Appendix A
Power spectra for first- and second-order autoregressive models of swarming insects
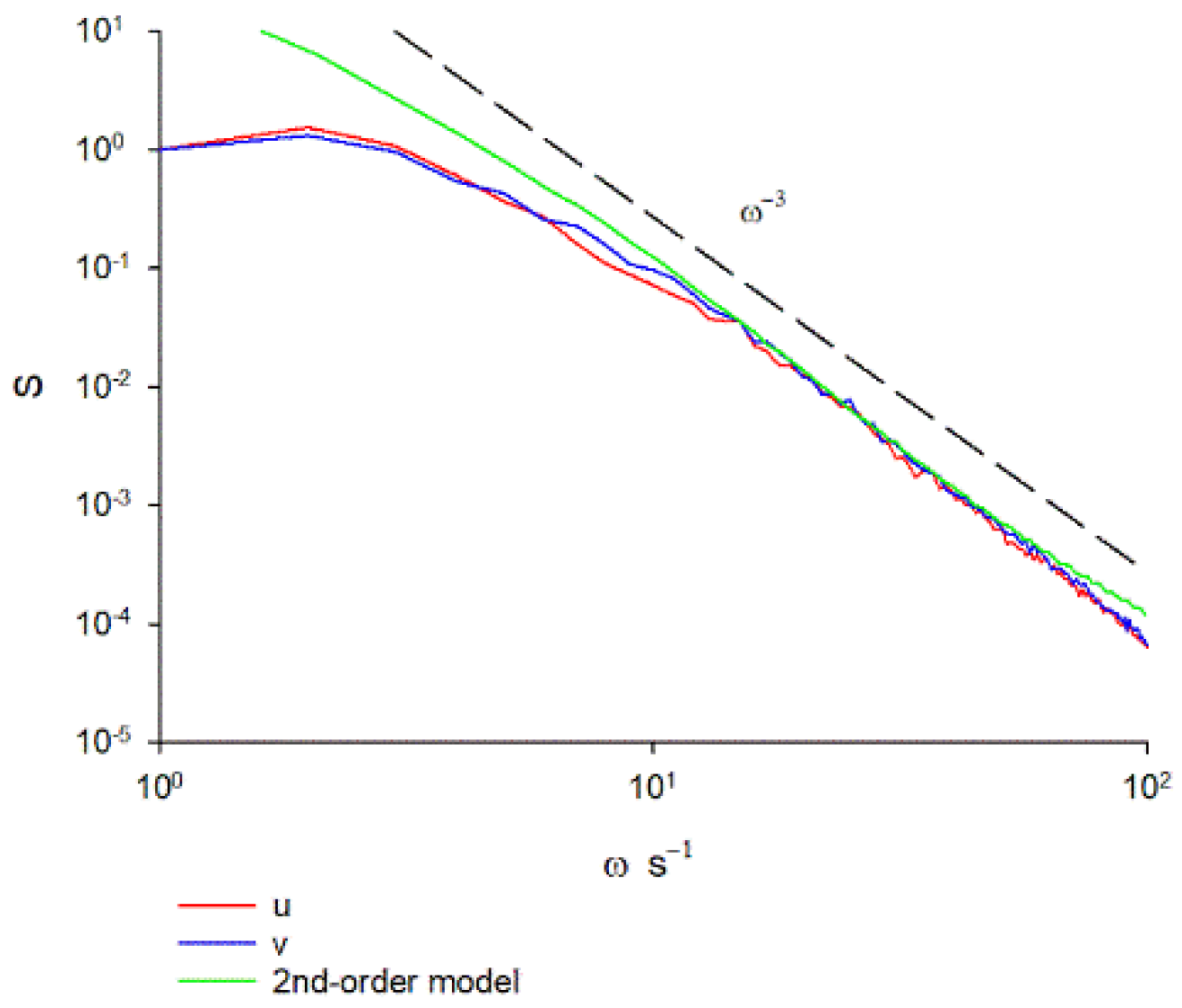
Here I show that the power spectra predicted by first- and second-order autoregressive models of the trajectories of swarming insects decrease respectively as
and
at high frequencies. That is, I show that confinement within a swarm does not upset the scaling behaviours obtained by Sawford [
19] for freely roaming trajectories.
First-order autoregressive models of swarming insects
Okubo [
1] classic one-dimensional model for the positions,
, and velocities,
, of swarming insects is given by
where
T is a velocity correlation timescale,
is the position variance,
is the velocity variance and
is an incremental Wiener process with correlation property
.
Okubo [
1] showed that according to this model the velocity autocorrelation function is given by
where
.
It follows that the velocity power spectra is given by
At sufficiently high frequencies .
Second-order autoregressive models of swarming insects
The simplest second-order one-dimensional model for the positions,
, velocities,
, and accelerations,
, of swarming insects is given by
where
is the acceleration variance and
is a timescale representative of the smallest scales of motion [
25].
It follows that for this model the velocity autocorrelation is a solution of the equation,
The simplest solutions are single exponentials
where the inverse timescales
are solutions of the equation,
Typically, one solution,
, is real whilst the other two,
, are a pair of complex conjugate pair. In this case the general solution to Equation (A5) therefore takes the form,
where
A and
B are weighting factors.
At sufficiently high frequencies
where
and
Because the complexity of the general analytic expressions for in terms of the model parameters, , , and precludes their application, here I present illustrative examples.
For
=
,
,
,
. The weighting factors
A and
B were found by minimizing the mean square difference between the predicted form of velocity autocorrelation, Equation (A7), and form obtained from simulation data obtained using Equation (A4) (
Figure A1). This gave
and
Consequently,
and
. The same scaling was obtained for
=
,
and for
=
,
.
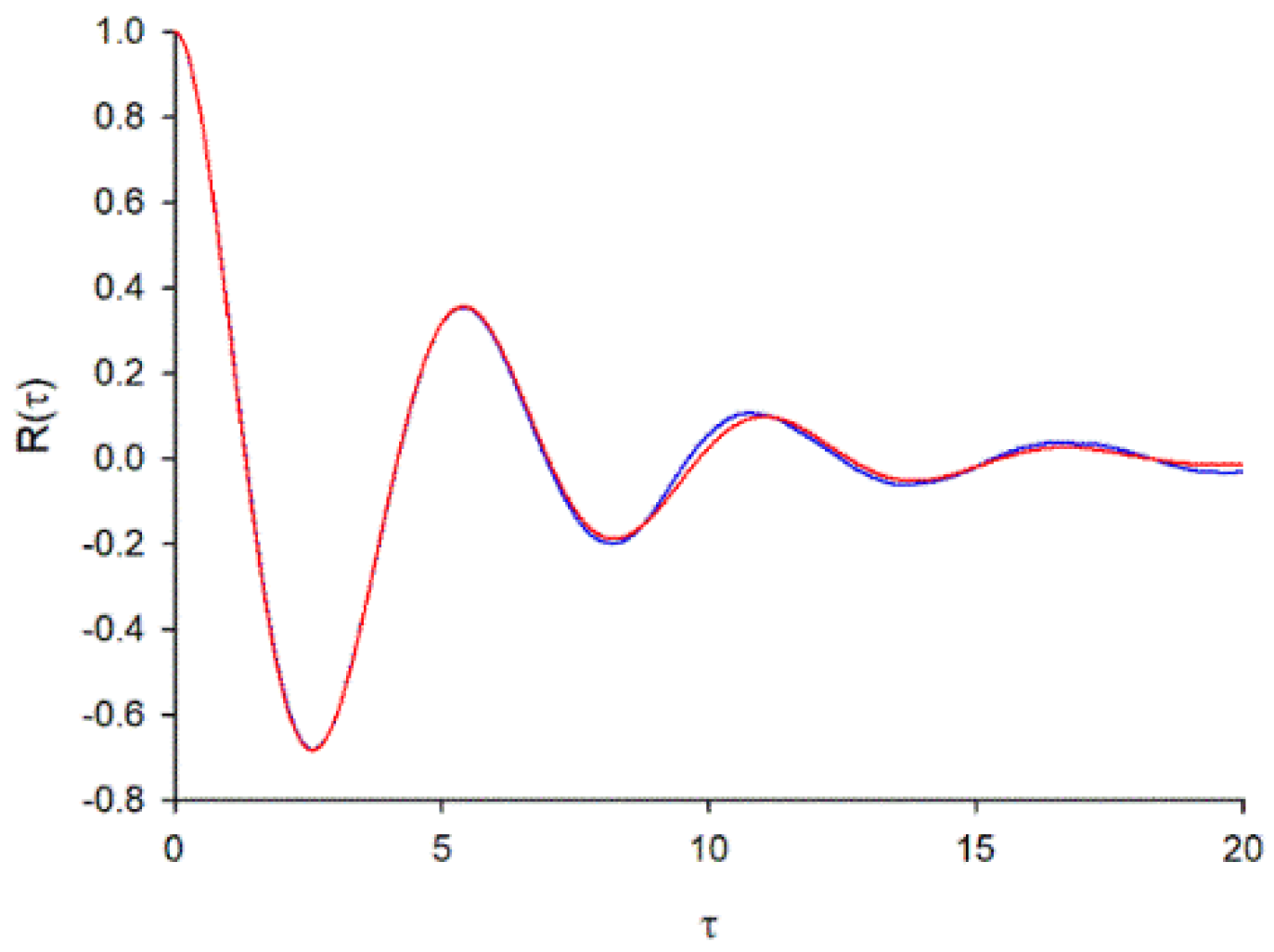
Figure A1.
Predicted form the velocity autocorrelation, Equation (A7), matches that obtained in numerical simulations. The trajectories of 10000 swarming insects were simulated by numerically integrating Equation (A4). Results are shown for = , a.u.
Figure A1.
Predicted form the velocity autocorrelation, Equation (A7), matches that obtained in numerical simulations. The trajectories of 10000 swarming insects were simulated by numerically integrating Equation (A4). Results are shown for = , a.u.
Appendix B
Jerks may destabilize small swarms
Reynolds [
26] showed that the positions of the non-biting midge
Chironomus riparius in laboratory swarms are maximally anticorrelated. In this case the average strength of the jerk experienced by the
ith midge is given by
where
are elements of the inverse of the normalized position covariance matrix, the subscripts denote different individuals, and where there is summation of over repeated indices. The average strength of the jerk experienced by the
ith midge therefore depends on the velocities of all individuals in the swarm, and so is unlikely to be aligned with the direction of travel (the major axis of the insect). If it were aligned, then the impact that jerks can have on flight dynamics would be minimized (see main text). This misalignment is, however, small in asymptotically large swarms which on average contain 10 or more individuals [
5]. This is because
and because
when
and
when
. Consequently,
Despite their disruptive influence in small swarms, the maximal positional anticorrelations appear to dictate the approach to the asymptotic state (
Appendix C).
Appendix C
Maximal anticorrelated positions and the asymptotic regime
Puckett and Ouellette [
27] reported that once swarms contain order 10 individuals, all statistics saturate, and that the swarms enter an asymptotic regime. Puckett and Ouellette [
27] also reported that the influence of the swarm marker (a visually prominent feature over which swarms nucleate) on the swarm morphology decays on a similar scale. Their results provide a strong constraint on how rapidly swarm models must produce collective states. Here I show that the observations of Puckett and Ouellette [
27] together with the occurrence of maximal anticorrelated positions [
26] are consistent with each individual in the swarm being on average located at a position that is mirror opposite to the average position of all other individuals, so that the average position of the
ith individual in a swarm containing
N individuals is given by
. With this specification the least biased (maximum entropy) choice for the distribution of positions is the multivariant Gaussian
where
is a measure of the mean square size of the swarm. This can be rewritten as
where
if
and
if
, and where there is summation of repeated subscripts. The distribution, Equation (A11), is realizable (
is positive definite) when
.
Normalized positional covariances are, to good approximation, given by if
and if , which is indicative of maximal anticorrelated positions.
If the volume of the swarm,
, is proportional to the population size,
N, of the swarm then the volume per individual is predicted to saturate when swarms contain order 10 individuals, as observed by Puckett and Ouellette [
27]. Puckett and Ouellette [
27] found that the approach to the asymptotic state can be accurately represented by a decaying exponential function of the form
where the characteristic scale
quantifies the rate of approach with increasing swarm size. Like the observations, model predictions are found to be accurately represented by the decaying exponential function albeit with
.But in the presence of a swarm marker of size
(a.u.), the modelling predicts that
(
Figure A2). In such cases the distribution of positions becomes
Puckett and Ouellette [
27] also reported on a related though distinct measure of how the midges arrange themselves in space, namely the average distance from an individual to its nearest neighbour. Puckett and Ouellette [
27] found that like the volume per individual, the nearest-neighbour distance falls off rapidly with swarm size for small swarms, but eventually saturates. This behaviour is predicted by stochastic models of the 3-dimensional trajectories of swarming insects which by construction are exactly consistent with the multivariant distributions of positions, Equation (11). As observed [
27] the characteristic scale,
, is larger than for the volume per individual but is less than the observed characteristic scale,
. But in the presence of a swarm marker of size
(a.u.) the modelling predicts that
(
Figure A2).
Finally, the stochastic modelling predicts that the anticorrelation of spatial positions results in correlated accelerations, as evidenced in an analysis of pre-existing data (
Figure A3). Such correlations have until now gone unnoticed, despite numerous attempts to uncover order in the dynamics of swarming insects [
2,
3,
15,
28,
29].
This analysis leaves open the question as to how the velocity statistics approach the asymptotic regime.
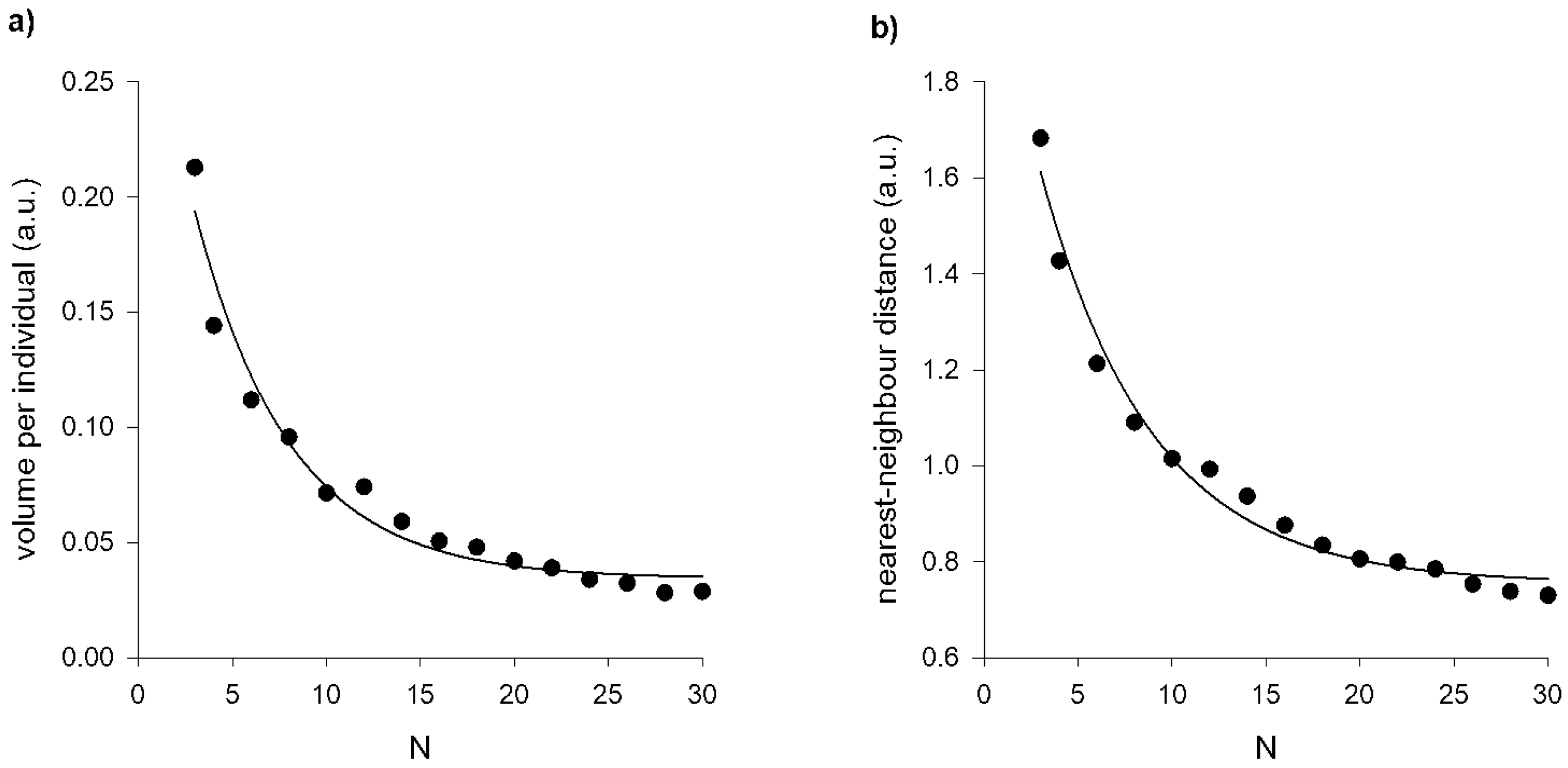
Figure A2.
a) Predicted volume per individual as a function of the number of individuals in the swarm. The solid line is an exponential fit,
with
. Puckett and Ouellette [
27] reported that
.
b) Predicted average distance from a given individual to its nearest neighbour as a function of the number of individuals in the swarm. The solid line is an exponential fit,
with
. Puckett and Ouellette [
27] reported that
.
Figure A2.
a) Predicted volume per individual as a function of the number of individuals in the swarm. The solid line is an exponential fit,
with
. Puckett and Ouellette [
27] reported that
.
b) Predicted average distance from a given individual to its nearest neighbour as a function of the number of individuals in the swarm. The solid line is an exponential fit,
with
. Puckett and Ouellette [
27] reported that
.
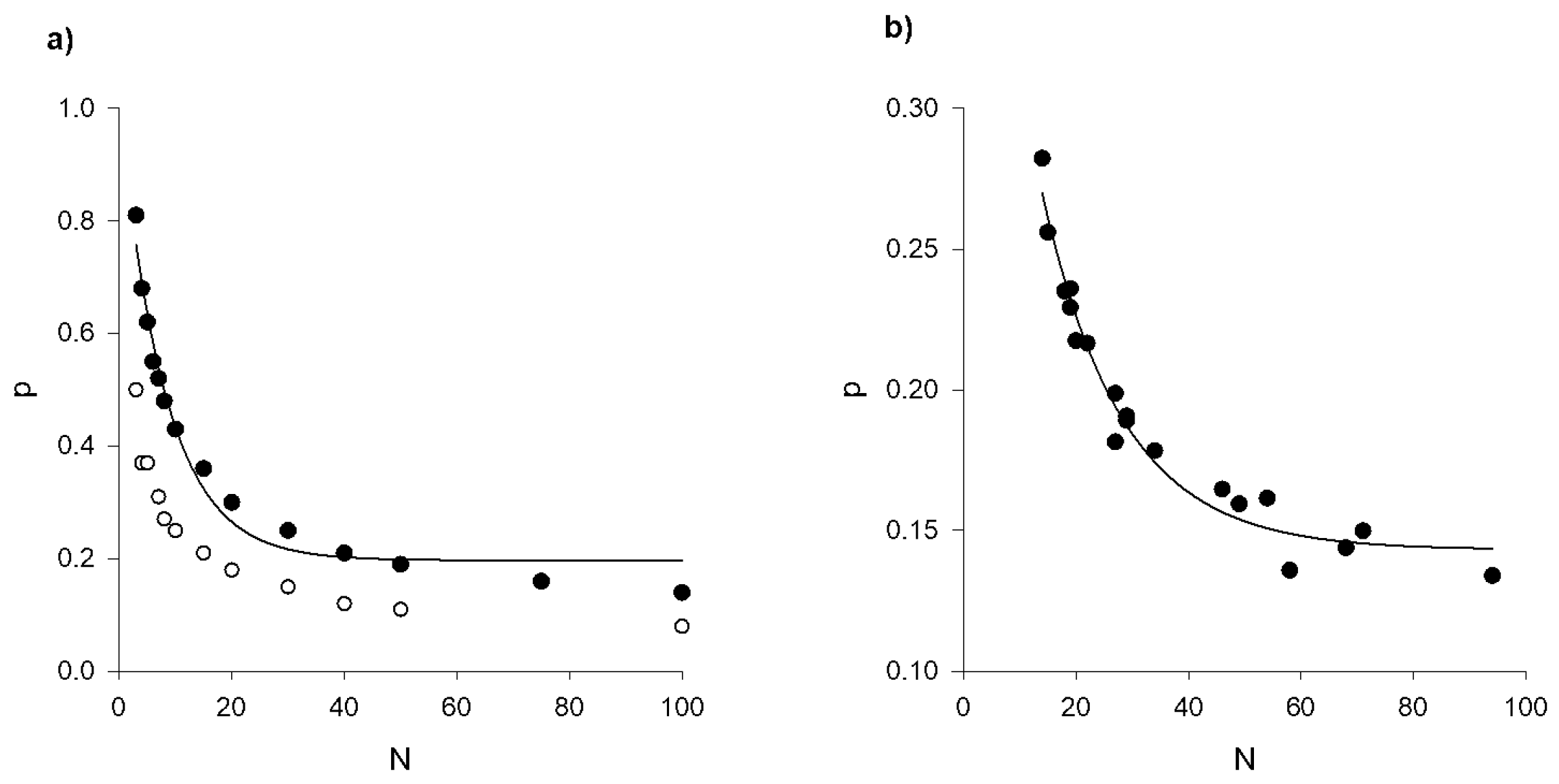
Figure A3.
a) Predicted and b) observed net polarizations of acceleration as a function of the number individuals in the swarm (●). The polarization
where
is the acceleration of the
ith individual and where the angular brackets denote a time average. Predictions were obtained using the stochastic model of Reynolds [
26]. The experimental data is taken from Sinhuber et al. [
24]. The solid lines are exponential fits,
with a)
and b)
. The average polarizations are significantly larger than the average polarizations that are predicted to arise sporadically in swarms of individuals with uncorrelated positions (o).
Figure A3.
a) Predicted and b) observed net polarizations of acceleration as a function of the number individuals in the swarm (●). The polarization
where
is the acceleration of the
ith individual and where the angular brackets denote a time average. Predictions were obtained using the stochastic model of Reynolds [
26]. The experimental data is taken from Sinhuber et al. [
24]. The solid lines are exponential fits,
with a)
and b)
. The average polarizations are significantly larger than the average polarizations that are predicted to arise sporadically in swarms of individuals with uncorrelated positions (o).