1. Introduction
Pancreatic cancer is one of the most lethal malignancies world-wide. Its poor prognosis and high mortality rate are due to diagnosis at a usually late stage, as well as an aggressive biological character [
1]. Data of pancreatic cancer incidence showed that in 2020 there were 495773 new cases and 466003 deaths [
2]. For patients having resectable pancreatic tumors the guidelines recommend surgery as standard of care management of their local disease [
3,
4,
5,
6]. However, significant morbidity and mortality are associated with pancreatic resection [
3,
7,
8]. One of the most challenging complications following pancreatic surgery is the development of a postoperative pancreatic fistula (POPF) [
9]. A pancreatic fistula is defined as an abnormal communication between the pancreatic ductal system and another epithelial surface, leading to the leakage of pancreatic fluid around the pancreas [
10,
11]. Specific diagnosis of POPF is defined by three-fold higher amylase concentration in the peritoneal drainage fluid compared to the upper limit serum amylase level range, or by amylase drain fluid surpassing of more than 300 IU/L the normal serum amylase [
11]. This complication can significantly affect patient outcomes, prolong hospital stays, and increase healthcare costs [
12].
The prevalence of pancreatic fistula following pancreatic resection varies widely in the literature. Reported rates range from 10% to 30% depending on the type of surgery and the criteria used for diagnosis [
8,
13,
14]. The International Study Group on Pancreatic Fistula (ISGPF) has provided standardized definitions and grading of pancreatic fistula, according to severity, which have helped in comparing outcomes across different studies [
12,
15]. Despite advances in surgical techniques and perioperative care, pancreatic fistulas remain a common and serious complication, underscoring the need for reliable predictive markers and effective management strategies [
16].
Several risk factors have been identified for the development of pancreatic fistula after pancreatic surgery [
17,
18]. These include patient-related factors such as age, male gender, nutritional status, elevated BMI, smoking and comorbidities, as well as disease-related factors such as tumor location and size [
11,
19]. Surgical factors also play a critical role, including the type of resection performed (e.g., pancreatoduodenectomy vs. distal pancreatectomy), the texture of the pancreatic remnant, the diameter of the main pancreatic duct and the technique used for pancreatic anastomosis [
20]. Soft pancreatic texture and a small pancreatic duct diameter have been consistently associated with a higher risk of fistula formation [
11,
21]. Fistula Risk Score (FRS) is a predictive model established to estimate patients risk according to compilation of several factors such as pancreatic duct diameter, gland texture, intraoperative blood loss and a pathology report [
22].
The development of a pancreatic fistula can lead to a cascade of adverse outcomes, including intra-abdominal abscess, systemic inflammation, sepsis, hemorrhage (due to pancreatic juice causing vessel erosion), and delayed gastric emptying [
11,
13]. These complications not only increase postoperative morbidity but also adversely impact long-term survival and quality of life [
8,
23]. Management of pancreatic fistulas often requires prolonged hospital stays, readmissions, and additional interventions, which contribute to increased healthcare costs and resource utilization [
24,
25]. Thus, identifying patients at high risk for fistula development and implementing targeted preventive strategies is of paramount importance [
8,
13,
22].
C-reactive protein (CRP) is an acute-phase reactant synthesized by the liver in response to inflammation [
26,
27]. It is a widely used biomarker for the detection and monitoring of inflammatory conditions due to its rapid and substantial increase in response to tissue injury and infection [
28,
29,
30]. In the context of pancreatic surgery, elevated postoperative CRP levels have been investigated as a potential early marker for complications, including pancreatic fistula [
31,
32]. Studies have shown that persistently high CRP levels in the early postoperative period may indicate ongoing inflammation and predict the development of clinically significant complications [
33,
34,
35].
The aim of this retrospective study is to investigate whether early postoperative CRP levels can serve as a reliable predictor for the development of POPF in patients with pancreatic cancer who underwent surgical resection at Bnai Zion Medical Center between August 2018 and December 2023. By identifying predictive markers and risk factors for the development of POPF, the early diagnosis and management of patients at risk may be improved, thus ultimately enhancing surgical outcomes and reducing the burden of postoperative complications.
The study will address the following specific objectives:
1. Determine the prevalence of pancreatic fistula in the patient cohort.
2. Identify patient, disease, and surgical factors associated with the development of pancreatic fistula.
3. Assess the correlation between postoperative CRP levels and the occurrence of pancreatic fistula.
4. Develop a predictive model for pancreatic fistula using CRP levels and other identified risk factors.
Achieving these objectives could contribute to the existing body of knowledge on pancreatic fistulas and provide valuable insights for clinical practice. The findings from this study may help promote the development of targeted interventions and personalized management strategies for patients undergoing pancreatic surgery, ultimately improving patient outcomes and reducing healthcare costs.
2. Materials and Methods
2.1. Study Design and Patient Population
This retrospective study included patients (age 18 years and above) diagnosed with pancreatic cancer who underwent surgical resection at Bnai Zion Medical Center between August 2018 and December 2023. The study was approved by the institutional review board (ethics committee approval protocol number: 0019-24-BNZ on 6 March 2024) and informed consent was obtained from all patients.
2.2. Inclusion and Exclusion Criteria
Patients were included if they had undergone partial pancreatic resection (e.g., pancreatoduodenectomy, distal pancreatectomy) for pancreatic cancer during the study period. Patients with incomplete medical records, patients undergoing total pancreatectomy and patients undergoing surgery for benign pancreatic conditions were excluded from the analysis.
2.3. Data Collection
Clinical and demographic data were collected (data was accessed for research purposes between August 2018 and May 2023) from medical records, including age, gender, smoking status, body mass index (BMI), serum albumin levels, and details of the surgical procedure. Postoperative outcomes, including the development of pancreatic fistula, were recorded. Pancreatic fistulas were classified according to the International Study Group on Pancreatic Fistula (ISGPF) criteria into grades A, B, and C [
12]. The authors had no access to information that could identify individual participants during or after data collection.
2.4. Measurement of CRP levels
CRP levels were measured on postoperative days 1 to 7. The CRP levels were analyzed using a standard immunoturbidimetric assay [
35].
2.5. Statistical Analysis
Descriptive statistics in terms of mean, standard deviation, median, percentages and ranges are presented for all the variables in this study. Normal distribution of continuous parameters was tested by Kolmogorov-Smirnov test. As a result of this test a t-test or Mann Whitney U test were used for analyzing differences between the two groups of the study (pancreatic fistula vs. without Pancreatic fistula). For categorical parameters, Fisher exact test or Pearson chi square were used. Repeated measures models examined changes in CRP levels over time for pancreatic fistula group vs. non-fistula group. Paired Receiver Operating Characteristic (ROC) model with Youden index was used to find the best cut-off for CRP measurements according to pancreatic fistula; while plotting sensitivity versus specificity. P<0.05 was considered as significant. SPSS version 29 was used for the statistical analysis.
3. Results
3.1. Patient Chracteristics
A total of ninety-four patients were included in the study (
Table 1). Among them, forty-six (48.9%) developed a pancreatic fistula postoperatively. The stratification of fistula grades was as follows: thirty-nine patients (84.8%) had grade A, five patients (10.9%) had grade B, and one patient (2.2%) had grade C fistula. The management of pancreatic fistula included observation in forty-one patients (89.1%), percutaneous drainage in three patients (6.5%), and surgical intervention in one patient (2.2%). There were no deaths related to pancreatic fistula.
3.2. Comparison of Patients with and without Pancreatic Fistula
There were no significant differences between patients who developed a pancreatic fistula and those who did not in terms of demographic characteristics such as gender, smoking status, BMI, and clinical features e.g. serum albumin levels, chemotherapy protocol, surgical technique, pathological data regarding lymph node involvement, sample margins and hospitalization period (
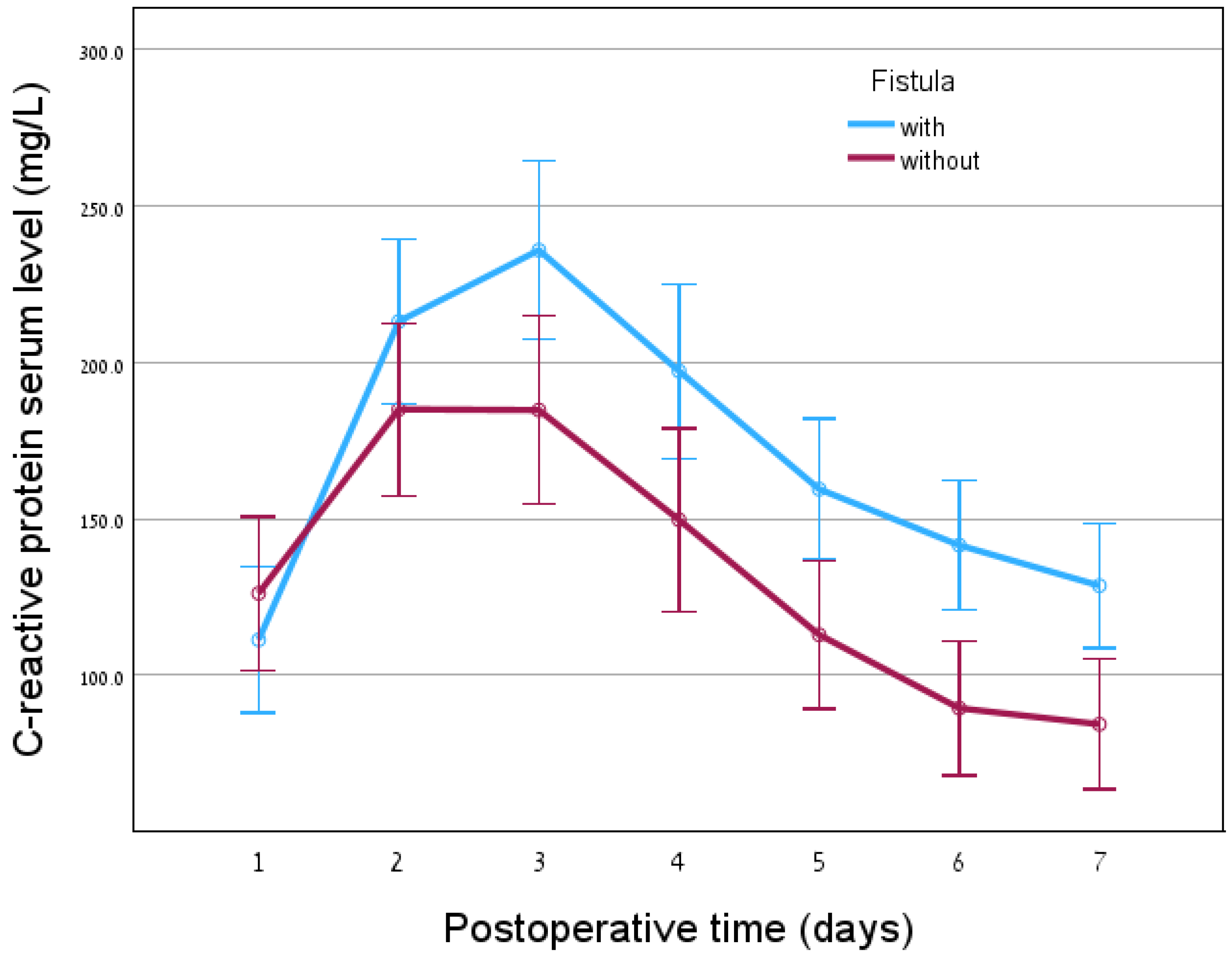
Table 1). Moreover, no association was found between WBC levels and pancreatic fistula presence. However, a significant difference was observed in CRP levels starting from postoperative day 3 (
p=0.002), upon comparison of the group of patients having pancreatic fistula vs. the cohort without pancreatic fistula. Higher CRP levels were observed in patients who developed a pancreatic fistula (
Figure 1). Receiver-operating characteristic (ROC) model with area under the curve (AUC) calculations showed that CRP levels on day 1 and day 2 are unable to predict pancreatic fistula formation. However, from day three on, following surgery, there is a statistical significance of CRP levels as a predicting factor for pancreatic fistula presence (
Table 2). Moreover, the high sensitivity and specificity of the tests exhibit the validity of the statistics showing the positive findings attributed to patients having fistula and designating the patients without fistula as negative (
Table 3). Due to small sample size of the different POPF grades, no statistical analysis could be done.
4. Discussion
4.1. Prevalence and Management of Pancreatic Fistula
In this retrospective study, the prevalence of pancreatic fistula following pancreatic surgery was 48.9%. This rate is consistent with other studies, which reported a prevalence of 10% to 41% depending on the criteria used for diagnosis and the type of surgery performed [
8,
14,
15,
36]. Most pancreatic fistulas in the current cohort were classified as grade A, and were managed conservatively by observation. This finding aligns with previous studies indicating that a significant proportion of pancreatic fistulas are low-grade and can be managed without invasive interventions [
8,
13,
19].
In contrast to grade A POPF, characterized by biochemical changes without impacting the clinical post-surgery recovery, grade B and grade C pancreatic fistulas require intensive care intervention therapy including drainage, re-operation and prolonged antibiotic therapy, resulting in extension of hospitalization and association with higher mortality risk [
11]. The current study findings show that 13% of the patients had POPF grades B and C. In Fukada’s et al. research, 24.6% of patients were recorded having grade B and C POPF [
37]. Five patients in the present study were characterized having grade B POPF, three of them needed additional intervention by drainage and two were kept under close monitoring. The single case of POPF diagnosed as grade C in the current study was treated by surgery. This patient represents 2% of the cohort which is in line with published reports stating an incidence between 2% and 11% [
11,
38]. Similarly, Martins Torres et al. reported that Grade C POPF after pancreatoduodenectomy were surgically managed [
38]. Xiang et al. also recorded surgical procedure such as reconstruction for treatment of grade C POPF [
39]. A recommended intervention is a percutaneous catheter drainage to promote fistula closure in 77% of cases [
11]. Meierhofer et al. have developed a gradual treatment protocol addressing all possible steps occurring post-surgery as management of pancreatic fistula [
11].
4.2. Risk Factors and Predictive Markers
The present study analysis revealed no significant differences in demographic and clinical characteristics between patients who developed a fistula and those who did not, except for CRP levels. This suggests that traditional risk factors such as gender, smoking status, BMI, and serum albumin levels may not be reliable predictors of pancreatic fistula in the cohort patient population. The significant association between elevated CRP levels on postoperative day 3 and the development of pancreatic fistula highlights the potential role of CRP as an early predictive marker [
28,
31,
40].
Schouten et al. validated risk models for POPF in Netherlands patients’ cohort [
41]. Independent factors predicting POPF included male gender, minimally invasive resection, elevated body mass index, small pancreatic duct diameter and soft pancreatic texture. However, that study questioned the accuracy of the predictive models.
4.3. Role of CRP in Predicting Pancreatic Fistula
CRP is an acute-phase protein that increases in response to inflammation and tissue injury. Its rapid and substantial rise in the postoperative period makes it a valuable marker for detecting complications [
28]. In the present study, patients who developed a pancreatic fistula had higher CRP levels compared to patients without pancreatic fistula starting from postoperative day 3. This indicates that CRP could serve as an early warning signal for the development of this complication. Previous studies have reported similar findings, suggesting that elevated CRP levels may reflect ongoing inflammation and predict the occurrence of clinically significant complications [
33,
34].
Additional early markers predicting risk for inflammation are clinically used post-surgery to avoid severe complications [
42,
43]. Interleukin-6 was reported to predict infection as an early marker on day three following laparoscopic cancer resection [
42]. This cytokine as well as procalcitonin and C-reactive protein blood levels were assessed and found to be increased in patients who developed infection [
42]. C-reactive protein/albumin ratio has recently been reported as the useful inflammatory marker predictor in unresectable pancreatic cancer treated by chemotherapy FOLFIRINOX or gemcitabine plus nab-paclitaxel [
43]. Moreover, Fukada’s et al. research suggests that following distal pancreatectomy, the ratio between drain fluid and the concentration of amylase in the blood serum, plays a role as an early factor indicating formation of POPF [
37]. Their analysis of the receiver operating characteristic (ROC) curve used as a predictor tool for POPF, resulted in the highest area AUC on postoperative day 3 (AUC 0.77) and cutoff value 22, while in the current study investigating CRP, the AUC was 0.680 and the cutoff value 202.45.
4.4. Implications for Clinical Practice
The identification of CRP as a predictive marker for pancreatic fistula has important clinical implications. Monitoring CRP levels in the early postoperative period could help identify patients at high risk for developing a fistula, allowing for timely interventions and closer monitoring. This could potentially reduce the incidence of severe complications, shorten hospital stays, and improve overall patient outcomes [
23,
24].
Predictive models assessing risk for post-operative pancreatic fistula used radiometric features, such as preoperative computed tomography CT and clinical data, e.g. BMI [
44]. According to a study by Bhasker et al., a clinically relevant POPF risk stratification, was available through combination of radiomic and clinical signature. Lee et al. intended to establish a preoperative artificial intelligence-based prediction model using CT imaging data [
45].
The current study has several limitations, including its retrospective design and the relatively small sample size. The single-center nature of the study may limit the generalizability of the findings. Additionally, while CRP levels were significantly associated with pancreatic fistula, the study did not evaluate other potential biomarkers or factors that could influence the development of this complication. Future studies with larger, multicenter cohorts and prospective designs are needed to validate the present findings and explore additional predictive markers.
5. Conclusions
In conclusion, the current study demonstrates a high prevalence of pancreatic fistula following pancreatic surgery. Most cases were classified as low-grade and managed conservatively. Elevated CRP levels on postoperative day 3 were significantly associated with the development of pancreatic fistula, suggesting that CRP could serve as an early predictive marker for this complication. Further research is warranted to confirm these findings and to develop comprehensive predictive models that incorporate CRP and other potential risk factors. Preventive approach should lead to better success in managing post-pancreatic surgery troubling complication outcome.
Author Contributions
Conceptualization, Abed Agbarya, M.M., and S.F.; methodology, Abed Agbarya; software, Abed Agbarya; validation, Abed Agbarya; formal analysis, Abed Agbarya; investigation, Abed Agbarya; resources, Abed Agbarya, M.M., R.E., I.M., Ahmad Asadi, O.K., V.S., D.R., R.S., S.B. and S.F.; data curation, Abed Agbarya, M.M., and S.F.; writing—original draft preparation, Abed Agbarya; writing—review and editing, M.M., S.F. R.E., I.M., Ahmad Asadi, O.K., V.S., D.R., R.S., S.B.; visualization, Abed Agbarya.; supervision, Abed Agbarya, M.M., and S.F.; project administration, Abed Agbarya.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Bnai Zion Medical Center (protocol code 0019-24-BNZ on 6 March 2024).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Hu, J.X.; Zhao, C.F.; Chen, W.B.; Liu, Q.C.; Li, Q.W.; Lin, Y.Y.; Gao, F. Pancreatic cancer: A review of epidemiology, trend, and risk factors. World J. Gastroenterol. 2021, 27, 4298–4321. [Google Scholar] [CrossRef] [PubMed]
- Ilic, I.; Ilic, M. International patterns in incidence and mortality trends of pancreatic cancer in the last three decades: A joinpoint regression analysis. World J. Gastroenterol. 2022, 28, 4698–4715. [Google Scholar] [CrossRef] [PubMed]
- Clancy, T.E. Surgery for Pancreatic Cancer. Hematol. Oncol. Clin. North Am. 2015, 29, 701–716. [Google Scholar] [CrossRef] [PubMed]
- Regmi, P.; Yang, Q.; Hu, H.J.; Liu, F.; Karn, H.R.; Ma, W.J.; Ran, C.D.; Li, F.Y. Overall Postoperative Morbidity and Pancreatic Fistula Are Relatively Higher after Central Pancreatectomy than Distal Pancreatic Resection: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2020, 7038907. [Google Scholar] [CrossRef]
- Connoy, T.; Pfeiffer, P.; Vilgrain, V.; Lamarca, A.; Seufferlein, T.; O’Reilly, E.M.; Hackert, T.; Golan, T.; Prager, G.; Haustermans, K.; et al. Pancreatic cancer: ESMO clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Onc. 2023, 34, P987–1002. [Google Scholar] [CrossRef]
- Turner, K.M.; Wilson, G.C.; Patel, S.H.; Ahmad, S.A. ASO Practice Guidelines Series: Management of Respectable, Borderline Respectable, and Locally Advanced Pancreas Cancer. Ann. Surg. Onc. 2024, 31, 1884–1897. [Google Scholar] [CrossRef]
- Bliss, L.A.; Witkowski, E.R.; Yang, C.J.; Tseng, J.F. Outcomes in operative management of pancreatic cancer. J. Surg. Oncol. 2014, 110, 592–598. [Google Scholar] [CrossRef]
- Nahm, C.B.; Connor, S.J.; Samra, J.S.; Mittal, A. Postoperative pancreatic fistula: a review of traditional and emerging concepts. Clin. Exp. Gastroenterol. 2018, 11, 105–118. [Google Scholar] [CrossRef]
- Cardini, B.; Primavesi, F.; Maglione, M.; Oberschmied, J.; Guschlauer, L.; Gasteiger, S.; Kuscher, S.; Resch, T.; Oberhuber, R.; Margreiter, C.; et al. Outcomes following pancreatic resections – results and challenges of an Austrian university hospital compared to nationwide data and international centres. Eur. Surg. 2019, 51, 81–89. [Google Scholar] [CrossRef]
- Unegbu, F.C.; Anjum, F. Pancreatic Fistula. [Updated 2023 Jul 31]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK560871/.
- Meierhofer, C.; Fuegger, R.; Spaun, G.O.; Wundsam, H.V.; Kichweger, P.; Biebl, M.; Schoefl, R. Endoscopic Transmural Therapy of Pancreatic Fistulas in an Interdisciplinary Setting-a Retrospective Data Analysis. J. Clin. Med. 2023, 12, 4531. [Google Scholar] [CrossRef]
- Bassi, C.; Dervenis, C.; Butturini, G.; Fingerhut, A.; Yeo, C.; Izbicki, J.; Neoptolemos, J.; Sarr, M.; Traverso, W.; Buchler, M.; et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Meierhofer, C.; Fuegger, R.; Biebl, M.; Schoefl, R. Pancreatic Fistulas: Current Evidence and Strategy-A Narrative Review. J. Clin. Med. 2023, 12, 5046. [Google Scholar] [CrossRef] [PubMed]
- Van Buren, G. 2nd, Bloomston, M.; Hughes, S.J.; Winter, J.; Behrman, S.W.; Zyromski, N.J.; Vollmer, C.; Velanovich, V.; Riall, T.; Muscarella, P.; et al. A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann. Surg. 2014, 259, 605–612. [Google Scholar] [CrossRef] [PubMed]
- Strasberg, S.M.; Linehan, D.C.; Hawkins, W.G. The accordion severity grading system of surgical complications. Ann. Surg. 2009, 250, 177–186. [Google Scholar] [CrossRef]
- Kawaida, H.; Kono, H.; Hosomura, N.; Amemiya, H.; Itakura, J.; Fuji, H.; Ichikawa, D. Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J. Gastroenterol. 2019, 25, 3722–3737. [Google Scholar] [CrossRef]
- Xu, H.; Mengm, Q.C.; Hua, J.; Wang, W. Identifying the risk factors for pancreatic fistula after laparoscopic pancreaticoduodenectomy in patients with pancreatic cancer. World J. Gastrointest. Surg. 2024, 16, 1609–1617. [Google Scholar] [CrossRef]
- Ke, Z.; Cui, J.; Hu, N.; Yang, Z.; Chen, H.; Hu, J.; Wang, C.; Wu, H.; Nie, X.; Xiong, J. Risk factors for postoperative pancreatic fistula: Analysis of 170 consecutive cases of pancreaticoduodenectomy based on the updated ISGPS classification and grading system. Medicine (Baltimore) 2018, 97, e12151. [Google Scholar] [CrossRef]
- Kamarajah, S.K.; Bundred, J.R.; Lin, A.; Halle-Smith, J.; Pande, R.; Sutcliffe, R.; Harrison, E.M.; Roberts, K.J. ; PARANOIA Study Group. Systematic review and meta-analysis of factors associated with post-operative pancreatic fistula following pancreatoduodenectomy. ANZ J. Surg, 91. [CrossRef]
- Shrikhande, S.V.; Sivasanker, M.; Vollmer, C.M.; Friess, H.; Besselink, M.G.; Fingerhut, A.; et al. Pancreatic anastomosis after pancreatoduodenectomy: A position statement by the International Study Group of Pancreatic Surgery. Surgery 2017, 161(5), 1221–1234. [Google Scholar] [CrossRef]
- Niu, C.; Chen, Q.; Liu, S.; Zhang, W.; Jiang, P.; Liu, Y. Clinical validation of the risk scoring systems of postoperative pancreatic fistula after laparoscopic pancreatoduodenetomy in Chinese cohorts: A single center retrospective study. Surgery 2022, 171, 1051–1057. [Google Scholar] [CrossRef]
- Ellis, R.J.; Brock Hewitt, D.; Liu, J.B.; Cohen, M.E.; Merkow, R.P.; Bentrem, B.J.; Bilimoria, K.Y.; Yang, A.D. Preoperative risk evaluation for pancreatic fistula after pancreaticoduodenectomy. J. Surg. Oncol. 2019, 119, 1128–1134. [Google Scholar] [CrossRef]
- Chen, J.S.; Liu, G.; Li, T.R.; Chen, J.Y.; Xu, Q.M.; Guo, Y.Z.; Li, M.; Yang, L. Pancreatic fistula after pancreaticoduodenectomy: Risk factors and preventive strategies. J. Cancer Res. Ther. 2019, 15, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Bonaroti, J.W.; Zenati, M.S.; Al-abbas, A.I.; Rieser, C.J.; Zureikat, A.H.; Hogg, M.E.; Zeh, H.J.; Boone, B.A. Impact of postoperative pancreatic fistula on long-term oncologic outcomes after pancreatic resection. HPB 2021, 23, 1269–1276. [Google Scholar] [CrossRef] [PubMed]
- Khalid, A.; Amini, N.; Pasha, S.A.; Deyman, L.; Newman, E.; King, D.A; DePeralta, D.; Gholami, S.; Deutsch, G.B.; Melis, M.; et al. Impact of postoperative pancreatic fistulas on outcomes in pancreatoduodeuoctomy: a comprehensive analysis of American College of Surgeons National Surgical Quality Improvement Program Data. J. Gastrointest. Surg. 2024; S1091-255X(24)00483-9. [Google Scholar] [CrossRef]
- Nehring, S.M.; Goyal, A.; Patel, B.C. C Reactive Protein [Updated 2023 Jul 10]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan. Available from: https://www.ncbi.nlm.nih.gov/books/NBK441843/. 4418. [Google Scholar]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Pepys, M.B.; Hirschfield, G.M. C-reactive protein: a critical update. J. Clin. Invest. 2003, 111, 1805–1812. [Google Scholar] [CrossRef]
- Luan, Y.Y.; Yao, Y.M. The clinical Significance and Potential Role of C-Reactive Protein in Chronic Inflammatory and Neurodegenerative Diseases. Front. Immuno. 2018, 9, 1302. [Google Scholar] [CrossRef]
- Plat, V.D.; Voeten, D.M.; Daams, F.; van der Peet, D.L.; Straatman, J. C-reactive protein after major abdominal surgery in daily practice. Surgery 2021, 170, 1131–1139. [Google Scholar] [CrossRef]
- Scepanovic, M.S.; Kovacevic, B.; Cijan, V.; Antic, A.; Petrovic, Z.; Asceric, R.; Krdzic, I.; Cuk, V. C-reactive protein as an early predictor for anastomotic leakage in elective abdominal surgery. Tech. Coloproctol. 2013, 17, 541–547. [Google Scholar] [CrossRef]
- Ma, J.; Jiang, P.; Ji, B.; Song, Y.; Liu, Y. Post-operative procalcitonin and C-reactive protein predict pancreatic fistula after laparoscopic pancreatoduodenectomy. BMC Surg. 2021, 21, 171. [Google Scholar] [CrossRef]
- Welsch, T.; Frommhold, K.; Hinz, U.; Weigand, M.A.; Kleeff, J.; Friess, H.; Buchler, M.W.; Schmidt, J. Persisting elevation of C-reactive protein after pancreatic resections can indicate developing inflammatory complications. Surgery 2008, 143, 20–28. [Google Scholar] [CrossRef]
- Adamina, A.; Steffen, T.; Tarantino, I.; Beutner, U.; Schmied, B.M.; Warschkow, R. Meta-analysis of the predictive value of C-reactive protein for infectious complications in abdominal surgery. BJS. 2015, 102, 590–98. [Google Scholar] [CrossRef]
- Kartik, A.; Muller, C.; Acs, M.; Piso, P.; Starlinger, P.; Bachleitner-Hoffman, T.; Grotz, T.E. Early postoperative CRP predicts major complications following cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Pleura Peritoneum. 2023, 8, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Van Buren, G.; Vollmer, C.M. The Landmark Series: Mitigation of the Postoperative Pancreatic Fistula. Ann. Surg. Oncol. 2021, 28, 1052–1059. [Google Scholar] [CrossRef] [PubMed]
- Fukada, M.; Murase, K.; Higashi, T.; Yasufuku, I.; Sato, Y.; Tajima, J.Y.; Kiyama, S.; Tanaka, Y.; Okumura, N.; Takahashi, T.; et al. Drain fluid and serum amylase concentration ratio is the most reliable indicator for predicting postoperative pancreatic fistula after distal pancreatectomy. BMC Surg. 2023, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Martins Torres, O.J.; Assuncao Moraes, J.M.; de Souza Martins Fernandes, E.; Hackert, T. Surgical Management of Postoperative Grade C Pancreatic Fistula following Pancreatoduodenectomy. Visc. Med. 2022, 38, 233–42. [Google Scholar] [CrossRef]
- Xiang, C.; Chen, Y.; Liu, X.; Zheng, Z.; Zhang, H.; Tan, C. Prevention and Treatment of Grade C Postoperative Pancreatic Fistula. J. Clin. Med. 2022, 11, 7516. [Google Scholar] [CrossRef]
- Matsunaga, T.; Saito, H.; Murakami, Y.; Kuroda, H.; Fukumoto, Y.; Osaki, T. Serum level of C-reactive protein on postoperative day 3 is a predictive indicator of postoperative pancreatic fistula after laparoscopic gastrectomy for gastric cancer. Asian J. Endosc. Surg. 2017, 10, 382–387. [Google Scholar] [CrossRef]
- Schouten, T.J.; Henry, A.C.; Smits, F.J.; Besselink, M.C.; Bonsing, B.A.; Bosscha, K.; Busch, O.R.; van Dam, R.M.; van Eijck, C.H.; Festen, S.; et al. Risk Models for Developing Pancreatic Fistula After Pancreatoduodenectomy: Validation in a Nationwide Prospective Cohort. Ann. Surg. 2023, 278, 1001–1008. [Google Scholar] [CrossRef]
- Huang, Y.; Yang, L.; Yang, W.; Zhou, P.; Jiang, Q.; Liu, W.; Yin, Y.; Zeng, X.; Zhang, P.; Tao, K. Interleukin-6 on postoperative day three as an early predictor of infections following laparoscopic gastric cancer resection. BMC Surgery 2024, 24, 92. [Google Scholar] [CrossRef]
- Shirakawa, T.; Makiyama, A.; Shimokawa, M.; Otsuka, T.; Shinohara, Y.; Koga, F.; Ueda, Y.; Nakazawa, J.; Otsu, S.; Komori. ; et al. C-reactive protein/albumin ratio is the most significant inflammatory marker in unresectable pancreatic cancer treated with FOLFIRINOX or gemcitabine plus nab-paclitaxel. Sci. Rep. 2023, 13, 8815. [Google Scholar] [CrossRef]
- Bhasker, N.; Kolbinger, F.R.; Skorobohach, N.; Zwanenburg, A.; Lock, S.; Weitz, J.; Hoffmann, R.T.; Distler, M.; Speidel, S.; Leger, S.; et al. Prediction of clinically relevant postoperative pancreatic fistula using radiomic features and preoperative data. Sci. Rep. 2023, 13, 7506. [Google Scholar] [CrossRef]
- Lee, W.; Park, H.J.; Lee, H.J.; Song, K.B.; Hwang, D.K.; Lee, J.H.; Lim, K.; Ko, Y.; Kim, H.J.; Kim, K.W.; et al. Deep learning-based prediction of post-pancreaticoduodenectomy pancreatic fistula. Sci. Rep. 2024, 14, 5089. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).