Submitted:
30 September 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Ethic
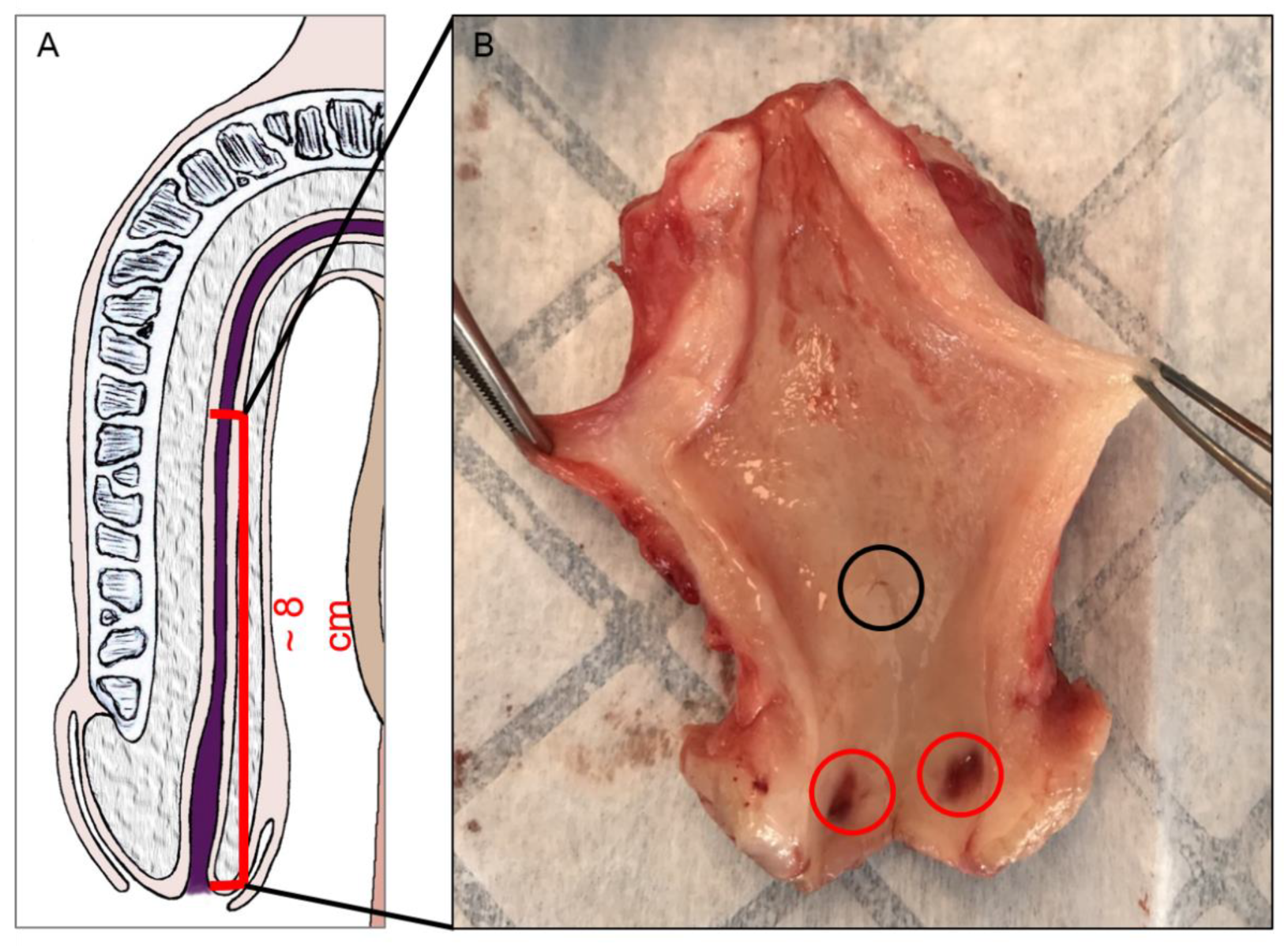
2.2. Organ Collect and Preparation
2.3. Histological Studies
2.3.1. Staining
2.3.2. Microscope Observation
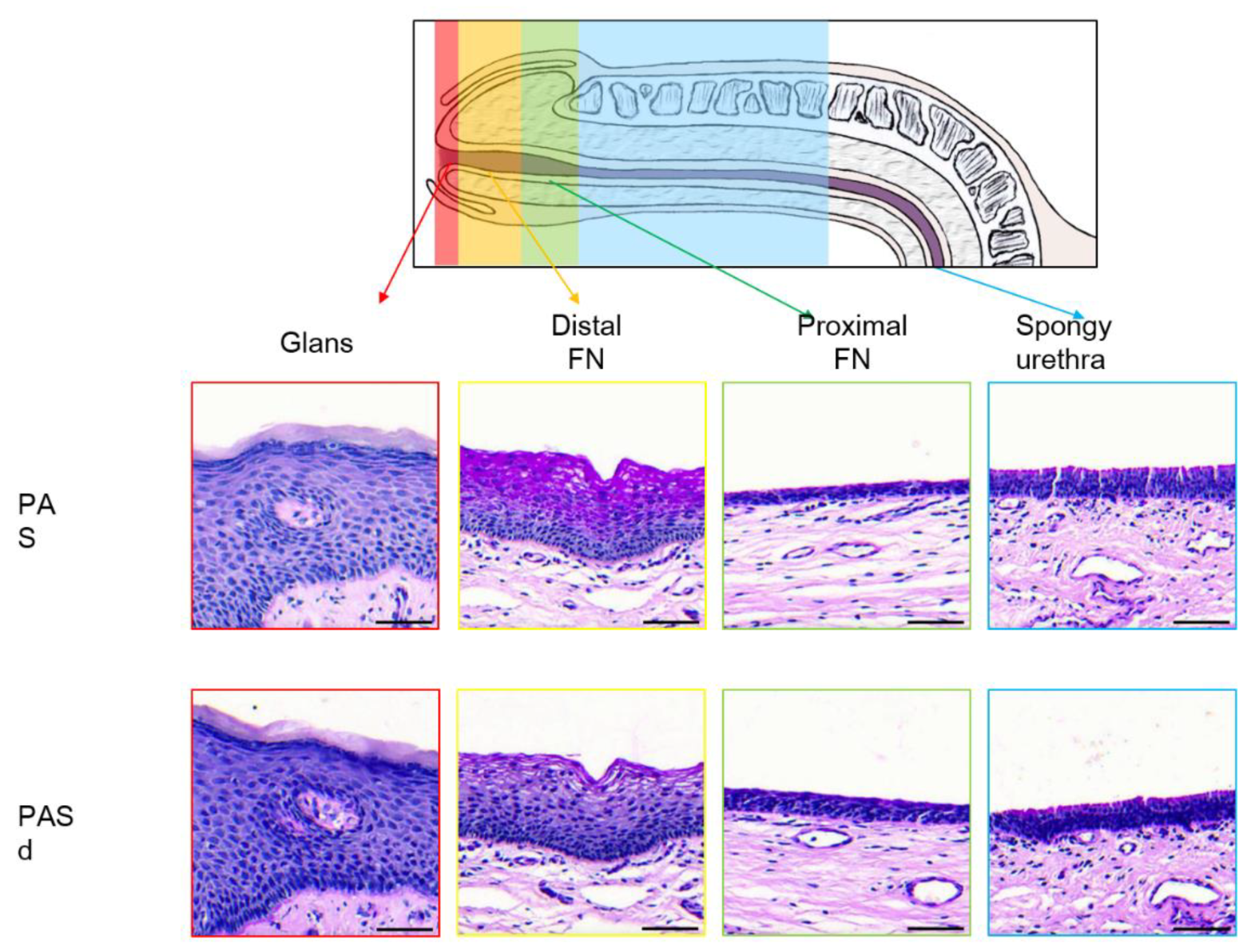
3. Results
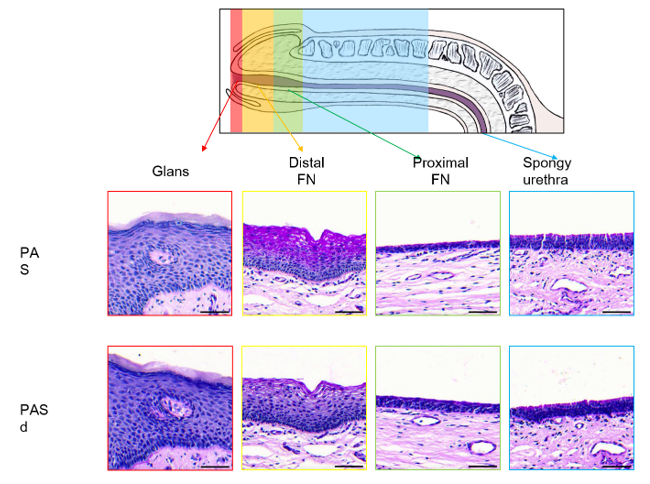
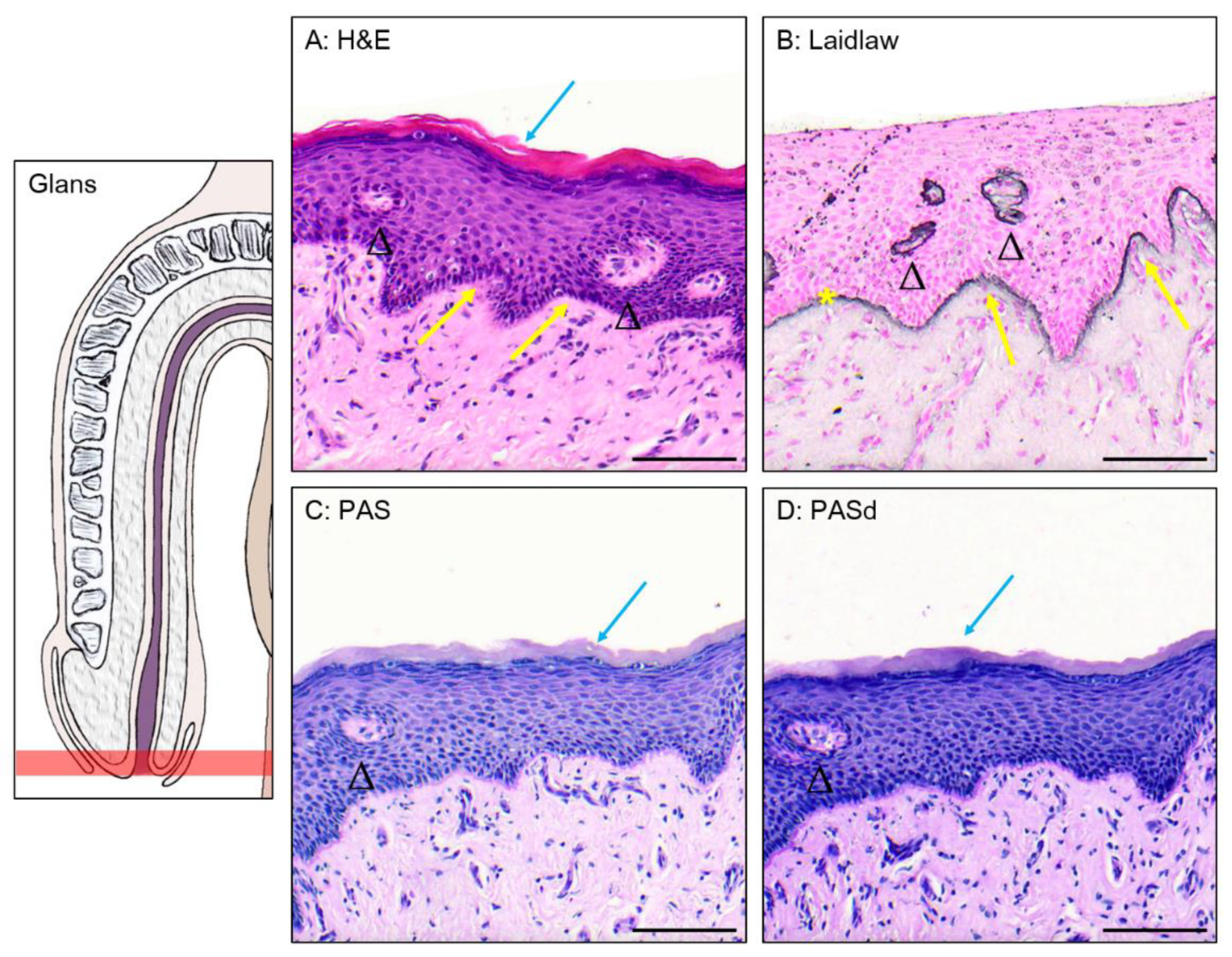
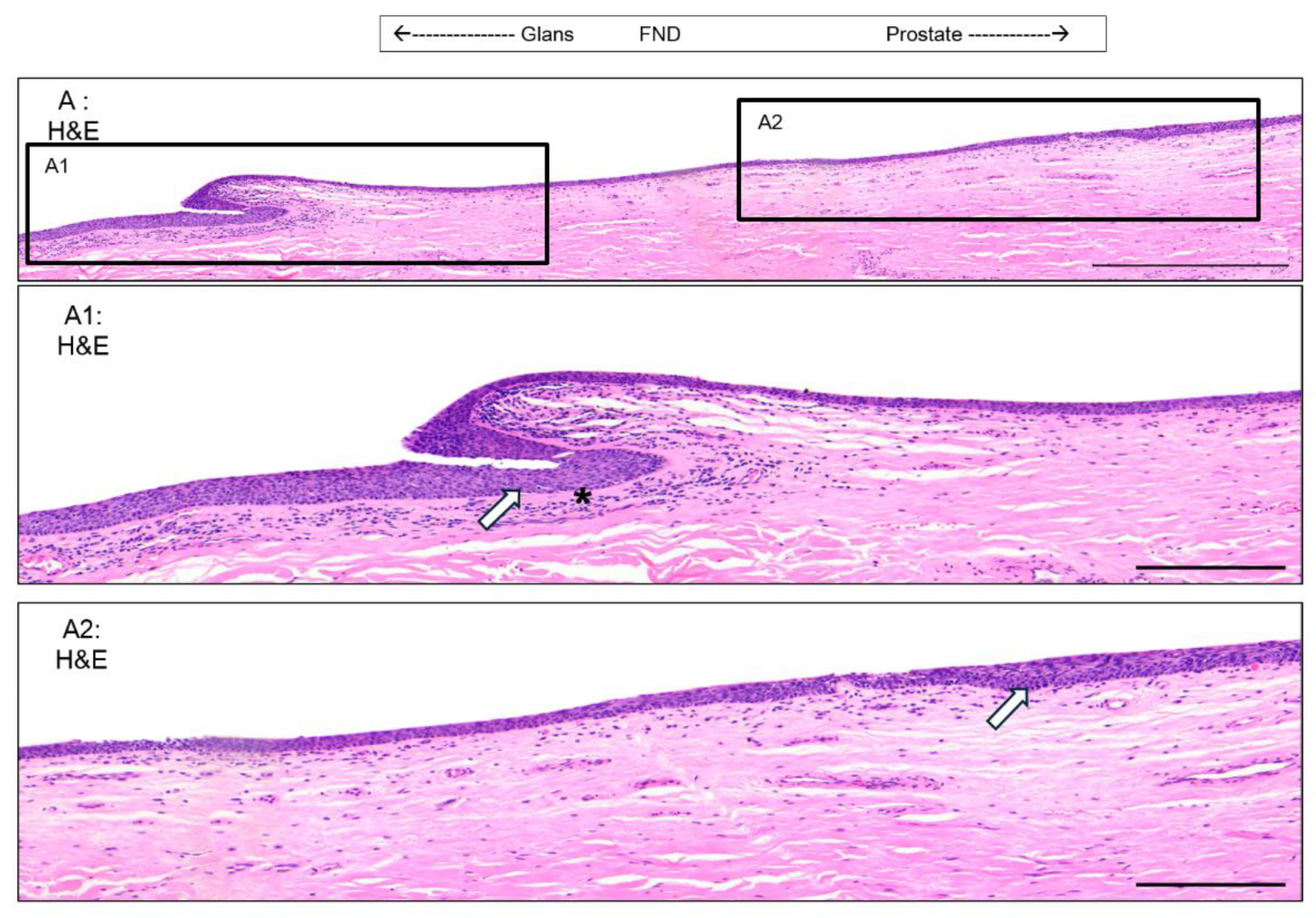
3.1. Epithelium of the Glans
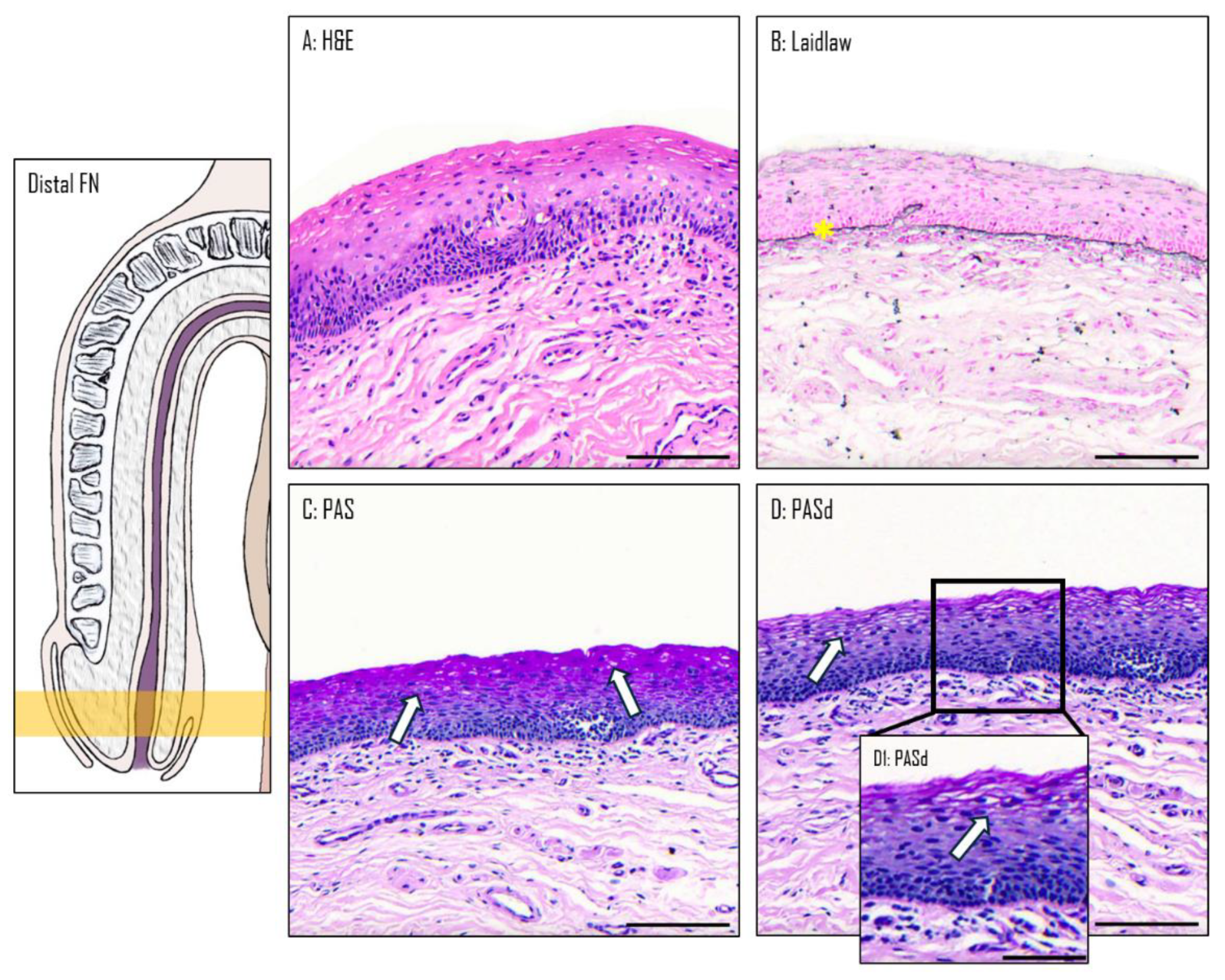
3.2. Epithelium of the Distal Fossa Navicularis (DFN)
3.3. Epithelium of the Proximal Fossa Navicularis (PFN)
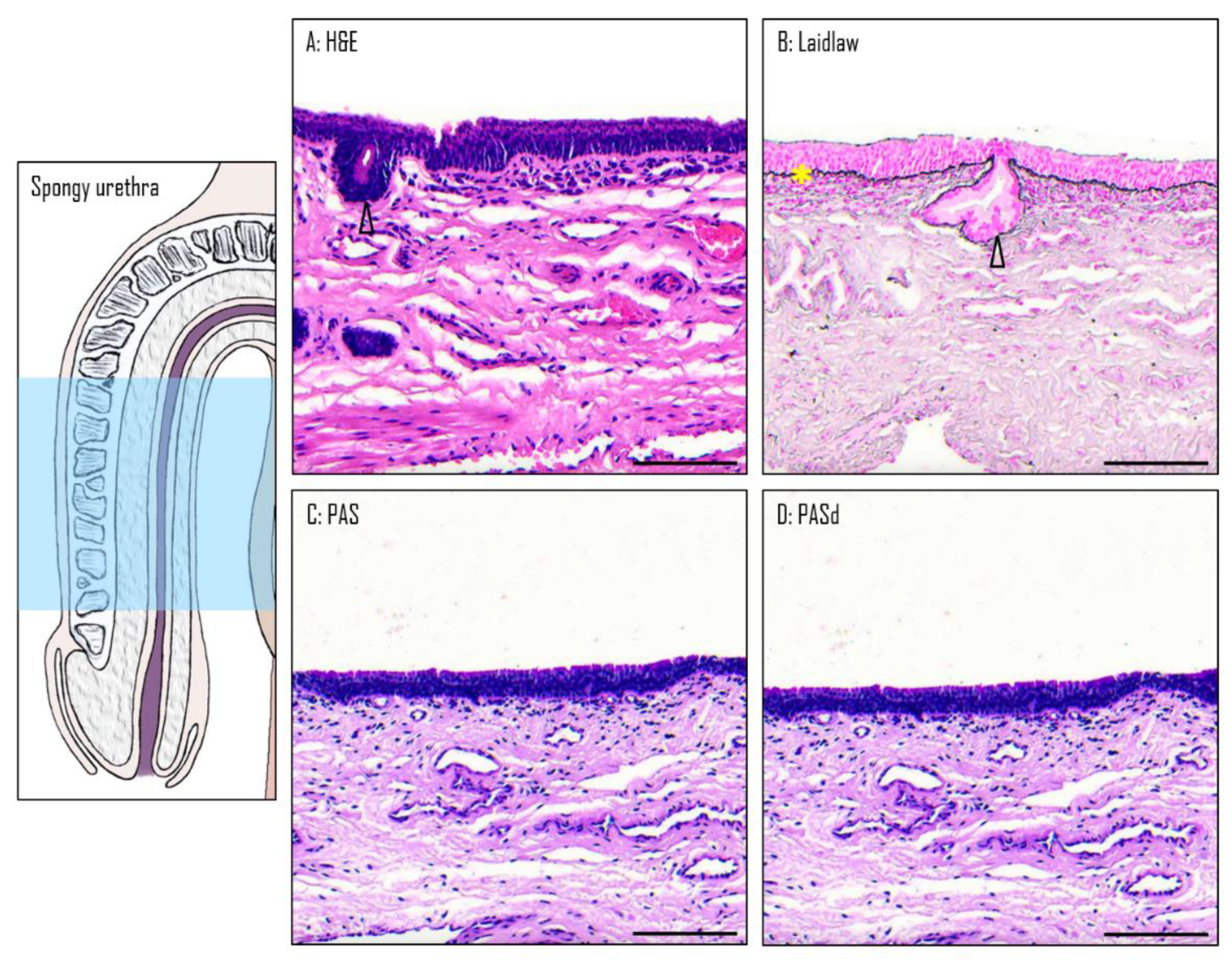
3.4. Epithelium of the Spongy Urethra
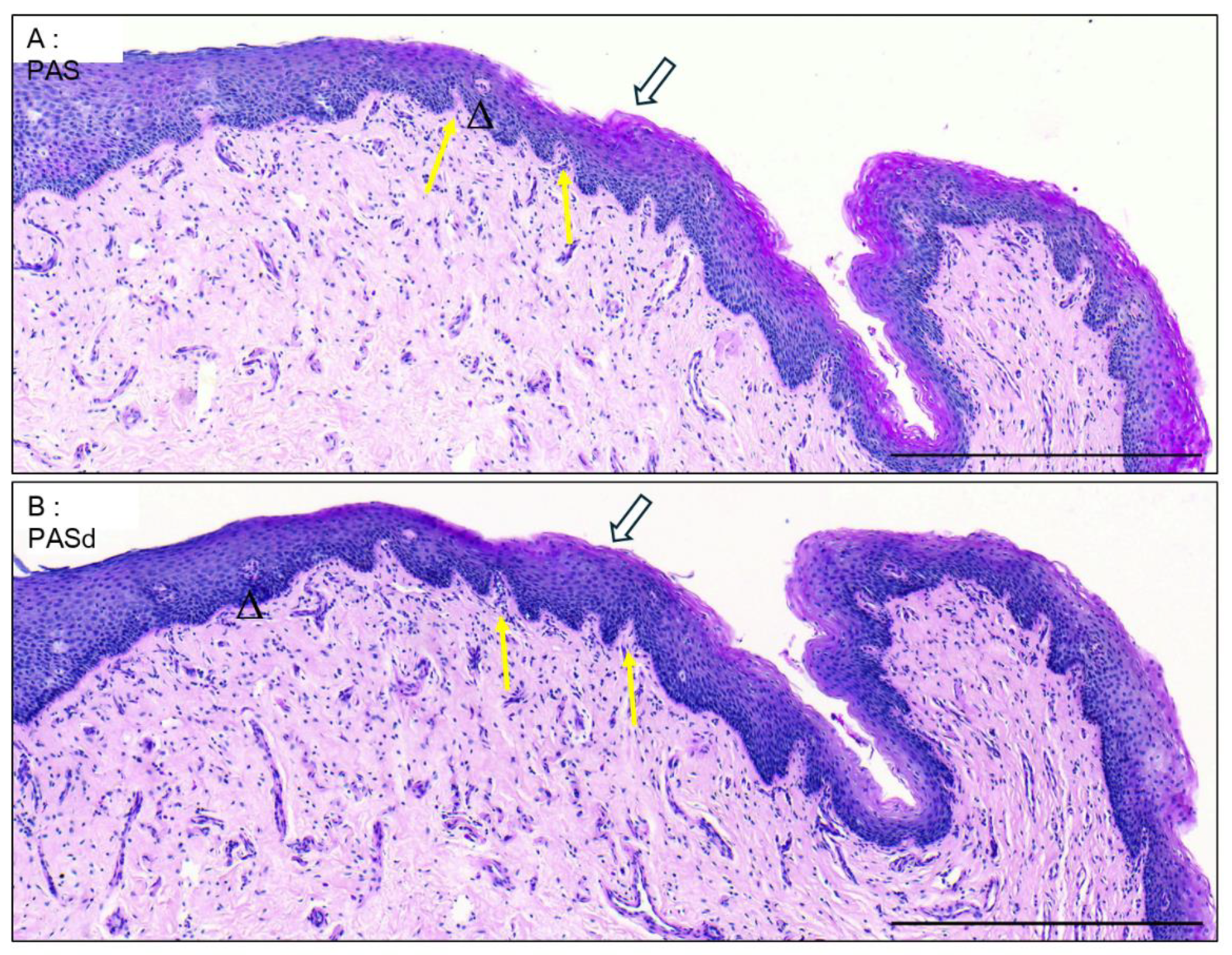
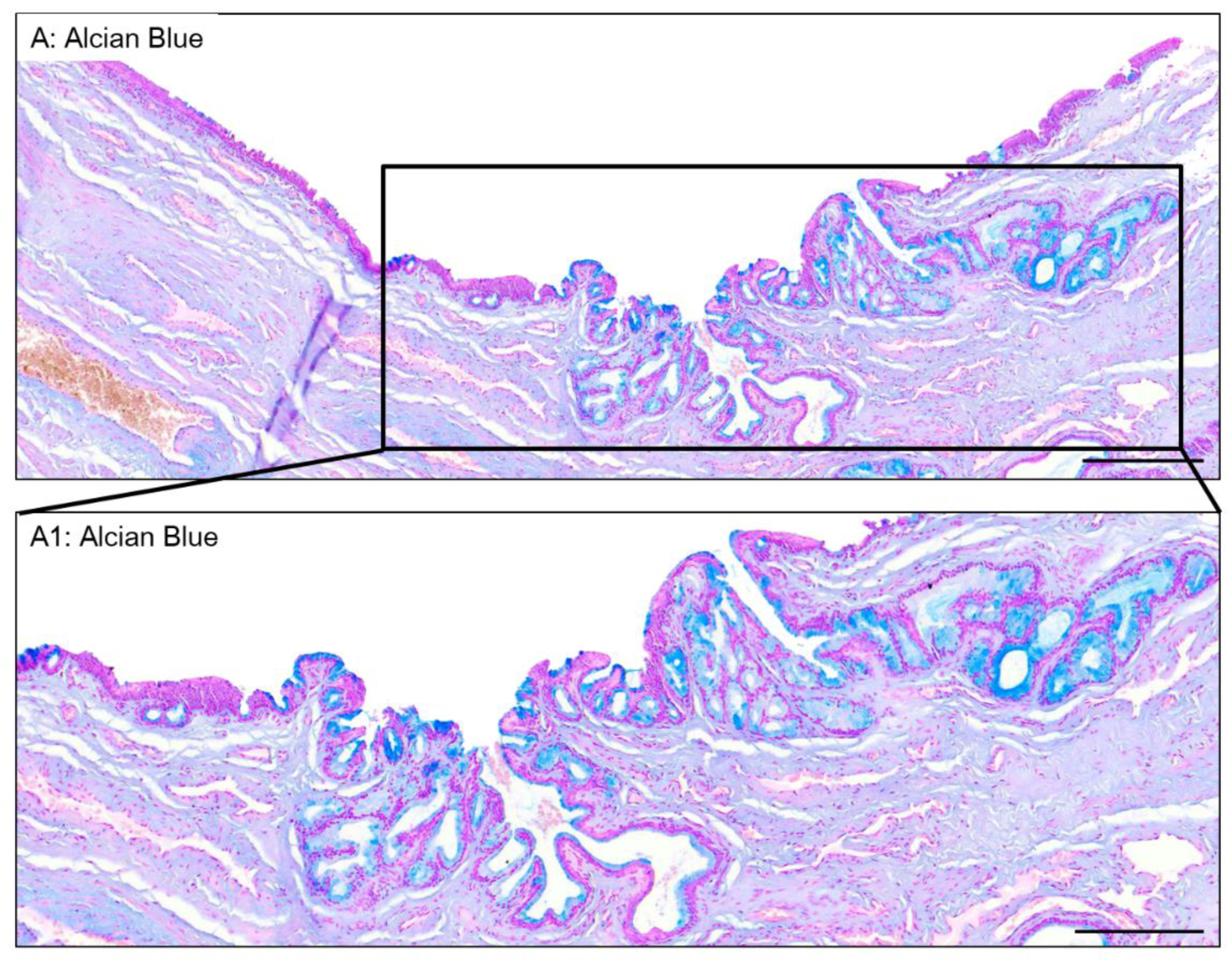
3.5. Valvule-Like Structure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elia E, Caneparo C, McMartin C, Chabaud S and Bolduc S 2024 Tissue Engineering for Penile Reconstruction. Bioengineering (Basel) 11.
- Caneparo C, Brownell D, Chabaud S and Bolduc S 2021 Genitourinary Tissue Engineering: Reconstruction and Research Models. Bioengineering (Basel) 8.
- Dessanti A, Rigamonti W, Merulla V, Falchetti D and Caccia G 1992 Autologous buccal mucosa graft for hypospadias repair: an initial report. J Urol 147 1081–3; discussion 1083-4.
- Cruz-Diaz O, Castellan M and Gosalbez R 2013 Use of buccal mucosa in hypospadias repair. Curr Urol Rep 14 366–72.
- Saad S, Osman N I and Chapple C R 2021 Tissue engineering: recent advances and review of clinical outcome for urethral strictures. Curr Opin Urol 31 498–503.
- Barbagli G, Heidenreich A, Zugor V, Karapanos L and Lazzeri M 2020 Urothelial or oral mucosa cells for tissue-engineered urethroplasty: A critical revision of the clinical outcome. Asian J Urol 7 18–23.
- Bédard P, Gauvin S, Ferland K, Caneparo C, Pellerin È, Chabaud S and Bolduc S 2020 Innovative Human Three-Dimensional Tissue-Engineered Models as an Alternative to Animal Testing. Bioengineering (Basel) 7.
- Saba I, Jakubowska W, Bolduc S and Chabaud S 2018 Engineering Tissues without the Use of a Synthetic Scaffold: A Twenty-Year History of the Self-Assembly Method Biomed Res Int 2018 1–13.
- Roy V, Magne B, Vaillancourt-Audet M, Blais M, Chabaud S, Grammond E, Piquet L, Fradette J, Laverdière I, Moulin V J, Landreville S, Germain L, Auger F A, Gros-Louis F and Bolduc S 2020 Human Organ-Specific 3D Cancer Models Produced by the Stromal Self-Assembly Method of Tissue Engineering for the Study of Solid Tumors. Biomed Res Int 2020 6051210.
- Pederzoli F, Joice G, Salonia A, Bivalacqua T J and Sopko N A 2019 Regenerative and engineered options for urethroplasty Nat Rev Urol 16 453–64.
- Holstein A F, Davidoff M S, Breucker H, Countouris N and Orlandini G 1991 Different epithelia in the distal human male urethra Cell Tissue Res 264 23–32.
- Hausmann R and Schellmann B 1994 Forensic value of the Lugol’s staining method: further studies on glycogenated epithelium in the male urinary tract Int J Legal Med 107 147–51.
- Hossler F E . 2014 Ultrastructure atlas of human tissues.
- Krstić R V. 1991 Urogenital Apparatus Human Microscopic Anatomy (Berlin, Heidelberg: Springer Berlin Heidelberg) pp 295–437.
- Barbieri J S, Wanat K and Seykora J 2014 Skin: Basic Structure and Function Pathobiology of Human Disease (Elsevier) pp 1134–44.
- Saba I, Barat C, Chabaud S, Reyjon N, Leclerc M, Jakubowska W, Orabi H, Lachhab A, Pelletier M, Tremblay M J and Bolduc S 2021 Immunocompetent Human 3D Organ-Specific Hormone-Responding Vaginal Mucosa Model of HIV-1 Infection Tissue Eng Part C Methods 27 152–66.
- Mohtashami M, Mohamadi M, Azimi-Nezhad M, Saeidi J, Nia FF, Ghasemi A. Lactobacillus bulgaricus and Lactobacillus plantarum improve diabetic wound healing through modulating inflammatory factors. Biotechnol Appl Biochem. 2021 Dec;68(6):1421-1431.
- Takada K, Komine-Aizawa S, Kuramochi T, Ito S, Trinh QD, Pham NTK, Sasano M, Hayakawa S. Lactobacillus crispatus accelerates re-epithelialization in vaginal epithelial cell line MS74. Am J Reprod Immunol. 2018 Sep;80(3):e13027.
- Bowie WR, Pollock HM, Forsyth PS, Floyd JF, Alexander ER, Wang SP, Holmes KK. Bacteriology of the urethra in normal men and men with nongonococcal urethritis. J Clin Microbiol. 1977 Nov;6(5):482-8.
- O’Hanlon DE, Moench TR, Cone RA. Vaginal pH and microbicidal lactic acid when lactobacilli dominate the microbiota. PLoS One. 2013 Nov 6;8(11):e80074.
- Aldunate M, Tyssen D, Johnson A, Zakir T, Sonza S, Moench T, Cone R, Tachedjian G. Vaginal concentrations of lactic acid potently inactivate HIV. J Antimicrob Chemother. 2013 Sep;68(9):2015-25.
- Atassi F, Servin AL. Individual and co-operative roles of lactic acid and hydrogen peroxide in the killing activity of enteric strain Lactobacillus johnsonii NCC933 and vaginal strain Lactobacillus gasseri KS120.1 against enteric, uropathogenic and vaginosis-associated pathogens. FEMS Microbiol Lett. 2010 Mar;304(1):29-38.
- Spurbeck RR, Arvidson CG. Lactobacilli at the front line of defense against vaginally acquired infections. Future Microbiol. 2011 May;6(5):567-82.
- Unemo M, Bradshaw CS, Hocking JS, de Vries HJC, Francis SC, Mabey D, Marrazzo JM, Sonder GJB, Schwebke JR, Hoornenborg E, Peeling RW, Philip SS, Low N, Fairley CK. Sexually transmitted infections: challenges ahead. Lancet Infect Dis. 2017 Aug;17(8):e235-e279.
- Petrova MI, van den Broek M, Balzarini J, Vanderleyden J, Lebeer S. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol Rev. 2013 Sep;37(5):762-92.
- Martin, DH. The microbiota of the vagina and its influence on women’s health and disease. Am J Med Sci. 2012 Jan;343(1):2-9.
- Schwebke, JR. Abnormal vaginal flora as a biological risk factor for acquisition of HIV infection and sexually transmitted diseases. J Infect Dis. 2005 Oct 15;192(8):1315-7.
- Schwebke JR, Desmond R. A randomized trial of metronidazole in asymptomatic bacterial vaginosis to prevent the acquisition of sexually transmitted diseases. Am J Obstet Gynecol. 2007 Jun;196(6):517.e1-6.
- Caneparo C, Chabaud S and Bolduc S 2021 Reconstruction of Vascular and Urologic Tubular Grafts by Tissue Engineering Processes 9 513.
- Dictionnaire médical de l’Académie de Médecine.
- Isola M, CossuM, DeLisa A, Isola R, Massa D, Casti A, Solinas P and Lantini M S 2010 Oxytocin Immunoreactivity in the Human Urethral (Littre’s) Glands Journal of Reproduction and Development 56 94–7.
- Zechhi-Orlandini S, Gulisano M, Orlandini G E and Holstein A F 2009 Scanning Electron Microscopic Observations on the Epithelium of the Human Spongy Urethra Andrologia 20 132–7.
- Serbanoiu A, Ion R T, Filipoiu F, Tulin A and Enyedi M 2024 Dissection of the Male Urethra Demonstrating Its Topographical Specificity. Cureus 16 e65946.
- Kajbafzadeh A 2005 Congenital Urethral Anomalies in Boys. Part II Urol J 2 125–31.
- Shenoy S P, Marla P K, Venugopal P, Adappa K K, Tantry T P, Shankar M and Rai G D 2011 An Endoscopic Study of the Lacuna Magna and Reappraisal of Its Clinical Significance in Contemporary Urological Practice Urology 78 1009–15.
- Pariser J J and Kim N 2019 Transgender vaginoplasty: Techniques and outcomes Transl Androl Urol 8 241–7.
- Dessy L A, Mazzocchi M, Corrias F, Ceccarelli S, Marchese C and Scuderi N 2014 The use of cultured autologous oral epithelial cells for vaginoplasty in male-to-female transsexuals: A feasibility, safety, and advantageousness clinical pilot study Plast Reconstr Surg 133 158–61.
- Sueters J, Groenman F A, Bouman M-B, Roovers J P W, de Vries R, Smit T H and Huirne J A F 2023 Tissue Engineering Neovagina for Vaginoplasty in Mayer–Rokitansky–Küster–Hauser Syndrome and Gender Dysphoria Patients: A Systematic Review Tissue Eng Part B Rev 29 28–46.
- Bustos S S, Bustos V P, Mascaro A, Ciudad P, Forte A J, Del Corral G and Manrique O J 2021 Complications and Patient-reported Outcomes in Transfemale Vaginoplasty: An Updated Systematic Review and Meta-analysis Plast Reconstr Surg Glob Open 9 e3510.

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




