1. Introduction
Everolimus is an oral inhibitor of the mammalian target of rapamycin (mTOR) with anti-cancer activity in multiple tumor types, including well-differentiated neuroendocrine neoplasms, named neuroendocrine tumors (NETs). Molecularly, well-differentiated NETs often depend on the activation of the mTOR pathway and angiogenesis [
1,
2,
3]. Everolimus is an effective therapy in second or further lines for patients with gastrointestinal (GI), lung, or pancreatic NETs. As compared with placebo, everolimus significantly prolonged median progression-free survival (PFS) times in patients with progressive advanced pancreatic NETs or GI/lung NETs in monotherapy, and, by investigator assessment (but not by central evaluation) in combination with octreotide in advanced functioning GI or lung NETs [
4,
5,
6].
Everolimus, at its approved dose of 10 mg once daily, can cause clinically relevant drug-related adverse events in approximately half of patients. Among the most common toxicities, of any degree, are stomatitis (65%) and skin rash (50%), and approximately one-third of patients experience infections, diarrhea, nausea, and fatigue.[
4,
5,
6] Data from randomized clinical trials suggest that grade 3 or higher adverse events consist of stomatitis (9%), diarrhea (7%), infections (7%), anemia (6%), hyperglycemia (5%), and fatigue (3%) [
4,
5]. Studies of real-world patients have reported even higher rates of severe toxicities, with all-grade everolimus-induced pneumonitis occurring in nearly 20% of patients and grade 3-4 in about 8% of cases [
7,
8,
9]. In a retrospective multicenter study from our group, 22% of patients had grade 3-4 infections, with 6% being opportunistic infections, and 3.6% related deaths [
10]. Such higher incidence of severe toxicity among our real-world patients likely reflects a more comorbid and fragile population and a longer follow up for the onset of adverse events, when compared with phase III trials (nearly the double, from 17 to 33 months) [
10].
In phase 1 clinical trials, everolimus 5 mg orally per day efficiently inhibited mTOR and offered better tolerance. A phase I study with everolimus evaluated oral doses of 5 and 10 mg/day and intravenous doses of 10 to 30 mg/week in patients with refractory solid tumors [
11]. After evaluating pharmacokinetic and pharmacodynamic effects (phosphorylation of S6 kinase, an efferent protein of the mTOR pathway) in peripheral mononuclear cells, the authors recommended oral doses starting from 5 mg/day or from 20 mg/week for future studies. Another phase I trial evaluated everolimus plasma levels and performed pre- and on-treatment tumor and skin biopsies to immunohistochemically analyze the expression of mTOR-related proteins with different dose schedules. Although a dose-dependent inhibition of the mTOR pathway was found, grade 3 limiting toxicity occurred in five patients due to stomatitis (10 mg/day cohort) and in four patients who received 70 mg/week intravenously. No grade 3 toxicity occurred with the dose of 5 mg/day [
12].
Anticipating toxicities and consistent with routine clinical practice, phase III trials usually anticipated two dose reductions from 10 mg to 5 mg per day and, subsequently, to 5 mg every other day. In addition, treatment interruption for a maximum of 4 weeks was allowed to manage adverse events. Even so, everolimus-related adverse events resulted in treatment discontinuation rates in phase III trials ranging from 12% to 24% [
4,
5,
6].
Because the approved dose of everolimus 10 mg daily leads to significant toxicity and everolimus 5 mg daily efficiently inhibited mTOR in phase I trials, we conducted a multicenter retrospective study to evaluate the efficacy of lower doses of everolimus in patients with advanced NETs.
2. Materials and Methods
Consecutive patients with a NET diagnosis and previous use of everolimus were identified retrospectively through medical records at A.C.Camargo Cancer Center (São Paulo, Brazil) and Hospital Moinhos de Vento (Porto Alegre, Brazil). The protocol received approval from the local ethics committee by each participant institution. Informed signed consent from individual patients was not required.
Eligible patients were 18 years or older with histologically confirmed locally advanced or metastatic unresectable NET of gastroenteropancreatic, lung, or unknown origins, well-differentiated histology of any grade according to the 2019 World Health Organization (WHO) classification, had measurable disease by the Response Evaluation Criteria in Solid Tumours (RECIST) v1.1, radiologically progressive tumors, and received at least one dose of everolimus [
13,
14]. Mixed neuroendocrine non-neuroendocrine neoplasms were excluded. Patients with functioning NETs were allowed to have received somatostatin analogs concurrently.
Clinical data were collected from electronic medical charts. The best overall response to everolimus was defined as the best response recorded from the start until the end of everolimus use as documented in medical charts.
The primary endpoint was time to treatment failure (TTF) in patients who received a mean daily dose of 7 – 10 mg (higher dose [HD]) or 6 mg (lower dose [LD]) of everolimus. Considering that many patients who start at 10 mg daily will require dose reductions, we stratified dose groups according to the mean dose administered for each patient throughout the treatment duration – instead of categorizing patients based on the starting dose. TTF was defined as the duration of everolimus treatment, from the first day of the first cycle (C1D1) until tumor progression (as defined by the treating oncologist in medical charts), treatment change for toxicity/intolerance, or death by any cause. TTF was compared between the dose groups (LD vs. HD) and adjusted for prognostic variables (age at C1D1 of everolimus, tumor grade, and line of everolimus. Overall survival (OS), a secondary endpoint, was calculated from C1D1 of everolimus to the date of death or last follow-up. Cox regression proportional hazard multivariable analyses for TTF and OS were performed to adjust for the above prognostic variables.
Dose reductions were decided by the treating physician, and they could be upfront (for frailty, older age) or during treatment due to toxicities. The evaluation of toxicities associated with everolimus in real-word settings was not the aim of this study, as this was already reported by our group and others [
7,
8,
10].
Descriptive statistics were used to report means, medians (range), and frequencies. The Chi2 nonparametric test was used to compare categorical variables, and the Mann-Whitney U test was used to compare medians. The Kaplan– Meier method was used to estimate all time-to-event data, and differences in survival (TTF, OS) times were evaluated using the log-rank test. Median survival and median follow-up time were summarised. Multivariable analyses were conducted using Cox-regression models, which were used to adjust the dependent variables of TTF and OS for the prognostic effects of age (continuous variable), tumor grade (1 and 2 vs. 3), and line of everolimus (1 or 2 vs. 3). Two-sided p values < 0.05 were considered significant. All statistical analyses were performed using the STATA IC/16.0 software (StataCorp, College Station, TX, USA).
3. Results
From August 2011 to September 2023, 92 patients were included: 74 (80%) received a mean daily of 10 mg (6.8 – 10) and were classified as the HD group, and 18 (20%) received a median of 5.1 mg (4.7 – 6), being classified as the LD group. Because of toxicities, 12 (16%) patients in the HD and 10 (55%) patients in the LD group required dose reductions during treatment (not upfront). Overall, eight (9%) patients started everolimus 5 mg because of older age and/or frailty, and among these, one patient required further reduction to 5 mg every other day.
Treatment discontinuation was observed in 19 (25.7%) patients in the HD group: 16 because of severe toxicities, one case due to logistic issues from the health care provider, one patient stopped everolimus because he wished so, and one stopped therapy after surgical intervention. Among 16 patients who discontinued treatment because of adverse events, five had grade 3/4 infections, four had grade 3 pneumonitis, one had grade 3 stomatitis, one had both grade 2 stomatitis and persistent grade 2 diarrhea, three had grade 3 skin rash, with one also presenting grade 2 diarrhea, one had severe hepatic steatosis with grade 3 transaminases elevation and grade 3 dyslipidemia, one had grade 3 anemia/thrombocytopenia. Four (22.3%) patients in the LD group stopped everolimus because of toxicities: one because of stomatitis and three because of myelotoxicity. There were no toxic-related deaths.
Both dose groups had similar characteristics at baseline, except for more grade 3 tumors and older median age in the LD group (
Table 1).
Abbreviations: ECOG, Eastern Cooperative Oncology Group; GI, gastrointestinal; HD, high-dose; LD, low-dose. & Mann Whitney U test; all other comparisons, Chi2. * Percentages might not add up to 100 because of rounding.
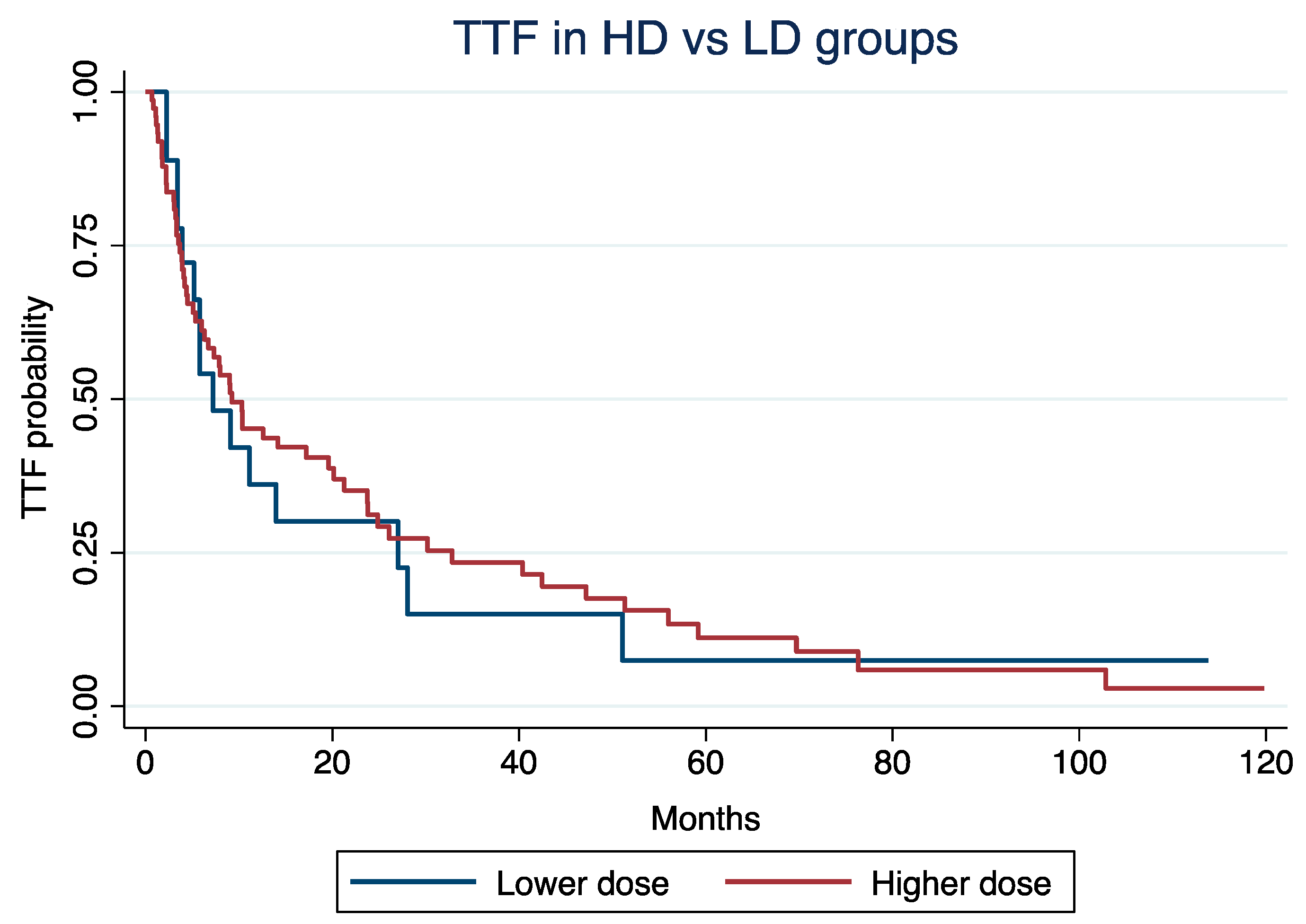
At a median follow-up time of 4.2 years, the median TTF was 9.2 months (Interquartile Range [IQR]: 3.7 – 32) for patients in HD and 7.2 months (IQR: 3.9 – 27) for those in LD groups, respectively (log-rank p = 0.85;
Figure 1).
TTF was not significantly different according to LD vs. HD (Hazard Ratio [HR]: 1.24, 95% Confidence Interval [CI]: 0.68 - 2.25; p = 0.47), even after adjustment for age at C1D1 of everolimus (HR: 1.02; 95% CI: 1.01 - 1.04; p = 0.002), NET grade (grade 3 vs. 1/2; HR: 1.27, 95% CI: 0.95 - 1.71; p = 0.11), or treatment line (3rd or more vs. 1st or 2nd ; HR: 1.55, 95% CI: 0.92 – 2.62; p = 0.09).
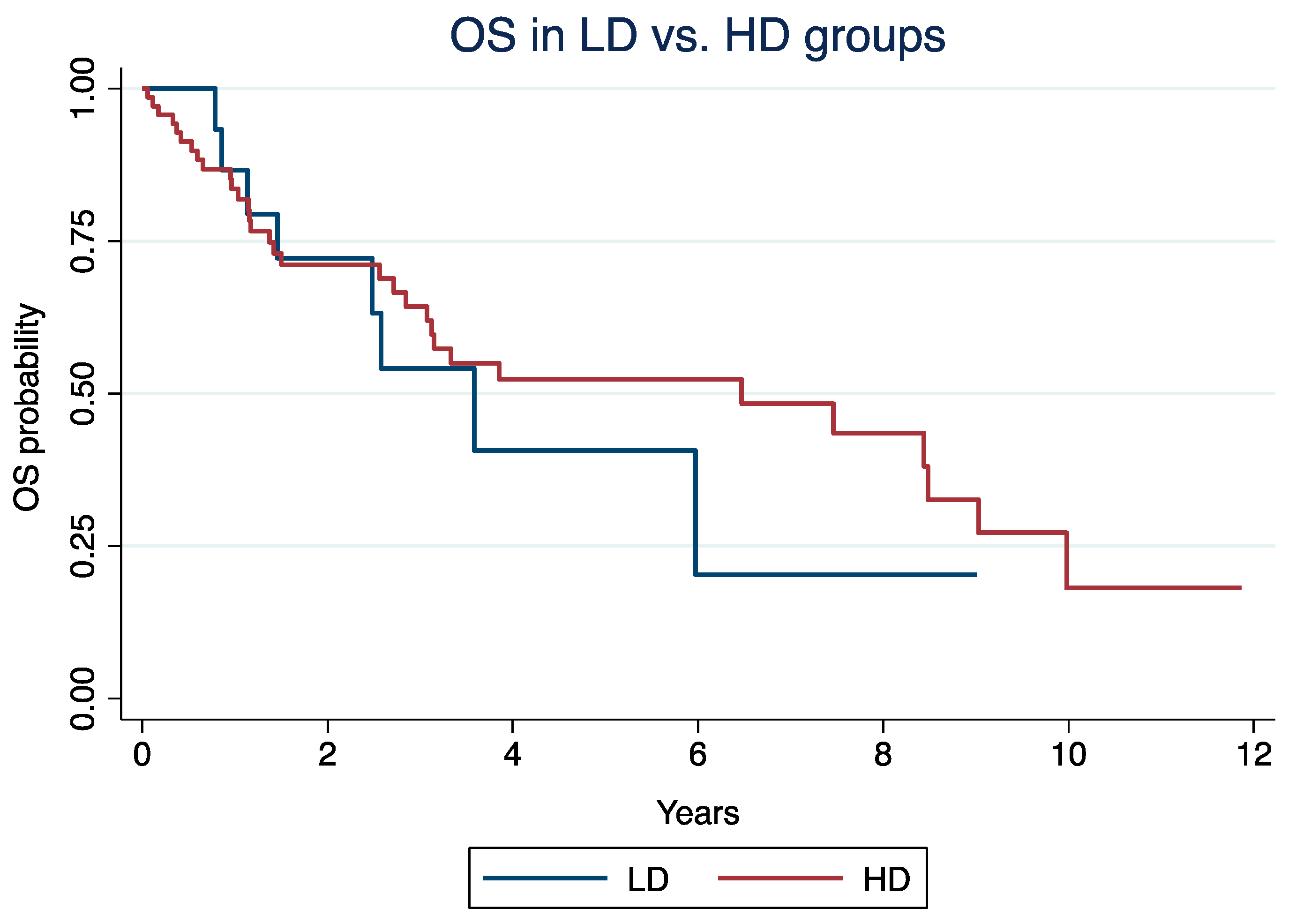
In the LD vs. HD groups, the median OS was 3.6 years (IQR: 1.4 – 6) and 6.5 years (IQR: 1.37 - 9.98; log-rank p = 0.57), respectively. In the Cox model, older age (HR: 1.03, 95% CI: 1.01 - 1.05; p = 0.007), grade 3 NET (HR: 1.68, 95% CI: 1.15 – 2.47; p = 0.008), and everolimus in 3rd or more lines (HR: 2.1; 95% CI: 1.05 - 4.13; p = 0.036), but not everolimus mean daily dose (HR: 1.32, 95% CI: 0.56 – 3.13; p = 0.53), were independently associated with OS.
4. Discussion
This multicenter retrospective study showed no significant differences in TTF or OS according to LD or HD of everolimus in patients with advanced and progressive NETs. This potentially offers advantages in terms of reduced toxicity and lower treatment-related costs.
Given the significant side effects associated with everolimus 10 mg daily, including severe adverse events experienced by a notable proportion of patients in both clinical trials and real-world settings, the prospect of using a lower dose is particularly appealing. Reducing adverse events enhances patients’ quality of life and improves treatment adherence, as patients are often more willing to continue therapies that they tolerate well. Additionally, lower drug costs increase the accessibility of treatments, which is especially relevant when there are limited financial resources for health care, such as in developing and underdeveloped countries. Fewer costs from lower doses of everolimus include the reduced cost from the drug itself (e.g., Brazil, where everolimus price is linear with dosage) and those associated with health care utilization (e.g., hospitalizations, laboratory/imaging tests to diagnose and monitor adverse events, and medications to treat them).
The ideal dose of everolimus for each patient remains to be defined, and it certainly involves pharmacokinetics parameters such as nutrition status, renal and hepatic functions. In that sense, pharmacokinetic studies could guide the appropriate dose levels to avoid undesirable adverse events while offering an effective dose. A prospective cohort study in Japanese patients with renal cell carcinoma observed that patients who developed adverse events had blood concentrations of everolimus after eight days of treatment twice as high as those who did not experience toxicities [
15]. In a pivotal phase I clinical trial of everolimus in solid tumors patients, toxicities were more frequent in the 10 mg daily group when compared with patients who received 5mg daily, with thrombocytopenia, hyperglycemia and diarrhea occurring only in the 10 mg cohort [
11]. Associations between higher blood level concentrations of everolimus and toxicity have also been reported in studies with thyroid, breast cancer, and mixed solid tumors patients, but not exclusively in patients with NETs. Patients with NETs likely represent a unique population in terms of pharmacokinetic parameters when compared with other tumor types as they often present good renal function (unlike patients with renal cell carcinoma), and more commonly have extensive liver metastases (unlike hormone-positive breast cancer patients) [
16,
17]. Nevertheless, it is improbable that therapeutic drug monitoring will be investigated in patients with NETs, that being a less common neoplasia and because everolimus is wordly available as a generic drug. Therefore, studies such ours are important to help clinicians evaluate the appropriateness of lower doses of everolimus for NET patients.
Another relevant aspect is how lower doses of everolimus could negatively impact on disease control. Our study did not find significant differences in TTF or OS according to dose groups, although a numerical gain was observed for the HD group for both outcomes. Yet such differences were lost when we adjusted the analyses for known prognostic factors such as age, tumor grade, and line of therapy. A recent retrospective single-center study which evaluated the efficacy and toxicity of everolimus in 52 NET patients also observed an inferior numerical, albeit nonsignificant, median progression free survival (7.5 months versus 12.4 months) among patients who started 5 mg daily versus those who initiated 10 mg daily [
18]. However, the authors did not adjust the progression free survival to other prognostic factors, and it was not clear how the authors analyzed the patients who required dose reductions from 10 mg daily.
The limitations of this study are primarily associated with its retrospective design, which inherently carries the risk of bias, including selection bias and confounding factors that may have influenced treatment outcomes. Markedly, the main limitation of our study relies on the reasons for patients to start everolimus at 5 mg daily: older age and frailty. Such observation explains the inferior OS of the LD group in the unadjusted analysis. Our LD group was represented by 55% of patients who used a lower dose because they needed dose reduction from 10 mg due to adverse events, and 45% used lower dose upfront due to frailty/comorbidities. Therefore, the LD inherently had a worse prognosis. We tried to cumbersome this selection bias by adjusting TTF and OS for age, treatment line, and NET grade. Yet, unmeasured variables could have impacted our results. Additionally, TTF, unlike progression-free survival, is not a standard endpoint to evaluate cancer-directed therapies in solid tumors. But we chose TTF as our primary endpoint because it represents a combined measure of treatment effectiveness when it considers both disease control and patient ability to tolerate treatment; additionally, it is difficult to properly estimate progression-free survival in a retrospective study because of the variability of intervals of imaging tests and difficulties to objectively measure radiological tumor response and progression. Lastly, our sample size of 92 patients may have contributed to our inability to detect small significant differences in outcomes between HD and LD groups.
Based on the present findings, we think that older and frailer patients should start everolimus at 5 mg daily. For fit patients, clinicians should be more flexible with dose intensity, starting treatment with 10 mg daily but reducing to 5 mg daily at the onset of grade 2 adverse events. In these cases, we do not think clinicians should maintain 10 mg daily until a grade 3 or 4 occurs. Yet, our results are hypothesis-generating and set the rationale to investigate the antitumor effects of LD of everolimus in a controlled study. Indeed, we have launched the EVENET trial, a near-equivalence randomized phase II trial, which tests the efficacy of 10 mg daily versus 5 mg daily of everolimus in patients with advanced and pre-treated grade 1 or 2 gastroenteropancreatic or lung NETs (NCT06472388). In this trial, the primary endpoint is the progression-free survival rate at 12 months.
In conclusion, this study suggests that everolimus 5 mg daily offers similar efficacy to 10 mg daily in patients with advanced NETs, while it likely decreases toxicity and possibly treatment-related costs. Our study provides insights into the prospective validation of a strategy that could effectively treat patients with NETs with reduced toxicity, lower costs, and increase drug access to patients world-wide.
Author Contributions
Conceptualization and supervision, R.R.; methodology, R.R., R.T.; clinical data collection, R.T., A.B., A.N.S., and R.W..; statistical analysis, R.R. and R.T.; writing—original draft preparation, R.R. and R.T.; writing—review and editing, R.T., A.B., A.N.S., R.W., and R.R. All authors have read and agreed to the published version of the manuscript.
Funding
This study was not supported by any funding.
Institutional Review Board Statement
The study received local ethic committee approval by each participant Institution.
Informed Consent Statement
Informed signed consent from individual patients was not required.
Data Availability Statement
The data are available upon reasonable request.
Conflicts of Interest
Rodrigo Taboada declares travel and educational support from IPSEN. Rachel P. Riechelmann has received honoraria for lectures and advisory boards from IPSEN. All the other authors declared no conflict of interest related to the submitted work.
References
- Jiao, Y.; Shi, C.; Edil, B.H.; de Wilde, R.F.; Klimstra, D.S.; Maitra, A.; Schulick, R.D.; Tang, L.H.; Wolfgang, C.L.; Choti, M.A.; et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science 2011, 331, 1199–1203. [Google Scholar] [CrossRef] [PubMed]
- Scarpa, A.; Chang, D.K.; Nones, K.; Corbo, V.; Patch, A.M.; Bailey, P.; Lawlor, R.T.; Johns, A.L.; Miller, D.K.; Mafficini, A.; et al. Whole-genome landscape of pancreatic neuroendocrine tumours. Nature 2017, 543, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Puccini, A.; Poorman, K.; Salem, M.E.; Soldato, D.; Seeber, A.; Goldberg, R.M.; Shields, A.F.; Xiu, J.; Battaglin, F.; Berger, M.D.; et al. Comprehensive Genomic Profiling of Gastroenteropancreatic Neuroendocrine Neoplasms (GEP-NENs). Clin Cancer Res 2020, 26, 5943–5951. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Shah, M.H.; Ito, T.; Bohas, C.L.; Wolin, E.M.; Van Cutsem, E.; Hobday, T.J.; Okusaka, T.; Capdevila, J.; de Vries, E.G.; et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med 2011, 364, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.C.; Fazio, N.; Singh, S.; Buzzoni, R.; Carnaghi, C.; Wolin, E.; Tomasek, J.; Raderer, M.; Lahner, H.; Voi, M.; et al. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016, 387, 968–977. [Google Scholar] [CrossRef] [PubMed]
- Pavel, M.E.; Hainsworth, J.D.; Baudin, E.; Peeters, M.; Horsch, D.; Winkler, R.E.; Klimovsky, J.; Lebwohl, D.; Jehl, V.; Wolin, E.M.; et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011, 378, 2005–2012. [Google Scholar] [CrossRef] [PubMed]
- Taboada, R.G.; Riechelmann, R.P.; Mauro, C.; Barros, M.; Hubner, R.A.; McNamara, M.G.; Lamarca, A.; Valle, J.W. Everolimus-Induced Pneumonitis in Patients with Neuroendocrine Neoplasms: Real-World Study on Risk Factors and Outcomes. Oncologist 2022, 27, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Panzuto, F.; Rinzivillo, M.; Fazio, N.; de Braud, F.; Luppi, G.; Zatelli, M.C.; Lugli, F.; Tomassetti, P.; Riccardi, F.; Nuzzo, C.; et al. Real-world study of everolimus in advanced progressive neuroendocrine tumors. Oncologist 2014, 19, 966–974. [Google Scholar] [CrossRef] [PubMed]
- Nishino, M.; Brais, L.K.; Brooks, N.V.; Hatabu, H.; Kulke, M.H.; Ramaiya, N.H. Drug-related pneumonitis during mammalian target of rapamycin inhibitor therapy in patients with neuroendocrine tumors: a radiographic pattern-based approach. Eur J Cancer 2016, 53, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Mauro, C.; de Jesus, V.H.F.; Barros, M.; Costa, F.P.; Weschenfelder, R.F.; D'Agustini, N.; Angel, M.; Luca, R.; Nunez, J.E.; O'Connor, J.M.; et al. Opportunistic and Serious Infections in Patients with Neuroendocrine Tumors Treated with Everolimus: A Multicenter Study of Real-World Patients. Neuroendocrinology 2021, 111, 631–638. [Google Scholar] [CrossRef] [PubMed]
- O'Donnell, A.; Faivre, S.; Burris, H.A., 3rd; Rea, D.; Papadimitrakopoulou, V.; Shand, N.; Lane, H.A.; Hazell, K.; Zoellner, U.; Kovarik, J.M.; et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol 2008, 26, 1588–1595. [Google Scholar] [CrossRef] [PubMed]
- Tabernero, J.; Rojo, F.; Calvo, E.; Burris, H.; Judson, I.; Hazell, K.; Martinelli, E.; Ramon y Cajal, S.; Jones, S.; Vidal, L.; et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol 2008, 26, 1603–1610. [Google Scholar] [CrossRef] [PubMed]
-
WHO Classification of Endocrine and Neuroendocrine Tumors; IARC: Lyon, France, 2022.
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, S.; Yamaguchi, H.; Kawasaki, Y.; Kikuchi, M.; Tanaka, M.; Ito, A.; Mano, N. Long-term relationship between everolimus blood concentration and clinical outcomes in Japanese patients with metastatic renal cell carcinoma: a prospective study. J Pharm Health Care Sci 2019, 5, 6. [Google Scholar] [CrossRef] [PubMed]
- de Wit, D.; Schneider, T.C.; Moes, D.J.; Roozen, C.F.; den Hartigh, J.; Gelderblom, H.; Guchelaar, H.J.; van der Hoeven, J.J.; Links, T.P.; Kapiteijn, E.; et al. Everolimus pharmacokinetics and its exposure-toxicity relationship in patients with thyroid cancer. Cancer Chemother Pharmacol 2016, 78, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Deppenweiler, M.; Falkowski, S.; Saint-Marcoux, F.; Monchaud, C.; Picard, N.; Laroche, M.L.; Tubiana-Mathieu, N.; Venat-Bouvet, L.; Marquet, P.; Woillard, J.B. Towards therapeutic drug monitoring of everolimus in cancer? Results of an exploratory study of exposure-effect relationship. Pharmacol Res 2017, 121, 138–144. [Google Scholar] [CrossRef] [PubMed]
- Kiesewetter, B.; Melhorn, P.; Macheiner, S.; Wolff, L.; Kretschmer-Chott, E.; Haug, A.; Mazal, P.; Raderer, M. Does the dose matter? Antiproliferative efficacy and toxicity of everolimus in patients with neuroendocrine tumors - Experiences from a tertiary referral center. J Neuroendocrinol 2023, 35, e13319. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).