1. Introduction
Chronic mountain sickness (CMS), also known as Monge’s disease, is a prevalent and progressive syndrome affecting many high-altitude regions around the world. Over 140 million people live above 2,500 meters globally, with an estimated 5%–10% at risk of developing CMS[
1]. This disorder, first described by Carlos Monge Medrano, primarily affects populations in the South American Andes and the North American Rockies[
2]. According to the 2018 Lake Louise Acute Mountain Sickness Score, diagnostic criteria for acute mountain sickness (AMS) include headache, weakness, gastrointestinal symptoms, and dizziness/light-headedness[
3]. Currently, the most effective treatment for CMS is relocation to lower elevations. For those unable or unwilling to migrate, medication may be prescribed. However, many of these drugs have significant side effects. For example, acetazolamide may cause sensory abnormalities in the limbs, frequent urination, difficulty swallowing, and taste disorders, while dexamethasone can lead to gastrointestinal irritation, hyperglycemia, and psychiatric disorders[
4]. Consequently, the search for safe, effective, and convenient alternative therapies remains a key focus of high-altitude medicine research due to the limitations of Western medicines in terms of accessibility and safety.
Nanoparticles (NPs) are versatile drug carriers developed to transport various therapeutic agents into cells. The advancement of nanomaterials offers powerful tools and new directions for medical diagnostics. Since the 21st century, nanotechnology has become a prominent area of research, with significant progress in the development and application of nanomaterials and nanodevices[
5]. Inorganic nanocarriers, as a novel class of drug carriers, show promising potential in achieving targeted drug delivery, controlled drug release, and improving the solubility and bioavailability of poorly soluble drugs, thereby enhancing drug efficacy[
6]. NPs possess significant anti-inflammatory and antioxidant properties and have demonstrated therapeutic effects in several chronic pathological conditions[
7]. Furthermore, nanomaterials can deliver drugs directly to targeted tissues and cells, offering potential for optimizing CMS treatment. This paper reviews recent advancements in nanodelivery strategies, focusing on inorganic nanocarriers, targeted molecules, and drug therapies, aiming to propose new therapeutic approaches for CMS in clinical settings. Additionally, future discussions will explore research on gene and viral nanocarriers.
2. Pathogenesis of CMS
Hypoxia levels in high-altitude areas can cause CMS, a clinical syndrome characterized by erythropoietic polycythemia (HAPC), hypoxia, and pulmonary hypertension[
8]. This condition primarily affects individuals residing above 2,500 meters above sea level. Clinical manifestations of CMS include symptoms such as dizziness, headache, nausea, vomiting, and dyspnea. In severe cases, it may progress to high-altitude pulmonary edema (HAPE), high-altitude cerebral edema (HACE), and other complex conditions[
9,
10,
11]. Long-term exposure to hypoxia can lead to pulmonary vasoconstriction and remodeling, increasing pulmonary circulatory resistance and pulmonary arterial pressure. This sequence of events often results in hypoxic pulmonary hypertension, which exacerbates the afterload of the right ventricle, causing largely irreversible pathological changes that significantly diminish the quality of life and life expectancy of high-altitude inhabitants[
12]. The hypoxic environment also contributes to myocardial hypoxia, oxidative stress, inflammatory responses, fibrosis, apoptosis, and other injuries, affecting cardiac structure and function[
13].
2.1. Hypoxia-induced factor accumulation and regulation
The development of hypoxic pulmonary arterial hypertension (HPAH) reduces the oxygen supply to pulmonary artery smooth muscle cells, decreasing intracellular mitochondrial hydrogen peroxide production and altering cellular redox signaling pathways. This change pathologically stimulates transcription factors. The hypoxia inducible factor (HIF), composed of heterodimers of HIF-1α, HIF-2α, HIF-3α, and HIF-β subunits, plays a critical role in cellular homeostasis as a major transcriptional regulator in hypoxic environments and cellular inflammatory responses. Under aerobic conditions, the HIF-α subunit is quickly inactivated by oxygen-dependent hydroxylation by HIF proline hydroxylase (HPH), leading to its ubiquitination and subsequent proteasomal degradation. In hypoxic conditions, HPH activity is inhibited, allowing stable accumulation of the HIF-α subunit and its dimerization with HIF-β, which triggers the transcription of genes that restore and maintain cellular oxygen availability. Animal studies have shown that mice deficient in HIF-1α exhibit reduced pulmonary vascular remodeling and pulmonary hypertension under chronic hypoxic conditions, but may experience worsened right ventricular hypertrophy and remodeling [
14]. Additionally, research by Hu et al. found that administering a HIF-2α inhibitor to hypoxic mice reduced mean pulmonary artery pressure and attenuated right ventricular remodeling, highlighting the significant role of HIF-2 in pulmonary vascular and ventricular remodeling responses to chronic hypoxia[
14].
2.2. Oxidative stress leads to CMS
Oxidative stress represents an imbalance between oxygenation and antioxidant effects in the body, leading to excessive production of reactive oxygen species (ROS) that surpasses the body’s capacity to eliminate them. ROS can easily react with various cellular structural components, affecting cellular differentiation, cellular apoptosis, and the immune system, thereby causing oxidative stress damage[
15]. SaradaS et al. observed significantly increased levels of ROS, lipid peroxidation, and superoxide dismutase (SOD) in rats exposed to a low-pressure hypoxic environment at an altitude of 7620 m[
16]. Studies have shown that exposure to high altitudes increases ROS production in capillary blood vessels while decreasing their antioxidant capacity [
17]. These pathophysiological changes are closely associated with various CMS conditions. Nuclear factor E2-related factor 2 (Nrf2) is a transcription factor that regulates the expression of several antioxidant proteins. Under physiological conditions, Nrf2 and Kelch-like ECH-associated protein 1 (Keap1) bind together in the cytoplasmic lysate. Upon the occurrence of oxidative stress, Nrf2 translocates to the nucleus and induces the expression of ROS detoxifying enzymes, effectively preventing ROS accumulation. This upregulation of Nrf2 in high-altitude environments has been confirmed as an effective alternative treatment for CMS[
18].
2.3. Inflammation leads to CMS
Rapid activation of inflammatory processes occurs during exposure to high altitude, low pressure, and low oxygen environments[
19]. Hartmann et al. examined inflammatory factors in the serum of volunteers exposed to sea level and high altitude (4350 m above sea level) at various time points. They found that only the level of interleukin-6 (IL-6) was consistently elevated, while other factors, such as interleukin-1β (IL-1β), tumor necrosis factor-alpha (TNF-α), and C-reactive protein (CRP), showed no significant changes. This suggests that IL-6 is more sensitive to high-altitude exposure. [
20]Additionally, serum levels of interleukin-2 (IL-2), interleukin-3 (IL-3), and macrophage chemotactic protein-1 (MCP-1), as well as IL-1β and TNF-α, have all been implicated in the pathogenesis of CMS and altitude adaptation. The altered inflammatory factors may serve as biomarkers for CMS[
21]. TNF-α release and NF-κB activation increased oxidative stress (OS) and inflammation, and inflamed tissues may also be hypoxic[
22,
23]. Furthermore, hypoxia promotes oxidative stress, a condition closely linked to the inflammatory process[
24]. Possible functions and mechanisms in the pathogenesis of chronic mountain sickness are showed in
Figure 1.
3. Properties of Inorganic Nanomaterials
Nanomaterials, due to their extremely small size, exhibit numerous exceptional properties that conventional materials lack, including unique optical, electromagnetic, thermodynamic, and quantum mechanical characteristics[
25]. These attributes make them highly promising for a wide range of applications, particularly in the biomedical field. Nanotechnology facilitates the transformation of drugs into nanoscale particles, enhancing interactions between these drugs and cells (
Figure 2 and
Table 1). Furthermore, these nanoparticles can be engineered for targeted delivery to specific lesion areas and controlled release, thereby increasing drug bioavailability, reducing required dosages and treatment duration, and minimizing adverse effects[
26]. Inorganic nanomaterials demonstrate high biocompatibility and the ability to traverse biological barriers, such as the blood-brain barrier (BBB) and the intestinal barrier, facilitating the transport of both hydrophilic and hydrophobic drugs while providing protection against degradation[
27]. Consequently, the unique properties of inorganic nanomaterials suggest their potential application in the treatment of CMS.
4. Inorganic Nanomaterials in CMS
As a novel class of drug carriers, inorganic nanocarriers show considerable promise in enhancing targeted drug delivery, facilitating slow-release mechanisms, and improving the solubility and bioavailability of poorly soluble drugs, thereby increasing therapeutic efficacy. Inorganic nanomaterials primarily consist of metals (such as gold, silver, platinum, and nickel), metal oxide nanoparticles (including titanium dioxide, manganese dioxide, magnetite, and alumina), and semiconductors (such as silicon and ceramics) [
28].
4.1. Carbon nanomaterials in CMS
Carbon nanomaterials (CNMs) are a class of materials characterized by unique structural and physicochemical properties, allowing for widespread applications in biosensors and tissue engineering. They exhibit excellent tensile strength (exceeding 100 GPa), high mechanical integrity, and a modulus of elasticity exceeding 1 TPa, along with electrical and thermal conductivity. CNMs have been extensively used in the treatment of hypoxia-induced hypoxic pulmonary arterial hypertension (HPAH) and cardiac injury. Generally, based on their structure and size, CNMs can be categorized into zero-dimensional (such as fullerenes, nanodiamonds, and carbon dots), one-dimensional (including carbon nanotubes, diamond nanorods, and carbon nanofibers), two-dimensional (like graphene and its derivatives, as well as diamond nanosheets), and three-dimensional nanomaterials (which comprise diamond-like carbon films, nanocrystalline diamond films, and fullerite) [
29].
4.1.1. CNMs in Pulmonary Arterial Hypertension
Hypoxic pulmonary arterial hypertension (HPAH) affects approximately 1% of the global population and is particularly prevalent among individuals over the age of 65, impacting up to 10% of this demographic. HPAH is classified as a pulmonary vascular disease characterized by progressive increases in pulmonary vascular resistance (PVR) and pulmonary artery pressure (PAP), resulting from pulmonary vascular contraction and remodeling.
Currently, carbon nanoparticles are widely used in the treatment of pulmonary arterial hypertension (PAH) [
30,
31,
32,
33]. You’s study reported a delivery nano-system utilizing programmed DNA self-assembling mammalian target of rapamycin (mTOR) siRNA-loaded DNA nanotubes (DNA-nts), which can be efficiently transfected into pulmonary artery smooth muscle cells (PASMCs) via endocytosis under both normal and hypoxic conditions. PASMCs are crucial in the pathogenesis of HPAH [
34]. The mTOR siRNA significantly induced autophagy and inhibited cell growth, suggesting its potential therapeutic value for diseases associated with abnormal autophagy in PASMCs [
35]. This study presents a safe, efficient, and cost-effective delivery platform that is easily scalable. Moreover, the assembled mTOR siRNA-loaded DNA nanotubes demonstrated relative stability, biocompatibility, and low cytotoxicity, while significantly inhibiting hypoxia-induced proliferation of PASMCs. This type of nanocarrier may serve as a promising delivery vector for managing HPAH and other chronic cardiopulmonary diseases[
35].
The mechanistic target of mTOR is a critical signaling pathway in the body, encompassing various biological processes, including gene transcription, protein translation, ribosome synthesis, and apoptosis. mTOR significantly regulates protein translation, cell growth, and proliferation by integrating signals from growth factors, nutrients, and energy levels[
36]. In pulmonary vascular cells, autophagy exhibits a dual regulatory mechanism in the occurrence and development of HPAH. Increased expressions of LC3B and Beclin1 were observed in pulmonary artery endothelial cells (PAECs). Experiments showed that active LC3B in vascular cells may partially inhibit proliferation associated with vascular remodeling. This finding confirms that the effect of autophagy on the regulation of hypoxic pulmonary hypertension (HPH) is bidirectional[
37,
38]. According to these results, autophagy may serve as a point of interaction for binding inorganic nanoparticles in HPH. Leifer's study designed an inhaled sustained-release formulation of treprostinil (TRE), a common treatment for HPH, utilizing a lipid nanoparticle (LNP) carrier. This carrier, prepared through a proprietary process, consists of TPD, the hydrocarbon squalane, phospholipid DOPC, and pegylated lipid cholesterol-PEG 2000, resulting in a highly stable nanoparticle that can be readily adapted for large-scale production. The findings indicated that TRE plasma coated with CNMs could prolong pulmonary vascular relaxation under hypoxia compared to conventional TRE solutions [
39].
As a metallic material, it can cause damage when introduced into an organism. MORIMOTO's study confirmed that exposure to fullerenes does not induce inflammation or causes only transient inflammation in the lungs. However, exposure to high concentrations of multi-walled carbon nanotubes (MWCNTs) and single-walled carbon nanotubes (SWCNTs) is associated with inflammation and granuloma formation in lung tissue[
40]. The application of carbon nanomaterials (CNMs) in low-oxygen environments can have similarly detrimental effects. An in vitro HPAH model study demonstrated that CNMs under hypoxic conditions lead to the production of reactive oxygen species (ROS), increased cellular production of nitrite and nitrate, and secretion of the pro-inflammatory mediator IL-6 [
41].
4.1.2. CNMs in Cardiac Hypertrophy
Sustained or periodic hypoxia in an organism can lead to irreversible physiological disturbances. Hypoxia can precipitate various metabolic diseases, myocardial hypertrophy, and myocardial ischemia. Consequently, monitoring and treating organisms in hypoxic environments is crucial for human health and development. Chronic exposure to hyperbaric hypoxia in high-altitude environments can cause structural and functional changes in the right ventricle of rats [
42]. Due to the interdependence of the left and right ventricles, changes in pressure and volume in one ventricle typically affect the other, ultimately resulting in total cardiac dysfunction[
43].
Lipid core nanoparticles (LDE) used as carriers for the antitumor agent methotrexate (MTX) significantly increase the cellular uptake of MTX. The literature suggests that combined LDE-MTX treatment reduces vascular inflammation and myocardial infarction (MI) area in hypoxic environments, while inhibiting the development of cardiac hypertrophy and decreasing apoptosis, macrophage infiltration, and ROS production [
44]. The combination of MTX and nanomaterials is often used in treating cardiac insufficiency. Natalia's study used paper nanoparticles encapsulating MTX to improve cardiac insufficiency and hypertrophy in rats[
45]. In addition to conventional CNMs, composite fibers prepared using carbon nanofibers and conductive polymers can theoretically combine the properties of both materials, improving their electrical properties. The functionality of nanoparticles (NPs) can be effectively integrated with the electrical conductivity of polyaniline (PANI) polymers, potentially endowing the materials with unique properties[
46,
47]. Composite nanomaterials are also applicable in hypoxic environments. Moradikhah's study used a carbon nanofiber polycaprolactone mat polymerized in situ with PANI as a cell-free antioxidant cardiac patch (CP). This reduced oxidative stress generated during ischemia-reperfusion (I/R) following MI in hypoxic environments, decreased intracellular ROS content and caspase-3 mRNA levels, and mitigated the hypertrophic effects of hydrogen peroxide on H9c2 cells [
48].
4.1.3. CNMs in Heart Failure
Prolonged hypoxia tends to cause a progressive increase in pulmonary vascular resistance and an increase in right heart afterload, leading to pathological dilatation of the right ventricle, reduced per-pulse output, decreased cardiac contractility, and impaired right ventricle-pulmonary artery coupling. Ultimately, this can result in right heart failure and death[
15,
49].
Cyclodextrin (CD) is a series of cyclic oligosaccharides composed of 6-12 D-glucopyranose units generated from straight-chain starch through the action of cyclodextrin glucosyltransferase. CD exhibits a unique property of having a hydrophilic exterior and a hydrophobic interior. Its hydrophobic cavity has a high capacity for molecular recognition and enrichment, enabling selective identification of various organic and inorganic molecules. The hydrophilic exterior, characterized by good water solubility, allows CD to function as a surface functionalization agent for nanomaterials, enhancing the dispersion of many non-water-soluble materials[
50,
51]. Currently, various forms of CD/CNT composites are widely used [
52,
53] CD preparations as oxygen nanocarriers have been shown to improve the survival of cardiomyoblasts in hypoxic environments (by 12%-20%), thus reducing the damage from heart failure and demonstrating the potential of CNMs for cardiovascular therapy[
54].
4.2. Silicon dioxide nanomaterials in CMS
In recent years, research and application of nanomaterials in the biomedical field have rapidly advanced, enhancing the sensitivity and targeting of drug therapies. Therapeutic platforms based on silica nanoparticles offer several advantages, including highly controllable size and shape, low toxicity, good biocompatibility, stability under physiological conditions, and biosafety, making them suitable for clinical applications[
55]. Silica nanoparticles can be classified into non-porous and mesoporous silica nanoparticles (MSNs). MSNs possess a large specific surface area and a large pore size structure, which endows them with unique biological properties[
56].
Due to the limitations of CNMs, such as short half-life and rapid carbon monoxide release leading to toxicity issues, MSNs are utilized as an alternative to enhance biological efficiency[
57]. The large surface area and pore volume of MSNs, along with their excellent biocompatibility, make them effective therapeutic carriers. Studies have investigated whether mesoporous silica (MSN-A-CORM-2) encapsulated with carbon monoxide-releasing molecule-2 (CORM-2) enhances its anti-hypoxic effect in cardiac cell lines H9 C2 (cardiomyoblasts) and 3 T3 (fibroblasts). Using scanning electron microscopy, transmission electron microscopy, and infrared spectroscopy, researchers have shown that encapsulation of hypoxic cardiac cells can significantly impact cell viability. Under hypoxic conditions, cell viability in H9 C2 and 3 T3 cells decreased by 46% and 54%, respectively. However, encapsulation of CORM-2 in mesoporous silica increased cell viability by 30% in H9 C2 cells and 29% in 3 T3 cells from hypoxia-injured and H/R-injured cells, respectively[
58]. The hypoxic environment at high altitudes stimulates sympathetic nerve activity and myocardial contractility, leading to increased hemodynamic load, which can induce acute cardiac decompensation, resulting in myocardial ischemia and potential myocardial infarction (MI)[
59]. Additionally, hypoxia can stimulate excessive reactive oxygen species (ROS) production in mitochondria, impairing the body's antioxidant capacity and disrupting the oxidative-antioxidant balance, potentially leading to lipid peroxidation and further affecting cardiac function [
60]. Liu's study utilized quercetin mesoporous silica nanoparticles (Q-MSNs) in a hypoxic environment on the myocardium of ischemia-reperfusion-injured rats. The study elucidated the mechanisms through the JAK2/STAT3 pathway, demonstrating that, compared to quercetin alone, Q-MSNs more effectively improved myocardial oxidative stress levels, inhibited myocardial cell apoptosis, reduced the infarcted area, and promoted cardiac blood flow recovery, thereby enhancing protective effects on the rat heart under hypoxic conditions[
61].
4.3. Gold nanomaterials in CMS
Gold was the first metallic element to be studied, and compared to other metals, it exhibits high resistance to oxidation and stability in chemical reactions[
62]. Gold nanoparticles (AuNPs) are defined as gold particles with at least one dimension measuring less than 100 nm in a three-dimensional structure. Their basic units consist of tiny-sized particles, granting them unique physicochemical properties that macroscopic particles lack, such as optical properties, plasmonic resonance, fluorescence, and adsorption capabilities [
63].
4.3.1. Gold Nanomaterials in Pulmonary Arterial Hypertension
Studies indicate that lung inflammation plays a significant role in the formation and development of hypoxic pulmonary arterial hypertension (HPAH). One study found that hypoxia increased HIF-1α mRNA expression over time, peaking after 24 hours, while HIF-2α expression also rose. However, NF-κB mRNA levels remained unchanged, and plasma levels of IL-1β and IL-6 stayed within the normal range. Prolonged exposure to hypoxia and low pressure resulted in a switch between HIF-1α and HIF-2α/nuclear factor erythroid 2-related factor 2 (NRF2), suggesting a close relationship between the development of HPAH, inflammation, and oxidative stress[
64].
AuNPs exhibit strong anti-inflammatory and antioxidant capacities. Ponnanikajamideen's study demonstrated that AuNPs can eliminate lipid peroxidation and nitric oxide[
65]. HPAH is characterized by airway hyperreactivity, which is associated with peribronchial fibrosis, mucus production, and the production of pro-inflammatory cytokines[
66]. The effects of nanocapsules on monocrotaline (MCT)-induced HPAH showed that nanoparticles increased the activities of statin (SH), superoxide dismutase (SOD), and nuclear factor erythroid 2-related factor (Nrf2), while decreasing oxidized glutathione (GSSG) levels. These changes significantly reduced the right ventricular hypertrophy index, indicating alleviation of HPAH symptoms[
7]. Free radicals play an important role in cellular responses under hypoxic conditions, with high concentrations leading to oxidative stress and cytotoxicity through the oxidation of nucleic acids, proteins, and lipids. Free radicals are primarily produced in the redox centers of the respiratory chain. Fan's study utilized fluorescent nanodiamonds (FNDs) to detect free radicals and employed quantum sensing to explore the relationships between hypoxia, free radicals, and cellular redox states, contributing to future investigations of AuNPs and cellular oxidative stress[
67].
4.3.2. Gold Nanomaterials in Myocardial Injury
Based on the structure and unique electrophysiological properties of myocardial tissue, improving existing myocardial tissue engineering materials to better mimic the microenvironment required for excitation-contraction coupling has become a key focus and challenge in the field. Recent studies indicate that novel conductive nanomaterials, with their excellent electrical conductivity, nanoscale size, and superior biocompatibility, can effectively enhance the electrical and mechanical microenvironment for seed cell survival. When combined with traditional natural or synthetic tissue engineering materials, these nanomaterials can support engineered cardiac tissue (ECT) transplantation, facilitating good electrophysiological integration between ECT and the in vivo myocardium after transplantation[
68].
An innovative Au-Se nanoplatform enhances the traditional Au-S bond by replacing it with a more stable Au-Se bond, while retaining the advantages of gold nanoparticles (AuNPs). This study simultaneously monitored the expression of myocardial apoptosis markers, Lon protease (Lon) and Caspase-3, in cardiomyocytes under hypoxic conditions with high precision using the new AuNPs. Additionally, the nanoprobe was coupled with the mitochondrial H₂O₂ probe MitoPY1, demonstrating the relationship between altered reactive oxygen species (ROS) and myocardial apoptosis[
69]. Apoptosis and oxidative stress are key factors contributing to organismal damage in high-altitude environments [
70]. As an important transcription factor, HIF-1α plays a significant role in the metabolic mechanisms of hypoxia. AuNPs can also be functionalized using sulfhydryl chemistry with HIF-1α junctions suitable for biological applications, significantly increasing their stability. By leveraging the peroxidase-like properties of gold nanoparticles, the expression of HIF-1α can be detected more sensitively in myocardial tissues of infarcted rats in hypoxic conditions [
71].
4.4. Magnetic nanomaterials in CMS
Unlike most existing biomedical nanomaterials, magnetic nanoparticles, such as iron oxide nanoparticles, can mediate external fields to produce local magnetic, thermal[
72], and mechanical effects[
73], as well as intrinsic enzyme-like catalytic activities[
74]. Moreover, iron oxide nanoparticles are among the few inorganic nanomaterials approved by the U.S. Food and Drug Administration (FDA) for clinical use.
Magnetic nanoparticles can generate mechanical effects in gradient or rotating magnetic fields, a phenomenon referred to as "nanomagnetism." This can modulate cellular functions, such as disrupting the cell membrane or cytoskeleton, leading to apoptosis or necrosis. Shen et al. reported that magnetic nanoparticles combined with epidermal growth factor (EGF) can self-assemble under a low-frequency field to form a magnetic knife (MNPS-EDF), resulting in lysosomal membrane rupture and cellular necrosis. The formation of these magnetic knives led to the cleavage of lysosomal and cellular membranes, causing the death of more than 90% of the cells[
75]. Furthermore, nano-magnetism can promote stem cell differentiation and the formation of functional tissues. Kang et al. demonstrated that by applying external fields of varying frequencies, they could control the oscillation rate of iron oxide nanoparticles complexed with spermacetyl-glycyl-aspartic acid (SPIO-RGD), facilitating remote manipulation of macrophage adhesion and polarization phenotypes in vivo and ex vivo [
76].
Under hypoxic conditions, transcription factors activate the HIF-1α-bFGF pathway, enhancing epithelial cell production and survival. HIF-1α is a key promoter of hypoxia-induced gene transcription and regulates angiogenesis through vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) [
77,
78], both of which are crucial for promoting new blood vessel formation through adaptive transcriptional changes in response to hypoxia. Literature has shown that VEGF pretreatment can increase post-infarction blood vessel levels, enhance blood vessel density, and mitigate myocardial injury[
79]. Studies using superparamagnetic iron oxide (SPIO) nanoparticle-labelled adipose-derived stem cells (SPIOASCs) implanted in infarcted myocardium have shown that hypoxia culture increases HIF-1α and VEGF mRNA expression in both ASCs and SPIOASCs. The results confirm that interventions with hypoxia or SPIOASCs promote therapeutic angiogenesis and enhance cardiac function recovery in infarcted myocardium[
80]. Mesoporous iron oxide nanoparticles (MION) serve as novel nanoparticles for constructing a targeted release system for diallyl trisulfide (DATS) (DATS@MION-PEG-LF). This system extends circulation time and targets the brain, exhibiting excellent biocompatibility, a controllable release pattern of H2S, and protective effects against in vitro hypoxia/reoxygenation after brain and cardiac ischemia. The protective effects primarily involve anti-apoptotic, anti-inflammatory, and antioxidant mechanisms[
81].
Magnetic resonance imaging (MRI) faces limitations in accurate quantification and sensitivity. These limitations can be overcome by designing multimodal nanoprobes that integrate with other imaging modalities to improve diagnostic sensitivity, accuracy, and quantification [
82]. For example, Li et al. developed Fe3O4/Au nanoparticles through a hydrothermal method and utilized them for magnetic resonance-computer tomography (MRI-CT) dual-modality imaging[
83]. In another study by Hnatiuk, allogeneic bone marrow mesenchymal stem cells (BMMSCs) transfected with a microcyclic vector encoding mutant HIF1-a (BMMSC-HIF) were injected into myocardial infarcted sheep, resulting in a 71.3% reduction in infarct volume. Enhanced long-term retention of BMMSC-HIF, attributed to HIF-1 overexpression, increased new vessel formation, and heart protection [
84], was observed using paramagnetic iron nanoparticles for cellular tracking. Studies on CMR testing for CMS [
42,
85] suggest that nanomaterials could be integrated with CMS assessments in the future.
4.5. Nickel nanoparticles in CMS
Nickel nanoparticles (NiONPs) possess high specific surface area, surface energy, magnetic properties, and low melting and ignition points, making them suitable for various industrial applications such as lithium-ion batteries, conductive coatings, pigments, and catalysts[
86]. Research has linked air pollution in high-altitude environments with increased incidences of cardiovascular and lung diseases [
87], implicating NiONPs in crossing the pulmonary epithelial barrier to interact directly with endothelial cells lining the arteries [
88]. These cells regulate vascular pressure and coagulation [
89], potentially inducing cardiovascular disease. NiONPs have also been implicated in oxidative stress in vitro and interact with the mechanosensitive ion channel TRPV4 to increase endothelin-1 (ET-1), a mediator involved in pulmonary arterial remodeling and the development of HPAH [
90,
91]. Germande's study on the toxic effects of NiONPs on human pulmonary artery endothelial cells (HPAEC) under hypoxic conditions confirmed that such environments increase ROS, nitrite, and IL-6 secretion in HPAECs, exacerbating HPAH development and adversely affecting populations at cardiovascular risk [
92].
5. Nanocarriers in CMS
5.1. Liposomes
Liposomes, a type of tiny lipid vesicle, are increasingly popular nanocarriers featuring an aqueous core surrounded by one or more phospholipid bilayers. These layers mimic the structure of biological membranes, enhancing biocompatibility and biodegradability. Liposomes are advantageous for prolonging the half-life of drugs, controlling drug release, and improving biocompatibility and safety[
93]. Additionally, they can deliver their payload selectively to disease sites through passive and/or active targeting, reducing systemic side effects, increasing tolerated doses, and improving therapeutic outcomes [
94].
Currently, liposomal nanoparticles are employed in various medical settings. Vaghasiya et al. developed a topical nano spray gel (NSG) formulation combining liposomal heparin sodium and ibuprofen for treating frostbite in extremely cold, high-altitude conditions [
95]. Research indicates that heparin aids in endothelial cell and capillary microcirculation repair[
96]. This study demonstrated that heparin liposomes promote wound healing both in vitro (using a scratch test on fibroblasts) and in vivo (in Sprague Dawley rats), while also reducing inflammatory cytokines such as IL-4, IL-6, IL-10, and TNF-α in the bloodstream [
95]. High-altitude hypobaric hypoxia can lead to pulmonary vasoconstriction and capillary leakage, increasing pulmonary arterial pressure and causing fluid accumulation in alveolar cavities, resulting in high-altitude pulmonary edema (HAPE) [
18]. Pulmonary artery pressure (PAP) is a crucial physiological indicator, and systems that release medication in response to PAP changes can facilitate early medical intervention. Li's study showed that multivesicular liposomes loaded with the calcium channel inhibitor albenesulfonic acid could effectively treat HAPE, with the drug being released into the vasculature within one hour of elevated pulmonary pressures, quickly reducing PAP[
97].
5.2. Extracellular Vesicles
Extracellular vesicles are membranous vesicles with a lipid bilayer structure, released spontaneously or under specific conditions by various cells, including immune cells, mesenchymal stem cells, epithelial cells, cancer cells, and other subcellular components. These vesicles primarily include exosomes from the endosomal pathway, microvesicles from membrane outgrowths, apoptotic bodies from apoptosis, and oncosomes from tumor cell membrane outgrowths. Due to challenges in determining their origin post-release, they are typically classified by size, with those under 100 nm or 200 nm termed small extracellular vesicles[
98,
99].
In high-altitude environments, endothelial cells and platelets are susceptible to damage, contributing to microangiopathy. Olaf's study, which analyzed platelet- and endothelial-derived extracellular vesicles in plasma samples from 39 mountaineers via flow cytometry, found significant elevations in CD31 neg CD42 blow/neg levels, indicating that extracellular vesicles serve as an early and sensitive marker of vascular damage[
100].
5.3. Polylactic acid-hydroxyacetic acid (PLGA)
Polylactic acid-hydroxyacetic acid (PLGA) is a degradable polymer compound widely used in the pharmaceutical field and beyond. PLGA can encapsulate various organic or inorganic materials, including small molecule drugs, vaccines, proteins, and metal particles. This versatility makes PLGA an ideal drug delivery system with potential for targeted applications [
101,
102]. Studies have shown that PLGA-encapsulated traditional Chinese medicine polysaccharides can effectively enhance the body's immune response [
103].
Macrophages are intrinsic immune cells widely distributed throughout the body, derived from circulating monocytes. They possess strong constitutive and pluripotent properties, allowing them to respond to local microenvironmental stimuli through polarization processes and acquire specific functional phenotypes to perform particular immune functions. Macrophages play a crucial role in both innate and adaptive immune responses through polarization [
104]. Typically, immune cells such as macrophages contribute to HPAH by altering lung tissue remodeling, influencing cell survival, proliferation, migration, and immunomodulation. An imbalance in macrophage M1 and M2 polarization is also a key factor in the progression of HPAH, leading to perivascular inflammation in the lung. In an animal model of HPAH, macrophage polarization exhibited dynamic changes over time; M1 macrophages were predominantly polarized in the early phase, while M2 macrophages became more prominent in the late phase. It was hypothesized that M1 macrophages are involved in the initial phase of inflammation by accelerating endothelial cell apoptosis, whereas M2 macrophages dominate in the reparative phase of inflammation and the subsequent phase of aberrant tissue remodeling by promoting the proliferation of smooth muscle and endothelial cells [
105]. Liu et al. reported an inhalable platform designed to overcome hypoxia-induced immune changes and alveolar damage. This platform utilizes encapsulated PLGA nanoparticles (NPs) with macrophage apoptotic vesicle membranes (cMAB)[
106]. The cMAB is pre-loaded with peroxidase nanocomplexes (NCs) and modified with pathology-responsive chains of the macrophage growth factor colony-stimulating factor (CSF), which exhibit a high affinity for alveolar epithelial cells (AECs). In a mouse model of hypoxic acute lung injury, these NCs can be efficiently translocated into mitochondria, thereby inhibiting inflammatory vesicle-mediated injury to AECs. This platform effectively targets hypoxia-induced immune changes and alveolar damage, and could be applied to various hypoxia-induced inflammatory lung injuries. Another study encapsulated HIF-1α siRNA in PLGA NPs using a complex emulsion method, which helped reveal mechanisms of bone marrow mesenchymal stem cell trafficking and the feasibility of nanomedicine delivery in a low-oxygen environment [
107]. These findings confirm the advantages of PLGA nanomaterials applied to a high-altitude environment.
5.4. Micelles
Micelles, a type of polymer-based nanomaterial, are nanoclusters with a shell/core structure formed by the self-assembly of amphiphilic block copolymers in aqueous solutions. Their hydrophobic core can encapsulate various hydrophobic anticancer drugs without altering their chemical structures, enhancing the stability and solubility of the drugs. Meanwhile, their hydrophilic shell and nanoscale size facilitate drug release and accumulation in vivo[
108]. However, micellar carriers are susceptible to internal environmental factors, which can cause structural damage, premature drug release, and other problems. Micelle NPs are formed through self-assembly of surfactants or amphiphilic polymers in water, and they are widely used as carriers for targeted drug delivery [
109].
One study developed hypoxia-responsive nano-micelles loaded with carmustine, a typical antitumor drug that acts by damaging DNA at the guanine O-6 position. In this nanosystem, hyaluronic acid acts as a tumor-targeting ligand, binding to CD44 receptors overexpressed on tumor cells, enhancing the stability of the nanoparticles [
110].
Tilianin-based nanomicelles covalently attach polyethylene glycol (PEG) to arylsulphide, forming amphiphilic diblock polymers that address Tilianin's water insolubility. These nanomicelles are highly effective hydrogen peroxide scavengers and inhibit Caspase-3 activity, thereby protecting cells from hypoxia-induced cytotoxicity. They also reduce levels of malondialdehyde (MDA), IL-1, and TNF-α, and suppress the expression of apoptosis, TLR 4, and nuclear transcription factor NF-κB p65 in hypoxia model rats[
111]. The mechanism of action of our nanocarrier-carried drugs on chronic mountain sickness is summarized in
Table 2
6. Nanomaterials for CMS
Currently, short-term exposure to high-altitude environments does not require specific treatment, as symptoms typically resolve within 2 to 3 days. The most effective preventive measures for CMS include continuous oxygen inhalation, a stepwise ascent to higher altitudes, or returning to lower altitudes for treatment. Severe cases can be managed with chemosynthetic drug therapy [
112]. Studies have shown that nanomaterials possess the largest specific surface area among all known materials, enabling them to effectively transport and deliver drugs. They can also be used for radioactivity labeling or modification, making them valuable for targeted drug delivery, cell imaging, molecular detection, and more. Additionally, nanomaterials exhibit good biocompatibility and degradability, allowing them to accumulate in specific organs with fewer side effects. Their slow-release properties can lower drug concentrations, thereby reducing toxic side effects. Furthermore, their stability makes them suitable for applications in bioimaging, antioxidants, and enhanced sustainability. These characteristics form the foundation for the use of nanomaterials in the medical field[
113].
6.1. Nanomaterials for HPAH
Although many drugs can improve the quality of life for patients with HPAH, they have several drawbacks, including short half-lives, instability, and lack of specificity, which limit their suitability for widespread clinical application[
114]. Nanoparticles (NPs) serve as a novel drug delivery system designed to optimize drug efficacy and minimize side effects. Intravenous prostacyclin agonists are effective in treating HPAH; however, they come with side effects and administration challenges. ONO 1301 is a molecule that provides long-lasting prostacyclin activity by inhibiting thromboxane synthase and exhibits a high binding affinity for the prostacyclin receptor[
115]. In a study involving an HPAH rat model, injecting ONO 1301 nanospheres resulted in decreased levels of IL-6, IL-1β, and TGF-α, as well as significant improvements in the percentage of intima-media thickness in the pulmonary vasculature and a notable decrease in right ventricular pressure compared to the control group. This demonstrates the potential application of NPs in hypoxic environments[
116]. Akagi et al. investigated the efficacy and safety of intratracheal administration of the prostacyclin analogue bezoprost (BPS), which was encapsulated in NPs for use in a hypoxic and MCT HPAH rat model, as well as in human HPAH pulmonary artery smooth muscle cells (PASMCs). Their findings indicated that a single administration of BPS NPs significantly reduced right ventricular pressure, right ventricular hypertrophy, and HPAH in the rats, improved survival rates, and did not cause inflammatory cell infiltration, hemorrhage, or fibrosis in the liver, kidney, spleen, or heart following administration of BPS NPs[
117].
Chinese medicines, known for their multi-component, multi-pathway, and multi-target properties, are safe and effective in treating HPAH. Combining them with NPs creates innovative drug formulations that overcome the limitations of current treatments. Research by Haddad et al. highlights the use of epigallocatechin gallate (EGCG), a key green tea component[
118], in NP-based treatments for HPAH. EGCG is noted for its broad pharmacological benefits, including protection against cardiovascular, neurodegenerative diseases, diabetes, and cancer[
119]. It also addresses iron accumulation and apoptotic injury in the hippocampus caused by high altitudes, while enhancing Nrf 2 and HO-1 expression to protect cardiac function[
85,
120].
6.2. Nanomaterials for Cardiac Injury
Most heart diseases, such as arrhythmias, MI, and myocardial hypertrophy, are caused by prolonged hypoxia of cardiomyocytes, primarily due to myocardial ischemia resulting from vascular obstruction caused by lipid deposits in the walls of coronary arteries. This leads to abnormal cardiac functioning[
121]. Human cardiac regeneration is limited by the lack of studies on cellular models of hypoxic cardiac tissue. Nanofibrous structures are suitable for transplanting and culturing cells, as they can accurately mimic the three-dimensional structure of human cardiac tissue using various mats with the appropriate physicochemical properties. These properties include sufficient elasticity to allow for cell contraction, a high surface area-to-volume ratio, and biocompatibility. Polyurethane nanofibrous mats have been shown to improve the expression of HIF-1α in human cardiomyocytes under hypoxic/reoxygenated conditions while simultaneously inhibiting cardiomyocyte apoptosis. Such studies support the search for an in vitro cardiac model and confirm the feasibility of NPs to intervene in cardiomyocytes exposed to hypoxic environments[
122]. Nitric oxide-releasing nanofibers have also been extensively investigated as potential therapeutic agents. In a study by Lee et al., biocompatible NO-releasing nanofibers were prepared from a mixture of polymers and methylaminopropyltrimethoxysilane (MAP 3) to assess their protective effect against HR injury in H9c2 cells. The results similarly demonstrated that NPs could protect the myocardium from HR injury [
123].
Growth hormone-releasing hormone (GHRH) agonists promote the repair of damaged cardiac tissues and hold significant potential in the treatment of MI. Xiang et al. designed a ROS-sensitive amphiphilic polymer, poly(ethylene glycol)-polypropylene sulfide-poly(ethylene glycol) (PEGPPS-PEG), which was combined with @MR409 nanobubbles to observe the effect of this composite nanomaterial on ROS levels in MI cells under hypoxic conditions. The results indicated that the composite nanomaterials could promote the recovery of cardiac function, providing a new approach for the treatment of MI in hypoxic environments [
124]. A strong association exists between MI and hemorrhagic shock, with fluid resuscitation rapidly replenishing blood volume and improving systemic perfusion. However, the use of large volumes of fluids can be damaging to the organism, increasing the burden on the heart and leading to coagulation abnormalities, cellular edema, and mitochondrial dysfunction, which limits therapeutic efficacy[
125]. TPP@PAMAM-MR (TPP-MR) is a novel cardiac resuscitation fluid with strong targeting properties for myocardial mitochondria. This new nanostructure, formed by wrapping L-malic acid with triphenylphosphine modified with polyamidamine (PAMAM), significantly reduces myocardial damage by increasing myocardial output and improving pulmonary artery pressure. Further studies showed that TPP-MR improved mitochondrial function in cardiomyocytes, reduced ROS levels in myocardial tissues, and suppressed the expression of iron death-associated proteins GPX 4, ACSL 4, and COX 2, leading to enhanced antioxidant capacity[
126]. We summarize the mechanisms by which different nanomaterials act on CMS in
Figure 2E and
Table 3.
7. Future Research Advances in Nanomaterials in CMS
Based on the data presented, we have summarized the future research directions for nanomaterial-based drug delivery systems in CMS into four key areas. These areas have been categorized, summarized, and potential future directions proposed.
7.1. Summary of HIF-α in nanomaterials
Hypoxia is the primary causative factor of altitude sickness. It has been demonstrated that HIF-1α signaling is involved in angiogenesis, glucose metabolism, and cell proliferation. HIF-1α was significantly upregulated in vitro and in vivo following exposure to nickel oxide nanoparticles (NiONP), and the accumulation of HIF-1α in the nuclei was linked to the loss of tight junctions and increased chemokine expression[
127]. HIF-1α signalling leads to involvement in angiogenesis, glucose metabolism, and cell proliferation. HIF-1α was significantly up-regulated in vitro and in vivo after exposure to nickel, NiONP, and nickel oxide nanoparticles, and nanonickel-induced HIF-1α nuclei accumulation was involved in nanonickel-induced loss of tight junctions, and chemokine expression. Inorganic nanomaterials can mitigate myocardial injury, restore cardiomyocyte function, and inhibit mitochondrial autophagy in hypoxic environments by modulating or silencing the expression of specific genes associated with HIF-1α and HIF-2α[
64,
76]. Therefore, further exploration of other inorganic nanoparticles is warranted to study pulmonary hypertension and myocardial injury in high-altitude environments.
7.2. Summary of ROS in nanomaterials
NPs have been shown to act as antioxidants, providing cytoprotective and disease-modifying therapeutic effects by scavenging ROS and reducing cellular oxidative stress. They also serve as effective drug delivery platforms for cardiovascular protective medications, enhancing the short half-life of conventional drugs in vivo. Thus, scavenging ROS and eliminating oxidative stress are crucial in treating CMS. Several studies have investigated the relationship between NPs and ROS within the framework of nanomaterial drug delivery systems[
41,
44,
60,
69,
92]. These investigations indicate that both inorganic and organic nanomaterials can pose risks to organisms, with NPs potentially reaching pulmonary circulation and contributing to increased pulmonary artery pressure and vascular tension, which can lead to atherosclerosis and ischemia[
60,
92]. Currently, research on treating CMS through ROS reduction through nanomaterials is limited and requires further exploration to advance this therapeutic strategy.
7.3. Diagnosis of CMS
CMR imaging is widely used as a non-invasive diagnostic tool for patients with CMS. It avoids the use of radiography or radioactive contrast agents, resulting in minimal side effects and a favorable safety profile. Numerous nanomaterials can serve as MRI contrast agents, significantly enhancing the resolution and sensitivity of magnetic resonance imaging. These agents have been widely utilized in clinical diagnostics, including MRI nanoprobes that target endothelial cells and macrophages. Gadolinium complexes (Gd-chelates) are the most commonly employed T1 contrast agents in clinical settings. However, the free gadolinium ions released during the metabolism of these contrast agents can be nephrotoxic and may lead to tissue fibrosis[
128]. By contrast, magnetic iron oxide nanoparticles exhibit good biocompatibility and superparamagnetism, with a tendency to be phagocytosed by the liver, spleen, and monocyte macrophage system. This suggests that they accumulate in areas of inflammatory response, which can be effectively identified. In the future, this technique could see increased application in diagnosing altitude-related illnesses[
129].
8. Conclusions and Perspectives
Inorganic nanomaterials offer several advantages, including ease of preparation, controllable shape and size, and simple surface modification. Their unique optical, electrical, and magnetic properties provide potential applications in imaging, targeted delivery, and synergistic drug therapy. These properties can lead to prolonged therapeutic effects, reduced drug side effects, and improved therapeutic efficiency. This study reviews the recent research progress on nanomaterials in the treatment of CMS. It first summarizes the current applications of inorganic nanomaterials in the treatment and diagnosis of CMS, including gold nanomaterials, CNMs, silicon nanomaterials, and magnetic nanomaterials. These materials can intervene in HPAH and myocardial injury through various mechanisms. However, due to the general toxicity, environmental impact, and high production costs of metallic materials, they remain in the stage of in vitro or animal testing, and their antioxidant biological mechanisms require further investigation.
Nanoparticles serve as gene carriers by encapsulating gene therapy molecules, such as DNA and RNA, or adsorbing them onto their surfaces. Specific targeting molecules, such as ligands and monoclonal antibodies, can be coupled to the surface of these nanoparticles. These targeting molecules bind to specific receptors on the surface of cells, facilitating cellular uptake and enabling safe and effective gene therapy. For instance, degradable nanoparticles can enhance the intracellular transport of VEGF antisense oligonucleotides into human retinal pigment epithelial cells, inhibiting mRNA expression and VEGF secretion. Polyamidoamine (PAMAM) dendrimers can serve as plasmid DNA carriers in place of β-cyclodextrin, with the PAMAM dendrimer/DNA complex enhancing catalase expression by 200-fold in in vitro assays[
130]. Given their unique potential, nanoparticles are expected to see widespread use in gene therapy as nanotechnology continues to develop.
Gene therapy targets various diseases caused by genetic abnormalities at the nucleic acid level. This approach can introduce therapeutic genes to correct missing or mutated genes in situ, or inhibit the abnormal expression of endogenous genes for therapeutic purposes. Nucleic acid drugs used in gene therapy include plasmid DNA (pDNA) that encodes specific proteins, messenger RNA (mRNA), antisense oligonucleotides for regulating target gene expression, short hairpin RNA, and small interfering RNA (siRNA). Among these, siRNAs developed based on the RNA interference (RNAi) mechanism have garnered significant attention due to their high specificity and efficiency. siRNAs can be encapsulated or conjugated with natural or synthetic carriers, facilitating efficient delivery to specific sites, cellular internalization in targeted tissues, and protection from serum degradation and macrophage phagocytosis[
131]. These vectors encompass both viral and non-viral types. However, the use of viral vectors raises safety concerns, such as high immunogenicity and the risk of insertional mutagenesis, while their low drug-carrying capacity and high production costs limit clinical applications. Recently, researchers have increasingly favored safe cationic liposomes, polymers like polyethyleneimine (PEI), and inorganic nanomaterials, which possess large gene-loading capacities and are amenable to scale-up. With the ongoing advancements in nanotechnology and gene therapy, their future use is anticipated to be widespread.
Author Contributions
B.L. and Y.Z. collected the literature, performed the analysis and interpretation, and wrote the manuscript. Y.Q., X.L., S.Z. K.Y. and R.Z. contributed to the literature collection and the analysis and interpretation of the results. D.Q. and X.L. contributed to the literature collection and analysis and revised the manuscript interpretation of the results. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (82160923, 82374425, 82260929); Applied Basic Research Programs of Science and Technology Commission Foundation of Yunnan Province (202301AS070053); Key Laboratory of Traditional Chinese Medicine for Prevention and Treatment of Neuropsychiatric Diseases, Yunnan Provincial Department of Education; Scientific Research Projects for High-level Talents of Yunnan University of Chinese Medicine (2019YZG01); Young Top-Notch Talent in 10,000 Talent Program of Yunnan Province (YNWR-QNBJ-2019-235).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Villafuerte F C, Corante N. Chronic Mountain Sickness: Clinical Aspects, Etiology, Management, and Treatment[J]. High Alt Med Biol, 2016, 17(2): 61-9. [CrossRef]
- MONGE,C.LIFE IN THE ANDES AND CHRONIC MOUNTAIN SICKNESS[J].Science, 1942, 95(2456):79-84. [CrossRef]
- Roach R C, Hackett P H, Oelz O, et al. The 2018 Lake Louise Acute Mountain Sickness Score[J]. High Alt Med Biol, 2018, 19(1): 4-6.
- Luo H, Liao X, Tang Q, et al. Traditional Chinese medicine for acute mountain sickness prevention: A systematic review and meta-analysis of randomized controlled trials[J]. Journal of Traditional Chinese Medical Sciences, 2023, 10(1): 73-82.
- Green M, Chen X. Recent progress of nanomaterials for microwave absorption[J]. Journal of Materiomics, 2019, 5(4): 503-541.
- Allen T M, Cullis P R. Drug Delivery Systems: Entering the Mainstream[J]. Science, 2004, 303(5665): 1818-1822.
- Serra M F, Cotias A C, Pimentel A S, et al. Gold Nanoparticles Inhibit Steroid-Insensitive Asthma in Mice Preserving Histone Deacetylase 2 and NRF2 Pathways[J]. Antioxidants, 2022, 11(9).
- Gao X, Zhang Z, Li X, et al. Macitentan Attenuates Chronic Mountain Sickness in Rats by Regulating Arginine and Purine Metabolism[J]. J Proteome Res, 2020, 19(8): 3302-3314.
- Woods P, Alcock J. High-altitude pulmonary edema[J]. Evolution, Medicine, and Public Health, 2021, 9(1): 118-119.
- Wang X, Chen G, Wan B, et al. NRF1-mediated microglial activation triggers high-altitude cerebral edema[J]. Journal of Molecular Cell Biology, 2022, 14(5).
- Song Z, Zhang A, Luo J, et al. Prevalence of High-Altitude Polycythemia and Hyperuricemia and Risk Factors for Hyperuricemia in High-Altitude Immigrants[J]. High Altitude Medicine & Biology, 2023, 24(2): 132-138.
- Garrido E, Botella De Maglia J, Castillo O. Acute, subacute and chronic mountain sickness[J]. Rev Clin Esp (Barc), 2021, 221(8): 481-490.
- Chen H, Chen C, Qin Y, et al. Protective effects of epigallocatechin-3-gallate counteracting the chronic hypobaric hypoxia-induced myocardial injury in plain-grown rats at high altitude[J]. Cell Stress Chaperones, 2023, 28(6): 921-933.
- Hu C-J, Poth J M, Zhang H, et al. Suppression of HIF2 signalling attenuates the initiation of hypoxia-induced pulmonary hypertension[J]. European Respiratory Journal, 2019.
- Pena E, El Alam S, Siques P, et al. Oxidative Stress and Diseases Associated with High-Altitude Exposure[J]. Antioxidants (Basel), 2022, 11(2).
- Sarada S, Himadri P, Mishra C, et al. Role of Oxidative Stress and NFkB in Hypoxia-Induced Pulmonary Edema[J]. Experimental Biology and Medicine, 2008, 233(9): 1088-1098.
- Mrakic-Sposta S, Gussoni M, Dellanoce C, et al. Effects of acute and sub-acute hypobaric hypoxia on oxidative stress: a field study in the Alps[J]. European Journal of Applied Physiology, 2020, 121(1): 1-10.
- Wang Y, Shen Z, Pei C, et al. Eleutheroside B ameliorated high altitude pulmonary edema by attenuating ferroptosis and necroptosis through Nrf2-antioxidant response signaling[J]. Biomed Pharmacother, 2022, 156: 113982.
- Hartmann G, Tschop M, Fischer R, et al. High altitude increases circulating interleukin-6, interleukin-1 receptor antagonist and C-reactive protein[J]. Cytokine, 2000, 12(3): 246-52.
- Hartmann G, Tschöp M, Fischer R, et al. HIGH ALTITUDE INCREASES CIRCULATING INTERLEUKIN-6, INTERLEUKIN-1 RECEPTOR ANTAGONIST AND C-REACTIVE PROTEIN[J]. Cytokine, 2000, 12(3): 246-252.
- Yi H, Yu Q, Zeng D, et al. Serum Inflammatory Factor Profiles in the Pathogenesis of High-Altitude Polycythemia and Mechanisms of Acclimation to High Altitudes[J]. Mediators of Inflammation, 2021, 2021: 1-9.
- Mcgettrick A F, O'neill L a J. The Role of HIF in Immunity and Inflammation[J]. Cell Metab, 2020, 32(4): 524-536.
- Eltzschig H K, Carmeliet P. Hypoxia and inflammation[J]. N Engl J Med, 2011, 364(7): 656-65.
- Pena E, Brito J, El Alam S, et al. Oxidative Stress, Kinase Activity and Inflammatory Implications in Right Ventricular Hypertrophy and Heart Failure under Hypobaric Hypoxia[J]. Int J Mol Sci, 2020, 21(17).
- Fang R H, Gao W, Zhang L. Targeting drugs to tumours using cell membrane-coated nanoparticles[J]. Nature Reviews Clinical Oncology, 2022, 20(1): 33-48.
- Villanueva-Flores F, Castro-Lugo A, Ramírez O T, et al. Understanding cellular interactions with nanomaterials: towards a rational design of medical nanodevices[J]. Nanotechnology, 2020, 31(13).
- !!! INVALID CITATION !!! .
- Mitchell M J, Billingsley M M, Haley R M, et al. Engineering precision nanoparticles for drug delivery[J]. Nature Reviews Drug Discovery, 2020, 20(2): 101-124.
- Patrick B, Akhtar T, Kousar R, et al. Carbon Nanomaterials: Emerging Roles in Immuno-Oncology[J]. International Journal of Molecular Sciences, 2023, 24(7).
- Bonner J C, Card J W, Zeldin D C. Nanoparticle-Mediated Drug Delivery and Pulmonary Hypertension[J]. Hypertension, 2009, 53(5): 751-753.
- Mandler W K, Nurkiewicz T R, Porter D W, et al. Microvascular Dysfunction Following Multiwalled Carbon Nanotube Exposure Is Mediated by Thrombospondin-1 Receptor CD47[J]. Toxicological Sciences, 2018, 165(1): 90-99.
- Restani R B, Pires R F, Baptista P V, et al. Nano-in-Micro Sildenafil Dry Powder Formulations for the Treatment of Pulmonary Arterial Hypertension Disorders: The Synergic Effect of POxylated Polyurea Dendrimers, PLGA, and Cholesterol[J]. Particle & Particle Systems Characterization, 2020, 37(6).
- Kimura S, Egashira K, Chen L, et al. Nanoparticle-Mediated Delivery of Nuclear Factor κB Decoy Into Lungs Ameliorates Monocrotaline-Induced Pulmonary Arterial Hypertension[J]. Hypertension, 2009, 53(5): 877-883.
- Goncharov D A, Kudryashova T V, Ziai H, et al. Mammalian Target of Rapamycin Complex 2 (mTORC2) Coordinates Pulmonary Artery Smooth Muscle Cell Metabolism, Proliferation, and Survival in Pulmonary Arterial Hypertension[J]. Circulation, 2014, 129(8): 864-874.
- You Z, Qian H, Wang C, et al. Regulation of vascular smooth muscle cell autophagy by DNA nanotube-conjugated mTOR siRNA[J]. Biomaterials, 2015, 67: 137-150.
- Wullschleger S, Loewith R, Hall M N. TOR Signaling in Growth and Metabolism[J]. Cell, 2006, 124(3): 471-484.
- Lee S-J, Smith A, Guo L, et al. Autophagic Protein LC3B Confers Resistance against Hypoxia-induced Pulmonary Hypertension[J]. American Journal of Respiratory and Critical Care Medicine, 2011, 183(5): 649-658.
- Mirhadi E, Roufogalis B D, Banach M, et al. Resveratrol: Mechanistic and therapeutic perspectives in pulmonary arterial hypertension[J]. Pharmacological Research, 2021, 163.
- Konicek D, Leifer F, Chen K-J, et al. Inhaled Treprostinil-Prodrug Lipid Nanoparticle Formulations Provide Long-Acting Pulmonary Vasodilation[J]. Drug Research, 2018, 68(11): 605-614.
- Yasuo M, Masanori H, Norihiro K, et al. Inhalation toxicity assessment of carbon-based nanoparticles[J]. Accounts of chemical research, 2013, 46(3): 770-81.
- Deweirdt J, Ducret T, Quignard J F, et al. Effects of FW2 Nanoparticles Toxicity in a New In Vitro Pulmonary Vascular Cells Model Mimicking Endothelial Dysfunction[J]. Cardiovascular Toxicology, 2021, 22(1): 14-28.
- Fang X, Ji Y, Li S, et al. Paeoniflorin attenuates cuproptosis and ameliorates left ventricular remodeling after AMI in hypobaric hypoxia environments[J]. Journal of Natural Medicines, 2024.
- Naeije R, Badagliacca R. The overloaded right heart and ventricular interdependence[J]. Cardiovasc Res, 2017, 113(12): 1474-1485.
- Maranhao R, Guido M C, Derisio De Lima A, et al. Methotrexate carried in lipid core nanoparticles reduces myocardial infarction size and improves cardiac function in rats[J]. International Journal of Nanomedicine, 2017, Volume 12: 3767-3784.
- Natalia L M, Maria G C, Camila A, et al. Left Ventricle Dysfunction is Prevented by the Treatment With Methotrexate Carried in Lipid Nanoparticles in Lipopolysaccharide-injected Rats[J]. Circulation, 2020, 142(S3): A13040-A13040.
- Wu Z, Bai J, Lai F, et al. Atomically dispersed platinum supported onto nanoneedle-shaped protonated polyaniline for efficient hydrogen production in acidic water electrolysis[J]. Science China Materials, 2023, 66(7): 2680-2688.
- Huang Y-Y, Wu J. Preparation and Characterization of Graphene Oxide/Polyaniline/Carbonyl Iron Nanocomposites[J]. Materials, 2022, 15(2).
- Moradikhah F, Shabani I, Tafazzoli Shadpour M. Fabrication of a tailor-made conductive polyaniline/ascorbic acid-coated nanofibrous mat as a conductive and antioxidant cell-free cardiac patch[J]. Biofabrication, 2024, 16(3).
- Oldfield C J, Duhamel T A, Dhalla N S. Mechanisms for the transition from physiological to pathological cardiac hypertrophy[J]. Canadian Journal of Physiology and Pharmacology, 2020, 98(2): 74-84.
- Lajos S, Julianna S. Cyclodextrins in analytical chemistry: host-guest type molecular recognition[J]. Analytical chemistry, 2013, 85(17): 8024-30.
- Jingkai N, Chongxin S, Bin L, et al. Assembling of a functional cyclodextrin-decorated upconversion luminescence nanoplatform for cysteine-sensing[J]. Chemical communications (Cambridge, England), 2015, 51(74): 14054-6.
- Wei Y, Kong L-T, Yang R, et al. Electrochemical impedance determination of polychlorinated biphenyl using a pyrenecyclodextrin-decorated single-walled carbon nanotube hybrid[J]. Chemical Communications, 2011, 47(18).
- Niu X, Yang X, Mo Z, et al. Perylene-functionalized graphene sheets modified with β-cyclodextrin for the voltammetric discrimination of phenylalanine enantiomers[J]. Bioelectrochemistry, 2019, 129: 189-198.
- Femminò S, Penna C, Bessone F, et al. α-Cyclodextrin and α-Cyclodextrin Polymers as Oxygen Nanocarriers to Limit Hypoxia/Reoxygenation Injury: Implications from an In Vitro Model[J]. Polymers, 2018, 10(2).
- Rajesh S, Pramod K, Worapol N, et al. Stimuli-responsive mesoporous silica nanoparticles: A custom-tailored next generation approach in cargo delivery[J]. Materials Science & Engineering C, 2021, 124: 112084-112084.
- Thi T T N, Tran T V, Tran N Q, et al. Hierarchical self-assembly of heparin-PEG end-capped porous silica as a redox sensitive nanocarrier for doxorubicin delivery[J]. Materials Science & Engineering C, 2017, 70(P2): 947-954.
- Konstantin P, Ali J S, Ralph S, et al. The Comparative Toxic Impact Assessment of Carbon Nanotubes, Fullerene, Graphene, and Graphene Oxide on Marine Microalgae Porphyridium purpureum[J]. Toxics, 2023, 11(6).
- Ramanathan M, Boovarahan S R, Gandhi S, et al. Synthesis and characterization of mesoporous silica SBA 15 improved the efficacy of CORM-2 against hypoxia reoxygenation injury[J]. Journal of Porous Materials, 2021, 28(6): 1-9.
- Wang Y, Yin Y, Liu Y, et al. Notoginsenoside R1 treatment facilitated Nrf2 nuclear translocation to suppress ferroptosis via Keap1/Nrf2 signaling pathway to alleviated high-altitude myocardial injury[J]. Biomedicine & Pharmacotherapy, 2024, 175: 116793-.
- Qingshu W, Ling H, Yu H, et al. Carbon Monoxide-Saturated Hemoglobin-Based Oxygen Carriers Attenuate High-Altitude-Induced Cardiac Injury by Amelioration of the Inflammation Response and Mitochondrial Oxidative Damage[J]. Cardiology, 2017, 136(3): 180-191.
- Chenjie L, Li Y, Yamin H, et al. Effect of Quercetin-Loaded Mesoporous Silica Nanoparticles on Myocardial Ischemia-Reperfusion Injury in Rats and Its Mechanism[J]. International journal of nanomedicine, 2021, 16: 741-752.
- Sakthi Devi R, Girigoswami A, Siddharth M, et al. Applications of Gold and Silver Nanoparticles in Theranostics[J]. Applied Biochemistry and Biotechnology, 2022, 194(9): 4187-4219.
- Essa N, O'connell F, Prina-Mello A, et al. Gold nanoparticles and obese adipose tissue microenvironment in cancer treatment[J]. Cancer Letters, 2022, 525: 1-8.
- Malacrida S, Giannella A, Ceolotto G, et al. Transcription Factors Regulation in Human Peripheral White Blood Cells during Hypobaric Hypoxia Exposure: an in-vivo experimental study[J]. Scientific Reports, 2019, 9(1).
- Ponnanikajamideen M, Rajeshkumar S, Vanaja M, et al. In Vivo Type 2 Diabetes and Wound-Healing Effects of Antioxidant Gold Nanoparticles Synthesized Using the Insulin Plant Chamaecostus cuspidatus in Albino Rats[J]. Canadian Journal of Diabetes, 2019, 43(2): 82-89.e6.
- Simpson C E, Chen J Y, Damico R L, et al. Cellular sources of interleukin-6 and associations with clinical phenotypes and outcomes in pulmonary arterial hypertension[J]. European Respiratory Journal, 2020, 55(4).
- Fan S, Gao H, Zhang Y, et al. Quantum Sensing of Free Radical Generation in Mitochondria of Single Heart Muscle Cells during Hypoxia and Reoxygenation[J]. ACS Nano, 2024, 18(4): 2982-2991.
- Jin-Oh Y, Marjan R, C Y G J, et al. Nanoengineering the heart: conductive scaffolds enhance connexin 43 expression[J]. Nano letters, 2011, 11(9): 3643-8.
- Zhan R, Guo W, Gao X, et al. Real-time in situ monitoring of Lon and Caspase-3 for assessing the state of cardiomyocytes under hypoxic conditions via a novel Au–Se fluorescent nanoprobe[J]. Biosensors and Bioelectronics, 2021, 176.
- Huan Y, Quan H, Jia B, et al. High-altitude cerebral hypoxia promotes mitochondrial dysfunction and apoptosis of mouse neurons[J]. Frontiers in Molecular Neuroscience, 2023, 16.
- Wang Q-L, Huang W-X, Zhang P-J, et al. Colorimetric determination of the early biomarker hypoxia-inducible factor-1 alpha (HIF-1α) in circulating exosomes by using a gold seed-coated with aptamer-functionalized Au@Au core-shell peroxidase mimic[J]. Microchimica Acta, 2019, 187(1).
- Andersen H L, Frandsen B A, Gunnlaugsson H P, et al. Local and long-range atomic/magnetic structure of non-stoichiometric spinel iron oxide nanocrystallites[J]. IUCrJ, 2021, 8(1): 33-45.
- Stavilă C, Herea D D, Zară M C, et al. Enhancement of chemotherapy effects by non-lethal magneto-mechanical actuation of gold-coated magnetic nanoparticles[J]. Nanomedicine: Nanotechnology, Biology and Medicine, 2024, 60.
- Lin Y, Ren J, Qu X. Catalytically Active Nanomaterials: A Promising Candidate for Artificial Enzymes[J]. Accounts of Chemical Research, 2014, 47(4): 1097-1105.
- Shen Y, Wu C, Uyeda T Q P, et al. Elongated Nanoparticle Aggregates in Cancer Cells for Mechanical Destruction with Low Frequency Rotating Magnetic Field[J]. Theranostics, 2017, 7(6): 1735-1748.
- Kang S H, Revuri V, Lee S-J, et al. Oral siRNA Delivery to Treat Colorectal Liver Metastases[J]. ACS Nano, 2017, 11(10): 10417-10429.
- Giacca M, Zacchigna S. VEGF gene therapy: therapeutic angiogenesis in the clinic and beyond[J]. Gene Therapy, 2012, 19(6): 622-629.
- Liu Y-F, An T, Yu H, et al. Xiaozheng pill exerts an anti-mammary hyperplasia effect through Raf/ERK/ELK and HIF-1α/bFGF pathways[J]. Journal of Traditional and Complementary Medicine, 2023, 13(6): 600-610.
- Xingxing L, Rongpeng L, Wei L, et al. Panax quinquefolium L. and Salvia miltiorrhiza Bunge. Enhances Angiogenesis by Regulating the miR-155-5p/HIF-1α/VEGF Axis in Acute Myocardial Infarction[J]. Drug design, development and therapy, 2023, 17: 3249-3267.
- Wang J, Xiang B, Deng J-X, et al. Hypoxia enhances the therapeutic potential of superparamagnetic iron oxide-labeled adipose-derived stem cells for myocardial infarction[J]. Journal of Huazhong University of Science and Technology [Medical Sciences], 2017, 37(4): 516-522.
- Sun X, Wang Y, Wen S, et al. Novel controlled and targeted releasing hydrogen sulfide system exerts combinational cerebral and myocardial protection after cardiac arrest[J]. Journal of Nanobiotechnology, 2021, 19(1).
- Mamani J B, Borges J P, Rossi A M, et al. Magnetic Nanoparticles for Therapy and Diagnosis in Nanomedicine[J]. Pharmaceutics, 2023, 15(6).
- Li Y, Liu, Zhong Y, et al. Biocompatibility of Fe3O4@Au composite magnetic nanoparticles in vitro and in vivo[J]. International Journal of Nanomedicine, 2011.
- Hnatiuk A P, Ong S G, Olea F D, et al. Allogeneic Mesenchymal Stromal Cells Overexpressing Mutant Human Hypoxia-Inducible Factor 1-α (HIF1-α) in an Ovine Model of Acute Myocardial Infarction[J]. Journal of the American Heart Association, 2016, 5(7).
- Chen H, Chen C, Qin Y, et al. Protective effects of epigallocatechin-3-gallate counteracting the chronic hypobaric hypoxia-induced myocardial injury in plain-grown rats at high altitude[J]. Cell Stress Chaperones, 2023.
- Huang X, Zhang W, Peng Y, et al. A Multifunctional Layered Nickel Silicate Nanogenerator of Synchronous Oxygen Self-supply and Superoxide Radical Generation for Hypoxic Tumor Therapy[J]. ACS Nano, 2021, 16(1): 974-983.
- Zhu W, Wei X, Zhang L, et al. The effect and prediction of diurnal temperature range in high altitude area on outpatient and emergency room admissions for cardiovascular diseases[J]. International Archives of Occupational and Environmental Health, 2021, 94(8): 1783-1795.
- Miller M R, Raftis J B, Langrish J P, et al. Inhaled Nanoparticles Accumulate at Sites of Vascular Disease[J]. ACS Nano, 2017, 11(5): 4542-4552.
- Mishra R C, Rahman M M, Davis M J, et al. Alpha1-adrenergic stimulation selectively enhances endothelium-mediated vasodilation in rat cremaster arteries[J]. Physiological Reports, 2018, 6(9).
- Université De Bordeaux C D R C-T D B, Pessac, France., Inserm U C D R C-T D B, Bordeaux, France., Université De Bordeaux C D R C-T D B, Pessac, France., et al. TRPV4 channel mediates adventitial fibroblast activation and adventitial remodeling in pulmonary hypertension[J]. American journal of physiology. Lung cellular and molecular physiology, 2020, 318(1): L135-L146.
- Yu L, Bailin T, Hongxin W, et al. Otud6b induces pulmonary arterial hypertension by mediating the Calpain-1/HIF-1α signaling pathway[J]. Cellular and Molecular Life Sciences, 2024, 81(1): 258-258.
- Germande O, Ducret T, Quignard J-F, et al. NiONP-Induced Oxidative Stress and Mitochondrial Impairment in an In Vitro Pulmonary Vascular Cell Model Mimicking Endothelial Dysfunction[J]. Antioxidants, 2022, 11(5).
- Liu P, Chen G, Zhang J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives[J]. Molecules, 2022, 27(4). [CrossRef]
- Huihui Z, Yujie Q, Zheyu Z, et al. Nanomaterials toward the treatment of Alzheimer’s disease: Recent advances and future trends[J]. Chinese Chemical Letters, 2021, 32(6).
- Vaghasiya K, Sharma A, Kumar K, et al. Heparin-Encapsulated Metered-Dose Topical “Nano-Spray Gel” Liposomal Formulation Ensures Rapid On-Site Management of Frostbite Injury by Inflammatory Cytokines Scavenging[J]. ACS Biomaterials Science & Engineering, 2019, 5(12): 6617-6631.
- Chengrui Z, Yingjian L, Yina L, et al. Unfractionated Heparin Protects Microcirculation in Endotoxemic Rats by Antagonizing Histones[J]. The Journal of surgical research, 2022, 282: 84-92.
- Li H, Liu S, Dai W, et al. Pressure-sensitive multivesicular liposomes as a smart drug-delivery system for high-altitude pulmonary edema[J]. Journal of Controlled Release, 2024, 365: 301-316.
- Hade M D, Suire C N, Suo Z. Mesenchymal Stem Cell-Derived Exosomes: Applications in Regenerative Medicine[J]. Cells, 2021, 10(8).
- Skogberg G, Lundberg V, Berglund M, et al. Human thymic epithelial primary cells produce exosomes carrying tissue-restricted antigens[J]. Immunology & Cell Biology, 2015, 93(8): 727-734.
- Da Costa Martins P A, Utermöhlen O, Jakobshagen K, et al. Emergence of AnnexinVpos CD31neg CD42blow/neg extracellular vesicles in plasma of humans at extreme altitude[J]. Plos One, 2019, 14(8).
- Xiaoping G, Xu Z, Zhengjie Z, et al. PLGA-Based Micro/Nanoparticles: An Overview of Their Applications in Respiratory Diseases[J]. International Journal of Molecular Sciences, 2023, 24(5): 4333-4333.
- Souci L, Jaunet H, Diguerher G L, et al. Intranasal inoculations of naked or PLGA-PEI nanovectored DNA vaccine induce systemic and mucosal antibodies in pigs: A feasibility study[J]. Research in Veterinary Science, 2020, 132(prepublish): 194-201.
- Department of Immunology S O M, Mashhad University of Medical Sciences , Mashhad, Iran., Cellular, Molecular Research Center Q U O M S, Qazvin, Iran., et al. Application of PLGA nano/microparticle delivery systems for immunomodulation and prevention of allotransplant rejection[J]. Expert opinion on drug delivery, 2020, 17(6): 767-780.
- Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity[J]. Annual Review of Pathology: Mechanisms of Disease, 2020, 15(1): 123-147.
- Mao X, Li Y, Yang R, et al. Single-Cell RNA-Sequencing Reveals the Active Involvement of Macrophage Polarizations in Pulmonary Hypertension[J]. Disease Markers, 2022, 2022: 1-17.
- Liu C, Quan X, Tian X, et al. Inhaled Macrophage Apoptotic Bodies-Engineered Microparticle Enabling Construction of Pro-Regenerative Microenvironment to Fight Hypoxic Lung Injury in Mice[J]. ACS Nano, 2024, 18(20): 13361-13376.
- Zhang L, Wang H-Y, Li M-Q, et al. A Trojan horse biomimetic delivery system using mesenchymal stem cells for HIF-1α siRNA-loaded nanoparticles on retinal pigment epithelial cells under hypoxia environment[J]. International Journal of Ophthalmology, 2022, 15(11): 1743-1751.
- Xuanrong S, Guowei W, Hao Z, et al. The Blood Clearance Kinetics and Pathway of Polymeric Micelles in Cancer Drug Delivery[J]. ACS nano, 2018, 12(6): 6179-6192.
- Cagel M, Tesan F C, Bernabeu E, et al. Polymeric mixed micelles as nanomedicines: Achievements and perspectives[J]. European Journal of Pharmaceutics and Biopharmaceutics, 2017, 113: 211-228.
- Li D, Wang X, Han K, et al. Hypoxia and CD44 receptors dual-targeted nano-micelles with AGT-inhibitory activity for the targeting delivery of carmustine[J]. International Journal of Biological Macromolecules, 2023, 246.
- Wang Y, Wang Y, Wang X, et al. Tilianin-loaded Reactive Oxygen Species-Scavenging Nano-Micelles Protect H9c2 Cardiomyocyte Against Hypoxia/Reoxygenation-Induced Injury[J]. Journal of Cardiovascular Pharmacology, 2018, 72(1): 32-39.
- Luks A M, Auerbach P S, Freer L, et al. Wilderness Medical Society Clinical Practice Guidelines for the Prevention and Treatment of Acute Altitude Illness: 2019 Update[J]. Wilderness & Environmental Medicine, 2019, 30(4): S3-S18.
- Li Y, Yang Y, Qing Y A, et al. <p>Enhancing ZnO-NP Antibacterial and Osteogenesis Properties in Orthopedic Applications: A Review</p>[J]. International Journal of Nanomedicine, 2020, Volume 15: 6247-6262.
- Keshavarz A, Kadry H, Alobaida A, et al. Newer approaches and novel drugs for inhalational therapy for pulmonary arterial hypertension[J]. Expert Opinion on Drug Delivery, 2020, 17(4): 439-461.
- Uchida T, Hazekawa M, Yoshida M, et al. A Novel Long-Acting Prostacyclin Agonist (ONO-1301) With an Angiogenic Effect: Promoting Synthesis of Hepatocyte Growth Factor and Increasing Cyclic AMP Concentration via IP-Receptor Signaling[J]. Journal of Pharmacological Sciences, 2013, 123(4): 392-401.
- Kanaya T, Miyagawa S, Kawamura T, et al. Innovative therapeutic strategy using prostaglandin I2 agonist (ONO1301) combined with nano drug delivery system for pulmonary arterial hypertension[J]. Scientific Reports, 2021, 11(1).
- Satoshi A, Kazufumi N, Hiromi M, et al. Intratracheal Administration of Prostacyclin Analogue-incorporated Nanoparticles Ameliorates the Development of Monocrotaline and Sugen-Hypoxia-induced Pulmonary Arterial Hypertension[J]. Journal of cardiovascular pharmacology, 2016, 67(4): 290-8.
- Haddad F, Mohammed N, Gopalan R C, et al. Development and Optimisation of Inhalable EGCG Nano-Liposomes as a Potential Treatment for Pulmonary Arterial Hypertension by Implementation of the Design of Experiments Approach[J]. Pharmaceutics, 2023, 15(2).
- Marjan T, Mohsen T, Tahereh F, et al. New insights into the role of the Nrf2 signaling pathway in green tea catechin applications[J]. Phytotherapy research : PTR, 2021, 35(6): 3078-3112.
- Chen C, Li B, Chen H, et al. Epigallocatechin-3-Gallate Ameliorated Iron Accumulation and Apoptosis and Promoted Neuronal Regeneration and Memory/Cognitive Functions in the Hippocampus Induced by Exposure to a Chronic High-Altitude Hypoxia Environment[J]. Neurochem Res, 2022, 47(8): 2254-2262.
- M. S J, L. M. S J, L. M C, Milena B. Engineered models of the human heart: directions and challenges[J]. Stem Cell Reports, 2020, 16(9).
- Zuzanna I, Ewelina K, Aleksandra K, et al. Hypoxia and re-oxygenation effects on human cardiomyocytes cultured on polycaprolactone and polyurethane nanofibrous mats[J]. Journal of Biological Engineering, 2024, 18(1): 37-37.
- Department of Biomedical Engineering C O M, Kyung Hee University, Seoul 02447, South Korea., Department of Chemistry C O N S, Kwangwoon University, Seoul 01897, South Korea., Department of Chemistry C O N S, Kwangwoon University, Seoul 01897, South Korea., et al. Potential Protective Effect of Nitric Oxide-Releasing Nanofibers in Hypoxia/Reoxygenation-Induced Cardiomyocyte Injury[J]. Journal of nanoscience and nanotechnology, 2019, 19(10): 6539-6545.
- Xiang P, Liu Q, Jing W, et al. Combined ROS Sensitive PEG-PPS-PEG with Peptide Agonist for Effective Target Therapy in Mouse Model[J]. International Journal of Nanomedicine, 2024, Volume 19: 9109-9120.
- Zusman B E, Dixon C E, Jha R M, et al. Choice of Whole Blood versus Lactated Ringer's Resuscitation Modifies the Relationship between Blood Pressure Target and Functional Outcome after Traumatic Brain Injury plus Hemorrhagic Shock in Mice[J]. Journal of Neurotrauma, 2021, 38(20): 2907-2917.
- L T, H S, Y W, et al. Erratum: The New Nano-Resuscitation Solution (TPP-MR) Attenuated Myocardial Injury in Hemorrhagic Shock Rats by Inhibiting Ferroptosis [Corrigendum][J]. International journal of nanomedicine, 2024, 19: 8401-8402.
- E G-B E, J T A, C S B, et al. Nickel nanoparticles enhance platelet-derived growth factor-induced chemokine expression by mesothelial cells via prolonged mitogen-activated protein kinase activation[J]. American journal of respiratory cell and molecular biology, 2012, 47(4): 552-61.
- Mcdonald R J, Mcdonald J S, Kallmes D F, et al. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities[J]. Radiology, 2017, 285(2): 546-554.
- Talev J, Kanwar J R. Iron Oxide Nanoparticles as Imaging and Therapeutic Agents for Atherosclerosis[J]. Seminars in Thrombosis and Hemostasis, 2020, 46(05): 553-562.
- Li J, Liang H, Liu J, et al. Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy[J]. International Journal of Pharmaceutics, 2018, 546(1-2): 215-225.
- M H S, E B, D B, et al. An RNA-directed nuclease mediates post-transcriptional gene silencing in Drosophila cells[J]. Nature, 2000, 404(6775): 293-6.
Figure 1.
Possible functions and mechanisms in the pathogenesis of chronic mountain sickness. After plateau exposure occurs, the oxygen content of the air drops rapidly and oxidative stress occurs rapidly. Inhibition of KEAP-1 activation, which in turn affects the expression of the antioxidant factor NRF2 and downstream factors. Meanwhile, the hypoxic environment leads to the occurrence of inflammatory response and autophagy, activating the NF-kB and HIF signaling pathways to produce inflammatory molecules. ROS, Reactive Oxygen Species; KEAP-1, Kelch-like ECH-associated protein 1; NRF2, Nuclear factor erythroid 2-related factor 2; HO-1, Heme oxygenase-1; SOD, Superoxide dismutase; NF-κB, nuclear factor kappa-B; CAT, Catalase; iNOS, Inducible nitric oxide synthase; PI3K: Phosphatidyl-inositol 3-kinase; AKT, Serine-threonine kinase, mTOR, Mammalian target of rapamycin; HIF-1α, Hypoxia-inducible factor-1α, VEGF, Vascular endothelial growth factor; TNF-α, Tumor Necrosis Factor-α; IL-6, Interleukin- 6; IL-8, Interleukin-8, ARE, Anti-oxidative response element; NRE, Negative regulatory element; HRE, Hypoxia response element.
Figure 1.
Possible functions and mechanisms in the pathogenesis of chronic mountain sickness. After plateau exposure occurs, the oxygen content of the air drops rapidly and oxidative stress occurs rapidly. Inhibition of KEAP-1 activation, which in turn affects the expression of the antioxidant factor NRF2 and downstream factors. Meanwhile, the hypoxic environment leads to the occurrence of inflammatory response and autophagy, activating the NF-kB and HIF signaling pathways to produce inflammatory molecules. ROS, Reactive Oxygen Species; KEAP-1, Kelch-like ECH-associated protein 1; NRF2, Nuclear factor erythroid 2-related factor 2; HO-1, Heme oxygenase-1; SOD, Superoxide dismutase; NF-κB, nuclear factor kappa-B; CAT, Catalase; iNOS, Inducible nitric oxide synthase; PI3K: Phosphatidyl-inositol 3-kinase; AKT, Serine-threonine kinase, mTOR, Mammalian target of rapamycin; HIF-1α, Hypoxia-inducible factor-1α, VEGF, Vascular endothelial growth factor; TNF-α, Tumor Necrosis Factor-α; IL-6, Interleukin- 6; IL-8, Interleukin-8, ARE, Anti-oxidative response element; NRE, Negative regulatory element; HRE, Hypoxia response element.
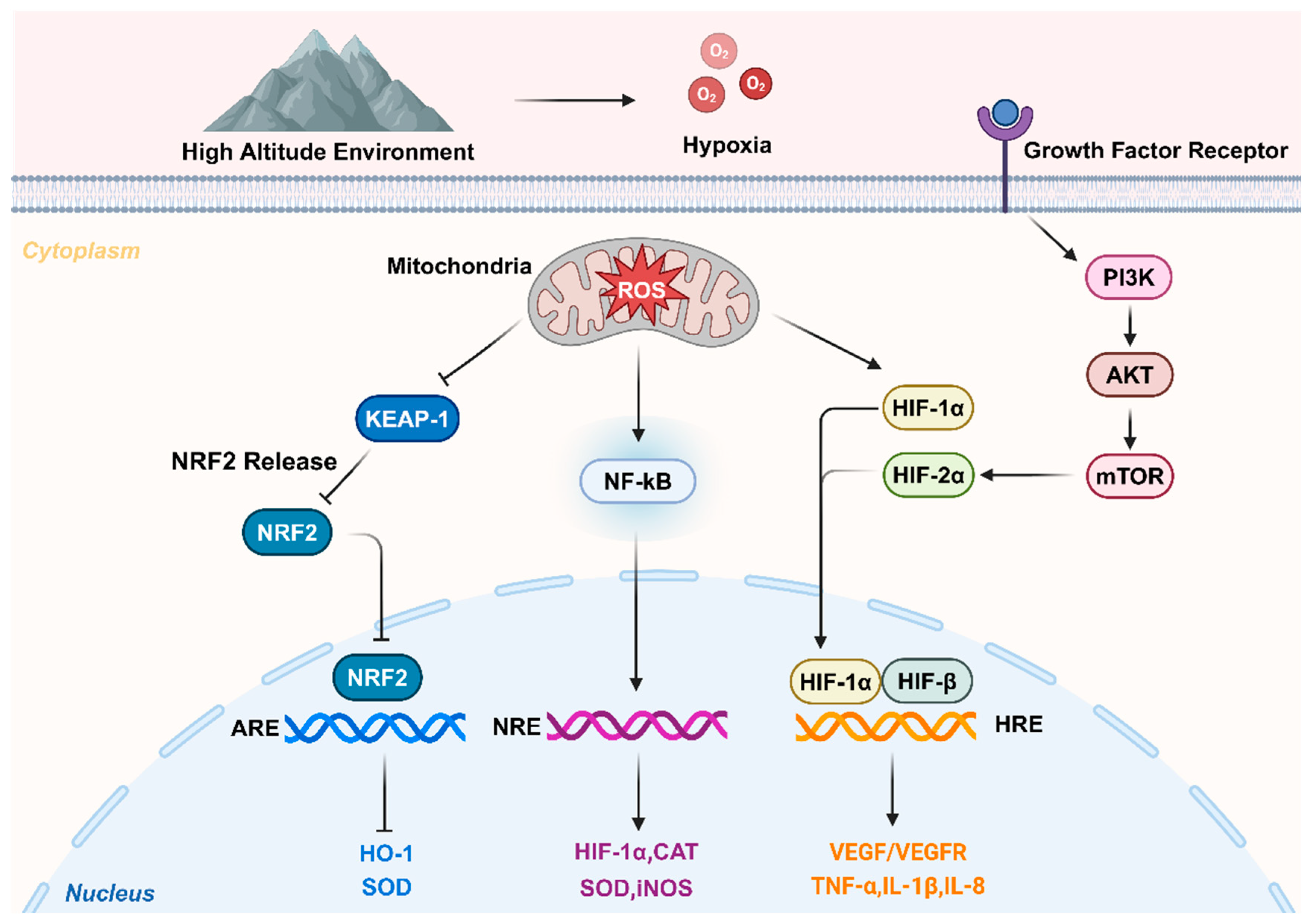
Figure 2.
The possible function and mechanism of nanomaterial-based drug delivery systems in chronic mountain sickness. (A) Several commonly used inorganic nanomaterials. (B) The preparation of nanoparticles mainly includes three steps, including the selection of nanocarriers, modification of surface molecules on nanocarriers, and agent encapsulation. (C) Nanomaterials effecting relevant targets in patients with chronic mountain sickness. (D) Nanoparticles are delivered to the target site by different injection modes, including: intraperitoneal injection, oral administration, nebulized administration. (E) Different nanoparticles can treat chronic mountain sickness by different mechanisms through drug delivery. ROS, Reactive Oxygen Species; KEAP-1, Kelch-like ECH-associated protein 1; NRF2, Nuclear factor erythroid 2-related factor 2; MDA, Malondialdehyde; NF-κB, nuclear factor kappa-B; HIF-1α, Hypoxia-inducible factor-1α; IL-1β, Interleukin-1β; IL-6, Interleukin- 6; Caspase-3, Cysteinyl aspartate specific proteinase-3.
Figure 2.
The possible function and mechanism of nanomaterial-based drug delivery systems in chronic mountain sickness. (A) Several commonly used inorganic nanomaterials. (B) The preparation of nanoparticles mainly includes three steps, including the selection of nanocarriers, modification of surface molecules on nanocarriers, and agent encapsulation. (C) Nanomaterials effecting relevant targets in patients with chronic mountain sickness. (D) Nanoparticles are delivered to the target site by different injection modes, including: intraperitoneal injection, oral administration, nebulized administration. (E) Different nanoparticles can treat chronic mountain sickness by different mechanisms through drug delivery. ROS, Reactive Oxygen Species; KEAP-1, Kelch-like ECH-associated protein 1; NRF2, Nuclear factor erythroid 2-related factor 2; MDA, Malondialdehyde; NF-κB, nuclear factor kappa-B; HIF-1α, Hypoxia-inducible factor-1α; IL-1β, Interleukin-1β; IL-6, Interleukin- 6; Caspase-3, Cysteinyl aspartate specific proteinase-3.

Table 1.
Advantages and challenges of commonly used inorganic nanomaterials.
Table 1.
Advantages and challenges of commonly used inorganic nanomaterials.
| No. |
Nanomaterials |
Advantages |
Challenges |
References |
| 1 |
Carbon |
High strength, high biocompatibility, good electrical and thermal conductivity |
Biological toxicity, release carbon monoxide |
[44] |
| 2 |
Silicon Dioxide |
Highly controllable treatment platform size and shape, low toxicity, good biocompatibility |
Easy to be internalised by cells, causing various diseases such as neurodegenerative diseases |
[56] |
| 3 |
Gold |
Optical properties, plasmon resonance properties, fluorescence properties and adsorption properties |
Lower circulation and tissue clearance |
[63] |
| 4 |
Magnetic |
Local magnetic, thermal and mechanical effects, intrinsic catalytic activity |
Biological toxicity |
[74] |
| 5 |
Nickel |
High specific surface area, surface energy and magnetic properties, |
Biological toxicity |
[86] |
Table 2.
Target molecules with their targets, properties, and carriers in chronic mountain sickness.
Table 2.
Target molecules with their targets, properties, and carriers in chronic mountain sickness.
| No. |
Ligands |
Targets |
Properties |
Carriers |
References |
| 1 |
Heparin sodium |
Endothelial cells and capillaries |
High stability and quick access to the target |
Liposomes |
[95] |
| 2 |
Amlodipine besylate |
Pulmonary capillaries |
Hydrostatic pressure sensitivity, carrying multiple drugs |
Pressure-sensitive multivesicular liposomes |
[97] |
| 3 |
ICAM-1, VCAM-1 and VE-cadherin |
Platelet and endothelial cell |
Early reflection of endothelial function damage from hypoxia |
Extracellular vesicles |
[100] |
| 4 |
Superoxidedismutase/catalase nanocomplexes |
Alveolar epithelialcells |
Altered macrophage phenotype, high affinity for targets |
Camouflaged PLGAmicroparticles with macrophage apoptotic body membrane |
[106] |
| 5 |
Plasmid and lentivirus |
Mesenchymalstem cell and retinal pigment epithelial cells |
Created a bionic delivery system that is highly targeted |
PLGA |
[107] |
| 6 |
Polyethylene glycol compound attached to propylene sulfide formed amphiphilic diblockpolymer |
cardiomyocyt |
Highly efficient hydrogen peroxide scavengers |
Tilianin loaded micelles |
[111] |
Table 3.
Relevant pathways and carries for nanomaterials therapy.
Table 3.
Relevant pathways and carries for nanomaterials therapy.
| No |
Drug |
Carries |
Pathway |
Effect |
References |
| 1 |
Methotrexate |
Lipid core nanoparticles |
ROS-VEGF |
MTX-LDE Increased antioxidant enzymes, reduced apoptosis, macrophages, ROS production, reduced hypoxic damage to myocardium |
[44] |
| 2 |
Polyaniline |
Nanofibrous polycaprolactone mats |
caspase-3- Bcl-2 |
Nanofibrous polymerised in situ with polyaniline reduce intracellular ROS content and caspase-3 mRNA expression and attenuate the hypertrophic effect of hypoxia on H9 c2 cells. |
[48] |
| 3 |
Carbon monoxide releasing molecule-2 |
Mesoporous silica nanoparticles |
Scanning electron microscopy and cell viability assay |
Mesoporous silica nanomaterials may lead to a sustained release of CO and thus hypoxia/reoxygenation resulting in minimisation of toxic effects. |
[58] |
| 4 |
Quercetin |
Mesoporous silica nanoparticles |
JAK2/STAT3 |
Q-MSNs elevated JAK 2 and STAT 3 protein expression, decreased Bax, caspase-3, Bim, and Bid protein expression, and ameliorated cardiomyocyte apoptosis, myocardial infarction, and ventricular remodelling in hypoxic environment |
[61] |
| 5 |
Heparin |
Liposomes |
IL-6-TNF-α |
Frostbite was significantly improved in rats after aerosolised administration of the nano-spray gel, with reductions in IL-6, TNF-α, IL-10, IL-4 |
[95] |
| 6 |
Prostacyclin |
ONO1301 |
IL-6, IL-1β, TGFβ |
ONO 1301 nanomaterials improved clinical outcomes in HPAH after administration of elevated HGF expression and significant reduction of IL-6,IL-1β, and TGFβ |
[116] |
| 7 |
Epigallocatechin gallate (EGCG) |
Liposome |
TGFβ |
EGCG nanoliposomes inhibit TGFβ signalling, by aerodynamic analysis, have all the properties required for inhalability, and are therefore expected to be a potential therapeutic approach for HPAH. |
[118] |
| 8 |
Growth hormone-releasing hormone |
nano PEG-PPS-PEG@MR409 vesicles |
ROS |
Nanomaterials encapsulating growth hormone-releasing hormone attenuate ROS content and apoptosis in posthypoxic myocardial infarction cells and restore cardiac function |
[124] |
| 9 |
Acetated Ringer’s (AR) and Lactate Ringer’s solution (LR) |
TPP@PAMAM-MR (TPP-MR) |
Glutathioneperoxidase 4 |
TPP@PAMAM-MR novel nanocrystal resuscitation solution improves cardiac and mitochondrial function in hypoxia-treated cardiomyocytes, attenuates ROS production, and inhibits iron-toxicity-associated GPX 4, ACSL 4, and COX 2 protein expression |
[124] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).







