Submitted:
01 October 2024
Posted:
02 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
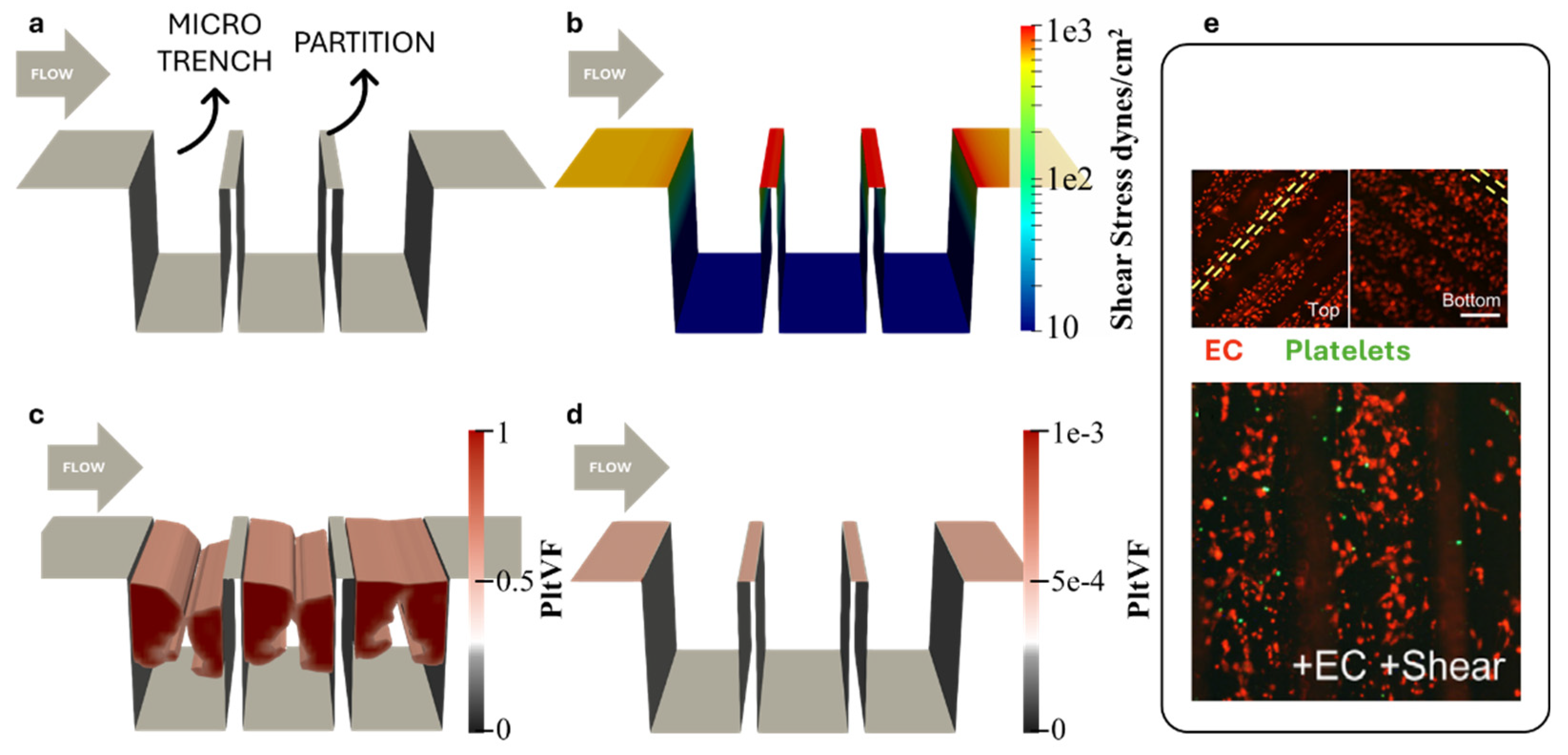
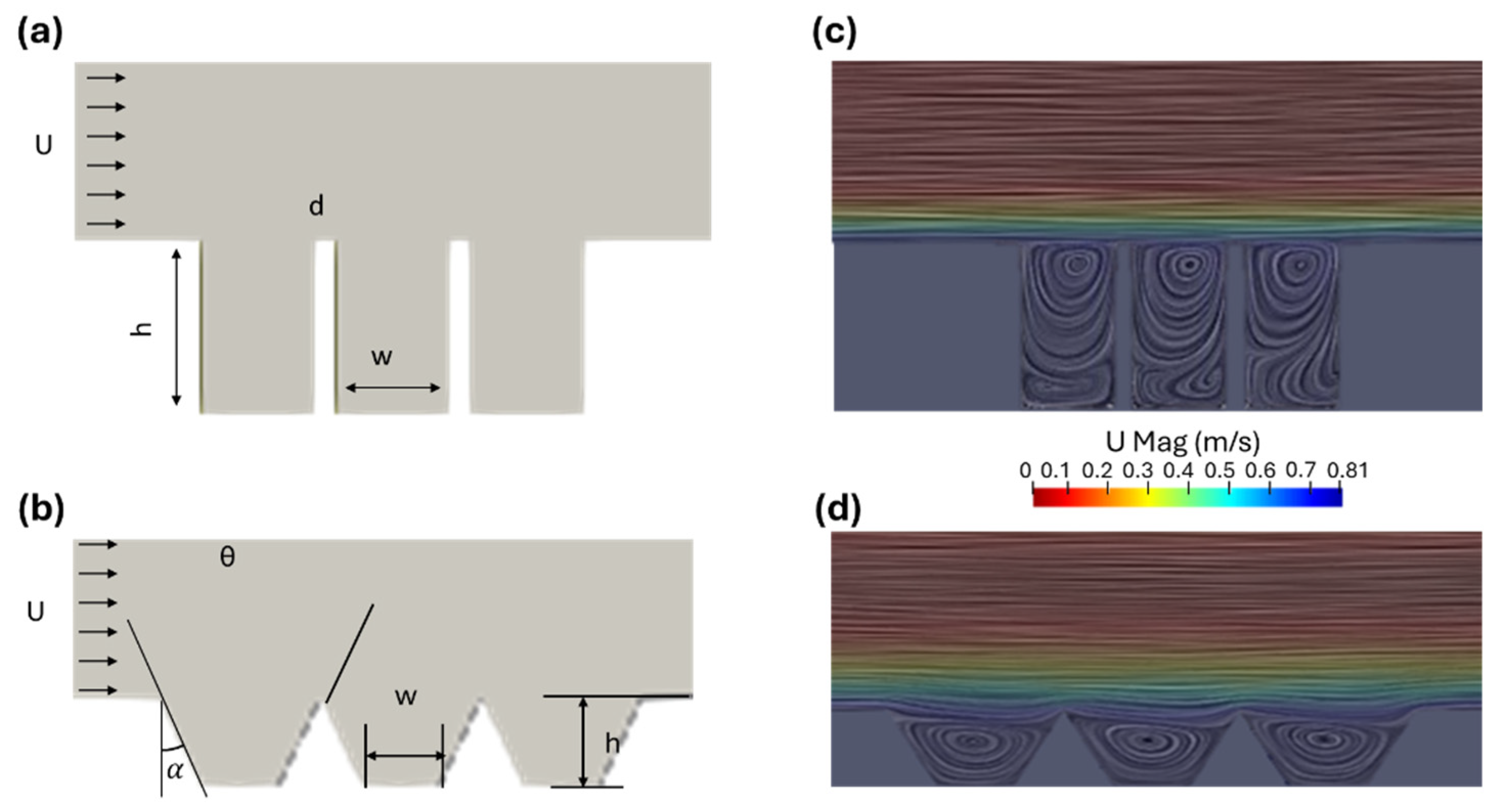
2.1. Flow Field Simulation and Optimization of the Microtrenches
3. Results
3.1. Simulation of Platelet Adhesion
3.2. Optimization of Microtrenches
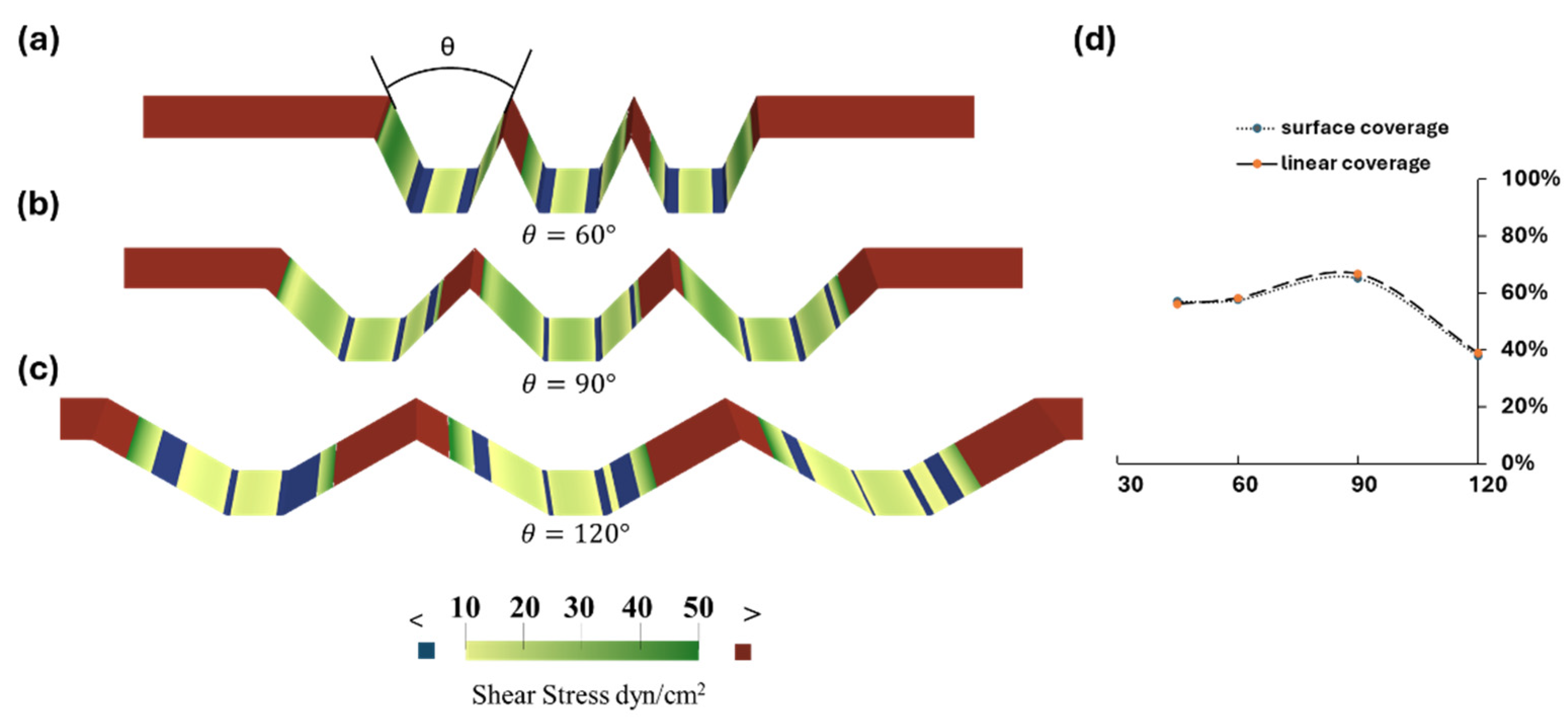
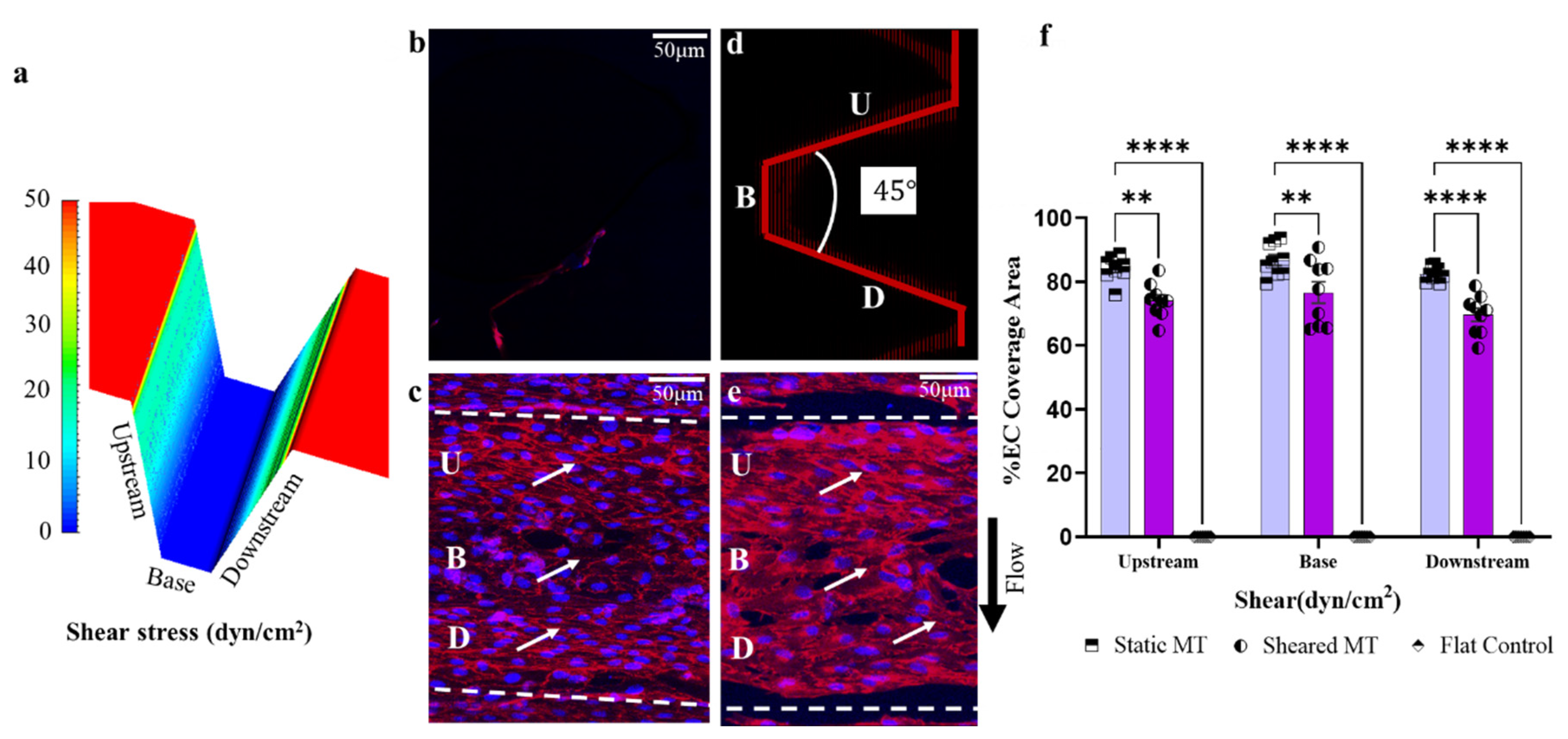
3.3. Optimization of Trapezoidal Trench Geometry
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gorbet, M.B.; Sefton, M. V Biomaterial-Associated Thrombosis: Roles of Coagulation Factors, Complement, Platelets and Leukocytes. In The Biomaterials: Silver Jubilee Compendium; 2006; Vol. 25, pp. 219–241 ISBN 9780080451541.
- Labarrere, C.A.; Dabiri, A.E.; Kassab, G.S. Thrombogenic and Inflammatory Reactions to Biomaterials in Medical Devices. Front. Bioeng. Biotechnol. 2020, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Aparicio, H.J.; Benjamin, E.J.; Callaway, C.W.; Carson, A.P.; Cheng, S.; Elkind, M.S. V; Evenson, K.R.; Ferguson, J.F.; Knutson, K.L.; Lee, C.D.; et al. Heart Disease and Stroke Statistics-2021 Update A Report from the American Heart Association. Circulation 2021, E254–E743. [Google Scholar] [CrossRef]
- Cannegieter, S.C.; Rosendaal, F.R.; Briët, E. Thromboembolic and Bleeding Complications in Patients with Mechanical Heart Valve Prostheses. Circulation 1994, 89, 635–641. [Google Scholar] [CrossRef] [PubMed]
- Mehra, M.R.; Uriel, N.; Naka, Y.; Joseph, C. Cleveland, J.; Yuzefpolskaya, M.; Salerno, C.T.; Walsh, M.N.; Milano, C.A.; Patel, C.B.; Hutchins, S.W.; et al. A Fully Magnetically Levitated Left Ventricular Assist Device — Final Report. N. Engl. J. Med. 2019, 380, 1618–1627. [Google Scholar] [CrossRef]
- Kuchinka, J.; Willems, C.; Telyshev, D. V.; Groth, T. Control of Blood Coagulation by Hemocompatible Material Surfaces—A Review. Bioengineering 2021, 8, 1–26. [Google Scholar] [CrossRef]
- Vogler, E.A.; Siedlecki, C.A. Contact Activation of Blood-Plasma Coagulation. Biomaterials 2009, 30, 1857–1869. [Google Scholar] [CrossRef]
- Furukawa, K.S.; Ushida, T.; Sugano, H.; Tamaki, T.; Ohshima, N.; Tateishi, T. Effect of Shear Stress on Platelet Adhesion to Expanded Polytetrafluoroethylene, a Silicone Sheet, and an Endothelial Cell Monolayer. ASAIO J. 2000, 46, 696–701. [Google Scholar] [CrossRef]
- Ahmann, K.A.; Johnson, S.L.; Hebbel, R.P.; Tranquillo, R.T. Shear Stress Responses of Adult Blood Outgrowth Endothelial Cells Seeded on Bioartificial Tissue. Tissue Eng.—Part A 2011, 17, 2511–2521. [Google Scholar] [CrossRef]
- Jin, R.C.; Voetsch, B.; Loscalzo, J. Endogenous Mechanisms of Inhibition of Platelet Function. Microcirculation 2005, 12, 247–258. [Google Scholar] [CrossRef]
- Dangas, G.D.; Weitz, J.I.; Giustino, G.; Makkar, R.; Mehran, R. Prostheticurrentc Heart Valve Thrombosis. J. Am. Coll. Cardiol. 2016, 68, 2670–2689. [Google Scholar] [CrossRef]
- Wolfe, J.T.; Shradhanjali, A.; Tefft, B.J. Strategies for Improving Endothelial Cell Adhesion to Blood-Contacting Medical Devices. Tissue Eng. Part B Rev. 2022, 28. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Butcher, J.T.; Rowlands, G.W.; Antaki, J.F. Biological Response to Sintered Titanium in Left Ventricular Assist Devices: Pseudoneointima, Neointima, and Pannus. ASAIO J. [CrossRef]
- Zapanta, C.M.; Griffith, J.W.; Hess, G.D.; Doxtater, B.J.; Khalapyan, T.; Pae, W.E.; Rosenberg, G. Microtextured Materials for Circulatory Support Devices: Preliminary Studies. ASAIO J. 2006, 52, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, T.; Nishinaka, T.; Mizuno, T.; Tatsumi, E.; Yamazaki, K. LVAD Inflow Cannula Covered with a Titanium Mesh Induces Neointimal Tissue with Neovessels. Int. J. Artif. Organs 2015, 38, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Robotti, F.; Franco, D.; Bänninger, L.; Wyler, J.; Starck, C.T.; Falk, V.; Poulikakos, D.; Ferrari, A. The Influence of Surface Micro-Structure on Endothelialization under Supraphysiological Wall Shear Stress. Biomaterials 2014, 35, 8479–8486. [Google Scholar] [CrossRef]
- Gong, X.; Yao, J.; He, H.; Zhao, X.; Liu, X.; Zhao, F.; Sun, Y.; Fan, Y. Combination of Flow and Micropattern Alignment Affecting Flow-Resistant Endothelial Cell Adhesion. J. Mech. Behav. Biomed. Mater. 2017, 74, 11–20. [Google Scholar] [CrossRef]
- Yoganathan, A.P.; Chaux, A.; Gray, R.J.; Woo, Y.R.; DeRobertis, M.; Williams, F.P.; Matloff, J.M. Bileaflet, Tilting Disc and Porcine Aortic Valve Substitutes: In Vitro Hydrodynamic Characteristics. J. Am. Coll. Cardiol. 1984, 3, 313–320. [Google Scholar] [CrossRef]
- Franco, D.; Milde, F.; Klingauf, M.; Orsenigo, F.; Dejana, E.; Poulikakos, D.; Cecchini, M.; Koumoutsakos, P.; Ferrari, A.; Kurtcuoglu, V. Biomaterials Accelerated Endothelial Wound Healing on Microstructured Substrates under Fl Ow. Biomaterials 2013, 34, 1488–1497. [Google Scholar] [CrossRef]
- Stefopoulos, G.; Robotti, F.; Falk, V.; Poulikakos, D.; Ferrari, A. Endothelialization of Rationally Microtextured Surfaces with Minimal Cell Seeding Under Flow. Small 2016, 12, 4113–4126. [Google Scholar] [CrossRef]
- Bachmann, B.J.; Giampietro, C.; Bayram, A.; Stefopoulos, G.; Michos, C.; Graeber, G.; Falk, M.V.; Poulikakos, D.; Ferrari, A. Honeycomb-Structured Metasurfaces for the Adaptive Nesting of Endothelial Cells under Hemodynamic Loads. Biomater. Sci. 2018, 6, 2726–2737. [Google Scholar] [CrossRef]
- Daxini, S.C.; Nichol, J.W.; Sieminski, A.L.; Smith, G.; Gooch, K.J.; Shastri, V.P. Micropatterned Polymer Surfaces Improve Retention of Endothelial Cells Exposed to Flow-Induced Shear Stress. Biorheology 2006, 43, 45–55. [Google Scholar]
- Frendl, C.M.; Tucker, S.M.; Khan, N.A.; Esch, M.B.; Kanduru, S.; Cao, T.M.; García, A.J.; King, M.R.; Butcher, J.T. Endothelial Retention and Phenotype on Carbonized Cardiovascular Implant Surfaces. Biomaterials 2014, 35, 7714–7723. [Google Scholar] [CrossRef]
- Ranjan, A.; Webster, T.J. Increased Endothelial Cell Adhesion and Elongation on Micron-Patterned Nano-Rough Poly(Dimethylsiloxane) Films. Nanotechnology 2009, 20. [Google Scholar] [CrossRef]
- Wu, W.-T. Theoretical and Computational Studies on Thrombosis Growth and Multiphase Characteristics of Blood Flow, Carnegie Mellon University, 2015.
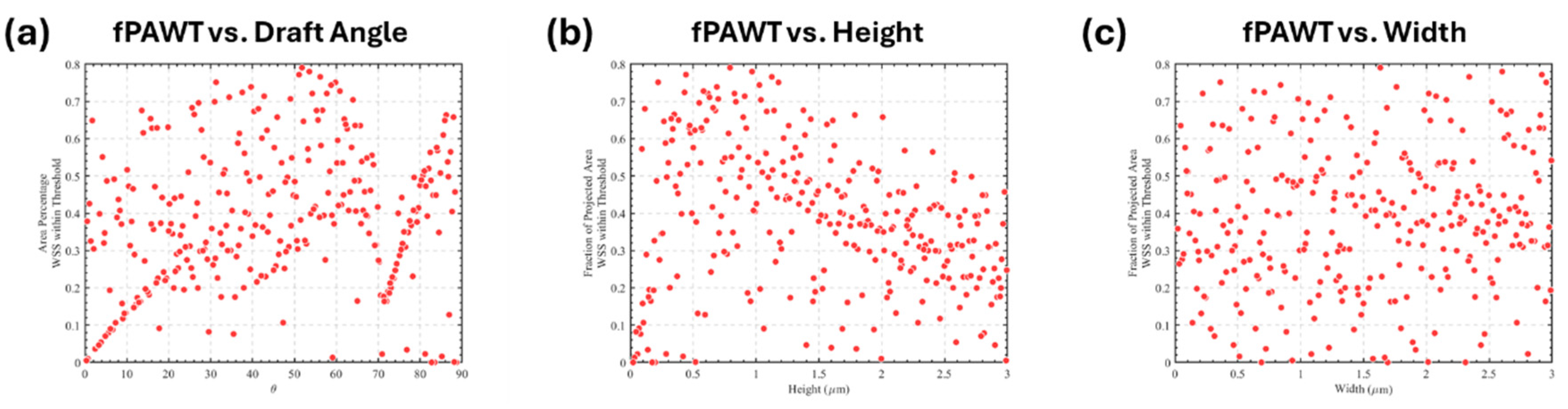
| Design Variables | Lower Bound | Upper Bound | Average Interval |
|---|---|---|---|
| Height (mm) | 0.01 | 3 | 0.1 |
| Width (mm) | 0.01 | 3 | 0.1 |
| Angle*(°) | 0 | 90 | 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





