Submitted:
02 October 2024
Posted:
03 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Data Collection
2.3. Metabolomic Analysis
2.4. Statistical Analysis
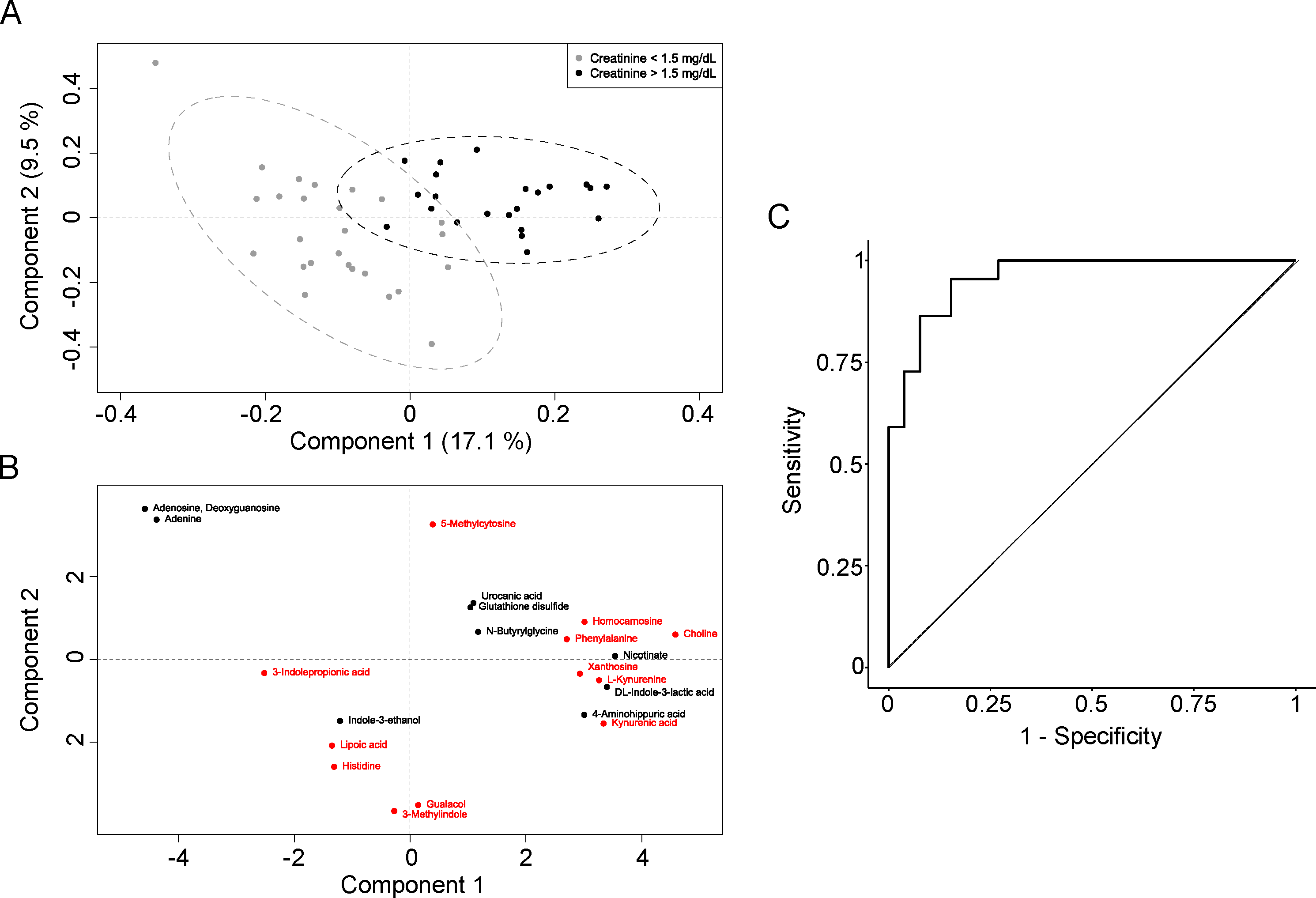
3. Results
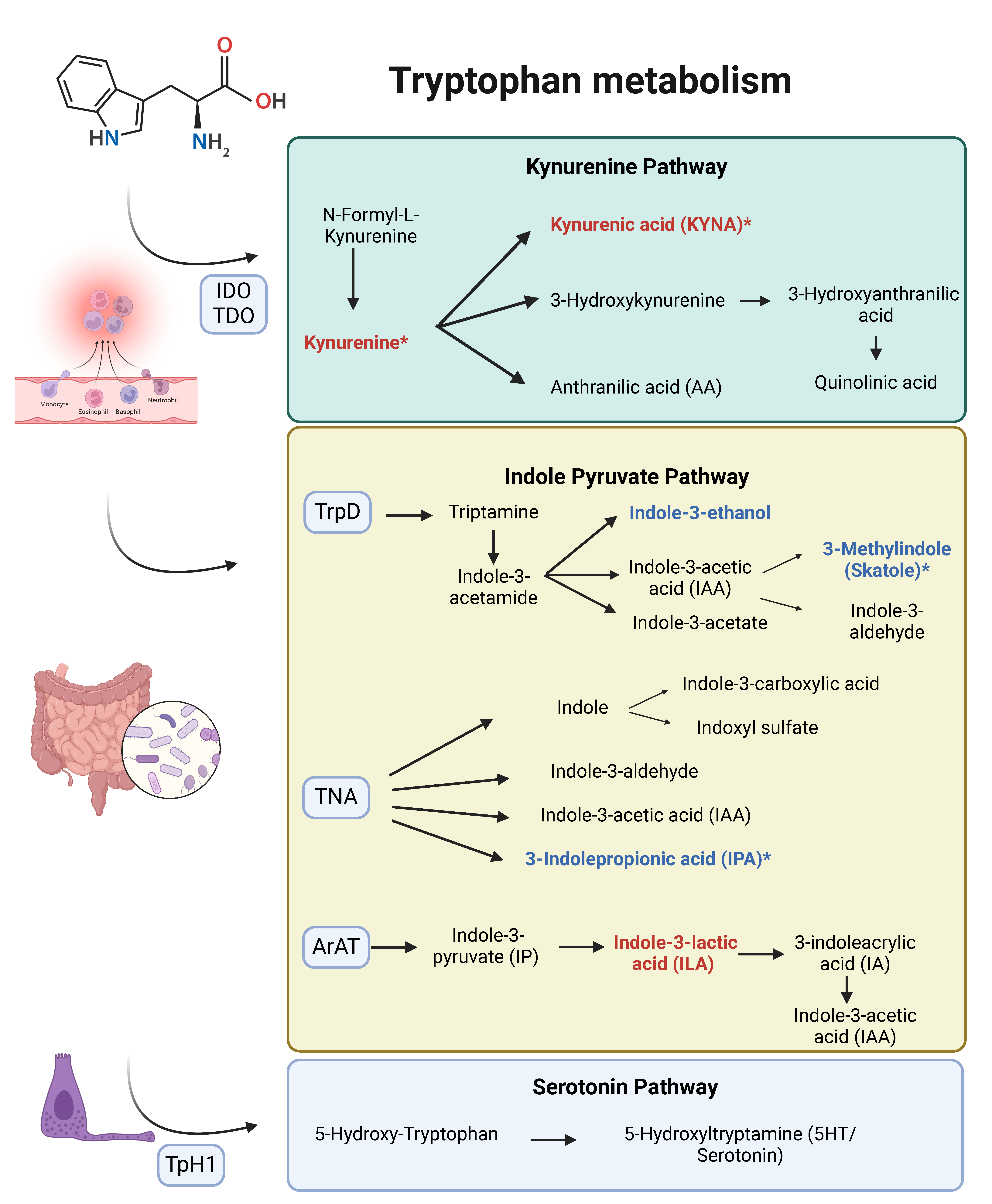
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD Chronic Kidney Disease Collaboration. Global, regional, and national burden of chronic kidney disease, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Yozgat I, Cakır U, Serdar M.A., et al. Longitudinal non-targeted metabolomic profiling of urine samples for monitoring of kidney transplantation patients. Ren Fail. 2024, 46, 2300736. [Google Scholar] [CrossRef]
- Stanimirova I, Banasik M, Ząbek A, et al. Serum metabolomics approach to monitor the changes in metabolite profiles following renal transplantation. Sci Rep. 2020, 10, 17223. [Google Scholar] [CrossRef]
- Peris-Fernández M, Roca-Marugán M, Amengual JL, et al. Uremic Toxins and Inflammation: Metabolic Pathways Affected in Non-Dialysis-Dependent Stage 5 Chronic Kidney Disease. Biomedicines. 2024, 12, 607. [Google Scholar]
- Hariharan S, Mcbride M.A., Cherikh W.S., Tolleris C.B., Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney International. 2002, 62, 311–318. [Google Scholar] [CrossRef]
- Legendre C, Canaud G, Martinez F. Factors influencing long-term outcome after kidney transplantation. Transpl Int. 2014, 27, 19–27. [Google Scholar] [CrossRef]
- Josephson, MA. Monitoring and Managing Graft Health in the Kidney Transplant Recipient. Clinical Journal of the American Society of Nephrology. 2011, 6, 1774. [Google Scholar] [CrossRef]
- Zhao X, Chen J, Ye L, Xu G. Serum metabolomics study of the acute graft rejection in human renal transplantation based on liquid chromatography-mass spectrometry. J Proteome Res. 2014, 13, 2659–2667. [Google Scholar] [CrossRef]
- Mao Y.Y., Bai J.Q., Chen J.H., et al. A pilot study of GC/MS-based serum metabolic profiling of acute rejection in renal transplantation. Transpl Immunol. 2008, 19, 74–80. [Google Scholar] [CrossRef]
- Blydt-Hansen T.D., Sharma A, Gibson I.W., et al. Urinary Metabolomics for Noninvasive Detection of Antibody-Mediated Rejection in Children After Kidney Transplantation. Transplantation. 2017, 101, 2553–2561. [Google Scholar] [CrossRef]
- Kostidis S, Bank J.R., Soonawala D, et al. Urinary metabolites predict prolonged duration of delayed graft function in DCD kidney transplant recipients. Am J Transplant. 2019, 19, 110–122. [Google Scholar] [CrossRef]
- Paeslack N, Mimmler M, Becker S, et al. Microbiota-derived tryptophan metabolites in vascular inflammation and cardiovascular disease. Amino Acids. 2022, 54, 1339–1356. [Google Scholar] [CrossRef]
- Hong S.H., Hong Y, Lee M, Keum B.R., Kim G.H. Natural Product Skatole Ameliorates Lipotoxicity-Induced Multiple Hepatic Damage under Hyperlipidemic Conditions in Hepatocytes. Nutrients. 2023, 15, 1490. [Google Scholar] [CrossRef]
- Madella A.M., Van Bergenhenegouwen J, Garssen J, Masereeuw R, Overbeek SA. Microbial-Derived Tryptophan Catabolites, Kidney Disease and Gut Inflammation. Toxins. 2022, 14, 645. [Google Scholar] [CrossRef]
- Zgarbová E, Vrzal R. Skatole: A thin red line between its benefits and toxicity. Biochimie. 2023, 208, 1–12. [CrossRef]
- Galano A, León-Carmona J.R., Alvarez-Idaboy J.R. Influence of the Environment on the Protective Effects of Guaiacol Derivatives against Oxidative Stress: Mechanisms, Kinetics, and Relative Antioxidant Activity. J Phys Chem B. 2012, 116, 7129–7137. [Google Scholar] [CrossRef]
- Premkumar J, Sampath P, Sanjay R, Chandrakala A, Rajagopal D. Synthetic Guaiacol Derivatives as Promising Myeloperoxidase Inhibitors Targeting Atherosclerotic Cardiovascular Disease. ChemMedChem. 2020, 15, 1187–1199. [Google Scholar] [CrossRef]
- Aqeel M.T., Rahman N ur, Khan A ullah, et al. Cardioprotective effect of 2-methoxy phenol derivatives against oxidative stress-induced vascular complications: An integrated in vitro, in silico, and in vivo investigation. Biomedicine & Pharmacotherapy 5240, 165, 115240.
- Holeček, M. Histidine in Health and Disease: Metabolism, Physiological Importance, and Use as a Supplement. Nutrients. 2020, 12, 848. [Google Scholar] [CrossRef]
- Watanabe M, Suliman ME, Qureshi A.R., et al. Consequences of low plasma histidine in chronic kidney disease patients: associations with inflammation, oxidative stress, and mortality1. The American Journal of Clinical Nutrition. 2008, 87, 1860–1866. [CrossRef]
- Vera-Aviles M, Vantana E, Kardinasari E, Koh N.L., Latunde-Dada G.O. Protective Role of Histidine Supplementation Against Oxidative Stress Damage in the Management of Anemia of Chronic Kidney Disease. Pharmaceuticals (Basel). 2018, 11, 111. [CrossRef]
- Jiang H, Chen C, Gao J. Extensive Summary of the Important Roles of Indole Propionic Acid, a Gut Microbial Metabolite in Host Health and Disease. Nutrients. 2022, 15, 151. [Google Scholar] [CrossRef]
- Yisireyili M, Takeshita K, Saito S, Murohara T, Niwa T. Indole-3-propionic acid suppresses indoxyl sulfate-induced expression of fibrotic and inflammatory genes in proximal tubular cells. Nagoya J Med Sci. 2017, 79, 477–486. [Google Scholar]
- Sun CY, Lin CJ, Pan HC, et al. Clinical association between the metabolite of healthy gut microbiota, 3-indolepropionic acid and chronic kidney disease. Clinical Nutrition. 2019, 38, 2945–2948. [Google Scholar] [CrossRef]
- Ambrosi N, Guerrieri D, Caro F, et al. Alpha Lipoic Acid: A Therapeutic Strategy that Tend to Limit the Action of Free Radicals in Transplantation. International Journal of Molecular Sciences. 2018, 19, 102. [Google Scholar] [CrossRef]
- Kamt, S.F. , Liu J, Yan L.J. Renal-Protective Roles of Lipoic Acid in Kidney Disease. Nutrients. 2023, 15, 1732. [Google Scholar] [CrossRef]
- Ding Y, Zhang Y, Zhang W, Shang J, Xie Z, Chen C. Effects of Lipoic Acid on Ischemia-Reperfusion Injury. Oxid Med Cell Longev. 2021, 2021, 5093216. [CrossRef]
- Wang J, Zhou C, Zhang Q, Liu Z. Metabolomic profiling of amino acids study reveals a distinct diagnostic model for diabetic kidney disease. Amino Acids. 2023, 55, 1563–1572. [Google Scholar] [CrossRef]
- Fox B.M., Gil H.W., Kirkbride-Romeo L, et al. Metabolomics assessment reveals oxidative stress and altered energy production in the heart after ischemic acute kidney injury in mice. Kidney Int. 2019, 95, 590–610. [CrossRef]
- Xiang X, Zhu J, Dong G, Dong Z. Epigenetic Regulation in Kidney Transplantation. Front Immunol. 2022, 13, 861498. [CrossRef]
- Heylen L, Thienpont B, Naesens M, et al. Ischemia-Induced DNA Hypermethylation during Kidney Transplant Predicts Chronic Allograft Injury. Journal of the American Society of Nephrology. 2018, 29, 1566. [Google Scholar] [CrossRef]
- Chen Y, Zelnick L.R., Wang K, et al. Kidney Clearance of Secretory Solutes Is Associated with Progression of CKD: The CRIC Study. J Am Soc Nephrol. 2020, 31, 817–827. [CrossRef]
- Granda M.L., Zelnick L.R, Prince D.K., Hoofnagle A, Young B.A., Kestenbaum B.R. Tubular Secretion and Estimated GFR Decline in the Jackson Heart Study. Kidney Int Rep. 2022, 7, 2668–2675. [CrossRef]
- Rhee E.P., Clish C.B., Ghorbani A, et al. A Combined Epidemiologic and Metabolomic Approach Improves CKD Prediction. J Am Soc Nephrol. 2013, 24, 1330–1338. [CrossRef]
- Saeb-Parsy K, Martin J.L., Summers D.M., Watson C.J.E, Krieg T, Murphy M.P. Mitochondria as Therapeutic Targets in Transplantation. Trends Mol Med. 2021, 27, 185–198.
- Farthing D.E., Farthing C.A., Xi L.Inosine and hypoxanthine as novel biomarkers for cardiac ischemia: From bench to point-of-care. Exp Biol Med (Maywood). 2015, 240, 821–831. [CrossRef]
- Rivera-Pérez C, Gaxiola-Robles R, Olguín-Monroy N.O., et al.Effect of hypoxia on purine metabolism in human skeletal muscle cells. Biotecnia. 2021, 23, 141–148.
- Zhang W, Miikeda A, Zuckerman J, et al. Inhibition of microbiota-dependent TMAO production attenuates chronic kidney disease in mice. Sci Rep. 2021, 11, 518. [Google Scholar] [CrossRef]
- Brühl A, Hafner G, Löffelholz K. Release of choline in the isolated heart, an indicator of ischemic phospholipid degradation and its protection by ischemic preconditioning: no evidence for a role of phospholipase D. Life Sci. 2004, 75, 1609–1620. [Google Scholar] [CrossRef]
- Lindeman J.H., Wijermars L.G., Kostidis S, et al.Results of an explorative clinical evaluation suggest immediate and persistent post-reperfusion metabolic paralysis drives kidney ischemia reperfusion injury. Kidney Int. 2020, 98, 1476–1488. [CrossRef]
- Møller N, Meek S, Bigelow M, Andrews J, Nair K.S. The kidney is an important site for in vivo phenylalanine-to-tyrosine conversion in adult humans: A metabolic role of the kidney. Proceedings of the National Academy of Sciences. 2000, 97, 1242–1246. [CrossRef]
- Kopple, J.D. Phenylalanine and tyrosine metabolism in chronic kidney failure. J Nutr. 2007, 137 (6 Suppl 1), 1586S-1590S; discussion 1597S-1598S. [Google Scholar] [CrossRef]
- Supavekin S, Zhang W, Kucherlapati R, Kaskel F.J, Moore L.C., Devarajan P. Differential gene expression following early renal ischemia/reperfusion. Kidney Int. 2003, 63, 1714–1724. [CrossRef]
- Wee H.N., Liu J.J., Ching J, Kovalik J.P., Lim S.C. The Kynurenine Pathway in Acute Kidney Injury and Chronic Kidney Disease. Am J Nephrol. 2021, 52, 771–787. [CrossRef]
- Zulpaite R, Miknevicius P, Leber B, Strupas K, Stiegler P, Schemmer P. Tryptophan Metabolism via Kynurenine Pathway: Role in Solid Organ Transplantation. International Journal of Molecular Sciences. 2021, 22, 1921. [Google Scholar] [CrossRef]
- Goek O.N., Prehn C, Sekula P, et al. Metabolites associate with kidney function decline and incident chronic kidney disease in the general population. Nephrol Dial Transplant. 2013, 28, 2131–2138. [CrossRef]
- Silva, R.E., Baldim J.L., Chagas-Paula D.A., et al. Predictive metabolomic signatures of end-stage renal disease: A multivariate analysis of population-based data. Biochimie.
- Vavrincova-Yaghi D, Seelen M.A., Kema I.P., et al. Early Posttransplant Tryptophan Metabolism Predicts Long-term Outcome of Human Kidney Transplantation. Transplantation. 2015, 99, e97–e104. [CrossRef]
- Lahdou I, Sadeghi M, Daniel V, et al. Increased pretransplantation plasma kynurenine levels do not protect from but predict acute kidney allograft rejection. Hum Immunol. 2010, 71, 1067–1072. [Google Scholar] [CrossRef]
- Dharnidharka, V.R, Al Khasawneh E, Gupta S, et al. Verification of association of elevated serum IDO enzyme activity with acute rejection and low CD4-ATP levels with infection. Transplantation. 2013, 96, 567–572. [CrossRef]
| Kidney transplant recipient | |
|---|---|
| Variable | Mean/Frequency |
| Age (years) (mean ± SD) | 53.98 ± 10.94 |
| Gender, n (%) | Male: 35 (70%) Female: 15 (30%) |
| Race, n (%) | Caucasian: 47 (94%) African American: 3 (6%) |
| Blood type, n (%) | 0+: 18 (36%) 0-: 1 (2%) A+: 19 (38%) A-: 6 (12%) B+: 5 (10%) B- 0 (0%). AB+: 1 (2%) AB-: 0 (0%) |
| Hypertension, n (%) | 41 (82%) |
| Type 2-diabetes, n (%) | 8 (16%) |
| Dyslipidemia, n (%) | 35 (70%) |
| BMI (mean ± SD) | 26.58 (3.89) |
| Obesity, n (%) | Underweight: 0 (0%) Normal weight: 18 (36%) Overweight: 18 (36%) Obesity: 14 (28%) |
| Hyperuricemia, n (%) | 17 (34%) |
| Smoking status, n (%) | Non-smoker: 21 (42%) Former smoker: 20 (40%) Current smoker: 9 (18%) |
| Physical activity, n (%) | Sedentary: 39 (78%) Moderately active: 5 (10%) Very active: 6 (12%) |
| Etiology of chronic kidney disease, n (%) | Glomerulonephritis: 5 (10%) Chronic pyelonephritis/tubulointerstitial: 7 (14%) Diabetes mellitus: 6 (12%) Hypertension/vascular diseases: 3 (6%) Hereditary/familial: 13 (26%) Systemic diseases: 4 (8%) Unclassified: 12 (24%) |
| Renal replacement therapy, n (%) | Hemodialysis: 37 (74%) Peritoneal dialysis: 13 (26%) |
| Time on dialysis (years), (mean ± SD) | 3.44 ± 2.32 |
| Residual diuresis, n (%) | <500 ml: 32 (64%) 500-1000 ml: 7 (14%) >1000 ml: 11 (22%) |
| Heart failure, n (%) | 2 (4%) |
| Coronary artery disease, n (%) | 7 (14%) |
| Vascular disease, n (%) | 4 (18%) |
| Previous transplant, n (%) | 6 (12%) |
| Transfusions history, n (%) | 17 (34%) |
| Pregnancy history, n (%) | 12 (24%) |
| Sensitization, n (%) | No: 41 (82%) < 98% PRAc: 6 (12%) >98% PRAc (PATHI): 3 (6%) |
| EPTS, (mean ± SD) | 41.96 ± 26.94 |
| Kidney Donor | |
| Variable | Mean/Frequency |
| Age, (mean ± SD) | 50.58 ± 16.30 |
| Gender, n (%) | Male: 15 (30%) Female: 35 (70%) |
| Donor type, n (%) | DBD: 29 (58%) DCD: 21 (42%) |
| Hypertension, n (%) | 14 (28%) |
| Diabetes mellitus, n (%) | 9 (18%) |
| BMI, (mean ± SD) | 26.29 ± 5.85 |
| Donor AKI, n (%) | 2 (4%) |
| Expanded criteria donor (EC), n (%) | 16 (32%) |
| KDPI, (mean ± SD) | 52.66 ± 28.72 |
| Transplant process | |
| Variable | Mean/Frequency |
| Cold ischemia time, (mean ± SD) | 17.30 ± 4.58 |
| Mismatch 6/6, (mean ± SD) | 4.3 ± 1.18 |
| Mismatch 10/10, (mean ± SD) | 7.2 ± 1.82 |
| One-week events | |
| Variable | Mean/Frequency |
| Overdose of calcineurin inhibitor, n (%) | 30 (60%) |
| Urinary infection, n (%) | 11 (22%) |
| Graft rejection, n (%) | 3 (6%) |
| Graft function, n (%) | Immediate graft function: 10 (20%) Slow graft function: 13 (26%) Delayed graft function: 27 (54%) |
| Six-month post-transplant events (Excluding week 1) | |
| Variable | Mean/Frequency |
| Graft rejection, n (%) | 1 (2%) |
| Urinary infection, n (%) | 18 (36%) |
| CMV infection, n (%) | 3 (6%) |
| BK infection, n (%) | Yes (viremia): 5 (10%) Nephropathy: 0 (0%) |
| MACE | 6 (12%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





