1. Introduction
Oral nicotine delivery products (ONDP) provide an alternate way of nicotine delivery to the conventional inhaled tobacco products [
1]. Some of the smokeless ONDPs currently available in the market are chewing tobacco, snus and moist snuff. These smokeless ONDPs are known to contain several harmful and potentially harmful constituents (HPHCs) such as tobacco-specific nitrosamines and polycyclic aromatic hydrocarbons (PAH
) [
2]. Nicotine replacement therapy (NRT) such as lozenges, gums and patches which predominantly deliver nicotine are widely used as a treatment for tobacco addiction. Recently, tobacco-free nicotine pouches, such as ZYN
R are also gaining popularity among teens and adults as novel ONDPs. Whether these tobacco-free oral nicotine pouches may also contain HPHCs is unclear. Back et al. demonstrated presence of low levels of ammonia, chromium, formaldehyde and nickel but absence of nitrosamines and PAH in the NRTs and ZYN
R [
3].
Various studies have described nicotine induced nephrotoxicity. Here we present a 19-year-old male with kidney transplant who presented with acute tubular necrosis after consumption of ZYNR. To the best of our knowledge, this association has not been described in the past.
2. Case Presentation
A 19-year-old Caucasian male underwent a living related kidney transplant from his mother 17 years ago for end stage kidney disease secondary to posterior urethral valve. He had a stable allograft function throughout the years with a baseline serum creatinine of 1.1-1.3 mg/dL. He presented to the emergency department with sudden onset right sided flank pain associated with nausea and dysuria. He denied history of gross hematuria, decreased oral fluid intake, changes in urine output, trauma, vomiting, diarrhea or fever. His medications consisted of tacrolimus 2 mg twice daily, mycophenolate 500 mg twice daily, prednisone 5 mg daily and losartan 25 mg daily. He reported compliance with these medications. He admitted started taking ZYNR pouches (Swedish Match, Stockholm, Sweden) several days prior to this presentation but denied smoking and usage of other tobacco containing ONDPs. He also denied usage of non-steroidal anti-inflammatory drugs.
His past medical history was significant for hospitalization three months ago for acute left eyelid swelling in the setting of history of chronic pansinusitis, nasal polyposis and asthma. The computed tomography scan of the orbit without contrast and magnetic resonance imaging of the sinuses with gadolinium showed evidence of extensive paranasal sinus disease with a dehiscence along the left cribriform plate with intracranial extension. This was consistent with allergic fungal chronic rhinosinusitis, with possible associated invasive fungal infection. He was treated with a three-day course of 60 mg daily prednisone followed by a taper to the baseline dose, ampicillin-sulbactam, levofloxacin, metronidazole and fluconazole for three weeks. Four weeks later, he underwent an extensive functional endoscopic sinus surgery along with bilateral ablation of nasal polyps.
Family history was not suggestive of kidney failure, dialysis or transplantation except for mother being the donor for his transplanted kidney.
Upon presentation, his initial vital signs showed temperature 98.4°F (36.8° C), heart rate 110 beats per minute, respiratory rate 20 per minute, blood pressure 104/66 mm Hg, oxygen saturation 95% on room air, and body mass index 24.1 kg/m². Physical examination was otherwise normal except for an ill-appearing young male with midline abdominal scar from prior kidney transplantation and mild right lower quadrant (RLQ) tenderness.
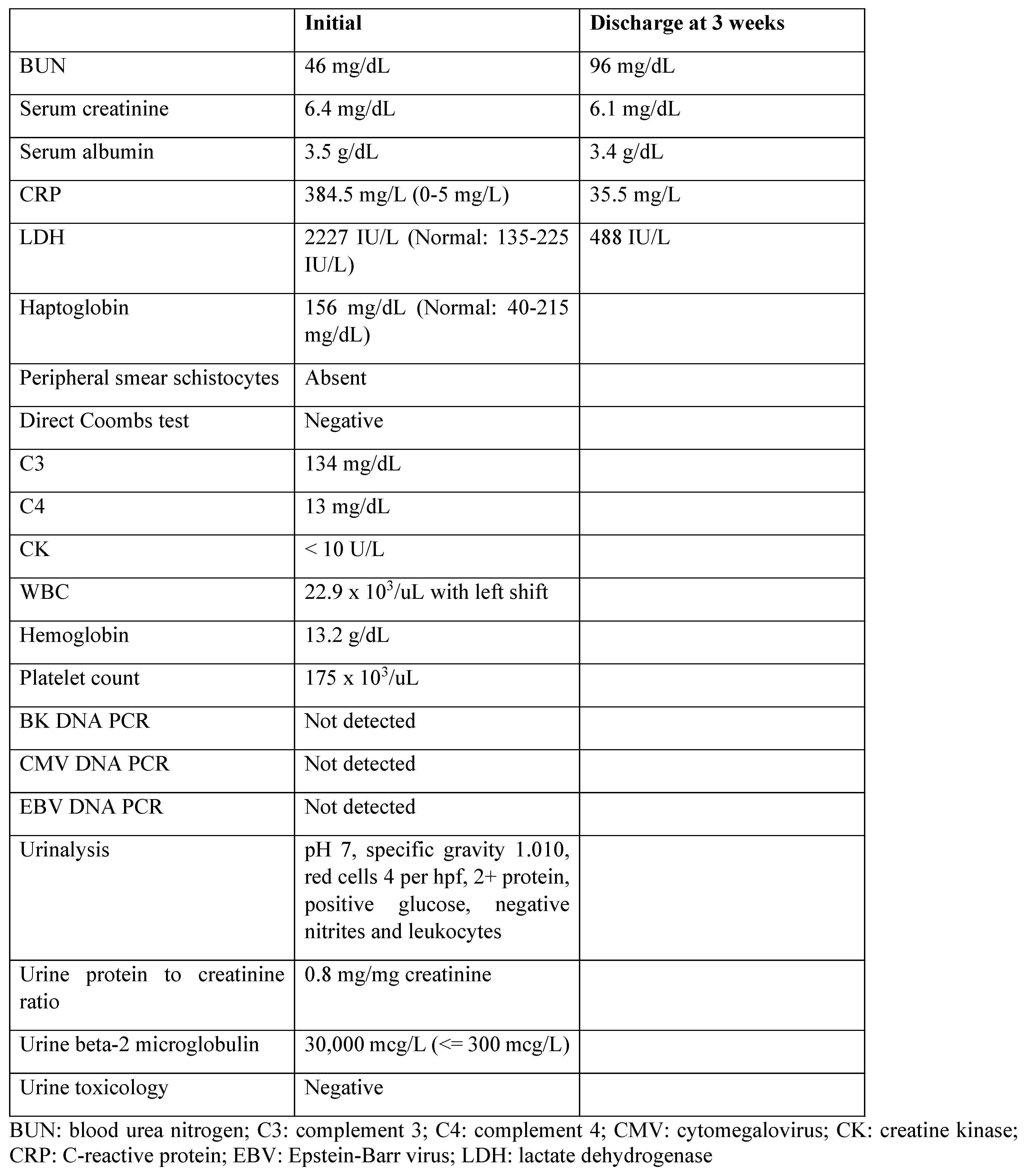
Initial investigations showed elevated C-reactive protein (CRP), increased serum creatinine and blood urea nitrogen, leukocytosis, neutrophilia and increased lactate dehydrogenase (LDH,
Table 1). Plasma haptoglobin was normal. Human rhino/enterovirus was positive. Blood and urine cultures were negative. Renal transplant sonogram with doppler showed an echogenic transplant kidney measuring 10.9 cm in length with no hydronephrosis or perinephric fluid collection, and patent waveforms in the renal artery and vein. Native kidneys were atrophic. Computed tomography scan of the abdomen without contrast showed a non-obstructing 3 mm stone in the mid pole of the RLQ transplant kidney Urinalysis showed glycosuria without associated hyperglycemia, 2+ proteinuria, absence of microscopic hematuria, dilute urine and no evidence of urinary tract infection. Random urine protein to creatinine ratio was 0.8. Urine beta-2 microglobulin was elevated (
Table 1).
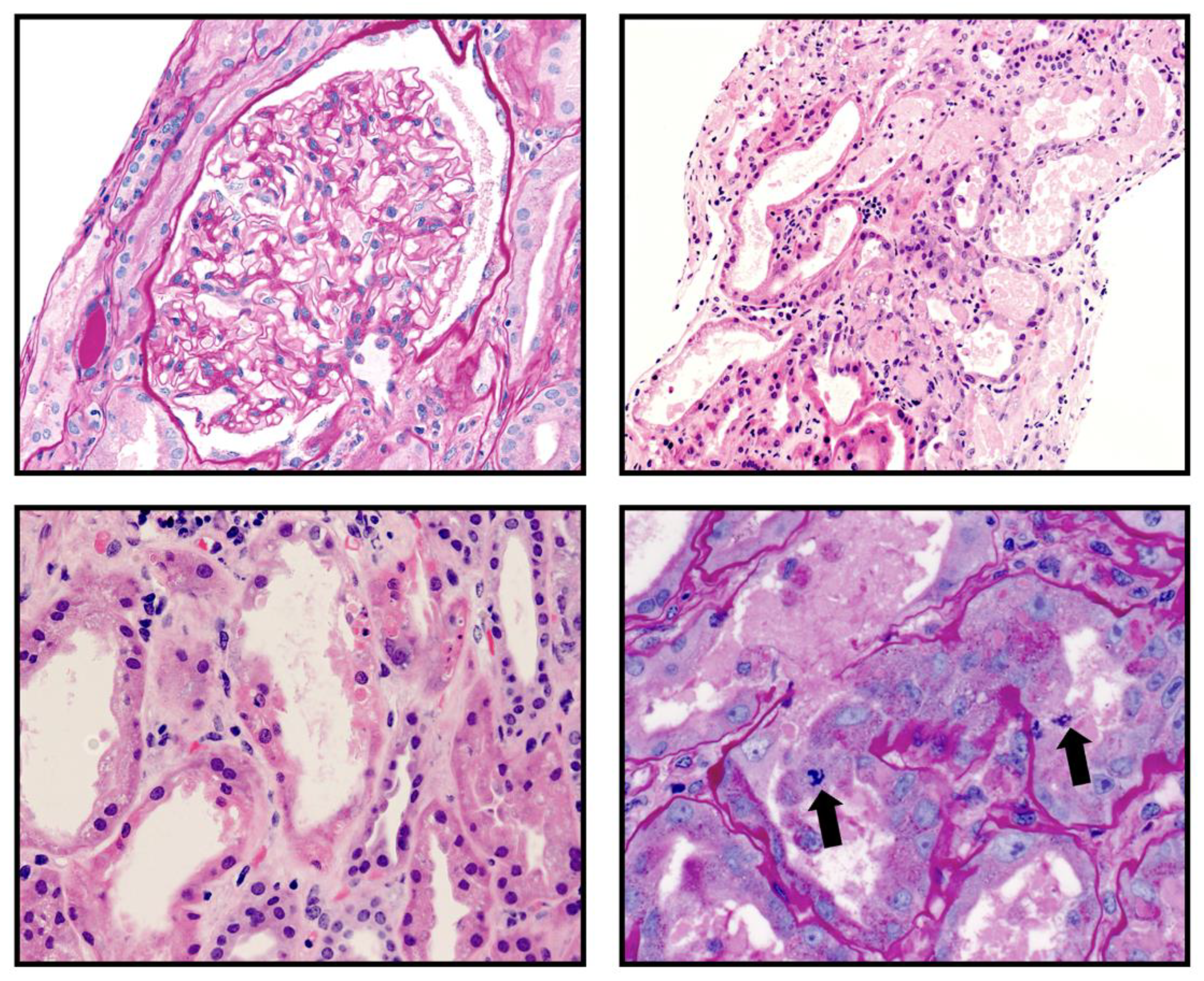
A percutaneous kidney transplant biopsy obtained three days after admission showed nine glomeruli which were normocellular with patent capillary lumens. There was no segmental sclerosis, necrosis or crescent formation. Two of the glomeruli were globally sclerotic. There was interstitial fibrosis and tubular atrophy involving 25% of the cortical parenchyma. There were a few scattered CD3 positive lymphocytes and CD68 immunoreactive mononuclear cells primarily within the interstitium (Figure). There was no peritubular capillaritis and tubulitis. There was severe acute tubular injury characterized by tubular cytoplasmic vacuolization, cell sloughing, individual cell necrosis and large regenerative-appearing nuclei, mitotic figures and luminal casts (
Figure 1A-D). There was prominent hyalinosis of the arterioles and a few small arteries. There were no definitive microthrombi and vasculitis identified. Immunostain for BK virus was negative. Immunofluorescence microscopy showed that glomeruli were negative for IgG, IgM, IgA, C3, C1q, albumin, kappa light chain and lambda light chain. Electron microscopy showed a renal cortex exhibiting acute tubular, and glomerular endothelial cytoplasmic swelling along with mild mesangial expansion without any electron dense deposits. The donor specific antibodies were negative.
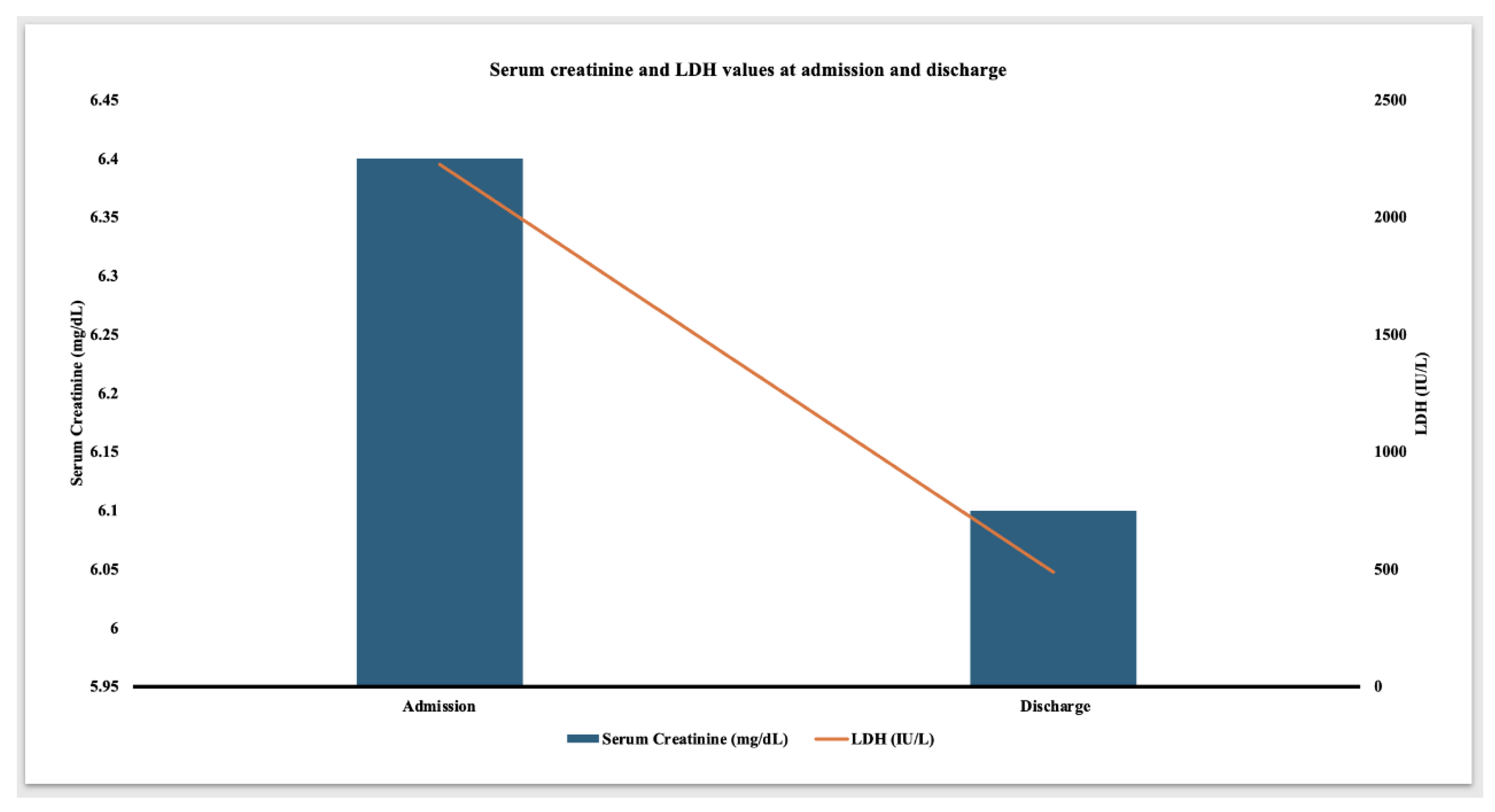
Given less likelihood of rejection, the steroid dose was converted to home baseline dose of 5 mg daily and he did not receive further anti-rejection therapies. Losartan was switched to amlodipine due to elevated serum creatinine. The patient remained afebrile and hemodynamically stable. CRP peaked at 389 mg/L on day four of presentation. Serum LDH showed a gradual decline. Serum trough tacrolimus levels remained therapeutic with a goal trough level of 3-5 ng/mL. The abdominal pain and hematuria resolved about a week after presentation. At the time of discharge, serum LDH decreased to 488 IU/L (
Figure 2) and normalized three weeks later. Serum creatinine was 6.1 mg/dL at the time of discharge and was followed up closely on an outpatient basis. The discharge medications consisted of tacrolimus 2 mg twice daily, mycophenolate 250 mg twice daily, prednisone 5 mg daily and amlodipine 10 mg daily.
A repeat transplant kidney biopsy four months after initial presentation showed evidence of severe interstitial fibrosis involving about 80% of the renal cortex, evidence of grade 1A T cell mediated rejection, negative BK virus staining, and severe interstitial fibrosis/tubular atrophy involving 80% of parenchyma. Given the age of transplant and severe chronicity, the clinical team and the family decided not to augment the immunosuppressive therapy. The most recent follow-up eight months after presentation showed a serum creatinine of 2.6 mg/dL with stable electrolytes with eGFR of 35 ml/min/1.73 m2.
3. Discussion
ZYN
R is a novel next generation non-tobacco based ONDP in a pouch formulation which do not burn tobacco or produce smoke. The ZYN
R pouch contains fillers (maltitol and microcrystalline cellulose), a stabilizer (hydroxypropyl cellulose), pH adjusters (sodium carbonate and sodium bicarbonate), a nicotine salt, food grade flavorings, and a sweetener (acesulfame K) [
3]. Studies have shown that there are no measurable levels of nitrosamines or PAHs in these products and that the number of quantified HPHCs were similar between ZYN
R and NRT products and found at low levels compared to tobacco-based products [
3]. Incidence of usage of nicotine in high schoolers and young adults remains substantial. In 2023, 22.2% of U.S. middle and high school students reported ever using any tobacco product and 10% of students reported current use of any tobacco product [
4]. With regards to the nicotine pouches, the current usage of such was 41.3% among the current smokeless tobacco users [
5].
Nicotine has been shown to be nephrotoxic [6-8]. The kidneys of chronic smokers contain high concentrations of nicotine via glomerular filtration and tubular secretion of nicotine [
7,
9]. The human proximal renal tubular epithelial cells (PRTECs) have prominent nicotinic acetylcholine receptors (nAChR) [10, 11]. One study showed that nicotine causes nephrotoxicity through the induction of NLRP6 inflammasome and alpha7 nAChRs [
10]. The mean renal tubular injury scores were higher in the nicotine group than in the normal group [
10]. In this study, the expression of kidney injury molecule-1 (KIM-1), a marker of tubular injury, was also significantly increased in the kidney tissue [
10]. Nicotine also has been shown to induce apoptosis by generating reactive oxygen species and cell cycle arrest at the G2/M phase, and by activating the MAPK and NF-κB signaling pathways in PRTECs [
12]. The PRTECs are highly susceptible to apoptosis, leading to acute tubular injury [
13]. The tubular injury seen in the patient described in this report could have been from one of these mechanisms. Repeated nicotine exposure from chronic smoking may sensitize the kidney to ischemic insults and may facilitates progression of acute kidney injury to chronic kidney injury [
14].
LDH is commonly measured in patients suspected with hemolysis. However, it is also a marker of cell injury and necrosis. A high concentration of LDH is found in the renal tubular cells [
15]. Hence, the elevated level of serum LDH may be a biomarker of renal tubular injury [16-18]. After kidney transplantation, ischemic ATN, not allograft rejection, leads to rise in LDH [
19,
20]. In delayed graft function, LDH may act as a good non-invasive marker of kidney injury and may serve as a better way to serially assess the clinical outcome or guide the management [
21,
22]. In kidney transplant recipients with preexistent tubular atrophy and interstitial fibrosis such as in this patient, ONDPSs may exacerbate the renal tubular injury leading to marked elevation in LDH. However, this needs to be studied further. Tacrolimus is a known cause of drug-induced hemolytic uremic syndrome (HUS). However, in the absence of peripheral schistocytes, thrombocytopenia, decreased plasma haptoglobin, absence of thrombi in the biopsy, and normal serum trough tacrolimus levels, tacrolimus-induced HUS leading to elevated LDH was unlikely 17 years after the transplantation. In the absence of anemia, normal plasma haptoglobin, and normal Coombs test, other causes of transient elevation in serum LDH were less likely in the patient described in this report.
4. Conclusions
This report highlights the importance of knowledge of possible association of ONDPs with the acute tubular injury. It remains to be studied whether the incidence of this specific renal toxicity is more prevalent in immunocompromised patients, as described in this report. Although we could not establish a definite cause and effect relationship between ONDP and renal tubular injury, this report suggests that clinicians will need to be vigilant in searching for a history of usage of ONDPs in patients presenting with unexplained rise in LDH and acute tubular injury. Given the widespread usage among high schoolers, further studies are necessary to determine the incidence of acute tubular injury in the users of ONDPs.
Conflict of Interest: The authors declare no conflict of interest.
Informed Consent: We obtained written informed consent from the patient for publication of this case report.
Author Contributions
Ratna Acharya was in charge of writing the case and structure/flow of the paper. Ratna Acharya was in charge of writing the conception and discussion. William Clapp was in charge of writing the pathology discussion and interpretation of the biopsy. Kiran Upadhyay oversaw the concept, writing, and edition of the manuscript.
Data Availability: The authors declare that data supporting the findings of this study are available within the article.
References
- Delnevo, C.D.; Hrywna, M.; Miller Lo, E.J.; Wackowski, O.A. Examining market trends in smokeless tobacco sales in the United States: 2011-2019. Nicotine Tob Res. 2021, 23, 1420–1424. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.T.; Taylor, K.M.; Holman, M.R.; Ding, Y.S.; Hearn, B.; Watson, C.H. Polycyclic aromatic hydrocarbons in the mainstream smoke of popular U.S. cigarettes. Chem Res Toxicol. 2015, 28, 1616–1626. [Google Scholar] [CrossRef] [PubMed]
- Back, S.; Masser, A.E.; Rutqvist, L.E.; Lindholm, J. Harmful and potentially harmful constituents (HPHCs) in two novel nicotine pouch products in comparison with regular smokeless tobacco products and pharmaceutical nicotine replacement therapy products (NRTs). BMC Chem. 2023, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Birdsey, J.; Cornelius, M.; Jamal, A.; Park-Lee, E.; Cooper, M.R.; Wang, J.; Sawdey, M.D.; Cullen, K.A.; Neff, L. Tobacco product use among U.S. middle and high school students - National Youth Tobacco Survey, 2023. MMWR Morb Mortal Wkly Rep. 2023, 72, 1173–1182. [Google Scholar] [CrossRef] [PubMed]
- Kramer, R.D.; Park-Lee, E.; Marynak, K.L.; Jones, J.T.; Sawdey, M.D.; Cullen, K.A. Nicotine pouch awareness and use among youth, National Youth Tobacco Survey, 2021. Nicotine Tob Res. 2023, 25, 1610–1613. [Google Scholar] [CrossRef] [PubMed]
- Rezonzew, G.; Chumley, P.; Feng, W.; Hua, P.; Siegal, G.P.; Jaimes, E.A. Nicotine exposure and the progression of chronic kidney disease: role of the α7-nicotinic acetylcholine receptor. Am J Physiol Renal Physiol. 2012, 303, F304–E312. [Google Scholar] [CrossRef]
- Hukkanen, J.; Jacob, P., 3rd; Benowitz, N.L. Metabolism and disposition kinetics of nicotine. Pharmacol Rev. 2005, 57, 79–115. [Google Scholar] [CrossRef]
- Jaimes, E.A.; Tian, R.X.; Joshi, M.S.; Raij, L. Nicotine augments glomerular injury in a rat model of acute nephritis. Am J Nephrol. 2009, 29, 319–326. [Google Scholar] [CrossRef]
- Urakawa, N.; Nagata, T.; Kudo, K.; Kimura, K.; Imamura, T. Simultaneous determination of nicotine and cotinine in various human tissues using capillary gas chromatography/mass spectrometry. Int J Legal Med. 1994, 106, 232–236. [Google Scholar] [CrossRef]
- Zheng, C.M.; Lee, Y.H.; Chiu, I.J.; Chiu, Y.J.; Sung, L.C.; Hsu, Y.H.; Chiu, H.W. Nicotine causes nephrotoxicity through the induction of NLRP6 inflammasome and alpha7 nicotinic acetylcholine receptor. Toxics. 2020, 8, 92. [Google Scholar] [CrossRef]
- Yeboah, M.M.; Xue, X.; Duan, B.; Ochani, M.; Tracey, K.J.; Susin, M.; Metz, C.N. Cholinergic agonists attenuate renal ischemia-reperfusion injury in rats. Kidney Int. 2008, 74, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Choi, J.S.; Joo, S.Y.; Bae, E.H.; Ma, S.K.; Lee, J.; Kim, S.W. Nicotine-induced apoptosis in human renal proximal tubular epithelial cells. PLoS One. 2016, 11, e0152591. [Google Scholar] [CrossRef] [PubMed]
- Vickers, A.E.; Rose, K.; Fisher, R.; Saulnier, M.; Sahota, P.; Bentley, P. Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol Pathol. 2004, 32, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Arany, I.; Grifoni, S.; Clark, J.S.; Csongradi, E.; Maric, C.; Juncos, L.A. Chronic nicotine exposure exacerbates acute renal ischemic injury. Am J Physiol Renal Physiol. 2011, 301, F125–F133. [Google Scholar] [CrossRef] [PubMed]
- Thiele, K.G.; Mattenheimer, H. The isoenzymes of lactate dehydrogenase in the nephron of the healthy human kidney. Z Klin Chem Klin Biochem. 1968, 6, 132–8. [Google Scholar]
- Roses, J.; Woods, J.E.; Zincke, H. The value of lactic dehydrogenase as a predictor of early allograft survival. Am J Surg. 1977, 133, 726–8. [Google Scholar] [CrossRef]
- Anderson, C.B.; Groce, M.A.; Mohapatra, R.N.; Codd, J.E.; Graff, R.J.; Gregory, J.G.; Newton, W.T. Serum lactic dehydrogenase and irreversible renal allograft rejection. Surgery. 1976, 79, 161–5. [Google Scholar]
- Koyama, Y.; Miyazato, T.; Tsuha, M.; Goya, M.; Kagawa, H.; Miyakawa, A.; Sugaya, K.; Hatano, T.; Ogawa, Y.; Shiraishi, M. Does the high level of lactate dehydrogenase predict renal function and outcome after renal transplantation from non-heart-beating cadaver donors? Transplant Proc. 2000, 32, 1604–5. [Google Scholar] [CrossRef]
- Green, H.; Tobar, A.; Gafter-Gvili, A.; Leibovici, L.; Klein, T.; Rahamimov, R.; Mor, E.; Grossman, A. Serum lactate dehydrogenase is elevated in ischemic acute tubular necrosis but not in acute rejection in kidney transplant patients. Prog Transplant. 2017, 27, 53–57. [Google Scholar] [CrossRef]
- Sándor, Z.; Katics, D.; Varga, Á.; Kalmár Nagy, K.; Szakály, P. Interpretation of LDH values after kidney transplantation. J Clin Med. 2024, 13, 485. [Google Scholar] [CrossRef]
- Truche, A.S.; Trocme, C.; Vergnaud, S.; Janbon, B.; Giovannini, D.; Malvezzi, P.; Moreau-Gaudry, X.; Rostaing, L.; Tetaz, R. Early prediction of graft outcomes after kidney transplantation from donors after circulatory death: biomarkers and transplantation characteristics. Transplant Proc. 2019, 51, 3234–3243. [Google Scholar] [CrossRef]
- Mezzolla, V.; Pontrelli, P.; Fiorentino, M.; Stasi, A.; Pesce, F.; Franzin, R.; Rascio, F.; Grandaliano, G.; Stallone, G.; Infante, B.; Gesualdo, L.; Castellano, G. Emerging biomarkers of delayed graft function in kidney transplantation. Transplant Rev (Orlando). 2021, 35, 100629. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).