Introduction
The gastrointestinal tract consists of a complex ecosystem in which the constant exposure to multiple microbes induces unique responses in the immune subsets residing in the gut [
1]. Recent studies indicated that the interaction between the mycobiome (fungal community) and the microbiome (bacterial community) plays a critical role in the pathogenesis of inflammatory bowel disease (IBD) [
2,
3], particularly causing the production of polymicrobial biofilms which in turn allow an uncontrolled microbial growth in the gut, giving pathogens multiple advantages, such as antibiotic resistance [
4], protection against the host immune system [
5] and increased surface adhesion [
6]. The resulting dysbiosis caused by the unsuppressed microbial growth is particularly relevant in IBD pathogenesis, as innate and adaptive immune responses can be altered by the microbiome dysregulation leading to unresolved local inflammation[
7].
Gut-residing microorganisms can have not only a detrimental impact on the host health. Specific microbial communities in fact play a pivotal role in maintaining gut homeostasis by modulating immune cells responses. Such responses help controlling the tolerance to commensal microbes, involving both adaptive and innate immune systems [
8], leading to health-promoting effects [
9]. The improvement obtained by this immune cell-commensal bacteria interaction induces boosted immunologic responses particularly towards inflammatory [
10] and infectious conditions [
11].
Hence, it is reasonable to suggest that the administration of beneficial microorganisms (probiotics) may help to ameliorate the gastrointestinal manifestations in IBD patients by modulating immune responses and therefore contributing to resolve chronic inflammation. As specified by the World Health Organization, probiotics are defined as live microorganisms which, when administered in adequate amounts, confer health benefits to the host [
12]. Probiotics are represented by various bacterial strains that, when administered at a functional dose, can be used as supplements in the general population [
13], positively affecting the gut microbiome composition and interacting with diverse immune subsets, thus strengthening the response of the immune system [
14] and preventing the progression of multiple diseases, such as cancer [
15] and obesity [
16]. Therefore, probiotics may represent a cost-efficient alternative solution in regard to managing multiple types of pathologies[
17,
18].
Although multiple studies demonstrated probiotics beneficial effects particularly in irritable bowel syndrome [
19], the molecular mechanisms driving the interaction between probiotics and the immune system that lead to immunomodulatory effects remain unclear.
We have previously shown that a probiotic blend recently developed in our lab containing bacterial and fungal strains (
Lactobacillus (
L.)
rhamnosus,
L.
acidophilus,
Bifidobacterium (
B.)
breve and
Saccharomyces (
S.)
boulardii) coupled with hydrolytic enzyme amylase is capable of inhibiting germination of pathogenic biofilm-producing microbial species in vitro [
20], and ameliorates CD-like ileitis in SAMP1/YitFc (SAMP) mouse model [
21]. One of the main factors causing attenuation of ileal disease is represented by the probiotics ability to increase β-diversity and avoid dysbiosis in the gut.
The probiotic and amylase combination has, in fact, been shown to have a positive effect by altering the intestinal microbiome and avoiding dysbiosis. However, the mechanisms underlying its interaction with specific intestinal immune populations have not yet been fully investigated.
Thus, in the present study we analyzed how the probiotics and amylase blend affects the distinct immune subsets present in the gut of SAMP mice, and what intracellular pathways are particularly induced or inhibited.
Herein we show that probiotics blend and amylase are both necessary to decrease ileitis in SAMP mice, as the experimental groups administered only amylase or only probiotics presented higher levels of inflammation similar to the ileitis present in the vehicle-treated control group. Mechanistically, we report that the combination of amylase and probiotic is able to promote significant alterations in distinct immune subsets resident in the intestinal mucosa, particularly affecting dendritic cells, innate lymphoid cells (ILC)s macrophages and neutrophils.
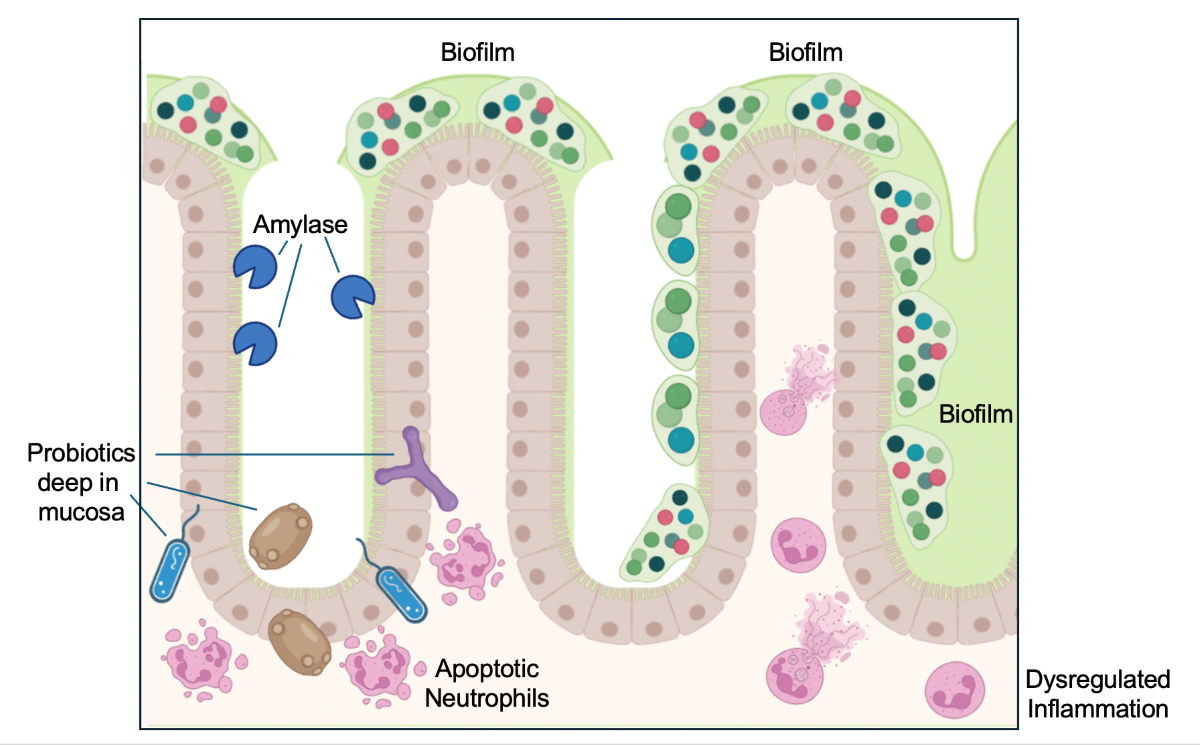
Finally, our data suggest that amylase anti-biofilm activity allows probiotics to infiltrate the intestinal mucosa and contribute to the apoptosis of some innate immune cell populations present in the lamina propria, such as neutrophils, thus facilitating resolution of the inflammatory process.
In conclusion, our study shows evidence of the immunomodulatory properties of the probiotic and amylase combination promoting apoptosis in immune subsets (most likely in neutrophils) present in the lamina propria of SAMP mice, thus facilitating the resolution of inflammation in the ileum.
Our data support the benefit of testing the recently developed probiotic blend as a therapeutic supplement in clinical trials focused on the management of IBD symptoms.
Materials and Methods
Experimental Animals
The SAMP mouse colony (species: Mus musculus) was maintained at Case Western Reserve University in the Animal Resource Center. 7-week-old mice, sex- and age-matched between the experimental cohorts were used to conduct the experiments. Mice were kept in micro-isolator cages (Allentown Inc, Allentown, NJ) with 1/8-inch corn bedding and cotton nestlets used for environmental enhancement (Envigo, Indianapolis, IN). Mice had access to laboratory diet P3000 (Harlan Teklad, Indianapolis, IN) and water during the whole experiments. All studies were performed in a blind fashion, and mice were subject to randomization and identified using a numerical code known only to the animal caretaker. The identification code was revealed only at the end of the experiments.
Test Materials and Administration Protocol
The probiotic strains and amylase enzyme were supplied by BIOHM Health, LLC (Cleveland, OH). Mice were gavaged every day for 56 days with: 1) probiotic blend + amylase (concentration: 0.25 mg/100 uL PBS); 2) only probiotics; 3) only amylase; PBS (vehicle-treated controls).
Histology
Experimental mice were sacrificed, and terminal ilea were collected, opened longitudinally, rinsed in PBS and fixed in formalin. Tissues were then processed, embedded in paraffin, sectioned (5 μm thick),stained with hematoxylin and eosin (H&E) and histologically evaluated by a trained gastrointestinal pathologist in a blinded manner, using a validated semiquantitative scoring system as previously described [
22]. Briefly, histology scores ranging from 0 (normal) to 3 (maximum level of changes) were used to analyze five histologic parameters: (1) villus distortion, (2) chronic inflammation (lymphocytes in the mucosal layer), (3) active inflammation (infiltration with neutrophils), (4) mononuclear inflammation (monocytes and macrophages) and (5) transmural inflammation.
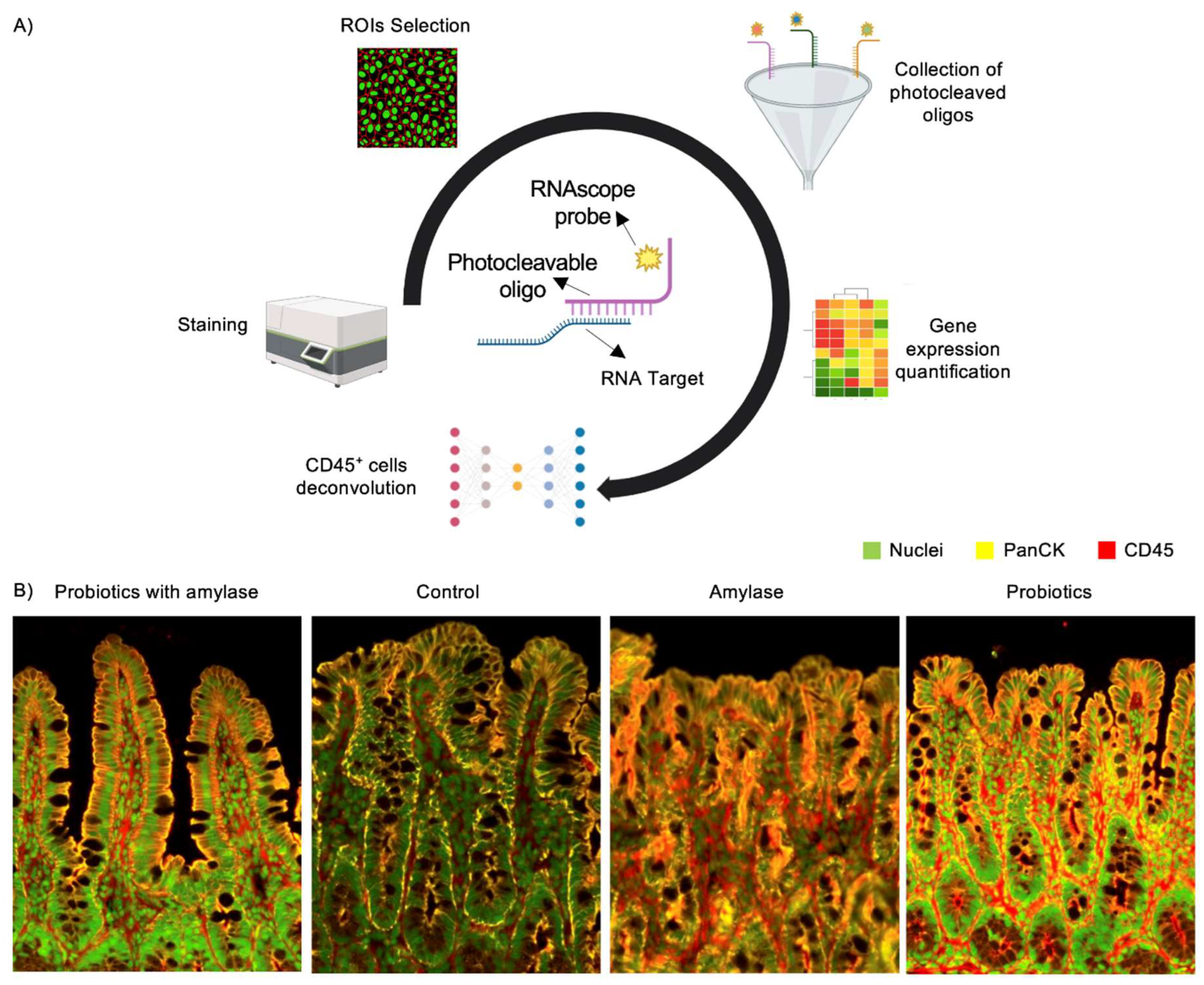
GeoMx NanoString Data Processing and Analysis
Paraffin embedded slides were incubated at 65°C for 2 hours and then rehydrated in EtOH and ddH2O, treated with proteinase K (1.0 μ g/ml at 37°C for 15 minutes). Epitope retrieval (ER2 at 100°C for 20 minutes) was also performed. Next, the sections underwent a process of hybridization with the CTA probes at 37°C for 24 hours. In order to identify tissue morphology landmarks, samples were washed for five minutes with buffer & formamide solution (1:1), and then, using the TME Morphology Kit (catalog #121300301, NanoString), were stained for CD45 (NBP234528AF647, Novus Biologicals) and pan-cytokeratin (PanCK) (NBP2-33200AF488, Novus Biologicals) to target immune and epithelial cells respectively. Syto83 (S11364, Invitrogen) was used for nuclear detection. Next, the slides were loaded into the DSP machine and scanned for subsequent immunofluorescent imaging. Regions of Interests (ROI)s on the mucosal layer of the ilea were selected by a pathologist based on CD45 staining. The FASTQ files obtained through sequencing were processed using a GeoMx NGS Pipeline to generate gene count data for each ROI. Measurement of the number of CD45
+ cells in the ROIs was performed using the Digital Spatial Profiler (DSP) platform [
23] and the acquired data were analyzed using the R software [
24]. Graphs were created using ComplexHeatmap package. Filtering and quality control were performed following manufacturer’s protocol (NGS Data Analysis, MAN-10119-01). Spike-in probes for RNA targets were used to quantify the expression of 22,000 murine genes in a high-throughput manner from ileum. Next, the geometric mean for each target and the limit of quantification (LOQ: value obtained calculating two standard deviations above the negative control probe counts’ geometric mean) were quantified for each ROI. The next data filtration step consisted in excluding from subsequent analysis the target values that show no measurement exceeding the LOQ value. The next step included a third quartile (Q3) normalization method performed following the GeoMx NanoString directions. Principal component analysis, heatmap dendrograms and volcano plots representing pathways analysis were obtained using custom scripts SpatialDecon_plugin.R and DimReduction.R.
TUNEL Assay
The degree of apoptosis in ileum tissues was evaluated utilizing the deoxynucleotidyl transferase (TdT)- deoxyuridine triphosphate (dUTP)-biotin nick end labeling (TUNEL) technique. Tissue sections were double stained with anti-neutrophil antibody (NP212439A94, Novus Biologicals) and Click-iTTM Plus TUNEL Assay kit (C10617, Thermofisher Scientific) following the manufacturer’s instructions.
Statistical Analysis
Experiments were executed and repeated in duplicate, and the acquired data were employed for multivariate analyses. Unpaired Student’s t test was used to compare data obtained from the experimental mouse cohorts and determine possible differences. Data were represented as mean + standard error of measurements (SEM)s, with 95% confidence intervals being reported. An alpha level value of 0.05 was regarded as significant. GraphPad software (San Diego, CA) was used to perform the analyses.
Study Approval
All the experiments were performed following the guidelines provided by the Association for Assessment and Accreditation of Laboratory Animal Care and reviewed by the Institutional Animal Care and Use Committee (Case Western Reserve University).
Results
Amylase and Probiotic Blend Are Both Necessary to Ameliorate Inflammation in SAMP Mice
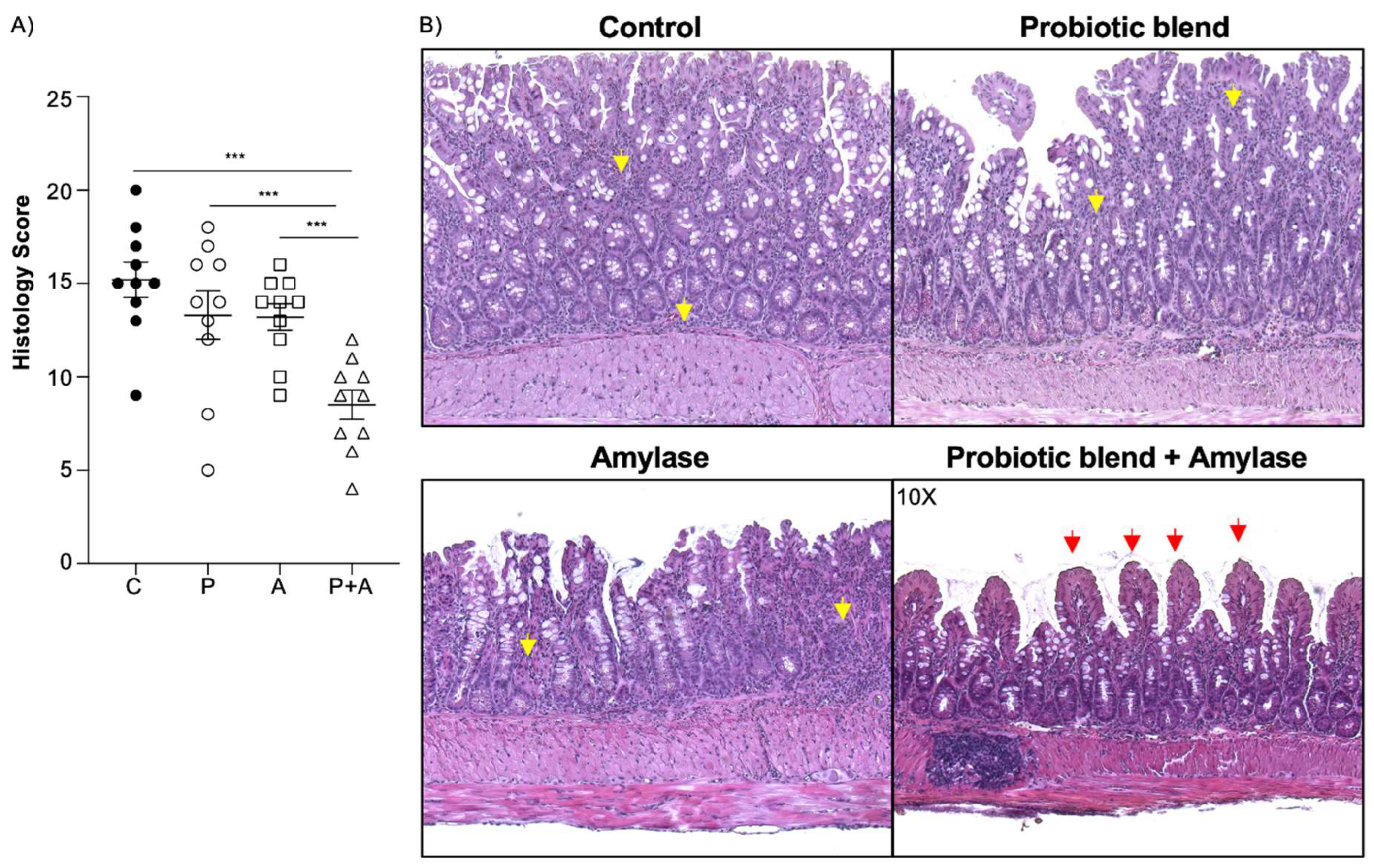
First, we tested the ability of the amylase alone or probiotic blend alone to decrease the inflammation in the ileum of SAMP mice by oral administration. We employed four experimental cohorts: mice treated with 1) only amylase, 2) only probiotic blend, 3) amylase plus probiotic blend and 4) phosphate buffer saline (PBS) as negative control. Histological analysis revealed that the only effective treatment in ameliorating ileitis was the amylase combined with probiotic blend in comparison with the other three groups (
Figure 1A) as indicated by the ileal tissue presenting better preserved villi architecture and attenuated transmural and active inflammation (
Figure 1B).
Probiotics Mix Coupled with Amylase Exerts an Enhanced Immunomodulatory Effect in the Mucosal Layer
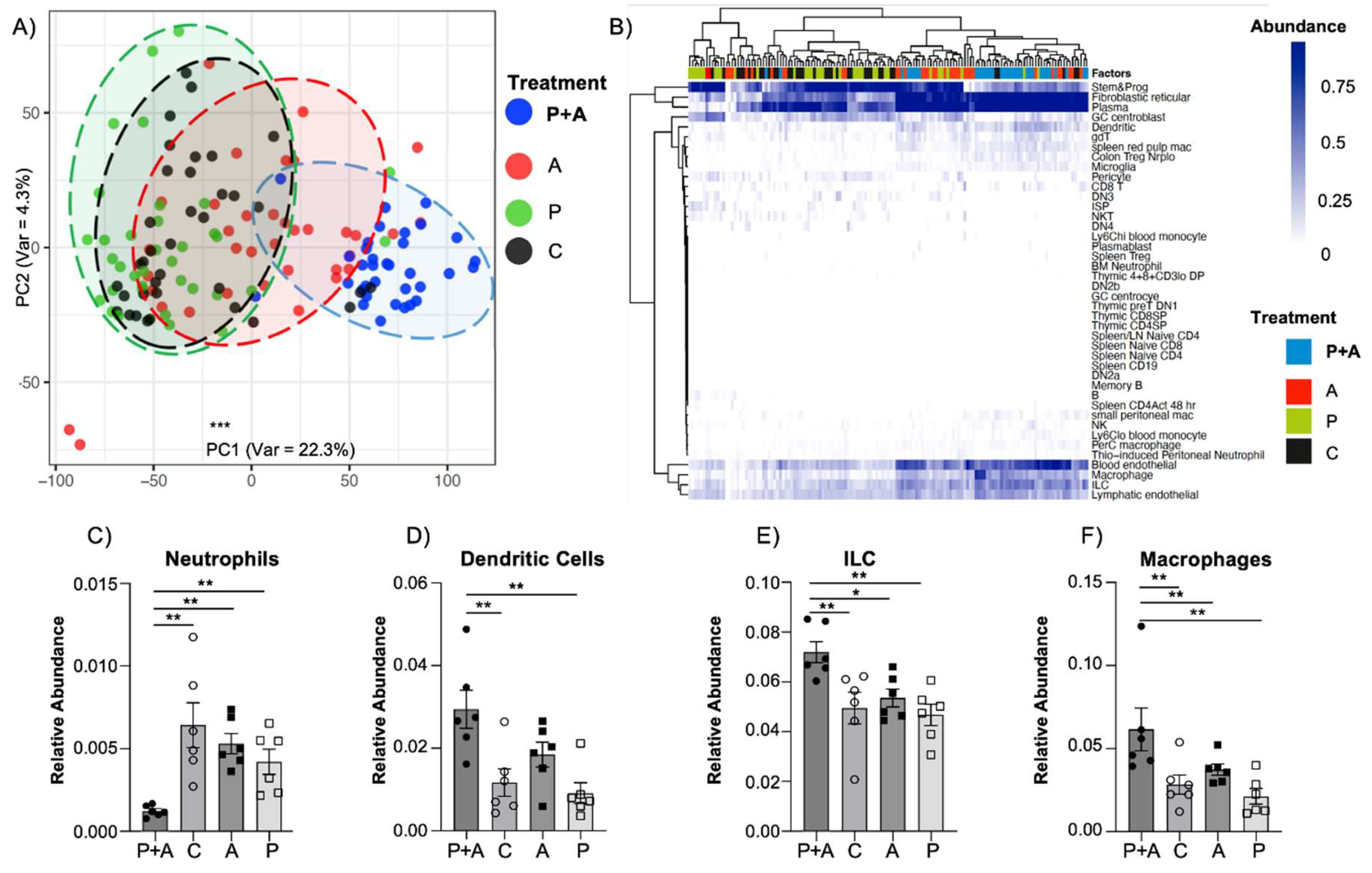
The biological clustering of ROIs, visualized by Principal Component Analysis (PCA) dimensional reduction plot, highlighted a clear separation of the different ROIs based on CD45
+ marker (
Figure 3A). Moreover, the marker genes of leukocyte subsets which were differentially expressed across the dataset revealed clustering of certain ROI groups (
Figure 3B). Among the analyzed immune cell populations, neutrophils were markedly reduced with the probiotics plus amylase treatment compared to all the other experimental groups, whereas dendritic cells, innate immune lymphoid cells (ILC)s and macrophages showed a completely opposite and statistically significant trend. (
Figure 3C-F).
Probiotic Blend Plus Amylase Stimulate Apoptosis Signaling in Immune Subsets
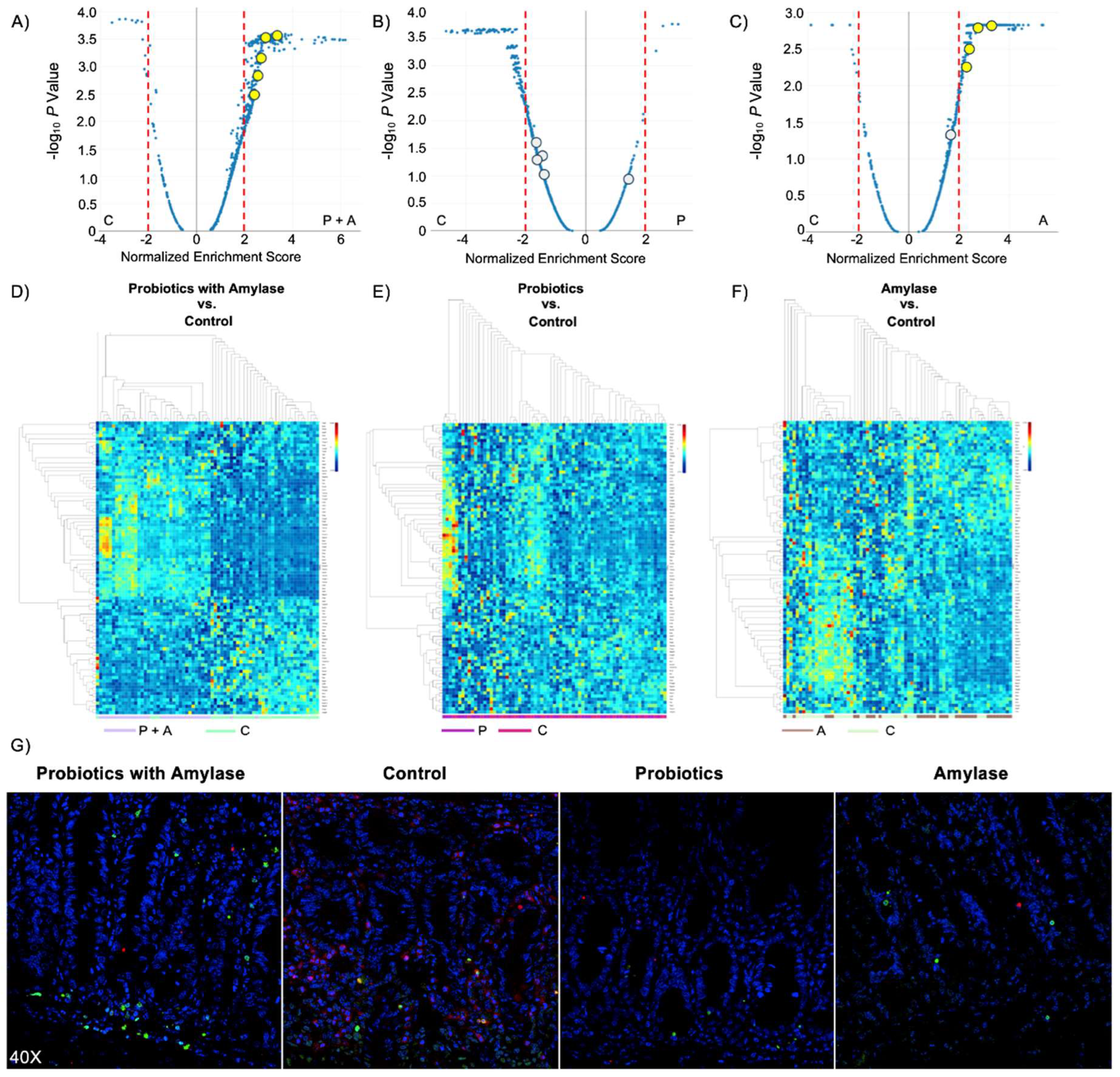
In order to highlight the signaling pathways that were inhibited or induced within the ROIs we compared the three experimental groups (probiotics+amylase, only probiotics, only amylase) with the control group (PBS), and charted gene expression values grouped by gene sets using volcano plots (
Figure 4A). The resulting data show that signaling pathways in CD45
+ cells were the most affected in the mice treated with probiotic+amylase combination, showing 469 significantly differentially expressed pathways compared to controls, while the probiotic-treated and the amylase-treated groups had respectively 247 and 389 significantly differentially expressed pathways compared to controls (
Figure 4B-C). A complete list of significantly differentially expressed pathways among groups, including normalized enrichment score, adjusted
P value and target genes involved in each pathway, is present in
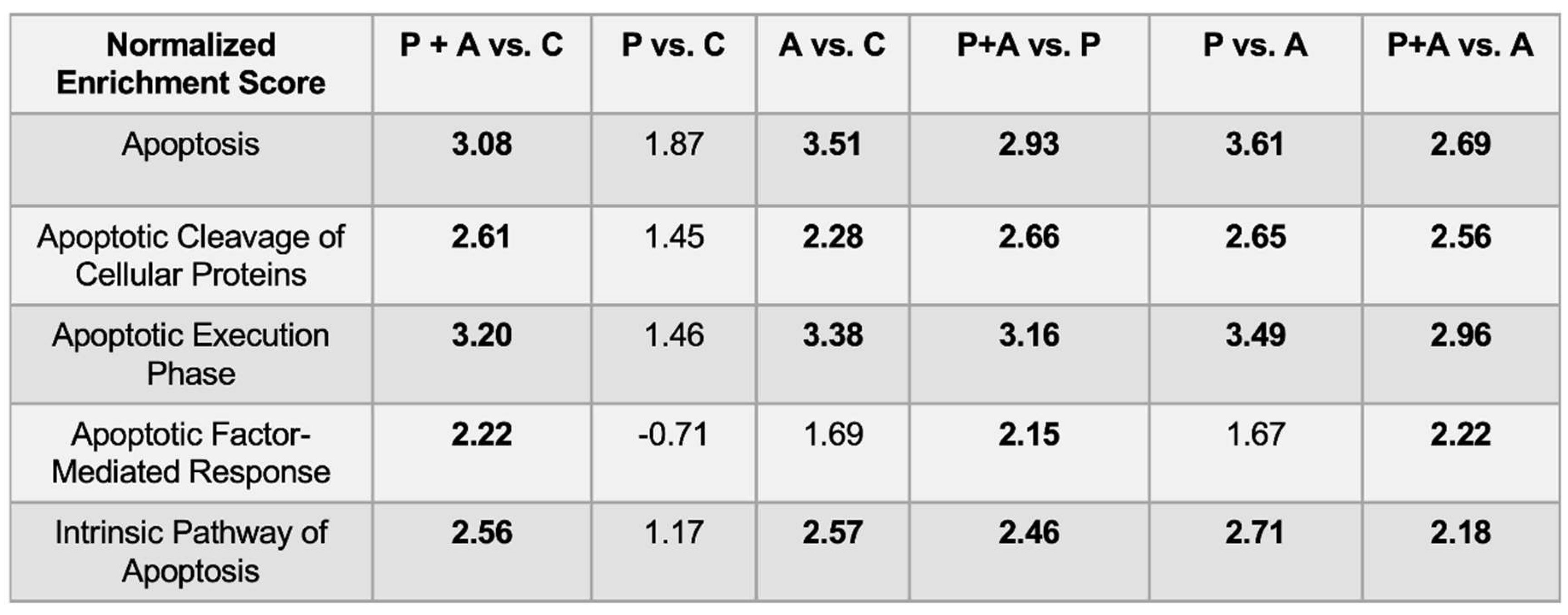
Figure S1. Interestingly, the lowest number of significantly differentially expressed pathways were found when comparing the “probiotics only” and the control groups, confirming the pivotal role of amylase in disrupting the biofilm present in the lumen of the intestine, allowing bacteria to infiltrate the ileal mucosa and promoting immunomodulatory responses. Moreover, the comparison of the ROIs in the four experimental groups showed enrichment of five pathways related to apoptosis signaling in the mice gavaged with probiotics and amylase (
Table 1). We analyzed the genes related to apoptosis signaling, and the heatmap revealed a clear distinct expression of those genes only between the “probiotic+amylase group” compared to the control group. Specifically, among the 91 genes related to apoptosis signaling analyzed, 70 of them showed a statistically significant differential expression in the “probiotic+amylase group” compared to control, within the selected ROIs of the two cohorts (
Figure 4D). In comparison, only eight genes were differentially expressed between the probiotic-treated group and the control group (
Figure 4E). Finally, 43 out of the 91 apoptosis-related genes analyzed were differentially expressed between the amylase-treated mice and the control group (
Figure 4F), corroborating our data-based hypothesis that amylase alone can break down biofilm in the gut, allowing commensal and pathogenic bacteria to infiltrate the intestinal mucosa and induce immune responses in the gut. A complete list of genes differentially expressed in the ROIs of the three experimental cohorts vs. the control group is present in
Figure S2.
Lastly, considering that neutrophils are the most abundant immune cells present in the lamina propria during inflammation ([
25]), and based on our GeoMx results indicating that neutrophils are the only immune subsets significantly decreased in the probiotic-amylase group compared to the other experimental groups, we evaluated the frequency of neutrophils undergoing the process of apoptosis. In order to do so we performed fluorescence imaging on ileal tissues from our experimental groups with a TUNEL assay, for detecting apoptosis, and neutrophils staining. Our results clearly indicate an increased number of cells actively undergoing the apoptotic process (green) and fewer neutrophils (red) in the mucosa of mice gavaged with probiotic and amylase mix compared to the control group (
Figure 4G). These data suggest that probiotics are able to infiltrate in the ileal mucosa possibly thanks to the anti-biofilm activity of amylase, inducing a downstream immune response promoting apoptosis mostly in neutrophils.
Together, the GeoMx Digital Spatial Profiling and TUNEL assay data identify spatially distinct changes in immune subsets within distinct ROIs in the four experimental groups, particularly emphasizing differences related to apoptosis signaling in immune subsets.
Discussion
In the present study, we performed transcriptome DSP analysis to investigate the interaction between a probiotic blend coupled with amylase, recently developed in our lab, and the immune cell subsets residing in the gut mucosa of the SAMP mouse model of CD-like ileitis. Firstly, we showed that amylase and probiotic blend are both necessary to ameliorate inflammation, since mice administered amylase alone or probiotic mix alone did not show any significant amelioration compared to the PBS-administered control group.
Moreover, our NanoString GeoMx data indicated that 453 cellular pathways were significantly differentially expressed in the mucosal layer of the probiotic+amylase treated mice compared to group administered only probiotics, suggesting that the anti-biofilm activity provided by the amylase addition facilitated the infiltration in the intestinal mucosa of the probiotic blend inducing an enhanced immunomodulatory response on mucosal immune cell subsets, resulting in decreased ileitis. This evidence is particularly relevant, considering that multiple microbiome studies have highlighted a cooperative interaction between different microbial populations resulting in biofilm development [
26,
27]. In particular, in a condition of dysbacteriosis, biofilm formation enables fungi and bacteria to escape immune detection [
28] and increase the production of oxygen reactive species which lead to disruption of the gastrointestinal barrier and leaky gut [
29]. In contrast, the number of significantly different pathways between probiotic+amylase treated mice and the control group was 469, indicating almost no difference between the control and the group administered only probiotic blend, as also confirmed by histological analysis of the ileal tissues showing similar degree of inflammation.
Furthermore, GeoMx analysis showed that a significant alteration in the expression of 77% of the apoptosis-related analyzed genes was associated with decreased abundance of neutrophils and increased abundance of dendritic cells, ILCs and macrophages in the lamina propria of probiotic+amylase treated mice compared to the control group. In comparison, the expression of only 8.8% of the apoptosis-related genes was significantly altered in the mice gavaged with only probiotics compared to the control group, with no observed difference in the abundance of neutrophils, dendritic cells, ILCs or macrophages. Conversely, expression of 47% of the apoptosis-related analyzed gene was significantly modified in the mice gavaged with only amylase compared to the control group, although no difference was observed in the abundance of neutrophils, dendritic cells, ILCs or macrophages. This amount of differentially expressed genes can be explained by the fact that amylase alone is capable of disrupting the biofilm present on the surface of the intestinal lumen, hence altering the abundance of microorganisms already present in the gut and their consequent interaction with the immune subsets present in the mucosa. Our hypothesis is corroborated by 16S rRNA analysis published by our team in a previous paper indicating changes in relative bacterial abundance after probiotic and amylase treatment, specifically affecting species belonging to the genus
Lachnoclostridium and the species
Mucispirillum schaedleri (increased), and species belonging to the family
Lactobacillaceae and the species
Selenomonas lacticifex (decreased) [
21].
Interestingly, comparing the differentially expressed genes between probiotic+amylase group and amylase group, we noticed a consistent increased expression in the probiotic+amylase mice of gene family members encoding for linker histone H1 proteins such as
H1f0,
H1f1,
H1f3 and
H1f5, which normally are responsible for chromatin condensation. Essential steps during early apoptotic process involve DNA cleavage and H1 histone-dependent induction of chromatin condensation [
30]. Multiples studies reported an increased expression of genes encoding for linker histone H1 proteins [
31] during early apoptosis only observed in correlation with the event of DNA fragmentation, thus possibly indicating a prerequisite for accessibility to DNA and endonuclease activity. Therefore, we speculate that the probiotic strains utilized in our experiment were responsible for inducing the expression of genes encoding for linker histone H1 proteins in mucosal immune cell subsets, and ,as a consequence, triggering a higher degree of programmed cell death, downstream promoting resolution of inflammation. The downregulated expression of genes encoding for linker histone H1 proteins observed in the amylase-treated group might be the reason for the observed decreased apoptosis and the consequent similar level of inflammation compared to the control group.
The probiotics strains used in our experiment have been documented in literature to exhibit pro-apoptotic activity towards neutrophils and other cell types. Specifically, Sustrova et al. demonstrated that
L. rhamnosus exerts pro-apoptotic effects on human neutrophils in vitro [
32]. Moreover,
S. boulardii [33] and B. breve [34] have also been confirmed to have pro-apoptotic properties in vitro, as both probiotics have been tested on human cancer cells. Since dysregulated infiltration of neutrophils into the gut mucosal layer can enhance severe tissue damage, neutrophil apoptosis needs to be tightly regulated to maintain mucosal homeostasis [
35]. A direct interaction between probiotic strains and immunocompetent cells in the intestine (including lymph nodes, Payer’s patches and crypts) can promote and modulate apoptosis in immune cells [
36,
37]. These evidence clearly indicate that in our experiment, biofilm disruption caused by amylase allowed the probiotics strains to infiltrate the mucosa, causing a higher degree of apoptosis in immune cell populations (particularly in neutrophils), facilitating resolution of inflammation.
Moreover, DSP analysis showed that both
Caspase (
Casp)3 and
Fas genes were significantly upregulated in the probiotic+amylase-treated mice compared to the control group. Our data concurred with a previous work by Luo et al. [
38] showing that decreased
Casp3 expression was correlated with delayed Fas-mediated apoptosis in neutrophils present in the umbilical cord, contributing to chronic inflammation. Besides
Casp3, we also found increased
Casp7 expression in leukocytes of probiotic+amylase treated mice compared to the remaining three groups. Our results are in line with a study by Akhter et al. [
39], showing that
Casp7-/- mice harbor macrophages unable to inhibit the replication of intracellular bacterial pathogens such as
Legionella pneumophila due to delayed induction of macrophage apoptosis, leaving them more susceptible to infection. The fact that both
Casp3 and
Casp7 were both consistently increased in the ROIs of the probiotic+amylase mice compared to the other three groups is not surprising, since both genes code for proteases which have multiple endogenous substrates in common and are both activated during apoptosis by the caspases-8 and -9 [
40].
In addition, the data related to increased expression of
Tnfsf10,
Tnfrsf10b,
Ripk1,
Fadd and
Casp8 in CD45
+ cells suggest that programmed cell death was triggered in immune cells also through the RIPK1-dependent apoptosis (RDA) pathway. In fact, this pathway starts with activation of Tumor Necrosis Factor Receptor 1 (TNFR1), which in turn activates the formation of the TNFR1 complex, leading to the recruitment of multiple E3 ubiquitin ligases, which contribute to the ubiquitination of RIPK1. The subsequent deubiquitination of RIPK1 promotes the production of the pro-apoptotic complex-IIb formed by FADD, Caspase-8 and RIPK1, which mediates the RDA[
41]. These findings highlight the importance of the mucosal layer as a functional hotspot in IBD where distinct interactions between gut-residing microorganisms and innate immune cells may contribute to aggressive CD manifestation characterized by high relapse rate, need of recurrent surgeries and penetrating disease[
42]. Interestingly, a co-evolution theory between immune cells and their microenvironment has been already suggested [
43], and our data support the idea that this phenomenon may occur preferentially in the lamina propria.
Finally, the TUNEL assay data coupled with IHC staining confirmed the presence of an increased number of immune cells in active phase of apoptosis and decreased number of neutrophils in the mucosal layer of probiotic+amylase treated mice compared to the control group, confirming our theory that amylase’ anti-biofilm activity allows probiotic strains to infiltrate deeper in the intestinal mucosa, triggering apoptosis in immune cells in the lamina propria, particularly affecting neutrophils.
In conclusion, our data demonstrate a beneficial role of our recently developed probiotic and amylase blend in ameliorating ileitis in the SAMP mice. The main beneficial effect was derived by an increased induction of apoptosis in neutrophils present in the lamina propria which in turn facilitate the resolution of inflammation. Chronic non-resolving intestinal mucosal inflammation, involving innate immune responses, plays a crucial role in IBD pathophysiology [
44], therefore, we think that our recently developed probiotic blend may be suited to be tested in CD clinical trials.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on
Preprints.org.
Author Contributions
Writing – Original Draft Preparation, Formal Analysis, Carlo De Salvo, Methodology, Formal Analysis, Abdullah Some, Writing – Review & Editing, Mahmoud Ghannoum, Supervision, Funding Acquisition, Writing – Review & Editing, Fabio Cominelli, Conceptualization, Funding Acquisition, Writing – Review & Editing, Luca Di Martino. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Institutes of Health grants DK042191, DK055812, DK091222 and DK097948 to Fabio Cominelli, DK125526 and 5P30DK097948 to Luca Di Martino and AI145289 to Mahmoud Ghannoum.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, LDM, upon reasonable request. Data will be stored for a long-term period (minimum 5 years) in the Box storage service (hosted in the cloud) that enables Case Western Reserve University to store, access and share files securely. Box is the only approved platform for storing restricted data in the cloud at Case Western Reserve University.
Acknowledgements
The authors acknowledge the services of the Histology/Imaging Core and the Mouse Models core of the NIH Cleveland Digestive Diseases Research Core Center.
Conflicts of Interest
Mahmoud Ghannoum is a co-founder of BIOHM health LLC.
Ethical Considerations
This manuscript is an original contribution not previously published (except as an abstract), and is not under consideration for publication elsewhere.
References
- Perez-Lopez, A.; Behnsen, J.; Nuccio, S.P.; Raffatellu, M. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol 2016, 16, 135-148. [CrossRef]
- Hoarau, G.; Mukherjee, P.K.; Gower-Rousseau, C.; Hager, C.; Chandra, J.; Retuerto, M.A.; Neut, C.; Vermeire, S.; Clemente, J.; Colombel, J.F.; et al. Bacteriome and Mycobiome Interactions Underscore Microbial Dysbiosis in Familial Crohn’s Disease. mBio 2016, 7. [CrossRef]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Li, N.; Li, J. Dysbiosis of gut fungal microbiota is associated with mucosal inflammation in Crohn’s disease. J Clin Gastroenterol 2014, 48, 513-523. [CrossRef]
- Aleksandrowicz, A.; Carolak, E.; Dutkiewicz, A.; Blachut, A.; Waszczuk, W.; Grzymajlo, K. Better together-Salmonella biofilm-associated antibiotic resistance. Gut Microbes 2023, 15, 2229937. [CrossRef]
- Ellermann, M.; Sartor, R.B. Intestinal bacterial biofilms modulate mucosal immune responses. J Immunol Sci 2018, 2, 13-18.
- Zheng, S.; Bawazir, M.; Dhall, A.; Kim, H.E.; He, L.; Heo, J.; Hwang, G. Implication of Surface Properties, Bacterial Motility, and Hydrodynamic Conditions on Bacterial Surface Sensing and Their Initial Adhesion. Front Bioeng Biotechnol 2021, 9, 643722. [CrossRef]
- Thoo, L.; Noti, M.; Krebs, P. Keep calm: the intestinal barrier at the interface of peace and war. Cell Death Dis 2019, 10, 849. [CrossRef]
- Lee, J.Y.; Tsolis, R.M.; Baumler, A.J. The microbiome and gut homeostasis. Science 2022, 377, eabp9960. [CrossRef]
- Kechagia, M.; Basoulis, D.; Konstantopoulou, S.; Dimitriadi, D.; Gyftopoulou, K.; Skarmoutsou, N.; Fakiri, E.M. Health benefits of probiotics: a review. ISRN Nutr 2013, 2013, 481651. [CrossRef]
- Chen, Y.; Cui, W.; Li, X.; Yang, H. Interaction Between Commensal Bacteria, Immune Response and the Intestinal Barrier in Inflammatory Bowel Disease. Front Immunol 2021, 12, 761981. [CrossRef]
- Aas, J.; Gessert, C.E.; Bakken, J.S. Recurrent Clostridium difficile colitis: case series involving 18 patients treated with donor stool administered via a nasogastric tube. Clin Infect Dis 2003, 36, 580-585. [CrossRef]
- Morelli, L.; Capurso, L. FAO/WHO guidelines on probiotics: 10 years later. J Clin Gastroenterol 2012, 46 Suppl, S1-2. [CrossRef]
- Smug, L.N.; Salminen, S.; Sanders, M.E.; Ebner, S. Yoghurt and probiotic bacteria in dietary guidelines of the member states of the European Union. Benef Microbes 2014, 5, 61-66. [CrossRef]
- Maldonado Galdeano, C.; Cazorla, S.I.; Lemme Dumit, J.M.; Velez, E.; Perdigon, G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann Nutr Metab 2019, 74, 115-124. [CrossRef]
- Zitvogel, L.; Pietrocola, F.; Kroemer, G. Nutrition, inflammation and cancer. Nat Immunol 2017, 18, 843-850. [CrossRef]
- Aron-Wisnewsky, J.; Warmbrunn, M.V.; Nieuwdorp, M.; Clement, K. Metabolism and Metabolic Disorders and the Microbiome: The Intestinal Microbiota Associated With Obesity, Lipid Metabolism, and Metabolic Health-Pathophysiology and Therapeutic Strategies. Gastroenterology 2021, 160, 573-599. [CrossRef]
- Benton, D.; Williams, C.; Brown, A. Impact of consuming a milk drink containing a probiotic on mood and cognition. Eur J Clin Nutr 2007, 61, 355-361. [CrossRef]
- Kadooka, Y.; Sato, M.; Imaizumi, K.; Ogawa, A.; Ikuyama, K.; Akai, Y.; Okano, M.; Kagoshima, M.; Tsuchida, T. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr 2010, 64, 636-643. [CrossRef]
- So, D.; Quigley, E.M.M.; Whelan, K. Probiotics in irritable bowel syndrome and inflammatory bowel disease: review of mechanisms and effectiveness. Curr Opin Gastroenterol 2023, 39, 103-109. [CrossRef]
- Hager, C.L.; Isham, N.; Schrom, K.P.; Chandra, J.; McCormick, T.; Miyagi, M.; Ghannoum, M.A. Effects of a Novel Probiotic Combination on Pathogenic Bacterial-Fungal Polymicrobial Biofilms. mBio 2019, 10. [CrossRef]
- Di Martino, L.; Osme, A.; Ghannoum, M.; Cominelli, F. A Novel Probiotic Combination Ameliorates Crohn’s Disease-Like Ileitis by Increasing Short-Chain Fatty Acid Production and Modulating Essential Adaptive Immune Pathways. Inflamm Bowel Dis 2023. [CrossRef]
- Corridoni, D.; Kodani, T.; Rodriguez-Palacios, A.; Pizarro, T.T.; Xin, W.; Nickerson, K.P.; McDonald, C.; Ley, K.F.; Abbott, D.W.; Cominelli, F. Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc Natl Acad Sci U S A 2013, 110, 16999-17004. [CrossRef]
- Hernandez, S.; Lazcano, R.; Serrano, A.; Powell, S.; Kostousov, L.; Mehta, J.; Khan, K.; Lu, W.; Solis, L.M. Challenges and Opportunities for Immunoprofiling Using a Spatial High-Plex Technology: The NanoString GeoMx((R)) Digital Spatial Profiler. Front Oncol 2022, 12, 890410. [CrossRef]
- Dessau, R.B.; Pipper, C.B. [‘‘R”--project for statistical computing]. Ugeskr Laeger 2008, 170, 328-330.
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil apoptosis: relevance to the innate immune response and inflammatory disease. J Innate Immun 2010, 2, 216-227. [CrossRef]
- Luo, A.; Wang, F.; Sun, D.; Liu, X.; Xin, B. Formation, Development, and Cross-Species Interactions in Biofilms. Front Microbiol 2021, 12, 757327. [CrossRef]
- Oliveira, M.; Cunha, E.; Tavares, L.; Serrano, I. P. aeruginosa interactions with other microbes in biofilms during co-infection. AIMS Microbiol 2023, 9, 612-646. [CrossRef]
- Cangui-Panchi, S.P.; Nacato-Toapanta, A.L.; Enriquez-Martinez, L.J.; Salinas-Delgado, G.A.; Reyes, J.; Garzon-Chavez, D.; Machado, A. Battle royale: Immune response on biofilms - host-pathogen interactions. Curr Res Immunol 2023, 4, 100057. [CrossRef]
- Ikeda, Y.; Saigo, N.; Nagasaki, Y. Direct evidence for the involvement of intestinal reactive oxygen species in the progress of depression via the gut-brain axis. Biomaterials 2023, 295, 122053. [CrossRef]
- Kijima, M.; Yamagishi, H.; Hara, Y.; Kasai, M.; Takami, Y.; Takemura, H.; Miyanari, Y.; Shinkai, Y.; Mizuta, R. Histone H1 quantity determines the efficiency of chromatin condensation in both apoptotic and live cells. Biochem Biophys Res Commun 2019, 512, 202-207. [CrossRef]
- Kratzmeier, M.; Albig, W.; Meergans, T.; Doenecke, D. Changes in the protein pattern of H1 histones associated with apoptotic DNA fragmentation. Biochem J 1999, 337 ( Pt 2), 319-327.
- Sustrova, T.; Ondrackova, P.; Leva, L.; Kolarova, M.; Kulich, P.; Sladek, Z. Effect of probiotics on the viability of porcine and human neutrophils in vitro. Veterinární medicína 2017, 62.
- Pakbin, B.; Pishkhan Dibazar, S.; Allahyari, S.; Javadi, M.; Farasat, A.; Darzi, S. Probiotic Saccharomyces cerevisiae var. boulardii supernatant inhibits survivin gene expression and induces apoptosis in human gastric cancer cells. Food Sci Nutr 2021, 9, 692-700. [CrossRef]
- Li, Q.; Li, Y.; Wang, Y.; Xu, L.; Guo, Y.; Wang, Y.; Wang, L.; Guo, C. Oral administration of Bifidobacterium breve promotes antitumor efficacy via dendritic cells-derived interleukin 12. Oncoimmunology 2021, 10, 1868122. [CrossRef]
- Martin, C.J.; Peters, K.N.; Behar, S.M. Macrophages clean up: efferocytosis and microbial control. Curr Opin Microbiol 2014, 17, 17-23. [CrossRef]
- Galdeano, C.M.; Perdigon, G. The probiotic bacterium Lactobacillus casei induces activation of the gut mucosal immune system through innate immunity. Clin Vaccine Immunol 2006, 13, 219-226. [CrossRef]
- Angulo, S.; Morales, A.; Danese, S.; Llacuna, L.; Masamunt, M.C.; Pultz, N.; Cifone, M.G.; De Simone, C.; Delgado, S.; Vila, J.; et al. Probiotic sonicates selectively induce mucosal immune cells apoptosis through ceramide generation via neutral sphingomyelinase. PLoS One 2011, 6, e16953. [CrossRef]
- Luo, D.; Schowengerdt, K.O., Jr.; Stegner, J.J.; May, W.S., Jr.; Koenig, J.M. Decreased functional caspase-3 expression in umbilical cord blood neutrophils is linked to delayed apoptosis. Pediatr Res 2003, 53, 859-864. [CrossRef]
- Akhter, A.; Gavrilin, M.A.; Frantz, L.; Washington, S.; Ditty, C.; Limoli, D.; Day, C.; Sarkar, A.; Newland, C.; Butchar, J.; et al. Caspase-7 activation by the Nlrc4/Ipaf inflammasome restricts Legionella pneumophila infection. PLoS Pathog 2009, 5, e1000361. [CrossRef]
- Lamkanfi, M.; Kanneganti, T.D. Caspase-7: a protease involved in apoptosis and inflammation. Int J Biochem Cell Biol 2010, 42, 21-24. [CrossRef]
- Ju, E.; Park, K.A.; Shen, H.M.; Hur, G.M. The resurrection of RIP kinase 1 as an early cell death checkpoint regulator-a potential target for therapy in the necroptosis era. Exp Mol Med 2022, 54, 1401-1411. [CrossRef]
- Yarur, A.J.; Strobel, S.G.; Deshpande, A.R.; Abreu, M.T. Predictors of aggressive inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2011, 7, 652-659.
- Mazmanian, S.K.; Liu, C.H.; Tzianabos, A.O.; Kasper, D.L. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell 2005, 122, 107-118. [CrossRef]
- Drury, B.; Hardisty, G.; Gray, R.D.; Ho, G.T. Neutrophil Extracellular Traps in Inflammatory Bowel Disease: Pathogenic Mechanisms and Clinical Translation. Cell Mol Gastroenterol Hepatol 2021, 12, 321-333. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).