1. Introduction
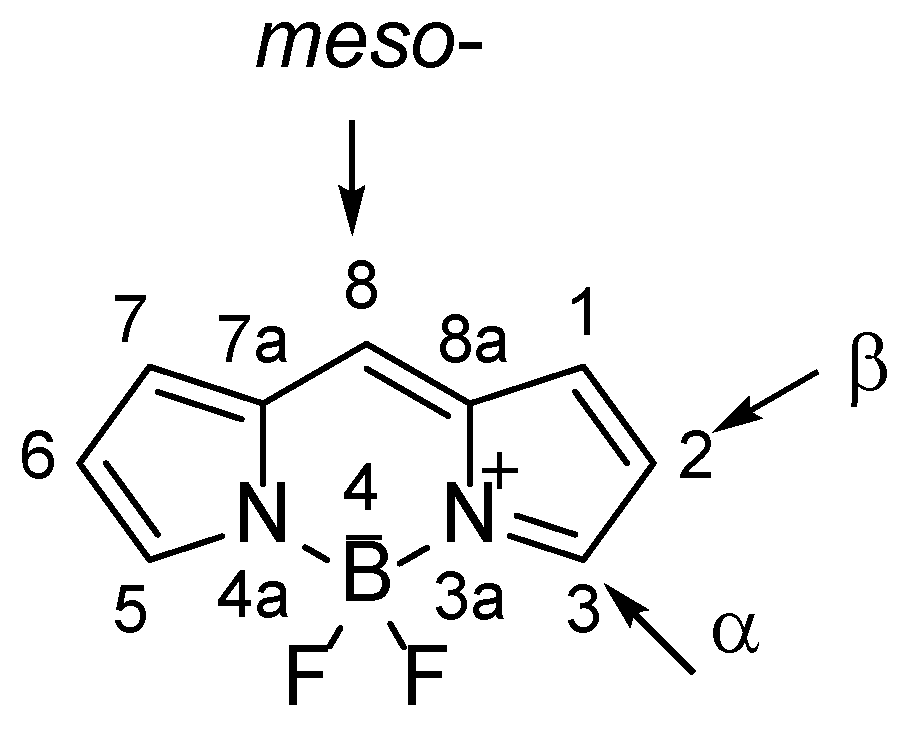
BODIPY is the commercial name for a class of heterocyclic fluorescent compounds with the IUPAC name 4,4-difluoro-4-bora-3a,4a-diaza-
s-indacene (
Figure 1) [
1], that were discovered by A. Treibs and F.H. Kreuzer in 1968 [
2]. To date they have received a significant scientific interest owing to their exceptional photophysical properties and widespread use in material science [
3], fluorescence sensing [
4], molecular biology and medicine where they have been used as fluorescent markers [
5] and phototherapeutics [
6]. Their use in different applications has been primarily enabled by their easy structural modifications which allow for tuning of spectral and photophysical properties [
7]. Consequently, the chemistry of BODIPY compounds has been reviewed on several occasions [
8], albeit the reviews on the reactions on boron in BODIPYs are scarce [
9].
BODIPY compounds generally show chemical and photochemical stability, good solubility in most organic solvents, low tendency to self-aggregation in solution and stability in physiological conditions. They are characterized by intense and narrow absorption bands in the visible part of the electromagnetic spectrum that correspond to the 0-0 transition and the population of the S
1 state, and a less pronounced shoulder attributed to the 0-1 vibrational transition [
4,
7,
8]. The absorption maxima,
λabs, are most often in the range 470–530 nm and they weakly depend on the polarity of solvent [
4,
7,
8]. The values of their molar absorption coefficients are high, in the range of 40000-110000 M
-1 cm
-1 [
4,
7,
8]. Most of them have large values of fluorescence quantum yields, with singlet excited state lifetimes of 1-10 ns, and negligible radiationless deactivation and the population of the triplet excited states [
10].
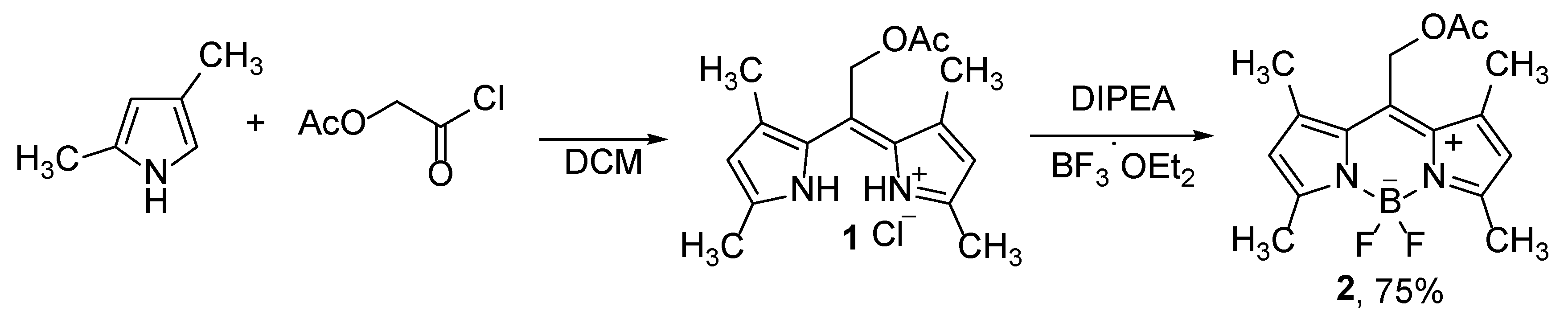
The synthesis of BODIPY derivatives is based on the preparation of dipyrromethenes, which are subsequently complexed with BF
3 in the presence of a base [
2,
8]. By using substituted pyrrole and suitable acid chloride, in the first steps of the synthesis, alkylated dipyrromethene
1 is prepared which is converted into BODIPY compound
2 (
Scheme 1) [
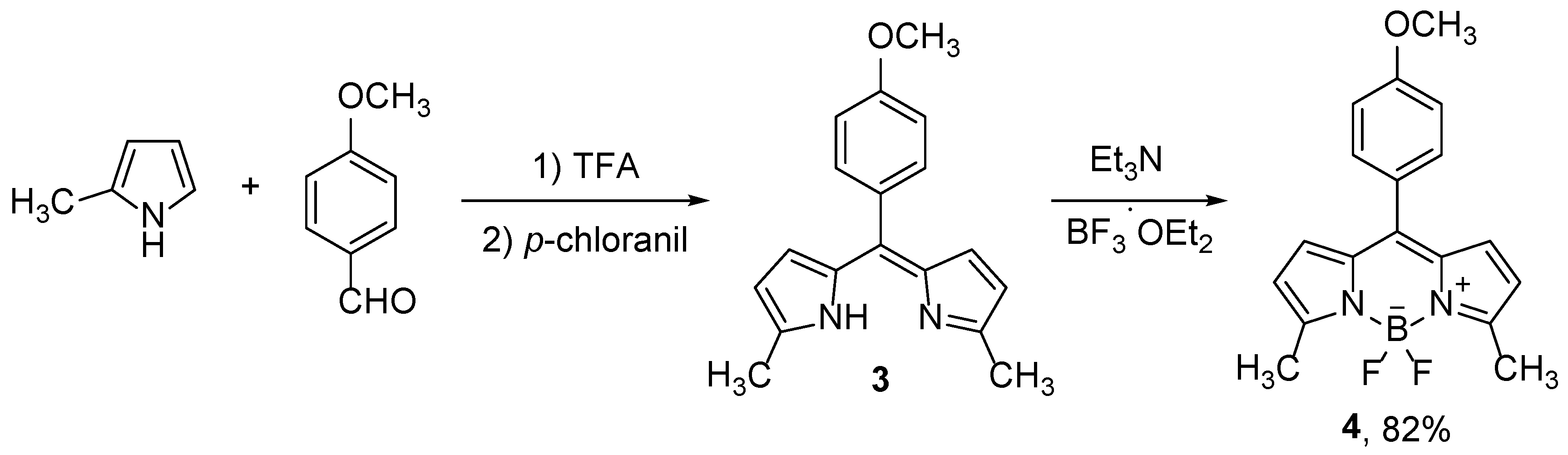
11]. The influence of the alkyl groups on spectroscopic and photophysical properties is negligible, but alkyl substituents are useful for further functionalization. The other often used procedure for the preparation of differently substituted dipyrromethenes is based on the MacDonald method, from pyrrole and pyrrole carbaldehyde derivatives, usually in the presence of acid such as HBr [
12,
13]. Derivatives with the desired aryl groups at the position 8 are easily prepared by choosing an appropriate aromatic aldehyde in the synthesis of dipyrromethane [
14], which are subsequently oxidized, typically with DDQ or
p-chloranil (
Scheme 2) [
8,
15]. Substituents in the
meso-position do not have a significant effect on the wavelengths of absorption and emission maxima, but they can modify the fluorescence quantum yields [
4,
8]. If the aromatic ring additionally contains electron-donating groups such as tertiary amines, or electron-withdrawing groups such as nitro- or cyano-groups, additional photophysical processes in the excited state, such as photoinduced electron transfer (PET) or charge transfer (CT), affect the fluorescence [
4]. Furthermore, phenyl groups in the
meso-position greatly reduce fluorescence quantum yields if their rotation is not sterically hindered [
16], which was applied in the development of molecular rotors – fluorescent probes for microviscosity and temperature [
17].
2. Structural modifications of BODIPY chromophore
The great advantage of BODIPY compounds compared to other fluorophores is a large number of possible synthetic modifications of the chromophore, which lead to the changes of spectral and photopysical properties [
7]. It is possible to introduce the desired substituents and carry out reactions on the entire chromophore – pyrrolic carbon atoms,
meso-carbon and the boron atom.
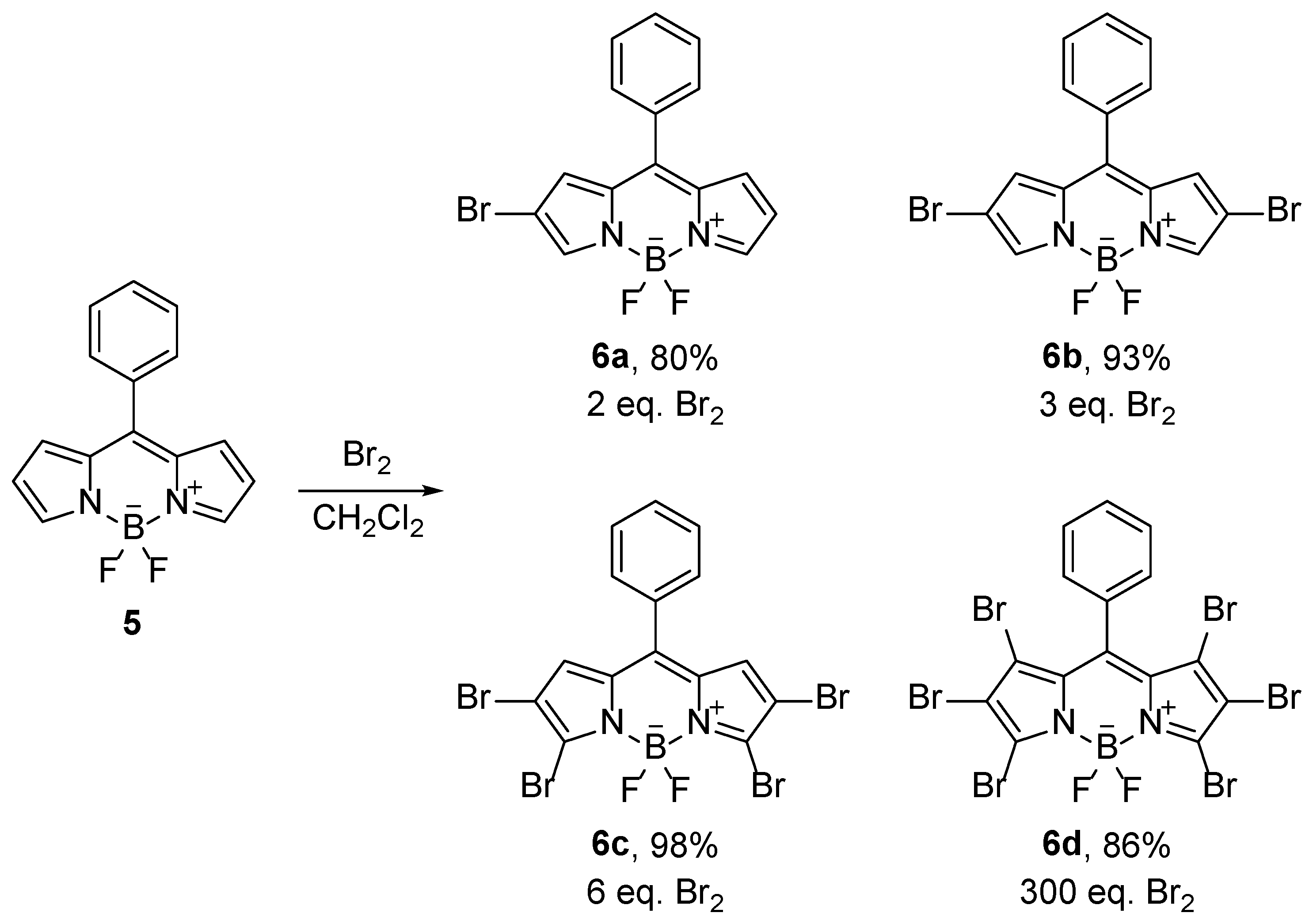
A frequent modification of BODIPY compounds is the introduction of halogen atoms as reactive groups. Halogenated derivatives are suitable because they enable the introduction of target functional groups in the late steps of the synthesis [
18]. A large number of halogenation reactions have been described: bromination with elemental bromine [
19],
N-bromosuccinimide [
20], and CuBr
2 [
21], chlorination with
N-chlorosuccinimide [
22], and CuCl
2·2H
2O [
23], iodination with ICl [
24] and I
2/HIO
3 [
25], or
N-iodosuccinimide [
26]. The electrophilic halogenation of compound
5 with bromine first takes place at the positions 2- and 6-, and then 3- and 5-, and finally at positions 1- and 7- (
Scheme 3). On the other hand, the use of copper halogenides gives rise to selective halogenation. Thus, CuBr
2 yields the brominated positions 2 and 6, whereas by use of CuCl
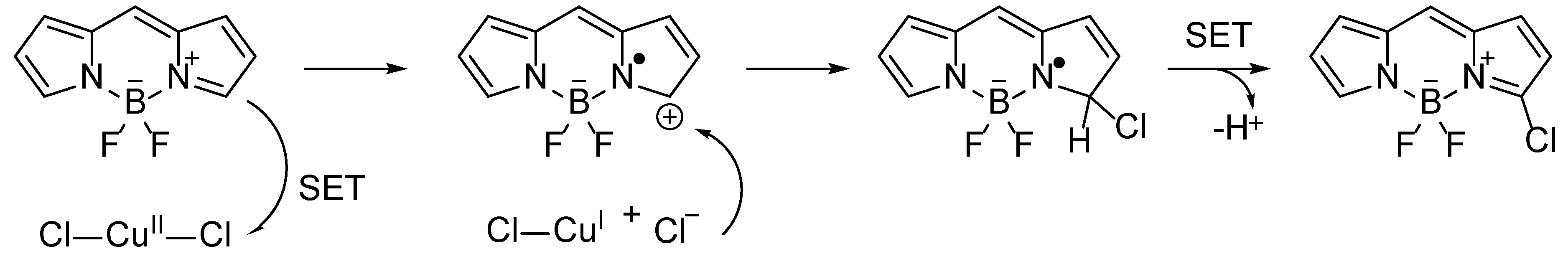
2, chlorines enter the positions 3 and 5. The difference in regioselectivity is due to different reaction mechanism in the case of CuCl
2, where single-electron transfer takes place (
Scheme 4).
The presence of bromine or iodine atoms in the BODIPY structures increases the rate of intersystem crossing due to greater spin-orbital coupling owing to the heavy atom effect, and consequently, there is a decrease in fluorescence quantum yields [
27]. The population of triplet excited state enables the generation of singlet oxygen, which is the main condition for the use of BODIPY compounds in photodynamic therapy where the iodo-derivatives show the greatest potential [
6]. Moreover, halogenated BODIPY derivatives can undergo reactions characteristic of halogenated aromatic heterocycles, such as nucleophilic aromatic substitution (
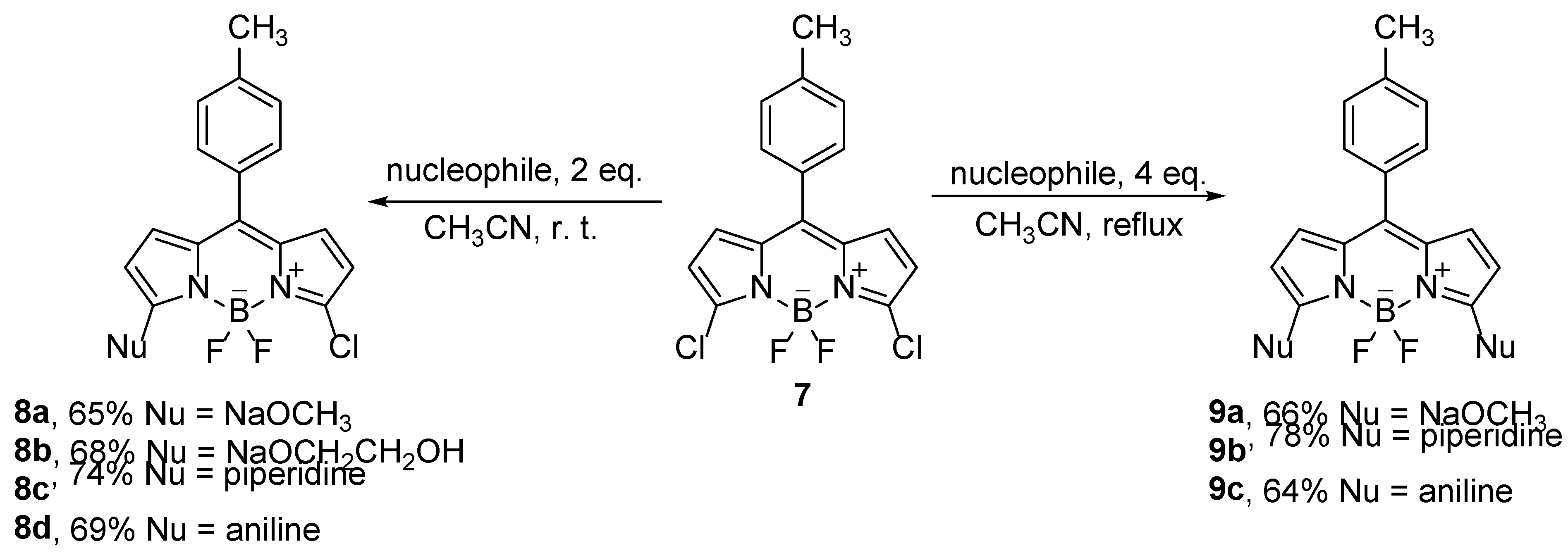
Scheme 5). Substitution reactions can be carried out with carbon, oxygen and nitrogen nucleophiles [
28].
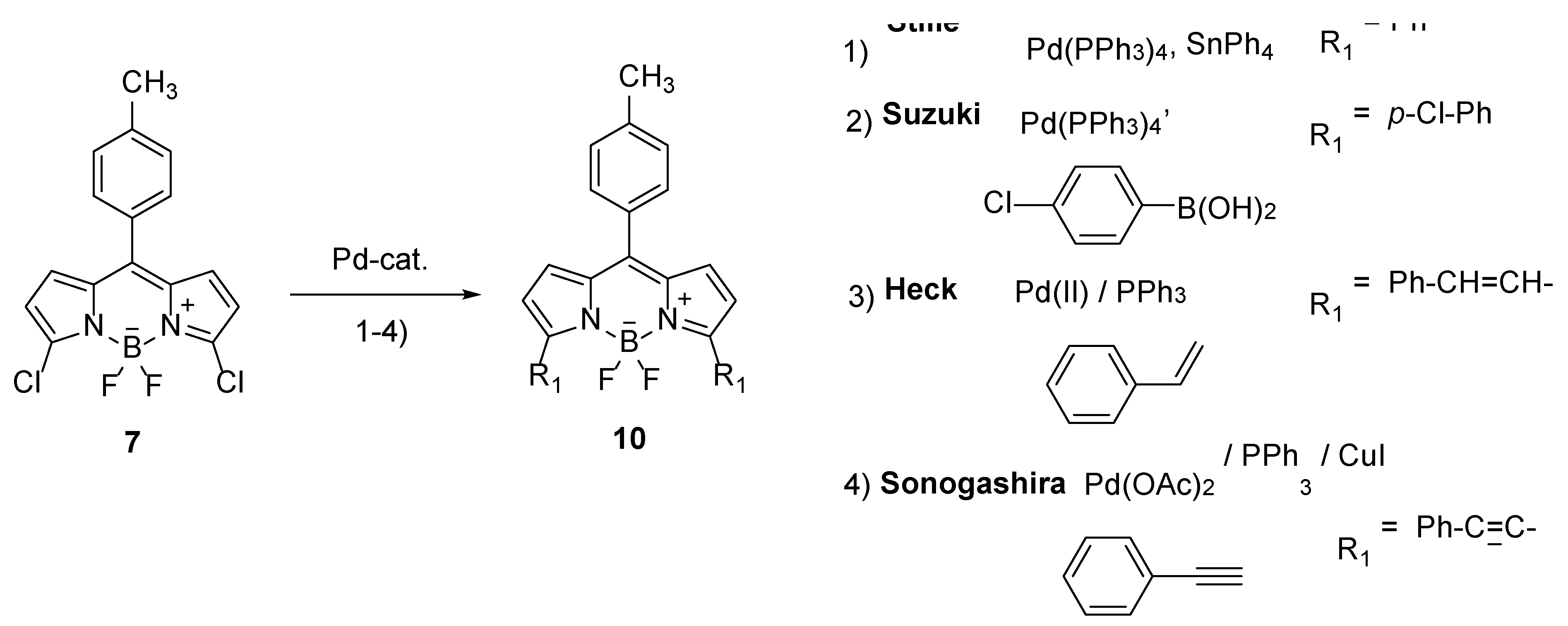
Halogenated BODIPY compounds, including chloro-derivatives, undergo palladium-catalyzed cross-coupling reactions, including - Suzuki, Stille, Heck and Sonogashira reactions (
Scheme 6) [
18,
29], which have been extensively used to enlarge chromophoric system and shift it bathochromically to red and infrared regions [
7,
8]. The smallest bathochromic shift in the absorption spectrum is caused by a phenyl substituent, and the largest by a styryl substituent. Furthermore, alkenyl derivatives are more often prepared from BODIPY compounds bearing methyl substituents by Knoevenagel reactions with aromatic aldehydes (
Scheme 7) [
30]. It is interesting to note that BODIPY compounds with alkenyl substituents generally have high fluorescence quantum yields and long singlet excited state lifetimes, even though it is known that alkenes very effectively deactivate by photochemical
E-
Z isomerization [
31]. Namely, the excitation of alkenyl BODIPY derivatives to S
1 does not lead to a decrease in the electron density between the C-atom of the double bond, which would result in the torsional relaxation on the S
1 surface [
32].
3. Reactivity of BODIPY compounds on boron
As BODIPY is the name for derivatives of 4,4-difluoro-4-bora-3a,4a-diaza-
s-indacene, it is understood that two fluorine atoms are attached to the boron atom in the structures. However, reactions have been developed by which it is possible to derivatize the 4- position, that is, the substitution reactions of fluorine on the boron atom have also been developed [
7,
9]. It is known that the substitution of fluorines affects the fluorescence quantum yields, chemical and photochemical stability, solubility, and redox properties, all without significant effects on the wavelengths of absorption and emission maxima [
7,
9,
34]. Most BODIPY compounds are poorly soluble in polar solvents, especially water. Because of the planarity of the molecules, they aggregate due to π-π stacking [
35]. By introducing hydrophilic, electronically charged or sterically large groups on the boron atom, which has a tetrahedral geometry, it is possible to effectively increase solubility and reduce aggregation [
36].
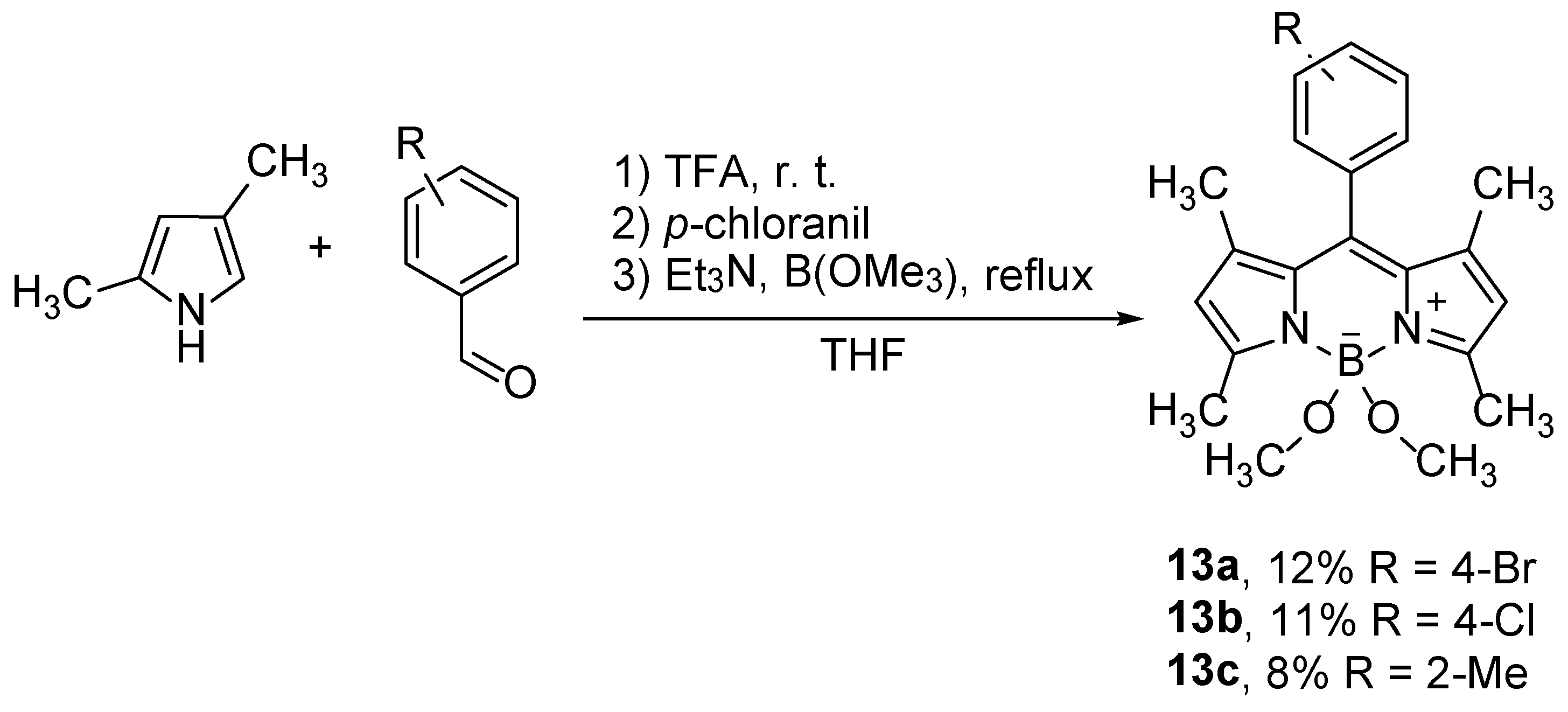
BODIPY compounds substituted on the boron can be prepared already in the first steps of the synthesis. The BODIPY prepared from 2,4-dimethylpyrrole and aromatic aldehydes, with subsequent oxidation with
p-chloranil to dipyrromethene and the treatment with triethylamine and trimethylborate as Lewis acid (instead of BF
3), gave compounds
13 in moderate yields (
Scheme 8) [
37].
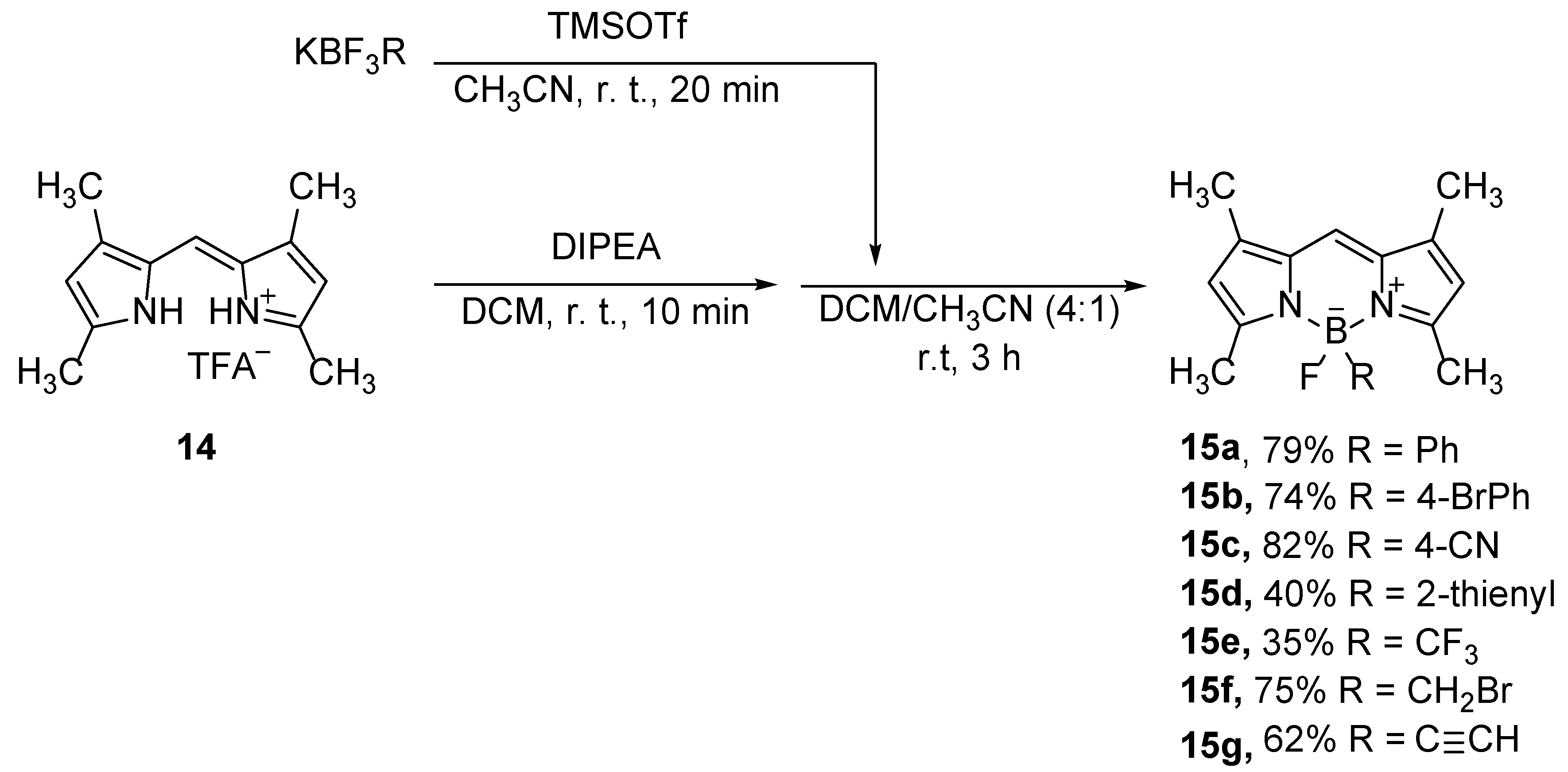
Better yields on B-substituted products, and wider scope of the B-substituted substrates can be obtained in the reactions of dipyrromethenes with potassium salts of B-substituted trifluoroborates (
Scheme 9) [
38]. The reaction afforded B-monoalkylated or monoarylated compounds
15 in moderate to good yields, as well as perfluoroalkyl derivatives, which were used in the live cell imaging [
39] or inhibition of tau amyloid formation [
39].
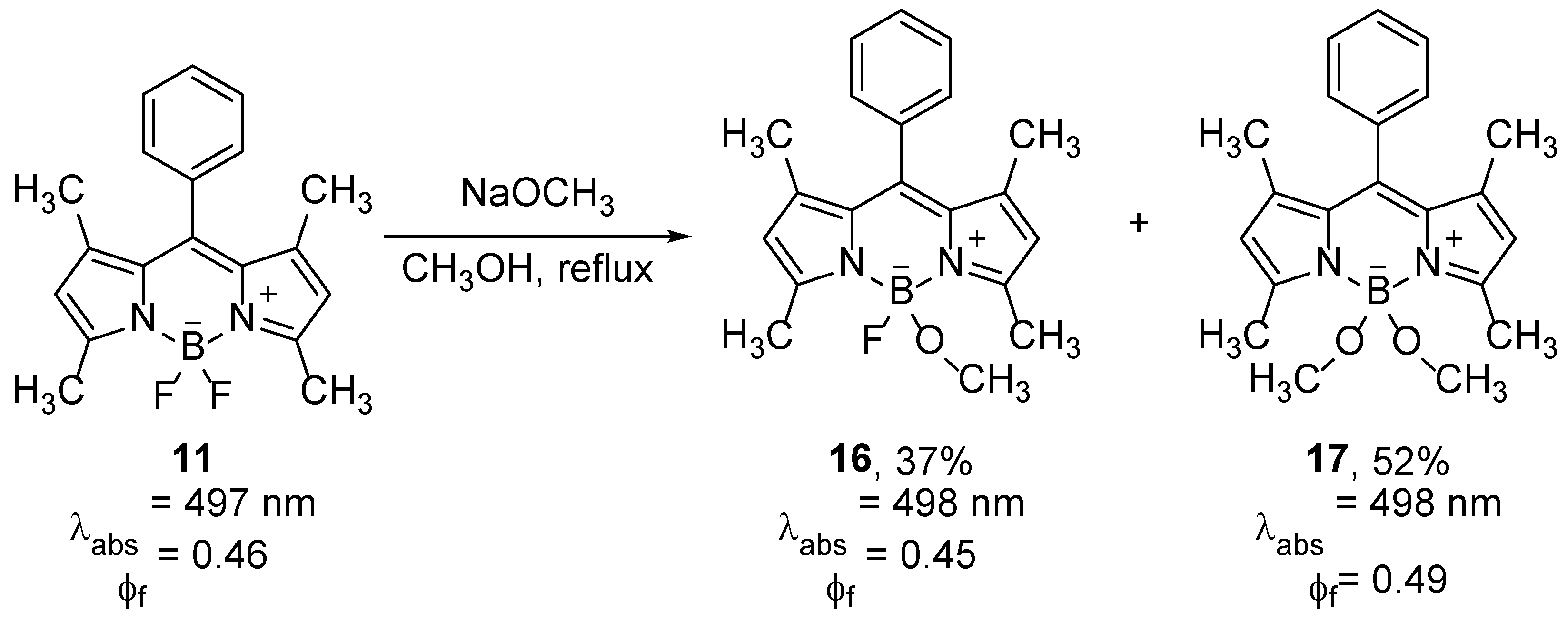
Oxygen nucleophiles can substitute fluorine at the boron atom under basic conditions, often at high temperatures. Thus, the reaction of compound
11 with sodium methoxide in methanol gave a mixture of compounds
16 and
17 (
Scheme 10) [
40]. The BODIPY compounds with alkoxy substituents at the boron have similar spectroscopic properties as the unsubstituted BF
2 compound, but their oxidation potentials differ. By replacing the more electronegative fluorine with a methoxide group, the inductive effect of fluorine is weakened, and the oxidation potentials have lower values by about 0.1 V per OCH
3 group. Therefore, the substitutions on boron have potential to tune properties of molecules for PET [
40].
Fluorine substitution reactions can also be conducted with sterically demanding nucleophiles, e.g. potassium
tert-butoxide, but they are often limited to monosubstitution and have low reaction yields [
41]. The use of a large excess of nucleophiles in the reaction does not give better yields on the substitution products, but results in the elimination of BF
2 and release of the free dipyrromethenes.
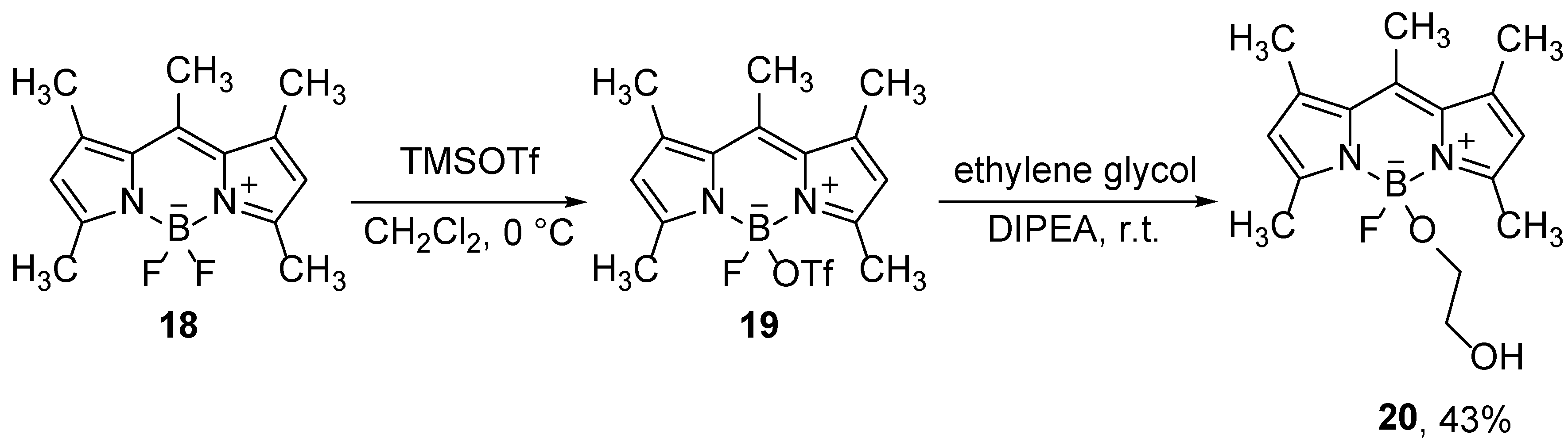
3.1. Lewis acid promoted formation of the B-O bonds
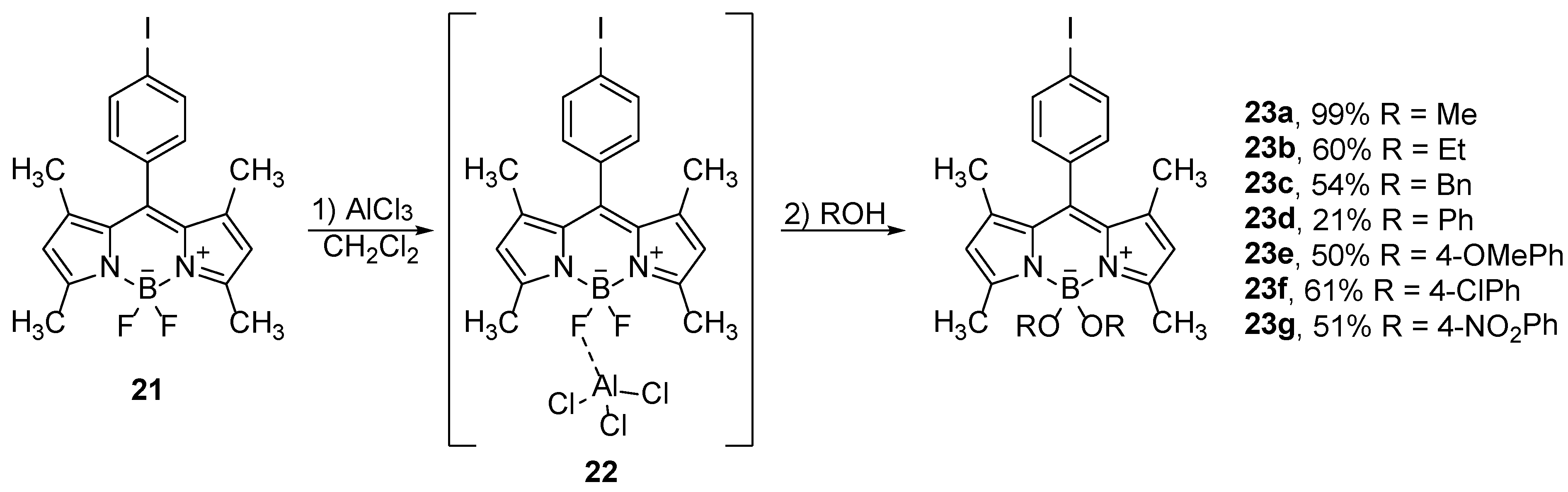
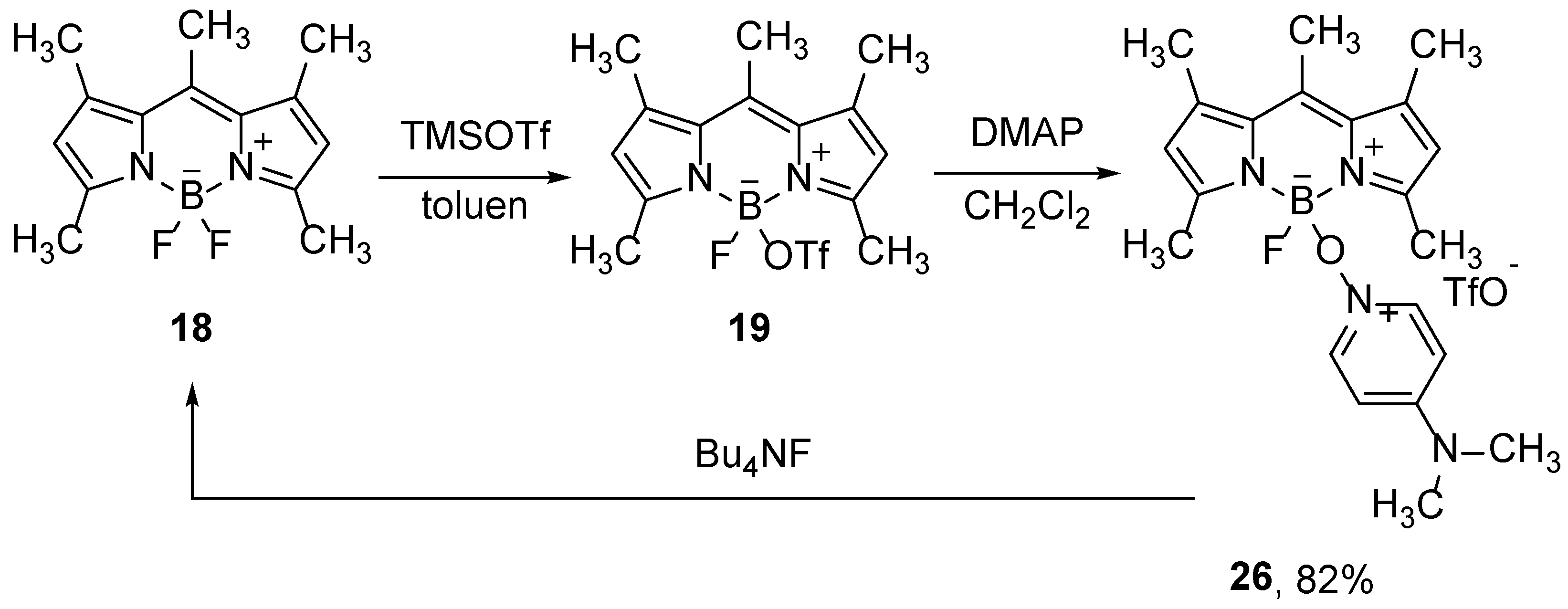
The use of Lewis acids, which interact with the boron atom or fluorines in BODIPY compounds, allows for the substitution of fluorine with different groups. Thus, Mazitschek and co-workers developed a two-step one-pot method for the synthesis of a series of 4-alkoxy-BODIPY derivatives. Selective monosubstitution is carried out on BODIPY
18 by use of trimethylsilyl-trifluoromethanesulfonate (TMSOTf), which binds to boron and forms an activated intermediate
19, which reacts with alcohols (
Scheme 11). The intermediate product is not stable under the reaction conditions and dissociates slowly, forming free dipyrromethene, so an alcohol must be added to the reaction mixture at the appropriate time [
42].
A convenient substitution method for the activation of the B–F bonds is based on the use of AlCl
3, which proved to be compatible with a large number of functional groups. Such a synthetic protocol was used for the preparation of 4,4-dialkoxy and 4,4-diaryloxy-BODIPY compounds
23 in the reactions with alcohols and phenolic derivatives (
Scheme 12). The reaction conditions were tolerant for the aldehydes, esters or amino groups [
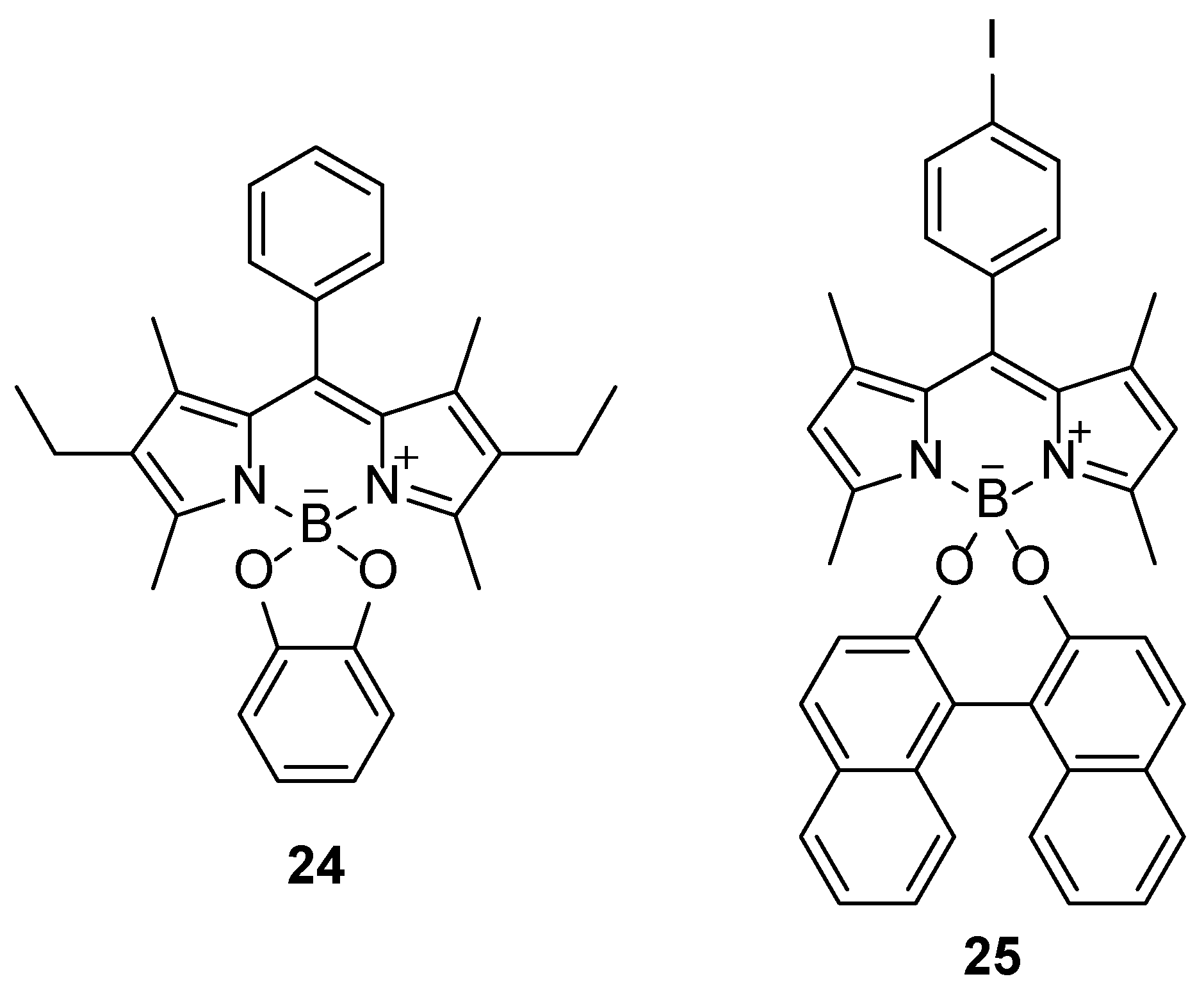
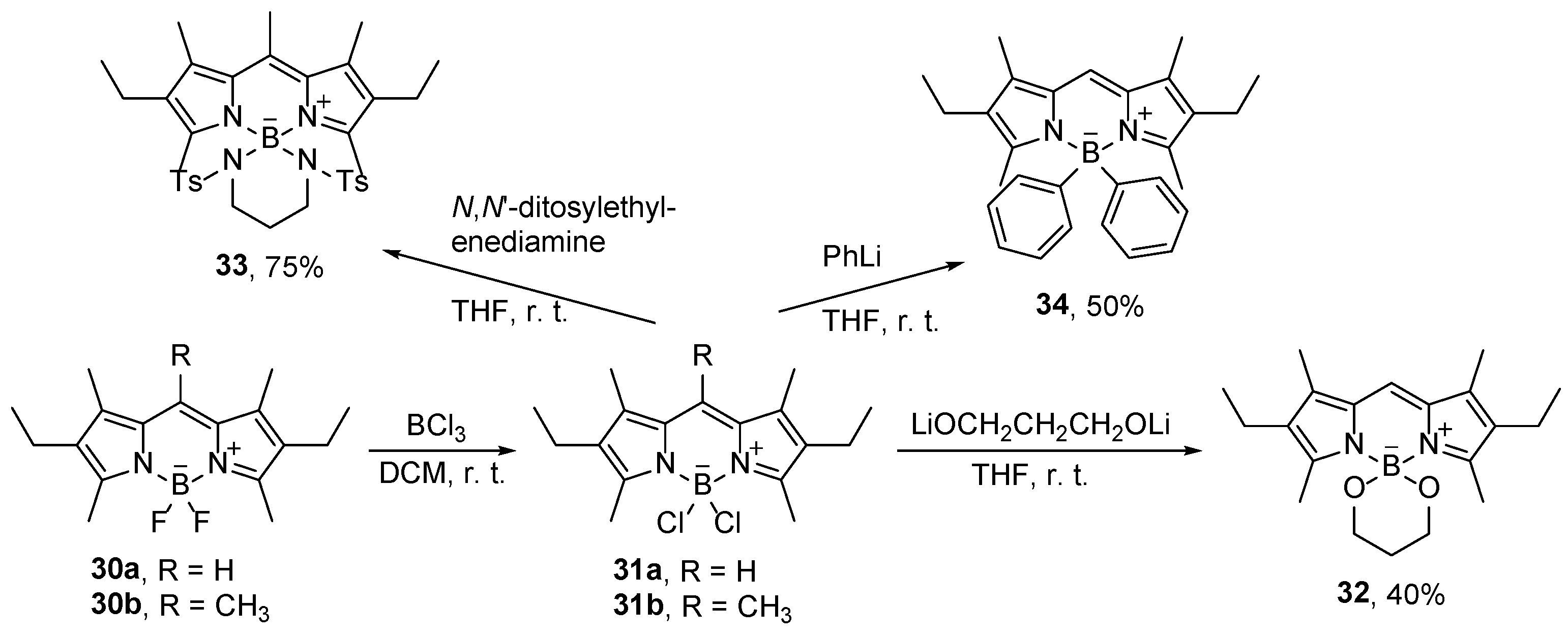
43]. If dialcohols or dihydroxybenzene derivatives such as catechol are used for the substitution, both fluorine atoms can be substituted giving cyclic derivatives (
Figure 2) [
44]. Furthermore, the authors reported that the fluorescence of the catechol derivative
24 (
Figure 2) was quenched, by very efficient PET from the catechol unit to the BODIPY chromophore, which can be restored by substitution of the catechol by methoxides.
Nonfluorescent alkoxy BODIPY derivatives, such as
26, can be used as a PET sensor for fluoride anion. In the presence of F
-, the parent BODIPY compound
18 is restored, which results in a significant increase in fluorescence (
Scheme 13) [
45].
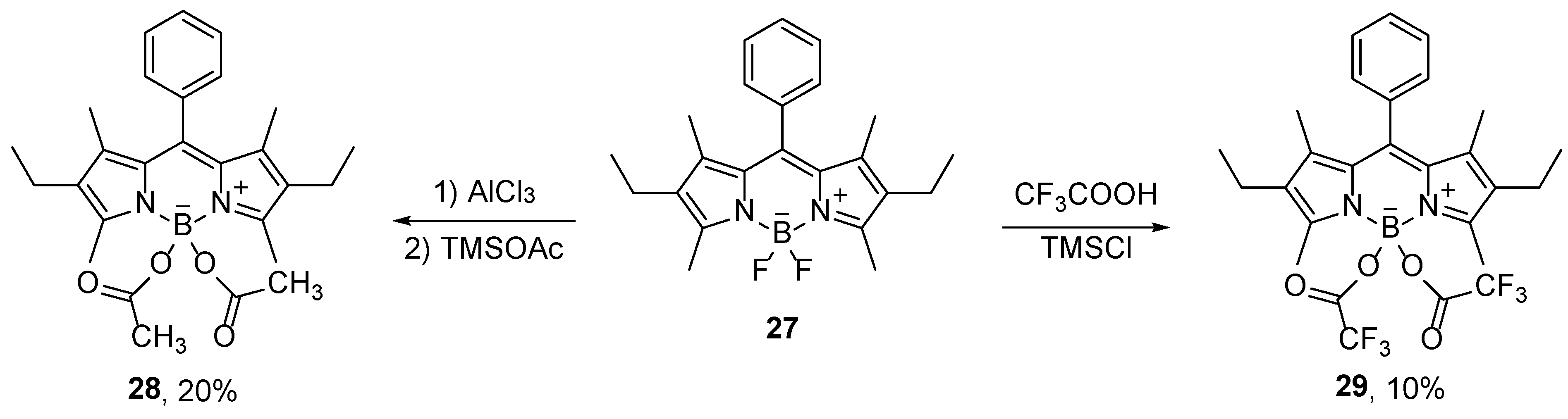
In addition to alcohols, carboxyl groups can also be attached to the boron atom. Substitutions with carboxyl groups are carried out using trimethylsilyl carboxylates generated in situ from carboxylic acids and trimethylsilyl chloride [
46]. Thus, 4,4-diacetoxy-BODIPY compounds were prepared in good yields in the reactions with trimethylsilyl acetate in the presence of AlCl
3 as a Lewis acid (
Scheme 14) [
46]. The acyloxy groups are located perpendicular to the plane defined by the planar BODIPY structure. Therefore, this substitution does not affect the absorption and emission maxima, but it significantly improves the solubility in polar solvents [
47].
3.2. Reactivity with BCl3 and subsequent reactions
A. Thompson and co-workers described reactivity of BODIPY compounds with BCl
3 [
48]. The addition of boron trichloride to the solutions of BODIPY
30 quantitatively results in the formation of 4,4-dichloro-BODIPY compound
31 (
Scheme 15). The chlorinated BODIPY derivatives are very reactive compounds, but can be isolated under inert conditions. Fluorescence quantum yields of the chlorinated derivatives are lower than fluorinated analogues, plausibly due to the heavy atom effect. The lower strength of the B–Cl bonds makes them more susceptible to nucleophilic attacks and enables fast and efficient substitutions with C-, N- or O-nucleophiles (
Scheme 15) [
48,
49].
The use of BCl
3 can also lead to the elimination of BF
2 from BODIPY and formation of the free base dipyrromethene [
50]. Such a strategy was used to prepare natural compounds prodigiosene derivatives
37 (
Scheme 16) [
51].
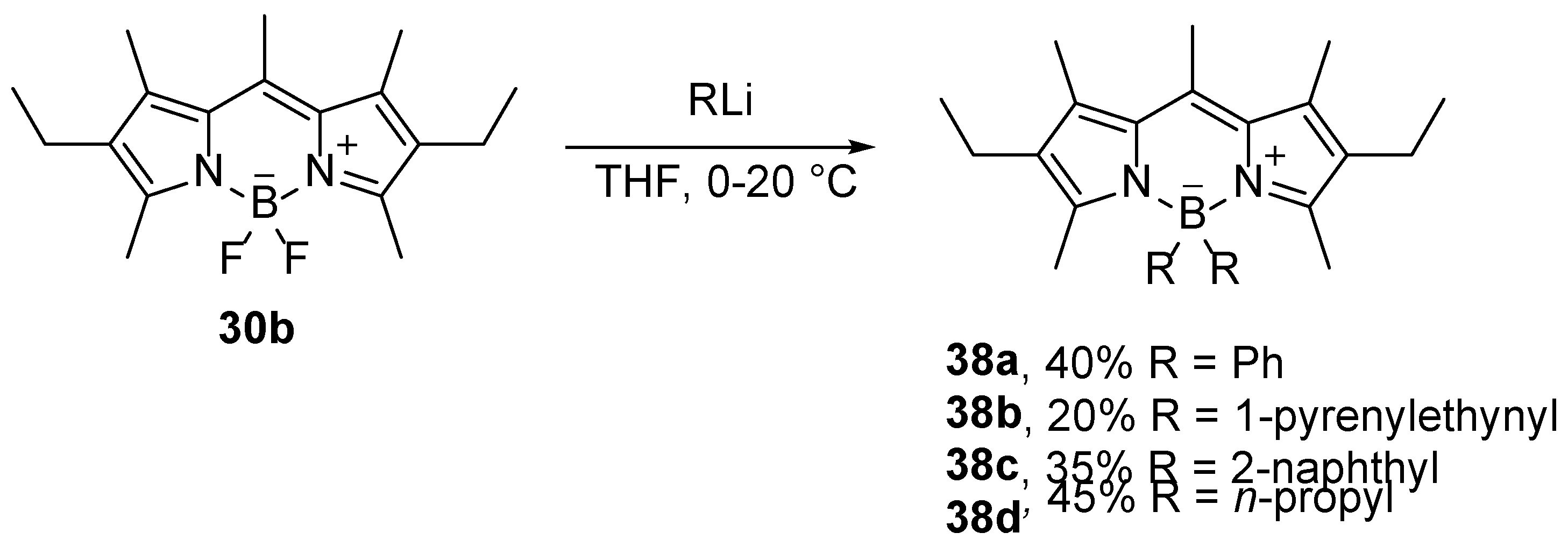
3.3. Organometallic alkylation and arylation
Strong carbon nucleophiles, such as organolithium compounds, attack the boron center of BODIPY compounds and substitute the fluorine atoms. Such a methodology was used for the synthesis of 4-alkyl [
52], 4-aryl [
52,
53], and 4-alkynyl [
52,
54] BODIPY derivatives (
Scheme 17). Organolithium reagents enable efficient substitution even under mild reaction conditions, but due to vigorous reactivity, monosubstituted derivatives cannot be formed [
52].
The absorption spectrum of the disubstituted compound
38b is similar to the overlapped absorption spectra of the BODIPY chromophore in
30b and the pyrenylalkyne, indicating no electron delocalization over the boron center. Furthermore, the photoexcitation of the pyrenylalkynyl groups gives rise to the emission from the BODIPY fluorophore. A good overlap of the S
0→S
2 bands of the BODIPY absorption and the pyrene emission enables efficient Förster resonance energy transfer (FRET) from the pyrene to the BODIPY [
54].
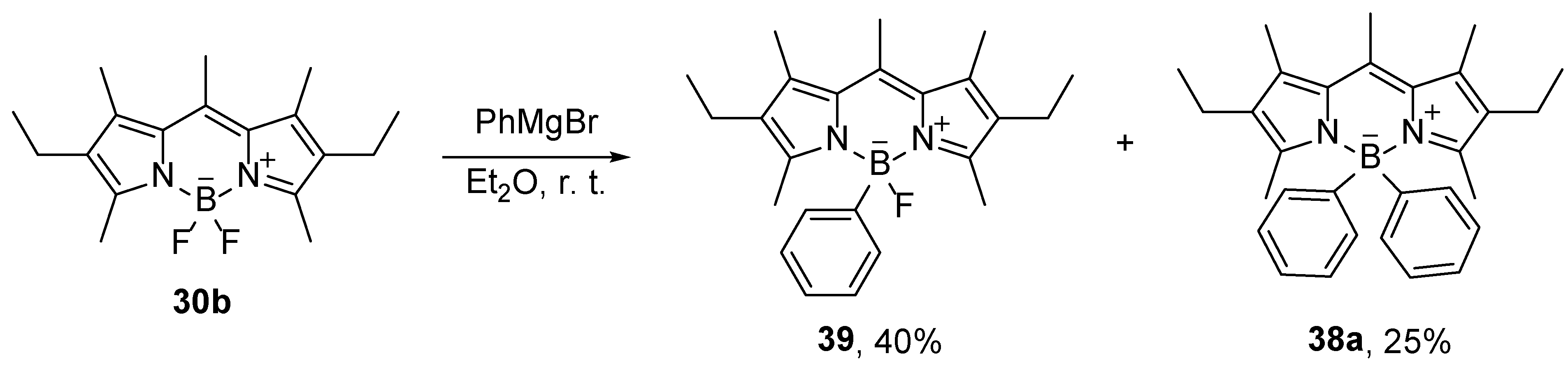
4-Monosubstituted BODIPY compounds were prepared with less reactive Grignard reagents [
53]. The reaction of compound
30b with phenylmagnesium bromide at 0 ℃ produces monosubstituted compound
39 in 40% yield (
Scheme 18) [
53]. At higher temperatures and larger amounts of the Grignard reagents, the yields on monosubstituted products decrease, while the disubstituted products are formed.
4. Photochemical reactivity of BODIPY compounds on boron
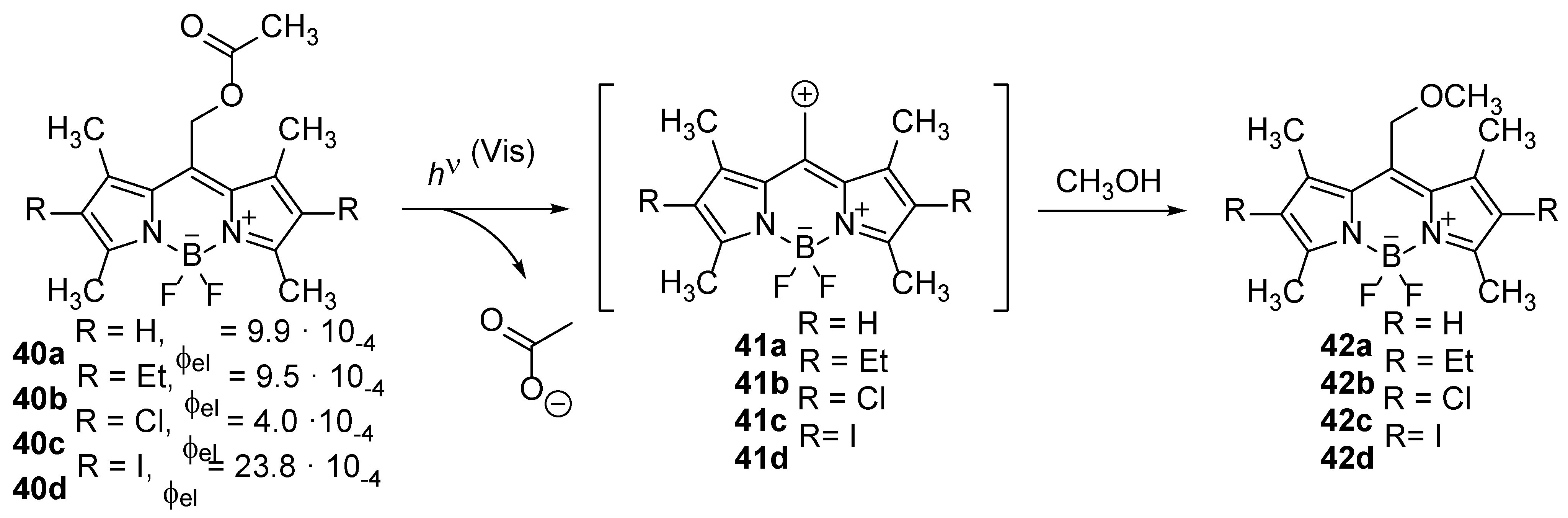
The number of described BODIPY compounds to date is very large, and most of them are characterized by exceptional photochemical stability. Therefore, the number of reports on photochemically reactive BODIPY compounds is limited, which are mostly connected to the development of photo-cleavable protective groups, known as photocages [
55] Photocages are used in biological research because they enable the manipulation of the activity of covalently bound substrates of interest and their temporally and spatially controlled release. While the appropriate functional group of the biologically active substrate is bound to the photocage, its biological activity is disabled, and it is activated by the release of the substrate after photoexcitation [
56]. Weinstein, Winter, Klan and Slanina synthesized a number of BODIPY compounds
40 which are photo-cleavable at the
meso-methy group (
Scheme 19) [
57,
58]. The advantage of BODIPY photocages compared to most organic chromophores is that the release of the substrate can be triggered by visible light, which is not harmful to healthy tissues and has a greater ability to penetrate through the tissue. They devoted a significant endeavor to elucidate the reaction mechanism and it was proposed that the reaction mostly takes place via the triplet excited states and proceeds via the BODIPY carbocation, which is in its triplet ground state. The efficiency for the elimination depends on the groups on BODIPY which facilitate the intersystem crossing and stabilize the carbocation and the leaving groups [
34,
57,
58].
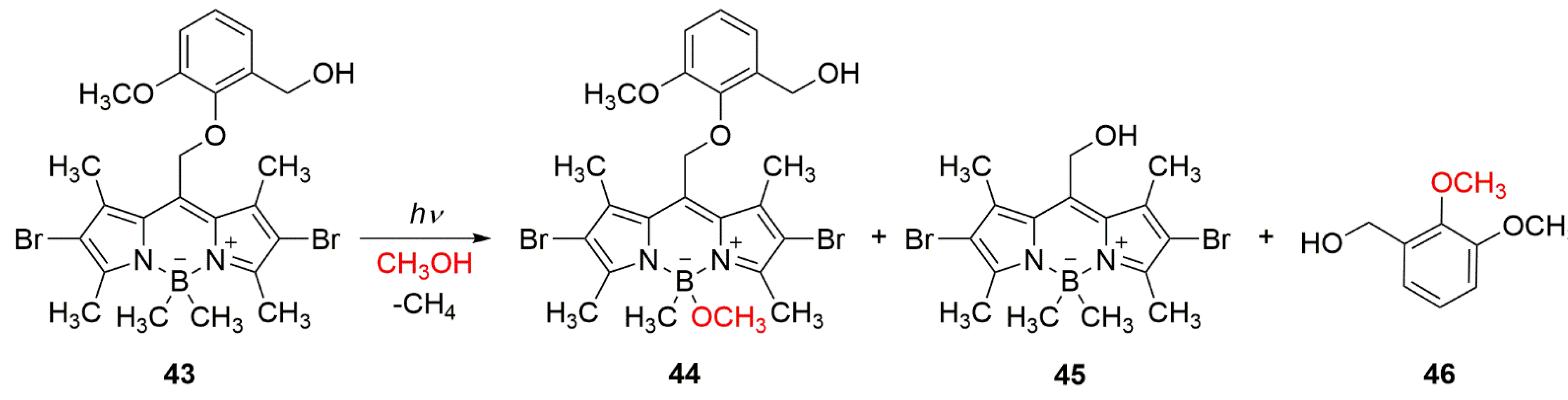
When the BODIPY photocage has a poor leaving group at the
meso-methyl position, such as phenoxyl, the cleavage at the boron competes with the elimination of the group from the
meso-position. Thus, upon irradiation of
43, two competing photoreactions take place (
Scheme 20) [
60].
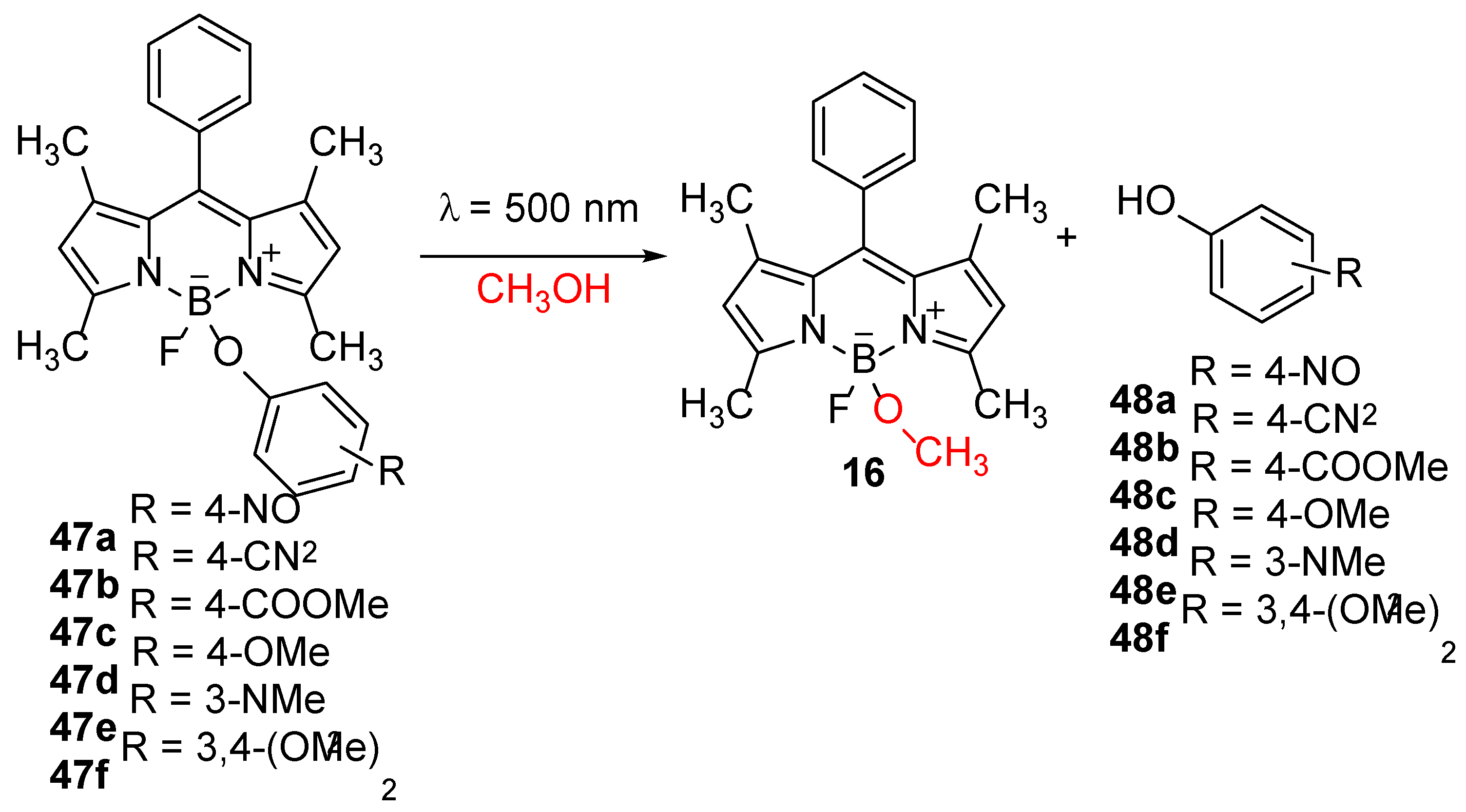
The photocleavage at the boron atom was also reported by Y. Urano et al. Upon excitation by visible light BODIPY compounds
47 with phenolic substituents on the boron atom underwent photoelimination reactions of the phenolic group (
Scheme 21) [
61]. The authors reported that the fluorescence quantum yields of these derivatives decrease with the increase in the energy of the HOMO orbitals of the phenolic groups, which they explained by PET from the phenolic groups to the BODIPY chromophore. It was assumed that photoexcitation gave rise to the radical cation of the phenolic groups and the radical anion of the BODIPY fluorophore, which were followed by the subsequent solvolysis of the B–O bonds [
61].
The parameters affecting the elimination of phenolic groups from boron were additionally investigated. It was demonstrated that the efficiency of PET can be affected by the substitution of the BODIPY at the 2- and 6-positions or by substitution of the
para-position of the phenol [
62]. PET is more efficient if there are electronegative substituents at the positions 2 and 6 of the BODIPY and if phenols bear electron-donating substituents in the
para-position. Furthermore, the efficiency of the elimination depends on the polarity of the solvent, and is higher in non-polar solvents.
Photochemically reactive BODIPY compounds on boron were also used as self-immolative molecules, first described by J. A. Katzenellenbogen and co-workers [
63]. Such systems consist of reactive carriers, scavenging links and substrates. The carriers can react with acids and bases, enzymes, or they can be excited by light and then release the linker and substrate. The reasons for the irreversible self-destruction of the linker are the increase in entropy of the system and the formation of thermodynamically stable products [
64]. The most commonly used linkers are structures containing an aromatic ring with an electron-donating group (hydroxy-, amino- or thiol) which is in the
ortho- or
para-conjugation with a leaving group attached to the benzylic position of the ring. The presence of the electron-donating groups is necessary in order to reduce the energy barrier of dearomatization, and to form reactive intermediates: quinone methides, azaquinone methides and tiaquinone methides. The destruction of the linker leads to the final release of the substrate and enables its biological actions [
64].
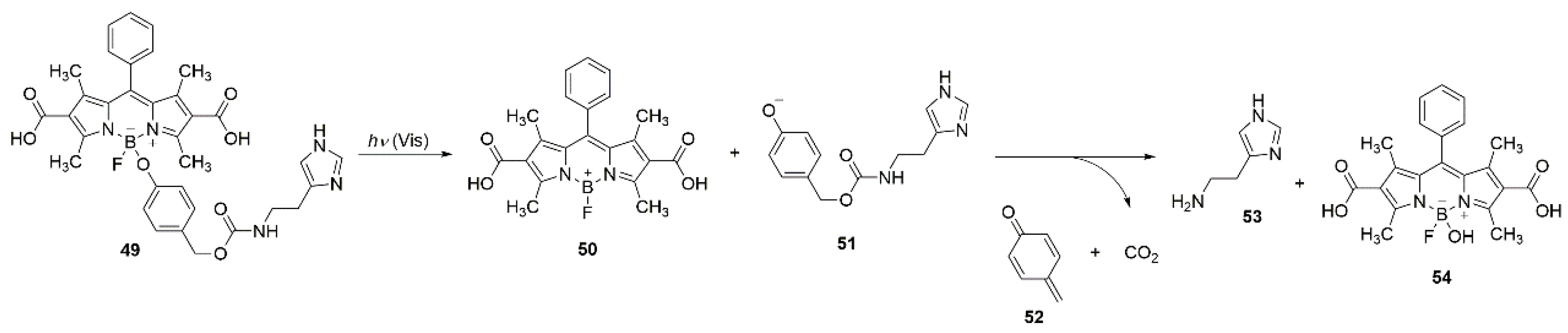
An example of a self-immolative system, which is based on the photoreactivity of BODIPY on boron is molecule
49. The fluorophore is a reactive carrier to which the biologically active substrate histamine is bound by a
para-hydroxybenzyloxycarbonyl self-destructing linker. The irradiation of
49 with visible light in living cells results in the release of phenol
51, which then decomposes into histamine (
53), CO
2 and quinone methide
52 (
Scheme 22) [
61].
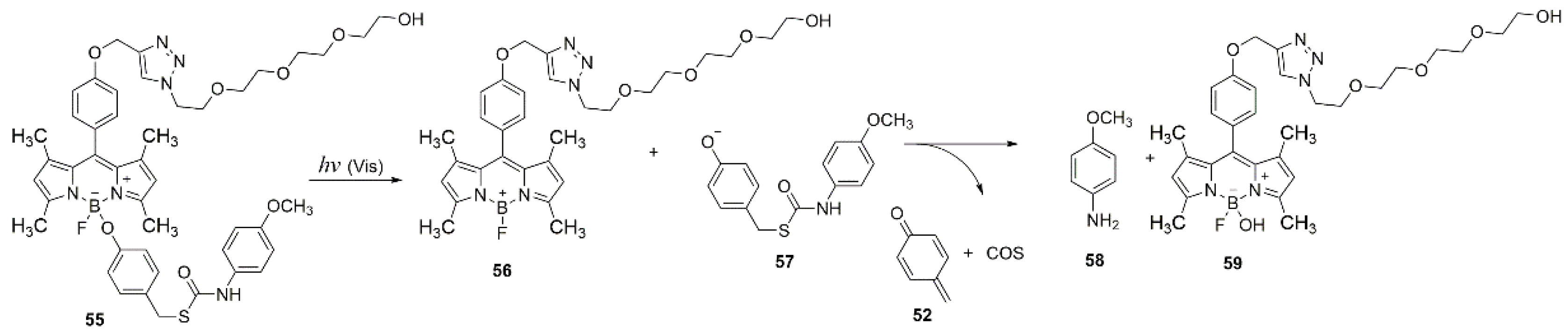
A similar approach for the photo-release in living cells is based on the photoreaction on boron of BODIPY compound
55. It was used for the controlled release of COS, which is then decomposed to H
2S (
Scheme 23) [
65].
5. Summary and Perspective
This mini-review article highlights reactions on the boron atom in BODIPY compounds, which hitherto have not received significant attention. However, structural modification on the boron atom allow for tuning of photophysical properties and different applications of new chromophoric systems. The reactions on the boron can be conducted under basic conditions, with strong nucleophiles such as alkoxides or organometallic reagents. Furthermore, the use of Lewis acids, which complex with the BF2 moiety, allow for different substitution protocols of the fluorines by nucleophiles. The use of BCl3 opens further avenues for the transformations, which include decomplexation and formation of the free dipyrines, the formation of the BCl2 complexes and subsequent substitutions with N- C- or O-nucleophiles. The photochemical reactivity on boron is also possible and the photo-solvolysis reactions open opportunities for the development of new photocages and tools for photo-pharmacology. Consequently, the modifications of the BODIPY position 4 has yet to flourish, opening new horizons for further development of BODIPY chromophore in different scientific disciplines.
Author Contributions
writing—original draft preparation, M.B.; writing—review and editing, all; supervision, I.Š. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Croatian Science Foundation (HRZZ grant no. HRZZ-IP-2019-04-8008).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study and in the writing of the manuscript.
References
- Haugland, R.P. The Handbook. A Guide to Fluorescent Probes and Labeling Technologies, 10th ed.; Molecular Probes, Inc.: Eugene, Oregon, USA, 2005. [Google Scholar]
- Treibs, A.; Kreuzer, F. -H.; Difluorboryl-Komplexe von Di- und Tripyrrylmethenen. Justus Liebigs Ann. Chem. 1968, 718, 208–223. [Google Scholar] [CrossRef]
- Yadav, I.S.; Misra, R. ; Design, synthesis and functionalization of BODIPY dyes: applications in dye-sensitized solar cells (DSSCs) and photodynamic therapy (PDT). J. Mater. Chem. C 2023, 11, 8688–8723. [Google Scholar] [CrossRef]
- Boens, N. , Leen, V.; Dehaen, W.; Fluorescent indicators based on BODIPY. Chem. Soc. Rev. 2012, 41, 1130–1172. [Google Scholar] [CrossRef]
- Kowada, T.; Maeda, H.; Kikuchi, K. ; BODIPY-based probes for the fluorescence imaging of biomolecules in living cells. Chem. Soc. Rev. 2024, 44, 4953–4972. [Google Scholar] [CrossRef]
- Awuah, S.G.; You, Y. ; Boron dipyrromethene (BODIPY)-based photosensitizers for photodynamic therapy. RSC Advances 2021, 2, 11169–11183. [Google Scholar] [CrossRef]
- Boens, N.; Verbelen, B.; Ortiz, M.J.; Jiao, L.; Dehaen, W. ; Synthesis of BODIPY dyes through postfunctionalization of the boron dipyrromethene core. Coord. Chem. Rev. 2014, 399, 213024. [Google Scholar] [CrossRef]
- Loudet, A.; Burgess, K. ; BODIPY Dyes and Their Derivatives: Syntheses and Spectroscopic Properties. Chem. Rev. 2020, 107, 4891–4932. [Google Scholar] [CrossRef]
- Ziessel, R.; Ulrich, G.; Harriman, A. ; The chemistry of BODIPY: A new El Dorado for fluorescence tools. New J. Chem. 2007, 31, 496–501. [Google Scholar] [CrossRef]
- Bassan, E.; Gualandi, A.; Cozzi, P.G.; Ceroni, P. ; Design of BODIPY dyes as triplet photosensitizers: electronic properties tailored for solar energy conversion, photoredox catalysis and photodynamic therapy. Chem. Sci., 2021, 12, 6607–6628. [Google Scholar] [CrossRef]
- Krumova, K.; Cosa, G. ; BODIPY dyes with tunable redox potentials and functional groups for further tethering: Preparation, electrochemical, and spectroscopic characterization. J. Am. Chem. Soc. 2010, 132, 17560–17569. [Google Scholar] [CrossRef]
- Wood, T.E.; Thompson, A. ; Advances in the chemistry of dipyrrins and their complexes. Chem. Rev. 2007, 107, 1831–1861. [Google Scholar] [CrossRef] [PubMed]
- Arsenault, G.P.; Bullock, E.; MacDonald, S.F. ; Pyrromethanes and Porphyrins Therefrom. J. Am. Chem. Soc. 1960, 82, 4384–4389. [Google Scholar] [CrossRef]
- Litter, B.J.; Miller, M.A.; Hung, C.-H.; Wagner, R.W.; O’Shea, D.F.; Boyle, P.D.; Lindsey, J.S. ; Refined Synthesis of 5-Substituted Dipyrromethanes. J. Org. Chem. 1999, 64, 1391–1396. [Google Scholar] [CrossRef]
- Qin, W.; Baruah, M.; Van der Auweraer, M.; De Schryver, F.C.; Boens, N. ; Photophysical Properties of Borondipyrromethene Analogues in Solution. J. Phys. Chem. A 2005, 109, 7371–7384. [Google Scholar] [CrossRef] [PubMed]
- Kee, H.L.; Kirmaier, C.; Yu, L.H.; Thamyongkit, P.; Youngblood, W.J.; Calder, M.E.; Ramos, L.; Noll, B.C.; Bocian, D.F.; Scheidt, W.R.; Birge, R.R.; Lindsey, J.S.; Holten, D.; Structural Control of the Photodynamics of Boron-Dipyrrin Complexes, J. Phys. Chem. B 2005, 109, 20433 – 20443. (b) Lincoln, R.; Greene, L.E.; Bain, C.; Flores-Rizo, J.O.; Bohle, D.S.; Cosa, G.; When Push Comes to Shove: Unravelling the Mechanism and Scope of Nonemissive meso-Unsaturated BODIPY Dyes. J. Phys. Chem. B 2020, 119, 4758–4765. [Google Scholar]
- Dent, M.R.; López-Duarte, I.; Dickson, C.J.; Geoghegan, N.D.; Cooper, J.M.; Gould, I.R.; Krams, R.; Bull, J.A.; Brooks, N.J.; Kuimova, M.K. ; Imaging phase separation in model lipid membranes through the use of BODIPY based molecular rotors. Phys. Chem. Chem. Phys. 2017, 17, 18393–18402. [Google Scholar] [CrossRef] [PubMed]
- Frank, F.J.; Waddell, P.G.; Hall, M.J.; Knight, J.G. ; Synthesis and Reactivity of 3,5-Diiodo-BODIPYs via a Concerted, Double Aromatic Finkelstein Reaction. Org. Lett. 2021, 23, 8595–8599. [Google Scholar] [CrossRef]
- Jiao, L.; Pang, W.; Zhou, J.; Wei, Y.; Mu, X.; Bai, G.; Hao, E. ; Regioselective Stepwise Bromination of Boron Dipyrromethene (BODIPY) Dyes. J. Org. Chem. 2011, 76, 9988–9996. [Google Scholar] [CrossRef]
- Hayashi, Y.; Yamaguchi, S.; Cha, W.Y.; Kim, D.; Shinokubo, H. ; Synthesis of Directly Connected BODIPY Oligomers through Suzuki - Miyaura Coupling. Org. Lett. 2011, 13, 2992–2995. [Google Scholar] [CrossRef]
- Wang, G.; Huang, C.; Hu, Z.; Zhang, W.; Zhang, Y. ; An Efficient and Convenient Bromination of BODIPY Derivatives with Copper (II) Bromide. Synthesis (Stuttg). 2011, 44, 104–110. [Google Scholar]
- Duran-Sampedro, G.; Agarrabeitia, A.R.; Garcia-Moreno, I.; Costela, A.; Bañuelos, J.; Arbeloa, T.; LópezArbeloa, I.; Chiara, J.L.; Ortiz, M.J. ; Chlorinated BODIPYs: Surprisingly Efficient and Highly Photostable Laser Dyes. Eur. J. Org. Chem. 2012, 6335–6350. [Google Scholar] [CrossRef]
- Zhou, X.; Yu, C.; Feng, Z.; Yu, Y.; Wang, J.; Hao, E.; Wei, Y.; Mu, X.; Jiao, L. ; Highly Regioselective α-Chlorination of the BODIPY Chromophore with Copper(II) Chloride. Org. Lett. 2015, 17, 4632–4635. [Google Scholar] [CrossRef] [PubMed]
- Bonardi, L.; Ulrich, G.; Ziessel, R. ; Tailoring the Properties of Boron - Dipyrromethene Dyes with Acetylenic Functions at the 2, 6, 8 and 4-B Substitution Positions. Org. Lett. 2008, 10, 2183–2186. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Zhang, J.; Vegesna, G.; Tiwari, A.; Luo, F. – T.; Zeller, M.; Luck, R.; Li, H.; Green, S.; Liu, H.; Controlled Knoevenagel reactions of methyl groups of 1,3,5,7-tetramethyl BODIPY dyes for unique BODIPY dyes. RSC Adv. 2012, 2, 404–407. [Google Scholar] [CrossRef]
- Peterson, J.A.; Fischer, L.J.; Gehrmann, E.J.; Shrestha, P.; Yuan, D.; Wijesooriya, C.S.; Smith, E.A.; Winter, A.H. ; Direct Photorelease of Alcohols from Boron-Alkylated BODIPY Photocages. J. Org. Chem. 2020, 85, 5712–5717. [Google Scholar] [CrossRef]
- Slanina, T.; Sharestha, P.; Palao, E.; Kand, D.; Peterson, J.A.; Dutton, A.S.; Rubinstein, N.; Weinstain, R.; Winter, A.H.; Klán, P. ; In Search of the Perfect Photocage: Structure-Reactivity Relationships in meso-Methyl BODIPY Photoremovable Protecting Groups. J. Am. Chem. Soc. 2017, 139, 15168–15175. [Google Scholar] [CrossRef]
- Rohand, T.; Baruah, M.; Qin, W.; Boens, N.; Dehaen, W. ; Functionalisation of fluorescent BODIPY dyes by nucleophilic substitution. Chem. Commun. 2006, 266–267. [Google Scholar] [CrossRef]
- Rohand, T.; Qin, W.; Boens, N.; Dehaen, W. ; Palladium-catalyzed coupling reactions for the functionalization of BODIPY dyes with fluorescence spanning the visible spectrum. European J. Org. Chem. 2010, 20, 4658–4663. [Google Scholar] [CrossRef]
- Deniz, E.; Isbasar, G.C.; Bozdemir, O.A.; Yildirim, L.T.; Siemiarczuk, A.; Akkaya, E.U. ; Bidirectional switching of near IR emitting boradiazaindacene fluorophores. Org. Lett. 2012, 10, 3401–3403. [Google Scholar] [CrossRef]
- Waldeck, D.H. ; Photoisomerization Dynamics of Stilbenes. Chem. Rev. 1991, 91, 415–436. [Google Scholar] [CrossRef]
- Baruah, M.; Qin, W.; Flors, C.; Hofkens, J.; Vallée, R.A.L.; Beljonne, D.; van der Auweraer, M.; de Borgggraeve, W.M.; Boens, N. ; Solvent and pH dependent fluorescent properties of a dimethylaminostyryl borondipyrromethene dye in solution. J. Phys. Chem. A 2006, 110, 5998–6009. [Google Scholar] [CrossRef] [PubMed]
- Zlatić, K.; Bogomolec, M.; Cindrić, M.; Uzelac, L.; Basarić, N. ; Synthesis, photophysical properties, anti-Kasha photochemical reactivity and biological activity of vinyl- and alkynyl-BODIPY derivatives. Tetrahedron 2022, 124, 132995. [Google Scholar] [CrossRef]
- Dissanayake, K.C.; Yuan, D.; Winter, A.H. ; Structure-Photoreactivity Studies of BODIPY Photocages: Limitations of the Activation Barrier for Optimizing Photoreactions. J. Org. Chem. 2024, 89, 6740–6748. [Google Scholar] [CrossRef]
- Niu, S.L.; Ulrich, G.; Retailleau, P.; Harrowfield, J.; Ziessel, R. ; New insights into the solubilization of BODIPY dyes. Tetrahedron Lett. 2009, 50, 3840–3844. [Google Scholar] [CrossRef]
- Romieu, A.; Massif, C.; Rihn, S.; Ulrich, G.; Ziessel, R.; Renard, P. – Y.; The first comparative study of the ability of different hydrophilic groups to water-solubilise fluorescent BODIPY dyes. New J. Chem. 2013, 37, 1016–1027. [Google Scholar] [CrossRef]
- Liu, K.M.; Tsai, M.S.; Jan, M.S.; Chau, C.M.; Wang, W.J. ; Convenient one-pot procedure for synthesizing 4,4′-dimethoxy- boradiaza-s-indacene dyes and their application to cell labeling. Tetrahedron 2011, 67, 7919–7922. [Google Scholar] [CrossRef]
- Sawazaki, T.; Shimizu, Y.; Oisaki, K.; Sohma, Y.; Kanai, M. ; Convergent and Functional-Group-Tolerant Synthesis of B-Organo BODIPYs. Org. Lett. 2018, 20, 7767–7770. [Google Scholar] [CrossRef]
- Taniguchi, A.; Sawazaki, T.; Shimizu, Y.; Sohma, Y.; Kanai, M. ; Photophysical properties and application in live cell imaging of B,B-fluoro-perfluoroalkyl BODIPYs. Med. Chem. Commun. 2019, 10, 1121–1125. [Google Scholar] [CrossRef]
- Gabe, Y.; Ueno, T.; Urano, Y. ; Tunable design strategy for fluorescence probes based on 4-substituted BODIPY chromophore: improvement of highly sensitive fluorescence probe for nitric oxide. Anal. Bioanal. Chem. 2006, 386, 621–626. [Google Scholar] [CrossRef]
- Smithen, D.A.; Baker, A.E.G.; Offman, M.; Crawford, S.M.; Cameron, T.S.; Thompson, A. ; Use of F-BODIPYs as a protection strategy for dipyrrins: Optimization of BF2 removal. J. Org. Chem. 2012, 77, 3439–3453. [Google Scholar] [CrossRef]
- Courtis, M.A.; Santos, S.A.; Guan, Y.; Hendricks, J.A.; Ghosh, B.; Miklos Szantai-Kis, D.; Reis, S.A.; Shah, J.V.; Mazitschek, R. ; Monoalkoxy BODIPYs-A fluorophore class for bioimaging. Bioconjug. Chem. 2014, 25, 1043–1051. [Google Scholar] [CrossRef] [PubMed]
- Tahtaoui, C.; Thomas, C.; Rohmer, F.; Klotz, P.; Duportail, G.; Mély, Y.; Bonnet, D.; Hibert, M. ; Convenient Method to Access New 4,4-Dialkoxy- and 4,4-Diaryloxy-diaza-s-indacene Dyes: Synthesis and Spectroscopic Evaluation. J. Org. Chem. 2006, 72, 269–272. [Google Scholar] [CrossRef] [PubMed]
- Shaban Ragab, S.; Swaminathan, S.; Deniz, E.; Captain, B.; Raymo, F.M. ; Fluorescence photoactivation by ligand exchange around the boron center of a BODIPY chromophore. Org. Lett. 2007, 15, 3154–3157. [Google Scholar] [CrossRef]
- Hudnall, T.W.; Gabbaï, F.P. ; A BODIPY boronium cation for the sensing of fluoride ions. Chem. Commun. 2008, 4596–4597. [Google Scholar] [CrossRef]
- Durán-Sampedro, G.; Agarrabetia, A.R.; Cerdán, L.; Perez-Ojeda Rodriguez, M.E.; Costela, A.; García-Moreno, I.; Esnal, I.; Bañuelos, J.; López-Arbeloa, Í.; Ortiz, M.J. ; Carboxylates versus fluorines: Boosting the emission properties of commercial BODIPYs in liquid and solid media. Adv. Funct. Mater. 2013, 23, 4195–4205. [Google Scholar] [CrossRef]
- Manzano, H.; Esnal, I.; Marqués-Matesanz, T.; Bañuelos, J.; López-Arbeloa, Í.; Ortiz, M.J.; Cerdán, L.; Costela, A.; García-Moreno, I.; Chiara, J.L. ; Unprecedented J-Aggregated Dyes in Pure Organic Solvents. Adv. Funct. Mater. 2016, 26, 2756–2769. [Google Scholar] [CrossRef]
- Lundrigan, T.; Crawford, S.M.; Cameron, T.S.; Thompson, A. ; Cl-BODIPYs: A BODIPY class enabling facile B-substitution. Chem. Commun. 2013, 48, 1003–1005. [Google Scholar] [CrossRef]
- Ray, C.; Díaz-Casado, L.; Avellanal-Zaballa, E.; Bañuelos, J.; Cerdán, L.; García-Moreno, I.; Moreno, F.; Maroto, B.L.; López-Arbeloa, Í.; de la Moya, S. ; N-BODIPYs Come into Play: Smart Dyes for Photonic Materials. Chem. - A Eur. J. 2017, 23, 9383–9390. [Google Scholar] [CrossRef] [PubMed]
- Travis Lundrigan, T.; Cameron, S.; Thompson, A. ; Activation and deprotection of F-BODIPYs using boron trihalides. Chem. Commun. 2014, 50, 7028–7031. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Q.; Yin, J.; Yu, C.; Cheng, K.; Wei, Y.; Hao, E.; Jiao, L. ; Metal-Free and Versatile Synthetic Routes to Natural and Synthetic Prodiginines from Boron Dipyrrin. Org. Lett. 2016, 18, 5696–5699. [Google Scholar] [CrossRef]
- Ulrich, G.; Goze, C.; Goeb, S.; Retailleau, P.; Ziessel, R. ; New fluorescent aryl- or ethynylaryl-boron-substituted indacenes as promising dyes. New J. Chem. 2006, 30, 982–986. [Google Scholar] [CrossRef]
- Goze, C.; Ulrich, G.; Mallon, L.J.; Allen, B.D.; Harriman, A.; Ziessel, R. ; Synthesis and Photophysical Properties of Borondipyrromethene Dyes Bearing Aryl Substituents at the Boron Center. J. Am. Chem. Soc. 2006, 128, 10231–10239. [Google Scholar] [CrossRef] [PubMed]
- Goze, C.; Ulrich, G.; Ziessel, R. ; Tetrahedral Boron Chemistry for the Preparation of Highly Efficient “Cascatelle” Devices. J. Org. Chem. 2007, 72, 313–322. [Google Scholar] [CrossRef]
- Klan, P.; Šolomek, T.; Bochet, C.G.; Blanc, A.; Givens, R.; Rubina, M.; Popik, V.; Kostikov, A.; Wirz, J. ; Photoremovable Protecting Groups in Chemistry and Biology: Reaction Mechanisms and Efficacy. Chem. Rev. 2020, 113, 119–191. [Google Scholar] [CrossRef]
- Ellis-Davies, G.C.R. ; Reverse Engineering Caged Compounds: Design Principles for their Application in Biology. Angew. Chem. Int. Ed. 2023, 62, e202206083. [Google Scholar] [CrossRef]
- Shrestha, P.; Kand, D.; Weinstain, R.; Winter, A.H. ; meso-Methyl BODIPY Photocages: Mechanisms, Photochemical Properties, and Applications. J. Am. Chem. Soc. 2021, 145, 32–17497. [Google Scholar] [CrossRef]
- Slanina, T.; Shrestha, P.; Palao, E.; Kand, D.; Peterson, J.A.; Dutton, A.S.; Rubinstein, N.; Weinstain, R.; Winter, A.H.; Klán, P. ; In Search of the Perfect Photocage: Structure-Reactivity Relationships in meso-Methyl BODIPY Photoremovable Protecting Groups. J. Am. Chem. Soc. 2022, 139, 15168–15175. [Google Scholar] [CrossRef]
- Goswami, P.P.; Syed, A.; Beck, C.L.; Albright, T.R.; Mahoney, K.M.; Unash, R.; Smith, E.A.; Winter, A.H. ; BODIPY-Derived Photoremovable Protecting Groups Unmasked with Green Light. J. Am. Chem. Soc. 2015, 137, 3783–3786. [Google Scholar] [CrossRef] [PubMed]
- Zlatić, K.; Popović, M.; Uzelac, L.; Kralj; M. ; Basarić, N.; Antiproliferative activity of meso-substituted BODIPY photocages: Effect of electrophiles vs singlet oxygen. Eur. J. Med. Chem. 2023, 259, 115705. [Google Scholar] [CrossRef]
- Umeda, N.; Takahashi,, H. ; Kamiya, M.; Ueno, T.; Komatsu, T.; Terai, T.; Hanaoka, K.; Nagano, T.; Urano, Y.; Boron dipyrromethene as a fluorescent caging group for single-photon uncaging with long-wavelength visible light. ACS Chem. Biol. 2014, 9, 2242–2246. [Google Scholar] [CrossRef]
- Kawatani, M.; Kamiya, M.; Takahashi, H.; Urano, Y. ; Factors affecting the uncaging efficiency of 500 nm light-activatable BODIPY caging group. Bioorg. Med. Chem. Lett. 2018, 28, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Carl, P.L.; Chakravarty, P.K.; Katzenellenbogen, J.A. ; A Novel Connector Linkage Applicable in Prodrug Design. J. Med. Chem. 1981, 24, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Gavriel, A.G.; Sambrook, M.R.; Russell, A.T.; Hayes, W. ; Recent advances in self-immolative linkers and their applications in polymeric reporting systems. Polym. Chem. 2014, 13, 3188–3269. [Google Scholar] [CrossRef]
- Sharma, A.K.; Nair, M.; Chauhan, P.; Gupta, K.; Saini, D.K.; Chakrapani, H. ; Visible-Light-Triggered Uncaging of Carbonyl Sulfide for Hydrogen Sulfide (H2S) Release. Org. Lett. 2017, 19, 4822–4825. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).