Submitted:
01 October 2024
Posted:
02 October 2024
You are already at the latest version
Abstract
Keywords:
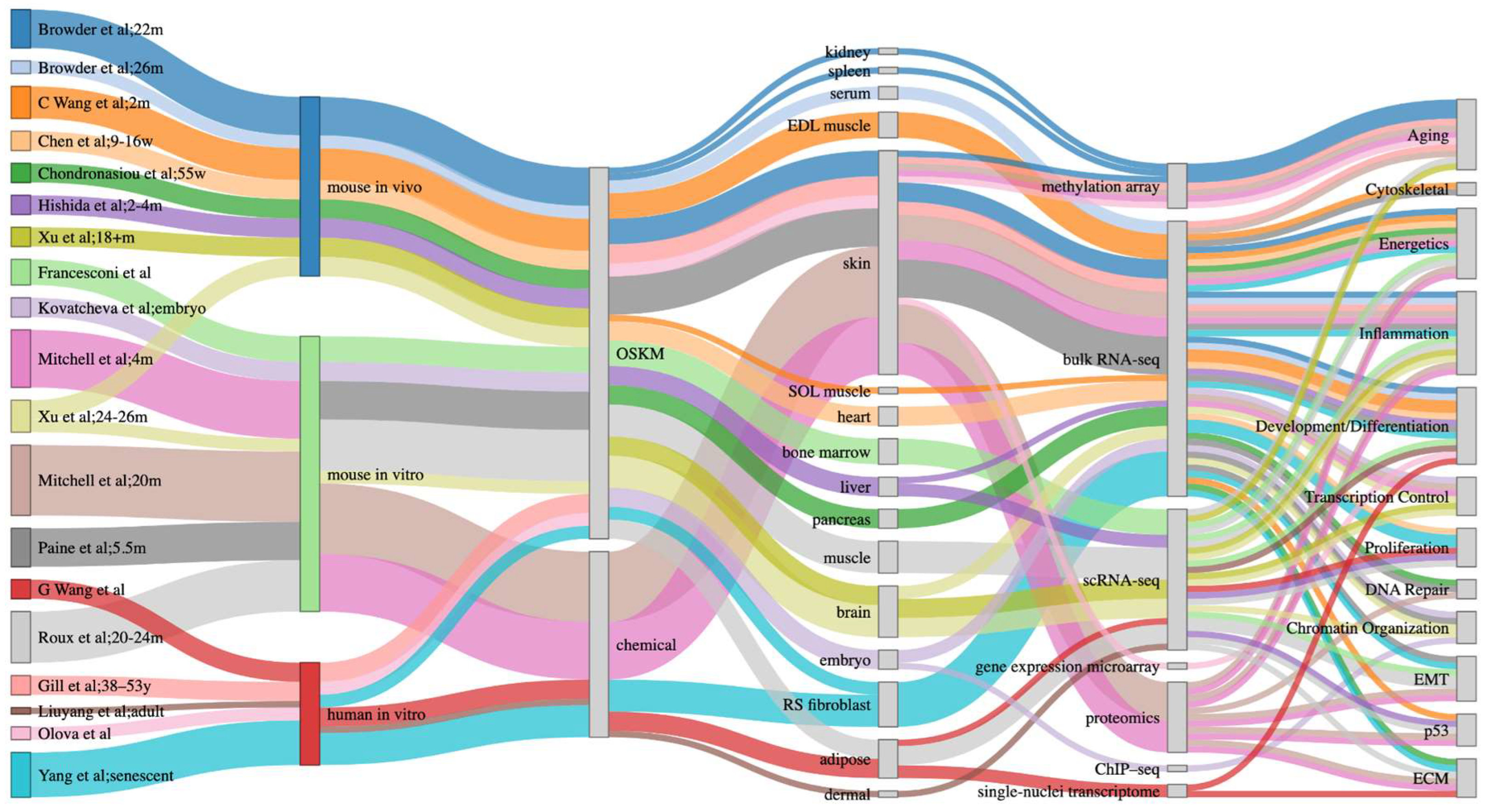
1. Introduction
1.1. Partial Reprogramming Overview
1.2. Temporal Dynamics of Reprogramming
1.3. Epigenetic Memory
1.4. Impact of Partial Reprogramming on Surrounding Tissue
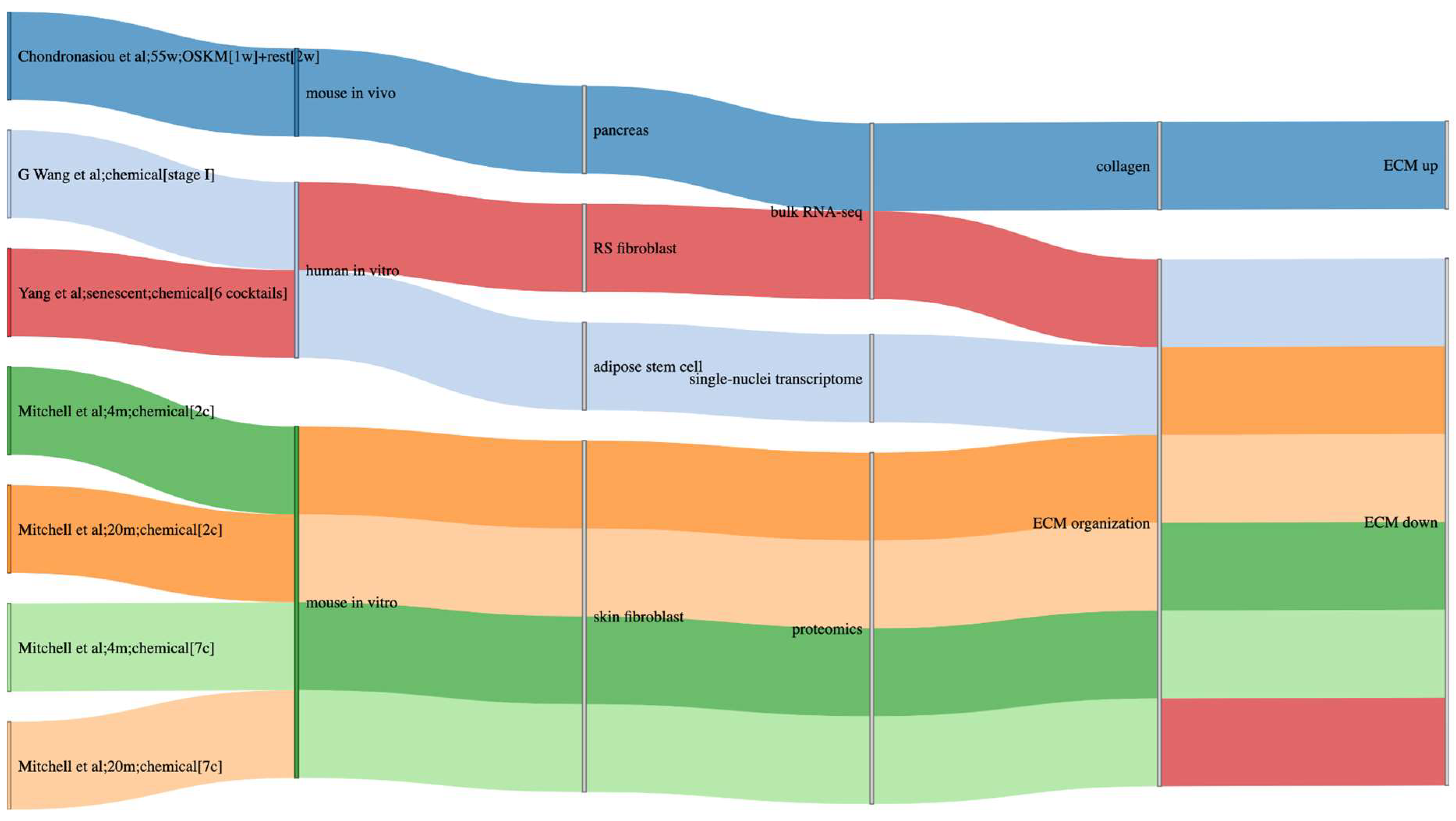
1.5. Cancer and Safety Concerns
1.6. Differences in “Reprogrammability”
2. Health Benefits and Age Reversal Effects
2.1. Aging Clocks
2.2. Rejuvenation
2.3. Stress Responses
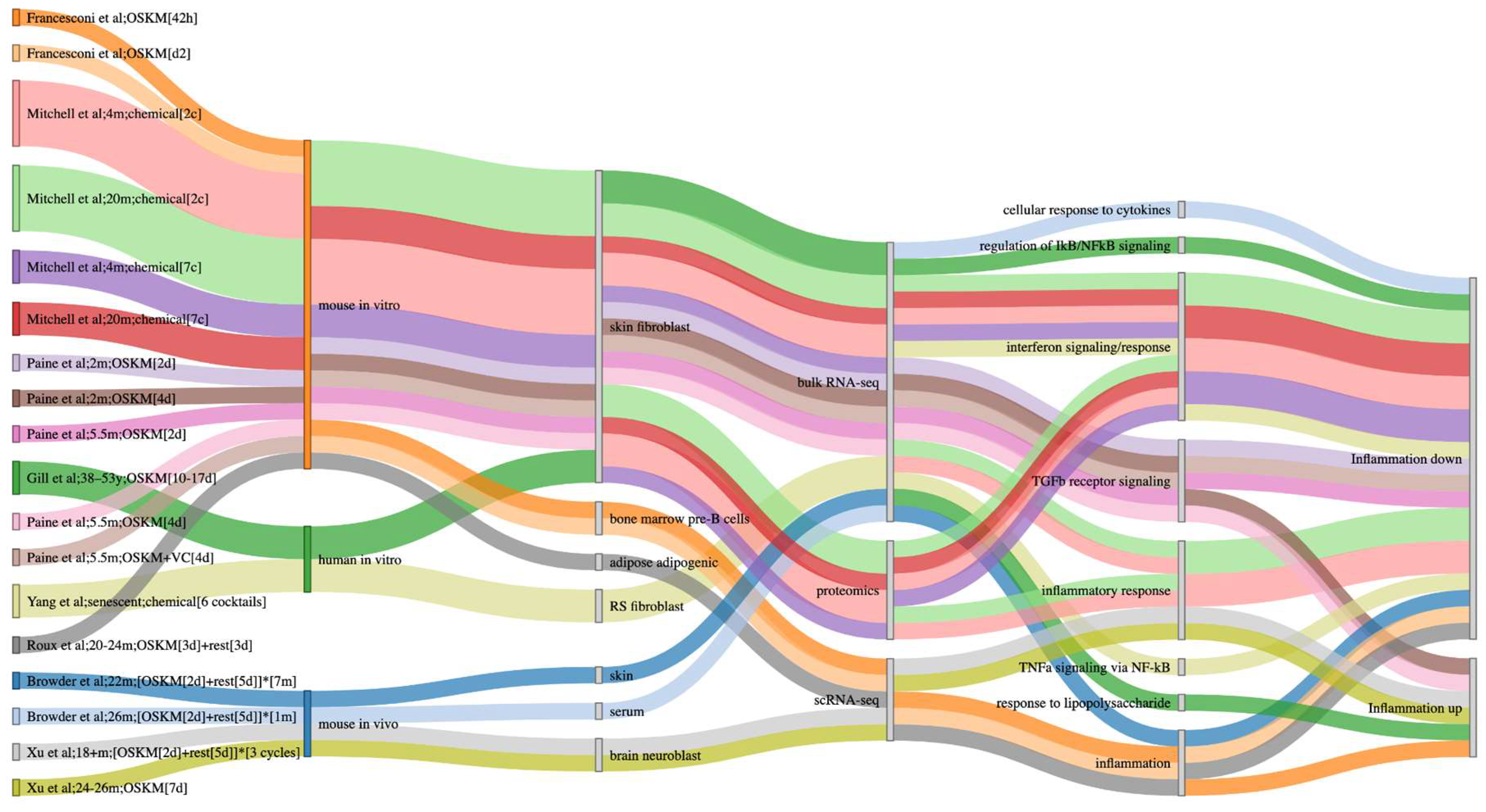
2.3.1. Inflammation
2.3.2. Cellular Senescence
2.3.3. Autophagy and mTOR Pathways
2.3.4. DNA Repair
2.4. Metabolic Changes
2.4.1. Mitochondrial Function and Energetic Pathways
2.4.2. Metabolic Changes
2.5. Similarities between Partial Reprogramming and Aging
Concluding Remarks
Supplementary Materials
Author Contributions
Acknowledgments
Conflict of Interest
References
- Abad, M.; Mosteiro, L.; Pantoja, C.; Canamero, M.; Rayon, T.; Ors, I.; Grana, O.; Megias, D.; Dominguez, O.; Martinez, D. , et al . ( 2013). Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature 502, 340–345. [CrossRef]
- Acosta, J.C.; O'Loghlen, A.; Banito, A.; Guijarro, M.V.; Augert, A.; Raguz, S.; Fumagalli, M.; Da Costa, M.; Brown, C.; Popov, N. , et al. ( 2008). Chemokine signaling via the CXCR2 receptor reinforces senescence. Cell 133, 1006–1018. [CrossRef]
- Anton-Fernandez, A.; Roldan-Lazaro, M.; Valles-Saiz, L.; Avila, J.; Hernandez, F. (2024). In vivo cyclic overexpression of Yamanaka factors restricted to neurons reverses age-associated phenotypes and enhances memory performance. Commun Biol 7, 631. [CrossRef]
- Avelar, R.A.; Ortega, J.G.; Tacutu, R.; Tyler, E.J.; Bennett, D.; Binetti, P.; Budovsky, A.; Chatsirisupachai, K.; Johnson, E.; Murray, A. , et al. ( 2020). A multidimensional systems biology analysis of cellular senescence in aging and disease. Genome Biol 21, 91. [CrossRef]
- Balistreri, C.R.; Candore, G.; Accardi, G.; Colonna-Romano, G.; Lio, D. (2013). NF-kappaB pathway activators as potential ageing biomarkers: targets for new therapeutic strategies. Immun Ageing 10, 24.
- Banito, A.; Rashid, S.T.; Acosta, J.C.; Li, S.; Pereira, C.F.; Geti, I.; Pinho, S.; Silva, J.C.; Azuara, V.; Walsh, M. , et al. ( 2009). Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev 23, 2134–2139. [CrossRef]
- Browder, K.C.; Reddy, P.; Yamamoto, M.; Haghani, A.; Guillen, I.G.; Sahu, S.; Wang, C.; Luque, Y.; Prieto, J.; Shi, L. , et al. (2022). In vivo partial reprogramming alters age-associated molecular changes during physiological aging in mice. Nat Aging 2, 243-253. [CrossRef]
- Carosi, J.M.; Fourrier, C.; Bensalem, J.; Sargeant, T.J. (2022). The mTOR-lysosome axis at the centre of ageing. FEBS Open Bio 12, 739-757.
- Chen, Y.; Luttmann, F.F.; Schoger, E.; Scholer, H.R.; Zelarayan, L.C.; Kim, K.P.; Haigh, J.J.; Kim, J.; Braun, T. (2021). Reversible reprogramming of cardiomyocytes to a fetal state drives heart regeneration in mice. Science 373, 1537-1540. [CrossRef]
- Cheng, F.; Wang, C.; Ji, Y.; Yang, B.; Shu, J.; Shi, K.; Wang, L.; Wang, S.; Zhang, Y.; Huang, X. , et al. ( 2022). Partial reprogramming strategy for intervertebral disc rejuvenation by activating energy switch. Aging Cell 21, e13577. [CrossRef]
- Chiche, A.; Le Roux, I.; von Joest, M.; Sakai, H.; Aguin, S.B.; Cazin, C.; Salam, R.; Fiette, L.; Alegria, O.; Flamant, P. , et al. (2017). Injury-Induced Senescence Enables In Vivo Reprogramming in Skeletal Muscle. Cell Stem Cell 20, 407-414 e404. [CrossRef]
- Chondronasiou, D.; Gill, D.; Mosteiro, L.; Urdinguio, R.G.; Berenguer-Llergo, A.; Aguilera, M.; Durand, S.; Aprahamian, F.; Nirmalathasan, N.; Abad, M. , et al. (2022a). Multi-omic rejuvenation of naturally aged tissues by a single cycle of transient reprogramming. Aging Cell 21, e13578. [CrossRef]
- Chondronasiou, D.; Martinez de Villarreal, J.; Melendez, E.; Lynch, C.J.; Pozo, N.D.; Kovatcheva, M.; Aguilera, M.; Prats, N.; Real, F.X.; Serrano, M. (2022b). Deciphering the roadmap of in vivo reprogramming toward pluripotency. Stem Cell Reports 17, 2501-2517. [CrossRef]
- Coppe, J.P.; Desprez, P.Y.; Krtolica, A.; Campisi, J. (2010). The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol 5, 99-118. [CrossRef]
- Coppe, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Munoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. (2008). Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6, 2853-2868. [CrossRef]
- Cribb, L.; Hodge, A.M.; Yu, C.; Li, S.X.; English, D.R.; Makalic, E.; Southey, M.C.; Milne, R.L.; Giles, G.G.; Dugue, P.A. (2022). Inflammation and Epigenetic Aging Are Largely Independent Markers of Biological Aging and Mortality. J Gerontol A Biol Sci Med Sci 77, 2378-2386. [CrossRef]
- Dhanasekaran, R.; Deutzmann, A.; Mahauad-Fernandez, W.D.; Hansen, A.S.; Gouw, A.M.; Felsher, D.W. (2022). The MYC oncogene - the grand orchestrator of cancer growth and immune evasion. Nat Rev Clin Oncol 19, 23-36. [CrossRef]
- Doeser, M.C.; Scholer, H.R.; Wu, G. (2018). Reduction of Fibrosis and Scar Formation by Partial Reprogramming In Vivo. Stem Cells 36, 1216-1225. [CrossRef]
- Ferrucci, L.; Fabbri, E. (2018). Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15, 505-522. [CrossRef]
- Francesconi, M.; Di Stefano, B.; Berenguer, C.; de Andres-Aguayo, L.; Plana-Carmona, M.; Mendez-Lago, M.; Guillaumet-Adkins, A.; Rodriguez-Esteban, G.; Gut, M.; Gut, I.G. , et al. (2019). Single cell RNA-seq identifies the origins of heterogeneity in efficient cell transdifferentiation and reprogramming. Elife 8. [CrossRef]
- Gill, D.; Parry, A.; Santos, F.; Okkenhaug, H.; Todd, C.D.; Hernando-Herraez, I.; Stubbs, T.M.; Milagre, I.; Reik, W. (2022). Multi-omic rejuvenation of human cells by maturation phase transient reprogramming. Elife 11. [CrossRef]
- Gonzalez-Gualda, E.; Baker, A.G.; Fruk, L.; Munoz-Espin, D. (2021). A guide to assessing cellular senescence in vitro and in vivo. FEBS J 288, 56-80.
- Guan, J.; Wang, G.; Wang, J.; Zhang, Z.; Fu, Y.; Cheng, L.; Meng, G.; Lyu, Y.; Zhu, J.; Li, Y. , et al. ( 2022). Chemical reprogramming of human somatic cells to pluripotent stem cells. Nature 605, 325–331. [CrossRef]
- Haridhasapavalan, K.K.; Raina, K.; Dey, C.; Adhikari, P.; Thummer, R.P. (2020). An Insight into Reprogramming Barriers to iPSC Generation. Stem Cell Rev Rep 16, 56-81. [CrossRef]
- Heffernan, C.; Sumer, H.; Malaver-Ortega, L.F.; Verma, P.J. (2012). Temporal Requirements of cMyc Protein for Reprogramming Mouse Fibroblasts. Stem Cells Int 2012, 541014. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; de Jong, T.V.; Melov, S.; Guryev, V.; Campisi, J.; Demaria, M. (2017). Unmasking Transcriptional Heterogeneity in Senescent Cells. Curr Biol 27, 2652-2660 e2654. [CrossRef]
- Hishida, T.; Yamamoto, M.; Hishida-Nozaki, Y.; Shao, C.; Huang, L.; Wang, C.; Shojima, K.; Xue, Y.; Hang, Y.; Shokhirev, M. , et al. ( 2022). In vivo partial cellular reprogramming enhances liver plasticity and regeneration. Cell Rep 39, 110730. [CrossRef]
- Horvath, S. (2013). DNA methylation age of human tissues and cell types. Genome Biol 14, R115. [CrossRef]
- Horvath, S.; Lacunza, E.; Mallat, M.C.; Portiansky, E.L.; Gallardo, M.D.; Brooke, R.T.; Chiavellini, P.; Pasquini, D.C.; Girard, M.; Lehmann, M. , et al. (2024). Cognitive rejuvenation in old rats by hippocampal OSKM gene therapy. Geroscience. [CrossRef]
- Huang, W.; Hickson, L.J.; Eirin, A.; Kirkland, J.L.; Lerman, L.O. (2022). Cellular senescence: the good, the bad and the unknown. Nat Rev Nephrol 18, 611-627. [CrossRef]
- Jadhav, U.; Cavazza, A.; Banerjee, K.K.; Xie, H.; O'Neill, N.K.; Saenz-Vash, V.; Herbert, Z.; Madha, S.; Orkin, S.H.; Zhai, H. , et al. (2019). Extensive Recovery of Embryonic Enhancer and Gene Memory Stored in Hypomethylated Enhancer DNA. Mol Cell 74, 542-554 e545. [CrossRef]
- Kosar, M.; Bartkova, J.; Hubackova, S.; Hodny, Z.; Lukas, J.; Bartek, J. (2011). Senescence-associated heterochromatin foci are dispensable for cellular senescence, occur in a cell type- and insult-dependent manner and follow expression of p16(ink4a). Cell Cycle 10, 457-468.
- Kovatcheva, M.; Melendez, E.; Chondronasiou, D.; Pietrocola, F.; Bernad, R.; Caballe, A.; Junza, A.; Capellades, J.; Holguin-Horcajo, A.; Prats, N. , et al. ( 2023). Vitamin B(12) is a limiting factor for induced cellular plasticity and tissue repair. Nat Metab 5, 1911–1930. [CrossRef]
- Kriukov, D.; Kuzmina, E.; Efimov, E.; Dylov, D.V.; Khrameeva, E.E. (2024). Epistemic uncertainty challenges aging clock reliability in predicting rejuvenation effects. Aging Cell, e14283. [CrossRef]
- Lapasset, L.; Milhavet, O.; Prieur, A.; Besnard, E.; Babled, A.; Ait-Hamou, N.; Leschik, J.; Pellestor, F.; Ramirez, J.M.; De Vos, J.; et al. (2011). Rejuvenating senescent and centenarian human cells by reprogramming through the pluripotent state. Genes Dev 25, 2248-2253. [CrossRef]
- Li, H.; Collado, M.; Villasante, A.; Strati, K.; Ortega, S.; Canamero, M.; Blasco, M.A.; Serrano, M. (2009). The Ink4/Arf locus is a barrier for iPS cell reprogramming. Nature 460, 1136-1139.
- Li, H.Y.; Chien, Y.; Chen, Y.J.; Chen, S.F.; Chang, Y.L.; Chiang, C.H.; Jeng, S.Y.; Chang, C.M.; Wang, M.L.; Chen, L.K. , et al. (2011). Reprogramming induced pluripotent stem cells in the absence of c-Myc for differentiation into hepatocyte-like cells. Biomaterials 32, 5994-6005. [CrossRef]
- Liuyang, S.; Wang, G.; Wang, Y.; He, H.; Lyu, Y.; Cheng, L.; Yang, Z.; Guan, J.; Fu, Y.; Zhu, J. , et al. (2023). Highly efficient and rapid generation of human pluripotent stem cells by chemical reprogramming. Cell Stem Cell 30, 450-459 e459. [CrossRef]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. (2013). The hallmarks of aging. Cell 153, 1194-1217.
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. (2023). Hallmarks of aging: An expanding universe. Cell 186, 243-278. [CrossRef]
- Lu, Y.; Brommer, B.; Tian, X.; Krishnan, A.; Meer, M.; Wang, C.; Vera, D.L.; Zeng, Q.; Yu, D.; Bonkowski, M.S. , et al. ( 2020). Reprogramming to recover youthful epigenetic information and restore vision. Nature 588, 124–129. [CrossRef]
- Martinez-Lopez, N.; Athonvarangkul, D.; Singh, R. (2015). Autophagy and aging. Adv Exp Med Biol 847, 73-87.
- Meena, J.K.; Cerutti, A.; Beichler, C.; Morita, Y.; Bruhn, C.; Kumar, M.; Kraus, J.M.; Speicher, M.R.; Wang, Z.Q.; Kestler, H.A. , et al. (2015). Telomerase abrogates aneuploidy-induced telomere replication stress, senescence and cell depletion. EMBO J 34, 1371-1384.
- Miliotou, E.; de Lazaro, I. (2024). A Youthful Touch: Reversal of Aging Hallmarks by Cell Reprogramming. Cells Tissues Organs, 1-13.
- Mitchell, W.; Goeminne, L.J.E.; Tyshkovskiy, A.; Zhang, S.; Chen, J.Y.; Paulo, J.A.; Pierce, K.A.; Choy, A.H.; Clish, C.B.; Gygi, S.P. , et al. (2024). Multi-omics characterization of partial chemical reprogramming reveals evidence of cell rejuvenation. Elife 12.
- Mosteiro, L.; Pantoja, C.; Alcazar, N.; Marion, R.M.; Chondronasiou, D.; Rovira, M.; Fernandez-Marcos, P.J.; Munoz-Martin, M.; Blanco-Aparicio, C.; Pastor, J. , et al. (2016). Tissue damage and senescence provide critical signals for cellular reprogramming in vivo. Science 354. [CrossRef]
- Mosteiro, L.; Pantoja, C.; de Martino, A.; Serrano, M. (2018). Senescence promotes in vivo reprogramming through p16(INK)(4a) and IL-6. Aging Cell 17.
- Mylonas, A.; O'Loghlen, A. (2022). Cellular Senescence and Ageing: Mechanisms and Interventions. Front Aging 3, 866718. [CrossRef]
- Niimi, P.; Gould, V.; Thrush-Evensen, K.; Levine, M.E. (2024). The Latent Aging of Cells. bioRxiv.
- Ocampo, A.; Reddy, P.; Martinez-Redondo, P.; Platero-Luengo, A.; Hatanaka, F.; Hishida, T.; Li, M.; Lam, D.; Kurita, M.; Beyret, E. , et al. (2016). In Vivo Amelioration of Age-Associated Hallmarks by Partial Reprogramming. Cell 167, 1719-1733 e1712. [CrossRef]
- Ohnishi, K.; Semi, K.; Yamamoto, T.; Shimizu, M.; Tanaka, A.; Mitsunaga, K.; Okita, K.; Osafune, K.; Arioka, Y.; Maeda, T. , et al. ( 2014). Premature termination of reprogramming in vivo leads to cancer development through altered epigenetic regulation. Cell 156, 663–677. [CrossRef]
- Olova, N.; Simpson, D.J.; Marioni, R.E.; Chandra, T. (2019). Partial reprogramming induces a steady decline in epigenetic age before loss of somatic identity. Aging Cell 18, e12877. [CrossRef]
- Paine, P.T.; Rechsteiner, C.; Morandini, F.; Desdin-Mico, G.; Mrabti, C.; Parras, A.; Haghani, A.; Brooke, R.; Horvath, S.; Seluanov, A. , et al. ( 2023). Initiation phase cellular reprogramming ameliorates DNA damage in the ERCC1 mouse model of premature aging. Front Aging 4, 1323194. [CrossRef]
- Qian, Y.; Chen, X. (2013). Senescence regulation by the p53 protein family. Methods Mol Biol 965, 37-61.
- Roux, A.E.; Zhang, C.; Paw, J.; Zavala-Solorio, J.; Malahias, E.; Vijay, T.; Kolumam, G.; Kenyon, C.; Kimmel, J.C. (2022). Diverse partial reprogramming strategies restore youthful gene expression and transiently suppress cell identity. Cell Syst 13, 574-587 e511. [CrossRef]
- Sarkar, T.J.; Quarta, M.; Mukherjee, S.; Colville, A.; Paine, P.; Doan, L.; Tran, C.M.; Chu, C.R.; Horvath, S.; Qi, L.S. , et al. (2020). Transient non-integrative expression of nuclear reprogramming factors promotes multifaceted amelioration of aging in human cells. Nat Commun 11, 1545. [CrossRef]
- Shibata, H.; Komura, S.; Yamada, Y.; Sankoda, N.; Tanaka, A.; Ukai, T.; Kabata, M.; Sakurai, S.; Kuze, B.; Woltjen, K. , et al. (2018). In vivo reprogramming drives Kras-induced cancer development. Nat Commun 9, 2081. [CrossRef]
- Shimamoto, A.; Yokote, K.; Tahara, H. (2015). Werner Syndrome-specific induced pluripotent stem cells: recovery of telomere function by reprogramming. Front Genet 6, 10. [CrossRef]
- Takahashi, A.; Ohtani, N.; Yamakoshi, K.; Iida, S.; Tahara, H.; Nakayama, K.; Nakayama, K.I.; Ide, T.; Saya, H.; Hara, E. (2006). Mitogenic signalling and the p16INK4a-Rb pathway cooperate to enforce irreversible cellular senescence. Nat Cell Biol 8, 1291-1297. [CrossRef]
- Teshigawara, R.; Cho, J.; Kameda, M.; Tada, T. (2017). Mechanism of human somatic reprogramming to iPS cell. Lab Invest 97, 1152-1157. [CrossRef]
- Utikal, J.; Polo, J.M.; Stadtfeld, M.; Maherali, N.; Kulalert, W.; Walsh, R.M.; Khalil, A.; Rheinwald, J.G.; Hochedlinger, K. (2009). Immortalization eliminates a roadblock during cellular reprogramming into iPS cells. Nature 460, 1145-1148. [CrossRef]
- Waddington, C.H. (2014). The strategy of the genes (Routledge).
- Wang, C.; Rabadan Ros, R.; Martinez-Redondo, P.; Ma, Z.; Shi, L.; Xue, Y.; Guillen-Guillen, I.; Huang, L.; Hishida, T.; Liao, H.K. , et al. ( 2021). In vivo partial reprogramming of myofibers promotes muscle regeneration by remodeling the stem cell niche. Nat Commun 12, 3094. [CrossRef]
- Wang, G.; Wang, Y.; Lyu, Y.; He, H.; Liuyang, S.; Wang, J.; Sun, S.; Cheng, L.; Fu, Y.; Zhu, J. , et al. (2023). Chemical-induced epigenome resetting for regeneration program activation in human cells. Cell Rep 42, 112547. [CrossRef]
- Wang, L.; Su, Y.; Huang, C.; Yin, Y.; Chu, A.; Knupp, A.; Tang, Y. (2019). NANOG and LIN28 dramatically improve human cell reprogramming by modulating LIN41 and canonical WNT activities. Biol Open 8. [CrossRef]
- Wernig, M.; Meissner, A.; Cassady, J.P.; Jaenisch, R. (2008). c-Myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2, 10-12. [CrossRef]
- Xu, L.; Ramirez-Matias, J.; Hauptschein, M.; Sun, E.D.; Lunger, J.C.; Buckley, M.T.; Brunet, A. (2024). Restoration of neuronal progenitors by partial reprogramming in the aged neurogenic niche. Nat Aging 4, 546-567. [CrossRef]
- Yang, J.H.; Petty, C.A.; Dixon-McDougall, T.; Lopez, M.V.; Tyshkovskiy, A.; Maybury-Lewis, S.; Tian, X.; Ibrahim, N.; Chen, Z.; Griffin, P.T. , et al. ( 2023). Chemically induced reprogramming to reverse cellular aging. Aging (Albany NY) 15, 5966–5989. [CrossRef]
- Yousefzadeh, M.J.; Zhao, J.; Bukata, C.; Wade, E.A.; McGowan, S.J.; Angelini, L.A.; Bank, M.P.; Gurkar, A.U.; McGuckian, C.A.; Calubag, M.F. , et al. ( 2020). Tissue specificity of senescent cell accumulation during physiologic and accelerated aging of mice. Aging Cell 19, e13094. [CrossRef]
- Yucel, A.D.; Gladyshev, V.N. (2024). The long and winding road of reprogramming-induced rejuvenation. Nat Commun 15, 1941. [CrossRef]
- Zviran, A.; Mor, N.; Rais, Y.; Gingold, H.; Peles, S.; Chomsky, E.; Viukov, S.; Buenrostro, J.D.; Scognamiglio, R.; Weinberger, L. , et al. (2019). Deterministic Somatic Cell Reprogramming Involves Continuous Transcriptional Changes Governed by Myc and Epigenetic-Driven Modules. Cell Stem Cell 24, 328-341 e329. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




