronic kidney failure stage III and higher with the rate of glomerular filtration below 60 ml/min/1.73 m
2; decompensated diabetes mellitus type I or type II with levels of glycated hemoglobin over 7.5%; left ventricular ejection fraction below 40%; any oncological disease; familial hypercholesterolemia; any hematological disease; and immune and autoimmune diseases. Additional details have been provided in our previous publications [
7,
8]. Blood pressure and heart rate were measured as described previously [
7]. Angiography was performed as described previously and the extent of atherosclerotic lesions and calculation of the Gensini score was performed using Advantage Workstation software version 4.4 [
9,
10]. Peripheral atherosclerosis of the brachiocephalic and femoral arteries was diagnosed and quantified by the B-mode ultrasound using a duplex sonography scanner with high-frequency (9-11 MHz) linear probes (GE Vivid 7 with TruScan raw data). The volume of the brachiocephalic and femoral plaques was assessed. A detailed description of the duplex scanning technique has been presented elsewhere [
11]. Lesions of the brachiocephalic arteries were assessed as follows: intact brachiocephalic arteries (no lesions), mild stenosis with the degree of stenosis <60%, and severe or hemodynamically significant stenosis ≥60% according to the guidelines of the European Society of Cardiology [
12]. Lesions of the femoral arteries were assessed as follows: intact femoral arteries (no lesions), mild stenosis with the degree of stenosis <70%, and severe stenosis ≥70% [
13]. Smoking status was assessed as follows: 0, never smoked; 1, smoking in the past; 2, present smoker. Statin treatment was recorded both before and after hospital admission. All patients were on the same diet pattern in a hospital setting. The phone survey of the participants of this cohort was conducted after a 3-year follow-up.
A written informed consent had been signed by all patients who participate in the study. The Independent Ethics Committee of NRCPM approved the protocol of the study according to Helsinki Declaration (approval no. 09-05/19).
Blood Sampling
Blood was withdrawn from the cubital vein at the baseline of the study. The serum and citrate plasma were separated by centrifugation (1,000g, 15 min, 4°С). The samples were processed as described previously [
5].
Routine Blood Analysis
Routine blood tests were conducted in NRCPM according to guidelines approved by Center for External Quality Control of Clinical Laboratory Testing of Russian Federation (
www.fsvok.ru). The analysis of total cholesterol, low density lipoprotein (LDL)-cholesterol, high density lipoprotein (HDL)-cholesterol, fibrinogen, C-reactive protein (CRP), insulin, glucose, leptin, endothelin, and adiponectin have been described previously [
10,
14] using commercial kits from Diagnostics Biochem Canada, Inc., and Invitrogen, Thermofisher Scientific, Austria.
Levels of serum NOx (nitrates and nitrites) were analyzed under diet in a hospital setting using Griess reaction after reduction of nitrate with vanadium (III) chloride [
15] as described previously [
16,
17]. Reagents for the assays were from Sigma-Aldrich (St Louis, MO, USA).
Microarray Analysis in Serum
Microarray analysis of serum proteome was performed in four serum samples of group A (severe coronary stenosis with high Gensini score) and of group B (no coronary stenosis according to Gensini score), using antibody microarrays with 656 antibodies per slide in two replicates (ASB 600, Full Moon BioSystems, USA) as described previously [
5]. All of eight serum samples were selected from the study cohort.
Indirect ELISA of CDH3
The samples were adjusted to the same protein concentration based on protein assay performed as presented in previous publication [
5]. The values for possible nonspecific cross reactivity were estimated by two types of control samples. Control 1 (background absorbance) comprised the samples containing immobilized serum (10 µg/mL) without the addition of primary antibodies. Control 2 (maximum binding) comprised the samples of immobilized recombinant human P-cadherin (40 pg/mL) in 100 µL of coating buffer. The optical density (OD) values were recalculated for each assayed batch according to the following equation: OD corrected = (OD450 of control 2 - OD450 of control 1)/OD450 of serum sample. The corrections and calibration curves were calculated by Magellan software (Tecan, Switzerland).
Statistical Analysis
Statistica software version 8.0 and SPSS IBM statistics version 23 was used for statistical analyzes. Sample size and power were estimated using the online calculator Sampsize
https://sampsize.sourceforge.net/iface/s2.html#nm (accessed on 10 September 2021) for estimation of sufficient numbers of outcomes for analysis. Kolmogorov-Smirnov criterion was used for test of normality of the distributions. The data are shown as the mean (SD) (standard deviation). Two-tailed non-parametric analysis of variance, Kruskal-Wallis and Mann-Whitney tests, were used to compare the groups. Odds ratio (OR) and area under curve (AUC) with 95% confidence interval (CI) were calculated, as appropriate. Multivariate logistic regression was performed with Wald test. The chi-squared statistic corresponded to the difference in -2 log-likelihoods between the final model and a reduced model. Reduced model was formed by omitting an effect from the final model. The null hypothesis was that all parameters of that effect are zero. The P values < 0.05 were considered significant.
Results
The cohort recruited 218 patients aged 63±10.9 years (54% men) who never smoked (N=98; 45%), smoked in the past (N=28; 12.8%), or were smokers at the time of the study (N=92; 42.2%), with Gensini score 57.0±38.4 (mean±SD). According to the data of coronary angiography, Gensini score of zero (no coronary lesions) was observed in 76 (34.9%) patients; coronary stenosis was less than 50% in 71 (32.6%) patients, and multiple vessel lesions, including left main disease with stenosis over 50% of at least one coronary artery was detected in 71 (32.6%) patients. Thus, coronary artery disease was demonstrated in 65,2% of patients.
A total of 71 (32.6%) patients out of all 218 patients subjected to coronary angiography received planned revascularization at the baseline. Only 5.2% (N=4) of patients with Gensini score of zero (total N=76) received planned revascularization. A total of 71 patients (50%) with Gensini score more than 1 (total N=142) received planned revascularization. Overall, 97.3% of these patients were classified to the group of patients with severe coronary lesions with Gensini score more than 10. Patients who underwent any type of repeated revascularization, including coronary artery bypass grafting, percutaneous coronary angioplasty, coronary and peripheral arteries during 3-year follow up, had multiple vascular lesions in multiple vessels, with very high average Gensini score of 59.3 (upper quartile).
A total of 58% of patients were treated with statins before blood withdrawal (
Table 1).
A total of 176 (80.8%) out of 218 included participants were followed up by phone survey after 3 year follow-up, and 42 participants (19.3%) were unavailable for follow up. The survey included 99 (56%) cardiovascular events: four cardiovascular death; four ischemic stroke; one acute myocardial infarction; 45 unplanned revascularization of coronary and peripheral arteries, including eight coronary artery bypass grafting , percutaneous coronary angioplasty, coronary angiography at least thee months after discharge from hospitalization due to deterioration of the symptoms and 37 hospitalizations related to deterioration of coronary artery disease for noninvasive treatment in a cardiology department. A total of 95 cardiovascular events (excluding cardiovascular death), were united as cardiovascular outcomes for further analysis. A cardiovascular death was not included because of low number of incidents. Thus, only alive participants with the cardiovascular outcomes were taken in calculation. General characteristics of the groups with and without cardiovascular events are presented in
Table 1.
Coronary lesions were quantified as Gensini score according to coronary angiography. Gensini score diversified form null (N=125, 44%) to one hundred ninety seven points. Angiographic characteristics of the total cohort were as follows. For all circulation pools: no lesions (N=42; 15%), mild lesions (N= 95; 34%), and severe hemodynamically significant lesions (N=144; 51%). For coronary circulation: no coronary lesions (Gensini score=0; N=76; 35%); Gensini score >0 with mild lesions (0>Gensini score>36, median Gensini score; N=63; 29%), and severe lesions (≥36 median Gensini score; N=79; 36%). For brachiocephalic circulation: no lesions (N=41; 19%), mild lesions (<60%; N= 156; 72%), and severe lesions (≥ 60%; N=21; 10%). For femoral circulation: no lesions (N=69; 32%), mild lesions (<70%; N=66; 30%), and severe lesions (≥70%; N=83; 38%). A total of 85% of patients of the cohort had a single or multiple plaques in any (coronary, brachiocephalic, or femoral) circulation pools; 82% of patients had a single or multiple plaques in the brachiocephalic vessels, and 68% of patients had a single or multiple plaques in the femoral vessels. Notably, a high correlation between the femoral and coronary lesions (quantified as the corresponding Gensini score) was detected (r = 0.8, P < 0.05), similar to the data reported previously [
18]. A correlation between the brachiocephalic and coronary lesions was also significant; however, the correlation coefficients were lower (r = 0.5, P < 0.05).
Serum proteome profiling was performed in small groups A with coronary stenosis and B with coronary vessels without lesions, and the status of patients were validated by coronary angiography. The levels of cadherin-P (CADH3; UniProtKB P22223) were higher in A-group compared with B-group (
Figure 2). Then, indirect ELISA was used for analysis of CDH3 concentrations in all serum samples of the cohort.
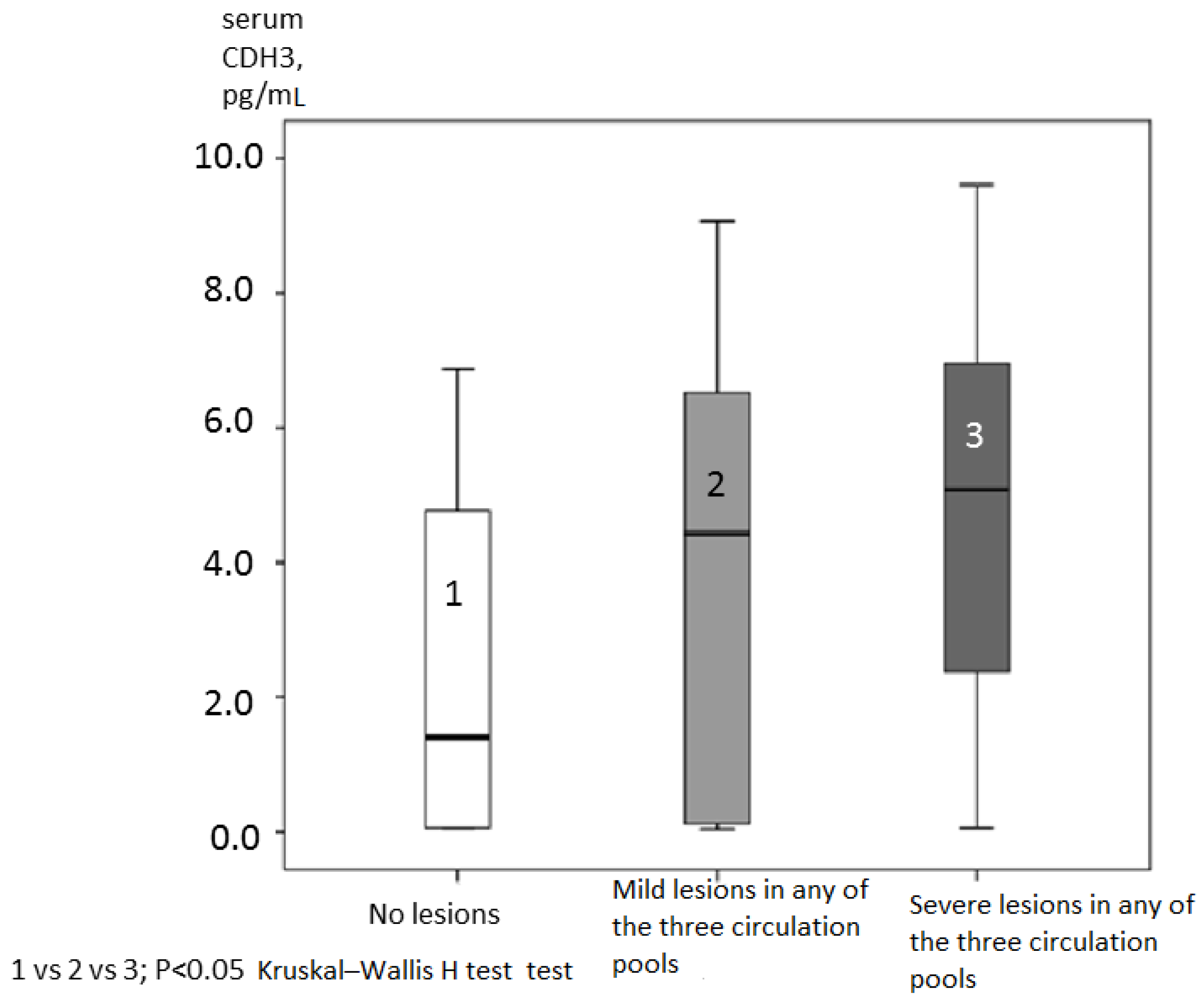
High levels of СHD3 were associated with mild and severe hemodynamically significant atherosclerotic lesions in any of the three types of circulation: coronary, brachiocephalic, and femoral (
Figure 3). СHD3 content in patients without lesions (1), with mild lesions (2), and with severe (3) lesions in any of these types of circulation was gradually higher: 2.47±2.39; 3.93±2.96; and 4.69±2.69 pg/mL, respectively (
Figure 3), and the differences between these groups were significant (P
1=0.009; P
2=0.0003; and P
3=0.045 according to Kruskal-Wallis test, respectively). СHD3 content was associated with coronary stenosis with Gensini score 0/1 as a binary variable defined by ROC-analysis: AUC 0.69; 95%CI (0.64-0.75); P=0.0001. Optimal cut-off for CDH3 was 0.52 pg/mL according to the AUC values. СHD3 content was also associated with brachiocephalic lesions at the cut off 0/1 Gensini score (AUC=0.58; 95%CI (0.51-0.64); P=0.047. In the case of brachiocephalic lesions, an optimal cut-off for CDH3 was 3.9 pg/mL. CDH3 content in the groups split according to the status of brachiocephalic atherosclerotic lesions was as follows: patients with no brachiocephalic lesions had the CDH3 levels of 3.43±2.6 pg/mL, and patients with any brachiocephalic lesions had the CDH3 levels of 4.15±2.9 pg/mL (P= 0.046; Mann-Whitney test). No associations were observed between serum CDH3 and atherosclerotic lesions in the femoral circulation if other types of circulation were not considered. Thus, the levels of serum CDH3 in patients who had coronary (
Table 1) and brachiocephalic atherosclerotic lesions were significantly different from those in patients with no coronary and brachiocephalic lesions, respectively.
Then, the cohort was split based on Gensini score and CV-events, and CDH3 levels are shown in
Table 1. No associations between CDH3 levels and statin treatment before blood withdrawal were observed in the total cohort or in patients who responded to the survey calls.
Association between CDH3 and CV-outcomes was evaluated by comparing the serum concentrations of CDH3, which were different in patients with CV-outcomes versus patients with no outcomes (
Table 1). CDH3 optimal cut-off concentration (4.6 pg/mL) for the 3-year follow up of the outcomes was estimated using ROC analysis (
Table 2). Serum levels of CDH3>4.6 pg/mL were associated with a higher number of the incidents of CV-outcomes (OR=1.81; 95%CI: 1.07-3.72; P=0.0022) (
Table 2).
Binomial regression analysis was performed after a 3-year follow up period to determine whether CDH3 concentrations measured using indirect ELISA are independently associated with CV-outcomes. Binomial logistic regression adjusted for conventional CV-risk factors (age, sex, smoking, and blood pressure) and coronary lesions (Gensini score as a continuous variable) demonstrated that serum CDH3 was independently associated with combined CV-outcomes (P=0.013) (
Table 3).
Classification tables were generated as a variant of binomial regression to evaluate whether inclusion of CDH3 in the list of parameters improves the prediction of CV-outcomes over the standard or base model with routine cardiac rick factors (sex, age, smoking, systolic blood pressure, diastolic blood pressure, and total cholesterol levels). These risk factors are the components of SCORE (Systematic Coronary Risk Evaluation) index, which is used as a predictor for CV-death in 10-year follow-up. Classification tables (
Table 4) were generated using dichotomous value fitting, including aggregated routine cardiac risk factors (sex, age at a median cut-off of 65 years, smoking, family history of CVD, systolic blood pressure at a median cut-off of 130 mm Hg, diastolic blood pressure at a median cut-off of 70 mm Hg, and total cholesterol at a median cut-off of 4.2 mmol/L). The upper part of
Table 4 corresponds to the base model, and the lower part of
Table 4 corresponds to the base model plus CDH3 (a cut-off of 4.5 pg/mL). Net reclassification improvement (NRI) was calculated using the data of
Table 4 and was approximately 2%. OR for the base model plus CDH3 was calculated using the data of
Table 4 (OR=3.23; P=0.0006) (
Table 5). In contrast, OR for the base model without CDH3 was relatively high but was characterized by somewhat lower statistical significance (OR=2.26; P=0.017) (
Table 5).
Associations of various biomarkers in the cohort, including lipid profile parameters such as total cholesterol, triglycerides, HDL-cholesterol and LDL-cholesterol, energy metabolism characteristics such as insulin, HOMA-IR, glucose, leptin , and adiponectin, inflammation such as fibrinogen and C-reactive protein, and endothelial functional markers such as endothelin-1 and NOx, with CDH3 were analyzed. Serum content of СHD3 was associated with NOx and endothelin-1 (
Table 6). However, an association of NOx with CDH3 was not independent according to the data of multivariate logistic regression (
Table 7). The level of NOx was measured in a hospital setting under a diet. Therefore, NOx can be considered a biomarker of endothelial function [
19]. Notably, serum CDH3 was associated with diastolic blood pressure (
Table 6), which had an independent reverse association with endothelin-1 (P=0.034) in a linear regression (
Table 8). Endothelin-1 is a well-known regulator of blood pressure. Notably, in contrast to CDH3 and endothelin-1, NOx was associated with systolic blood pressure (P=0.045). Other assessed biomarkers (total cholesterol, triglycerides, LDL-cholesterol, HDL-cholesterol, glucose, insulin, HOMA-IR, C-reactive protein, fibrinogen, adiponectin, and leptin) were not associated with serum CDH3 (
Table 6).
Thus, our results indicated that high concentrations of CDH3 were associated with an increase in the incidents of cardiovascular outcomes, diastolic blood pressure, and severity of coronary lesions. Elevated serum СDH3 was the predictor of cardiovascular outcomes adjusted for conventional CV-risk factors and the presence of coronary plagues. Elevated serum СDH3 was associated with lesions predominantly in the coronary and brachycephalic circulation. Endothelin-1 and NOx were associated with high levels of СDH3. These biomarkers point out toward possible signal transduction pathways that regulate cellular processes participating in the formation of atherosclerotic plaques.
Discussion
The data of the present study indicated that high CDH3 was associated with cardiovascular outcomes adjusted for the presence of plaques in the coronary circulation, suggesting a role of CDH3 in plaque biology. Additionally, we demonstrated significant associations between CDH3 and severity of coronary stenosis.
Revascularization is defined as a major factor that determines cardiovascular outcomes. Thus, it is important to discriminate between the role of CDH3 as a predictor of severe artery disease, thus de facto being a predictor of revascularization and CV-outcomes, and the role of CDH3 as an independent predictor of CV-outcomes. This discrimination may be possible because our analysis used only unplanned coronary angiography at least 3 months after discharge from hospitalization, while the downstream procedures based on the presence of significant coronary stenosis were not considered in the present study.
Moreover, binomial regression analysis was performed to discriminate between the predicting roles of CDH3 in terms of severity of coronary stenosis and CV-outcomes. CDH3 had an independent impact on CV-outcomes adjusted by the plaque lesions (assessed as Gensini score) in coronary circulation (
Table 3), clearly indicating that CDH3 was an independent predictor of CV-outcomes. Calculations of NRI for base model of CV-outcomes included only routine cardiac risk factors and CV-heredity. However, addition of CDH3 to this base model resulted in NRI>0, gaining 2% over the base model without CDH3. This result indicated that CDH3 optimized the prediction of CV-outcomes by the base model of conventional cardiac risk factors.
NRI is widely used to assess relative ability of 2 risk models to distinguish between low- and high-risk individuals. However, the validity and usefulness of NRI have been questioned. The main critical points emphasize that NRI has substantial variability that heavily depends on the risk cutoff values, which makes it unstable for comparison of miscalibrated models. Moreover, the results of NRI may be challenging to interpret correctly. Additional criticism includes a possibility of falsely useful noninformative models and problematic evaluation of confidence intervals [
20]. Moreover, a meaningful range of improvement for NRI has not been established [
21], which is one of the main limitations of NRI. NRI depends on the selection and number of categories [
21].
In the present study, 58% of patients of the cohort were treated with statins before blood withdrawal. Thus, associations between statin therapy and CDH3 levels were examined. These parameters were not associated in the total cohort and in patients who responded to the phone survey. Thus, statin therapy is highly unlikely to influence the results of the present study.
Similar to other members of the cadherin family, cadherin-P regulates embryonic development, adult tissue architecture, differentiation, cellular shape, and polarity, growth, and migration of the cells [
22,
23,
24]
. Binding of various adhesion molecules to activated endothelium influences the interactions with leukocytes and monocytes important for atherosclerosis [
24]
. For example, increased secretion of cadherin-VE (vascular endothelial cadherin-5) from epicardial arteries is associated with the severity of coronary atherosclerosis [
25,
26]. Changes in the levels of cadherin-VE in vivo may influence vascular permeability, induce cell growth, and promote vascular fragility [
27].
The data of the present study indicated that endothelial biomarkers (endothelin-1 and NOx) were associated with serum content of CDH3, and CDH3 was associated with the degree of atherosclerotic lesions (
Figure 3). These biomarkers are involved in generally interconnected signaling pathways (
Figure 4). Although CDH3 was associated with the presence of atherosclerotic plaques, we have no evidence of the associations of CDH3 with plaque stability or anatomic characteristics because these data were not collected in the present study.
Cadherins are known to have important mechanical functions, which are required during the development and maintenance of epithelial or endothelial barriers [
28]. For example,
endothelin-1 is released in response to a number of stimuli, including acute and chronic stress, hyperosmolality [
29]
, high sodium intake [
29]
, and hypoxia [
30,
31]
. Tissue hypoxia increases the production of endothelin-1 by endothelial cells or adipocytes, leading to an increase in the levels of endothelin-1 [
31]
. Activation of the endothelin B receptor (ETB) has been extensively investigated in vascular endothelium [
31]. ETB activation results in the release of prostaglandins and NO, inducing vascular smooth muscle relaxation [
32].
Moreover, plasma endothelin-1 level is positively correlated with insulin resistance in humans [
33,
34]. The effects of this increase in the levels of endothelin-1 in combination with elevated insulin resistance and decreased glucose uptake depend on the target tissue because the expression and activation of endothelin receptor subtypes are tissue-specific. Additional effects of insulin on the cardiovascular system are mediated by sympathetic nervous system and the L-arginine/NO signaling pathway [
35,
36]. Thus,
a defect of NO synthesis may facilitate sympathetic activation because NO is known to inhibit central neural vasoconstrictor outflow in animals and humans [
36,
37]. Additional metabolic effects of insulin target endothelium [
38]. Insulin upregulates endothelial NO synthesis and thus enhances blood flow [
39]. Hence, a decrease in NO levels blocks this effect of insulin on local circulation and on glucose uptake [
40]. This effect is mediated by the activation of the phosphatidylinositol-3-kinase signaling pathway and changes in the phosphorylation of endothelial NO-synthase.
Overall, the present study demonstrated that NOx and endothelin-1 were associated with the content of CDH3 in the serum and systolic and diastolic blood pressure, respectively (
Table 7). We suggest that NOx and endothelin-1 may act as the regulators of the serum content of CDH3, and this hypothesis is in agreement with known effects of these mediators on vascular tone [
41]. The findings of the present study are supported by a scheme that illustrates the relevant signaling pathways in atherogenesis (
Figure 4).
Figure 1.
Flowchart of the study.
Figure 1.
Flowchart of the study.
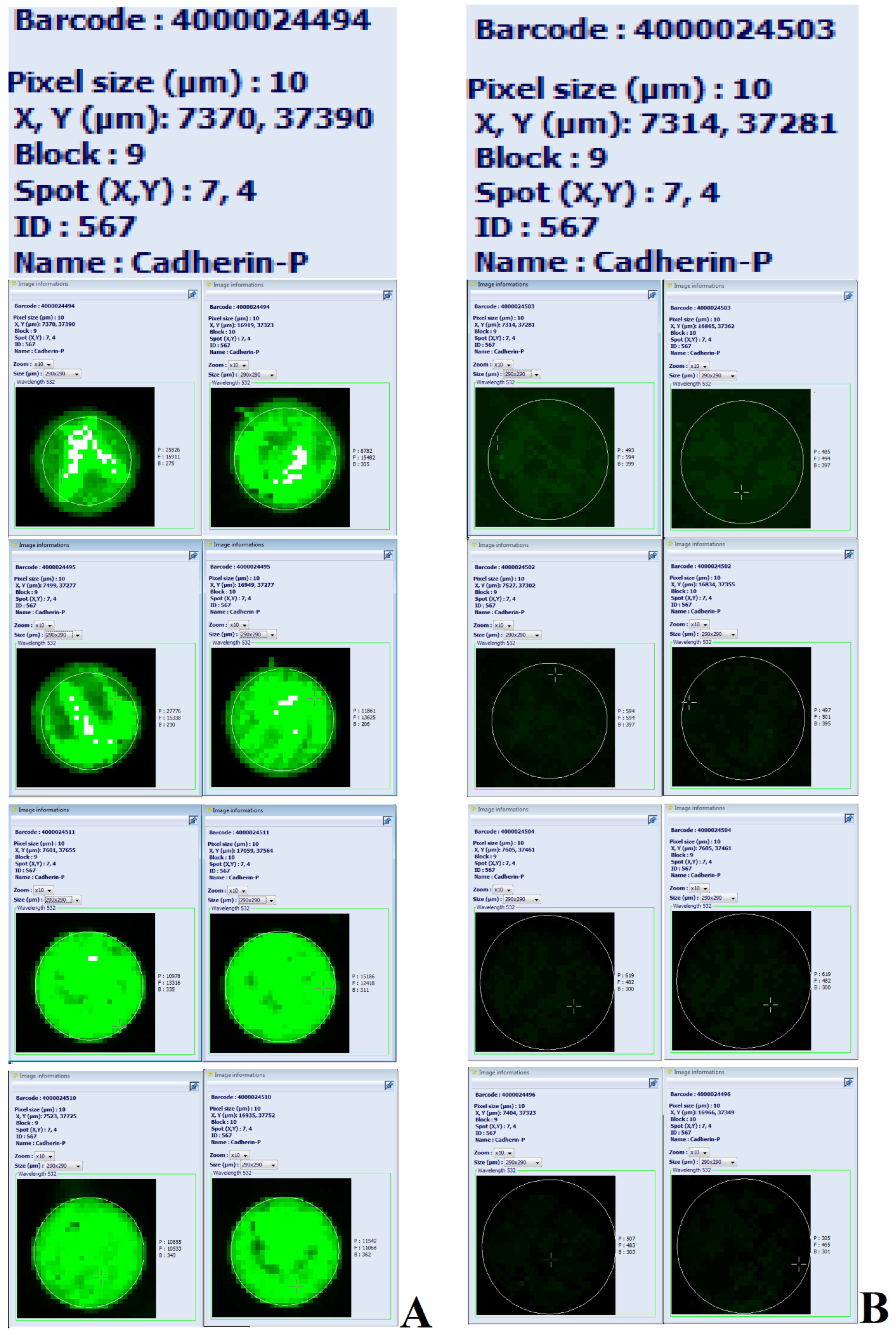
Figure 2.
Image of eight Explorer antibody microarrays (ASB600, Full Moon Biosystems, USA) with 656 antibodies per slide in two replicates for each antibody. The load of labeled serum proteins was 500 ng; scan settings: velocity: 20 l/s; laser power: 5.0; detector gain: 100; pixel size: 10; wavelength: 532 nm. Enlarged images illustrate the differences in the levels of cadherin-P in the serum of four patients with coronary stenosis in two replicates (A on the left) versus the serum of four patients with no coronary lesions in two replicates (B on the right).
Figure 2.
Image of eight Explorer antibody microarrays (ASB600, Full Moon Biosystems, USA) with 656 antibodies per slide in two replicates for each antibody. The load of labeled serum proteins was 500 ng; scan settings: velocity: 20 l/s; laser power: 5.0; detector gain: 100; pixel size: 10; wavelength: 532 nm. Enlarged images illustrate the differences in the levels of cadherin-P in the serum of four patients with coronary stenosis in two replicates (A on the left) versus the serum of four patients with no coronary lesions in two replicates (B on the right).
Figure 3.
CDH3 content (pg/mL) in patients without lesions, with mild lesions, and with hemodynamically significant lesions in any of the tree circulation pools (coronary, brachycephalic, and femoral).
Figure 3.
CDH3 content (pg/mL) in patients without lesions, with mild lesions, and with hemodynamically significant lesions in any of the tree circulation pools (coronary, brachycephalic, and femoral).
Figure 4.
Scheme of the interactions of NO synthesis and endothelin-1 with CDH3 in the context of vascular and sympathetic abnormalities in cardiovascular diseases. Tissue hypoxia increases the production of endothelin-1, leading to an increase in the tissue and plasma levels of endothelin-1. The activation of the endothelin receptor B (ETB) leads to the activation of the pathway, resulting in the release of NO that causes vascular smooth muscle relaxation and activation of the L-arginine/NO pathway. Overexpression of cadherin-P is linked to oncogenic signaling of NO in human breast tumors. This mechanism involves the activation of the Ras/MEK/ERK signaling pathway by NO, in turn leading to the activation of Ets-1, which is a transcription factor implicated in metastasis and tumor progression and causes cadherin-P upregulation. The cadherin-P signaling pathway in malignant tumors influences the invasion, motility, stemness, and metastastic potential of various tissues. ET-1, endothelin-1; ETB, endothelin-1 receptor type B; PI3K, phosphatidylinositol-3-kinase; Gq, GTP-binding protein Gq; NO, nitric oxide.
Figure 4.
Scheme of the interactions of NO synthesis and endothelin-1 with CDH3 in the context of vascular and sympathetic abnormalities in cardiovascular diseases. Tissue hypoxia increases the production of endothelin-1, leading to an increase in the tissue and plasma levels of endothelin-1. The activation of the endothelin receptor B (ETB) leads to the activation of the pathway, resulting in the release of NO that causes vascular smooth muscle relaxation and activation of the L-arginine/NO pathway. Overexpression of cadherin-P is linked to oncogenic signaling of NO in human breast tumors. This mechanism involves the activation of the Ras/MEK/ERK signaling pathway by NO, in turn leading to the activation of Ets-1, which is a transcription factor implicated in metastasis and tumor progression and causes cadherin-P upregulation. The cadherin-P signaling pathway in malignant tumors influences the invasion, motility, stemness, and metastastic potential of various tissues. ET-1, endothelin-1; ETB, endothelin-1 receptor type B; PI3K, phosphatidylinositol-3-kinase; Gq, GTP-binding protein Gq; NO, nitric oxide.

Table 1.
General characteristics of the participants (N=218) and the levels of biochemical markers in the total cohort, in the groups with or without cardiovascular (CV) events* within a 3-year follow-up, and in the groups with (Gensini score>0) and without (Gensini score=0) according to the data of coronary angiography. The groups were compared by Mann-Whitney U test.
Table 1.
General characteristics of the participants (N=218) and the levels of biochemical markers in the total cohort, in the groups with or without cardiovascular (CV) events* within a 3-year follow-up, and in the groups with (Gensini score>0) and without (Gensini score=0) according to the data of coronary angiography. The groups were compared by Mann-Whitney U test.
| |
Stratification after 3-year follow up |
|
Baseline characteristics |
|
| Parameter |
Patients with no CV-events* |
Patients with CV-events |
|
Total cohort |
Patients with Gensini score=0 |
Patients with Gensini score>0 |
|
| N |
77 |
99 |
|
218 |
76 |
142 |
|
| |
Mean (SD) |
P |
Mean (SD) |
P |
| General characteristic |
| Sex |
1.48 |
1.46 |
NS |
1.39 |
1.57 |
1.42(0.49) |
0.036 |
| Age |
59.7 (12.1) |
63.7 (9.3) |
NS |
63.2 (10.9) |
60.5 (11.9) |
63.5(10.1) |
NS |
| Smoking (0/1/2)**, % |
53/14/33 |
41/13/47 |
NS |
45/13/42 |
50/14/36 |
42/9/46 |
NS |
| Patients with CV- family history ***, % |
59.7 |
75 |
0.01 |
67.8 |
47.4 |
81 |
>0.0001 |
| BMI, kg/m2
|
28.8(4.7) |
30.1 (4.8) |
NS |
29.9 (5.8) |
29.4 (5.6) |
30.1(5.1) |
NS |
| WS, cm |
90.4 (9.9) |
94.9 (13.2) |
NS |
93.7 (12.5) |
92.1 (12.2) |
93.7(12.3) |
NS |
| SBP, mm Hg |
128.5 (13.2) |
130.1 (14.1) |
NS |
128.5 (12.7) |
126.53(11.5) |
131.1(14.3) |
0.02 |
| DBP, mm Hg |
73.1 (7.4) |
72.3 (8.7) |
NS |
71.8 (7.8) |
71.8 (7.6) |
73.5(8.6) |
NS |
| Biochemical markers |
|
| NOx, µM |
42.62 (23.9) |
39.62 (26.74) |
NS |
41.29 (25.30) |
51.73(32.03) |
35.50(18.35) |
0.000 |
| Endothelin-1, pg/ml |
1.72 (0.8) |
1.73 (0.36) |
NS |
1.68(0.63) |
1.72 (0.84) |
1.66(0.47) |
0.005 |
| TC, mmol/L |
4.30 (1.1) |
4.23 (1.04) |
NS |
4.13 (1.08) |
4.45(1.08) |
4.24(1.08) |
NS |
| Triglycerides, mmol/L |
1.55 (0.96) |
1.57 (0.61) |
NS |
1.58 (0.90) |
1.54(0.85) |
1.50(0.72) |
NS |
| LDL-cholesterol, mmol/L |
2.45 (0.92) |
2.46 (0.88) |
NS |
2.33 (0.90) |
2.62(0.97) |
2.43(0.91) |
NS |
| HDL-cholesterol, mmol/L |
1.20 (0.31) |
1.06 (0.27) |
NS |
1.10 (0.31) |
1.18(0.32) |
1.12(0.30) |
NS |
| Glucose, mmol/L |
6.12 (1.53) |
6.53 (1.91) |
0.000 |
6.32 (1.79) |
5.85(1.35) |
6.60(1.78) |
0.000 |
| Insulin, µIU/ml |
12.01 (10.7) |
14.99 (14.22) |
0.000 |
12.90 (9.82) |
10.94(10.18) |
14.55(12.89) |
0.002 |
| HOMA-IR |
3.58 (4.21) |
4.75 (5.83) |
0.002 |
3.82 (3.66) |
2.96(3.26) |
4.64(5.4) |
0.000 |
| CRP, mg/L |
5.70 (16.3) |
7.98 (12.6) |
0.000 |
9.46 (22.23) |
6.80(21.62) |
7.48(15.72) |
NS |
| Fibrinogen, g/L |
4.60 (1.35) |
4.88 (1.21) |
0.01 |
5.03 (1.40) |
4.59(1.40) |
4.87(1.25) |
0.01 |
| Adiponectin, µg/mL |
8.80 (3.61) |
8.55 (4.72) |
NS |
8.86 (5.01) |
9.30(4.55) |
9.08(5.93) |
NS |
| Leptin, ng/mL |
44.18 (46.2) |
29.04 (38.21) |
NS |
34.00 (42.50) |
39.14(43.99) |
34.78(41.94) |
NS |
| CDH3, pg/mL |
3.50 (2.68) |
4.29 (2.96) |
0.016 |
4.02 (2.88) |
2.88 (2.72) |
4.72 (2.76) |
0.000 |
|
Statin treatment before blood withdrawal, % |
49.3 |
65.2 |
0.006 |
58.2 |
32.3 |
72.3 |
0.000 |
Table 2.
Associations between the levels of CDH3 and combined CV-outcomes* defined by ROC-analysis and OR evaluation.
Table 2.
Associations between the levels of CDH3 and combined CV-outcomes* defined by ROC-analysis and OR evaluation.
| |
Statistical method |
|
P |
|
|
N total=172;
CV-outcomes* N=95;
CDH3 (continuous) |
AUC (95%CI)
|
0.58 (0.52-0.64)
|
0.017
|
Optimal cut-off for CDH3 = 4.6 pg/mL
|
Sensitivity 0.55
Specify 0.63 |
CV-outcomes N (50/45);
CDH3 (binary) N (53/24);
cut-off 4.6 pg/mL
|
OR (95%CI) |
1.81 (1.07-3.72) |
0.022 |
|
|
Table 3.
Binomial logistic regression for associations between cardiovascular outcomes* (dependent variable) and serum CDH3 adjusted to routine cardiovascular risk factors in patients after a 3-year follow-up.
Table 3.
Binomial logistic regression for associations between cardiovascular outcomes* (dependent variable) and serum CDH3 adjusted to routine cardiovascular risk factors in patients after a 3-year follow-up.
| Parameters |
B |
Wald
(chi-squared) |
P |
| |
Sex |
1.251 |
0.720 |
0.396 |
| Age |
0.430 |
30.021 |
0.082 |
| Smoking |
-0.038 |
40.713 |
0.030 |
| Patients with CV- family history ** |
-0.544 |
0.781 |
0.377 |
| Gensini score |
0.407 |
70.346 |
0.007 |
| Systolic blood pressure, mm Hg |
-0.021 |
40.058 |
0.044 |
| Diastolic blood pressure, mm Hg |
-0.039 |
50.353 |
0.021 |
| Total cholesterol, mmol/L |
0.078 |
0.846 |
0.358 |
| Triglycerides, mmol/L |
590.197 |
0.840 |
0.359 |
| LDL-cholesterol, mmol/L |
-270.063 |
0.856 |
0.355 |
| HDL-cholesterol, mmol/L |
-590.539 |
0.764 |
0.382 |
| Glucose, mmol/L |
-560.314 |
30.088 |
0.079 |
| C-reactive protein, mg/L |
-0.211 |
0.889 |
0.346 |
| CDH3, pg/mL |
0.013 |
30.726 |
0.049 |
Table 4.
Associations of CDH3 on fitting quality assessed by classification tables. The tables were obtained by binomial fitting and calculations of the aggregated dichotomous values, which included routine cardiac risk and CV-outcomes* (base model) or base model plus CDH3 at a cut-off of 4.5 pg/mL (base model plus CDH3).
Table 4.
Associations of CDH3 on fitting quality assessed by classification tables. The tables were obtained by binomial fitting and calculations of the aggregated dichotomous values, which included routine cardiac risk and CV-outcomes* (base model) or base model plus CDH3 at a cut-off of 4.5 pg/mL (base model plus CDH3).
| Classification table for base model including sex, age at a median cut-off of 65 years, Patients with CV- family history (yes/no), smoking, systolic blood pressure at a median cut-off of 130 mm Hg, diastolic blood pressure at a median cut-off of 70 mm Hg, and total cholesterol at a median cut-off of 4.2 mmol/L |
| Observed |
Predicted |
|
| |
0 |
1 |
% of corrected observations |
| 0 |
29 |
48 |
37.7% |
| 1 |
20 |
75 |
78.9% |
| Total % |
28.5% |
71.5% |
60.5% |
| Classification table for base model plus CDH3 |
| |
0 |
1 |
% of corrected observations |
| 0 |
37 |
36 |
50.7% |
| 1 |
21 |
66 |
75.9% |
| Total % |
36.2% |
63.7% |
64.4% |
| |
|
|
|
Table 5.
Risk reclassification for evaluating prediction models for CV-outcomes* and traditional cardiac risk factors (base model) and CV-outcomes and CDH3 added to the base model. For details see
Table 4.
Table 5.
Risk reclassification for evaluating prediction models for CV-outcomes* and traditional cardiac risk factors (base model) and CV-outcomes and CDH3 added to the base model. For details see
Table 4.
| Parameters |
N |
OR (95%CI) |
P |
| Routine cardiac risk factors** |
29/48
20/75 |
2.26 (1.15-4.45) |
0.017 |
| Routine cardiac risk factors plus CDH3 (cut-off 4.6 pg/mL) |
37/36
21/66 |
3.23 (1.64-6.32) |
0.0006 |
Table 6.
Associations between the CDH3 levels as binary variable (cut-off 4.6 pg/mL) and various parameters in the total cohort defined by ROC-analysis.
Table 6.
Associations between the CDH3 levels as binary variable (cut-off 4.6 pg/mL) and various parameters in the total cohort defined by ROC-analysis.
| |
AUC (95%CI)
for binary CDH3 (cut-off 4.6 pg/mL) |
Optimal cut-off for the corresponding parameters according to AUC |
P |
| General characteristics |
|
|
|
| Sex |
- |
|
NS |
| Age |
- |
|
NS |
| Smoking (0/1/2)**, % |
|
|
NS |
| Patients with CV- family history. |
0.61 (0.52-0.69) |
|
0.016 |
| BMI, kg/m2
|
- |
|
NS |
| WS, cm |
- |
|
NS |
| SBP, mm Hg |
- |
|
NS |
| DBP, mm Hg |
0.58 (0.53-0.63) |
71.0 |
0.006 |
| Biochemical markers |
|
|
|
| NOx, µM and endothelin-1 in model |
0.64 (0.54-0.74) |
33.01 |
0.005 |
| Endothelin-1, pg/mL |
0.66(0.57-0.75) |
1.67 |
0.001 |
| TC, mmol/L |
- |
|
|
| Triglycerides, mmol/L |
- |
|
NS |
| LDL-cholesterol, mmol/L |
- |
|
NS |
| HDL-cholesterol, mmol/L |
- |
|
NS |
| Glucose, mmol/L |
- |
|
NS |
| Insulin, µIU/mL |
- |
|
NS |
| HOMA-IR |
- |
|
NS |
| CRP, mg/L |
- |
|
NS |
| Fibrinogen, g/L |
- |
|
NS |
| Adiponectin, µg/mL |
- |
|
NS |
| Leptin, ng/mL |
- |
|
NS |
|
Statins treatment before blood withdrawal, % |
|
|
NS |
Table 7.
Binomial logistic regression for the associations of NOx and endothelin-1 with CDH3 as binary variable at a cut-off of 0.52 pg/mL.
Table 7.
Binomial logistic regression for the associations of NOx and endothelin-1 with CDH3 as binary variable at a cut-off of 0.52 pg/mL.
| Parameter |
B |
S.E. |
Wald |
df |
P |
Exp(B) |
| |
NOх, µM |
0.006 |
0.007 |
0.855 |
1 |
0.355 |
1.006 |
| Endothelin-1 (pg/mL) |
0.854 |
0.301 |
8.055 |
1 |
0.005 |
2.349 |
Table 8.
Linear regression analysis for systolic and diastolic blood pressure associations with endothelin-1, NOx, and CDH3.
Table 8.
Linear regression analysis for systolic and diastolic blood pressure associations with endothelin-1, NOx, and CDH3.
| |
|
Unstandardized coefficients |
Standardized coefficients |
P |
| Dependent variable |
Models |
B |
S.E. |
Beta |
|
| Systolic blood pressure, mm Hg |
Endothelin-1, pg/mL |
-0.687 |
1.382 |
-0.041 |
0.620 |
| NOx, µM |
-0.054 |
0.027 |
-0.162 |
0.045 |
| CDH3, pg/mL |
0.158 |
0.315 |
0.041 |
0.616 |
| Diastolic blood pressure, mm Hg |
Endothelin-1, pg/mL |
-1.995 |
0.931 |
-0.172 |
0.034 |
| |
NOx, µM |
-0.016 |
0.018 |
-0.069 |
0.382 |
| |
CDH3, pg/mL |
0.561 |
0.212 |
0.212 |
0.009 |