Submitted:
01 October 2024
Posted:
02 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Background
2. Methodology
2.1. Systematic Review Reporting and Protocol Registration
2.2. Data Sources and Search 2010-2023 Was Searched Using Multiple Scientific Databases, Including Cochrane Library, Scopus, PubMed and Global Index Medicus. The Search Strategy Consisted of Terms Describing the Intervention and Outcomes
2.3. Eligibility (Inclusion and Exclusion) Criteria
2.4. Data Extraction
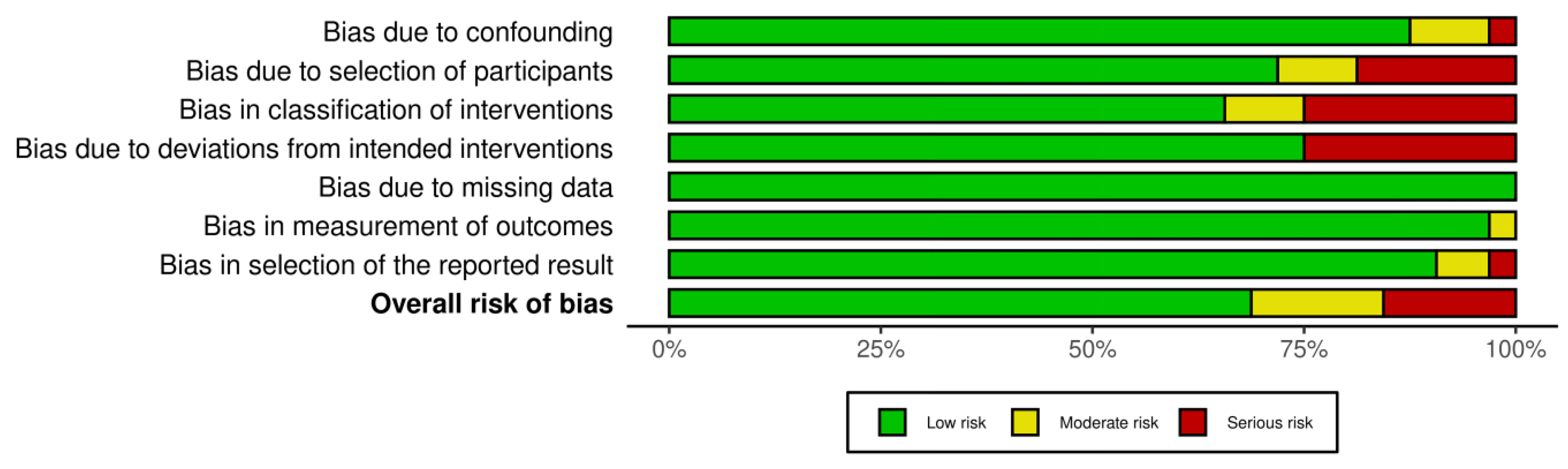
2.5. Analysis of Risk of Bias

3. Results
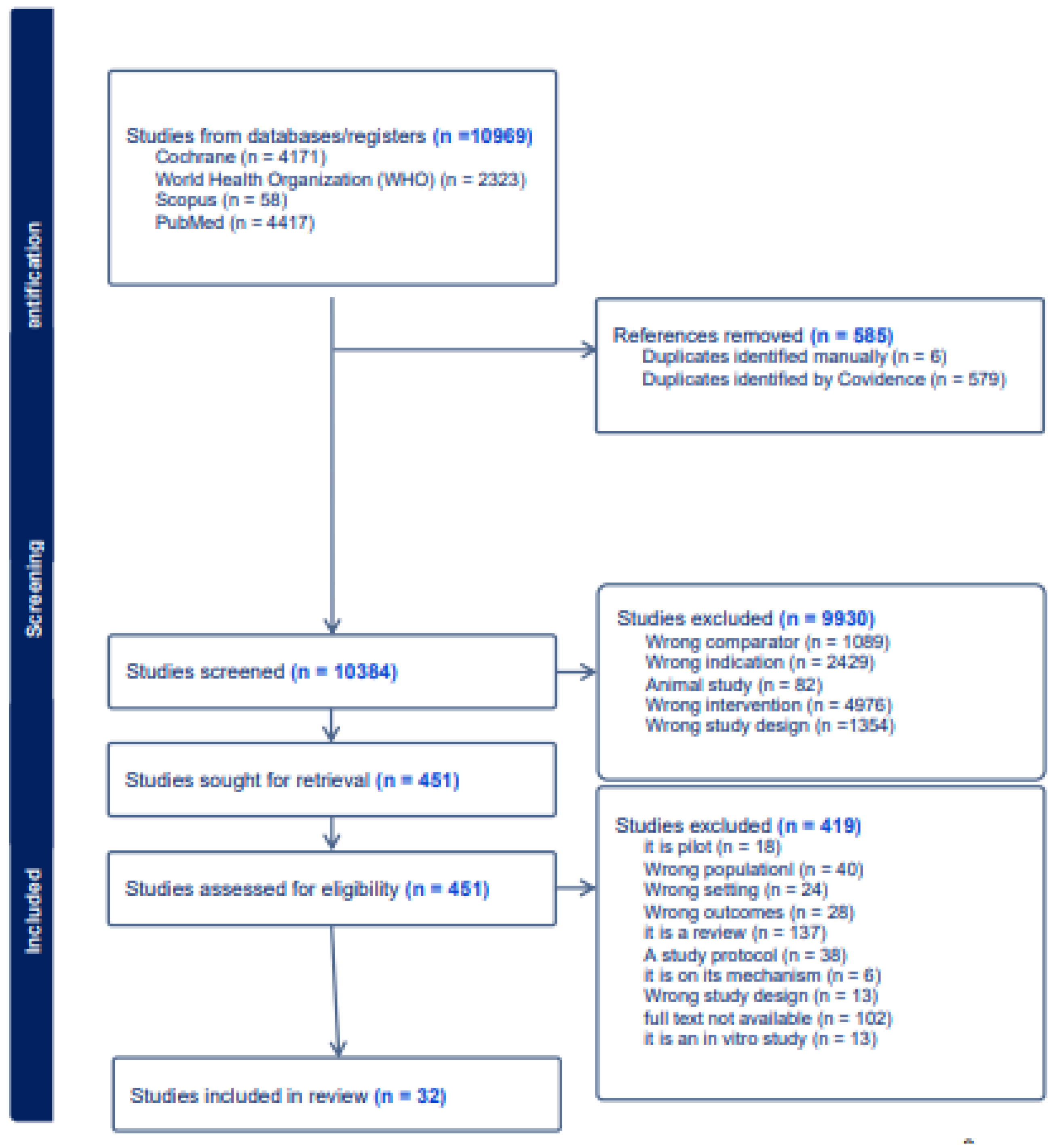
3.1. Search Results
3.2. Quality Assessment
3.3. Characteristics of Study
3.4. Meta-Analysis Result
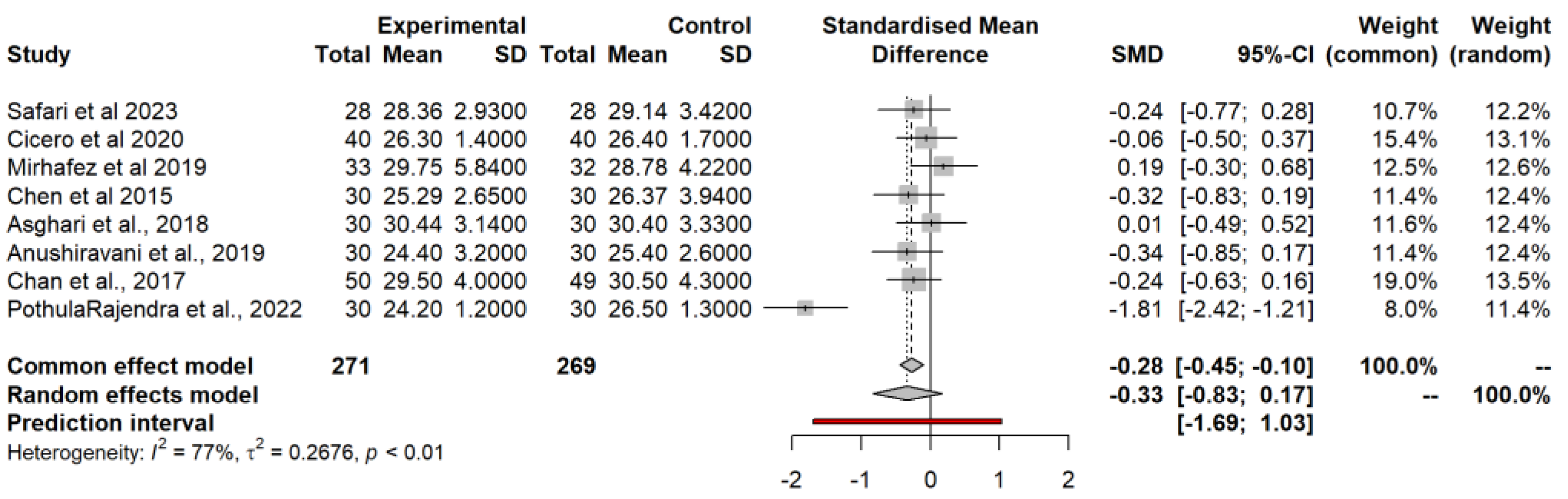
3.4.1. Body Mass Index (BMI) Model
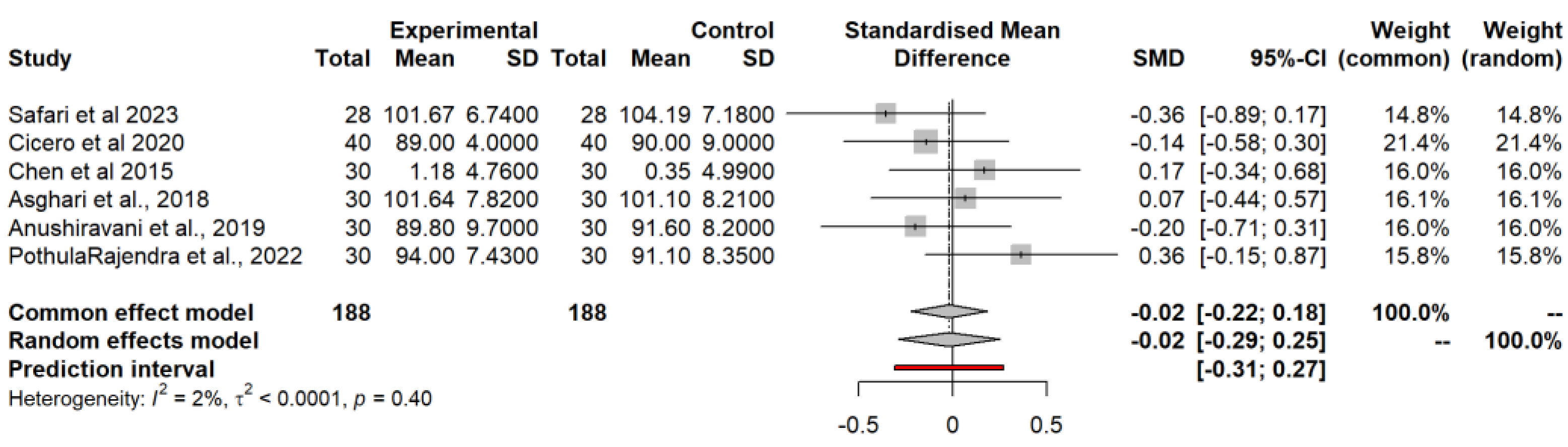
3.4.2. Waist Circumference (WC) Model
3.4.3. Low-Density Lipoprotein (LDL) Model
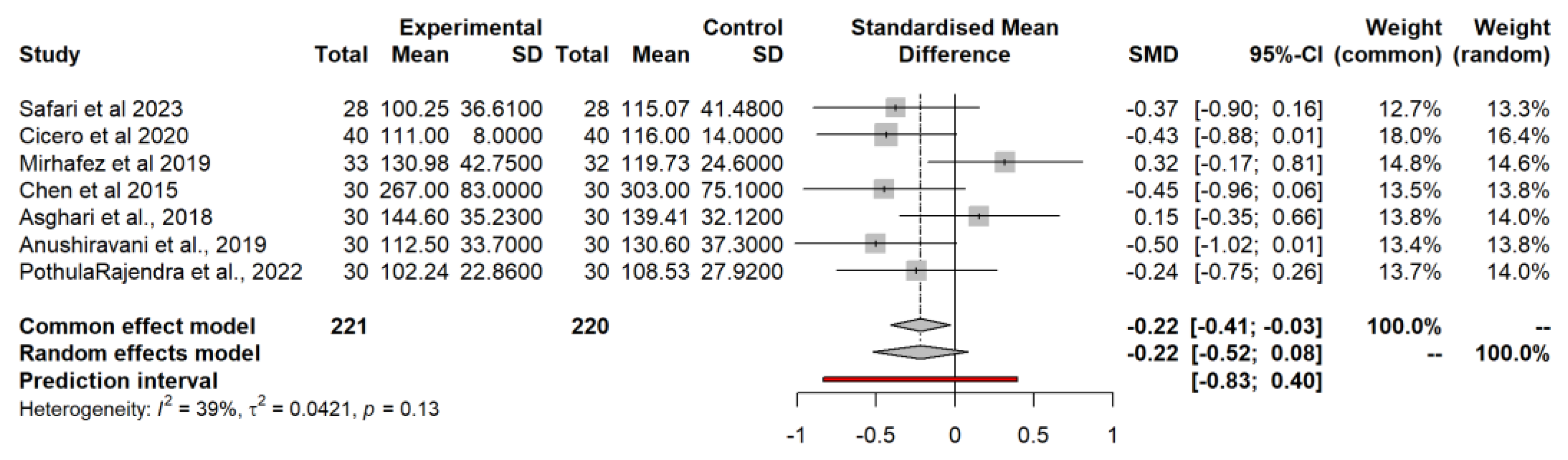
3.4.4. High-Density Lipoprotein (HDL) Model
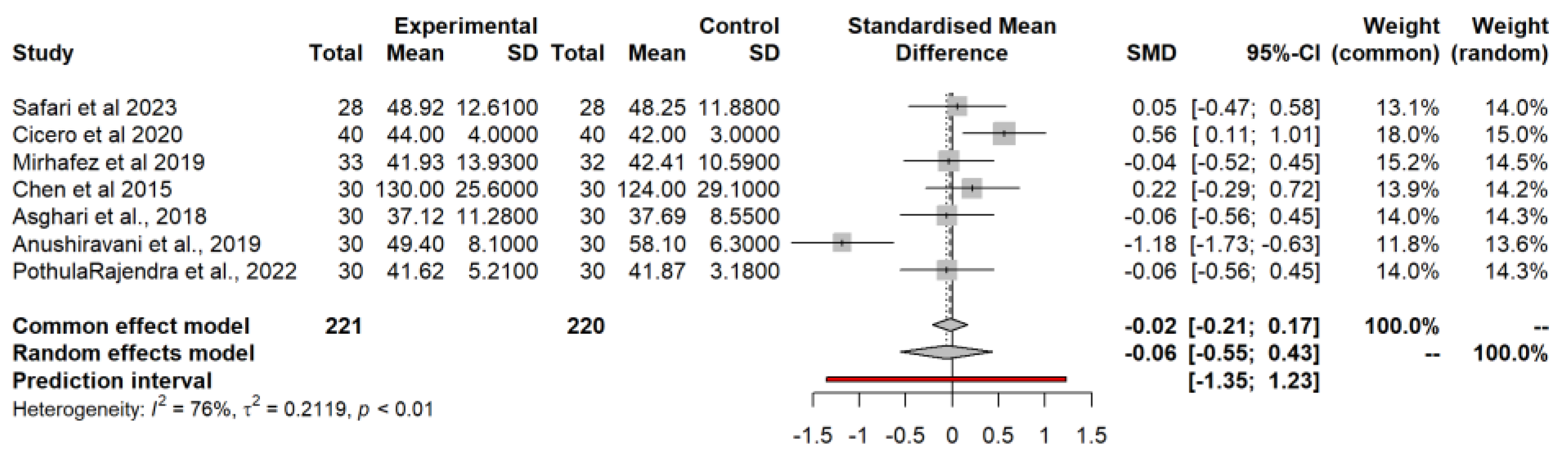
3.4.5. Homeostatic Model Assessment for Insulin Resistance (HOMA-IR)
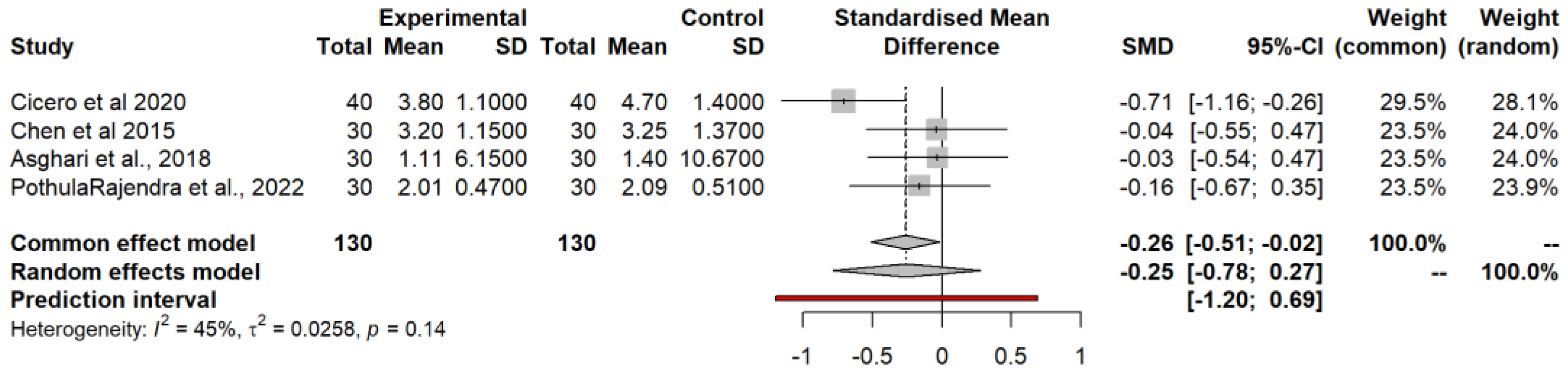
3.4.6. Total Cholesterol (TC)
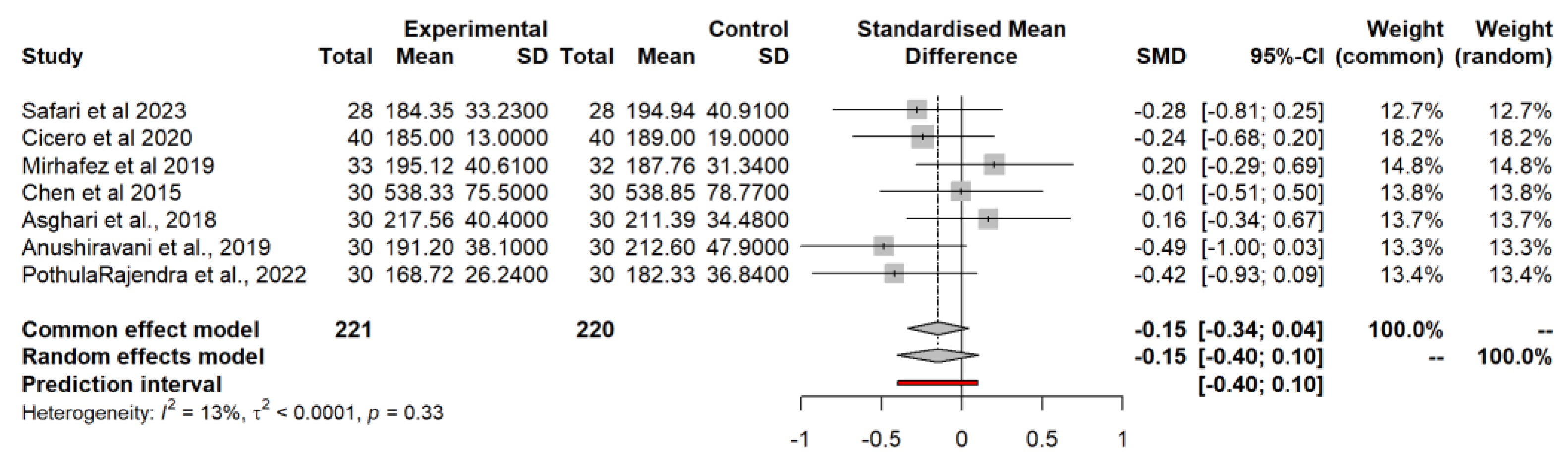
3.4.7. Aspartate Aminotransferase (AST)
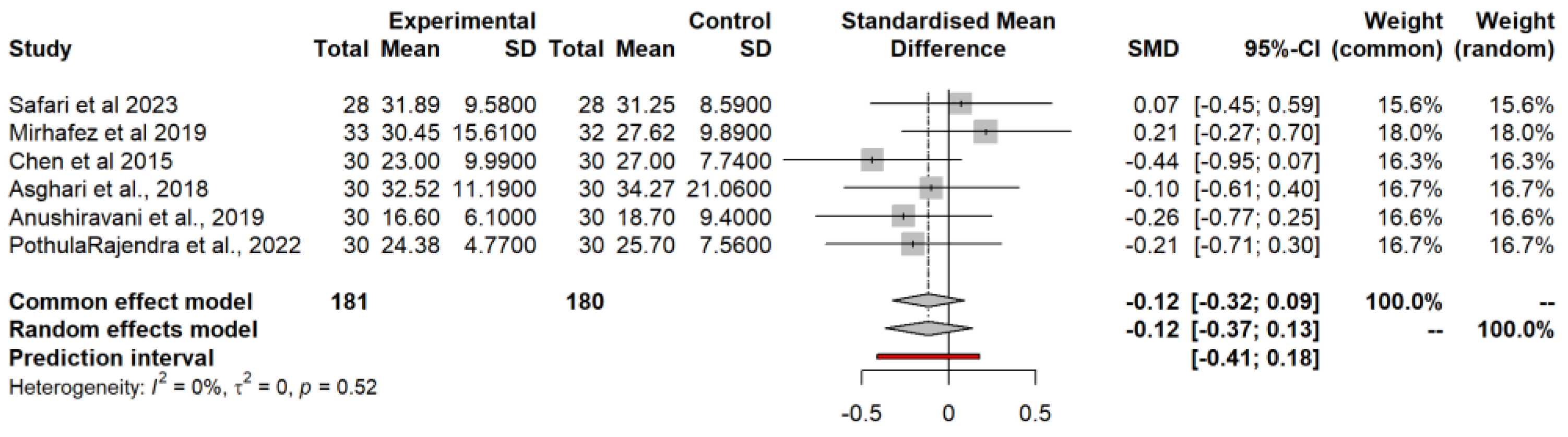
3.4.8. Alanine Aminotransferase (ALT)
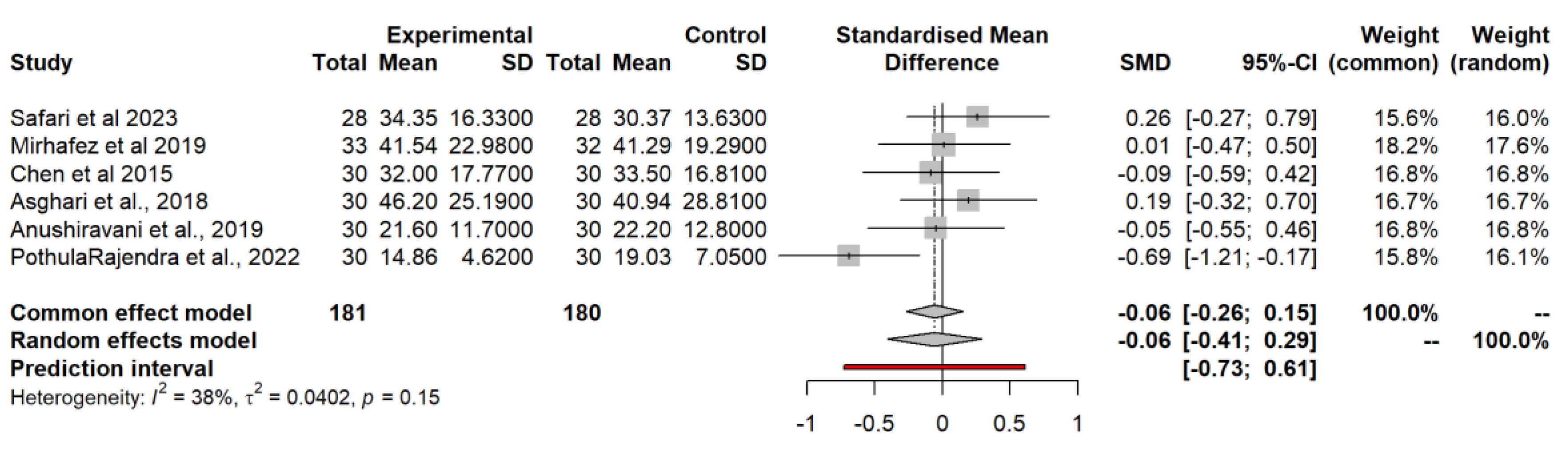
3.4.9. Triglycerides (TG)
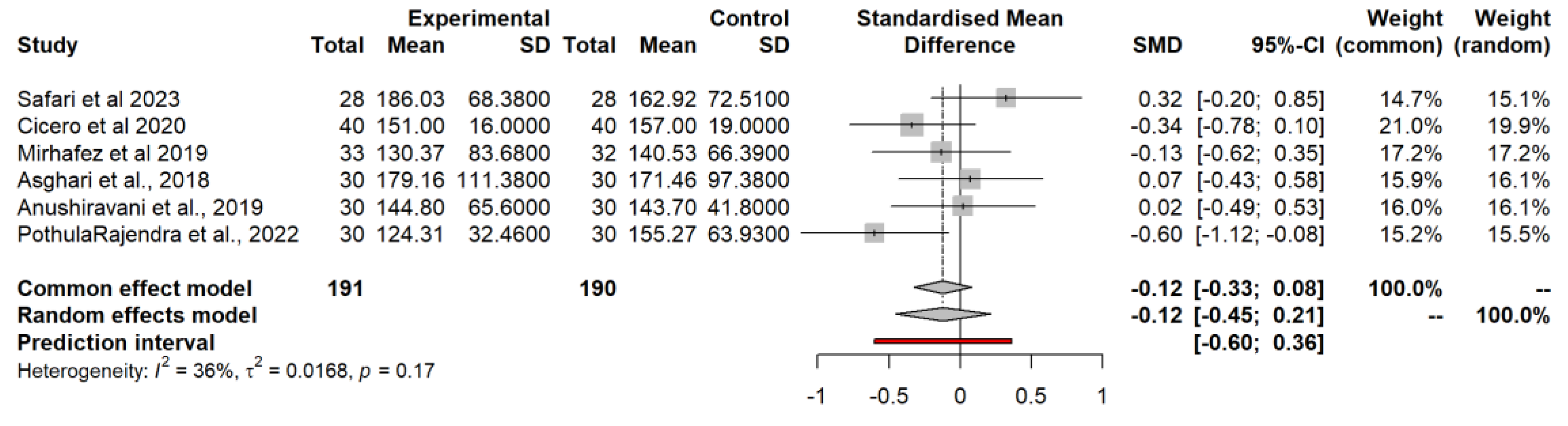
3.5. Food-Bioactive Outcomes
3.5.1. Curcumin
3.5.2. Silybin
3.5.3. Silymarin
3.5.4. Nutraceutical Supplementation
3.5.5. Resveratrol
3.5.6. Diet-Related
3.5.7. Turmeric
3.5.8. Other Food-Bioactive Outcomes
4. Discussion
4.4. Strengths, Limitations and Gaps
5. Conclusion
References
- Abenavoli, L.; Greco, M.; Nazionale, I.; Peta, V.; Milic, N.; Accattato, F.; Foti, D.; Gulletta, E.; Luzza, F. Effects of Mediterranean diet supplemented with silybin–vitamin E–phospholipid complex in overweight patients with non-alcoholic fatty liver disease. Expert Review of Gastroenterology & Hepatology 2015, 9, 519–527. [Google Scholar] [CrossRef]
- Afsharinasab, M.; Mohammad-Sadeghipour, M.; Reza Hajizadeh, M.; Khoshdel, A.; Mirzaiey, V.; Mahmoodi, M. The effect of hydroalcoholic Berberis integerrima fruits extract on the lipid profile, antioxidant parameters and liver and kidney function tests in patients with nonalcoholic fatty liver disease. Saudi Journal of Biological Sciences 2020, 27, 2031–2037. [Google Scholar] [CrossRef] [PubMed]
- Amerikanou, C.; Kanoni, S.; Kaliora, A.C.; Barone, A.; Bjelan, M.; D’Auria, G.; Gioxari, A.; Gosalbes, M.J.; Mouchti, S.; Stathopoulou, M.G.; Soriano, B.; Stojanoski, S.; Banerjee, R.; Halabalaki, M.; Mikropoulou, E.V.; Kannt, A.; Lamont, J.; Llorens, C.; Marascio, F.; Marascio, M. Effect of Mastiha supplementation on NAFLD: The MAST4HEALTH Randomised, Controlled Trial. Molecular Nutrition & Food Research 2021, 65, 2001178. [Google Scholar] [CrossRef]
- Anushiravani, A.; Haddadi, N.; Pourfarmanbar, M.; Mohammadkarimi, V. Treatment options for nonalcoholic fatty liver disease. European Journal of Gastroenterology & Hepatology 2019, 31, 613–617. [Google Scholar] [CrossRef]
- Asghari, S.; Asghari-Jafarabadi, M.; Somi, M.-H.; Ghavami, S.-M.; Rafraf, M. Comparison of Calorie-Restricted Diet and Resveratrol Supplementation on Anthropometric Indices, Metabolic Parameters, and Serum Sirtuin-1 Levels in Patients With Nonalcoholic Fatty Liver Disease: A Randomized Controlled Clinical Trial. Journal of the American College of Nutrition 2018, 37, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Asghari, S.; Rafraf, M.; Farzin, L.; Asghari-Jafarabadi, M.; Ghavami, S.-M.; Somi, M.-H. Effects of Pharmacologic Dose of Resveratrol Supplementation on Oxidative/Antioxidative Status Biomarkers in Nonalcoholic Fatty Liver Disease Patients: A Randomized, Double-Blind, Placebo-Controlled Trial. Advanced Pharmaceutical Bulletin 2018, 8, 307–317. [Google Scholar] [CrossRef]
- Chen, S.; Zhao, X.; Ran, L.; Wan, J.; Wang, X.; Qin, Y.; Shu, F.; Gao, Y.; Yuan, L.; Zhang, Q.; Mi, M. Resveratrol improves insulin resistance, glucose and lipid metabolism in patients with non-alcoholic fatty liver disease: A randomized controlled trial. Digestive and Liver Disease 2015, 47, 226–232. [Google Scholar] [CrossRef]
- Cheng, I.; Weng, S.; Wu, M.; Suk, F.; Lien, G.; Chen, C. Low-molecular-weight fucoidan and high-stability fucoxanthin decrease serum alanine transaminase in patients with nonalcoholic fatty liver disease—A double-blind, randomized controlled trial. Advances in Digestive Medicine 2019, 6, 116–122. [Google Scholar] [CrossRef]
- Chiurazzi, M.; Cacciapuoti, N.; Di Lauro, M.; Nasti, G.; Ceparano, M.; Salomone, E.; Guida, B.; Lonardo, M.S. The Synergic Effect of a Nutraceutical Supplementation Associated to a Mediterranean Hypocaloric Diet in a Population of Overweight/Obese Adults with NAFLD. Nutrients 2022, 14, 4750. [Google Scholar] [CrossRef]
- Cicero, A. F. G.; Sahebkar, A.; Fogacci, F.; Bove, M.; Giovannini, M.; Borghi, C. Effects of phytosomal curcumin on anthropometric parameters, insulin resistance, cortisolemia and non-alcoholic fatty liver disease indices: a double-blind, placebo-controlled clinical trial. European Journal of Nutrition 2019. [Google Scholar] [CrossRef]
- Ebrahimi-Mameghani, M.; Sadeghi, Z.; Abbasalizad Farhangi, M.; Vaghef-Mehrabany, E.; Aliashrafi, S. Glucose homeostasis, insulin resistance and inflammatory biomarkers in patients with non-alcoholic fatty liver disease: Beneficial effects of supplementation with microalgae Chlorella vulgaris : A double-blind placebo-controlled randomized clinical trial. Clinical Nutrition 2017, 36, 1001–1006. [Google Scholar] [CrossRef] [PubMed]
- Fogacci, F.; Rizzoli, E.; Giovannini, M.; Bove, M.; D’Addato, S.; Borghi, C.; Cicero, A. F. Effect of Dietary Supplementation with Eufortyn® Colesterolo Plus on Serum Lipids, Endothelial Reactivity, Indexes of Non-Alcoholic Fatty Liver Disease and Systemic Inflammation in Healthy Subjects with Polygenic Hypercholesterolemia: The ANEMONE Study. Nutrients 2022, 14, 2099–2099. [Google Scholar] [CrossRef]
- Federico, A.; Dallio, M.; Masarone, M.; Gravina, A.G.; Di Sarno, R.; Tuccillo, C.; Cossiga, V.; Lama, S.; Stiuso, P.; Morisco, F.; Persico, M.; Loguercio, C. Evaluation of the Effect Derived from Silybin with Vitamin D and Vitamin E Administration on Clinical, Metabolic, Endothelial Dysfunction, Oxidative Stress Parameters, and Serological Worsening Markers in Nonalcoholic Fatty Liver Disease Patients. Oxidative Medicine and Cellular Longevity 2019, 2019, 1–12. [Google Scholar] [CrossRef]
- Ferro, Y.; Pujia, R.; Mazza, E.; Lascala, L.; Lodari, O.; Maurotti, S.; Pujia, A.; Montalcini, T. A new nutraceutical (Livogen Plus®) improves liver steatosis in adults with non-alcoholic fatty liver disease. Journal of Translational Medicine 2022, 20, 377. [Google Scholar] [CrossRef]
- Haidari, F.; Hojhabrimanesh, A.; Helli, B.; Seyedian, S.-S.; Ahmadi-Angali, K. An energy-restricted high-protein diet supplemented with β-cryptoxanthin alleviated oxidative stress and inflammation in nonalcoholic fatty liver disease: a randomized controlled trial. Nutrition Research 2020, 73, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Kim, S.-M.; Nam, G.E.; Kim, S.-H.; Park, S.-J.; Park, Y.-K.; Baik, H.W. A Randomized, Double-Blind, Placebo-Controlled, Multi-Centered Clinical Study to Evaluate the Efficacy and Safety of Artemisia annua L. Extract for Improvement of Liver Function. Clinical Nutrition Research 2020, 9, 258–270. [Google Scholar] [CrossRef]
- Fateh, H. L.; Rashid, S. A.; Muhammad, S. S.; Al-Jaf, S. H.; Ali, A. M. Comparing effects of beetroot juice and Mediterranean diet on liver enzymes and sonographic appearance in patients with non-alcoholic fatty liver disease: a randomized control trials. Frontiers in Nutrition 2023. [Google Scholar] [CrossRef]
- Mojiri-Forushani, H.; Hemmati, A.; Khanzadeh, A.; Zahedi, A. Effectiveness of Grape Seed Extract in Patients with Nonalcoholic Fatty Liver: A Randomized Double-Blind Clinical Study. Hepatitis Monthly 2022. [Google Scholar] [CrossRef]
- jarhahzadeh, M.; Alavinejad, P.; Farsi, F.; Husain, D.; Rezazadeh, A. The effect of turmeric on lipid profile, malondialdehyde, liver echogenicity and enzymes among patients with nonalcoholic fatty liver disease: a randomized double blind clinical trial. Diabetology & Metabolic Syndrome 2021. [Google Scholar] [CrossRef]
- Jinato, T.; Chayanupatkul, M.; Dissayabutra, T.; Chutaputti, A.; Tangkijvanich, P.; Chuaypen, N. Litchi-Derived Polyphenol Alleviates Liver Steatosis and Gut Dysbiosis in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized Double-Blinded, Placebo-Controlled Study. Nutrients 2022, 14, 2921. [Google Scholar] [CrossRef]
- Illnait, J.; Rodríguez, I.; Mendoza, S.; Fernández, Y.; Mas, R.; Miranda, M.; Piñera, J.; Fernández, J.C.; Mesa, F.; Fernández, L.; Carbajal, D.; Gámez, R. Effects of D-002, a mixture of high molecular weight beeswax alcohols, on patients with nonalcoholic fatty liver disease. The Korean Journal of Internal Medicine/Korean Journal of Internal Medicine 2013, 28, 439–439. [Google Scholar] [CrossRef] [PubMed]
- Mirhafez, S.R.; Farimani, A.R.; Dehhabe, M.; Bidkhori, M.; Hariri, M.; Ghouchani, B.F.; Abdollahi, F. Effect of Phytosomal Curcumin on Circulating Levels of Adiponectin and Leptin in Patients with Non-Alcoholic Fatty Liver Disease: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Journal of Gastrointestinal and Liver Diseases 2019, 28, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Navarro, V.J.; Belle, S.H.; D’Amato, M.; Adfhal, N.; Brunt, E.M.; Fried, M.W.; Reddy, K.R.; Wahed, A.S.; Harrison, S. Silymarin in non-cirrhotics with non-alcoholic steatohepatitis: A randomized, double-blind, placebo controlled trial. PLOS ONE 2019, 14, e0221683. [Google Scholar] [CrossRef]
- Pervez, M.A.; Khan, D.A.; Gilani ST, A.; Fatima, S.; Ijaz, A.; Nida, S. Hepato-Protective Effects of Delta-Tocotrienol and Alpha-Tocopherol in Patients with Non-Alcoholic Fatty Liver Disease: Regulation of Circulating MicroRNA Expression. International Journal of Molecular Sciences 2022, 24, 79. [Google Scholar] [CrossRef] [PubMed]
- Rajendra VK, P.; Kurapati, S.; Balineni, S.K.; Gogineni NT, T. A blend of Sphaeranthus indicus flower head and Terminalia chebula fruit extracts reduces fatty liver and improves liver function in non-alcoholic, overweight adults. Functional Foods in Health and Disease 2022, 12, 361. [Google Scholar] [CrossRef]
- Rashidmayvan, M.; Mohammadshahi, M.; Seyedian, S.S.; Haghighizadeh, M.H. The effect of Nigella sativa oil on serum levels of inflammatory markers, liver enzymes, lipid profile, insulin and fasting blood sugar in patients with non-alcoholic fatty liver. Journal of Diabetes & Metabolic Disorders 2019, 18, 453–459. [Google Scholar] [CrossRef]
- Saadati, S.; Sadeghi, A.; Mansour, A.; Yari, Z.; Poustchi, H.; Hedayati, M.; Hatami, B.; Hekmatdoost, A. Curcumin and inflammation in non-alcoholic fatty liver disease: a randomized, placebo controlled clinical trial. BMC Gastroenterology 2019. [Google Scholar] [CrossRef]
- Safari, Z.; Bagherniya, M.; Khoram, Z.; Ebrahimi Varzaneh, A.; Heidari, Z.; Sahebkar, A.; Askari, G. The effect of curcumin on anthropometric indices, blood pressure, lipid profiles, fasting blood glucose, liver enzymes, fibrosis, and steatosis in non-alcoholic fatty livers. Frontiers in Nutrition 2023. [Google Scholar] [CrossRef]
- Mazloomi, S. M.; Samadi, M.; Davarpanah, H.; Davarpanah, H.; Babajafari, S.; Clark, C.C.; Ghaemfar, Z.; Rezaiyan, M.; Mosallanezhad, A.; Shafiee, M.; Rostami, H. The effect of Spirulina sauce, as a functional food, on cardiometabolic risk factors, oxidative stress biomarkers, glycemic profile, and liver enzymes in nonalcoholic fatty liver disease patients: A randomized double-blinded clinical trial. Food Science and Nutrition 2021, 10, 317–328. [Google Scholar] [CrossRef]
- Šmíd, V.; Dvořák, K.; Šedivý, P.; Kosek, V.; Leníček, M.; Dezortová, M.; Hajšlová, J.; Hájek, M.; Vítek, L.; Bechyňská, K.; Brůha, R. Effect of Omega-3 Polyunsaturated Fatty Acids on Lipid Metabolism in Patients With Metabolic Syndrome and NAFLD. Hepatology Communications 2022. [Google Scholar] [CrossRef]
- Theodotou, M.; Fokianos, K.; Moniatis, D.; Kadlenic, R.; Chrysikou, A.; Aristotelous, A.; Mouzouridou, A.; Diakides, J.; Stavrou, E. Effect of resveratrol on non-alcoholic fatty liver disease. Experimental and Therapeutic Medicine 2019. [Google Scholar] [CrossRef] [PubMed]
- Wah Kheong, C.; Nik Mustapha, N.R.; Mahadeva, S. A Randomized Trial of Silymarin for the Treatment of Nonalcoholic Steatohepatitis. Clinical Gastroenterology and Hepatology 2017, 15, 1940–1949e8. [Google Scholar] [CrossRef] [PubMed]
- Kwatra, B. A review on potential properties and therapeutic applications of grape seed extract. World J. Pharm. Res 2020, 9, 2519–2540. [Google Scholar]
- Kumar; et al. Curcumin and insulin sensitivity: A systematic review and meta-analysis. Nutrients 2018, 10, 1745. [Google Scholar]
- Li; et al. Silymarin and lipid profile: A systematic review and meta-analysis. Phytotherapy Research 2019, 33, 536–545. [Google Scholar]
- Panahi; et al. Curcumin and body weight: A systematic review and meta-analysis. Journal of Medicinal Food 2018, 21, 1039–1048. [Google Scholar]
- Zhang; et al. Silymarin and HDL cholesterol: A systematic review and meta-analysis. European Journal of Nutrition 2020, 59, 651–661. [Google Scholar]
- Aggarwal; et al. Curcumin: The Indian solid gold. Advances in Experimental Medicine and Biology 2010, 691, 1–75. [Google Scholar]
- Bhatt; et al. Resveratrol: A review of its pharmacological activities. Journal of Pharmacy and Pharmacology 2012, 64, 1345–1356. [Google Scholar]
- Pradhan; et al. Silymarin: A review of its pharmacological and clinical uses. Journal of Pharmacy and Pharmacology 2016, 68, 851–866. [Google Scholar]
- Higgins; et al. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, 2019. [Google Scholar]
- Guyatt; et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2011, 343, d4088. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.L.; Ng, C.H.; Huang, D.Q.; Chan, K.E.; Tan, D.J.; Lim, W.H.; Yang, J.D.; Tan, E.; Muthiah, M.D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clinical and Molecular Hepatology 2022, 29, S32–S42. [Google Scholar] [CrossRef] [PubMed]
- Kudaravalli, P.; John, S. Nonalcoholic Fatty Liver; StatPearls Publishing, 2021; Available online: https://www.ncbi.nlm.nih.gov/books/NBK541033/.
- El-Zayadi, A.-R. Hepatic steatosis: A benign disease or a silent killer. World Journal of Gastroenterology 2008, 14, 4120. [Google Scholar] [CrossRef]
- Li, A.-N.; Li, S.; Zhang, Y.-J.; Xu, X.-R.; Chen, Y.-M.; Li, H.-B. Resources and biological activities of natural polyphenols. Nutrients 2014, 6, 6020–6047. [Google Scholar] [CrossRef]
- Xu, K.; Liu, S.; Zhao, X.; Zhang, X.; Fu, X.; Zhou, Y.; Xu, K.; Miao, L.; Li, Z.; Li, Y.; Qiao, L.; Bao, J. Treating hyperuricemia related non-alcoholic fatty liver disease in rats with resveratrol. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 2019, 110, 844–849. [Google Scholar] [CrossRef]
- Xia, H.-M.; Wang, J.; Xie, X.-J.; Xu, L.-J.; Tang, S.-Q. Green tea polyphenols attenuate hepatic steatosis, and reduce insulin resistance and inflammation in high-fat diet-induced rats. International Journal of Molecular Medicine 2019, 44, 1523–1530. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Yang, T.; Heng, C.; Zhou, Y.; Jiang, Z.; Qian, X.; Du, L.; Mao, S.; Yin, X.; Lu, Q. Quercetin improves nonalcoholic fatty liver by ameliorating inflammation, oxidative stress, and lipid metabolism in db/db mice. Phytotherapy Research : PTR 2019, 33, 3140–3152. [Google Scholar] [CrossRef]
- Teng, M. L. P.; Ng, C. H.; Huang, D. Q.; Chan, K. E.; Tan, D. J. H.; Lim, W. H.; Yang, J. D.; Tan, E.; Muthiah, M. D. Global incidence and prevalence of nonalcoholic fatty liver disease. Clinical and Molecular Hepatology 2023. [Google Scholar] [CrossRef]
- Tomeno, W.; Imajo, K.; Takayanagi, T.; Ebisawa, Y.; Seita, K.; Takimoto, T.; Honda, K.; Kobayashi, T.; Nogami, A.; Kato, T.; Honda, Y.; Kessoku, T.; Ogawa, Y.; Kirikoshi, H.; Sakamoto, Y.; Yoneda, M.; Saito, S.; Nakajima, A. Complications of Non-Alcoholic Fatty Liver Disease in Extrahepatic Organs. Diagnostics (Basel, Switzerland) 2020, 10, E912. [Google Scholar] [CrossRef]
- Huh, Y.; Cho, Y.J.; Nam, G.E. Recent Epidemiology and Risk Factors of Nonalcoholic Fatty Liver Disease. Journal of Obesity and Metabolic Syndrome 2022, 31, 17–27. [Google Scholar] [CrossRef]
- Younossi, Z.; Anstee, Q.M.; Marietti, M.; Hardy, T.; Henry, L.; Eslam, M.; George, J.; Bugianesi, E. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nature Reviews Gastroenterology & Hepatology 2017, 15, 11–20. [Google Scholar]
| Study Author and Year | Food Bioactive Compounds | ALT | AST | CRP | FIBRINOGEN | GGT | INSULIN | HOMA IR | LDL | HDL | BMI | WC | LIVER FIBROSIS | HEPATIC STEATOSIS | TC | |
| 1 | Abenavoli et al., 2015 | silybin–vitamin E–phospholipidcomplex | ↑ | ↓ | NA | NA | ↑ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | NA | ↓ | ↓ |
| 2 | Afsharinasab et al., 2020 | hydroalcoholic Berberis integerrima fruit extract | ↓ | ↓ | ALP ↓ | ↓ | NA | NA | NA | ↓ | ↑ | ↓ | ↓ | NA | NA | ↓ |
| 3 | Amerikanou et al., 2021 | Mastiha supplementation | = | = | NA | NA | = | = | = | = | = | = | = | = | NA | = |
| 4 | Anushiravani et al., 2019 | silymarin 140 mg/day, | ↓ | ↓ | NA | ↓ | NA | NA | NA | ↓ | ↑ | ↓ | ↓ | NA | NA | ↓ |
| 5 | Asghari et al., 2018 | Resveratrol Supplementation | ↓ | ↓ | NA | ↑ | NA | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | NA | = | ↑ |
| 6 | HAN et al., 2020 | Artemisia annua L. Extract | ↓ | ↓ | NA | ↑ | NA | NA | NA | NA | NA | = | NA | NA | NA | NA |
| 7 | Chen et al., 2017 | Silymarin | ↓ | ↓ | NA | ↑ | ↓ | NA | ↑ | ↓ | ↑ | ↓ | = | NA | NA | ↓ |
| 8 | Cheng et al., 2019 | fucoidan-fucoxanthin mixture | = | = | NA | NA | NA | = | NA | = | = | ↓ | = | = | = | |
| 9 | Chen et al., 2015 | Resveratrol | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↑ | NA | NA | ↓ | |
| 10 | Rajendra et al., 2022 | blend of Sphaeranthus indicus flower head and Terminalia chebula fruit extracts | ↓ | ↓ | ALP ↓ | NA | ↓ | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | ↓ | ↓ | |
| 11 | Chiurazzi et al. (2022) | Vitamin E, L-glutathione, silymarin and hepato-active | ↓0 | ↓ | NA | NA | NA | NA | NA | ↓ | NA | ↓ | ↓ | NA | ↓ | ↓ |
| 12 | Cicero et al. (2019) | Curcumin | NA | NA | NA | NA | NA | ↓ | ↓ | ↓ | ↑ | ↓ | ↓ | NA | ↓ | ↓ |
| 13 | Ebrahimi-Mameghani et al. (2017) | Chlorella vulgaris | ↓ | ↓ | ↓ | NA | NA | ↓ | ↓ | NA | NA | NA | ↓ | NA | NA | NA |
| 14 | Hawal Lateef Fateh et al. (2023) | Beetroot juice | ↓ | ↓ | NA | NA | NA | NA | NA | ↓ | ↑ | ↓ | ↓ | NA | NA | ↓ |
| 15 | Federico et al. (2019) | Silybin | ↓ | = | ↓ | NA | NA | ↑ | ↓ | = | NA | = | = | NA | ↓ | = |
| 16 | Ferro et al. (2022) | Livogen Plus® | = | = | = | NA | NA | ↓ | ↓ | NA | ↑ | NA | NA | NA | NA | ↑ |
| 17 | Federica Fogacci et al. (2022) | a nutraceutical compound (Eufortyn® Colesterolo Plus) | ↓ | ↑ | ↓ | NA | NA | NA | NA | ↓ | ↓ | ↓ | ↓ | NA | ↓ | ↓ |
| 18 | Haidari et al. (2020) | β -Cryptoxanthin | NA | NA | ↓ | NA | NA | NA | NA | NA | NA | ↓ | ↓ | NA | NA | NA |
| 19 | José Illnait et al. (2013) | Beeswax Alcohol | = | = | NA | NA | = | ↓ | ↓ | ↓ | = | = | NA | NA | ↓ | ↓ |
| 20 | jarhahzadeh et al. (2021) | Turmeric | ↓ | ↓ | NA | NA | ↓ | NA | NA | ↓ | ↑ | NA | NA | NA | NA | = |
| 21 | Jinato et al. (2022) | Litchi-Derived Polyphenol | ↓ | = | NA | NA | NA | NA | NA | = | = | ↓ | = | NA | NA | = |
| 22 | Mohammad et al., (2021) | Spirulina sauce | ↓ | ↓ | NA | NA | NA | ↓ | ↓ | = | ↑ | ↓ | ↓ | NA | NA | = |
| 23 | Mirhafez et al., (2019) | Phytosomal Curcumin | ↓ | ↓ | NA | NA | NA | ↓ | NA | ↓ | ↑ | ↓ | NA | NA | NA | ↑ |
| 24 | Hoda Mojiri-Forushani et al. (2022) | Grape Seed Extract | ↓ | ↓ | NA | NA | NA | = | NA | = | = | NA | NA | NA | NA | = |
| 25 | Navarro et al. (2019) | Sylimarin | ↓ | ↓ | NA | NA | ↓ | ↓ | NA | NA | NA | NA | NA | NA | ||
| 26 | Pervez et al. (2022) | Delta-Tocotrienol and Alpha-Tocopherol | NA | NA | NA | NA | NA | ↓ | NA | NA | NA | NA | NA | NA | NA | |
| 27 | Rejandra et al. (2022) | Sphaeranthus Indicus flower head and Terminalia chebula fruit extracts | ↓ | ↓ | ALP ↓ | NA | ↓ | NA | ↓ | ↓ | ↑ | NA | NA | FLI ↓ | NA | ↓ |
| 28 | Rashidmayvan et al. (2019) | Nigella sativa oil | ↓ | ↓ | ALP↓ | NA | = | NA | NA | NA | ↑ | ↓ | ↓ | NA | NA | ↓ |
| 29 | Saadati et al., 2019) | Curcumin | ↓ | ↓ | NA | NA | NA | NA | NA | NA | NA | ↓ | ↓ | ↓ | NA | NA |
| 30 | Safari et al. (2023) | Phytosomal Curcumin | ↑ | ↑ | ↑ | ↑ | NA | NA | NA | NA | = | ↓ | ↓ | NA | NA | |
| 31 | Šmíd et al. (2022) | Omega-3 Polyunsaturated Fatty Acids | ↓ | ↓ | NA | NA | ↓ | NA | NA | ↑ | ↓ | ↑ | ↑ | NA | NA | NA |
| 32 | Theodotou et al. (2019) | Resveratrol | NA | NA | AL P↓ | NA | NA | ↓ | NA | ↓ | ↓ | NA | NA | NA | NA | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





