Submitted:
30 September 2024
Posted:
01 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
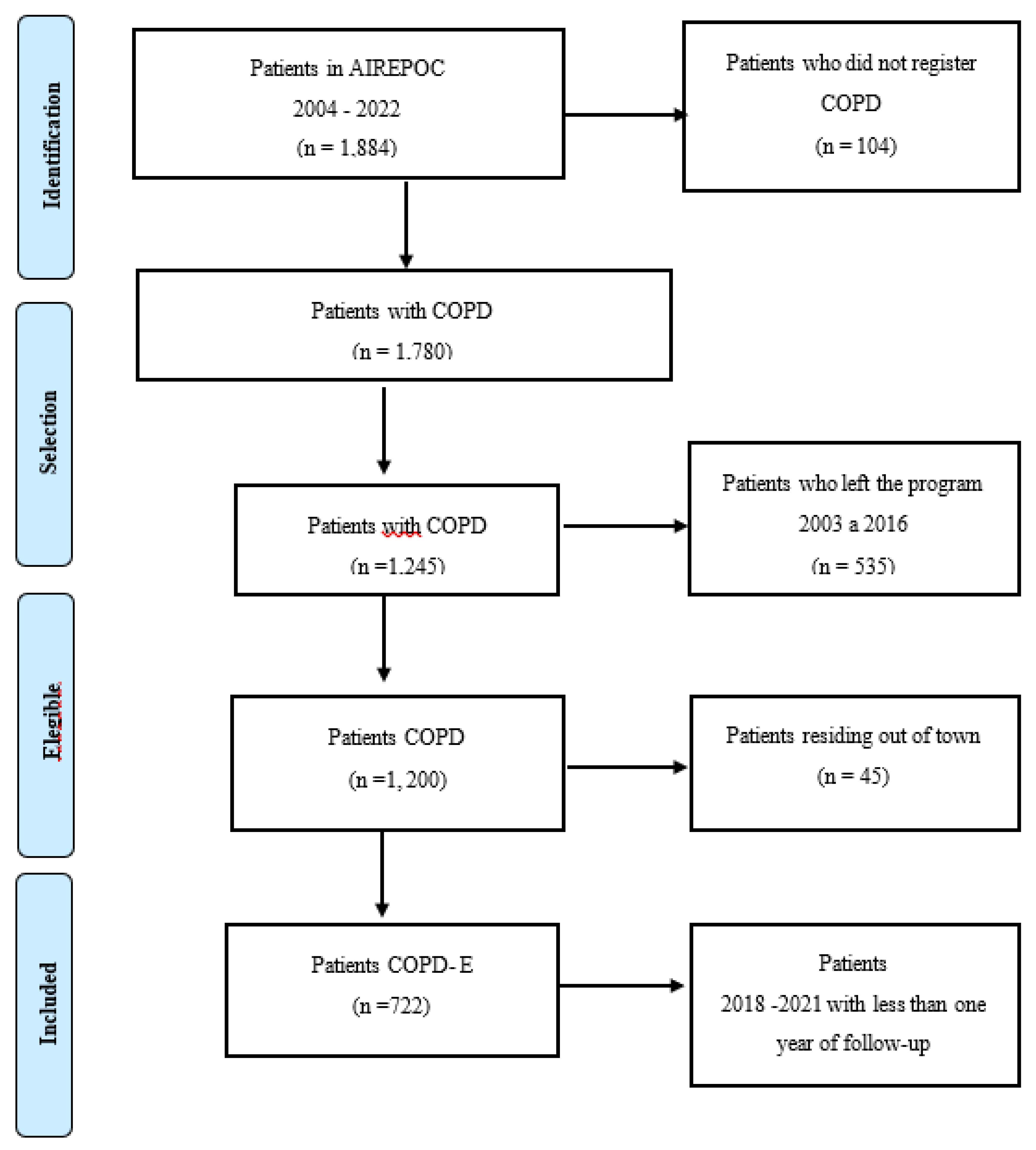
2.1. Study Site and Design
2.2. Patient Cohort Assembly
2.3. Source of Patient Data
2.4. Sources of Air Pollutant and Meteorological Data
2.5. Exposure Estimation
2.6. Study Outcome Variable
2.7. Statistical Analysis
3. Results
3.1. Characteristics of the Participants in the Cohort
3.2. Exposure to Air Pollution
3.3. Survival
3.4. Air Pollutants and the Risk of COPD-E
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- World Health Organization WHO. Chronic Obstructive Pulmonary Disease (COPD).
- Institute for Health Metrics and Evaluation. Global Burden of Disease (GBD). GBD Compare 2019. https://vizhub.healthdata.org/gbd-compare/ (2022, accessed 20 June 2022).
- World Health Organization WHO. WHO The top 10 causes of death.
- Sleeman, K.E.; de Brito, M.; Etkind, S.; et al. The escalating global burden of serious health-related suffering: projections to 2060 by world regions, age groups, and health conditions. Lancet Glob Heal 2019, 7, e883–e892. [Google Scholar] [CrossRef]
- Cohen, A.J.; Brauer, M.; Burnett, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. Lancet (London, England) 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; et al. Prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Respir Med 2020, 8, 585–596. [Google Scholar] [CrossRef]
- Soriano, J.B.; Kendrick, P.J.; Paulson, K.R.; Gupta, V.; Abrams, E.M.; Adedoyin, R.A.; et al. Supplementary appendix 2 of prevalence and attributable health burden of chronic respiratory diseases, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 8.
- Roque, A.; Taborda-Barata, L.; Cruz, Á.A.; et al. COPD treatment – a conceptual review based on critical endpoints. Pulmonology Epub ahead of print. 2023. [CrossRef]
- Stolz, D.; Mkorombindo, T.; Schumann, D.M.; et al. The Lancet Commissions Towards the elimination of chronic obstructive pulmonary disease : a Lancet Commission. Lancet 2022, 400, 921–972. [Google Scholar] [CrossRef]
- Hendryx, M.; Luo, J.; Chojenta, C.; et al. Air pollution exposures from multiple point sources and risk of incident chronic obstructive pulmonary disease (COPD) and asthma. Environ Res 2019, 179, 108783. [Google Scholar] [CrossRef]
- Lee, Y.-G.; Lee, P.-H.; Choi, S.-M.; et al. Effects of Air Pollutants on Airway Diseases. Int J Environ Res Public Health 18. Epub ahead of print September 2021. [CrossRef]
- Cavailles, A.; Melloni, B.; Motola, S.; et al. Identification of Patient Profiles with High Risk of Hospital Re-Admissions for Acute COPD Exacerbations (AECOPD) in France Using a Machine Learning Model. Int J Chron Obstruct Pulmon Dis 2020, 15, 949–962. [Google Scholar] [CrossRef]
- Kwon, S.O.; Hong, S.H.; Han, Y.-J.; et al. Long-term exposure to PM(10) and NO(2) in relation to lung function and imaging phenotypes in a COPD cohort. Respir Res 2020, 21, 247. [Google Scholar] [CrossRef]
- Hurst, J.R.; Skolnik, N.; Hansen, G.J.; et al. Understanding the impact of chronic obstructive pulmonary disease exacerbations on patient health and quality of life. Eur J Intern Med 2020, 73, 1–6. [Google Scholar] [CrossRef]
- Stanford, R.H.; Engel-Nitz, N.M.; Bancroft, T.; et al. The Identification and Cost of Acute Chronic Obstructive Pulmonary Disease Exacerbations in a United States Population Healthcare Claims Database. COPD 2020, 17, 499–508. [Google Scholar] [CrossRef]
- Kang, S.; Hong, Y.S.; Park, J.; et al. Air pollution and mortality in patients with chronic obstructive pulmonary disease: a cohort study in South Korea. Ther Adv Chronic Dis 2023, 14, 20406223231176176. [Google Scholar] [CrossRef]
- Thurston, G.D.; Balmes, J.R.; Garcia, E.; et al. Outdoor Air Pollution and New-Onset Airway Disease. An Official American Thoracic Society Workshop Report. Ann Am Thorac Soc 2020, 17, 387–398. [Google Scholar] [CrossRef]
- Concato, J.; Stein, P.; Corrigan-curay, J.; et al. Randomized, observational, interventional, and real-world—What’s in a name? 2020, 1514–1517. [Google Scholar]
- Simoni, M.; Baldacci, S.; Maio, S.; et al. Adverse effects of outdoor pollution in the elderly. J Thorac Dis 2015, 7, 34–45. [Google Scholar]
- Emmendorfer, L.R.; Dimuro, G.P. A Novel Formulation for Inverse Distance Weighting from Weighted Linear Regression BT - . In Computational Science – ICCS 2020; Krzhizhanovskaya, V.V., Závodszky, G., Lees, M.H., et al., Eds.; Springer International Publishing: Cham, 2020; pp. 576–589. [Google Scholar]
- World Health Organization WHO. WHO global air quality guidelines Particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide; 2021; Epub ahead of print 2021; ISBN 978-92-4-003422-8. [Google Scholar]
- Andersen, Z.J.; Hvidberg, M.; Jensen, S.S.; et al. Chronic obstructive pulmonary disease and long-term exposure to traffic-related air pollution: a cohort study. Am J Respir Crit Care Med 2011, 183, 455–461. [Google Scholar] [CrossRef]
- De Matteis, S.; Forastiere, F.; Baldacci, S.; et al. Issue 1 - ‘Update on adverse respiratory effects of outdoor air pollution’. Part 1): Outdoor air pollution and respiratory diseases: A general update and an Italian perspective. Pulmonology 2022, 28, 284–296. [Google Scholar] [CrossRef]
- Badida, P.; Krishnamurthy, A.; Jayaprakash, J. Meta analysis of health effects of ambient air pollution exposure in low- and middle-income countries. Environ Res 2023, 216, 114604. [Google Scholar] [CrossRef]
- Dąbrowiecki, P.; Chciałowski, A.; Dąbrowiecka, A.; et al. Air pollution and long-term risk of hospital admission due to chronic obstructive pulmonary disease exacerbations in Poland: a time-stratified, case-crossover study. Polish Arch Intern Med. Epub ahead of print February 2023. [CrossRef]
- Guo, C.; Zhang, Z.; Lau, A.K.H.; Lin, C.Q.; Chuang, Y.C.C.J.; et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Heal. [CrossRef]
- Huh, J.-Y.; Hong, J.; Han, D.-W.; et al. The Impact of Air Pollutants and Meteorological Factors on Chronic Obstructive Pulmonary Disease Exacerbations: A Nationwide Study. Ann Am Thorac Soc 2022, 19, 214–226. [Google Scholar] [CrossRef]
- Li, Q.; Yi, Q.; Tang, L.; et al. Influence of Ultrafine Particle Exposure on Asthma Exacerbation in Children: a Meta-Analysis. Curr Drug Targets 2019, 20, 412–420. [Google Scholar] [CrossRef]
- Tran, H.M.; Chen, T.T.L.Y.; et al. Climate-mediated air pollution associated with COPD severity. 2022; 843–156969.
- Doiron, D.; Bourbeau, J.; de Hoogh, K.; et al. Ambient air pollution exposure and chronic bronchitis in the Lifelines cohort. Thorax 2021, 76, 772–779. [Google Scholar] [CrossRef]
- Ferrari, U.; Exner, T.; Wanka, E.R.; et al. Influence of air pressure, humidity, solar radiation, temperature, and wind speed on ambulatory visits due to chronic obstructive pulmonary disease in Bavaria, Germany. Int J Biometeorol 2012, 56, 137–143. [Google Scholar] [CrossRef]
- Zilli Vieira, C.L.; Koutrakis, P.; Liu, M.; et al. Intense solar activity reduces urinary 6-sulfatoxymelatonin in patients with COPD. Respir Res 2023, 24, 91. [Google Scholar] [CrossRef]
- Cowan, K.N.; Wyatt, L.H.; Luben, T.J.; et al. Effect measure modification of the association between short-term exposures to PM(2.5) and hospitalizations by longs-term PM(2.5) exposure among a cohort of people with Chronic Obstructive Pulmonary Disease (COPD) in North Carolina, 2002-2015. Environ Health 2023, 22, 49. [Google Scholar] [CrossRef]
- Liang, L.; Cai, Y.; Barratt, B.; et al. Associations between daily air quality and hospitalisations for acute exacerbation of chronic obstructive pulmonary disease in Beijing, 2013-17: an ecological analysis. Lancet Planet Heal 2019, 3, e270–e279. [Google Scholar] [CrossRef]
- de F C Lichtenfels, A.J.; van der Plaat, D.A.; de Jong, K.; et al. Long-term Air Pollution Exposure, Genome-wide DNA Methylation and Lung Function in the LifeLines Cohort Study. Environ Health Perspect 2018, 126, 27004. [Google Scholar] [CrossRef]
- Yan, P.; Liu, P.; Lin, R.; et al. Effect of ambient air quality on exacerbation of COPD in patients and its potential mechanism. Int J Chron Obstruct Pulmon Dis 2019, 14, 1517–1526. [Google Scholar] [CrossRef]
- Liu, X.; Dong, M.; Wang, J .; et al. A weekly time-weighted method of outdoor and indoor individual exposure to particulate air pollution. Methodsx 2019, 6, 2439–2443. [Google Scholar] [CrossRef]
- IQAir. World Air Quality Report Region and city PM2.5 Ranking 2023. www.iqair.com (2024).
- Kang, J.; Kim, H.-C.; Jang, Y.; et al. Randomised controlled trial of a behavioural intervention to reduce exposure to PM2.5 in patients with COPD. Environ Int 2023, 181, 108286. [Google Scholar] [CrossRef]
- Liu, S.; Jørgensen, J.T.; Ljungman, P.; et al. Long-term exposure to low-level air pollution and incidence ofchronic obstructive pulmonary disease: The ELAPSE project. Env Int 146.
| Characteristics | ALL | No COPD-E | COPD -E | p | N |
|
N=722 (CI95%: LI – LS) |
N=340 (CI95%: LI – LS) |
N=382 (CI95%: LI – LS) |
|||
| Exacerbation of COPD (%) 1 | 53 [50;57] | 0 [0;0] | 100 [96;99.9] | 0.000 | 722 |
| Sex | |||||
| Men | 60 [57;64] | 64 [59;69] | 57 [52;62] | 0.038 | 722 |
| Woman | 39.9 [36.3;43.6] | 35.9 [30.8;41.2] | 43.5 [38.4;48.6] | 0.046 | 722 |
| Current age () | 75.1 [74.4;75.8] | 74.4 [73.4;75.5] | 75.6 [74.7;76.6] | 0.086 | 722 |
| History of COPD-E (%) | |||||
| COPD-E mild | 12 [9; 16] | 8 [4;12] | 16 [10;22] | 0.031 | 722 |
| COPD-E moderate | 39 [32; 45] | 19 [13;25] | 56 [45;66] | <0.001 | 722 |
| COPD-E severe | 40 [0.34;0.45] | 24 [17;30] | 54 [46;62] | <0.001 | 722 |
| COPD classification according to GOLD 2019: (%) | <0.001 | 722 | |||
| Grade A | 28.0 [24.7;31.4] | 30.0 [25.2;35.2] | 26.2 [21.8;30.9] | ||
| Grade B | 39.5 [35.9;43.1] | 51.2 [45.7;56.6] | 29.1 [24.6;33.9] | ||
| Grade C | 10.9 [8.76;13.4] | 5.29 [3.17;8.24] | 16.0 [12.4;20.0] | ||
| Grade D | 21.6 [18.7;24.8] | 13.5 [10.1;17.6] | 28.8 [24.3;33.6] | ||
| Pulmonary function () | |||||
| FVC_PRE 2 | 2.66 [2.46;2.86] | 2.81 [2.39;3.22] | 2.52 [2.43;2.61] | 0.189 | 702 |
| FEV1_PRE 3 | 1.30 [1.26;1.34] | 1.33 [1.27;1.39] | 1.27 [1.21;1.32] | 0.136 | 702 |
| FEV1/FVCPRE 4 | 0.82 [0.46;1.19] | 0.77 [0.27;1.27] | 0.88 [0.36;1.40] | 0.762 | 697 |
| FVC_POS 5 | 3.14 [2.43;3.85] | 3.77 [2.54;5.01] | 2.58 [1.82;3.34] | 0.106 | 702 |
| FEV1POSPRED 6 | 1.81 [1.73;1.88] | 2.00 [1.89;2.11] | 1.64 [1.54;1.74] | <0.001 | 693 |
| VE1/FVCPRED 7 | 44.1 [33.5;54.7] | 51.1 [39.7;62.5] | 37.7 [20.3;55.1] | 0.206 | 699 |
| LIN 8 | 68.8 [68.5;69.1] | 68.9 [68.5;69.4] | 68.7 [68.4;69.0] | 0.443 | 722 |
| FEV1/CV_FCRAPO | 69.5 [69.3;69.7] | 69.6 [69.3;69.9] | 69.5 [69.2;69.7] | 0.443 | 722 |
| FVC_POS0_2 9 | 0.90 [0.84;0.96] | 0.93 [0.80;1.06] | 0.87 [0.85;0.89] | 0.397 | 722 |
| FEV1_POS0 10 | 1.37 [1.34;1.41] | 1.39 [1.34;1.45] | 1.36 [1.30;1.41] | 0.394 | 722 |
| FEV1_POS0_2 11 | 0.58 [0.57;0.60] | 0.58 [0.56;0.60] | 0.58 [0.57;0.60] | 0.863 | 722 |
| FEV1/FVC0 12 | 0.52 [0.51;0.52] | 0.52 [0.50;0.53] | 0.52 [0.50;0.53] | 0.790 | 722 |
| Quality of Life -questionnaire Saint George 13 |
|||||
| Symptoms | 41.0 [39.5;42.5] | 36.0 [33.9;38.1] | 45.8 [43.7;47.9] | <0.001 | |
| Activity | 60.2 [58.3;62.1] | 58.5 [55.9;61.2] | 61.7 [59.0;64.4] | 0.103 | |
| Impact | 32.9 [31.3;34.5] | 31.4 [29.1;33.8] | 34.3 [32.0;36.5] | 0.087 | |
| Total | 42.4 [40.9;43.8] | 40.1 [38.1;42.2] | 44.4 [42.4;46.5] | 0.003 | |
| Used LTOT at admission (%) | 0.349 | ||||
| No | 32.8 [29.4;36.4] | 34.7 [29.7;40.0] | 31.2[26.5;36.1] | ||
| Yes | 67.2 [63.6;70.6] | 65.3 [60.0;70.3] | 68.8 [63.9;73.5] | ||
| Current prescribed hours | 15.3 [14.6;16.0] | 15.4 [14.4;16.4] | 15.3 [14.4;16.2] | 0.893 | 563 |
| Risk factor: (%) | 0.244 | 722 | |||
| Wood smoke | 12.9 [10.5;15.5] | 13.5 [10.1;17.6] | 12.3 [9.18;16.0] | ||
| No risk factor | 1.11 [0.48;2.17] | 0.29 [0.01;1.63] | 1.83 [0.74;3.74] | ||
| Tobacco | 56.5 [52.8;60.2] | 59.4 [54.0;64.7] | 53.9 [48.8;59.0] | ||
| Firewood tobacco | 25.2 [22.1;28.5] | 22.9 [18.6;27.8] | 27.2 [22.8;32.0] | ||
| Passive tobacco | 2.77 [1.70;4.25] | 2.35 [1.02;4.58] | 3.14 [1.63;5.42] | ||
| Tobacco and other fumes | 1.52 [0.76;2.71] | 1.47 [0.48;3.40] | 1.57 [0.58;3.39] | ||
| Current smoking (%) | 0.692 | 709 | |||
| No | 90.8 [88.5;92.9] | 91.0 [87.4;93.9] | 90.7 [87.3;93.4] | ||
| Yes | 9.03 [7.02;11.4] | 8.68 [5.89;12.2] | 9.33 [6.59;12.7] | ||
| NA | 0.14 [0.00;0.78] | 0.30 [0.01;1.66] | 0.00 [0.00;0.98] | ||
| Index Package year (IPA) () | 35.0 [32.8;37.2] | 35.1 [31.9;38.2] | 34.9 [31.8;38.0] | 0.944 | 622 |
| Index Package year (IPA) categorized (%) | 0.979 | 722 | |||
| Mild < 5 | 13.9 [11.4;16.6] | 13.8 [10.3;18.0] | 13.9 [10.6;17.8] | ||
| Moderato 5-15 | 41.1 [37.5;44.8] | 40.6 [35.3;46.0] | 41.6 [36.6;46.7] | ||
| Severe 16-25 | 23.4 [20.4;26.7] | 24.1 [19.7;29.0] | 22.8 [18.7;27.3] | ||
| Very Severe >25 | 21.6 [18.7;24.8] | 21.5 [17.2;26.2] | 21.7 [17.7;26.2] | ||
| Hospitalization (%) | <0.001 | 637 | |||
| Never | 0.16 [0.00;0.87] | 0.00 [0.00;1.41] | 0.27 [0.01;1.47] | ||
| Before de 2018 | 61.4 [57.5;65.2] | 91.2 [87.0;94.3] | 40.8 [35.8;46.0] | ||
| Follow - up (2018-2021) | 38.5 [34.7;42.4] | 8.85 [5.69;13.0] | 58.9 [53.7;63.9] | ||
| Number of patients with COPD-E (%) before follow - up | 31 [27;34] | 14 [10;17] | 46 [41;51] | <0.001 | 722 |
| Hospitalization for respiratory cause | 0.31 [0.28;0.34] | 0.14 [0.10;0.18] | 0.46 [0.41;0.51] | <0.001 | 722 |
| Type of hospitalization (%) | <0.001 | 722 | |||
| No respiratory | 0,14 [0,00;0,77] | 0,29 [0,01;1,63] | 0,00 [0,00;0,96] | ||
| Respiratory | 30,7 [27,4;34,3] | 13,5 [10,1;17,6] | 46,1 [41,0;51,2] | ||
| No hospitalization | 69,1 [65,6;72,5] | 86,2 [82,0;89,7] | 53,9 [48,8;59,0] | ||
| ICU yes | 0,06 [0,03;0,08] | 0,04 [0,01;0,08] | 0,06 [0,03;0,09] | 0,435 | 374 |
| Mean Follow - up time in days | 719 [684;755] | 932 [881;984] | 530 [489;570] | <0,001 | 722 |
| Type de COPD-E (%) | <0,001 | 722 | |||
| No exacerbation | 47.1 [43.4;50.8] | 100 [98.9;100] | 0.00 [0.00;0.96] | ||
| Severe | 4.43 [3.05;6.20] | 0.00 [0.00;1.08] | 8.38 [5.80;11.6] | ||
| Mild | 11.2 [9.01;13.8] | 0.00 [0.00;1.08] | 21.2 [17.2;25.6] | ||
| Moderate | 37.3 [33.7;40.9] | 0.00 [0.00;1.08] | 70.4 [65.6;75.0] | ||
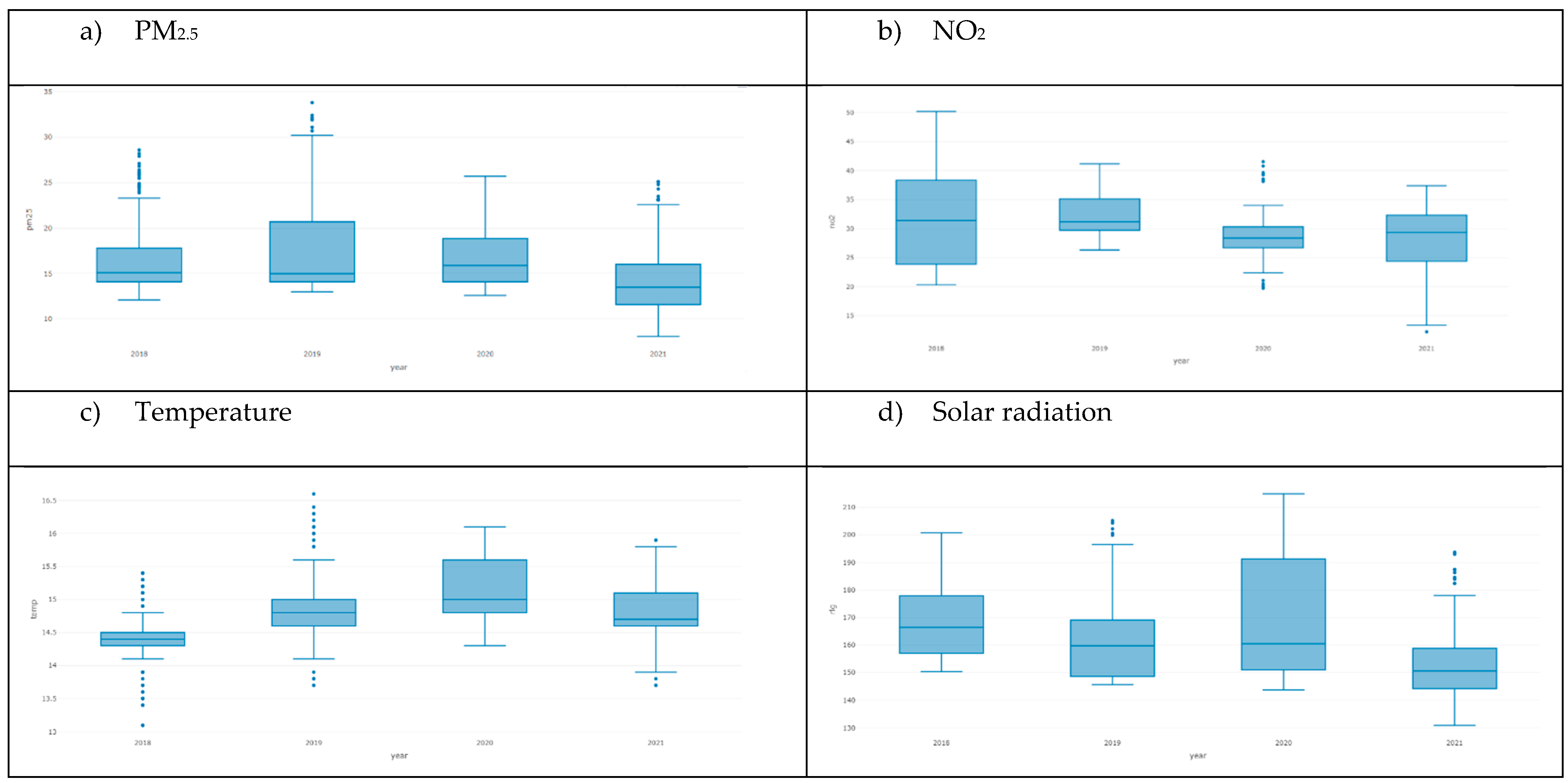
| PM2.5 (ug/m3) () 14 | 16.0 [15.7;16.3] | 15.4 [15.0;15.8] | 16.6 [16.1;17.0] | <0.001 | 722 |
| NO2 (ug/m3) () 15 | 30.3 [29.8;30.8] | 29.6 [28.9;30.3] | 31.0 [30.3;31.7] | 0.005 | 722 |
| Temperature (°C) () | 14.7 [14.7;14.8] | 14.7 [14.7;14.8] | 14.7 [14.7;14.8] | 0.827 | 722 |
| Precipitation (mm) () | 0.11 [0.11;0.12] | 0.13 [0.12;0.13] | 0.10 [0.10;0.11] | <0.001 | 722 |
| Solar Radiation (Wm2) () | 161 [160;162] | 158 [156;159] | 164 [163;166] | <0.001 | 722 |
| Relative Humidity () | 65.4 [65.3;65.6] | 66.0 [65.8;66.3] | 64.9 [64.7;65.1] | <0.001 | 722 |
| COVID19 (%) | 0.870 | 722 | |||
| No | 78.1 [74.9;81.1] | 78.5 [73.8;82.8] | 77.7 [73.2;81.8] | ||
| Yes | 21.9 [18.9;25.1] | 21.5 [17.2;26.2] | 22.3 [18.2;26.8] |
| Variable | Model with PM2.5 HR (CI95: LI – LS) |
Model with categorical variable for ≥ 15µg/m3 PM2.5 HR (CI 95: LI – LS) |
Model with NO2 HR (CI95: LI – LS) |
Model with categorical variable for ≥ 25µg/m3 NO2 HR (CI95: LI – LS) |
|---|---|---|---|---|
| n: 586 n events: 329 | ||||
| Pollutant | 1.03 (1.02- 1.05) ** | 1.19 (0.95-1.48) | 1.05 (1.03 -1.07) *** | 1.49 (1.12 -1.98) ** |
| FEV1 pre- bronchodilator | 0.81 (0.65- 1.003) | 0.80 (0.64 -0.99) * | 0.83 (0.67 -1.03) | 0.80 (0.64-0.99) * |
| Quality of the questionnaire Saint George (Symptoms) |
1.01 (1.01 - 1.02) *** | 1.01 (1.01 -1.018) *** | 1.01 (1.01 -1.02) *** | 1.01 (1.01-1.02) *** |
| Solar radiation | 1.02 (1.01- 1.03) *** | 1.01 (1.01-1.018) *** | 1.02 (1.02 -1.03) *** | 1.02 (1.01-1.02) *** |
| Number before of COPD-E | 1.02 (1.02- 1.024) *** | 1.02 (1.02 -1.024) *** | 1.02 (1.01 -1.024) *** | 1.02 (1.02-1.024) *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





