Submitted:
01 October 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. The Multifaceted Role and Expression of CBX3 in CVD
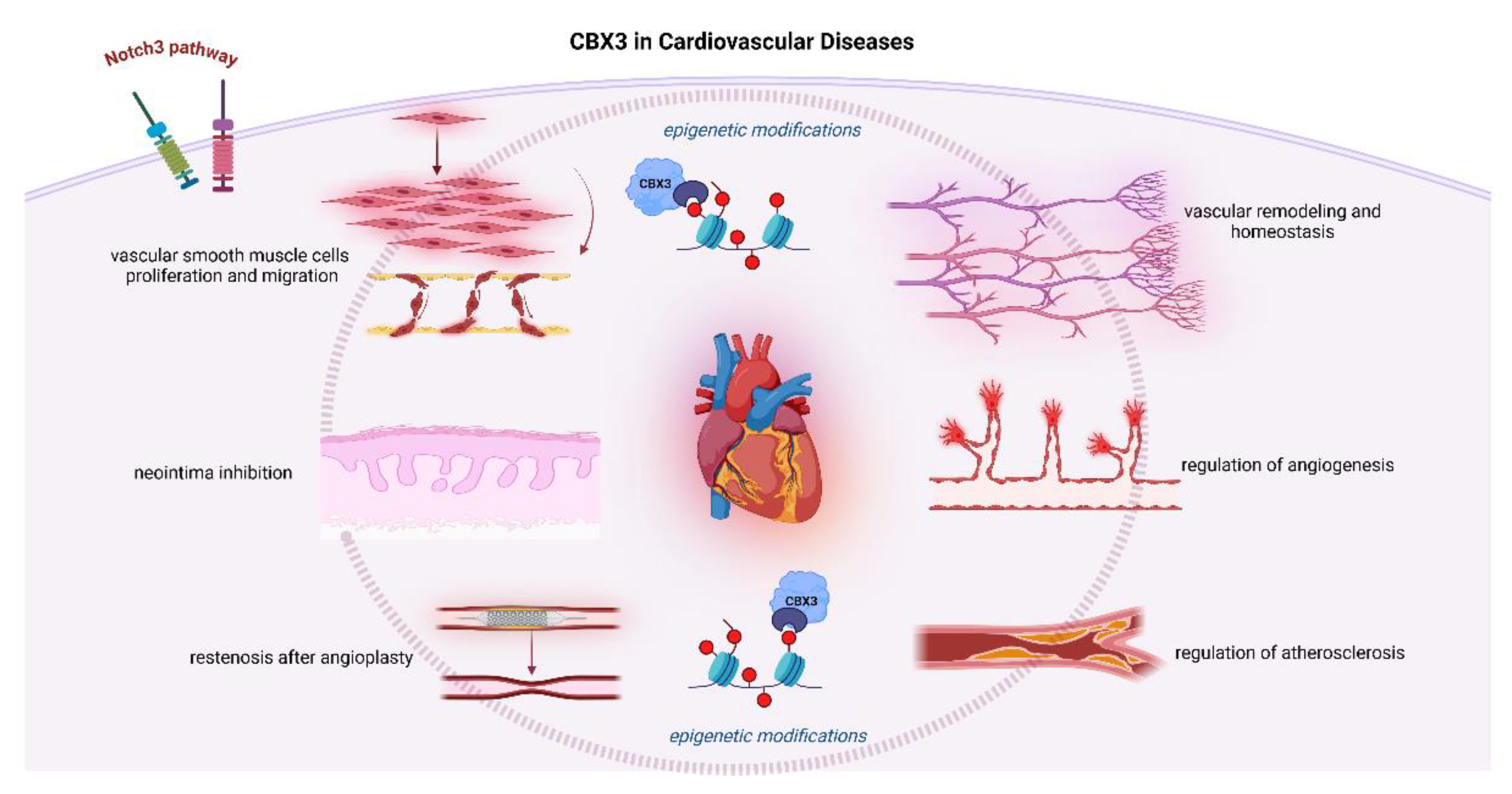
3. CBX3 Impacts Proliferation, Migration, and Formation of Neointima Via Notch3 Pathway
4. CBX3 is Associated with Lung Cancer Risk in CVD Patients
5. Dysregulation of CBX3 in the Immune Response and Its Impact in Heart Disease
6. The Potential Therapeutic Benefits of Targeting CBX3 in CVDs
7. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABCG1 ATP Binding Cassette Subfamily G Member 1 |
| CBX chromobox |
| CoQ coenzyme Q |
| CVD cardiovascular disease |
| DM diabetes mellitus |
| ICIs immune checkpoint inhibitors |
| MS metabolic syndrome |
| NLR nucleotide-binding leucine-rich repeat receptors |
| NLRP3 NLR family pyrin domain containing 3 |
| NPC Neural progenitor cells |
| SRF serum response factor |
| SMURF1 SMAD specific E3 ubiquitin protein ligase 1 |
| SMURF2 SMAD specific E3 ubiquitin protein ligase 2 |
| SMCs smooth muscle cells |
| SMAD TGF-β/Suppressor of Mothers against Decapentaplegic |
| tiRNA-Gly-GCC tRNA with the anticodon GCC |
| VSMCs vascular smooth muscle cells |
References
- Brown, J.C.; Gerhardt, T.E.; Kwon, E. Risk Factors for Coronary Artery Disease; 2024.
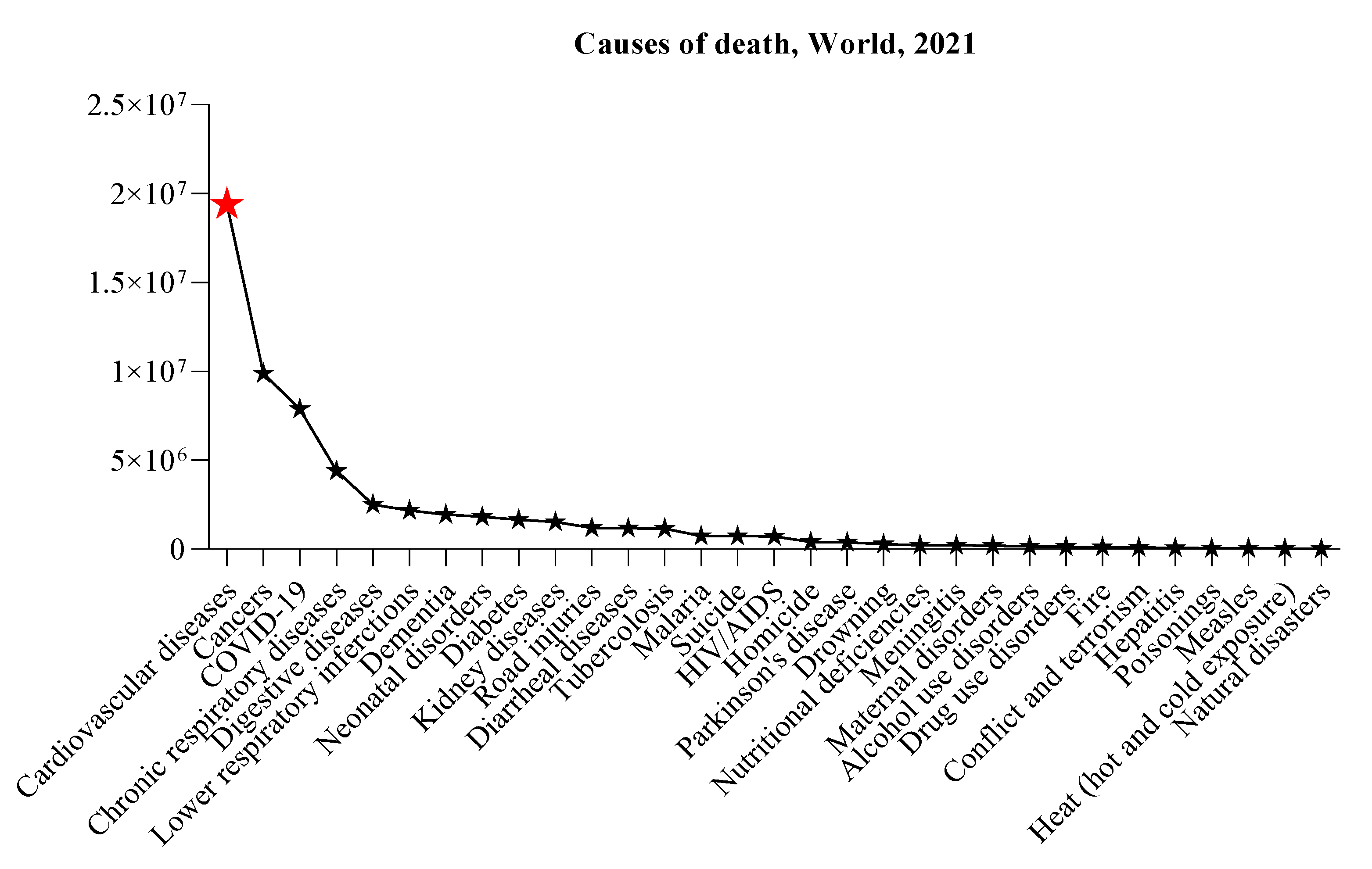
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990-2019: Update From the GBD 2019 Study. J Am Coll Cardiol 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The Heart of the World. Glob Heart 2024, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Bin, W.; Le, Z.; Mubarik, S.; Fu, G.; Wang, Y.; Nawsherwan. Prediction of Cardiovascular Diseases Mortality- and Disability-Adjusted Life-Years Attributed to Modifiable Dietary Risk Factors from 1990 to 2030 among East Asian Countries and the World. Front Nutr 2022, 9, 898978. [Google Scholar] [CrossRef]
- Gooding, H.C.; Gidding, S.S.; Moran, A.E.; Redmond, N.; Allen, N.B.; Bacha, F.; Burns, T.L.; Catov, J.M.; Grandner, M.A.; Harris, K.M.; et al. Challenges and Opportunities for the Prevention and Treatment of Cardiovascular Disease Among Young Adults: Report From a National Heart, Lung, and Blood Institute Working Group. J Am Heart Assoc 2020, 9, e016115. [Google Scholar] [CrossRef]
- Ullah, A.; Kumar, M.; Sayyar, M.; Sapna, F.; John, C.; Memon, S.; Qureshi, K.; Agbo, E.C.; Ariri, H.I.; Chukwu, E.J.; et al. Revolutionizing Cardiac Care: A Comprehensive Narrative Review of Cardiac Rehabilitation and the Evolution of Cardiovascular Medicine. Cureus 2023, 15, e46469. [Google Scholar] [CrossRef]
- Ordovás, J.M.; Smith, C.E. Epigenetics and Cardiovascular Disease. Nat Rev Cardiol 2010, 7, 510–519. [Google Scholar] [CrossRef]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Fiemotonghan, B.-E.; Okoye, O.K.; Agu-Ben, C.M.; Onyekweli, S.O.; Amapu, D.A.; Ikpegbu, R.; Asekhauno, M.; et al. A Comprehensive Review of Heart Failure: Unraveling the Etiology, Decoding Pathophysiological Mechanisms, Navigating Diagnostic Modalities, Exploring Pharmacological Interventions, Advocating Lifestyle Modifications, and Charting the Horizon of Emerging Therapies in the Complex Landscape of Chronic Cardiac Dysfunction. Medicine 2024, 103, e36895. [Google Scholar] [CrossRef]
- Crowson, C.S.; Liao, K.P.; Davis, J.M.; Solomon, D.H.; Matteson, E.L.; Knutson, K.L.; Hlatky, M.A.; Gabriel, S.E. Rheumatoid Arthritis and Cardiovascular Disease. Am Heart J 2013, 166, 622–628. [Google Scholar] [CrossRef]
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Kannel, W.B. Progression of Uncontrolled Hypertension and Implications for Managing Its Sequelae. Manag Care 2003, 12, 26–33. [Google Scholar]
- Zhang, C.; Chen, D.; Maguire, E.M.; He, S.; Chen, J.; An, W.; Yang, M.; Afzal, T.A.; Luong, L.A.; Zhang, L.; et al. Cbx3 Inhibits Vascular Smooth Muscle Cell Proliferation, Migration, and Neointima Formation. Cardiovasc Res 2018, 114, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.W.; Roubin, G.S.; King, S.B. Restenosis after Coronary Angioplasty. Potential Biologic Determinants and Role of Intimal Hyperplasia. Circulation 1989, 79, 1374–1387. [Google Scholar] [CrossRef] [PubMed]
- Niu, H.; Chen, P.; Fan, L.; Sun, B. Comprehensive Pan-Cancer Analysis on CBX3 as a Prognostic and Immunological Biomarker. BMC Med Genomics 2022, 15, 29. [Google Scholar] [CrossRef]
- Kunkiel, J.; Gödecke, N.; Ackermann, M.; Hoffmann, D.; Schambach, A.; Lachmann, N.; Wirth, D.; Moritz, T. The CpG-Sites of the CBX3 Ubiquitous Chromatin Opening Element Are Critical Structural Determinants for the Anti-Silencing Function. Sci Rep 2017, 7, 7919. [Google Scholar] [CrossRef]
- Huang, C.; Su, T.; Xue, Y.; Cheng, C.; Lay, F.D.; McKee, R.A.; Li, M.; Vashisht, A.; Wohlschlegel, J.; Novitch, B.G.; et al. Cbx3 Maintains Lineage Specificity during Neural Differentiation. Genes Dev 2017, 31, 241–246. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, H.; Ren, D.; Sun, Y.; Guo, F.; Cai, H.; Zhou, C.; Zhou, Y.; Jin, X.; Wu, H. CBX3 Regulated By YBX1 Promotes Smoking-Induced Pancreatic Cancer Progression via Inhibiting SMURF2 Expression. Int J Biol Sci 2022, 18, 3484–3497. [Google Scholar] [CrossRef]
- Koseler, A.; Ma, F.; Kilic, I.D.; Morselli, M.; Kilic, O.; Pellegrini, M. Genome-Wide DNA Methylation Profiling of Blood from Monozygotic Twins Discordant for Myocardial Infarction. In Vivo 2020, 34, 361–367. [Google Scholar] [CrossRef]
- Muka, T.; Koromani, F.; Portilla, E.; O’Connor, A.; Bramer, W.M.; Troup, J.; Chowdhury, R.; Dehghan, A.; Franco, O.H. The Role of Epigenetic Modifications in Cardiovascular Disease: A Systematic Review. Int J Cardiol 2016, 212, 174–183. [Google Scholar] [CrossRef]
- Lorenzen, J.M.; Martino, F.; Thum, T. Epigenetic Modifications in Cardiovascular Disease. Basic Res Cardiol 2012, 107, 245. [Google Scholar] [CrossRef]
- Yabe, K.; Kamio, A.; Oya, S.; Kakutani, T.; Hirayama, M.; Tanaka, Y.; Inagaki, S. H3K9 Methylation Regulates Heterochromatin Silencing through Incoherent Feedforward Loops. Sci Adv 2024, 10, eadn4149. [Google Scholar] [CrossRef]
- Ninova, M.; Fejes Tóth, K.; Aravin, A.A. The Control of Gene Expression and Cell Identity by H3K9 Trimethylation. Development 2019, 146. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Ouyang, H.; Amaya, M.F.; Ravichandran, M.; Loppnau, P.; Min, J.; Zang, J. Structural Basis of the Chromodomain of Cbx3 Bound to Methylated Peptides from Histone H1 and G9a. PLoS One 2012, 7, e35376. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, R.; Gonzales-Cope, M.; Chronis, C.; Bonora, G.; McKee, R.; Huang, C.; Patel, S.; Lopez, D.; Mishra, N.; Pellegrini, M.; et al. Proteomic and Genomic Approaches Reveal Critical Functions of H3K9 Methylation and Heterochromatin Protein-1γ in Reprogramming to Pluripotency. Nat Cell Biol 2013, 15, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.A.; Vondriska, T.M. Clinical Epigenomics for Cardiovascular Disease: Diagnostics and Therapies. J Mol Cell Cardiol 2021, 154, 97–105. [Google Scholar] [CrossRef]
- Kolbus, D.; Ljungcrantz, I.; Andersson, L.; Hedblad, B.; Fredrikson, G.N.; Björkbacka, H.; Nilsson, J. Association between CD8+ T-Cell Subsets and Cardiovascular Disease. J Intern Med 2013, 274, 41–51. [Google Scholar] [CrossRef]
- Russell-Hallinan, A.; Watson, C.J.; O’Dwyer, D.; Grieve, D.J.; O’Neill, K.M. Epigenetic Regulation of Endothelial Cell Function by Nucleic Acid Methylation in Cardiac Homeostasis and Disease. Cardiovasc Drugs Ther 2021, 35, 1025–1044. [Google Scholar] [CrossRef]
- Lozano-Velasco, E.; Inácio, J.M.; Sousa, I.; Guimarães, A.R.; Franco, D.; Moura, G.; Belo, J.A. MiRNAs in Heart Development and Disease. Int J Mol Sci 2024, 25. [Google Scholar] [CrossRef]
- Ha, T.-Y. MicroRNAs in Human Diseases: From Cancer to Cardiovascular Disease. Immune Netw 2011, 11, 135–154. [Google Scholar] [CrossRef]
- Nonaka, C.K.V.; Sampaio, G.L.; Silva, K.N.; Khouri, R.; Macedo, C.T.; Rogatto, S.R.; Ribeiro Dos Santos, R.; Souza, B.S. de F.; Soares, M.B.P.; Chagas Translational Research Consortium. Therapeutic MiR-21 Silencing Reduces Cardiac Fibrosis and Modulates Inflammatory Response in Chronic Chagas Disease. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef]
- Mierzejewski, B.; Ciemerych, M.A.; Streminska, W.; Janczyk-Ilach, K.; Brzoska, E. MiRNA-126a Plays Important Role in Myoblast and Endothelial Cell Interaction. Sci Rep 2023, 13, 15046. [Google Scholar] [CrossRef]
- Arderiu, G.; Peña, E.; Civit-Urgell, A.; Badimon, L. Endothelium-Released Microvesicles Transport MiR-126 That Induces Proangiogenic Reprogramming in Monocytes. Front Immunol 2022, 13, 836662. [Google Scholar] [CrossRef]
- Chen, J.; Lin, Y.; Zheng, S.; Chen, Q.; Tang, S.; Zhong, X. CBX3 Promotes Clear Cell Renal Carcinoma through PI3K/AKT Activation and Aberrant Immunity. J Transl Med 2023, 21, 600. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Teng, X.; Luo, C.; Kong, L. Mechanisms and Advances of Epigenetic Regulation in Cardiovascular Disease. Frontiers in Bioscience-Landmark 2024, 29, 205. [Google Scholar] [CrossRef] [PubMed]
- van der Laan, S.W.; Siemelink, M.A.; Haitjema, S.; Foroughi Asl, H.; Perisic, L.; Mokry, M.; van Setten, J.; Malik, R.; Dichgans, M.; Worrall, B.B.; et al. Genetic Susceptibility Loci for Cardiovascular Disease and Their Impact on Atherosclerotic Plaques. Circ Genom Precis Med 2018, 11, e002115. [Google Scholar] [CrossRef]
- Palou-Márquez, G.; Subirana, I.; Nonell, L.; Fernández-Sanlés, A.; Elosua, R. DNA Methylation and Gene Expression Integration in Cardiovascular Disease. Clin Epigenetics 2021, 13, 75. [Google Scholar] [CrossRef]
- Miroshnikova, V. V; Panteleeva, A.A.; Pobozheva, I.A.; Razgildina, N.D.; Polyakova, E.A.; Markov, A. V; Belyaeva, O.D.; Berkovich, O.A.; Baranova, E.I.; Nazarenko, M.S.; et al. ABCA1 and ABCG1 DNA Methylation in Epicardial Adipose Tissue of Patients with Coronary Artery Disease. BMC Cardiovasc Disord 2021, 21, 566. [Google Scholar] [CrossRef]
- Jin, J.; Zhao, X.; Zhu, C.; Li, M.; Wang, J.; Fan, Y.; Liu, C.; Shen, C.; Yang, R. Hypomethylation of ABCG1 in Peripheral Blood as a Potential Marker for the Detection of Coronary Heart Disease. Clin Epigenetics 2023, 15, 120. [Google Scholar] [CrossRef]
- Hedman, Å.K.; Mendelson, M.M.; Marioni, R.E.; Gustafsson, S.; Joehanes, R.; Irvin, M.R.; Zhi, D.; Sandling, J.K.; Yao, C.; Liu, C.; et al. Epigenetic Patterns in Blood Associated With Lipid Traits Predict Incident Coronary Heart Disease Events and Are Enriched for Results From Genome-Wide Association Studies. Circ Cardiovasc Genet 2017, 10, e001487. [Google Scholar] [CrossRef]
- Bauer, A.J.; Martin, K.A. Coordinating Regulation of Gene Expression in Cardiovascular Disease: Interactions between Chromatin Modifiers and Transcription Factors. Front Cardiovasc Med 2017, 4, 19. [Google Scholar] [CrossRef]
- Cao, G.; Xuan, X.; Zhang, R.; Hu, J.; Dong, H. Gene Therapy for Cardiovascular Disease: Basic Research and Clinical Prospects. Front Cardiovasc Med 2021, 8, 760140. [Google Scholar] [CrossRef]
- Alfaddagh, A.; Martin, S.S.; Leucker, T.M.; Michos, E.D.; Blaha, M.J.; Lowenstein, C.J.; Jones, S.R.; Toth, P.P. Inflammation and Cardiovascular Disease: From Mechanisms to Therapeutics. Am J Prev Cardiol 2020, 4, 100130. [Google Scholar] [CrossRef] [PubMed]
- Bairey Merz, C.N.; Dwyer, J.; Nordstrom, C.K.; Walton, K.G.; Salerno, J.W.; Schneider, R.H. Psychosocial Stress and Cardiovascular Disease: Pathophysiological Links. Behavioral medicine (Washington, D.C.) 2002, 27, 141–147. [Google Scholar] [CrossRef]
- De Rosa, S.; Arcidiacono, B.; Chiefari, E.; Brunetti, A.; Indolfi, C.; Foti, D.P. Type 2 Diabetes Mellitus and Cardiovascular Disease: Genetic and Epigenetic Links. Front Endocrinol (Lausanne) 2018, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Malka, K.; Liaw, L. NOTCH3 as a Modulator of Vascular Disease: A Target in Elastin Deficiency and Arterial Pathologies. J Clin Invest 2022, 132. [Google Scholar] [CrossRef]
- Rusanescu, G.; Weissleder, R.; Aikawa, E. Notch Signaling in Cardiovascular Disease and Calcification. Curr Cardiol Rev 2008, 4, 148–156. [Google Scholar] [CrossRef]
- Gomez, A.H.; Joshi, S.; Yang, Y.; Tune, J.D.; Zhao, M.-T.; Yang, H. Bioengineering Systems for Modulating Notch Signaling in Cardiovascular Development, Disease, and Regeneration. J Cardiovasc Dev Dis 2021, 8. [Google Scholar] [CrossRef]
- Kachanova, O.; Lobov, A.; Malashicheva, A. The Role of the Notch Signaling Pathway in Recovery of Cardiac Function after Myocardial Infarction. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef]
- Baeten, J.T.; Lilly, B. Notch Signaling in Vascular Smooth Muscle Cells. Adv Pharmacol 2017, 78, 351–382. [Google Scholar] [CrossRef]
- Jensen, L.F.; Bentzon, J.F.; Albarrán-Juárez, J. The Phenotypic Responses of Vascular Smooth Muscle Cells Exposed to Mechanical Cues. Cells 2021, 10. [Google Scholar] [CrossRef]
- Steffensen, L.B.; Rasmussen, L.M. A Role for Collagen Type IV in Cardiovascular Disease? Am J Physiol Heart Circ Physiol 2018, 315, H610–H625. [Google Scholar] [CrossRef]
- Jiang, Y.; Qian, H.-Y. Transcription Factors: Key Regulatory Targets of Vascular Smooth Muscle Cell in Atherosclerosis. Mol Med 2023, 29, 2. [Google Scholar] [CrossRef]
- Alva, J.A.; Iruela-Arispe, M.L. Notch Signaling in Vascular Morphogenesis. Curr Opin Hematol 2004, 11, 278–283. [Google Scholar] [CrossRef]
- Xiu, M.; Wang, Y.; Li, B.; Wang, X.; Xiao, F.; Chen, S.; Zhang, L.; Zhou, B.; Hua, F. The Role of Notch3 Signaling in Cancer Stemness and Chemoresistance: Molecular Mechanisms and Targeting Strategies. Front Mol Biosci 2021, 8, 694141. [Google Scholar] [CrossRef]
- Wang, K.; Liu, C.-Y.; Fang, B.; Li, B.; Li, Y.-H.; Xia, Q.-Q.; Zhao, Y.; Cheng, X.-L.; Yang, S.-M.; Zhang, M.-H.; et al. The Function and Therapeutic Potential of Transfer RNA-Derived Small RNAs in Cardiovascular Diseases: A Review. Pharmacol Res 2024, 206, 107279. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, K.; Zhao, C.; Liu, N.; Wang, Z.; Yang, W.; Cheng, Z.; Zhou, L.; Wang, K. The Function of TRNA-Derived Small RNAs in Cardiovascular Diseases. Mol Ther Nucleic Acids 2024, 35, 102114. [Google Scholar] [CrossRef]
- Rong, Z.; Li, F.; Zhang, R.; Niu, S.; Di, X.; Ni, L.; Liu, C. Inhibition of TiRNA-Gly-GCC Ameliorates Neointimal Formation via CBX3-Mediated VSMCs Phenotypic Switching. Front Cardiovasc Med 2023, 10, 1030635. [Google Scholar] [CrossRef]
- Xiao, Q.; Wang, G.; Yin, X.; Luo, Z.; Margariti, A.; Zeng, L.; Mayr, M.; Ye, S.; Xu, Q. Chromobox Protein Homolog 3 Is Essential for Stem Cell Differentiation to Smooth Muscles in Vitro and in Embryonic Arteriogenesis. Arterioscler Thromb Vasc Biol 2011, 31, 1842–1852. [Google Scholar] [CrossRef]
- Dorn, T.; Kornherr, J.; Parrotta, E.I.; Zawada, D.; Ayetey, H.; Santamaria, G.; Iop, L.; Mastantuono, E.; Sinnecker, D.; Goedel, A.; et al. Interplay of Cell-Cell Contacts and RhoA/MRTF-A Signaling Regulates Cardiomyocyte Identity. EMBO J 2018, 37. [Google Scholar] [CrossRef]
- Wang, C.; Lu, D.; Cronin-Fenton, D.; Huang, C.; Liew, Z.; Wei, D.; Qin, G.; Yu, Y.; Li, J. Cardiovascular Disease and Risk of Lung Cancer Incidence and Mortality: A Nationwide Matched Cohort Study. Front Oncol 2022, 12, 950971. [Google Scholar] [CrossRef]
- de Jesus, M.; Chanda, A.; Grabauskas, T.; Kumar, M.; Kim, A.S. Cardiovascular Disease and Lung Cancer. Front Oncol 2024, 14, 1258991. [Google Scholar] [CrossRef]
- Chianca, M.; Panichella, G.; Fabiani, I.; Giannoni, A.; L’Abbate, S.; Aimo, A.; Del Franco, A.; Vergaro, G.; Grigoratos, C.; Castiglione, V.; et al. Bidirectional Relationship Between Cancer and Heart Failure: Insights on Circulating Biomarkers. Front Cardiovasc Med 2022, 9, 936654. [Google Scholar] [CrossRef] [PubMed]
- Koene, R.J.; Prizment, A.E.; Blaes, A.; Konety, S.H. Shared Risk Factors in Cardiovascular Disease and Cancer. Circulation 2016, 133, 1104–1114. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, Z.; Shen, W.; Huang, G.; Sedivy, J.M.; Wang, H.; Ju, Z. Inflammation, Epigenetics, and Metabolism Converge to Cell Senescence and Ageing: The Regulation and Intervention. Signal Transduct Target Ther 2021, 6, 245. [Google Scholar] [CrossRef]
- Hatlen, P.; Langhammer, A.; Carlsen, S.M.; Salvesen, Ø.; Amundsen, T. Self-Reported Cardiovascular Disease and the Risk of Lung Cancer, the HUNT Study. Journal of Thoracic Oncology 2014, 9, 940–946. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, L.; Shi, S.; He, H.; Shen, Q.; Wang, H.; Qin, S.; Chang, J.; Zhong, R. Bidirectional Association Between Cardiovascular Disease and Lung Cancer in a Prospective Cohort Study. J Thorac Oncol 2024, 19, 80–93. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, L.; Yan, G.; Chen, Y.; Zhou, M.; Wu, Y.; Li, Y. Inflammation and Tumor Progression: Signaling Pathways and Targeted Intervention. Signal Transduct Target Ther 2021, 6, 263. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Behdarvand Dehkordi, F.; Chehelgerdi, M.; Kabiri, H.; Salehian-Dehkordi, H.; Abdolvand, M.; Salmanizadeh, S.; Rashidi, M.; Niazmand, A.; Ahmadi, S.; et al. Exploring the Promising Potential of Induced Pluripotent Stem Cells in Cancer Research and Therapy. Mol Cancer 2023, 22, 189. [Google Scholar] [CrossRef]
- Hebib, L.; Herraiz-Adillo, Á.; Higueras-Fresnillo, S.; Berglind, D.; Daka, B.; Wennberg, P.; Hagström, E.; Lenander, C.; Ahlqvist, V.H.; Östgren, C.J.; et al. Life’s Essential 8 Is Inversely Associated with High-Sensitivity C-Reactive Protein. Sci Rep 2024, 14, 15024. [Google Scholar] [CrossRef]
- Mossmann, M.; Wainstein, M.V.; Mariani, S.; Machado, G.P.; de Araújo, G.N.; Andrades, M.; Gonçalves, S.C.; Bertoluci, M.C. Increased Serum IL-6 Is Predictive of Long-Term Cardiovascular Events in High-Risk Patients Submitted to Coronary Angiography: An Observational Study. Diabetol Metab Syndr 2022, 14, 125. [Google Scholar] [CrossRef]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harb Perspect Biol 2014, 6, a016295. [Google Scholar] [CrossRef]
- Ridker, P.M. From C-Reactive Protein to Interleukin-6 to Interleukin-1: Moving Upstream To Identify Novel Targets for Atheroprotection. Circ Res 2016, 118, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, M.; Hong, D.; Zeng, M.; Zhang, X. The Paradoxical Role of Cellular Senescence in Cancer. Front Cell Dev Biol 2021, 9, 722205. [Google Scholar] [CrossRef] [PubMed]
- Kumari, R.; Jat, P. Mechanisms of Cellular Senescence: Cell Cycle Arrest and Senescence Associated Secretory Phenotype. Front Cell Dev Biol 2021, 9, 645593. [Google Scholar] [CrossRef] [PubMed]
- Faubert, B.; Solmonson, A.; DeBerardinis, R.J. Metabolic Reprogramming and Cancer Progression. Science 2020, 368. [Google Scholar] [CrossRef] [PubMed]
- Hammoudi, N.; Ahmed, K.B.R.; Garcia-Prieto, C.; Huang, P. Metabolic Alterations in Cancer Cells and Therapeutic Implications. Chin J Cancer 2011, 30, 508–525. [Google Scholar] [CrossRef]
- Dudley, A.C.; Griffioen, A.W. Pathological Angiogenesis: Mechanisms and Therapeutic Strategies. Angiogenesis 2023, 26, 313–347. [Google Scholar] [CrossRef]
- de Boer, R.A.; Hulot, J.-S.; Tocchetti, C.G.; Aboumsallem, J.P.; Ameri, P.; Anker, S.D.; Bauersachs, J.; Bertero, E.; Coats, A.J.S.; Čelutkienė, J.; et al. Common Mechanistic Pathways in Cancer and Heart Failure. A Scientific Roadmap on Behalf of the Translational Research Committee of the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur J Heart Fail 2020, 22, 2272–2289. [Google Scholar] [CrossRef]
- Ray, M.R.; Jablons, D.; He, B. Lung Cancer Therapeutics That Target Signaling Pathways: An Update. Expert Rev Respir Med 2010, 4, 631–645. [Google Scholar] [CrossRef]
- Gallucci, G.; Tartarone, A.; Lerose, R.; Lalinga, A.V.; Capobianco, A.M. Cardiovascular Risk of Smoking and Benefits of Smoking Cessation. J Thorac Dis 2020, 12, 3866–3876. [Google Scholar] [CrossRef]
- Kepka, L.; Bujko, K.; Orlowski, T.M.; Jagiello, R.; Salata, A.; Matecka-Nowak, M.; Janowski, H.; Rogowska, D. Cardiopulmonary Morbidity and Quality of Life in Non-Small Cell Lung Cancer Patients Treated with or without Postoperative Radiotherapy. Radiother Oncol 2011, 98, 238–243. [Google Scholar] [CrossRef]
- Grimaldi, A.M.; Affinito, O.; Salvatore, M.; Franzese, M. CBX Family Members in Two Major Subtypes of Renal Cell Carcinoma: A Comparative Bioinformatic Analysis. Diagnostics (Basel) 2022, 12. [Google Scholar] [CrossRef]
- Zhou, F.; Chen, L.; Lu, P.; Cao, Y.; Deng, C.; Liu, G. An Integrative Bioinformatics Investigation and Experimental Validation of Chromobox Family in Diffuse Large B-Cell Lymphoma. BMC Cancer 2023, 23, 641. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Lai, C.; Luo, J.; Shi, J.; Guo, K.; Hu, J.; Mulati, Y.; Xiao, Y.; Kong, D.; Liu, C.; et al. The Predictive Significance of Chromobox Family Members in Prostate Cancer in Humans. Cell Oncol (Dordr) 2024. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Jiang, C.; Chen, D.; Wu, Y.; Lu, J.; Zhong, L.; Yao, F. Analysis of Pan-Cancer Revealed the Immunological and Prognostic Potential of CBX3 in Human Tumors. Front Med (Lausanne) 2022, 9, 869994. [Google Scholar] [CrossRef]
- Xie, L.; Chen, J.; Wang, Y.; Jin, C.; Xie, Y.; Ma, H.; Xiang, M. Emerging Roles of Macrophages in Heart Failure and Associated Treatment Approaches. Ther Adv Chronic Dis 2023, 14, 20406223231168756. [Google Scholar] [CrossRef]
- Le, P.T.; Ha, N.; Tran, N.K.; Newman, A.G.; Esselen, K.M.; Dalrymple, J.L.; Schmelz, E.M.; Bhandoola, A.; Xue, H.-H.; Singh, P.B.; et al. Targeting Cbx3/HP1γ Induces LEF-1 and IL-21R to Promote Tumor-Infiltrating CD8 T-Cell Persistence. Front Immunol 2021, 12, 738958. [Google Scholar] [CrossRef]
- Sun, M.; Ha, N.; Pham, D.-H.; Frederick, M.; Sharma, B.; Naruse, C.; Asano, M.; Pipkin, M.E.; George, R.E.; Thai, T.-H. Cbx3/HP1γ Deficiency Confers Enhanced Tumor-Killing Capacity on CD8+ T Cells. Sci Rep 2017, 7, 42888. [Google Scholar] [CrossRef]
- Jansen, F.; Li, Q.; Pfeifer, A.; Werner, N. Endothelial- and Immune Cell-Derived Extracellular Vesicles in the Regulation of Cardiovascular Health and Disease. JACC Basic Transl Sci 2017, 2, 790–807. [Google Scholar] [CrossRef]
- Li, R.; Jiang, S.; Li, W.; Hong, H.; Zhao, C.; Huang, X.; Zhang, Z.; Li, H.; Chen, H.; Bo, X. Exploration of Prognosis-Related MicroRNA and Transcription Factor Co-Regulatory Networks across Cancer Types. RNA Biol 2019, 16, 1010–1021. [Google Scholar] [CrossRef]
- Asiaee, A.; Abrams, Z.B.; Pua, H.H.; Coombes, K.R. Transcriptome Complexity Disentangled: A Regulatory Molecules Approach. bioRxiv 2024. [Google Scholar] [CrossRef]
- Qin, S.; Xie, B.; Wang, Q.; Yang, R.; Sun, J.; Hu, C.; Liu, S.; Tao, Y.; Xiao, D. New Insights into Immune Cells in Cancer Immunotherapy: From Epigenetic Modification, Metabolic Modulation to Cell Communication. MedComm (Beijing) 2024, 5, e551. [Google Scholar] [CrossRef] [PubMed]
- Napiórkowska-Baran, K.; Schmidt, O.; Szymczak, B.; Lubański, J.; Doligalska, A.; Bartuzi, Z. Molecular Linkage between Immune System Disorders and Atherosclerosis. Curr Issues Mol Biol 2023, 45, 8780–8815. [Google Scholar] [CrossRef] [PubMed]
- Poels, K.; Neppelenbroek, S.I.M.; Kersten, M.J.; Antoni, M.L.; Lutgens, E.; Seijkens, T.T.P. Immune Checkpoint Inhibitor Treatment and Atherosclerotic Cardiovascular Disease: An Emerging Clinical Problem. J Immunother Cancer 2021, 9. [Google Scholar] [CrossRef]
- Tan, S.; Spear, E.; Sane, N.; Chan, J.; Nelson, A.J.; Alamgeer, M.; Nerlekar, N.; Segelov, E.; Nicholls, S.J. Atherosclerotic Cardiovascular Events in Cancer Patients Treated With Immune Checkpoint Inhibitors: A Retrospective Cohort Study. Heart Lung Circ 2024, 33, 721–729. [Google Scholar] [CrossRef]
- Khan, A.; Ley, K. Immunotherapy for Atherosclerosis by Targeting Pro-Inflammatory T Cells. Cell Res 2024, 34, 467–468. [Google Scholar] [CrossRef]
- Suero-Abreu, G.A.; Zanni, M. V; Neilan, T.G. Atherosclerosis With Immune Checkpoint Inhibitor Therapy: Evidence, Diagnosis, and Management: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol 2022, 4, 598–615. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Costache, A.-D.; Costache, I.-I. Therapeutic Strategies and Chemoprevention of Atherosclerosis: What Do We Know and Where Do We Go? Pharmaceutics 2022, 14. [Google Scholar] [CrossRef]
- Rajamäki, K.; Lappalainen, J.; Öörni, K.; Välimäki, E.; Matikainen, S.; Kovanen, P.T.; Eklund, K.K. Cholesterol Crystals Activate the NLRP3 Inflammasome in Human Macrophages: A Novel Link between Cholesterol Metabolism and Inflammation. PLoS One 2010, 5, e11765. [Google Scholar] [CrossRef]
- Megha, K.B.; Joseph, X.; Akhil, V.; Mohanan, P. V Cascade of Immune Mechanism and Consequences of Inflammatory Disorders. Phytomedicine 2021, 91, 153712. [Google Scholar] [CrossRef]
- Campos, J.H.; Soares, R.P.; Ribeiro, K.; Andrade, A.C.; Batista, W.L.; Torrecilhas, A.C. Extracellular Vesicles: Role in Inflammatory Responses and Potential Uses in Vaccination in Cancer and Infectious Diseases. J Immunol Res 2015, 2015, 832057. [Google Scholar] [CrossRef]
- Sidorkiewicz, M. Is MicroRNA-33 an Appropriate Target in the Treatment of Atherosclerosis? Nutrients 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Tavoosi, Z.; Moradi-Sardareh, H.; Saidijam, M.; Yadegarazari, R.; Borzuei, S.; Soltanian, A.; Goodarzi, M.T. Cholesterol Transporters ABCA1 and ABCG1 Gene Expression in Peripheral Blood Mononuclear Cells in Patients with Metabolic Syndrome. Cholesterol 2015, 2015, 682904. [Google Scholar] [CrossRef] [PubMed]
- Poznyak, A. V; Kashirskikh, D.A.; Sukhorukov, V.N.; Kalmykov, V.; Omelchenko, A. V; Orekhov, A.N. Cholesterol Transport Dysfunction and Its Involvement in Atherogenesis. Int J Mol Sci 2022, 23. [Google Scholar] [CrossRef] [PubMed]
- Apostolakis, S.; Spandidos, D. Chemokines and Atherosclerosis: Focus on the CX3CL1/CX3CR1 Pathway. Acta Pharmacol Sin 2013, 34, 1251–1256. [Google Scholar] [CrossRef]
- Duan, Q.; Flynn, C.; Niepel, M.; Hafner, M.; Muhlich, J.L.; Fernandez, N.F.; Rouillard, A.D.; Tan, C.M.; Chen, E.Y.; Golub, T.R.; et al. LINCS Canvas Browser: Interactive Web App to Query, Browse and Interrogate LINCS L1000 Gene Expression Signatures. Nucleic Acids Res 2014, 42, W449–60. [Google Scholar] [CrossRef]
- Russo, R.C.; Garcia, C.C.; Teixeira, M.M.; Amaral, F.A. The CXCL8/IL-8 Chemokine Family and Its Receptors in Inflammatory Diseases. Expert Rev Clin Immunol 2014, 10, 593–619. [Google Scholar] [CrossRef]
- Márquez, A.B.; van der Vorst, E.P.C.; Maas, S.L. Key Chemokine Pathways in Atherosclerosis and Their Therapeutic Potential. J Clin Med 2021, 10. [Google Scholar] [CrossRef]
- Herman, A.B.; Occean, J.R.; Sen, P. Epigenetic Dysregulation in Cardiovascular Aging and Disease. The journal of cardiovascular aging 2021, 1. [Google Scholar] [CrossRef]
- Shi, Y.; Zhang, H.; Huang, S.; Yin, L.; Wang, F.; Luo, P.; Huang, H. Epigenetic Regulation in Cardiovascular Disease: Mechanisms and Advances in Clinical Trials. Signal Transduct Target Ther 2022, 7, 200. [Google Scholar] [CrossRef]
- Sawalha, K.; Norgard, N.; López-Candales, A. Epigenetic Regulation and Its Effects on Aging and Cardiovascular Disease. Cureus 2023, 15, e39395. [Google Scholar] [CrossRef]
- Ray, K.K.; Nicholls, S.J.; Buhr, K.A.; Ginsberg, H.N.; Johansson, J.O.; Kalantar-Zadeh, K.; Kulikowski, E.; Toth, P.P.; Wong, N.; Sweeney, M.; et al. Effect of Apabetalone Added to Standard Therapy on Major Adverse Cardiovascular Events in Patients With Recent Acute Coronary Syndrome and Type 2 Diabetes: A Randomized Clinical Trial. JAMA 2020, 323, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Pagiatakis, C.; Di Mauro, V. The Emerging Role of Epigenetics in Therapeutic Targeting of Cardiomyopathies. Int J Mol Sci 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Garza, M.A.; Wason, E.A.; Zhang, J.Q. Cardiac Remodeling and Physical Training Post Myocardial Infarction. World J Cardiol 2015, 7, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Sopic, M.; Robinson, E.L.; Emanueli, C.; Srivastava, P.; Angione, C.; Gaetano, C.; Condorelli, G.; Martelli, F.; Pedrazzini, T.; Devaux, Y.; et al. Integration of Epigenetic Regulatory Mechanisms in Heart Failure. Basic Res Cardiol 2023, 118, 16. [Google Scholar] [CrossRef]
- Al-Hasani, K.; Mathiyalagan, P.; El-Osta, A. Epigenetics, Cardiovascular Disease, and Cellular Reprogramming. J Mol Cell Cardiol 2019, 128, 129–133. [Google Scholar] [CrossRef]
- Alghamdi, B.A.; Aljohani, I.M.; Alotaibi, B.G.; Ahmed, M.; Almazmomi, K.A.; Aloufi, S.; Alshamrani, J. Studying Epigenetics of Cardiovascular Diseases on Chip Guide. Cardiogenetics 2022, 12, 218–234. [Google Scholar] [CrossRef]
- Kapustin, A.; Tsakali, S.S.; Whitehead, M.; Chennell, G.; Wu, M.-Y.; Molenaar, C.; Kutikhin, A.; Bogdanov, L.; Sinitsky, M.; Rubina, K.; et al. Extracellular Vesicles Stimulate Smooth Muscle Cell Migration by Presenting Collagen VI. bioRxiv 2023. [Google Scholar] [CrossRef]
- Wagner, N.; Wagner, K.-D. Molecular Mechanisms of Cardiac Development and Disease. Int J Mol Sci 2023, 24. [Google Scholar] [CrossRef]
- Adhikary, S.; Pathak, S.; Palani, V.; Acar, A.; Banerjee, A.; Al-Dewik, N.I.; Essa, M.M.; Mohammed, S.G.A.A.; Qoronfleh, M.W. Current Technologies and Future Perspectives in Immunotherapy towards a Clinical Oncology Approach. Biomedicines 2024, 12. [Google Scholar] [CrossRef]
- Jyotsna, F.; Ikram, J.; Nageeta, F.; Komal, F.; Anjlee, F.; Patel, H.; Nassri, T.; Kumari, M.; Kumar, R.; Shah, S.U.; et al. Unlocking the Potential of Immunotherapy in Cardiovascular Disease: A Comprehensive Review of Applications and Future Directions. Cureus 2023. [Google Scholar] [CrossRef]
- Suzumura, E.A.; de Oliveira Ascef, B.; Maia, F.H. de A.; Bortoluzzi, A.F.R.; Domingues, S.M.; Farias, N.S.; Gabriel, F.C.; Jahn, B.; Siebert, U.; de Soarez, P.C. Methodological Guidelines and Publications of Benefit-Risk Assessment for Health Technology Assessment: A Scoping Review. BMJ Open 2024, 14, e086603. [Google Scholar] [CrossRef] [PubMed]
- Duffy, A.; Browne, F.; Connolly, M. Safeguarding Adults: A Concept Analysis. J Adv Nurs 2024. [Google Scholar] [CrossRef] [PubMed]
- Sapna, F.; Raveena, F.; Chandio, M.; Bai, K.; Sayyar, M.; Varrassi, G.; Khatri, M.; Kumar, S.; Mohamad, T. Advancements in Heart Failure Management: A Comprehensive Narrative Review of Emerging Therapies. Cureus 2023, 15, e46486. [Google Scholar] [CrossRef] [PubMed]
| Target | CBX3-mediated epigenetic effect |
Effect on CVD | Reference |
|---|---|---|---|
| DNA | binding | regulation of gene transcription and chromatin structure for cardiovascular health | [25] |
| CD8+ T cells | interaction with chromatin modifiers | cardiovascular disease progression and outcomes | [26] |
| miR-21 | repressed expression | cardiac fibrosis attenuation and remodeling in response to stress | [30] |
| miR-126 | expression regulation | maintenance of vascular integrity and promotion angiogenesis | [31,32] |
| Tnnt3, Tbx20, Tbx3, Hand1, PDGFRA | upregulation | cardiac lineage development | [16] |
| Sox1 | downregulation | circulatory system development | [16] |
| Wnt4 | up-regulation | circulatory system development | [16] |
| Cdk8 | decreased recruitment | cardiovascular system development | [33] |
| CPT1A, ABCG1 | negative/positive directions | lipid-associated CpGs | [34] |
| ABCG1 locus | expression | increased triglyceride levels and a heightened risk of new-onset coronary heart disease | [35,36] |
| GATA4 | modulation of chromatin environment | cardiac gene regulation | [40] |
| ApoC3 | altered activity | progression of atherosclerosis | [41] |
| Target/factor | CBX3-mediated mechanism | Effect on CVD | Reference |
|---|---|---|---|
| CD8+ effector T cells | increase of LEF-1 and IL-21R | immune response | [87,88] |
| B cells | modulation of the local immune response and the structural integrity of blood vessels | progression | [89] |
| immune checkpoints and chemokines |
immune response modulation |
cellular processes and disease states |
[92] |
| Genes in immune activation and suppression |
Interaction |
development of atherosclerosis | [92] |
| SMURF2 inhibition and TGF-β signaling activation |
selective inhibition | stabilization of atherosclerotic plaque | [17] |
| NLRP3 inflammasome | prevention of the activation | atherosclerotic lesions | [99] |
| IL-1β | reduction of the release | limiting the extent of inflammation and tissue damage | [100] |
| inflammatory signals | impedes the vesicular secretion | control of the inflammatory environment |
[101] |
| CoQ |
interaction | macrophage reverse cholesterol transport | [101,102] |
| CX3CL1 | interaction | modulates the inflammatory response within the plaques | [105,106] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





