Submitted:
29 September 2024
Posted:
30 September 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Experimental Design and Sampling
2.3. Assessment Total FMDV-Specific Antibodies
2.4. Assessment of FMDV-Specific Neutralizing Antibodies
2.5. Virus Strains
2.6. Indirect Reference Parameters for Assessment of the Challenge Protection
2.7. Statistical Analysis
3. Results
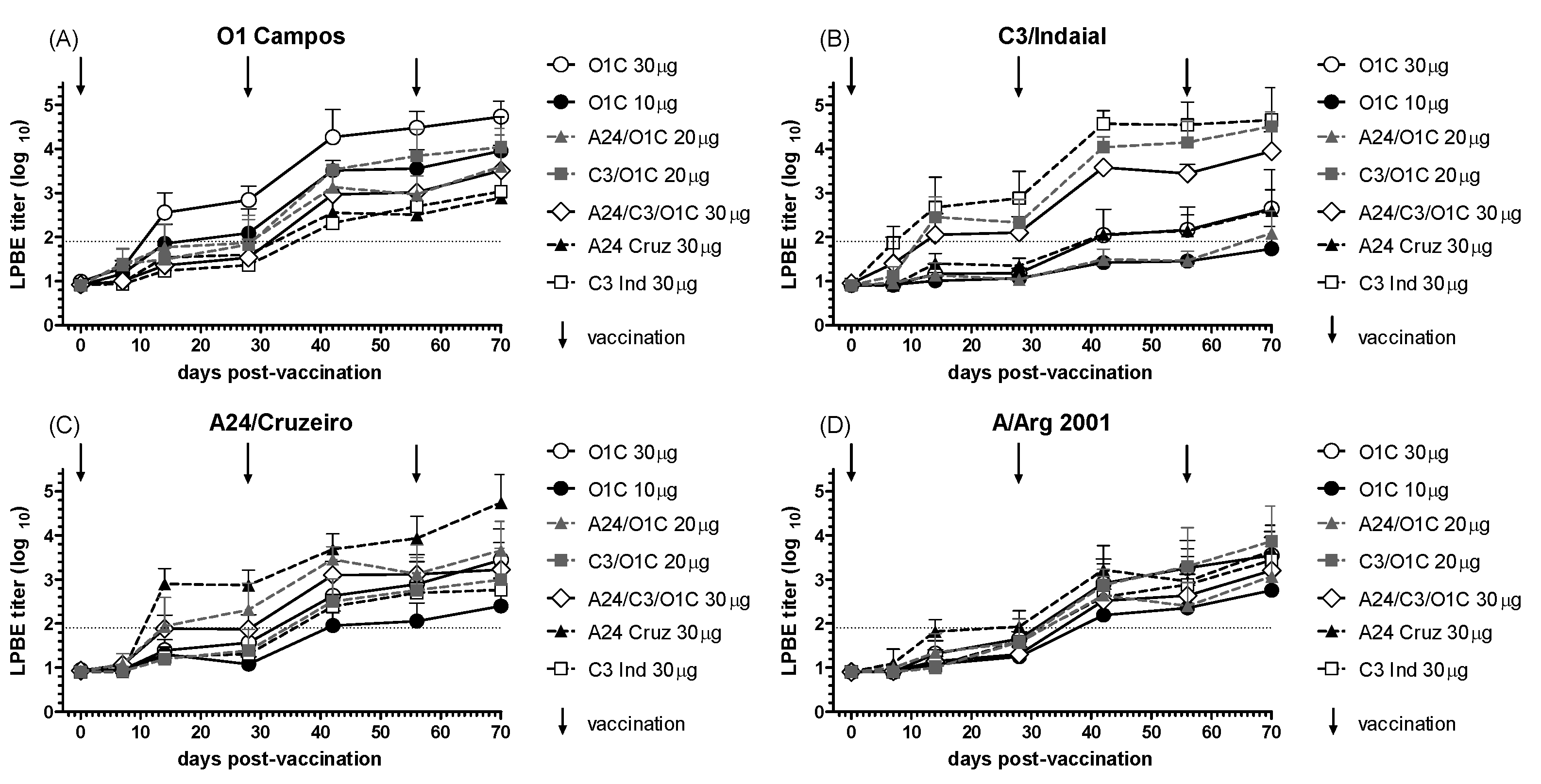
3.1. Induction of Total FMDV-Specific Antibodies
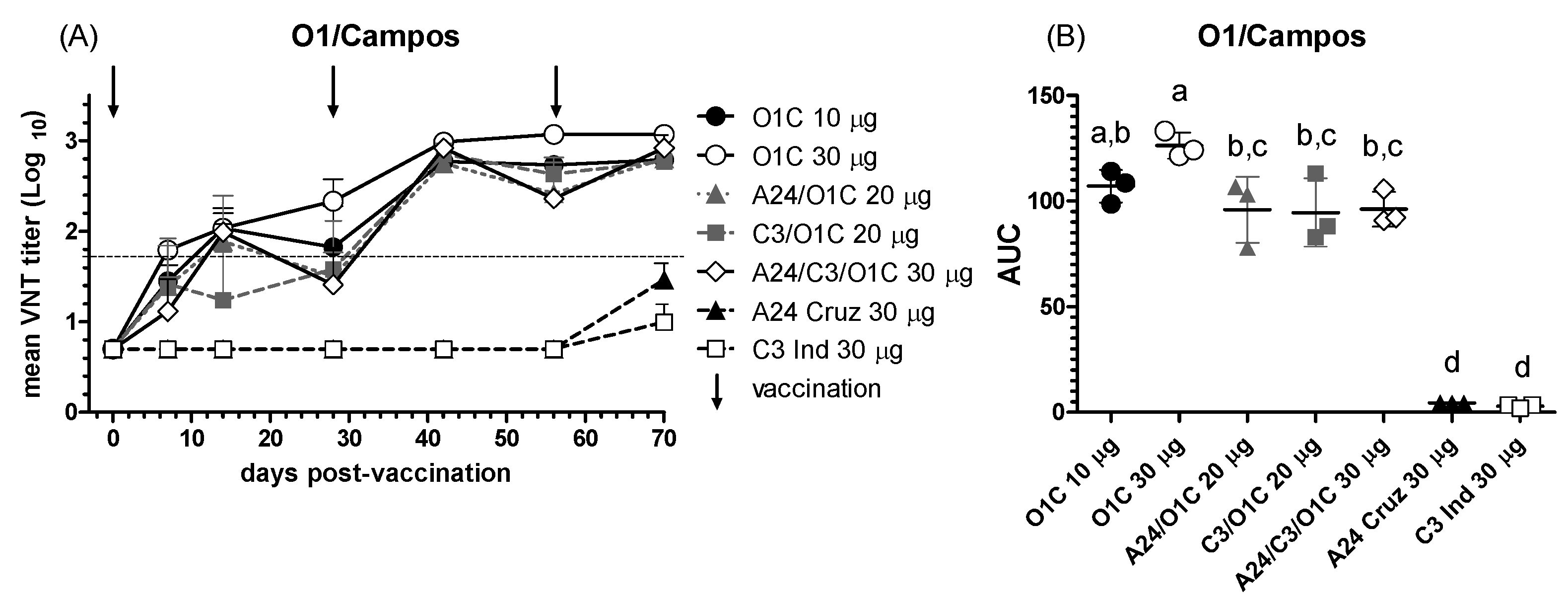
3.2. Induction of Neutralizing FMDV-Specific Antibodies
3.2.1. Antibody Responses Against the O1/Campos Strain
3.2.2. Antibody Responses Against Heterologous Serotype O South American Strains
3.2.3. Antibody Responses Against the Heterologous Serotype O Asian Strains
3.3. Effect of the Vaccine Schedule and Strain Composition in the Neutralizing Activity of the Immune Sera
3.3.1. Vaccination Schedule
3.3.2. Vaccine Formulation
3.4. Serological Relationship between a Heterologous Strains and Vaccine Virus
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WOAH Chapter 3.1.8 Foot and Mouth Disease. In Manual of Diagnostic Tests and Vaccines for Terrestrial Animals 2022; WOAH, Ed.; WOAH, 2022; Vol. 1, ISBN 9778-92-95108-18-9. [Google Scholar]
- Alexandersen, S.; Mowat, N. Foot-and-Mouth Disease: Host Range and Pathogenesis. Curr Top Microbiol Immunol 2005, 288, 9–42. [Google Scholar] [PubMed]
- Brito, B.P.; Rodriguez, L.L.; Hammond, J.M.; Pinto, J.; Perez, A.M. Review of the Global Distribution of Foot-and-Mouth Disease Virus from 2007 to 2014. Transbound. Emerg. Dis. 2017, 64, 316–332. [Google Scholar] [CrossRef] [PubMed]
- Knight-Jones, T.J.D.; McLaws, M.; Rushton, J. Foot-and-Mouth Disease Impact on Smallholders - What Do We Know, What Don’t We Know and How Can We Find Out More? Transbound. Emerg. Dis. 2017, 64, 1079–1094. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.; Muriel, P.; Russell, D.; Osborne, P.; Bromley, A.; Rowland, M.; Creigh-Tyte, S.; Brown, C. Economic Costs of the Foot and Mouth Disease Outbreak in the United Kingdom in 2001. Rev Sci Tech 2002, 21, 675–687. [Google Scholar] [CrossRef]
- Carpenter, T.E.; O’Brien, J.M.; Hagerman, A.D.; McCarl, B.A. Epidemic and Economic Impacts of Delayed Detection of Foot-and-Mouth Disease: A Case Study of a Simulated Outbreak in California. J Vet Diagn Invest 2011, 23, 26–33. [Google Scholar] [CrossRef]
- Perry, B.D.; Rich, K.M. Poverty Impacts of Foot-and-Mouth Disease and the Poverty Reduction Implications of Its Control. Vet Rec 2007, 160, 238–241. [Google Scholar] [CrossRef]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV Virus Taxonomy Profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef]
- Domingo, E.; Baranowski, E.; Escarmís, C.; Sobrino, F. Foot-and-Mouth Disease Virus. Comp. Immunol. Microbiol. Infect. Dis. 2002, 25, 297–308. [Google Scholar] [CrossRef]
- Drake, J.W.; Holland, J.J. Mutation Rates among RNA Viruses. Proc. Natl. Acad. Sci. U. S. A. 1999, 96, 13910–13913. [Google Scholar] [CrossRef]
- Domingo, E.; Escarmís, C.; Baranowski, E.; Ruiz-Jarabo, C.M.; Carrillo, E.; Núñez, J.I.; Sobrino, F. Evolution of Foot-and-Mouth Disease Virus. Virus Res. 2003, 91, 47–63. [Google Scholar] [CrossRef]
- Brooksby, J.B. Portraits of Viruses: Foot-and-Mouth Disease Virus. Intervirology 1982, 18, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Domingo, E.; Mateu, M.G.; Martínez, M.A.; Dopazo, J.; Moya, A.; Sobrino, F. Genetic Variability and Antigenic Diversity of Foot-and-Mouth Disease Virus. In Virus Variability, Epidemiology and Control; Kurstak, E., Marusyk, R.G., Murphy, F.A., Van Regenmortel, M.H.V., Eds.; Springer US: Boston, MA, 1990; pp. 233–266. ISBN 978-1-4757-9271-3. [Google Scholar]
- Pereira, H.G. Subtyping of Foot-and-Mouth Disease Virus. Dev. Biol. Stand. 1976, 35, 167–174. [Google Scholar] [PubMed]
- Ludi, A.B.; Horton, D.L.; Li, Y.; Mahapatra, M.; King, D.P.; Knowles, N.J.; Russell, C.A.; Paton, D.J.; Wood, J.L.N.; Smith, D.J.; et al. Antigenic Variation of Foot-and-Mouth Disease Virus Serotype A. J. Gen. Virol. 2014, 95, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Zhang, Z.; Nfon, C.; Yang, M. Genetic and Antigenic Relationship of Foot-and-Mouth Disease Virus Serotype O Isolates with the Vaccine Strain O1/BFS. Vaccine 2018, 36, 3802–3808. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.M.S.; Amin, R.; Rahman, M.Z.; Hossain, M.A.; Sultana, M. Antigenic Heterogeneity of Capsid Protein VP1 in Foot-and-Mouth Disease Virus (FMDV) Serotype Asia 1. Adv. Appl. Bioinforma. Chem. AABC 2013, 6, 37–46. [Google Scholar] [CrossRef]
- Maake, L.; Harvey, W.T.; Rotherham, L.; Opperman, P.; Theron, J.; Reeve, R.; Maree, F.F. Genetic Basis of Antigenic Variation of SAT3 Foot-And-Mouth Disease Viruses in Southern Africa. Front. Vet. Sci. 2020, 7, 568. [Google Scholar] [CrossRef]
- Sangula, A.K.; Siegismund, H.R.; Belsham, G.J.; Balinda, S.N.; Masembe, C.; Muwanika, V.B. Low Diversity of Foot-and-Mouth Disease Serotype C Virus in Kenya: Evidence for Probable Vaccine Strain Re-Introductions in the Field. Epidemiol. Infect. 2011, 139, 189–196. [Google Scholar] [CrossRef]
- Mattion, N.; Konig, G.; Seki, C.; Smitsaart, E.; Maradei, E.; Robiolo, B.; Duffy, S.; Leon, E.; Piccone, M.; Sadir, A.; et al. Reintroduction of Foot-and-Mouth Disease in Argentina: Characterisation of the Isolates and Development of Tools for the Control and Eradication of the Disease. Vaccine 2004, 22, 4149–4162. [Google Scholar] [CrossRef]
- Doel, T.R. FMD Vaccines. Virus Res 2003, 91, 81–99. [Google Scholar] [CrossRef]
- Pega, J.; Di Giacomo, S.; Bucafusco, D.; Schammas, J.M.; Malacari, D.; Barrionuevo, F.; Capozzo, A.V.; Rodriguez, L.L.; Borca, M.V.; Perez-Filgueira, M. Systemic Foot-and-Mouth Disease Vaccination in Cattle Promotes Specific Antibody-Secreting Cells at the Respiratory Tract and Triggers Local Anamnestic Responses upon Aerosol Infection. J Virol 2015, 89, 9581–9590. [Google Scholar] [CrossRef]
- Cox, S.J.; Parida, S.; Voyce, C.; Reid, S.M.; Hamblin, P.A.; Hutchings, G.; Paton, D.J.; Barnett, P.V. Further Evaluation of Higher Potency Vaccines for Early Protection of Cattle against FMDV Direct Contact Challenge. Vaccine 2007, 25, 7687–7695. [Google Scholar] [CrossRef] [PubMed]
- Paton, D.J.; Valarcher, J.F.; Bergmann, I.; Matlho, O.G.; Zakharov, V.M.; Palma, E.L.; Thomson, G.R. Selection of Foot and Mouth Disease Vaccine Strains--a Review. Rev. Sci. Tech. Int. Off. Epizoot. 2005, 24, 981–993. [Google Scholar] [CrossRef]
- Malirat, V.; Caldevilla, C.; Cardillo, S.; Espinoza, A.M.; Novo, S.G.; Taffarel, A.; Benito, M.B.; Bergmann, I.E. Broad Immunogenic Spectrum of Monovalent and Trivalent Foot-and-Mouth Disease Virus Vaccines Containing O1 Campos, A24 Cruzeiro and A Argentina 2001 Strains against Circulating Viral Lineages in Cattle and Pigs. Vaccine 2023, 41, 5782–5790. [Google Scholar] [CrossRef] [PubMed]
- Lombard, M.; Füssel, A.E. Antigen and Vaccine Banks: Technical Requirements and the Role of the European Antigen Bank in Emergency Foot and Mouth Disease Vaccination. Rev. Sci. Tech. Int. Off. Epizoot. 2007, 26, 117–134. [Google Scholar] [CrossRef]
- Paton, D.J.; Reeve, R.; Capozzo, A.V.; Ludi, A. Estimating the Protection Afforded by Foot-and-Mouth Disease Vaccines in the Laboratory. Vaccine 2019, 37, 5515–5524. [Google Scholar] [CrossRef]
- Hammond, J.M.; Maulidi, B.; Henning, N. Targeted FMD Vaccines for Eastern Africa: The AgResults Foot and Mouth Disease Vaccine Challenge Project. Viruses 2021, 13, 1830. [Google Scholar] [CrossRef]
- Ludi, A.B.; McLaws, M.; Armson, B.; Clark, J.; Di Nardo, A.; Parekh, K.; Henstock, M.; Muellner, P.; Muellner, U.J.; Rosso, F.; et al. PRAGMATIST: A Tool to Prioritize Foot-and-Mouth Disease Virus Antigens Held in Vaccine Banks. Front. Vet. Sci. 2022, 9, 1029075. [Google Scholar] [CrossRef]
- Gubbins, S.; Paton, D.J.; Dekker, A.; Ludi, A.B.; Wilsden, G.; Browning, C.F.J.; Eschbaumer, M.; Barnabei, J.; Duque, H.; Pauszek, L.L.; et al. Predicting Cross-Protection against Foot-and-Mouth Disease Virus Strains by Serology after Vaccination. Front. Vet. Sci. 2022, 9, 1027006. [Google Scholar] [CrossRef]
- Di Giacomo, S.; Bucafusco, D.; Schammas, J.M.; Pega, J.; Miraglia, M.C.; Barrionuevo, F.; Capozzo, A.V.; Perez-Filgueira, D.M. Assessment on Different Vaccine Formulation Parameters in the Protection against Heterologous Challenge with FMDV in Cattle. Viruses 2022, 14, 1781. [Google Scholar] [CrossRef]
- Sutmöller, P.; Costa, K.; Gomes, I. The Serum Microneutralization Test for Foot-and-Mouth Disease: Establishment of an Expected Percentage of Protection. Bol Cent Panam Fiebre Aft. 1980, 39–40, 37–42. [Google Scholar]
- Pay, T.W.F.; Hingley, P.J. Foot and Mouth Disease Vaccine Potency Test in Cattle: The Interrelationship of Antigen Dose, Serum Neutralizing Antibody Response and Protection from Challenge. Vaccine 1992, 10, 699–706. [Google Scholar] [CrossRef] [PubMed]
- PANAFTOSA. Subproyecto a Correlación de Las Técnicas de Control de Potencia de Las Vacunas Contra La Fiebre Aftosa En Los Países de La Cuenca Del Río de La Plata 1994.
- Hamblin, C.; Kitching, R.P.; Donaldson, A.I.; Crowther, J.R.; Barnett, I.T. Enzyme-Linked Immunosorbent Assay (ELISA) for the Detection of Antibodies against Foot-and-Mouth Disease Virus. III. Evaluation of Antibodies after Infection and Vaccination. Epidemiol Infect 1987, 99, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Periolo, O.H.; Seki, C.; Grigera, P.R.; Robiolo, B.; Fernandez, G.; Maradei, E.; D’Aloia, R.; La Torre, J.L. Large-Scale Use of Liquid-Phase Blocking Sandwich ELISA for the Evaluation of Protective Immunity against Aphthovirus in Cattle Vaccinated with Oil-Adjuvanted Vaccines in Argentina. Vaccine 1993, 11, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Kärber, G. Beitrag Zur Kollektiven Behandlung Pharmakologischer Reihenversuche. Archiv für experimentelle pathologie und pharmakologie 1931, 162, 480–483. [Google Scholar] [CrossRef]
- Maradei, E.; La Torre, J.; Robiolo, B.; Esteves, J.; Seki, C.; Pedemonte, A.; Iglesias, M.; D’Aloia, R.; Mattion, N. Updating of the Correlation between lpELISA Titers and Protection from Virus Challenge for the Assessment of the Potency of Polyvalent Aphtovirus Vaccines in Argentina. Vaccine 2008, 26, 6577–6586. [Google Scholar] [CrossRef]
- SENASA. Reglamentación que permite el control de las vacunas destinadas a la prevención de la Fiebre Aftosa 351/2006. 2006.
- Maradei, E.; Perez Beascoechea, C.; Malirat, V.; Salgado, G.; Seki, C.; Pedemonte, A.; Bonastre, P.; D’Aloia, R.; La Torre, J.L.; Mattion, N.; et al. Characterization of Foot-and-Mouth Disease Virus from Outbreaks in Ecuador during 2009-2010 and Cross-Protection Studies with the Vaccine Strain in Use in the Region. Vaccine 2011, 29, 8230–8240. [Google Scholar] [CrossRef]
- Mattion, N.; Goris, N.; Willems, T.; Robiolo, B.; Maradei, E.; Beascoechea, C.P.; Perez, A.; Smitsaart, E.; Fondevila, N.; Palma, E.; et al. Some Guidelines for Determining Foot-and-Mouth Disease Vaccine Strain Matching by Serology. Vaccine 2009, 27, 741–747. [Google Scholar] [CrossRef]
- Goris, N.; Maradei, E.; D’Aloia, R.; Fondevila, N.; Mattion, N.; Perez, A.; Smitsaart, E.; Nauwynck, H.J.; La Torre, J.; Palma, E.; et al. Foot-and-Mouth Disease Vaccine Potency Testing in Cattle Using Homologous and Heterologous Challenge Strains: Precision of the “Protection against Podal Generalisation” Test. Vaccine 2008, 26, 3432–3437. [Google Scholar] [CrossRef]
- Maradei, E.; Malirat, V.; Beascoechea, C.P.; Espinoza, A.M.; Novo, S.G.; Smitsaart, E.; Salgado, G.; Mattion, N.; Toledo, J.R.; Bergmann, I.E. Emergence of Antigenic Variants of Foot-and-Mouth Disease Virus Serotype O in Ecuador and Preliminary Evaluation of a Field Strain as a Vaccine Candidate. Vaccine 2014, 32, 2446–2451. [Google Scholar] [CrossRef]
- Galdo Novo, S.; Malirat, V.; Maradei, E.D.; Espinoza, A.M.; Smitsaart, E.; Pedemonte, A.R.; Mattion, N.; Bergmann, I.E. Antigenic and Immunogenic Spectrum of Foot-and-Mouth Disease Vaccine Strain O1 Campos against Representative Viruses of Topotypes That Circulated in Asia over the Past Decade. Vaccine 2017, 35, 2303–2307. [Google Scholar] [CrossRef]
- Samuel, A.R.; Knowles, N.J. Foot-and-Mouth Disease Type O Viruses Exhibit Genetically and Geographically Distinct Evolutionary Lineages (Topotypes). J. Gen. Virol. 2001, 82, 609–621. [Google Scholar] [CrossRef]
| Experimental groups | FMDV strains (µg/dose) | ||
|---|---|---|---|
| O1/Campos | A24/Cruzeiro | C3/Indaial | |
| 01C 10 μg | 10 | - | - |
| 01C 30 μg | 30 | - | - |
| A24 30 µg | - | 30 | - |
| C3I 30 μg | - | - | 30 |
| A24/O1C 20μg | 10 | 10 | - |
| C3I/O1C 20 μg | 10 | - | 10 |
| A24/C3I/O1C 30 μg | 10 | 10 | 10 |
| experimental groups | Heterologous FMDV strains | |||||
|---|---|---|---|---|---|---|
| O/ECU/46/2010 | O/SKR/84/YDM | O/Taiwan/1997 | ||||
| 14 dpv | 28 dpv | 14 dpv | 28 dpv | 14 dpv | 28 dpv | |
| 01C 10 μg | 0.07 | 0.11 | 0.20 | 0.28 | 0.10 | 0.15 |
| 01C 30 μg | 0.08 | 0.15 | 0.42 | 0.49 | 0.32 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





