1. Introduction
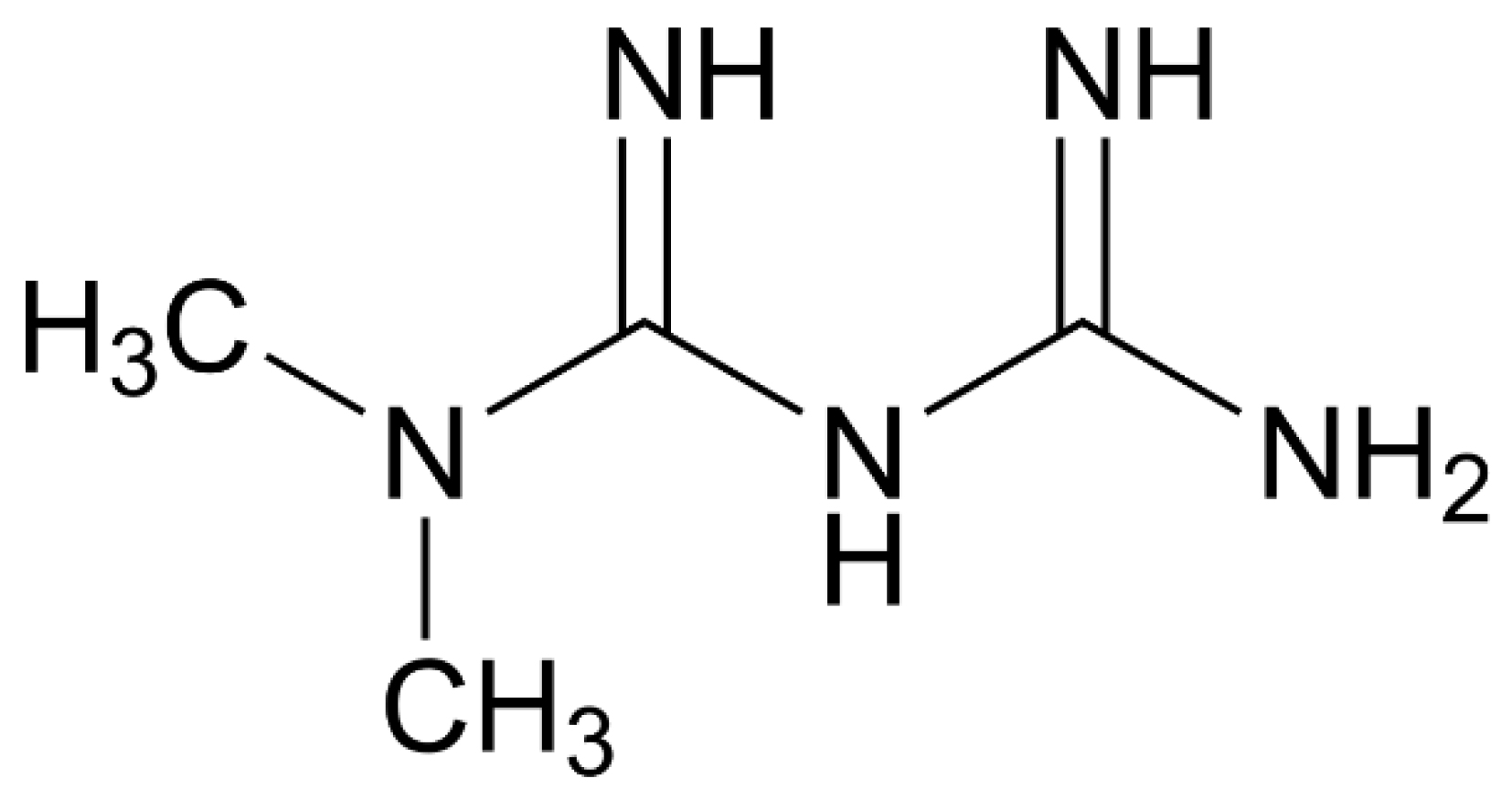
Metformin (
Figure 1), also known as 3-(diaminomethylidene)-1,1-dimethylguanidine (IUPAC name) or 1,1-Dimethylbiguanide or N,N-dimethylimidodicarbonimidic diamide is a member of the class of guanidines – a biguanide carrying two methyl substituents at position 1.
Metformin is primarily an antihyperglycemic agent and a first-line pharmacotherapy used in the management of type 2 diabetes mellitus, because it lowers blood glucose concentrations without causing hypoglycemia. Metformin inhibits the mitochondrial respiratory chain in the liver, leading to activation of AMP-activated protein kinase, enhancing insulin sensitivity for type 2 diabetes mellitus. The discovery of metformin traces back to medieval times when the use of Galega officinalis, a perennial herbaceous plant, in traditional herbal medicine was associated with treating symptoms of what is now known as type 2 diabetes mellitus. Other names for Galega officinalis include goat’s rue, French lilac, Spanish sainfoin, Italian fitch, or false indigo. Traditionally, goat’s rue was used for expelling parasites and treating snakebites. It is believed to have served as a diuretic and tonic in typhoid conditions and as a stimulant for the nervous system. It also exhibits fever-reducing effects. However, metformin provides also other medical benefits. Metformin is known to cause weight loss as a favourable side effect. Unfavourable side effects are mainly lactic acidosis, digestive problems, such as diarrhoea, vomiting, and flatulence. Common side effects are also abdominal discomfort, headaches and lack of energy. Alternatives of metformin are for example Dipeptidyl peptidase 4 (DPP-4) inhibitors. These medications offer the potential to enhance A1C scores without inducing hypoglycemia. Their mechanism involves inhibiting the enzyme DPP-4, thus preventing the degradation of glucagon-like peptide 1 (GLP-1) and gastric inhibitory peptide (GIP). GLP-1 and GIP are innate hormones that aid in lowering blood glucose levels. By prolonging the activity of these hormones, DPP-4 inhibitors facilitate glucose regulation. Generally, DPP-4 inhibitors are well tolerated. Other options are GLP-1 and dual GLP-1/GIP receptor agonists. Both GLP-1 and GIP are endogenous hormones crucial for glucose regulation. These medications mimic the actions of these natural hormones but possess resistance to degradation by DPP-4. Consequently, they facilitate blood glucose reduction and may additionally contribute to weight loss and the prevention of heart disease. Other possibilities are Sodium-glucose cotransporter 2 (SGLT2) inhibitors. Glucose present in the bloodstream undergoes filtration in the kidneys, where it’s either excreted in urine or reabsorbed into the blood. SGLT2 plays a role in the reabsorption of glucose in the kidneys. Consequently, SGLT2 inhibitors impede this process, facilitating the elimination of surplus glucose through urine. These medications offer the potential to enhance blood glucose control, promote weight loss, and reduce blood pressure. They may be prescribed by a physician for individuals with type 2 diabetes mellitus (T2DM) who also experience heart or kidney issues. However, it’s important to note that they may elevate the risk of genital yeast infections. Next availability are Sulfonylureas. These medications function by promoting the release of insulin from the beta cells in the pancreas. While all sulfonylurea drugs have comparable impacts on blood sugar levels, they vary in terms of side effects, interactions with other drugs, and dosing frequency. Common side effects may encompass hypoglycemia and weight gain. Last option are Thiazolidinediones (TZDs). These medications enhance the effectiveness of insulin in muscle and fat tissues while simultaneously decreasing glucose production in the liver. TZDs are associated with a reduced likelihood of hypoglycemia. However, individuals using drugs from this class may face an elevated risk of heart failure, and they may also experience fluid retention in the lower extremities.
Preclinical studies show that metformin has been identified as a potentially efficacious antitumor agent that acts collaboratively with other immunotherapeutic agents involved in tumor elimination [
1]. Metformin is also effective in treating several skin diseases, including acne vulgaris, hidradenitis suppurativa, psoriasis and hirsutism [
2], and is used to treat inflammatory diseases, endocrine-related dermatosis, and hyperpigmentation illnesses [
3]. According to Feng [
4], metformin can improve the dysfunction of macrophages that causes accelerated atherosclerosis. As observed by Hammad Uddin [
5] it showed also osteogenic, regenerative, anti-neoplastic and osseointegration properties in dentistry. Metformin is also classified as an antimicrobial agent [
6]. The antibacterial activity was studied against two highly resistant strains of Gram-positive and Gram-negative bacteria, namely methicillin-resistant
Staphylococcus aureus and multidrug resistant
Pseudomonas aeruginosa. It was found that metformin was able to reduce the resistance of these two strains to the tested antibiotics namely, doxycycline, levofloxacin, ampicillin, chloramphenicol, and rifampicin. Furthermore, these combinations (metformin-tested antibiotics) provided either a synergistic or an additive effect; suggesting that metformin efficiently enhances and potentiates the activity of these antibiotics. Metformin is also effective in treating osteoarthritis [
7,
8], neurological disorders [
9], inflammation [
10], endometriosis [
11], epilepsy [
12], vascular disease [
13], kidney disease [
14], renal diseases [
15], preeclampsia [
16], multiple sclerosis [
17], musculoskeletal disorders [
18], retinal diseases [
19] and venous thrombosis [
20]. Obtained data also suggest its effectiveness in targeting several age-related morbidities in humans [
21,
22]. Metformin is also very efficient in case of polycystic ovarian syndrome (PCOS) treatment. Typically, it impacts approximately 5 to 10% of the population, with ultrasound techniques easily revealing a range of cysts of various sizes in both ovaries. PCOS is strongly linked to a hyperglycemic condition, often tied to insulin resistance and type 2 diabetes. In PCOS patients, there is typically a consistent basal insulin secretion, leading to a state of hyperinsulinemia and consequently, relative dysfunction of pancreatic β-cells. Furthermore, metformin is expected to have an anticoagulant effect. However, its therapeutic use as an anticoagulant agent has not yet been approved, and further analysis is required before it can be fully endorsed.
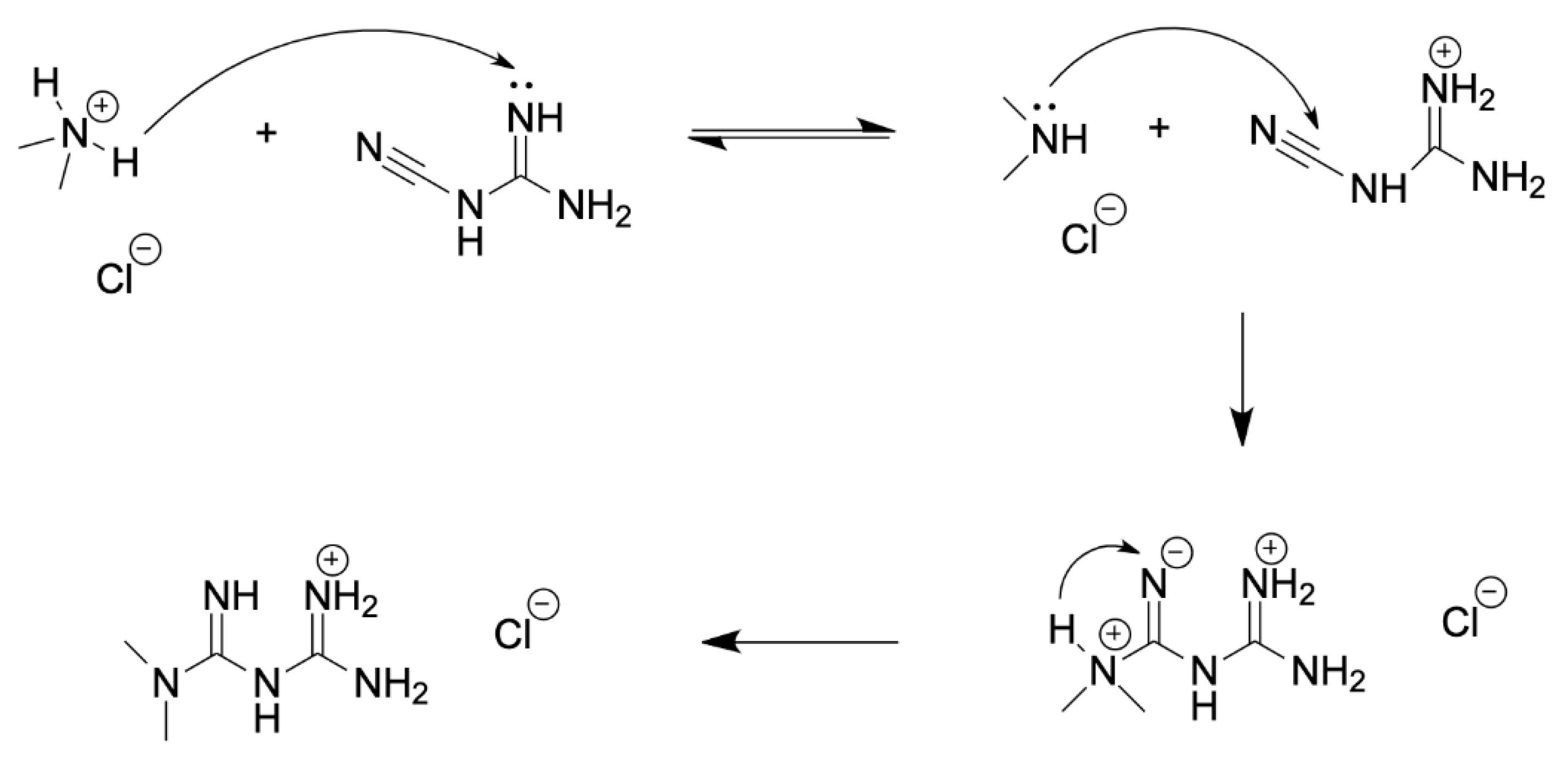
There are various patented procedures for metformin synthesis which differ in reaction conditions and purification strategy, but almost all involve reacting the hydrochloride salt of dimethylamine with cyanoguanidine. First, the dimethylamine hydrochloride is deprotonated by cyanoguanidine, forming a guanidinium cation. The dimethylamine nitrogen now has a free lone pair available for nucleophilic attack at the electrophilic nitrile carbon. Consequently, a proton transfer gives the metformin HCl salt. Reaction scheme of metformin synthesis is introduced in following
Figure 2.
Several clinical trials show that metformin inhibits tumor growth, invasion, and metastasis [
23,
24]. It is used in monotherapy or in combination with various chemotherapeutic or immunotherapeutic agents [
25]. For example, In the context of head and neck squamous cell carcinoma, metformin has been studied in combination with chemoradiotherapy and has demonstrated promising results. It was found that combining metformin with chemoradiotherapy resulted in high overall survival rates for patients with head and neck squamous cell carcinoma. Metformin is also known to enhance the effects of immune checkpoint inhibitors and other chemotherapeutic agents. Its ability to improve the immune response by increasing NK cell activity and inhibiting cancer-promoting pathways like CXCL1 suggests that it can synergize with other drugs to improve outcomes. The advantageous role of metformin was observed in cancer treatment for malignant skin tumors [
2], bone cancer [
26], breast cancer [
27], head and neck squamous cell carcinoma [
28], esophageal squamous cell carcinoma [
29], glioblastoma [
30], small cell lung cancer [
31], non-small cell lung cancer [
32,
33], lung adenocarcinoma [
34], endometrial cancer [
35], ovarian cancer [
36], urothelial cancer [
37], prostate cancer [
38], colorectal cancer [
39], pancreatic cancer [
40], hepatocellular carcinoma [
41], multiple myeloma [
42], acute lymphoblastic leukemia [
43] etc. However, despite all these findings, there are also numerous papers suggesting that there is no evidence for a decreased risk of various cancer types in association with metformin use [
44,
45,
46,
47]. There are proposed various mechanisms by which metformin exerts anticancer effect. Metformin inhibits oxidative respiration by acting on complex I of the mitochondrial respiratory chain, thus inhibiting the synthesis of ATP, increasing the adenosine diphosphate (ADP)/ATP ratio and adenosine monophosphate (AMP)/ATP ratio, and promoting the activation of AMPK. In addition, studies have shown that metformin-induced glucose starvation can also lead to the activation of AMPK through the lysosomal v-ATPase-Regulator complex. AMPK is a key signal integration factor crucial for the control of mitochondrial health and metabolism and is also closely related to cell senescence and cell fate [
48]. The potential anticancer impact of metformin might not solely rely on AMPK activation. Research suggests an alternative mechanism wherein metformin could hinder cell DNA damage by suppressing reactive oxygen species production. Even in the absence of AMPK activity, metformin can influence AKT/mTOR signaling by directly impeding mTORC1 signaling. Moreover, metformin demonstrates the ability to restrain cyclin D1, a pivotal regulator governing the cell cycle [
49]. Additionally, metformin can contribute to its anticancer properties by modulating the immune microenvironment and bolstering the immune response against cancerous cells.
The above-mentioned studies indicate a wide range of possible metformin applications, but novel advanced synthesized metformin derivatives could be more effective for treating each given disease and beneficial for the patient’s comfort. Moreover, preparation of novel metformin derivatives which diminish the secondary side effect of lactic acidosis is of equal importance. Therefore, the author presents all currently available data on the studied subject, revealing significant opportunities for future research in terms of novel derivatization strategies.
2. Metformin Derivates
Over the past few years, several metformin derivatives (
Figure 3) have been extensively studied. These include buformin, phenformin, imeglimin, lixumistat, IM176 derivatives, moroxydine, proguanil, cycloguanil, metformin sulfenamides and sulphonamides, polypeptide derivative LysMET, artesinate-metformin conjugate AM2. Additionally, salts such as metformin hydrochloride, metformin hydrobromide, metformin hydroiodide, metformin acetate, metformin embonate, metformin threonate, metformin tartrate, metformin citrate, metformin mesylate and metformin maleate have also been studied.
2.1. Buformin
Buformin, also called Butformin, 1-butylbiguanide, n-butylbiguanide, butyldiguanide or 2-butyl-1-(diaminomethylidene)guanidine (IUPAC name) is an anti-diabetic drug belonging to the biguanide class, chemically related to metformin. Buformin is a potent AMPK activator which acts as an orally active biguanide antidiabetic agent. Buformin decreases hepatic gluconeogenesis and lowers blood glucose production in vivo. Buformin also has anti-cancer activities It was withdrawn from the market in most countries due to its high risk of causing lactic acidosis. Recent studies have shown that buformin has tumor hypoxia depression capacity and exhibits potent antitumor activity in many malignant tumors [
50].
2.2. Phenformin
Phenformin or N-Phenethylbiguanide or 1-(diaminomethylidene)-2-(2-phenylethyl)guanidine (IUPAC name) is an older biguanide antidiabetic drug that was used in the past but has been largely discontinued due to its increased risk of lactic acidosis, a serious side effect. Phenformin (1-phenethylbiguanide) is an orally active antidiabetic and anticancer agent. Phenformin acts through acting APMK activation and blocking mTOR pathway. Phenformin is also a substrate of P-glycoprotein (P-gp), and an OXPHOS inhibitor. Phenformin induces cancer cell apoptosis. Metformin replaced phenformin as the preferred choice for diabetes treatment because it has a lower risk of this adverse effect. However, phenformin has been recognized as a drug possessing anti-cancer potential due to its anti-proliferative effect [
51]. This was confirmed for lung cancer [
52], liver cancer [
53], breast cancer [
54], squamous cell carcinoma [
55] and malignant glioma [
56]. According to Liu, phenformin, but not metformin, can efficiently suppress the inflammatory response induced by 12-O-tetradecanoylphorbol-13-acetate (TPA) in the skin. Inflammatory responses are mainly mediated by cytokines that are expressed by different types of cells, including immune cells, dermal fibroblasts and keratinocytes in the skin [
57]. Furthermore, phenformin has been shown to be a more potent mitochondrial inhibitor than metformin [
58].
2.3. Imeglimin
Imeglimin or (4R)-6-N,6-N,4-trimethyl-1,4-dihydro-1,3,5-triazine-2,6-diamine (IUPAC name) a recently approved drug and the first in a new class (novel mode of action) of type 2 diabetes mellitus medicines. Although not a biguanide, imeglimin shares a chemical moiety with metformin and also modulates mitochondrial complex I activity, a potential mechanism for metformin-mediated lactate accumulation. Imeglimin is an oral glucose-lowering agent. Imeglimin improves insulin sensitivity. Imeglimin also reduces reactive oxygen species production, increases mitochondrial DNA and improves mitochondrial function. The free form of the compound is prone to instability, it is advisable to consider the stable salt form Imeglimin hydrochloride that retains the same biological activity. Imeglimin improves pancreatic β-cell function and enhances insulin action in the liver and skeletal muscle [
59]. Imeglimin also reduces the production of reactive oxygen species that are harmful to the human body and improves the function of mitochondria and the endoplasmic reticulum, important in the synthesis, folding, modification, and transport of proteins [
60]. The safety profile of imeglimin seems to be very promising, despite long-term studies still being incomplete [
61]. A recent study by Hozumi reveals that imeglimin exerts biochemical effects similar to those of metformin on mitochondrial respiration, AMPK activity, and gene expression in hepatocytes at relatively high concentrations. However, effects on the expression of certain genes related to mitochondrial function differed between the two drugs [
62].
2.4. Lixumistat
Lixumistat, IM156, HL156A, HL271 or N′-[N′-[4-(trifluoromethoxy)phenyl]carbamimidoyl] pyrrolidine-1-carboximidamide (IUPAC name), is a novel biguanide with higher potency of AMP-activated protein kinase activation than metformin. Lixumistat is a novel biguanide mitochondrial protein complex 1 inhibitor of oxidative phosphorylation with anti-tumor activity. Lixumistat regulates oxidative phosphorylation to attenuate mitochondrial metabolic reprogramming and inhibit lung fibrosis. Lixumistat also suppresses B-cell activation to alleviate systemic lupus erythematosus. It has inhibitory activity against angiogenesis and cancer [
63]. Unlike metformin, which is relatively hydrophilic and requires active transport to enter cells, IM156 is more hydrophobic, which makes it potentially more bioavailable to cancer cells. In addition, at equal concentrations, IM156 was more potent at decreasing the oxygen consumption rate of tumor cells compared to phenformin and metformin. It was also more effective in reducing cellular ATP production versus phenformin without an increase in the extracellular acidification rate [
64]. Results clarified the beneficial effect of HL156A in reducing oral cancer development [
65]. Additionally, another study shows that inhibiting autophagy is a potential approach to increase the effectiveness of metformin and/or HL156A in treating oral squamous cell carcinoma cells [
66]. Moreover, published data demonstrate that HL156A suppresses multidrug resistance, which is a significant clinical crisis in cancer treatment and has been linked to the cellular expression of multidrug efflux transporters. Thus, HL156A, a derivative of the antidiabetic drug metformin, could be a new candidate as a potential chemotherapeutic agent in multidrug-resistant cancer cells [
67].
2.5. IM176
IM176, also called as IM176OUT05 is a novel biguanide derivative that has been reported to activate AMPK, inhibiting mTOR, androgen receptor, and prostate-specific antigen (PSA). IM176 is a high soluble biguanide and activates stem cell metabolism, promotes hair regrowth and increases stemness induction and maintenance during the pluripotent stem cell generation process. IM176 inhibits mitochondrial electron transport chain activity. It has superior anti-tumoral effects in prostate cancer [
68,
69]. IM176 showed antitumor effects comparable to those of metformin and phenformin, and may therefore be a novel candidate for the treatment of patients with prostate cancer, including castration-resistant prostate cancer [
69]. Nevertheless, the available literature is very limited as IM176 is still thoroughly studied.
2.6. Moroxydine
Moroxydine, called also as ABOB hydrochloride is a heterocyclic biguanide, also known as 4-morpholinocarboximidoylguanidine, morpholinobiguanide or N-(diaminomethylidene)morpholine-4-carboximidamide (IUPAC name). It is the metformin derivative that is mostly studied in its hydrochloride form. According to the literature, moroxydine has an antiviral effect [
70] with application potential as a pesticide for the management of plant viruses [
71,
72]. Moroxydine has multi-antiviral activities against DNA and RNA viruses including influenza symptoms, herpes simplex, varicellazoster, measles, mumps disease, hepatitis C virus, etc. [
73]. Moroxydine hydrochloride shows high anti-grass carp reovirus activity. Recent results also indicate moderate effectiveness of moroxydine for SARS-CoV-2 inhibition [
74].
2.7. Proguanil and Cycloguanil
Proguanil, also known as chlorguanide, chloroguanide or (1
E)-1-[amino-(4-chloroanilino)methylidene]-2-propan-2-ylguanidine (IUPAC name) and cycloguanil, often called chlorguanide triazine or 1-(4-chlorophenyl)-6,6-dimethyl-1,3,5-triazine-2,4-diamine (IUPAC name), are metformin derivatives with antimalarial effects [
70]. Proguanil is inactive, but its cyclic metabolite cycloguanil is active [
75]. Proguanil has gained attention due to its anti-tumor effects [
76], which is based on reducing tumor hypoxia, inducing mitochondrial dysfunction and oxidative stress, and causing DNA damage [
77]. The anticancer activity of cycloguanil was also observed [
78].
2.8. Metformin Sulfenamides and Sulfonamides
Metformin sulfenamides are derivatives of metformin with various promising effects. They differ in length and shape of the hydrocarbonthio chain. Metformin sulfenamides show more clearly marked anti-coagulant properties than metformin [
79,
80]. Another study has suggested that biguanides might have the potential in preventing brain disorders associated with diabetes complications in the future [
81]. A very interesting derivative is metformin cysteine, which has been reported to improve lipid profile and fasting blood sugar and fasting blood insulin better than metformin [
82]. Metformin sulfonamides show similar effects in anti-coagulation properties as metformin sulfenamides [
79,
80,
83,
84].
2.9. LysMET
LysMET, a novel polypeptide derivative of metformin, is synthesized through a one-step reaction involving poly-l-lysine and dicyandiamide under acidic conditions and heat. This prepared derivative has the potential to serve as both an anticancer therapeutic and a gene carrier. In experiments, LysMET showed similar effectiveness to MET in suppressing HT-29 colon cancer cells, highlighting the importance of the biguanide component. Furthermore, LysMET demonstrated favorable attributes for effectively controlling tumor progression, indicating its dual role as both a drug and gene carrier [
85].
2.10. Artesunate-Metformin Conjugate AM2
Artesunate-metformin conjugate AM2 was synthesized by Lin et al. [
86] by two steps. At the beginning, metformin hydrochloride reacted with sodium hydroxide in dichloromethane to obtain free metformin. Such prepared metformin was subjected to the reaction with artesunate, a derivative of artemisinin, in dichloromethane under the catalysis of 4-Dimethylaminopyridine and carbonyldiimide hydrochloride. The author found that the novel artesunate-metformin conjugate, AM2, shows significant potential as a highly effective anti-bladder cancer agent. It inhibits the proliferation, migration, and lipogenesis of bladder cancer cells, specifically targeting the Clusterin/SREBP1/FASN signaling pathway. AM2 was shown to be far more potent than both cisplatin and metformin alone, exhibiting minimal toxicity to normal cells. These results suggest that AM2 could become a promising therapeutic option for bladder cancer treatment.
2.11. Metformin Salts
Metformin hydrochloride is the most well-known salt of metformin. It is also called metformin extended-release, metformin ER, or metformin XR. While both forms of metformin contain the same active ingredient, they are taken differently and have some differences in side effects. Metformin is usually dosed as a 500 mg tablet taken twice daily with food. However, there is also an 850 mg tablet that can be taken once daily. Metformin hydrochloride is the extended-release version of metformin and only needs to be taken once daily with food. It also has fewer side effects and lasts longer than regular metformin. Both metformin and metformin hydrochloride are approved to manage type 2 diabetes mellitus. Studies comparing metformin and metformin hydrochloride for type 2 diabetes mellitus found that metformin hydrochloride is relatively comparable to metformin in effectiveness. In fact, metformin hydrochloride may be superior to regular metformin due to its lower side-effect profile and ease of application. Those with type 2 diabetes mellitus may be more inclined to take a once-daily metformin pill instead of a twice-daily pill. Study by Derosa et al. have found that metformin hydrochloride was more effective than metformin in treating patients with type 2 diabetes. Those taking metformin hydrochloride experienced better glycemic control and lipid metabolism compared to metformin [
87]. Another study demonstrated the therapeutic equivalence of metformin hydrochloride and metformin over a 24-week period for patients with type 2 diabetes mellitus. This confirms metformin hydrochloride as an important treatment option for patients in whom dosing frequency could affect medication compliance and compromise treatment outcomes [
88].
This study was confirmed by Akram, who found that metformin hydrochloride may improve the quality of therapy and the safety profile relative to a conventional dosage form in patients with type 2 diabetes mellitus [
89]. Similar results were observed by Tan [
90] with some limitations. The authors included only five randomized trials and one observational study, and long-term endpoints were not identified for metformin hydrochloride and metformin use. A more recent study [
91] explains, that the difference between the two formulations is not statistically significant. Metformin hydrochloride was statistically associated with a reduced cumulative incidence of dyspepsia compared to metformin. Moreover, metformin hydrochloride was found to improve total cholesterol and low-density lipoprotein cholesterol compared to metformin [
92]. The same observations were given in another literature [
93]. However, the use of metformin hydrochloride was associated with an increase in triglycerides
There is very limited literature related to metformin hydrobromide and hydroiodide. A recent study has provided only information about the metformin hydrobromide synthesis without any further thorough study [
94]. Surprisingly, the metformin hydroiodide is mentioned in literature only in the context of solar cell passivation [
95].
Metformin acetate belongs to biguanides which have been already almost forgotten. It was synthesized with antibacterial effect expectations [
96]. In this study, the authors synthesized also complexes with Cu (II), Ni (II), Mn (II) and Zn (II). These complexes were much more microbially active than metformin acetate. With respect to the above-mentioned, this led to a loss of interest in studying metformin acetate and recent literature is missing.
Metformin embonate, also known as metformin pamoate or 4-[(3-carboxy-2-hydroxynaphthalen-1-yl)methyl]-3-hydroxynaphthalene-2-carboxylic acid;3-(diaminomethylidene)-1,1-dimethylguanidine (IUPAC name), is a drug widely known for its use in anti-diabetic treatments [
97]. However, its anti-diabetic effectiveness is lower compared to metformin.
A series of novel derivatives, metformin threonate, metformin tartrate, metformin citrate, metformin mesylate and metformin maleate were synthesized. Among them, metformin threonate exhibited the strongest potency on AMP-activated protein kinase activation with a better safety profile [
94,
98].
3. Conclusions and Future Perspectives
Metformin has garnered significant attention due to its multispectral mechanism of action, which can be enhanced through derivatization. The review provides details on the types and importance of metformin derivatives, along with their advantages over metformin. Based on the published material the authors were able to gather, several tentative conclusions can be derived.
The formulation of metformin hydrochloride has been designed to facilitate a more gradual release of the drug in the primary absorption site, namely the upper gastrointestinal tract. This improvement enhances tolerability and patient compliance by reducing the frequency of administration and the occurrence of adverse events.
Phenformin, unlike metformin, has been demonstrated to effectively suppress the inflammatory response in the skin and exhibits greater potency as a mitochondrial inhibitor compared to metformin.
The unique mechanism of action and safety profile of Imeglimin, in comparison to metformin, potentially address the current gap in the treatment of type 2 diabetes mellitus.
Lixumistat is more hydrophobic than metformin, making it potentially more bioavailable to cancer cells.
Derivative IM176 shows antitumor effects comparable to those of metformin and phenformin.
Derivatives like moroxydine exhibit antiviral effects through a broad-spectrum action against both DNA and RNA viruses, and they are capable of inhibiting the SARS-CoV-2 virus as well.
Proguanil and cycloguanil, both derivatives of metformin, demonstrate antimalarial effects. Furthermore, their potential anti-tumor effects show promise.
Metformin sulfenamides and sulfonamides demonstrate more evident anti-coagulant properties than metformin itself.
Another derivative LysMET showed similar effectiveness to MET in suppressing HT-29 tumors.
Artesunate-metformin conjugate AM2 was shown to be far more potent than metformin alone, exhibiting minimal toxicity to normal cells. These results suggest that AM2 could become a promising therapeutic option for bladder cancer treatment.
These findings offer direct evidence that new metformin derivatives could serve as a promising foundation for designing and synthesizing novel biguanide-based compounds with more advanced effects than their parent drug, metformin. In practice, a broad spectrum of possibilities exists for the next synthesis work, as metformin derivatives have the potential to contribute advancements to the studied subject, despite some of the previously mentioned studies indicating no evidence for a decreased risk of various cancer types associated with metformin use.
The subsequent derivatives should meet crucial requirements, foremost among them being the mitigation of lactic acidosis, a serious side effect for some patients. Additionally, drug administration should be as comfortable as possible, facilitated by an extended release. In various clinical diagnoses, discernible targeted effects are essential, anticipating the potential use of metformin derivatives in adjuvant therapy post-cancer treatment. Controlled weight loss, as a favorable side effect of metformin derivatives, should also be investigated for its role in obesity prevention, thereby mitigating associated risks.
Author Contributions
Conceptualization M.L., K.Š., resources M.L., supervision M.L., validation S.Z., K.Š., M.L., writing – original draft S.Z., K.Š., M.L.
Funding
This study was financed by the Ministry of Education, Youth and Sports of the Czech Republic (RP/CPS/2024-28/005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No data are presented.
Acknowledgments
The work was supported by the Ministry of Education, Youth and Sports of the Czech Republic (RP/CPS/2024-28/005).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Ma, R.; Yi, B.; Riker, A.I.; Xi, Y. Metformin and Cancer Immunity. Acta Pharmacol Sin 2020, 41, 1403–1409. [Google Scholar] [CrossRef] [PubMed]
- Kadylak, D.; Polak, K.; Wojciechowska, K.; Bergler-Czop, B. Metformin in Skin Diseases. Przegl Dermatol 2021, 108, 27–37. [Google Scholar] [CrossRef]
- Hussein, R.; Abdelbasset, W.; Osama Mahfouz, O.M. Novel Metformin Indications and Skin Disorders. IJBM 2022, 12, 521–525. [Google Scholar] [CrossRef]
- Feng, X.; Chen, W.; Ni, X.; Little, P.J.; Xu, S.; Tang, L.; Weng, J. Metformin, Macrophage Dysfunction and Atherosclerosis. Frontiers in Immunology 2021, 12. [Google Scholar] [CrossRef]
- Hammad Uddin, M.K.; Khan Sadiq, M.S.; Ahmed, A.; Khan, M.; Maniar, T.; Mateen, S.M.; Saba, B.; Kashif, S.M.; Usman, S.; Najeeb, S.; et al. Applications of Metformin in Dentistry—A Review. Journal of Taibah University Medical Sciences 2023, 18, 1299–1310. [Google Scholar] [CrossRef]
- Masadeh, M.M.; Alzoubi, K.H.; Masadeh, M.M.; Aburashed, Z.O. Metformin as a Potential Adjuvant Antimicrobial Agent Against Multidrug Resistant Bacteria. CPAA 2021, 13, 83–90. [Google Scholar] [CrossRef]
- Lambova, S.N. Pleiotropic Effects of Metformin in Osteoarthritis. Life 2023, 13, 437. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Z.; Zhao, P. The Effects of Metformin in the Treatment of Osteoarthritis: Current Perspectives. Frontiers in Pharmacology 2022, 13. [Google Scholar] [CrossRef]
- Li, N.; Zhou, T.; Fei, E. Actions of Metformin in the Brain: A New Perspective of Metformin Treatments in Related Neurological Disorders. International Journal of Molecular Sciences 2022, 23, 8281. [Google Scholar] [CrossRef]
- Feng, Y.Y.; Wang, Z.; Pang, H. Role of Metformin in Inflammation. Mol Biol Rep 2023, 50, 789–798. [Google Scholar] [CrossRef]
- Kimber-Trojnar, Ż.; Dłuski, D.F.; Wierzchowska-Opoka, M.; Ruszała, M.; Leszczyńska-Gorzelak, B. Metformin as a Potential Treatment Option for Endometriosis. Cancers 2022, 14, 577. [Google Scholar] [CrossRef] [PubMed]
- Alnaaim, S.A.; Al-kuraishy, H.M.; Al-Gareeb, A.I.; Ali, N.H.; Alexiou, A.; Papadakis, M.; Saad, H.M.; Batiha, G.E.-S. New Insights on the Potential Anti-Epileptic Effect of Metformin: Mechanistic Pathway. Journal of Cellular and Molecular Medicine 2023, 27, 3953–3965. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Marei, I.; Ye, K.; Ding, H.; Anderson, T.J.; Hollenberg, M.D.; Hill, M.A. Repurposing Metformin for Vascular Disease. Current Medicinal Chemistry 2023, 30, 3955–3978. [Google Scholar] [CrossRef]
- Pan, Q.; Lu, X.; Zhao, C.; Liao, S.; Chen, X.; Guo, F.; Yang, C.; Liu, H. Metformin: The Updated Protective Property in Kidney Disease. Aging 2020, 12, 8742–8759. [Google Scholar] [CrossRef]
- Lv, Z.; Guo, Y. Metformin and Its Benefits for Various Diseases. Frontiers in Endocrinology 2020, 11. [Google Scholar] [CrossRef]
- Cheng, D.; Zhou, X.; Xu, X. The Role of Metformin in Treating Preeclampsia. Maternal-Fetal Medicine 2021, 03, 203–207. [Google Scholar] [CrossRef]
- Dziedzic, A.; Saluk-Bijak, J.; Miller, E.; Bijak, M. Metformin as a Potential Agent in the Treatment of Multiple Sclerosis. International Journal of Molecular Sciences 2020, 21, 5957. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Z.; Zhao, P. The Function of Metformin in Aging-Related Musculoskeletal Disorders. Frontiers in Pharmacology 2022, 13. [Google Scholar] [CrossRef]
- Amin, S.V.; Khanna, S.; Parvar, S.P.; Shaw, L.T.; Dao, D.; Hariprasad, S.M.; Skondra, D. Metformin and Retinal Diseases in Preclinical and Clinical Studies: Insights and Review of Literature. Exp Biol Med (Maywood) 2022, 247, 317–329. [Google Scholar] [CrossRef]
- Alqahtani, S.; Mahzari, M. Protective Effect of Metformin on Venous Thrombosis in Diabetic Patients: Findings From a Systematic Review. Journal of Endocrinology and Metabolism 2023, 12, 161–167. [Google Scholar] [CrossRef]
- Kulkarni, A.S.; Gubbi, S.; Barzilai, N. Benefits of Metformin in Attenuating the Hallmarks of Aging. Cell Metabolism 2020, 32, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Novelle, M.G.; Ali, A.; Diéguez, C.; Bernier, M.; Cabo, R. de Metformin: A Hopeful Promise in Aging Research. Cold Spring Harb Perspect Med 2016, 6, a025932. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Qi, Y.; Jiang, H.; Mi, T.; Zhang, Y.; Peng, C.; Li, W.; Zhang, Y.; Zhou, Y.; Zang, Y.; et al. The Development and Benefits of Metformin in Various Diseases. Front. Med. 2023, 17, 388–431. [Google Scholar] [CrossRef] [PubMed]
- Moldasheva, A.; Surov, V.; Aljofan, M. Editorial: New Lights Through Old Windows: Metformin and Derivatives as Anti-Cancer Treatments. Frontiers in Pharmacology 2022, 13. [Google Scholar] [CrossRef]
- Crist, M.; Yaniv, B.; Palackdharry, S.; Lehn, M.A.; Medvedovic, M.; Stone, T.; Gulati, S.; Karivedu, V.; Borchers, M.; Fuhrman, B.; et al. Metformin Increases Natural Killer Cell Functions in Head and Neck Squamous Cell Carcinoma through CXCL1 Inhibition. J Immunother Cancer 2022, 10, e005632. [Google Scholar] [CrossRef]
- Tseng, C.-H. Metformin and Primary Bone Cancer Risk in Taiwanese Patients with Type 2 Diabetes Mellitus. Bone 2021, 151, 116037. [Google Scholar] [CrossRef]
- Serageldin, M.A.; Kassem, A.B.; El-Kerm, Y.; Helmy, M.W.; El-Mas, M.M.; El-Bassiouny, N.A. The Effect of Metformin on Chemotherapy-Induced Toxicities in Non-Diabetic Breast Cancer Patients: A Randomised Controlled Study. Drug Saf 2023, 46, 587–599. [Google Scholar] [CrossRef]
- Curry, J.M.; Nwagu, U.; Harshyne, L.; Linnenbach, A.; Srivastava, N.; Cognetti, D.M.; Luginbuhl, A.; Zinner, R.; Axelrod, R.; Bar-Ad, V.; et al. 923P Immune Alterations in a Window of Opportunity for Durvalumab (MEDI4736) plus Metformin Trial in Squamous Cell Carcinoma of the Head and Neck (SCCHN). Annals of Oncology 2020, 31, S665–S666. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Santoni, G.; Lagergren, J. Diabetes, Metformin Use, and Survival in Esophageal Cancer: A Population-Based Cohort Study. JNCI Cancer Spectrum 2023, 7, pkad043. [Google Scholar] [CrossRef]
- Yoon, W.-S.; Chang, J.H.; Kim, J.H.; Kim, Y.J.; Jung, T.-Y.; Yoo, H.; Kim, S.-H.; Ko, Y.-C.; Nam, D.-H.; Kim, T.M.; et al. Efficacy and Safety of Metformin plus Low-Dose Temozolomide in Patients with Recurrent or Refractory Glioblastoma: A Randomized, Prospective, Multicenter, Double-Blind, Controlled, Phase 2 Trial (KNOG-1501 Study). Discov Oncol 2023, 14, 90. [Google Scholar] [CrossRef]
- Fan, H.; Bai, S.; Guan, X.; Ma, W.; Fu, Y.; Zhang, X.; Deng, L.; Tian, J. Metformin Improves Survival in Patients with Concurrent Diabetes and Small Cell Lung Cancer: A Meta-Analysis. Minerva Endocrinol (Torino) 2023, 48, 214–221. [Google Scholar] [CrossRef]
- Afzal, M.Z.; Dragnev, K.; Sarwar, T.; Shirai, K. Clinical Outcomes in Non-Small-Cell Lung Cancer Patients Receiving Concurrent Metformin and Immune Checkpoint Inhibitors. Lung Cancer Management 2019, 8, LMT11. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Chen, X.; Wang, L.; Yang, B.; Cai, S. Metformin Adjunct With Antineoplastic Agents for the Treatment of Lung Cancer: A Meta-Analysis of Randomized Controlled Trials and Observational Cohort Studies. Frontiers in Pharmacology 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Li, C.; Zheng, Q.; Fang, S.; Xu, J.; Wang, H.; Guo, H. Metformin Suppresses Lung Adenocarcinoma by Downregulating Long Non-Coding RNA (lncRNA) AFAP1-AS1 and Secreted Phosphoprotein 1 (SPP1) While Upregulating miR-3163. Bioengineered 2022, 13, 11987–12002. [Google Scholar] [CrossRef] [PubMed]
- Barczyński, B.; Frąszczak, K.; Kotarski, J. Perspectives of Metformin Use in Endometrial Cancer and Other Gynaecological Malignancies. Journal of Drug Targeting 2022, 30, 359–367. [Google Scholar] [CrossRef]
- Park, J.Y.; Lim, M.C.; Baek, M.H.; Park, Y.H.; Kim, S. Impact of Metformin on Survival Outcome in Ovarian Cancer: A Nationwide Population-Based Cohort Study. J Gynecol Oncol 2021, 32, e65. [Google Scholar] [CrossRef]
- Mlicka, A.; Mlicki, P.; Niewiadomski, P.; Zielińska, W.; Hałas-Wiśniewska, M.; Izdebska, M. Synergistic Effect of Metformin and Doxorubicin on the Metastatic Potential of T24 Cells. Acta Histochemica 2023, 125, 151975. [Google Scholar] [CrossRef]
- Olokpa, E.; Mandape, S.N.; Pratap, S.; Stewart, L.M.V. Metformin Regulates Multiple Signaling Pathways within Castration-Resistant Human Prostate Cancer Cells. BMC Cancer 2022, 22, 1025. [Google Scholar] [CrossRef]
- Xiao, Q.; Xiao, J.; Liu, J.; Liu, J.; Shu, G.; Yin, G. Metformin Suppresses the Growth of Colorectal Cancer by Targeting INHBA to Inhibit TGF-β/PI3K/AKT Signaling Transduction. Cell Death Dis 2022, 13, 1–14. [Google Scholar] [CrossRef]
- Gyawali, M.; Venkatesan, N.; Ogeyingbo, O.D.; Bhandari, R.; Botleroo, R.A.; Kareem, R.; Ahmed, R.; Elshaikh, A.O.; Gyawali, M.; Venkatesan, N.; et al. Magic of a Common Sugar Pill in Cancer: Can Metformin Raise Survival in Pancreatic Cancer Patients? Cureus 2021, 13. [Google Scholar] [CrossRef]
- Di Matteo, S.; Nevi, L.; Overi, D.; Landolina, N.; Faccioli, J.; Giulitti, F.; Napoletano, C.; Oddi, A.; Marziani, A.M.; Costantini, D.; et al. Metformin Exerts Anti-Cancerogenic Effects and Reverses Epithelial-to-Mesenchymal Transition Trait in Primary Human Intrahepatic Cholangiocarcinoma Cells. Sci Rep 2021, 11, 2557. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Chen, S.; Xiang, W.; Xiao, M.; Xiao, H. The Mechanism of Treatment of Multiple Myeloma with Metformin by Way of Metabolism. Arch Med Sci 2021, 17, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, F.; Guan, J.; Zhou, L.; Chen, B. Action Mechanism of Metformin and Its Application in Hematological Malignancy Treatments: A Review. Biomolecules 2023, 13, 250. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Jick, S.S.; Meier, C.R.; Bodmer, M. No Evidence for a Decreased Risk of Thyroid Cancer in Association with Use of Metformin or Other Antidiabetic Drugs: A Case-Control Study. BMC Cancer 2015, 15, 719. [Google Scholar] [CrossRef]
- Bodmer, M.; Becker, C.; Meier, C.; Jick, S.S.; Meier, C.R. Use of Metformin Is Not Associated with a Decreased Risk of Colorectal Cancer: A Case–Control Analysis. Cancer Epidemiology, Biomarkers & Prevention 2012, 21, 280–286. [Google Scholar] [CrossRef]
- de Jong, R.G.; Burden, A.M.; de Kort, S.; van Herk-Sukel, M.P.; Vissers, P.A.; Janssen, P.K.; Haak, H.R.; Masclee, A.A.; de Vries, F.; Janssen-Heijnen, M.L. No Decreased Risk of Gastrointestinal Cancers in Users of Metformin in The Netherlands; A Time-Varying Analysis of Metformin Exposure. Cancer Prevention Research 2017, 10, 290–297. [Google Scholar] [CrossRef]
- Tsilidis, K.K.; Capothanassi, D.; Allen, N.E.; Rizos, E.C.; Lopez, D.S.; van Veldhoven, K.; Sacerdote, C.; Ashby, D.; Vineis, P.; Tzoulaki, I.; et al. Metformin Does Not Affect Cancer Risk: A Cohort Study in the U.K. Clinical Practice Research Datalink Analyzed like an Intention-to-Treat Trial. Diabetes Care 2014, 37, 2522–2532. [Google Scholar] [CrossRef]
- Zhang, T.; Xu, D.; Trefts, E.; Lv, M.; Inuzuka, H.; Song, G.; Liu, M.; Lu, J.; Liu, J.; Chu, C.; et al. Metabolic Orchestration of Cell Death by AMPK-Mediated Phosphorylation of RIPK1. Science 2023, 380, 1372–1380. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, X. Research Progress on the Use of Metformin in Leukemia Treatment. Curr. Treat. Options in Oncol. 2024, 25, 220–236. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic Reprogramming Mediated PD-L1 Depression and Hypoxia Reversion to Reactivate Tumor Therapy. Journal of Controlled Release 2022, 352, 793–812. [Google Scholar] [CrossRef]
- Cao, Y.; Xu, S.; Xu, C.; Xiao, D.; Chen, Z.; Wang, W.; Wang, Z.; Yang, X. Synthesis, Anticancer Activity and Mechanism of Phenformin Derivatives. ChemistrySelect 2022, 7, e202104250. [Google Scholar] [CrossRef]
- Pereira-Nunes, A.; Ferreira, H.; Abreu, S.; Guedes, M.; Neves, N.M.; Baltazar, F.; Granja, S. Combination Therapy With CD147-Targeted Nanoparticles Carrying Phenformin Decreases Lung Cancer Growth. Advanced Biology 2023, 7, 2300080. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Zhou, S.; Qin, M.; Tang, J.; Yan, X.; Huang, L.; Huang, M.; Deng, J.; Xiao, D.; Hu, X.; et al. Phenformin and Ataxia-Telangiectasia Mutated Inhibitors Synergistically Co-Suppress Liver Cancer Cell Growth by Damaging Mitochondria. FEBS Open Bio 2021, 11, 1440–1451. [Google Scholar] [CrossRef]
- Totten, S.P.; Im, Y.K.; Cepeda Cañedo, E.; Najyb, O.; Nguyen, A.; Hébert, S.; Ahn, R.; Lewis, K.; Lebeau, B.; La Selva, R.; et al. STAT1 Potentiates Oxidative Stress Revealing a Targetable Vulnerability That Increases Phenformin Efficacy in Breast Cancer. Nat Commun 2021, 12, 3299. [Google Scholar] [CrossRef]
- Zhuang, D.; Wang, S.; Liu, G.; Liu, P.; Deng, H.; Sun, J.; Liu, C.; Leng, X.; Zhang, Q.; Bai, F.; et al. Phenformin Suppresses Angiogenesis through the Regulation of Exosomal microRNA-1246 and microRNA-205 Levels Derived from Oral Squamous Cell Carcinoma Cells. Frontiers in Oncology 2022, 12. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Y.; Zhang, S.; Wu, H.; Yang, D.; Nie, C.; Hu, Q. Phenformin and Metformin Inhibit Growth and Migration of LN229 Glioma Cells in Vitro and in Vivo. OTT 2018, 11, 6039–6048. [Google Scholar] [CrossRef]
- Liu, G.; Li, D.; Zhang, L.; Xu, Q.; Zhuang, D.; Liu, P.; Hu, L.; Deng, H.; Sun, J.; Wang, S.; et al. Phenformin Down-Regulates c-Myc Expression to Suppress the Expression of Pro-Inflammatory Cytokines in Keratinocytes. Cells 2022, 11, 2429. [Google Scholar] [CrossRef]
- Augustin, R.C.; Huang, Z.; Ding, F.; Zhai, S.; McArdle, J.; Santisi, A.; Davis, M.; Sander, C.; Davar, D.; Kirkwood, J.M.; et al. Metformin Is Associated with Improved Clinical Outcomes in Patients with Melanoma: A Retrospective, Multi-Institutional Study. Front Oncol 2023, 13, 1075823. [Google Scholar] [CrossRef]
- Chevalier, C.; Fouqueray, P.; Bolze, S. Imeglimin: A Clinical Pharmacology Review. Clin Pharmacokinet 2023, 62, 1393–1411. [Google Scholar] [CrossRef]
- Yanai, H.; Adachi, H.; Hakoshima, M.; Katsuyama, H. Glucose-Lowering Effects of Imeglimin and Its Possible Beneficial Effects on Diabetic Complications. Biology 2023, 12, 726. [Google Scholar] [CrossRef]
- Nowak, M.; Grzeszczak, W. Imeglimin: A New Antidiabetic Drug with Potential Future in the Treatment of Patients with Type 2 Diabetes. Endokrynol Pol 2022, 73, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Hozumi, K.; Sugawara, K.; Ishihara, T.; Ishihara, N.; Ogawa, W. Effects of Imeglimin on Mitochondrial Function, AMPK Activity, and Gene Expression in Hepatocytes. Sci Rep 2023, 13, 746. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Lee, S.K.; Yun, N.J.; Kim, Y.S.; Song, J.J.; Bae, Y.-S. IM156, a New AMPK Activator, Protects against Polymicrobial Sepsis. Journal of Cellular and Molecular Medicine 2022, 26, 3378–3386. [Google Scholar] [CrossRef]
- Janku, F.; Beom, S.-H.; Moon, Y.W.; Kim, T.W.; Shin, Y.G.; Yim, D.-S.; Kim, G.M.; Kim, H.S.; Kim, S.Y.; Cheong, J.-H.; et al. First-in-Human Study of IM156, a Novel Potent Biguanide Oxidative Phosphorylation (OXPHOS) Inhibitor, in Patients with Advanced Solid Tumors. Invest New Drugs 2022, 40, 1001–1010. [Google Scholar] [CrossRef]
- Lam, T.G.; Jeong, Y.S.; Kim, S.-A.; Ahn, S.-G. New Metformin Derivative HL156A Prevents Oral Cancer Progression by Inhibiting the Insulin-like Growth Factor/AKT/Mammalian Target of Rapamycin Pathways. Cancer Sci 2018, 109, 699–709. [Google Scholar] [CrossRef]
- Nguyen, M.T.; Choe, H.-C.; Kim, B.-H.; Ahn, S.-G. A New Link between Apoptosis Induced by the Metformin Derivative HL156A and Autophagy in Oral Squamous Cell Carcinoma. European Journal of Pharmacology 2022, 920, 174859. [Google Scholar] [CrossRef]
- Jeong, Y.S.; Lam, T.G.; Jeong, S.; Ahn, S.-G. Metformin Derivative HL156A Reverses Multidrug Resistance by Inhibiting HOXC6/ERK1/2 Signaling in Multidrug-Resistant Human Cancer Cells. Pharmaceuticals 2020, 13, 218. [Google Scholar] [CrossRef]
- Torunoglu, S.T.; Zajda, A.; Tampio, J.; Markowicz-Piasecka, M.; Huttunen, K.M. Metformin Derivatives – Researchers’ Friends or Foes? Biochemical Pharmacology 2023, 215, 115743. [Google Scholar] [CrossRef]
- Kim, Y.; Yoo, S.; Lim, B.; Hong, J.H.; Kwak, C.; You, D.; Hwang, J.J.; Kim, C.-S. A Novel Biguanide Derivative, IM176, Induces Prostate Cancer Cell Death by Modulating the AMPK-mTOR and Androgen Receptor Signaling Pathways. Prostate Int 2023, 11, 83–90. [Google Scholar] [CrossRef]
- Kathuria, D.; Raul, A.D.; Wanjari, P.; Bharatam, P.V. Biguanides: Species with Versatile Therapeutic Applications. European Journal of Medicinal Chemistry 2021, 219, 113378. [Google Scholar] [CrossRef]
- Yu, M.; Liu, H.; Guo, L.; Zhou, T.; Shan, Y.; Xia, Z.; Li, X.; An, M.; Wu, Y. Antiviral Modes of Action of the Novel Compound GLY-15 Containing Pyrimidine Heterocycle and Moroxydine Skeleton against Tobacco Mosaic Virus. Pest Management Science 2022, 78, 5259–5270. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huang, M.; Wang, L.; Xue, W.; Xie, X.; Li, X. Insights into a Rapid Screening Method for Anti-Cucumber Mosaic Virus Compounds. J Virol Methods 2022, 301, 114402. [Google Scholar] [CrossRef] [PubMed]
- Kandeel, M.; Abdelrahman, A.H.M.; Oh-Hashi, K.; Ibrahim, A.; Venugopala, K.N.; Morsy, M.A.; Ibrahim, M.A.A. Repurposing of FDA-Approved Antivirals, Antibiotics, Anthelmintics, Antioxidants, and Cell Protectives against SARS-CoV-2 Papain-like Protease. Journal of Biomolecular Structure and Dynamics 2021, 39, 5129–5136. [Google Scholar] [CrossRef] [PubMed]
- Min, J.S.; Kwon, S.; Jin, Y.-H. SARS-CoV-2 RdRp Inhibitors Selected from a Cell-Based SARS-CoV-2 RdRp Activity Assay System. Biomedicines 2021, 9, 996. [Google Scholar] [CrossRef]
- Arfeen, M.; Patel, D.S.; Abbat, S.; Taxak, N.; Bharatam, P.V. Importance of Cytochromes in Cyclization Reactions: Quantum Chemical Study on a Model Reaction of Proguanil to Cycloguanil. Journal of Computational Chemistry 2014, 35, 2047–2055. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, T.; Hu, X.; Xu, S.; Xiao, D.; Wu, J.; Yan, X.; Yang, X.; Li, G. Proguanil Synergistically Sensitizes Ovarian Cancer Cells to Olaparib by Increasing DNA Damage and Inducing Apoptosis. Int J Med Sci 2022, 19, 233–241. [Google Scholar] [CrossRef]
- Mudassar, F.; Shen, H.; O’Neill, G.; Hau, E. Targeting Tumor Hypoxia and Mitochondrial Metabolism with Anti-Parasitic Drugs to Improve Radiation Response in High-Grade Gliomas. J Exp Clin Cancer Res 2020, 39, 208. [Google Scholar] [CrossRef]
- Brown, J.I.; Wang, P.; Wong, A.Y.L.; Petrova, B.; Persaud, R.; Soukhtehzari, S.; Lopez McDonald, M.; Hanke, D.; Christensen, J.; Iliev, P.; et al. Cycloguanil and Analogues Potently Target DHFR in Cancer Cells to Elicit Anti-Cancer Activity. Metabolites 2023, 13, 151. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Mateusiak, Ł.; Mikiciuk-Olasik, E.; Sikora, J. Sulfenamide and Sulfonamide Derivatives of Metformin Can Exert Anticoagulant and Profibrinolytic Properties. Chemico-Biological Interactions 2018, 284, 126–136. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Huttunen, K.M.; Broncel, M.; Sikora, J. Sulfenamide and Sulfonamide Derivatives of Metformin – A New Option to Improve Endothelial Function and Plasma Haemostasis. Sci Rep 2019, 9, 6573. [Google Scholar] [CrossRef]
- Markowicz-Piasecka, M.; Sikora, J.; Mateusiak, Ł.; Mikiciuk-Olasik, E.; Huttunen, K.M. Metformin and Its Sulfenamide Prodrugs Inhibit Human Cholinesterase Activity. Oxidative Medicine and Cellular Longevity 2017, 2017, e7303096. [Google Scholar] [CrossRef] [PubMed]
- Javanmanesh, F.; Kashanian, M.; Rahimi, M.; Sheikhansari, N. A Comparison between the Effects of Metformin and N-Acetyl Cysteine (NAC) on Some Metabolic and Endocrine Characteristics of Women with Polycystic Ovary Syndrome. Gynecological Endocrinology 2016, 32, 285–289. [Google Scholar] [CrossRef] [PubMed]
- Zajda, A.; Sikora, J.; Huttunen, K.M.; Markowicz-Piasecka, M. Structural Comparison of Sulfonamide-Based Derivatives That Can Improve Anti-Coagulation Properties of Metformin. International Journal of Molecular Sciences 2022, 23, 4132. [Google Scholar] [CrossRef]
- Zajda, A.; Sikora, J.; Hynninen, M.; Tampio, J.; Huttunen, K.M.; Markowicz-Piasecka, M. Substituent Effects of Sulfonamide Derivatives of Metformin That Can Dually Improve Cellular Glucose Utilization and Anti-Coagulation. Chemico-Biological Interactions 2023, 373, 110381. [Google Scholar] [CrossRef]
- Ramasamy, T.; Ruttala, H.B.; Kaliraj, K.; Poudel, K.; Jin, S.G.; Choi, H.-G.; Ku, S.K.; Yong, C.S.; Kim, J.O. Polypeptide Derivative of Metformin with the Combined Advantage of a Gene Carrier and Anticancer Activity. ACS Biomater Sci Eng 2019, 5, 5159–5168. [Google Scholar] [CrossRef]
- Lin, P.; Yang, X.; Wang, L.; Zou, X.; Mu, L.; Xu, C.; Yang, X. Novel Artesunate-Metformin Conjugate Inhibits Bladder Cancer Cell Growth Associated with Clusterin/SREBP1/FASN Signaling Pathway. Korean J Physiol Pharmacol 2024, 28, 219–227. [Google Scholar] [CrossRef]
- Derosa, G.; D’Angelo, A.; Romano, D.; Maffioli, P. Effects of Metformin Extended Release Compared to Immediate Release Formula on Glycemic Control and Glycemic Variability in Patients with Type 2 Diabetes. Drug Des Devel Ther 2017, 11, 1481–1488. [Google Scholar] [CrossRef]
- Aggarwal, N.; Singla, A.; Mathieu, C.; Montanya, E.; Pfeiffer, A.F.H.; Johnsson, E.; Zhao, J.; Iqbal, N.; Bailey, C. Metformin Extended-Release versus Immediate-Release: An International, Randomized, Double-Blind, Head-to-Head Trial in Pharmacotherapy-Naïve Patients with Type 2 Diabetes. Diabetes, Obesity and Metabolism 2018, 20, 463–467. [Google Scholar] [CrossRef]
- Akram, A. Reactive Hypoglycemia From Metformin Immediate-Release Monotherapy Resolved by a Switch to Metformin Extended-Release: Conceptualizing Their Concentration-Time Curves. Cureus 2021, 13, e16112. [Google Scholar] [CrossRef]
- Tan, J.; Wang, Y.; Liu, S.; Shi, Q.; Zhou, X.; Zhou, Y.; Yang, X.; Chen, P.; Li, S. Long-Acting Metformin Vs. Metformin Immediate Release in Patients With Type 2 Diabetes: A Systematic Review. Front. Pharmacol. 2021, 12. [Google Scholar] [CrossRef]
- Abrilla, A.A.; Pajes, A.N.N.I.; Jimeno, C.A. Metformin Extended-Release versus Metformin Immediate-Release for Adults with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Diabetes Research and Clinical Practice 2021, 178, 108824. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Rivera, R.; D’Angelo, A.; Maffioli, P. Metformin: From Immediate Release to Extended Release Formula, Effectiveness, And Safety in Patients With Chronic Kidney Disease. EMJ 2020, 8, 70–78. [Google Scholar] [CrossRef]
- Tarry-Adkins, J.L.; Grant, I.D.; Ozanne, S.E.; Reynolds, R.M.; Aiken, C.E. Efficacy and Side Effect Profile of Different Formulations of Metformin: A Systematic Review and Meta-Analysis. Diabetes Ther 2021, 12, 1901–1914. [Google Scholar] [CrossRef]
- Zhang, G.; Chen, S.; Jia, J.; Liu, C.; Wang, W.; Zhang, H.; Zhen, X. Development and Evaluation of Novel Metformin Derivative Metformin Threonate for Brain Ischemia Treatment. Frontiers in Pharmacology 2022, 13. [Google Scholar] [CrossRef]
- Dong, H.; Shen, G.; Zou, Y.; Li, Y.; Lin, Z.; Cai, Q.; Xu, X.; Song, Q.; Duan, H.; Müller-Buschbaum, P.; et al. Synergistic Defect Passivation by Metformin Halides for Improving Perovskite Solar Cell Performance. J. Phys. Chem. C 2023, 127, 11845–11853. [Google Scholar] [CrossRef]
- Olar, R.; Badea, M.; Marinescu, D.; Chifiriuc, C.-M.; Bleotu, C.; Grecu, M.N.; Iorgulescu, E.E.; Bucur, M.; Lazar, V.; Finaru, A. Prospects for New Antimicrobials Based on N,N-Dimethylbiguanide Complexes as Effective Agents on Both Planktonic and Adhered Microbial Strains. European Journal of Medicinal Chemistry 2010, 45, 2868–2875. [Google Scholar] [CrossRef]
- Nanubolu, J.B.; Sridhar, B.; Ravikumar, K.; Sawant, K.D.; Naik, T.A.; Patkar, L.N.; Cherukuvada, S.; Sreedhar, B. Polymorphism in Metformin Embonate Salt – Recurrence of Dimeric and Tetrameric Guanidinium–Carboxylate Synthons. CrystEngComm 2013, 15, 4448–4464. [Google Scholar] [CrossRef]
- Zhen, X.; Liu, Z.; Qian, L. Application of Metformin in the Treatment of Cerebral Ischemic Stroke; China Patent No CN110604730A; China Ntational Intellectual Property Administration: Beijing, China, 2019. [Google Scholar]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).