1. Introduction
Ecologists often assume that habitat fragmentation negatively correlates with species diversity, as small populations and patch isolation reduce population survival probability [
1,
2]. At the same time, observations that many small patches can harbor higher species richness than large patches in the same total habitat area were quite frequent [
3]. Recently, more comprehensive empirical research [
4] makes a compelling case that landscapes support more species when the patches are many and small, given the same area and sampling effort. At the same time, a review by Riva et al. [
5] identified five studies (between 2017 and 2022) where habitat fragmentation per se affected species richness, with one suggesting a positive effect. Some other theoretical studies suggest that increased fragmentation and a decline in concomitant patch size may lead to higher landscape diversity under some circumstances. Possible mechanisms for higher species richness in small-patch landscapes include (a) higher heterogeneity, e.g., [
6], combined with (b) lower number of species on small patches, (c) opportunities for specialization, d) higher turnover, e.g., [
2], and (d) metapopulation dynamics leading to differential tracking of different spatial resources (this aspect complements “d”). For example, Campos et al. [
7] concluded that smaller, more numerous habitat patches support greater species diversity due to increased species dispersal and colonization opportunities, which involves several earlier processes. Others noted the edge effect increases with the patch size decline [
8] and likely offers additional habitat heterogeneity for some species to exploit. These findings provide a reasonable argument for a possible positive effect of small habitat size and landscape fragmentation on species richness, at least in a portion of examined landscapes. However, the trend distilled from the extensive data set reveals high noise and frequent departures associated with specific landscapes and taxa. It also leaves the conditions under which these departures occur and possible factors responsible for the departures largely unexplored, primarily due to a lack of relevant data. Because the discovered trend is superficially counterintuitive [
5], explanations inevitably grapple with substantial variability among individual studies and, possibly, circular logic, such as defining generalists as species not declining or increasing in abundance. The most recent theoretical study by Zhang et al. [
9] attempts to explain the diversity of patterns. It finds conditional evidence for a positive effect of habitat size reduction on biodiversity. This effect is not straightforward, however. In their model, it depends on the total amount of habitat.
Furthermore, the results do not appear definitive, as they rely on one type of interaction – competition - and a rather specific set of traits arising from adopting the competition-colonization model. Simulated landscapes do not include other interactions and several essential features of the landscape, such as interhabitat differences and species specialization. In short, the recent studies inspire further questions and suggest a need for a broader look. To answer some of these questions, we used an object-based metacommunity model. The model design attempted to include a broader set of general processes to avoid outcomes arising from specific model features.
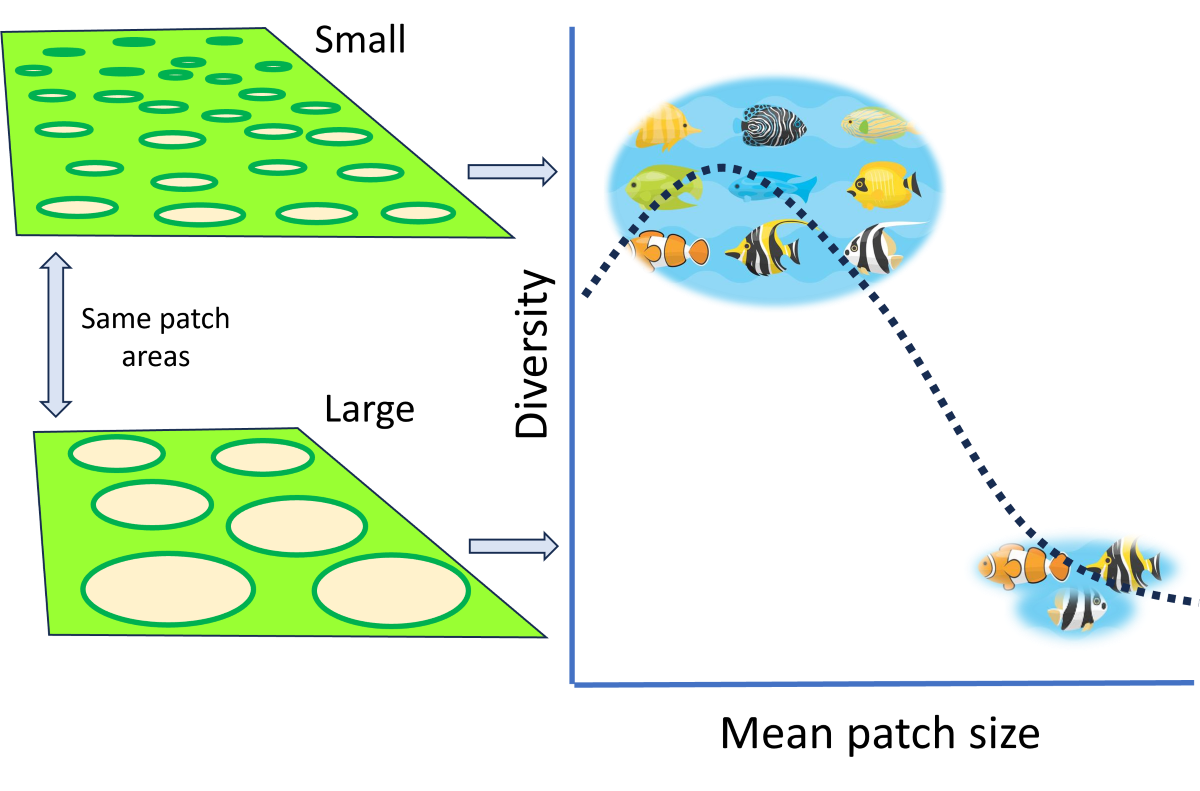
To explore the effect of patch size, we created small, medium, and large patch landscapes and monitored the patch connectivity. The dependent variables included species richness and population density. We used an agent-based, spatially explicit metacommunity model where species gain energy on ‘suitable’ patches, with dispersal and reproduction costing energy and species interactions involving costs and rewards on the gradient from negative to positive interactions. To examine the effect of patch size on species richness, we have focused on specific landscape features (see Methods: Model, Species, and Data Collection sections). Applying a metacommunity model allows for examining interactions of processes such as dispersal, various species interactions, level of heterogeneity, and population rescue on species diversity as a function of patch size – the variable of considerable theoretical and practical interest. By modeling landscapes of the same total area regardless of the size and number of habitat patches, the exercise suggests definitive answers to other hypotheses, such as the Habitat Amount or Multi-dimensional hypotheses, cf., [
10]. We focused on the hypothesis that the species richness will be higher in landscapes with smaller patches than in landscapes with larger patches as long as they feature similar spatial patterns, total habitat area, species movement probability, habitat heterogeneity, species interactions, dispersal networks, and reproduction rules.
3. Results
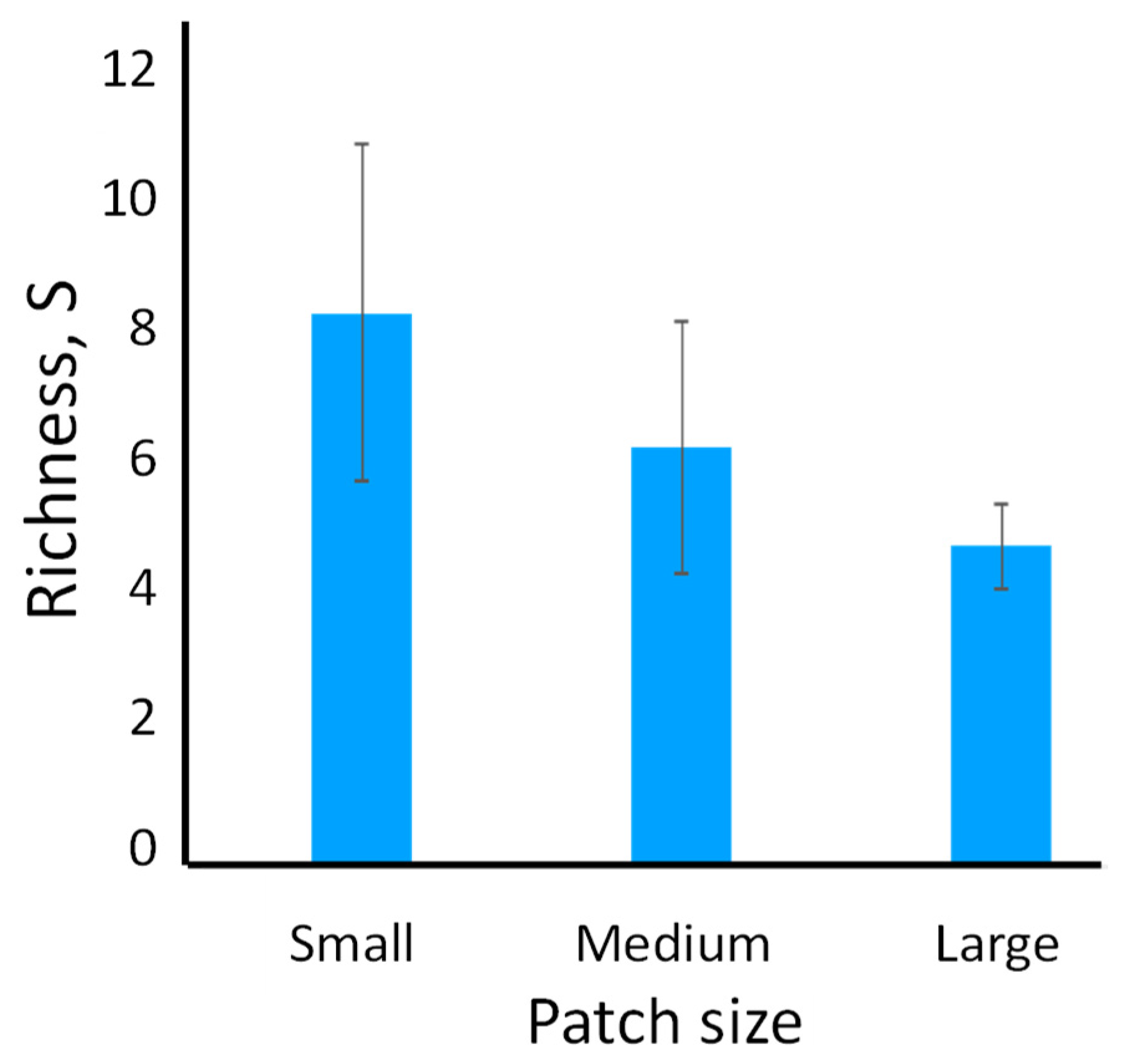
We found that landscapes with many small patches maintained twice as many species as landscapes with patches about twice as large in area (
Figure 1). The question arises regarding the mechanisms supporting higher richness in smaller patches. Riva and Fahrig [
4] reported a bewildering range of responses along the patch size axis. Interpreting the relationship between species richness and habitat size is likely to interact with other ecological factors. Some such factors may include habitat connectivity, depth of inter-habitat differences, and species specialization to habitat types.
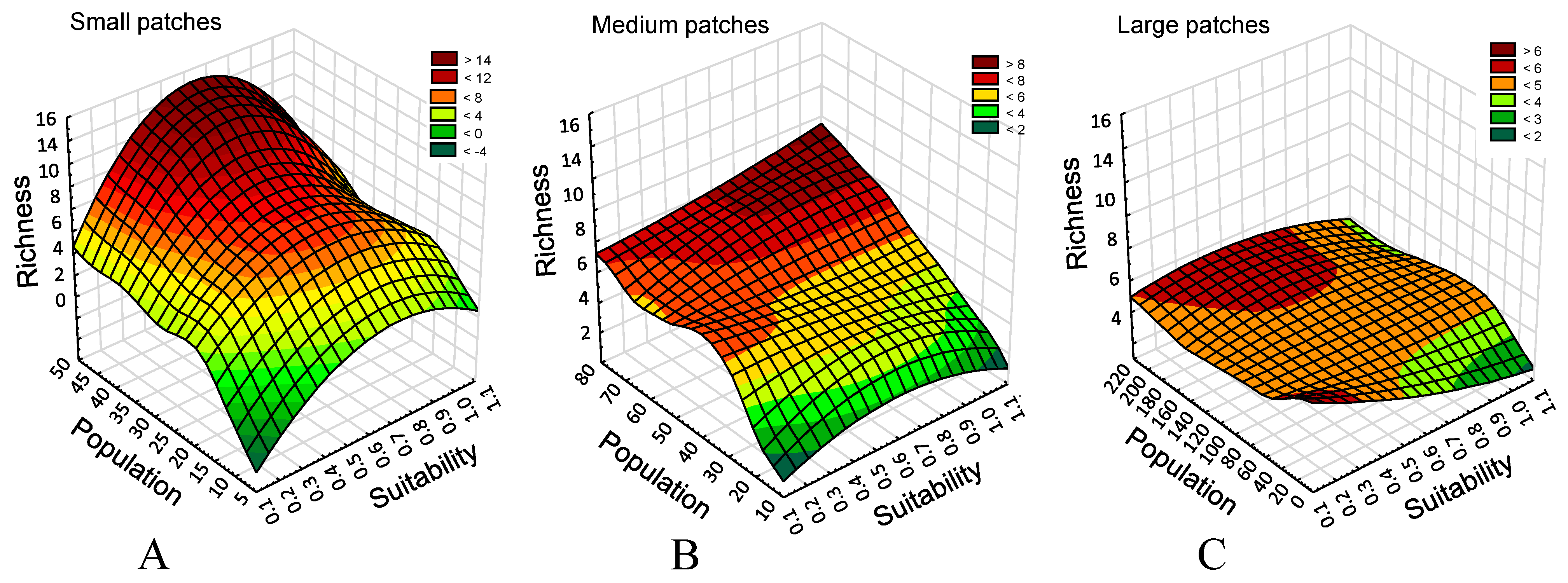
First, we notice that when the combined population of all species increases, the species richness declines (
Figure 2). As the total landscape population increases with the patch size, a new question arises about the causes. Do larger patches allow for greater total populations, a factor that could leave a few more successful species to dominate the landscape? Alternatively, does increasing fragmentation (and an ensuing patch size reduction) promote a tradeoff between colonization and extinction, cf. [
17], such that no species can monopolize most patches? The latter process might also interact with inter-habitat differences, where habitat specialists might succeed in only some locations.
The responses (
Figure 2) suggest that the most likely culprits are local patch processes rather than the landscape population size of combined species. Here, whether the population is low or high, species richness remains similar. Small patch landscapes offer more spatial patch diversity and support more specialists (
Figure 2A; the majority of species seeded in the model), while large patch landscapes appear to represent functional homogenization [
18], with few generalists dominating (
Figure 2C).
The relationship between habitat connectivity, a proxy metric for higher effective dispersal, and richness reveals no response in large-patch landscapes (
Figure 2C), 12% of variance explained in medium-patch landscapes (
Figure 2B), and 5% in small-patch landscapes. This is not to say that connectivity does not play a role. More likely, there is a shift in importance from small-patch landscapes (limited resources, increased variability in dispersal success) (Figs. 2A, 3) to large patch size (
Figure 2C) where generalist species appear to flood the landscape.
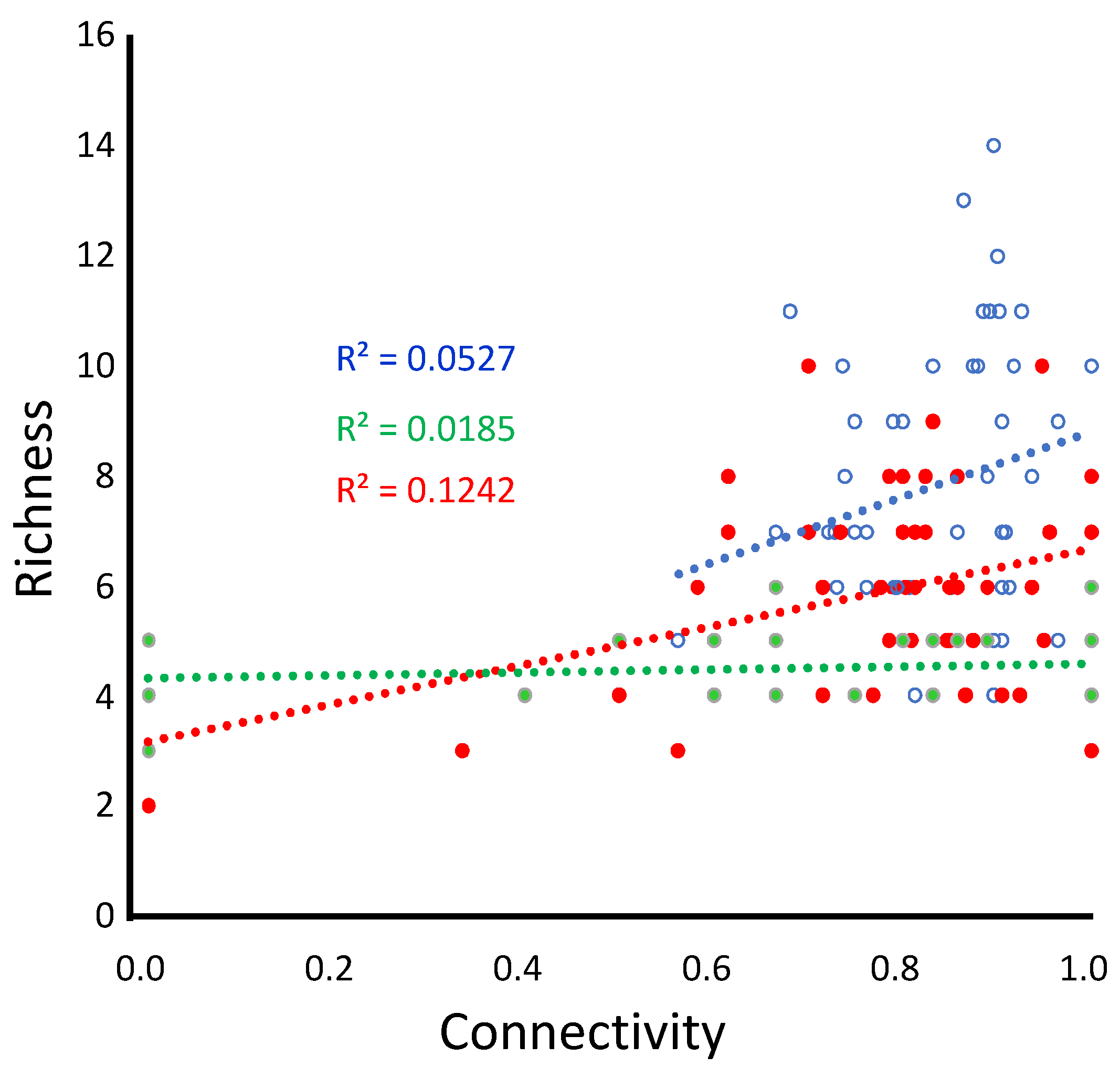
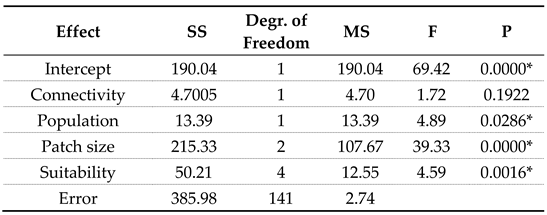
While the patterns associated with connectivity are weak, they are statistically significant (
Figure 3,
Table 1) and likely relevant in nature.
As we suspected, the ability to disperse appears to have contributed to higher landscape richness in small and medium patch landscapes. In large patch landscapes, dispersal may play a lesser role as movement among patches is less important for obtaining resources and reducing dispersal costs. This change in the playing field may allow habitat generalists to dominate numerically; see also [
19] for compatible results.
Connectivity did not contribute to the explanation of richness differences among landscapes, although it should not be dismissed (
Table 2). Low connectivity negatively impacted richness, particularly in small-patch landscapes where no species survived below the 0.5 value of the connectivity metric. Meta-analyses of empirical patterns likely include both situations, and depending on their mix, we should expect to see either type of effect.
A plausible interpretation is that a meta-habitat comprising many small and different patches (high heterogeneity) is conducive to the coexistence of many different species, e.g., [
20], but see also [
21]. Here, higher patch connectivity supports significantly higher richness by either protecting small populations from local extinction or successfully restoring locally extinct species such that in the long run (of the model) species seeded at the beginning of the simulations survived in the landscape. The effect of connectivity among large patches on richness is statistically insignificant. This dual effect of patch size and connectivity may create a stage for high richness and dispersal by more specialized species. While connectivity effects differ among landscapes of different patch sizes, they become marginally significant when evaluated in the combined context of more important factors such as patch size, suitability, and aggregated population of all species (
Table 2).
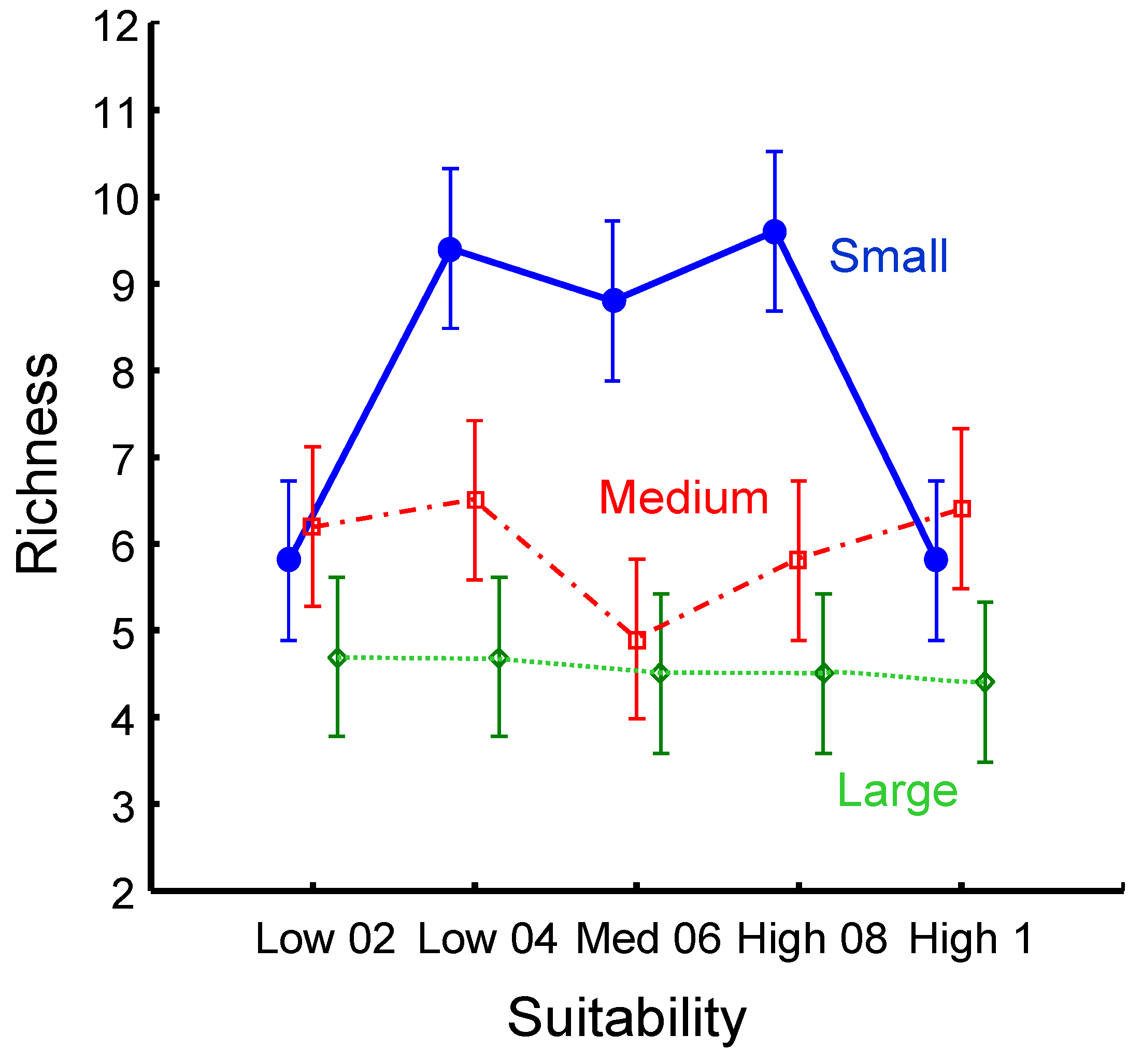
Maintaining a sufficient population size to track suitable habitats, especially when a species is a habitat specialist, can also provide insights into richness-promoting factors (
Figure 4). The suitability effect is unimodal-like and prominent when the patches are small. The effect disappears in medium and large patches. The low species richness in low-suitability habitats concurs with the low density of the aggregate population.
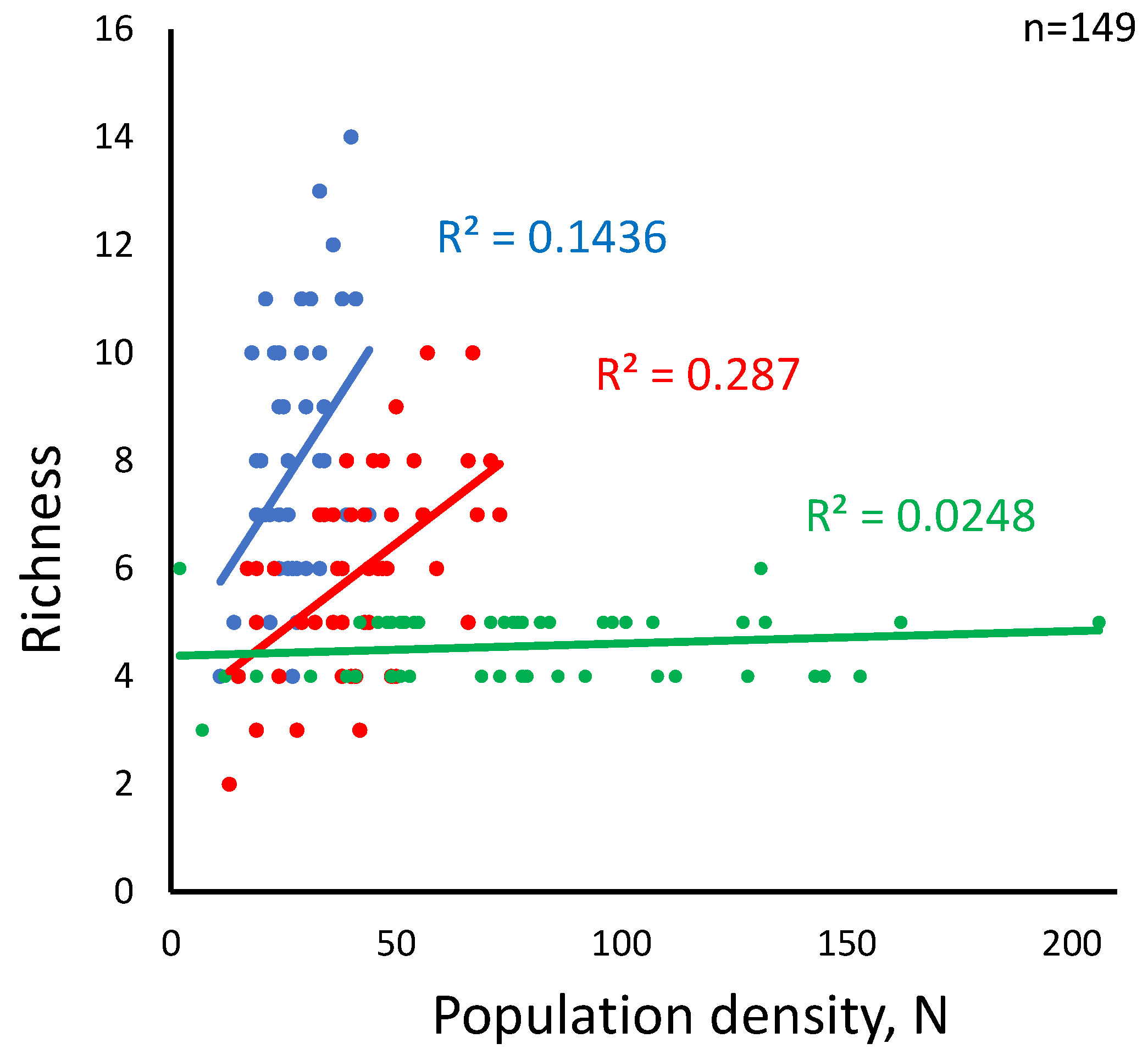
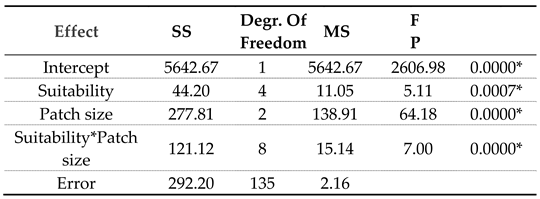
While differences among the three landscape types are clear (p<0.0000), the aggregate population size is unrelated to habitat suitability (ANCOVA, p=0.764). However, within treatments, the population size positively correlates with richness in medium and small-patch landscapes but shows no correlation in large-patch landscapes (
Figure 5). As high total population correlates negatively with richness in large-patch landscapes, a plausible explanation might be that in such landscapes, a decline of in spatial habitat turnover per unit distance reduces dispersal costs for a limited number of species at the expense of other, less competitive species. Another related interpretation might involve dispersal network heterogeneity if it varied as a function of the patch size. We cannot determine this directly at this stage, but we saw neither effect of patch size on mean connectivity among the landscape type (One-way ANOVA F(2, 147) =.60637, p = 0.547) nor any interaction effect of habitat suitability category and the patch size.
Nevertheless, the above result challenges a straightforward interpretation in ecological terms. This is because simulated data and natural systems allow additional factors to modulate the effects of the factors of interest. These range from static spatial configurations to dynamic biological interactions to generate many reasonable expectations. In our model, habitat suitability sheds some light on the unfolding relationships.
Habitat suitability strongly promotes species richness in the small-patch landscapes, although only in the middle of the suitability range. Its effect in other landscapes declines sharply, although it may have some influence in medium-sized patch landscapes when the population is relatively high (
Figure 5,
Table 2).
The pattern of declining effect of habitat suitability and population size is consistent with the idea that habitat fragmentation and diversity of habitat conditions form a combination of conditions that imposes sufficient limitations on the success of habitat generalists. This limitation may allow local yet highly uneven persistence of habitat specialists in small-patch landscapes. As the species number declines on a gradient from small to large patches, the ratio of specialists to generalists shrinks from 2.9, 1.3, and 1.1, respectively. The pattern supports expectations that generalists do better where the spectrum of different and isolated conditions does not protect specialists from negative interactions. Also, a much higher landscape population density may exacerbate the negative interactions through resource competition.
4. Discussion
The most significant finding is a confirmation that, in an unbiased model, small-patch landscapes can support higher species richness than other landscapes (in the model, this support is expressed as the most modest species losses over 500 generations in the simulated community). This support correlates with the lowest combined density of all species over the landscape. Empirical research shows that among many different factors, uneven dispersal of species (uneven spatial isolation, heterogeneity) may significantly influence species richness due to differential species abilities and uneven accessibility of patches [
22]. Our simulations allow for such effects implicitly, but we do not know how the habitat size affects them because the overall connectivity and habitat suitability distributions did not differ between the landscape types (Results).
The patterns exhibited by generalist species appear in line with the observations in nature, underscoring the generalists as being more abundant, less variable, and present in a broader range of habitat types (here defined by differences in suitability). However, the generalists’ contribution does not support the hypothesis suggesting they boost increasing biodiversity in small-patch landscapes, cf. [
4]. This result could arise from the model choice of allowing only 10% of species to have the broadest ranges of habitat use. The generalists' impact may, however, materialize differently. Specifically, the species diversity exhibits an unimodal relationship with habitat suitability in the small-patch landscapes. The unimodal response may suggest that the highest habitat suitability (in nature, it could be an abundance of resources) may increase extinction risks for unknown reasons, possibly translating into the high density of a few successful generalist species.
Our results support the recent empirical findings [
4] that local reduction of biodiversity in small habitats may allow an opposite trend for higher biodiversity at the landscape level. Recent models, e.g., [
9], support some features of our simulation, but others, e.g., [
23] show contradictory results. These similarities and differences may reflect variation based on observed data from natural systems. In contrast, different conclusions among simulated metacommunities are more likely due to specific assumptions and the choice of variables in the models. For example, Guo et al. [
23] examined food webs with competition-colonization tradeoffs among basal species, omnivores, and other secondary consumers and found a broad range of outcomes. Importantly, they show habitat loss would lead to topology oscillations and changes in species number dependent on the spread of omnivory, habitat loss, and basal species colonization success. By being more general in treating species interactions, our model may be missing some of the more specific processes they identified, e.g., the role of basal species. The differences among conclusions and underlying model premises strongly suggest a need for a general framework that is simple, integrated, and comprehensive. Without such a framework, the diversity of findings may end up more confusing than illuminating.
Our approach and data differ in some respects from those presented in the recent study of natural datasets [
4]. Some disparity is not surprising given the differing research questions in each study. Our model simulation focused on contrasts arising solely from habitat patch size. Also, all patches in a landscape had the same size and shape, and all the remaining landscape/patches/species settings were the same across landscape size types, which is not the case in natural systems.
Although the initial settings other than patch size were identical, this does not mean our simulated landscapes are identical. Differences emerge from random variation and may have some effect on landscape patch structure (connectivity), local and global species species composition, interactions, specialization, and dispersal. The Riva and Fahrig study [
4] included a variable mix of small and large patches. We examined the performance of identically constructed species sets across those three landscape times, while the empirical study [
4] compared one species set on small and one on large patches in many different landscapes where species specialization, interaction intensity, and dispersal rates were largely unknown.
The trends observed in individual data sets examined by Riva and Fahrig contribute to noise that may add to a significant scatter of correlations and mask the relationship between the species richness and patch size. This inevitable feature of the inductive approach generates uncertainty and vague answers because of many possible causes. One possibility stands out. Unless the patches analyzed by Riva and Fahrig [
4] had the same size structure distribution across the data sets, which they certainly could not, the patterns they found might be artefactual to some degree, i.e., biased by mechanisms prevailing in large or small patches in a particular landscape. Furthermore, the individual data sets may have had different species-habitat relationships, contingent on the species habitat specialization relative to habitat mosaic attributes, including the suitability of individual patches. In this context, a lack of support for the habitat amount hypothesis (cf., [
24]) in our results is relevant to establishing null expectations and providing a universal reference for past and future comparisons.
In our simulations, all patches were of the same size within the landscape type. While this is a software limitation, size homogeneity offers some advantages because size differences may exaggerate differences in species performance and mask the size effects. We will keep this potentially influential difference in mind when highlighting putative lessons from our analyses.
Earlier empirical studies also occasionally found that smaller patches, when representing more heterogeneity (habitat type diversity), can support more species than other patches [
25]. While our model had the same settings of habitat suitability for all three landscape types, the landscape with the smallest patches may exhibit the most significant heterogeneity for at least one reason. Smaller patches show a higher temporal variability of local richness and species identity, enhancing the heterogeneity of resources and unpredictability of interactions on individual patches of the same suitability level. An experimental study associated this variability with the variability of species interactions in fragmented landscapes [
26]. Another coexistence mechanism may possibly involve a local competitive advantage of good dispersers [
27].
Implications. If our findings reflect null trends adequately, habitat suitability models aiming at biodiversity conservation should consider a specific species class – one including species particularly adept at using small habitats. This implication should not be surprising because habitat suitability models successfully couple the distribution of suitable patches and species requirements [
28], while recognizing various sources of species/habitat mismatch and its consequences [
27,
29]. Other studies appear to emphasize the link and note that habitat quality may override the effect of habitat fragmentation (e.g., [
30,
31]). Both possibilities are relevant to biodiversity management and offer promising research pursuits.