1. Introduction
Since humanity began the process of domestication and cultivation of plants, thousands of species have spread beyond their natural areas and many of them have effectively naturalized in both anthropized and natural biotopes. This process has intensified in parallel with the improvement and increase of communications, commerce, urbanization, and climate change [
1,
2]. Today it is feasible to imagine the planet as a tight network of interconnected nodes, acting as donors or species receptors, along whose threads numerous taxa migrate from one point to another, artificially promoting the coexistence of species that until now had never lived together [
3]. Moreover, in the case of vegetal species it has being so important the active introduction for design of gardens, urban green spaces, and peri-urban landscapes [
4]. Urban areas not only create new conditions that can drive a fast entrance of alien species, but also favour their rapid evolution and to maintain high numbers of non-native species [
5].
It is from the great geographical discoveries of the XV and XVI centuries, including the America discovery, that the rate of plant introductions highly increased in terms of number of species, number of individuals and distance from original populations. Spanish and Portuguese navigators were responsible for high number of bidirectional introductions in Europe but also in the new discovered areas [
6].
Not all introduced species can trigger invasion processes and only a proportion of them become naturalized, and only a percentage of naturalized become invasive ones. These proportions are not constant and vary depending on the species, the receiving ecosystem, and the mode of introduction. The concept invasive plant usually applied to vegetal species that cause any inference to environmental or economic impact [
7]. Also, some authors have proposed terms like ‘pests’ and ‘weeds’ for the naturalized species causing substantial changes on ecosystems [
8]. It is estimated that a 50–80% of naturalized plant species have harmful effects in their new introduction areas and only a 10% them produce substantial changes on the character, condition, form, or nature of ecosystems [
9].
Plant invasions are a source of agricultural and economic problems around the world, but also a major ecological threat is the effect on biodiversity loss. Regarding to the invasiveness of species, the success of invasive species seems to be largely due to their superiority over native species in terms of growth rate and dissemination to recipient ecosystems; this superiority appeared to be related to higher values of fitness-related traits, such as growth rate, age of maturity, fertility, and seed dispersal.
In general, invasive species conform populations in both anthropic and natural systems. Although most of plant invasions occur in anthropic influenced areas, the registered cases in natural environments are increasing related to surrounding anthropized spaces. These ones are taking a special relevance due to the implications for the conservation of habitats, ecosystems, and their biological components [
3].
Plant species occupy a distribution area whose extension and conformation depend on the climatic and edaphic conditions to which each species is adapted, as well as the climatic and geological history of each region. In the Mediterranean Region, it is easier to adapt species from other areas with a Mediterranean climate around the globe, such as the Western United States, Western Chile, Southern Africa, or Southern Australia, or even from other warm and dry climates [
10].
Invasive alien species are one of the main causes of biodiversity loss in the world. In Europe, since 2005, members of the DAISIE (Delivering Alien Invasive Species Inventories for Europe) project have been creating an inventory that provides the first pan-European overview of more than 10,000 fish, birds, plants, insects, and other non-indigenous species that live among us. The inventory lists 10,822 non-native species present in Europe. Although not all of them are invasive, it is estimated that between 10% and 15% are potentially dangerous for European biodiversity. The European Environment Agency has compiled a list of 163 invasive alien species that threaten Europe’‘s ecosystems. Since 1950, one or more species are established each year, and there is no indication that this rate is slowing down. Most invasive species come from North America and Asia. However, a considerable number of them come from different European areas. European’‘s single market and the absence of border controls would favour this flow [
1].
Within the European Union, Regulation (EU) No. 1143/2014 of the European Parliament and of the Council, of October 22, 2014, on the prevention and management of the introduction and spread of invasive alien species establishes rules to prevent, minimize and mitigate the adverse effects of invasive alien species on biodiversity and associated ecosystem services, and on human health and security, as well as to reduce their social and economic consequences. For its part, Directive 92/43/EEC, of the Council, of May 21, 1992, on the conservation of natural habitats and of wild fauna and flora, establishes in Article 22 that the Member States shall guarantee that the intentional introduction into nature of a species that is not autochthonous to their territory is regulated in such a way that it does not harm the native wild fauna and flora or their natural habitats in their natural range, and if deemed necessary, they will prohibit such introduction. In this context, in 2008, the European Commission adopted the Communication” Towards a European Union Strategy on Invasive Species” [
11].
Spain counts with a large plant biodiversity, with around 9000 vascular plant species, with a percentage of endemicity of around 20%, greater than other Mediterranean countries as Greece (13%) or Italy (11%) and much higher than in Central Europe countries with an average of endemicity of around 1% [
12]. This fact, together with the good weather and the strategic position in southern mediterranean area, makes this country to have a high risk of foreigner plant entrance, naturalization, and invasion. In fact, the data of the Spanish Ministry for Ecological Transition and Demographic Challenge (METDC) reveals that 502 non-native introduced species (plants+fungi+animals) have been detected in our country in last years, where 306 are already stablished naturalized species, and near 200 are considered invasive alien species by the METDC [
13]. Regarding vegetal species, the official data of the Spanish Catalogue of Invasive Alien Species published in 2024 by METDC [
13], indicates the presence of 64 species of vascular plants considered invasive alien plant species, apart from 15 invasive algae species.
To face this problem, there was approved in 2007 the Law Number 42 of the Ministry of Agriculture, Food and Environment to regulate the Natural Heritage and Biodiversity Management in Spain. It considers invasive alien species such as “that which is introduced or established in an ecosystem or natural or semi-natural habitat and which is an agent of change and threat to biological diversity. native, either because of its invasive behaviour, or because of the risk of genetic contamination”. This law, in its article 64, defines the Spanish Catalogue of Invasive Alien Species, which include all those invasive alien species and subspecies that constitute, or may become a serious threat to autochthonous species, habitats or ecosystems, agronomy, or for economic resources associated with the use of natural heritage. With the approval of Royal Spanish Decree 630/2013, which regulates the Spanish Catalogue of Invasive Exotic Species, the list of taxa included in it was defined. The inclusion of one species in this catalogue prohibits its possession, transport, traffic, and trade of living specimens, including their remains, or propagules, that could survive or reproduce.
In the case of Andalusia region, placed at southern Spain, this is a large region with 87 591 km2 and with a high rate of plant biodiversity. Its vegetation spectrum counts with more than 25% of plant endemic species (around 450) and the 20% of its surface is protected under any environmental figure. Nevertheless, in this region there are more than 300 alien species (plant+animals) present that have been introduced by different ways, voluntarily or involuntarily [
3]. Of these, 180 are naturalized to a greater or lesser extent. In the case of vascular plants there are 172 species included in the Spanish List of Wildlife Species under Special Protection Regime and 49 species are included in any of the categories of the Spanish Catalogue of Threatened Species published by the METDC [
13].
It is remarkable that the list of the 100 Worst Invasive Alien Species in the World, made by the IUCN [
14] includes 2 plant species with a high rate of presence in Andalusia: Arundo donax L. and Eichhornia crassipes (Mart.) Solms. Also is especially abundant and invasive the “heaven tree” Ailanthus altissima (Mill.) Swingle, with a high increase in last years both in ruderal, urban and natural areas.
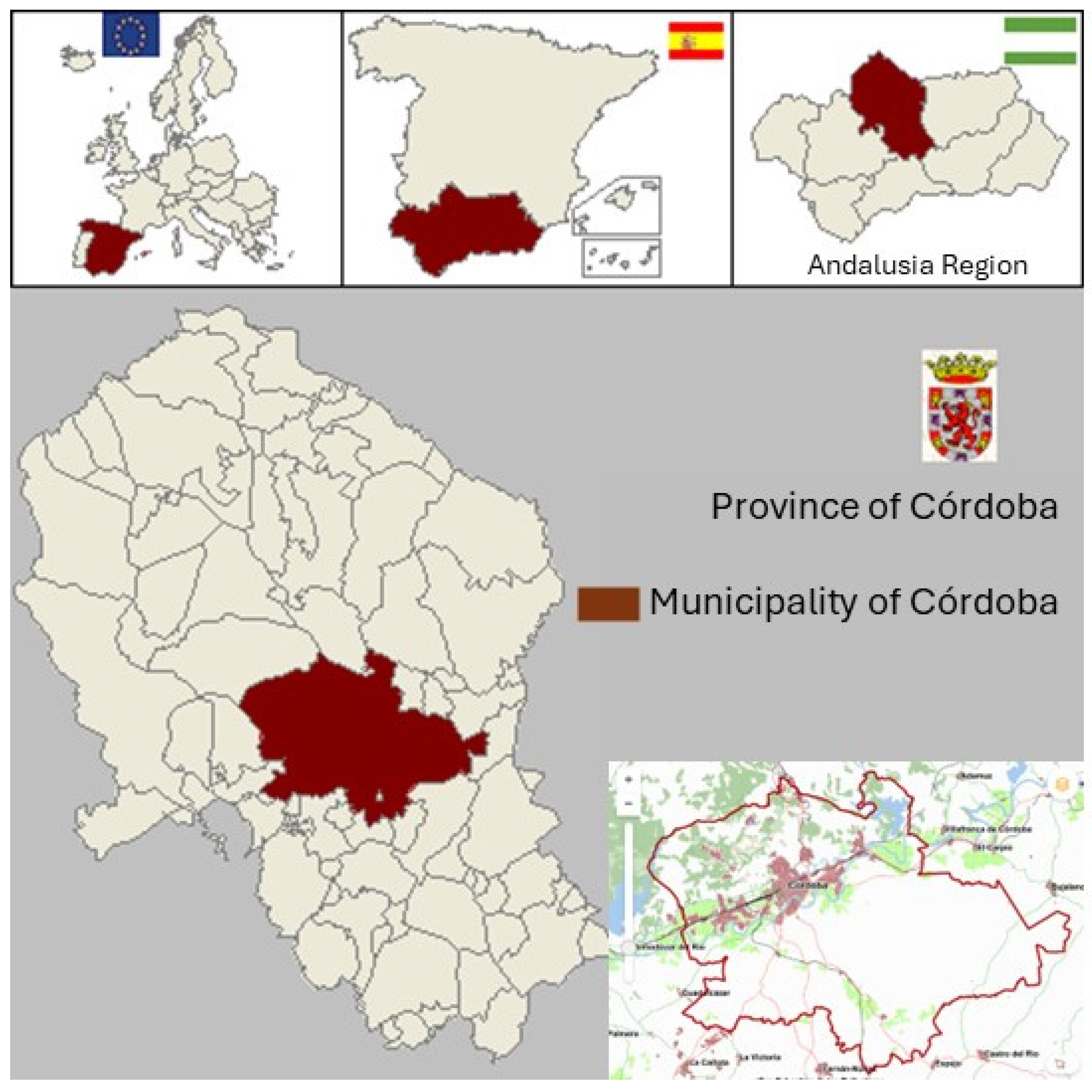
To fight against invasive plants, Andalusia Region is developing a program for the Control of Invasive Alien Species, which is a dynamic project made up of the sum of the various actions undertaken to manage them. It is based on three key elements: prevention, action and information. The present work takes part of this project evaluating the distribution range of vegetal invasive alien species in the largest municipality of Andalusia, Córdoba, Southern Spain in terms of surface (1.253 km²). It shows the results of a detailed monitoring in the city and surroundings of this city Córdoba to locate, take inventory and evaluate the presence of both foreign naturalized and invasive vascular plants in the area. The study includes urban, semi-natural and natural areas of all the municipality. The big extension of this municipality, the high importance of its surrounding natural ecosystems for economy and tourism, and its location in the high environmental threatened region of Andalusia, also highly affected by climate change, make an ideal emplacement for carry out this sort of work to obtain real information and a better understanding how biological invasions are currently developing and shifting natural vegetation in the Mediterranean area [
15].
The main objective of the present work is to carry out a detailed inventory on the presence of invasive plant species in the municipality of Córdoba (Southern Spain), indicating the degree of presence and location, with the aim to have a real idea of the actual scenario and to evaluate the possible repercussions that they may have on the native flora and the potential future evolution in order to help to design effective responses and restorative actions against the increasing biological invasions.
3. Results
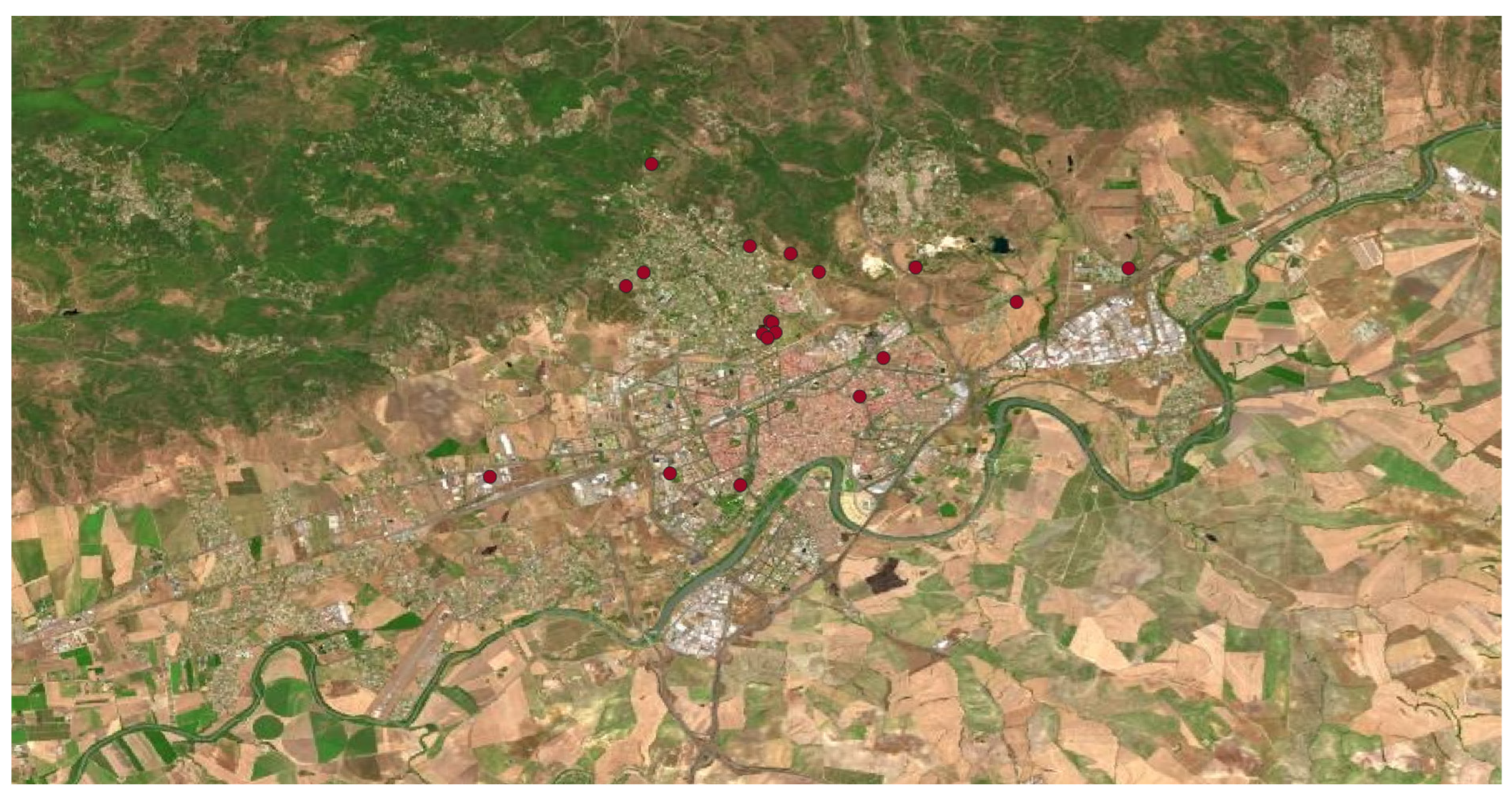
In base to the previous bibliographic and databases’ research, the field sampling was planning and carried out in an exhaustive way through all the grids that we divided the municipality of Córdoba. The field work confirmed the presence of 17 invasive alien plant species in different locations of the study area, it supposes the 35% of the invasive plant species of Andalusia, and the 27% of Spain. In
Table 1 are listed the located alien invasive plant species in the municipality of Córdoba during the study period (2021-2023). The name of the species, the total sampled points where it was found, the degree of ornamental use or naturalized way of life, the origin area, the stratum (tree, herbaceous, shrub), the Raunkiaer plant life-form, and if the alien species in included in the Spanish Catalogue of Invasive Alien Species (SCIAS).
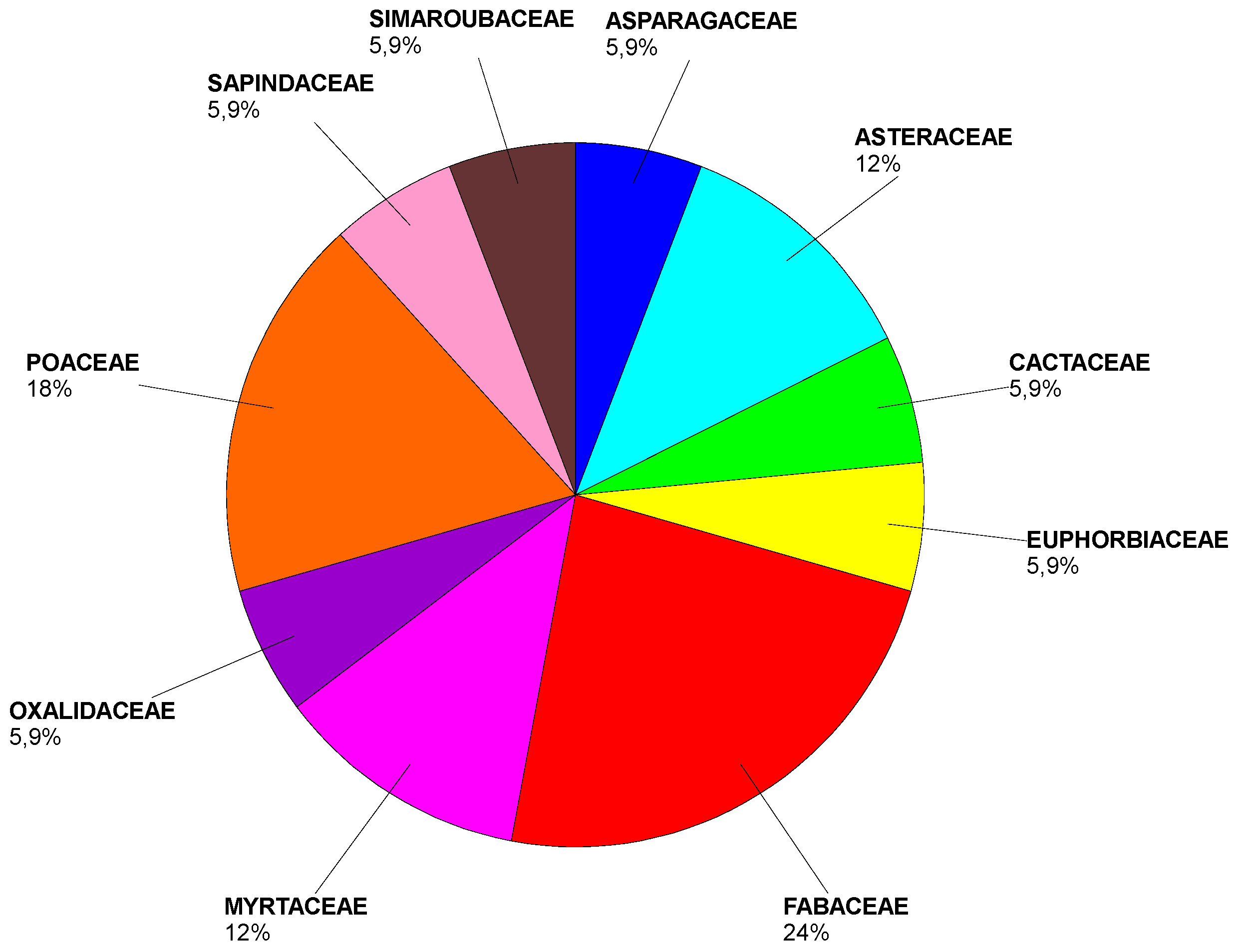
Analysing the frequency of the different species of invasive alien plants located at the area, in
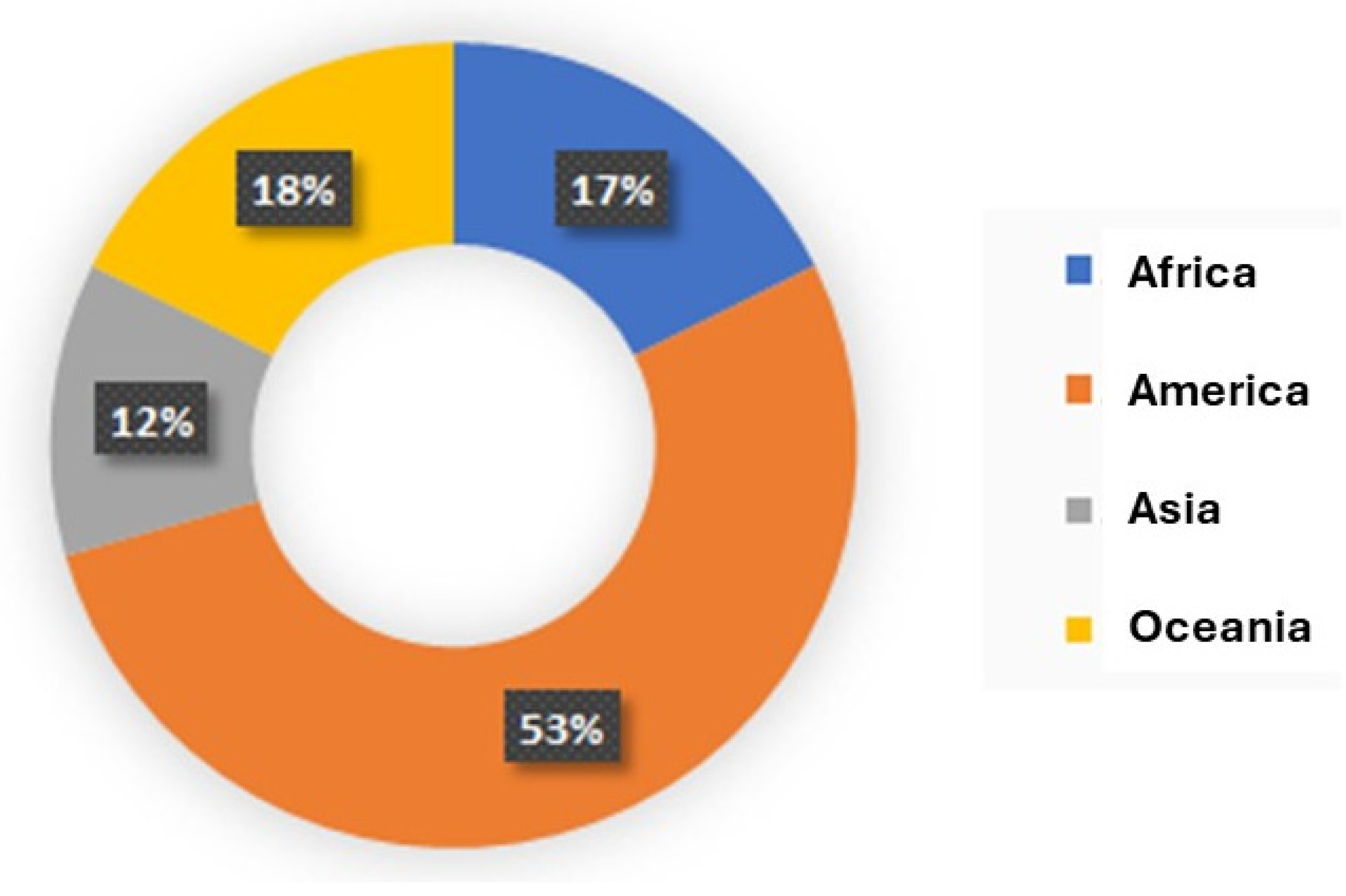
Table 1 appears both the number of populations and the coordinates where there were detected. On some occasions the number of populations do not coincide with the number of coordinates because in some locations there were more than one population. The obtained data revealed a great variety of origins and families in the detected alien species. The areas of origin belong to 4 different continents, being America the highest source, with a 53% of the species,
Figure 4.
On the other hand, about the plant phylogeny, it is so diverse, the 17 found species belong to 10 different families:
Asparagaceae,
Asteraceae,
Cactaceae,
Euphorbiaceae,
Fabaceae,
Myrtaceae,
Oxalidaceae,
Poaceae,
Sapindaceae and
Simaroubaceae, with
Fabaceae and
Poaceae standing out above the rest (
Figure 5).
From those families, the most abundant invasive species were Ailanthus altissima with 49 populations, followed by
Eucalyptus spp. with 39,
Robinia pseudoacacia, 23,
Agave americana, 19,
Arundo donax and
Parkinsonia aculeata with 16 and
Sorghum halepensis in 15 different locations. On the other hand, it appears other less abundant invasive species but considered of high invasive risk such as
Acer negundo with 9 populations,
Opuntia ficus-indica and
Oxalis pes-caprae 8,
Ricinus communis 6,
Xanthium strumarium and
Xanthium spinosum 4 and 1 respectively, and
Cortaderia selloana 2. Speaking about the features of the most abundant species,
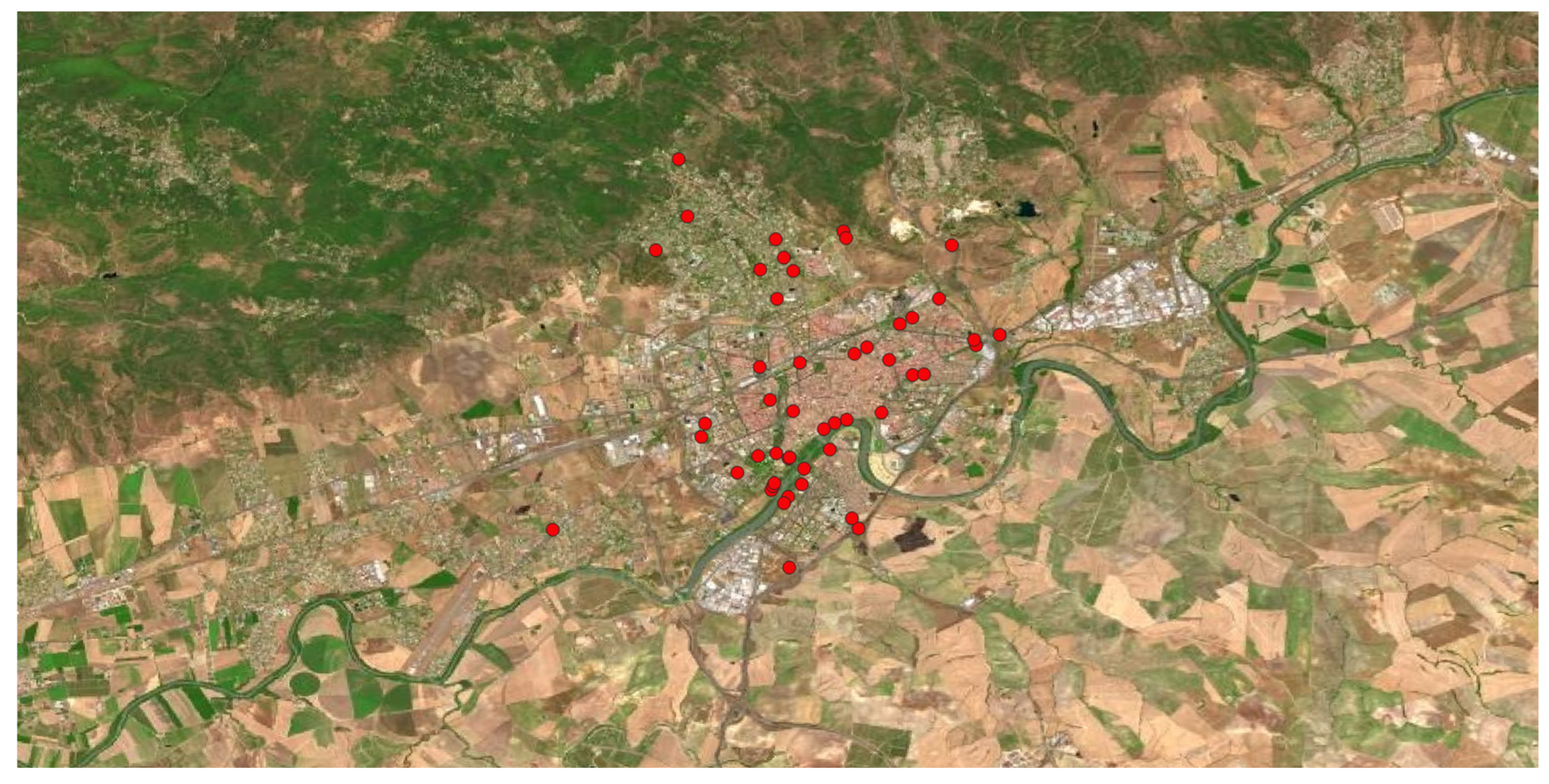
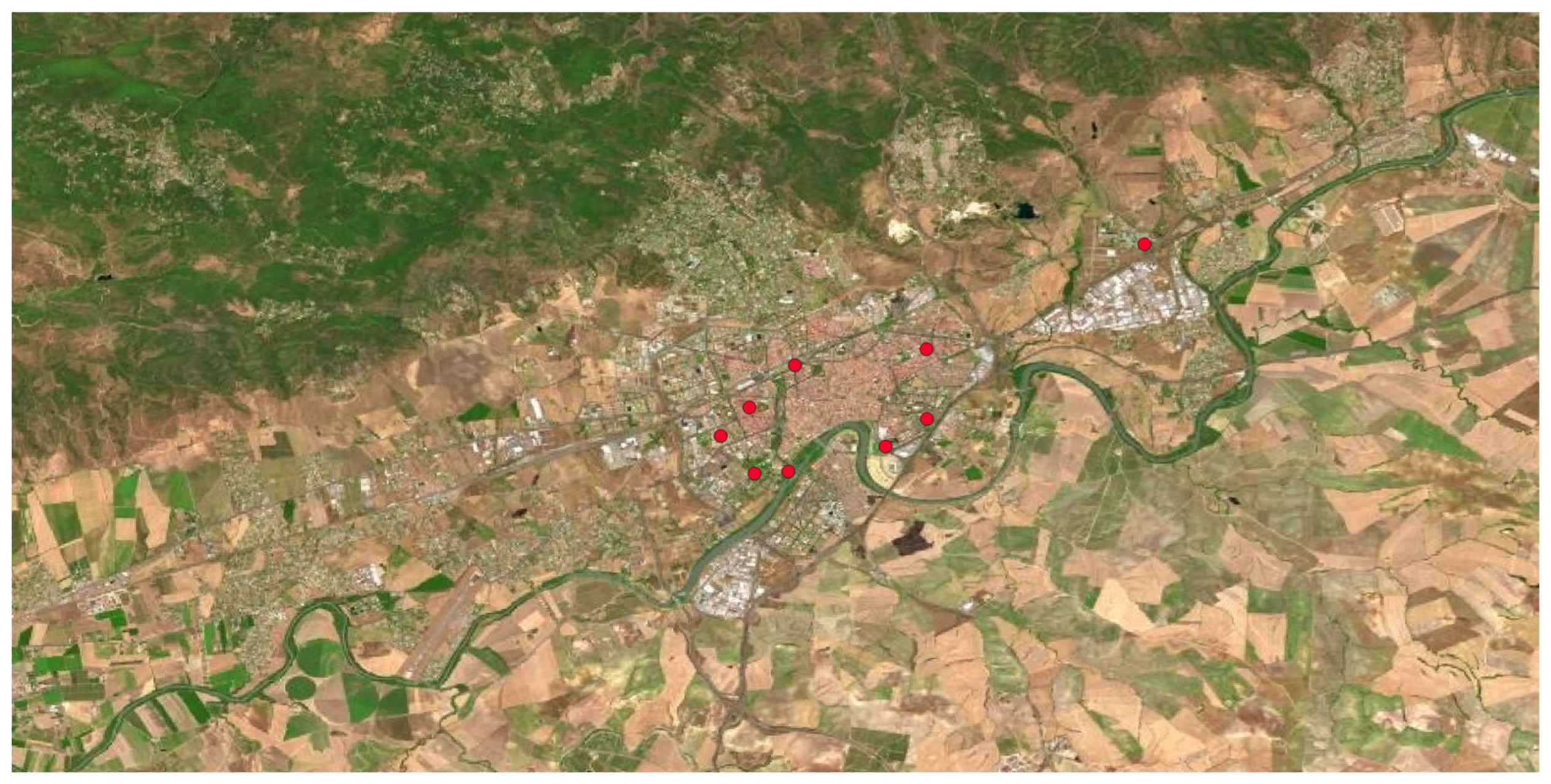
Ailanthus altissima, commonly known as “the heaven tree” due to its high size, is a species originally from China and Vietnam, introduced in many temperate countries as an ornamental tree in the Centuries XIX and XX. It was primary used for forming alignment green lines in streets and highways, as well as fixing unstable soils or building windbreaks. Currently, it is a very invasive plant since, due to its rapid growth and the allelopathic effects of roots and seeds. It colonises so fast ruderal areas or areas without vegetation after construction works.
Figure 6 shows its homogeneous distribution observed in the municipality, being present in all the environments.
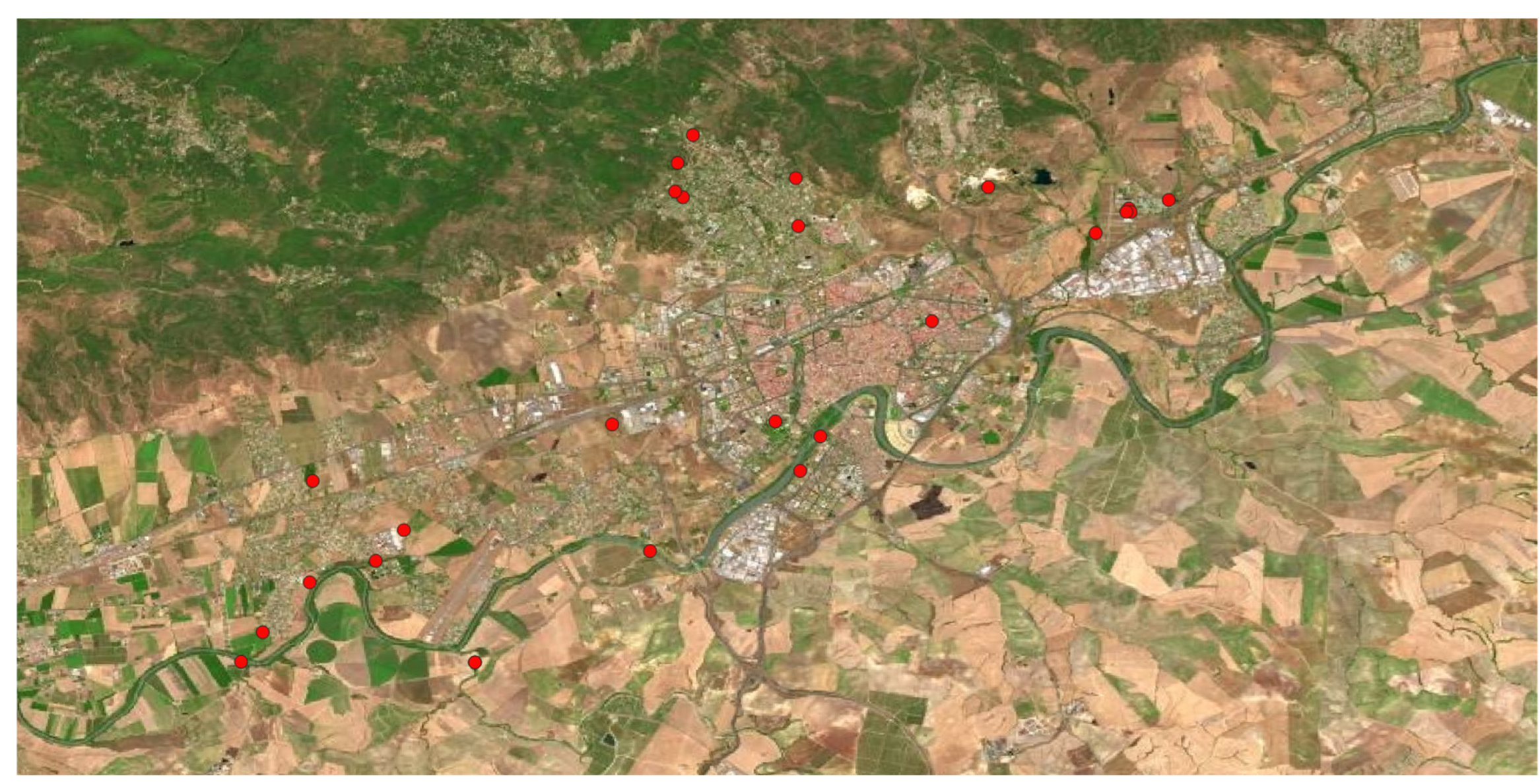
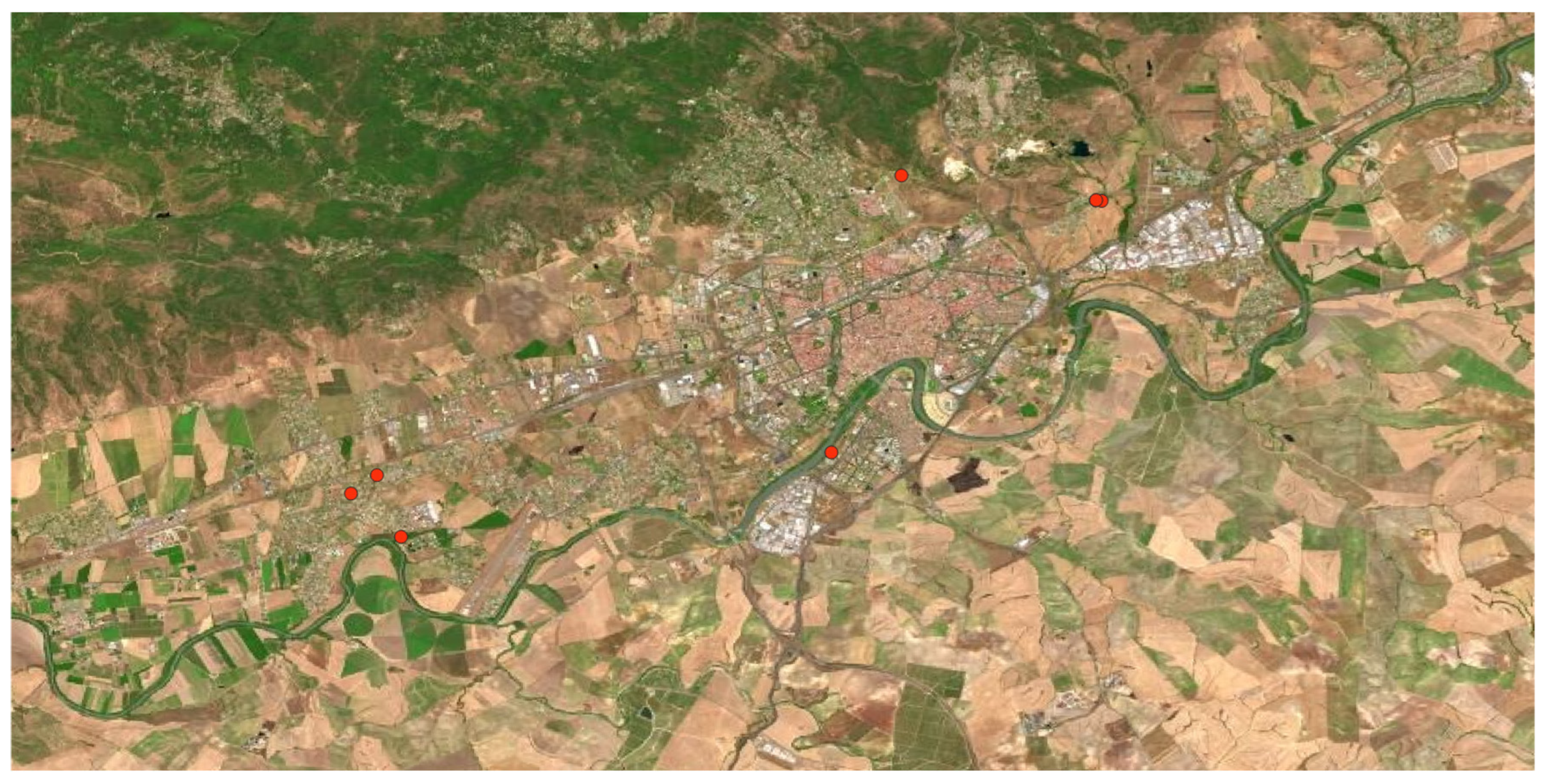
Belonging to the
Eucalyptus spp. genus, 2 species were found in the area:
E. camaldulensis and
E. globulus. Introduced from Australia in most Mediterranean and Atlantic regions of Spain, mainly for paper extraction purposes, they reproduce so fast, producing negative effects on the landscape, biodiversity, and soil due to the acidity of their leaves, provoking an allelopathic effects on the autochthonous flora, almost completely sterilizing the soil that remains in this situation even long after the eucalyptus have disappeared. In the study area were found many populations forming extensive tree masses colonizing all kinds of habitats, urban, peri-urban, and natural areas (
Figure 7).
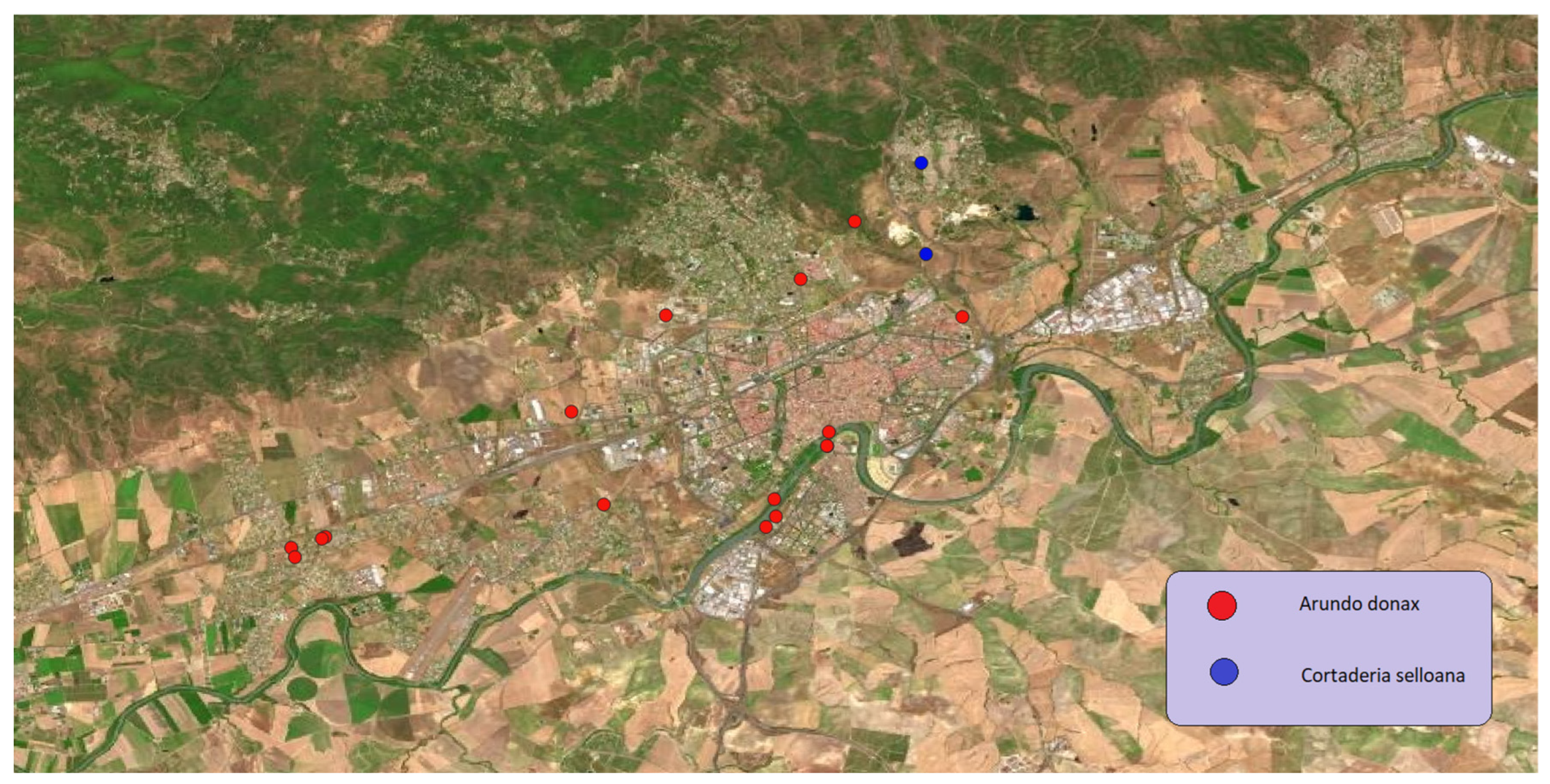
In the case of
Arundo donax (
Poaceae family), originally from Asia, it was introduced in Europe intentionally, as a cultivated species for the formation of barriers, hedges, or vegetable windbreaks. According to the IUCN, it is one of the most dangerous and harmful invasive alien plants in the world, making it part of the list of the 100 worst invasive biological species. Its impacts on the natural environment of the study area include the displacement of native riparian vegetation, such as
Phragmites australis (Cav.) Trin. ex Steud. Other so high invasive from the
Poaceae is
Cortaderia selloana, originally introduced from the Patagonia for ornamental purposes, it appears in less locations in the study area, but it has a strong ability to invade riverbanks and river areas which makes it a very dangerous species for these valuable ecosystems. In
Figure 8 we can see the distribution of both
Arundo donax, and
Cortaderia selloana in the Córdoba municipality.
Other
Poaceae species is
Sorghum halepense, presented in the area with 15 populations in different locations (
Figure 9). It comes from the east of the Mediterranean basin, North Africa and Southwest Asia. It appears as a summer weed within agricultural crops, especially those irrigated. In Córdoba this species appears especially in roadsides, agricultural areas, and riverbanks, forming extensive masses.
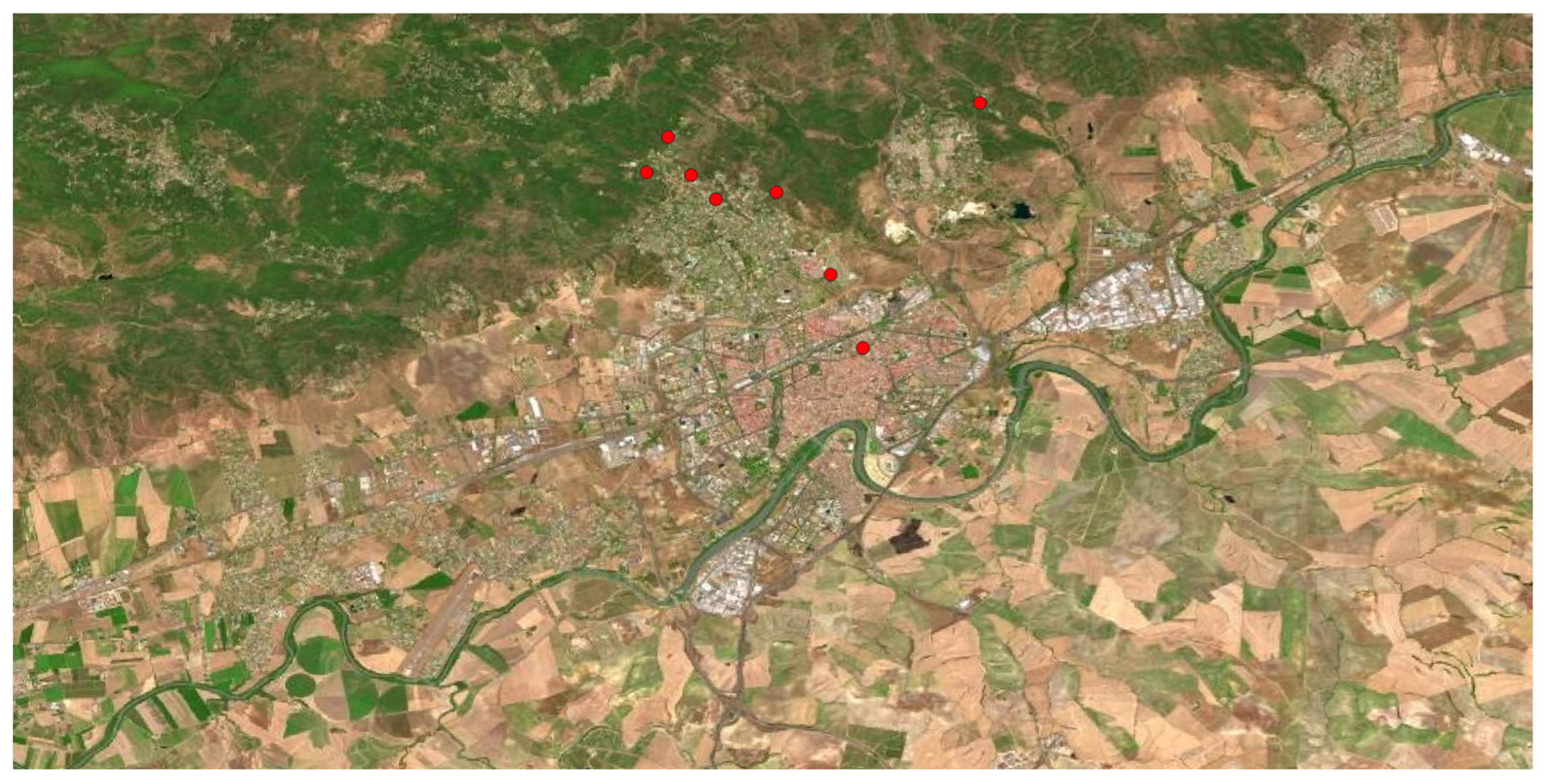
Speaking about the
Fabaceae family, it has many invasive tree species mainly introduced from America. In the area, we found
Robinia psudoacacia, a tree native to central and eastern area of the United States, introduced in Europe as ornamental, and nowadays naturalized in many scattered places on the Iberian Peninsula, including Córdoba municipality (
Figure 10). Due to its ecophysiological characteristics, it is very dangerous for natural ecosystems, especially in forestry ones.
Other
Fabaceae species to be remarked in the study area is
Parkinsonia aculeata, native to tropical America. In Spain, it is a very abundant invasive plant which forms thickets that threaten the conservation of wetlands and riparian forests. In our study 16 populations were detected, distributed through all the municipality. It appears associated with gardens and street ornamental tree lines, but also it was found in the river area replacing autochthonous species (
Figure 11).
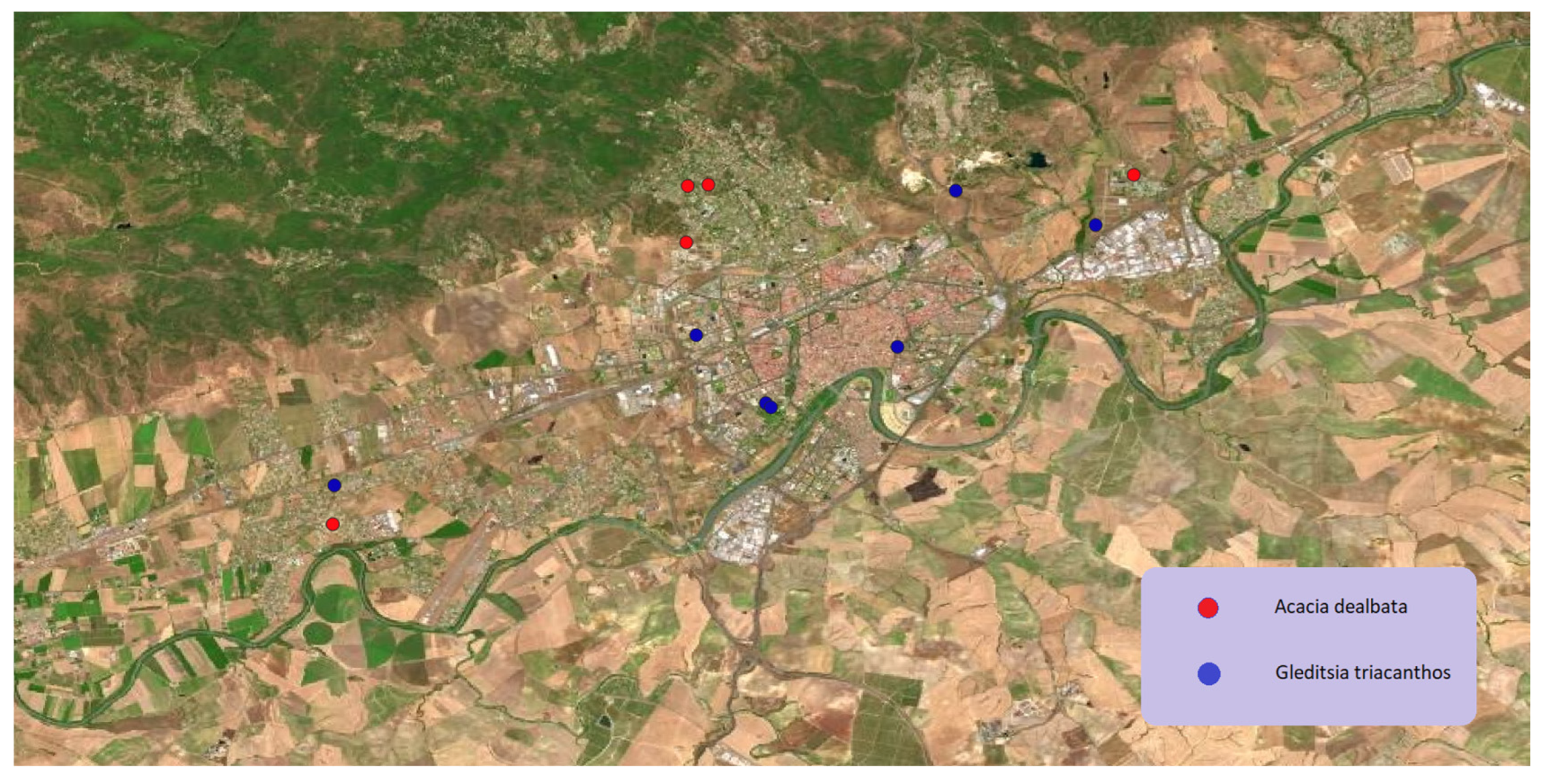
Also from the
Fabaceae family is
Gleditsia triacanthos which lives naturally in central and eastern North America. In its region of origin, it inhabits humid soils and riparian environments. Currently it is cultivated as an ornamental tree, in alignments of streets, walks and parks. Its appreciable ornamental value joins with its rusticity and its tolerance to the polluted atmospheres of cities, so it is frequent in urban areas of Spain. In Córdoba it has found both as ornamental tree in parks and gardens but also as invasive growing spontaneously in periurban locations (
Figure 12).
Together with the distribution of
Gleditsia triacanthos it is also possible to observe the distribution of other
Fabaceae tree in
Figure 12. It is
Acacia dealbata from Australia. This tree was introduced in many temperate zones of Europe for gardening, being currently the most widely used mimosa in this continent. Nowadays, both species are spreading as invasive species in several points of the Mediterranean ecosystems.
Finally, other abundant invasive species in the area is
Agave americana, from the
Asparagaceae family, and originally from eastern Mexico. It was introduced in Europe, in the XVI Century, by the conquerors of the New World. Firstly, it was used as ornamental plant in dry areas of Spain, and later it was planted for be used as a textile plant for obtaining coarse fibres. Nowadays it is widespread distributed, overall, in southern Spain and Canary Islands. Apart from a decorative use, it naturally appears on roadsides or borders of states in drier areas of the Mediterranean area. In the area it was observed at 19 populations both in gardens and natural and semi-natural environments (
Figure 13).
On the other hand, we also must comment the presence in the study area of other less abundant invasive alien, but also with importance due to their high risk of invasiveness and the consideration of strong threat by different official organisms. This is the case of
Acer negundo, presents in 9 locations of the Cordoba municipality (
Figure 14). This species, native to North America lives here in forests and riparian formations, always on humid substrates. In Europe was introduced as ornamental tree, being now naturalized because it frequently escapes from gardening areas. It usually grows subspontaneous or naturalized in ditches degraded peri-urban areas and riverside forests.
Other less abundant species is
Opuntia ficus-indica, presents in 8 locations of the Cordoba municipality (
Figure 15). This species, coming from tropical America, was intentionally introduced by the America conquerors in the XVI Century for agricultural proposes because their fruits and raising mealybugs for dye production, carmine cochineal. Nowadays is used as ornamental plant and thanks to their strong spines, to form protective hedges in arid areas. Nevertheless, it is important to note that nowadays many populations of Opuntia are affected by a phytophagous parasite,
Dactylopius opuntiae (Cockerell), a phytophagous parasite known as false carmine cochineal.
In the case of
Oxalis pes-caprae, it is an herbaceous plant native to South Africa. In Spain it abounds in the South area, and especially in coastal regions, penetrating towards the interior of the Iberian Peninsula through the Guadalquivir valley (Andalusia Region, South Spain). It produces economic and environmental damage in gardens and crops due to its strong and fast asexual reproduction, and the production of biochemical substances that inhibit the germination of other seeds. In the study area, these weeds form dense covers that monopolize light and space in 8 locations with natural vegetation, displacing native flora (
Figure 16).
Other less abundant species, but with a high risk of invasiveness is the castor plant,
Ricinus communis, presents at 6 locations of the study area (
Figure 17). Its origin is difficult to determine due to its domestication and cultivation since protohistoric times. It is widely naturalized through the Mediterranean coastal regions, penetrating towards warm inland enclaves. In the study area it is present as ornamental in some gardens but also living as naturalized in rough ruderal environments.
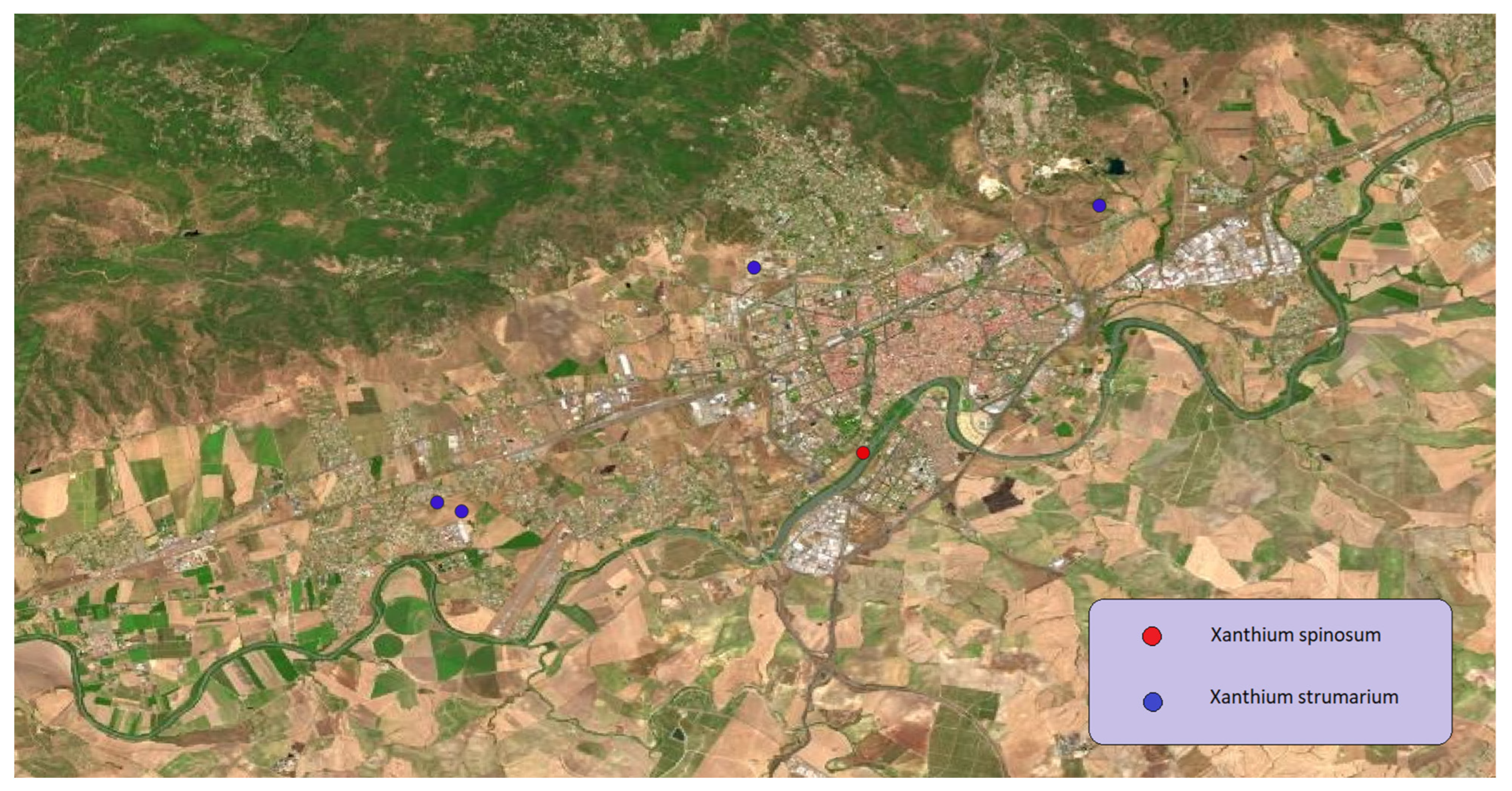
Belonging to the
Asteraceae family
Xanthium strumarium and
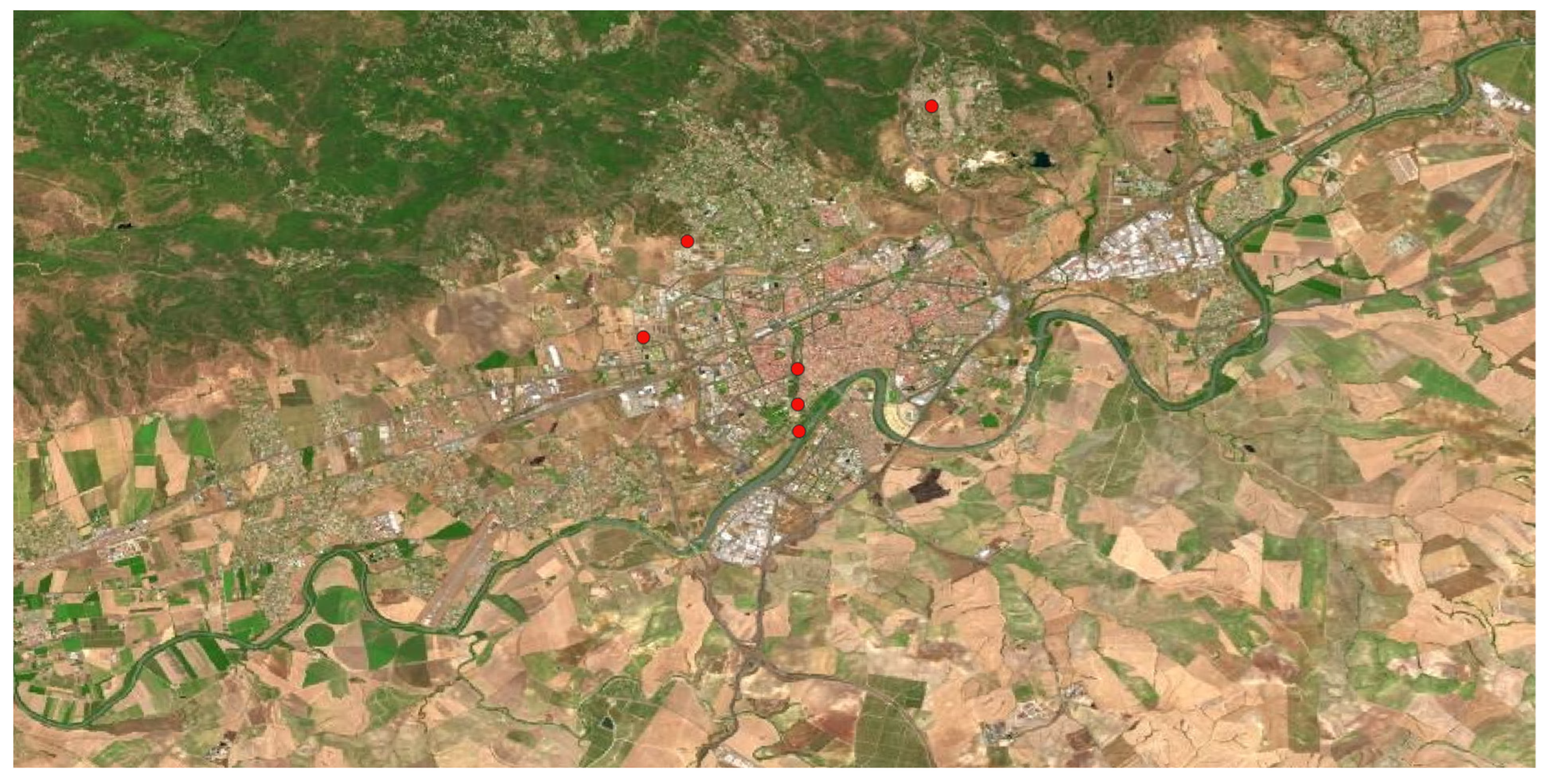
Xanthium spinosum are so strong invasive species from America. Within Spain, transhumance activity, moving flocks of sheep cross over the Peninsula looking for pastures, had to play a decisive role in their seeds’ dispersal. Nowadays they are frequently located by almost all the Iberian Peninsula environments. It is usually found in ruderal areas and. In Córdoba, not many populations were located (
Figure 18), mostly in ruderal areas as a summer weed.
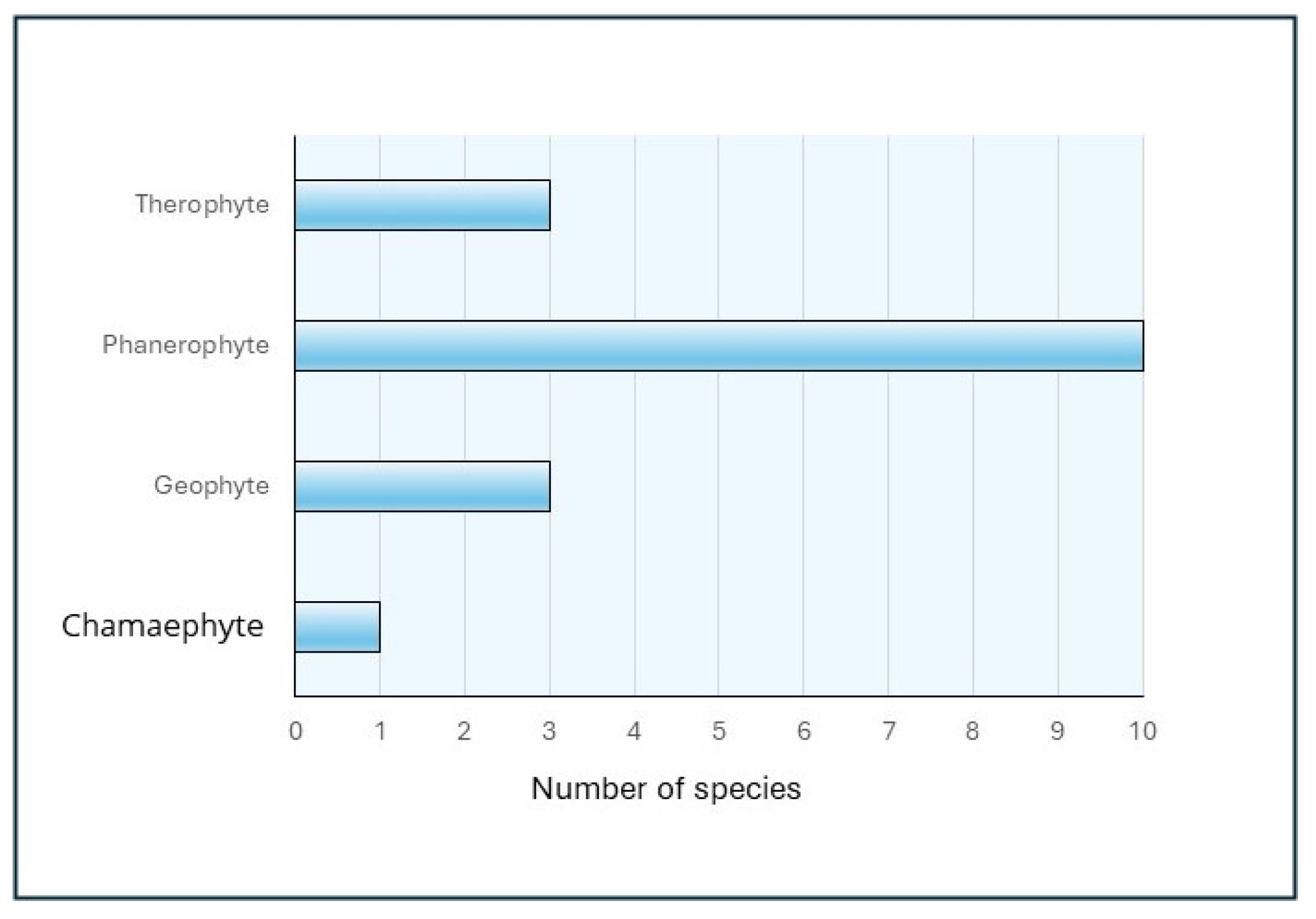
If we analysis together the data obtained, it is possible to obtain some interesting information about different aspects of the characteristics of the observed invasive species. Considering their form of development according to the Raunkiær classification system, which categorizes them according to the life-form of the plants, we have found 4 different types: Camephytes, Geophytes, Therophytes, and Phanerophytes, being this latter the most abundant type in the area (
Figure 19).
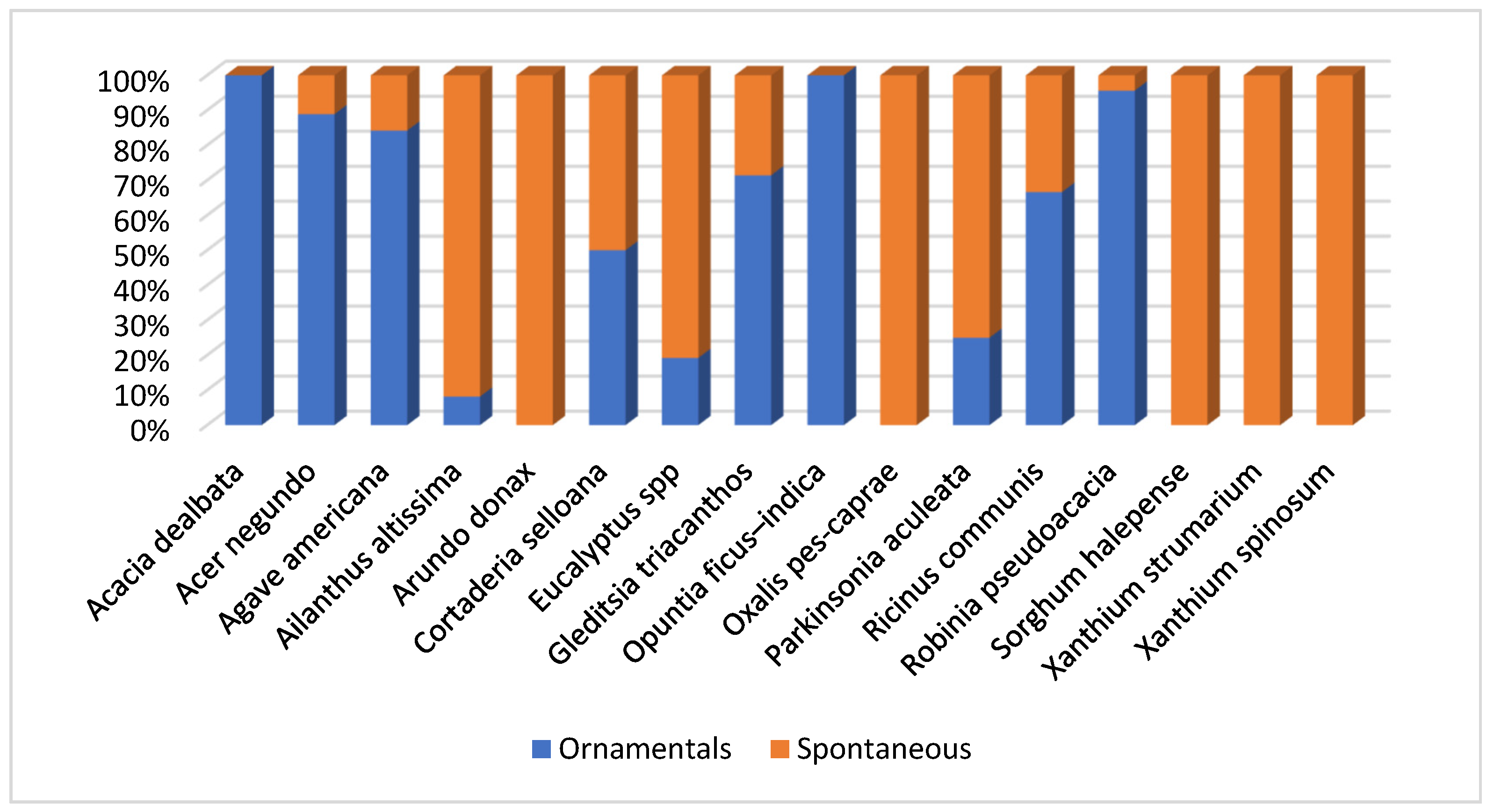
On the other hand, a graphical analysis of the way of life that we observed in the detected alien species was made. We annotated if the populations we found were planted as ornamental in gardens, parks, or private properties, or if they were growing spontaneously in the area.
Figure 20 shows the percentages per each species, being the
Fagaceae trees such as
Acacia dealbata,
Robinia pseudoacacia or
Gleditsia triacanthos the most used as ornamental. Other species as
Acer negundo,
Ailanthus altissima or
Agave americana also show elevated percentages.
In the case of spontaneous populations of the alien species observed in the area, we made a bar chart scattering the type of habitat where they were observed.
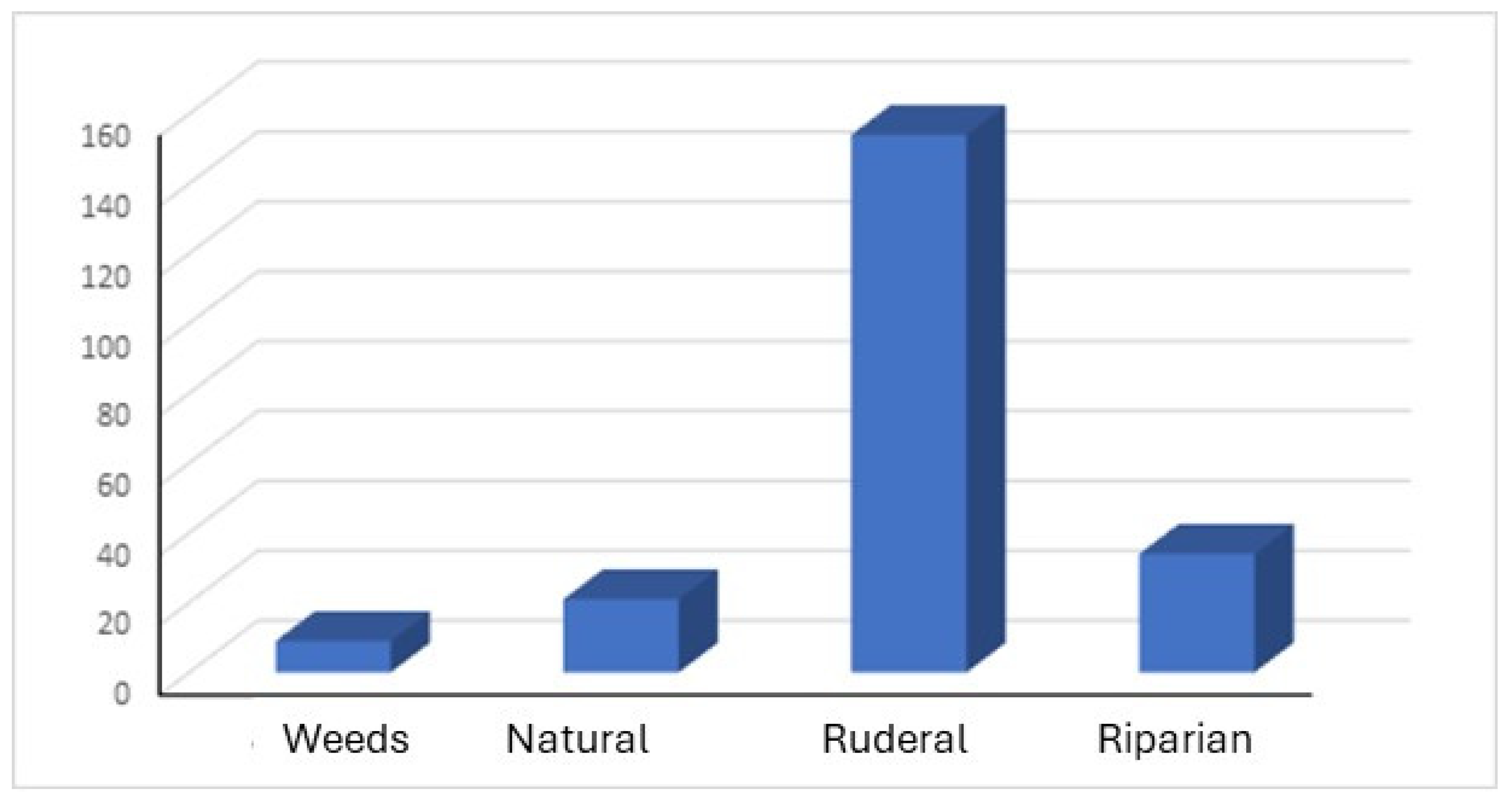
Figure 21 shows the results as the number of populations located at four different environments: crops’ weeds, natural, ruderal, and riparian vegetation areas.
The lowest proportion of species were weeds in crop areas. The species found in this sort of environment were Sorghum halepense, Xanthium strumarium, Xanthium spinosum, and Opuntia ficus-indica, occupying margin of paths and borders between properties. On the contrary, most of the species growing spontaneously were in ruderal areas, abandoned soils that usually conquest invasive species with a high rate of seed germination, some allelopathic strategies or fast asexual reproduction. Nevertheless, in the study area, it was also possible to observe some naturalized invasive species in riparian areas, mostly in the riverbanks of the Guadalquivir River that cross the city. There, it grows species such as Arundo donax, Ailanthus altissima, Ricinus communis, Parkinsonia aculeata or Acer negundo. Also, these species can be observed near other little streams or water channels of the study area, always in smaller populations.
4. Discussion
Invasive alien species are those species capable of surviving, establishing, and reproducing in a biogeographically different place from the original one, overcoming the biotic and abiotic limiting factors that may be encountered in their arrival, establishment, and reproduction [
1]. Biological invasions by plant species are a natural phenomenon, but the presence of humans and the climate change has accelerated this process exponentially [
2]. The main pathways of introduction include legal and illegal trade in ornamental species, tourism, and human migration. Today, these activities have increased and accelerated in a high percentage respecting to the past Century. Recent studies concluded that plant invasions are the result of complex interactions between the behaviour of alien species, the vulnerability of the receiving environment, and the history of introductions [
21].
In the case of Spain, the presence of alien invasive species is considered of a high risk and a strong threat for natural habitats [
13]. The location of our country at the Mediterranean basin, one of the most affected areas by biological invasions [
22], together with the combined effect with climate change that allows and favours the growing of species from warmer climates, make our country as a “hotspot” of alien invasive species [
23]. Apart from the negative ecological consequences, this scenario involves high economic loss for our country of around €232 million from 1997 to 2022 [
24]. This amount englobes different aspects, mostly management costs, damage costs and post-invasion management actions, being curious that the lowest percentage is inverted on prevention actions, contrary to all international guidelines, which recommend investing more in prevention. In fact, are the regional administrations that mostly pay the totally of the cost derivate of the alien invasive plant invasions, being a difficulty assumable cost. In this sense it is necessary to stablish the real territorial distribution range, frequency, and abundance of the different invasive alien plant species to determine their real impact and the responsibility for the different official institutions to carry out managing actions.
The present work has as one of the results the list of the invasive alien plant species in the municipality of Córdoba, southern Spain, Andalusia Region, one of the areas of Europe most affected by the biological invasion of alien plant species [
25]. We also determine the frequency, location, abundance, and distribution features of them as well as the potential impact in the different habitats and natural flora of the study area. Our study provides an updated species inventory with a focus on species that are currently occurring in the urban, peri-urban, natural, and semi-natural vegetation areas of Córdoba; this sets compile bibliographic data reported at books and databases but also a huge field work for 3 years, being today one of the best and more complete inventories of the Region. Our results reveal that the use as ornamental has brought to the area most of the alien species. The second step is the naturalization, and we have observed as all the 17 alien species detected in the area, are already naturalized growing by themselves out of the original gardens and properties. And the following step is the invasion, most of the naturalized species don’t cause alterations on the new areas but it is estimated that around 1% of these species become invasive, establish ecological interaction with the invaded community and displacing autochthonous flora in a non-return way [
2].
The species we looked for, were listed at the main red books of alien invasive species of Europe and Spain. This is the case of Ailanthus altissima that in Spain it is considered as a high-risk invasive species, with a sharp increase of its presence in natural areas in last years [
26], and that we found in many locations of the area. Other strong invasive tree species detected two belonging to the
Eucalyptus spp. genus. They are a threat for the ecosystem equilibrium of natural areas because they completely sterilize the soil due to the acidity of their leaves and fruits. It remains practically naked even long after the eucalyptus have disappeared [
18].
The Fagaceae family appears represented with three tree species, primary introduced as ornamental,
Acacia dealbata,
Robinia psudoacacia,
Parkinsonia aculeata and
Gleditsia triacanthos, that because their eco-physiological characteristics, are very dangerous for natural ecosystems, especially in forest ones. They scape from parks and gardens and invade forest clearings causing the displacement of native flora. their rapid root growing and their easy asexual reproduction, makes it very difficult to eliminate [
27]. Moreover,
Parkinsonia aculeata is an important threat difficult to control in riverbanks. This river ecosystems have a high ecological value as native flora corridors and as reservoirs of biodiversity, so the threat for invasiveness there is of a high importance and should represent a priority in any invasive management actions. [
28]. These cited
Fabaceae species are also considered as invasive species in several points of the Mediterranean ecosystems due to their fast-spreading rate overall, in irrigated or humid environments [
29].
In the study area, some species were found in many locations with populated groups while other were less abundant, being detected in less locations with few individuals. These are the cases of
Acer negundo,
Opuntia ficus-indica and
Oxalis pes-caprae,
Ricinus communis,
Xanthium strumarium and
Xanthium spinosum, and
Cortaderia selloana. Nevertheless, they represent a high invasive risk as it has been considered by different official organisms related to environmental legislation, as the Spanish Ministry for Ecological Transition and Demographic Challenge [
13]. One of them is
Acer negundo, native to North America that lives in the study area in forestry habitat and riparian formations. It is considered that if it finds adequate humidity conditions, it acquires a strong invasive character due to its higher phenotypic plasticity [
21]. On the other hand, we found other species adapted to dry soils, as
Agave americana and
Opuntia ficus-indica, both frequently associated at the field. Apart from a decorative use, they naturally appear on roadsides or borders of states as it has been observed in other locations of southern Spain or farther Mediterranean areas [
18]. Their presence in arid areas giving rise to a landscape that evokes the western deserts of North America [
30]. Fortunately, it is important to note that nowadays many populations of Opuntia are affected by a phytophagous parasite,
Dactylopius opuntiae (Cockerell), a phytophagous parasite known as false carmine cochineal, that today is playing an important role as a biological control agent of this invasive species [
31]. In the case of
Ricinus communis its origin is difficult to determine due to its domestication and cultivation since protohistoric times, although Africa has been traditionally established as its place of origin [
32]. It is widely naturalized through the Mediterranean coastal regions, penetrating towards warm inland enclaves. It can live in rough ruderal environments, being so difficult the eradication [
33]. Moreover, its ingestion by humans or animals can cause health problems as intestinal bleeding and organ damage. The poison by a prolongated ingestion can kill a person or an animal in a few in three days [
34]. Traditionally, farmers affected by this weed have largely relied on manual removal strategies. Today scientists are evaluating other types of eradication actions such as spray treatments or biological control measures [
35]. Also, other weed species such as
Oxalis pes-caprae, native to South Africa present a high risk in the area forming dense covers that monopolize light and space in gardening peri urban and natural areas of the municipality. It was introduced in the Mediterranean basin involuntarily, through the pathways of agricultural weeds [
36]. This garden and agricultural invasive is out of gardens and agricultural areas since years ago and it has strongly displaced displacing native flora in the Mediterranean area. Its eradication is a big challenge due this fast asexual reproduction, and nowadays has been not already achieve by any eradication method [
37].
In the case of the
Poaceae family, different alien species appear in the area,
Arundo donax, Sorghum halepense and
Cortadeira seollana.
Arundo donax, originally from East Asia, was introduced in Europe since the 16th Century as a cultivated species for the formation of barriers, hedges, or vegetable windbreaks [
38]. In our country it is present in most of the regions, especially in the south and east, and both archipelagos. According to the IUCN, it is one of the most dangerous and harmful invasive alien plants in the world, making it part of the list of the 100 worst invasive biological species [
39]. Its impacts on the natural environment of the study area include the displacement of native riparian vegetation, such as
Phragmites australis (Cav.) Trin. ex Steud.
Cortadeira seollana coming from Patagonia for ornamental purposes, it appears in a few locations in the study area, but it has a strong ability to invade riverbanks and river areas which makes it a very dangerous species for these valuable ecosystems [
40]. Finally,
Sorghum halepense is considered as one of the most damaging weeds for agriculture in warm temperate and subtropical areas [
41].
Belonging to the
Asteraceae family
Xanthium strumarium and
Xanthium spinosum are two so strong invasive species coming from America whose introduction into Europe took place involuntarily [
42]. Within Spain, transhumance activity, moving flocks of sheep cross over the Peninsula looking for pastures, played a decisive role in their seeds’ dispersal. Nowadays they are frequently located by almost all the Iberian Peninsula environments. It is usually found in ruderal areas and as a summer weed of different various crops [
43]. In Córdoba, not many populations were located, but its fast reproduction range make them a potential risk in seminatural and cultivated areas [
23].
Nevertheless, it is necessary to point out that we found indications about the existence of some other alien species in the municipality during the previous bibliographic research process, but that was not possible to verify their presence in our field work. This was the case of
Fallopia baldschuanica (Regel) J. Holub: from the
Polygonaceae family native to Asia and
Pennisetum setaceum (Forssk.) Chiov from the
Poaceae family and native to Africa that Almendro et al. [
44] located in a small, naturalized population within the Guadalquivir River banks, but that nowadays were not there, probably because its eradication in the clean works of the area carried out by the Council Environmental Department. Another example was
Ipomoea indica (Burm.) Merr, from the
Convolvulaceae family and native to America, although with a low risk of invasiveness, and not included in the Atlas of Invasive Alien Plants in Spain [
18], this species is included in the Spanish Catalogue of Invasive Exotic Species and was cited by López-Tirado [
45] on slopes of Sierra Morena, out of the municipality border, and seems to be already out of the study area. Finally, in the case of
Datura stramonium L., (
Solanaceae family, native to America), which appeared as present in the GBIF database in some parks and urban clearings of the municipality some years ago, today no specimen was found during our survey, probably because they were eliminated by the Council Environmental Department due to its high toxicity and risk for human health.
In general, the detected alien invasive species in the study area were located both in gardens and private properties for ornamental purposes but also out of them naturalised in semi-natural and natural areas of the peri urban surroundings. Our results fit with the consulted bibliography of the municipality council about plants species used in public gardens, where these species appears on the top list,
https://infraestructuras.cordoba.es/sec-parqujard/especieszv/listadoszv [
46]. The use of land plays an important role in the conquest of invasive species and the naturalization of ornamental species, for example after agricultural land abandonment or in degraded soil after a road or buildings’ work, they take advantage to germinate there and spread out their seeds. The lowest proportion of species were weeds in crop areas, since we were working in urban areas and surroundings, where the surface devoted to crops was not so high. Nevertheless, most of the observed species growing spontaneously in the area were found in ruderal areas, abandoned soils that conquest invasive species with a high rate of seed germination, allelopathic strategies or fast asexual reproduction. This is typical from alien invasive plant species, being the first step before introducing to natural or riparian environments [
47].
The successful establishment of a species in a new territory depends on its ability to reproduce. In the study area the situation is extremely varied since the reproduction mechanisms are very different. If a plant can multiply vegetatively, it multiplies the possibility of a successful colonization of a new territory. This is the case
of Oxalis pes- caprae, native to southern Africa and found at 8 locations in the area [
48]. The main invasive species that are characterized by being the most harmful are weeds, which cause problems in the production and management of crops. This is the case of several invaders from America, such as
Sorghum halepense [
49]. In natural areas, some naturalized species, when dominant, may displace native species (eg.
Acer negundo,
Robinia pseudoacacia,
Acacia dealbata and
Gleditsia triacanthos). However, the magnitude of its impact at the community and ecosystem level needs further investigation.
We must also mention the case of a very controversial species in the area, the eucalyptus. It was introduced in Spain in the XIX Century, and is currently used mainly to produce cellulose, although it admits various uses as a structural element or medicinal oils. The growing of this non-autochthonous species, characterized by its competitive nature and a great dispersal capacity, constitutes a limiting factor for the natural regeneration capacity of the autochthonous formations. The economic and ecological impact of this species in our region and country is so elevated, nevertheless any action is nowadays carried out against due to the paper industry dependence on it [
24].
Regards to the life-forms, Marini et al. [
50] observed that the relationship between species richness and environment for natives was strongly dependent on life-form, while aliens showed their own trend different from the area pattern. This occurs in our study are, where chamaephyte is the most abundant life-form for native species, as in all the Mediterranean area [
51], while alien invasive plants showed phanerophytes as the most abundant form in the study area, following by therophytes and geophytes, Similar trends across alien and native life-forms were found for the relationship between species richness and human impact along the whole area.
Finally, it is necessary to comment the effect of climate change in the area and its influence on the presence of alien invasive species. Probably the fact that we found them in more locations and populations than the indicated at bibliography and consulted databases is related with it. Córdoba area is suffering in last years the effect of climate change with especially high temperatures and an important lack of precipitations [
52,
53]. It could be favoring the increment in the presence of invasive species in all the monitored habitats, including natural ones, and displacing native flora, a phenomenon increasingly observed not only in the Mediterranean area [
22], but also in the rest of the climate areas [
54].
For an exotic species introduced into a new environment is not so easy to become invasive, and it must pass through different filters. Nevertheless, there is a percentage that arrive to escape and naturalize becoming invasive even to have a bigger knowledge of the management and use of foreign plant species with ornamental purposes. In the past, one of the most important were the biogeographic barriers, solved today by means of transport, voluntary introductions, etc. So, nowadays the worst difficulty for them is the biological adaptation together with the concurrence rates, determined by their own biological characteristics and the conditions of the receiving ecosystem [
4,
9,
20].
To fight against invasive plants, Andalusia Region is developing a program for the Control of Invasive Alien Species, which is a dynamic project made up of the sum of the various actions undertaken to manage them. It is based on three key elements: prevention, action, and information. The results from the present work add knowledge about the distribution range of vegetal invasive alien species in the largest municipality of Andalusia. Moreover, our data can draw the actual scenario for similar cities on the Mediterranean area, even trying to raise awareness of a more rational and integrative use of natural vegetation in urban gardening, and overall to give us an idea not only about the real actual situation but also about the possible future evolution to design responses and restorative actions against the increasing biological invasions. Up today, the ratio between theoretical and applied management actions indicates that there are more theoretical studies than applied research or management actions for invasive species [
55,
56]. For this reason, is needed an increasing effort in the effective dissemination of the protocols and results of real experiences in invasive alien species management to enhance current practical knowledge, including that undertaken by public institutions and environmental agencies.
Figure 1.
Localization of the Cordoba municipality. Source: Andalusian Multiterritorial Information System (SIMA).
Figure 1.
Localization of the Cordoba municipality. Source: Andalusian Multiterritorial Information System (SIMA).
Figure 2.
Part of the elaborated list with the vegetal invasive species with potential possibilities to be located in the study area.
Figure 2.
Part of the elaborated list with the vegetal invasive species with potential possibilities to be located in the study area.
Figure 3.
QGIS used interface of the gridding of a 1:10.000 scale map of Córdoba.
Figure 3.
QGIS used interface of the gridding of a 1:10.000 scale map of Córdoba.
Figure 4.
Diagram of percentages’ origin of the detected invasive plant species in the study area.
Figure 4.
Diagram of percentages’ origin of the detected invasive plant species in the study area.
Figure 5.
Percentage diagram of the represented families of the alien invasive plant species in the study area.
Figure 5.
Percentage diagram of the represented families of the alien invasive plant species in the study area.
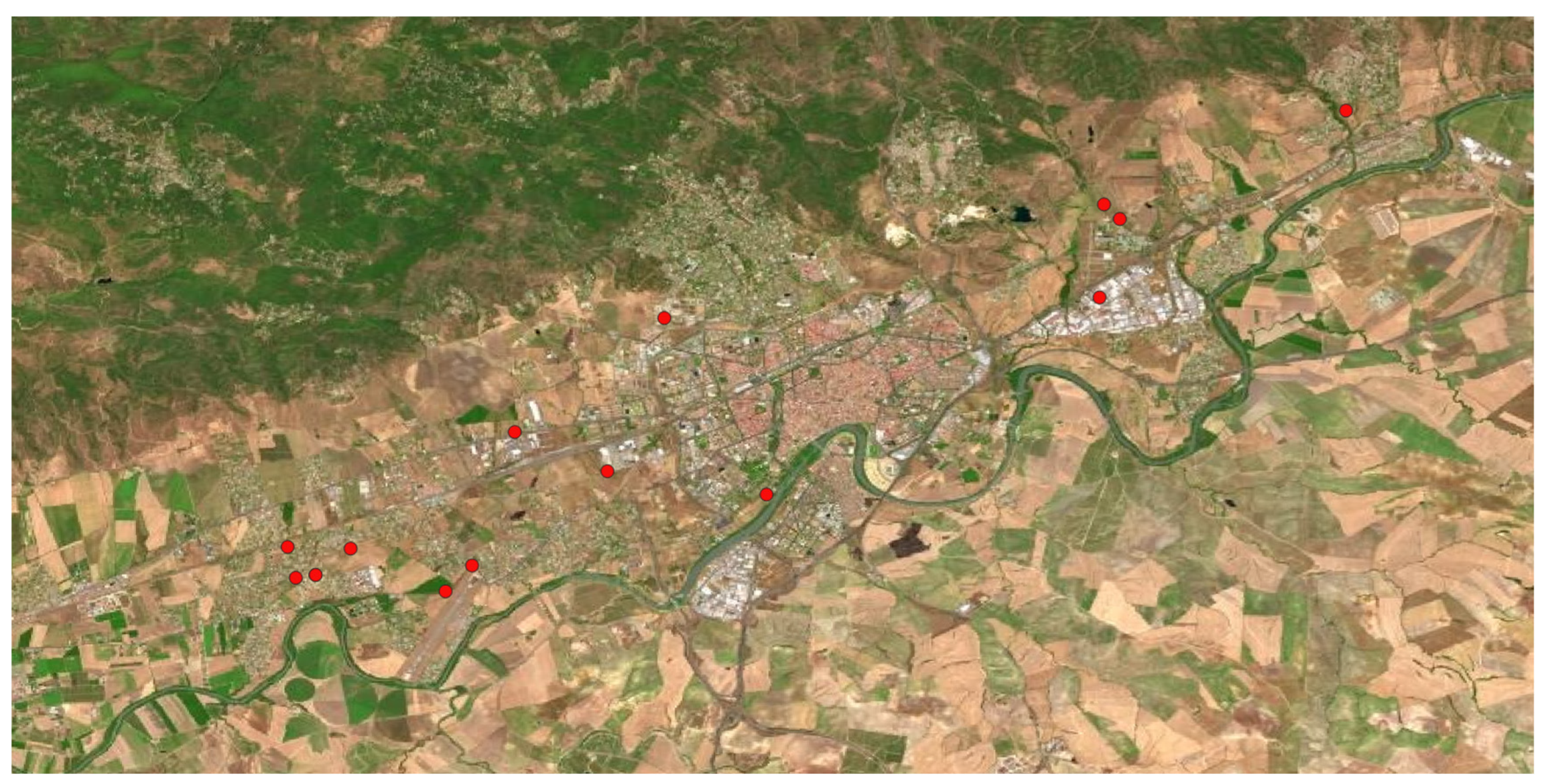
Figure 6.
Geographical distribution of Ailanthus altissima (Mill.) Swingle in Córdoba municipality.
Figure 6.
Geographical distribution of Ailanthus altissima (Mill.) Swingle in Córdoba municipality.
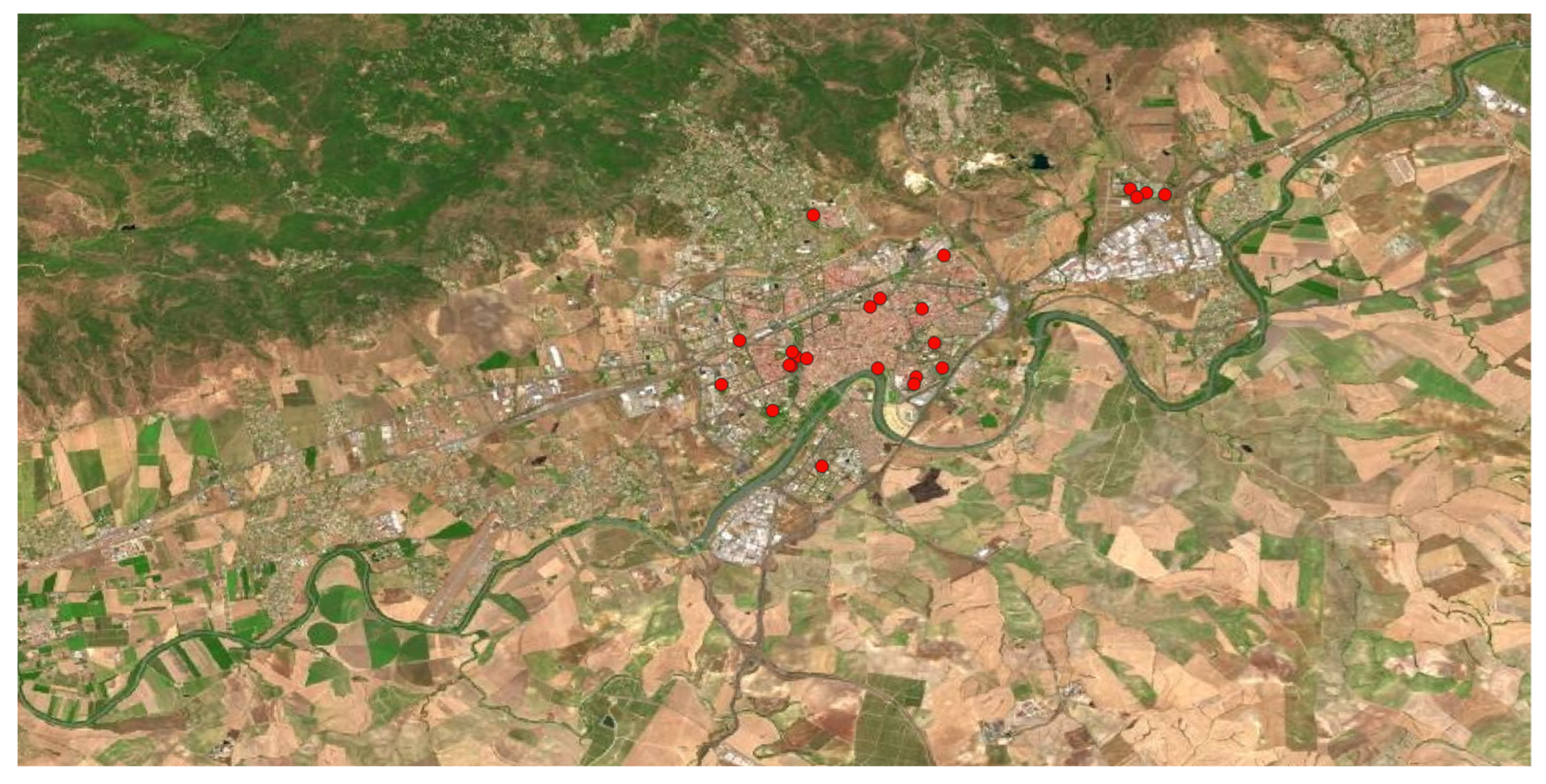
Figure 7.
Geographical distribution of Eucalyptus spp. in Córdoba municipality.
Figure 7.
Geographical distribution of Eucalyptus spp. in Córdoba municipality.
Figure 8.
Geographical distribution of Arundo donax L. and Cortaderia selloana (Schultes & Schultes Fil.) Ascherson & Graebner in Córdoba area.
Figure 8.
Geographical distribution of Arundo donax L. and Cortaderia selloana (Schultes & Schultes Fil.) Ascherson & Graebner in Córdoba area.
Figure 9.
Geographical distribution of Sorghum halepense (L.) Pers. in Córdoba municipality.
Figure 9.
Geographical distribution of Sorghum halepense (L.) Pers. in Córdoba municipality.
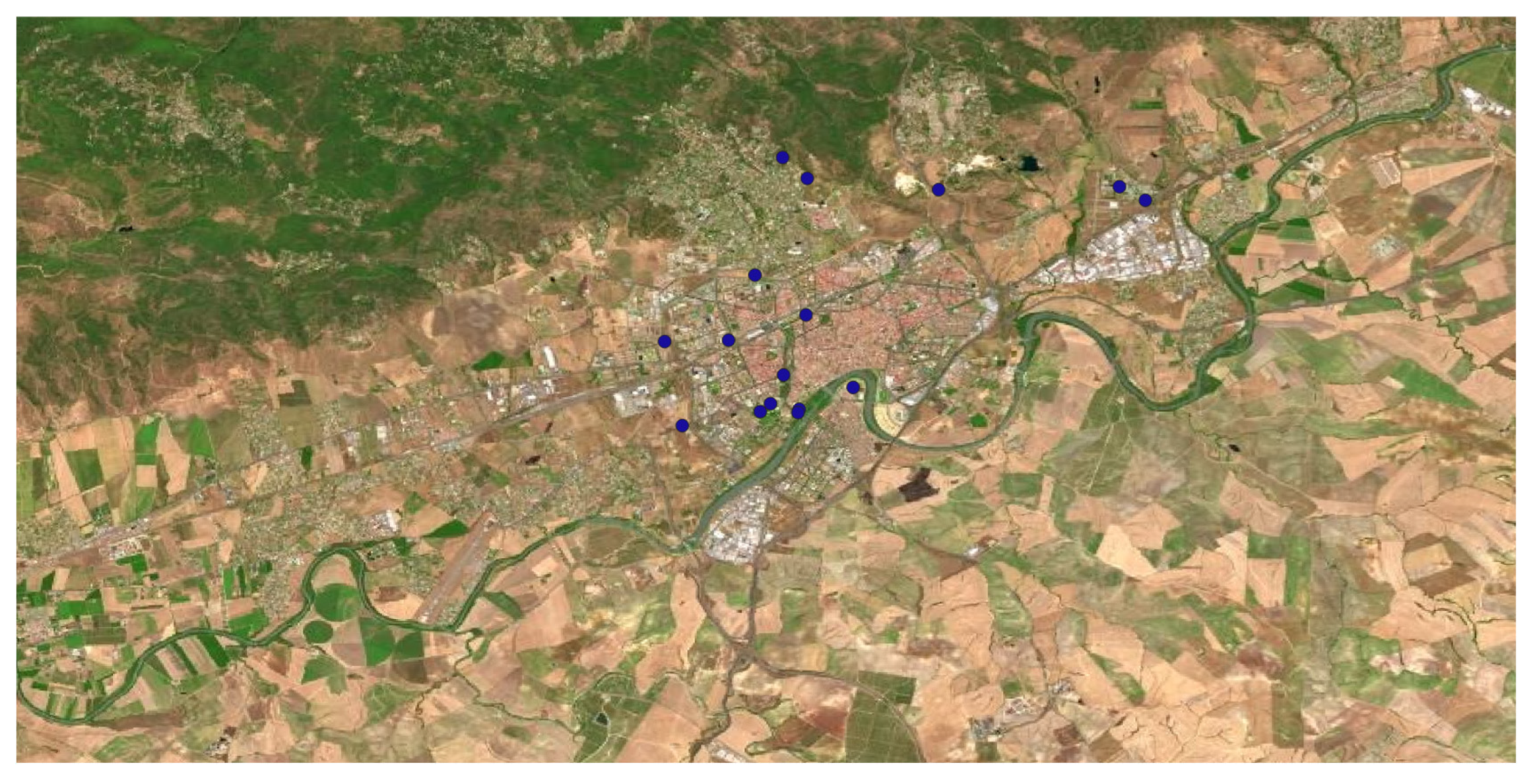
Figure 10.
Geographical distribution of Robinia pseudoacacia L. in Córdoba municipality.
Figure 10.
Geographical distribution of Robinia pseudoacacia L. in Córdoba municipality.
Figure 11.
Geographical distribution of Parkinsonia aculeata L in Córdoba area.
Figure 11.
Geographical distribution of Parkinsonia aculeata L in Córdoba area.
Figure 12.
Geographical distribution of Acacia dealbata Link and Gleditsia triacanthos L. in Córdoba municipality.
Figure 12.
Geographical distribution of Acacia dealbata Link and Gleditsia triacanthos L. in Córdoba municipality.
Figure 13.
Geographical distribution of Agave americana L. in Córdoba municipality.
Figure 13.
Geographical distribution of Agave americana L. in Córdoba municipality.
Figure 14.
Geographical distribution of Acer negundo L. in Córdoba.
Figure 14.
Geographical distribution of Acer negundo L. in Córdoba.
Figure 15.
Geographical distribution of Opuntia ficus-indica (L.) Miller in Córdoba area.
Figure 15.
Geographical distribution of Opuntia ficus-indica (L.) Miller in Córdoba area.
Figure 16.
Geographical distribution of Oxalis pes-caprae L. in Córdoba municipality.
Figure 16.
Geographical distribution of Oxalis pes-caprae L. in Córdoba municipality.
Figure 17.
Geographical distribution of Ricinus communis L.in Córdoba area.
Figure 17.
Geographical distribution of Ricinus communis L.in Córdoba area.
Figure 18.
Geographical distribution of Xanthium spinosum L. and Xanthium strumarium L. subsp. italicum (Moretti) D. Löve in Córdoba area.
Figure 18.
Geographical distribution of Xanthium spinosum L. and Xanthium strumarium L. subsp. italicum (Moretti) D. Löve in Córdoba area.
Figure 19.
Diagram of the biotypes (Raunkier life-forms) of the invasive plant species located in the study area.
Figure 19.
Diagram of the biotypes (Raunkier life-forms) of the invasive plant species located in the study area.
Figure 20.
Percentage of ornamental versus spontaneous plants observed in each alien species observed in the Córdoba area.
Figure 20.
Percentage of ornamental versus spontaneous plants observed in each alien species observed in the Córdoba area.
Figure 21.
Distribution in different environments of the alien species growing spontaneously in the study area.
Figure 21.
Distribution in different environments of the alien species growing spontaneously in the study area.
Table 1.
List of the located invasive alien species in the municipality of Córdoba. It is indicated the species name, the total sampled locations where each species it was found (Nº Loc.), the degree of ornamental use or naturalized way of life, the origin area, the stratum (tree, weed, shrub), the Raunkiaer plant life-form, if the alien species in included in the Spanish Catalogue of Invasive Alien Species (SCIAS) and the coordinates of the most important locations where the species was found.
Table 1.
List of the located invasive alien species in the municipality of Córdoba. It is indicated the species name, the total sampled locations where each species it was found (Nº Loc.), the degree of ornamental use or naturalized way of life, the origin area, the stratum (tree, weed, shrub), the Raunkiaer plant life-form, if the alien species in included in the Spanish Catalogue of Invasive Alien Species (SCIAS) and the coordinates of the most important locations where the species was found.
| Species |
Family |
Nº
Populations |
% Ornamental use |
%
Naturalized |
Origin |
Stratum |
Life-form |
SCIAS |
Locations’ Coordinates |
|
Acacia dealbata Link. |
Fabaceae |
5 |
100 |
0 |
Oceania |
Tree |
Phanerophyte |
YES |
37°54′‘53.6” N, 4°43′‘32.5” W 37°54′‘53.6” N, 4°48′‘19.5” W 37°54′‘54.4” N, 4°48′‘05.7” W 37°54′‘15.8” N, 4°48′‘20.8”W 37°51′‘05.4” N, 4°52′‘19.0” W |
|
Acer negundo L. |
Sapindaceae
|
9 |
88.9 |
11.1 |
America |
Tree |
Phanerophyte |
NO |
37°54′‘49.2”N, 4°43′‘03.9”W 37°52′‘52.0”N, 4°45′‘29.7”W 37°52′‘34.3”N; 4°45′‘58.0”W 37°53′34.2”N, 4°45′37.1”W 37°53′27.3”N, 4°46′57.8”W 37°52′39.4”N, 4°47′50.2”W 37°52′59.6”N, 4°47′28.4”W 37°52′15.3”N, 4°47′24.9”W 37°52′17.1”N; 4°47′02.7”W |
|
Agave americana L. |
Asparagaceae |
19 |
84.2 |
15.8 |
America |
Shrub |
Phanerophyte |
YES |
37°54′09.7” N, 4°46′57.6” W 37°56′00.2” N, 4º48′17.3” W 37°55′05.3” N, 4°46′42.6” W 37°54′56.4” N, 4° 45′20.5”W 37°55′10.3”N 4°47′09.8”W |
|
Ailanthus altissima (Mill.) Swingle |
Simaroubaceae
|
49 |
8.2 |
91.8 |
Asia |
Tree |
Phanerophyte |
YES |
37°52′39.6 “N 4°48′02.8” W 37°52′25.1”N 4°47′24.1 “W 37°53′27.5” N 4°46′57.0 “W 37°53′27.5” N 4°46′57.0 “W 37°54′54.2 “N 4°46′23.6” W 37°52′47.3 “N 4°46′32.7” W 37°53′17.7 “N 4°45′40.6” W 37°53′46.4 “N 4°44′42.4” W 37°51′36.3 “N 4°46′16.6” W 37°52′23.5”N 4°47′03.7”W 37°54′43.6”N 4°48′32.8”W |
|
Arundo donax L. |
Poaceae |
16 |
0 |
100 |
Asia |
Weed |
Geophyte |
YES |
37°51′56.0” N 4°49′05.7”W 37°53′59.9”N 4°45′07.0”W 37°55′05.4”N 4°46′19.3”W 37°52′35.1”N 4°46′36.5”W |
|
Cortaderia selloana (Schultes & Schultes Fil.) |
Poaceae |
2 |
50 |
50 |
America |
Weed |
Camephyte |
YES |
37°54′43.2 “N 4 ° 45′28.8” W 37º55′44.6 “N 4 ° 45′33.4” W |
Eucalyptus globulus Labill
|
Myrtaceae |
21 |
15.4 |
84,6 |
Oceania |
Tree |
Phanerophyte |
NO |
37°54′38.0” N 4°47′08.0” W 37°54′57.4” N 4°48′25.7” W 37°55′01.3” N 4°48′31.0” W 37°54′33.8” N 4°43′47.4” W |
Eucalyptus camaldulensis Dehnh
|
Myrtaceae |
18
|
10.3 |
89.7 |
Oceania |
Tree |
Phanerophyte |
NO |
37°50′11.4” N 4°50′47.7” W 37°54′58.2” N 4°45′18.7” W |
|
Gleditsia triacanthos L. |
Fabaceae |
7 |
71.4 |
28.6 |
America |
Tree |
Phanerophyte |
NO |
37°54′26.8”N 4°43′46.5”W 37°53′05.0”N 4°45′58.3”W 37°52′27.0”N 4°47′26.9”W 37°52′24.2”N 4°47′23.5”W 37°53′12.9”N 4°48′13.9”W 37°51′31.6”N 4°52′17.9”W 37°54′51.0”N 4°45′20.6”W
|
|
Opuntia ficus–indica (L.) Miller |
Cactaceae |
8 |
100 |
0 |
America |
Shrub |
Phanerophyte |
NO |
37°51′20.1 “N, 4°52′33.8” W 37°51′32.1 “N, 4°52′16.1” W 37°50′53.4 “N 4°51′59.9” W 37°51′47.0 “N 4°47′11.0” W 37°54′53.2” N, 4°46′24.2” W 37°54′35.9” N, 4°44′11.0” W 37°54′36.4” N, 4°44′13.3” W 37°54′37.2” N, 4°44′15.3” W |
|
Oxalis pes-caprae L. |
Oxalidaceae
|
8 |
0 |
100 |
Africa |
Weed |
Geophyte |
YES |
37°55′38.5” N, 4°48′29.1” W 37°56′01.8” N, 4°48′21.0” W 37°55′35.2” N, 4°48′06.1” W 37°55′19.0” N, 4°47′49.4” W 37°54′28.9” N, 4°46′31.0” W 37°55′23.8” N, 4°47′08.7” W 37°56′26.2”N, 4°44′52.2”W 37°53′38.3”N 4°46′10.9”W |
|
Parkinsonia aculeata L. |
Fabaceae |
16 |
25 |
75 |
America |
Shrub |
Phanerophyte |
NO |
37°55′01.3”N, 4°46′55.7”W 37°53′10.6”N 4°48′32.2”W 37°52′47.0”N 4°47′11.7”W 37°53′27.9”N 4°46′56.1”W 37°54′46.1”N 4°43′04.8”W 37°54′53.5”N 4°45′25.7”W 37°52′23.4”N 4°47′00.6”W 37°55′15.6”N 4°47′12.6”W |
|
Ricinus communis L. |
Euphorbiaceae |
6 |
66.7 |
33.3 |
Africa |
Shrub |
Therophyte |
YES* |
37°53′11.9” N, 4°48′51.3” W 37°53′11.9” N, 4º48′51.3” W 37°52′50.8” N, 4°47′07.2” W 37°55′47.4” N, 4°45′37.7” W 37°52′26.7” N, 4°47′07.4” W 37°52′8.6” N, 4°47′06.5” W |
|
Robinia pseudoacacia L. |
Fabaceae |
23 |
95.6 |
4.4 |
America |
Tree |
Phanerophyte |
NO |
37°54′46.7” N, 4°43′17.5” W 37°54′35.7” N, 4°46′56.3” W 37°52′45.3” N, 4°45′47.5” W 37°53′38.7” N, 4°46′11.9” W |
|
Sorghum halepense (L.) Pers. |
Poaceae |
15 |
0 |
100 |
Africa |
Shrub |
Geophyte |
NO |
37°54′15.3”N 4°43′31.6”W 37°51′27.1”N 4°52′36.4”W 37°51′15.2”N 4°50′33.1”W 37°55′31.0”N 4°43′37.1”W |
|
Xanthium strumarium L. subsp. italicum (Moretti) D. Löve |
Asteraceae |
4 |
0 |
100 |
America |
Weed |
Therophyte |
NO |
37°51′27.2” N, 4°51′56.2” W 37°51′21.4” N, 4°51′40.2” W 37°54′06.2” N, 4°48′24.9” W 37°54′45.6” N, 4°44′35.6” W |
|
Xanthium spinosum L. |
Asteraceae |
1 |
0 |
100 |
America |
Weed |
Therophyte |
NO |
37°52′00.1” N, 4°47′12.4” W |