Submitted:
03 October 2024
Posted:
07 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Diets, Animals, and Experimental Design
2.2. Sampling and Data Collection
2.3. HE Staining of Tissue Sections
2.4. Serum Biochemical Indexes and Antioxidant Enzymes
2.5. RNA Extraction, and Quantitative Real-Time PCR
2.5.1. RNA Isolation and cDNA Synthesis
2.5.2. Q-PCR (Quantitative Real-Time Polymerase Chain Reaction)
2.6. Statistical Analysis
3. Results
3.1. Growth Performance
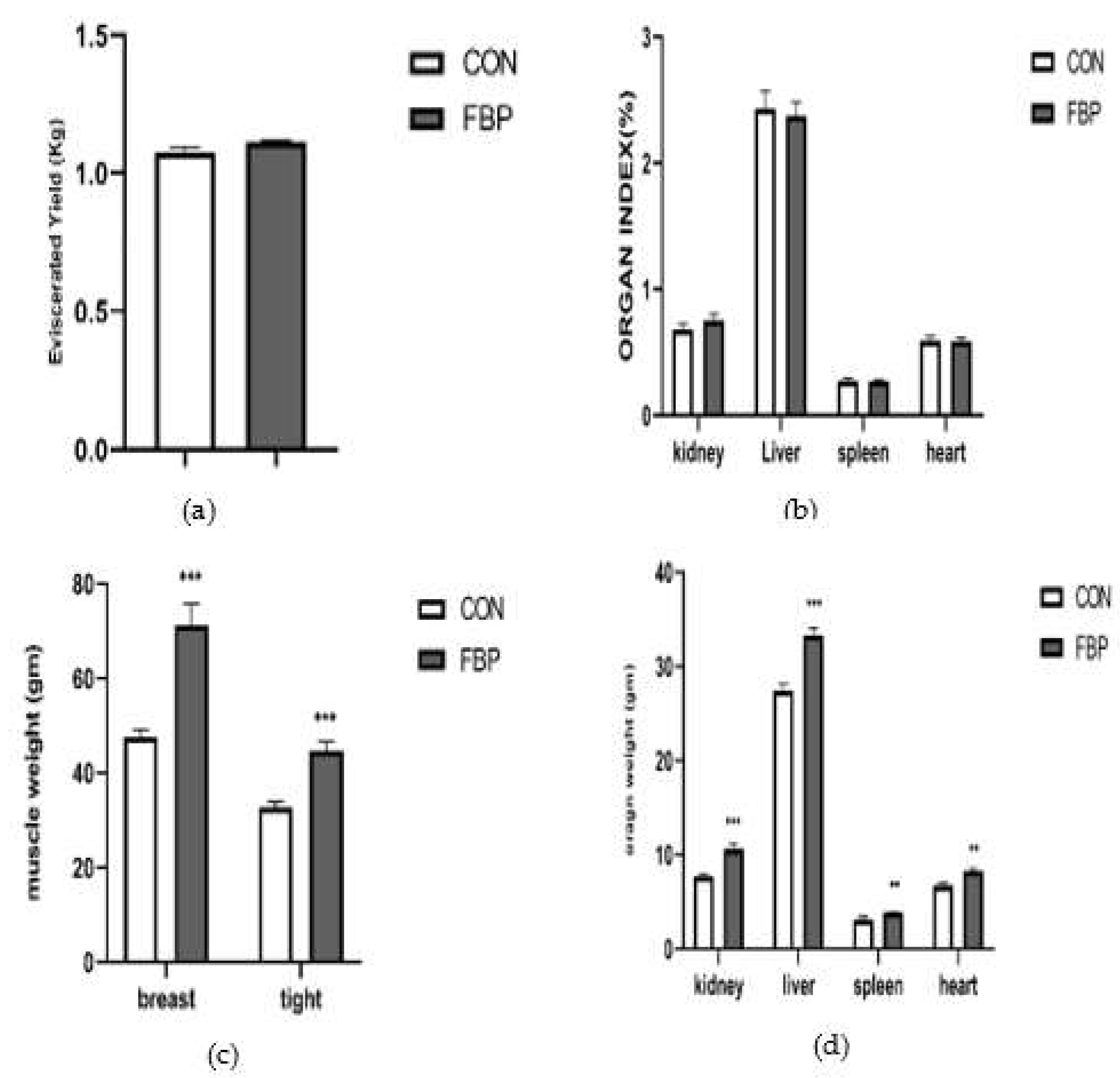
3.2. Carcass and Organ Weight
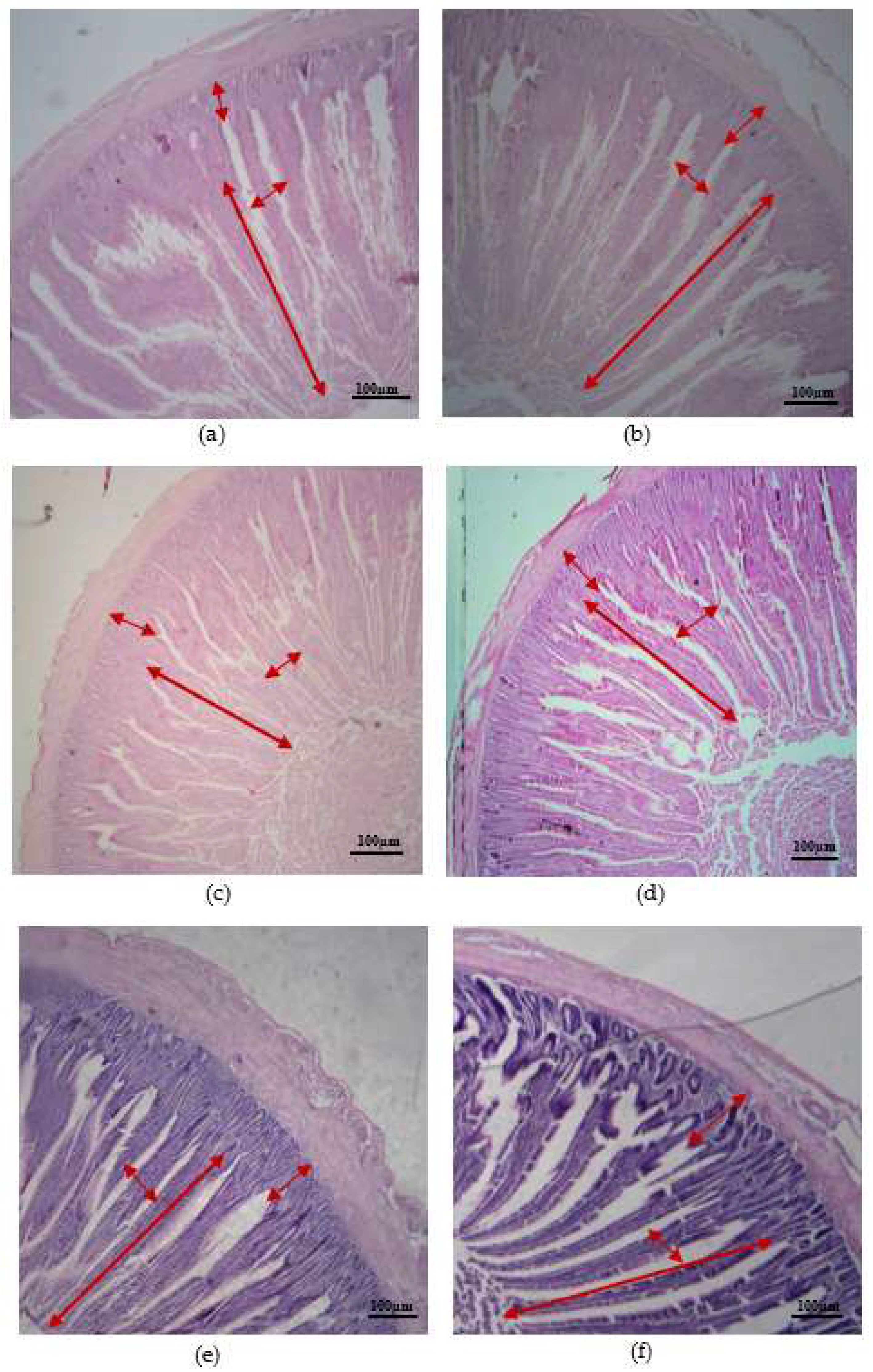
3.3. Intestinal Morphology
3.4. Serum Biochemical Index
3.5. Antioxidant Status
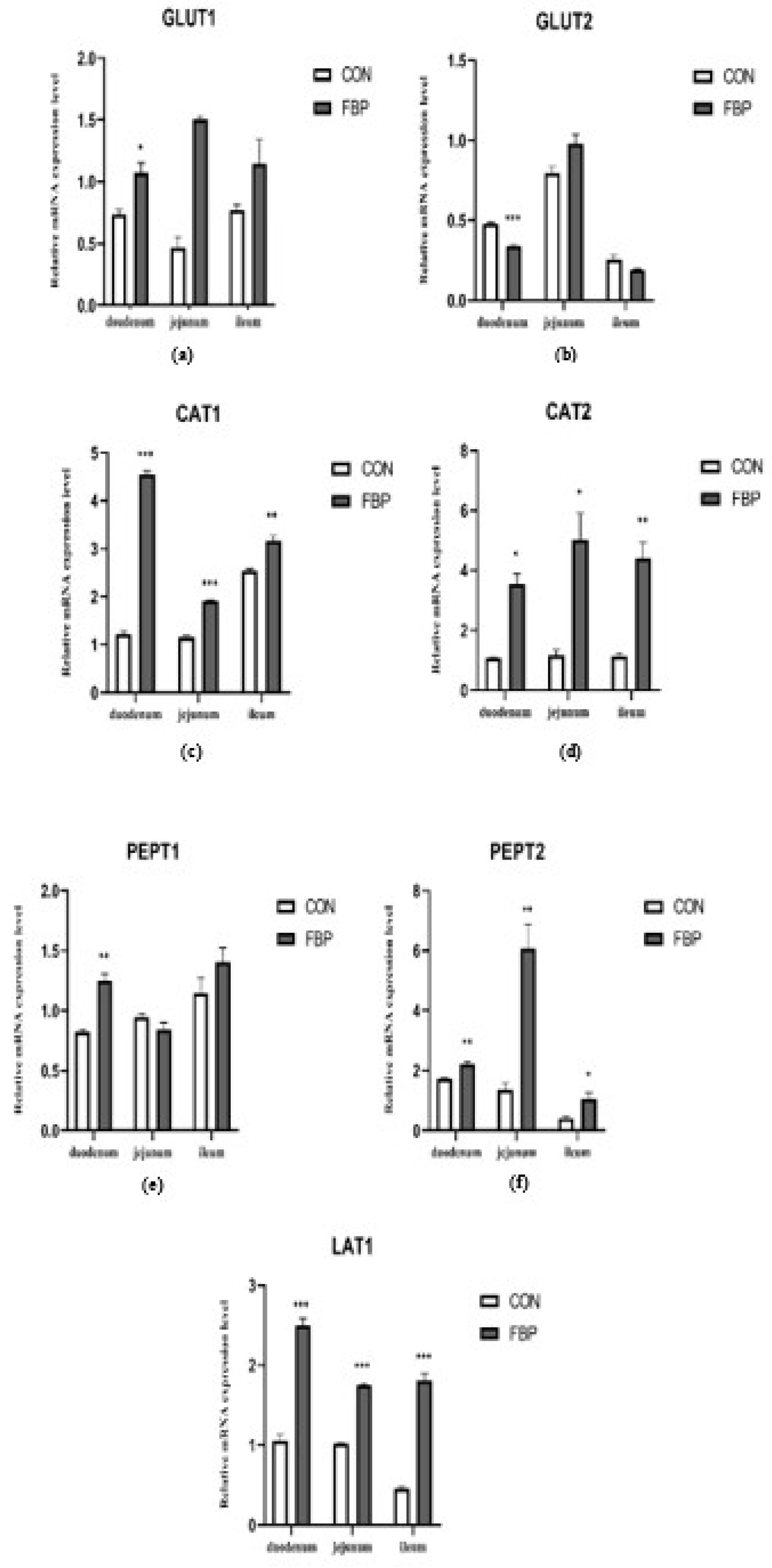
3.6. Effects of FBP on Relative mRNA Expression Levels of Nutrient Transporter Genes in Intestinal
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bian, F., et al., Bamboo - An untapped plant resource for the phytoremediation of heavy metal contaminated soils. Chemosphere, 2020. 246: p. 125750. [CrossRef]
- Qi, S., et al., Bamboo Forest Mapping in China Using the Dense Landsat 8 Image Archive and Google Earth Engine. Remote Sensing, 2022. 14(3). [CrossRef]
- Bisht, N.C.a.M.S., Bamboo: A Prospective Ingredient for Functional Food and Nutraceuticals. 10th World Bamboo Congress, Korea 2015, 2015.
- Cheng, Y., et al., Bamboo leaf: A review of traditional medicinal property, phytochemistry, pharmacology, and purification technology. J Ethnopharmacol, 2023. 306: p. 116166. [CrossRef]
- Rattanawut, J., O. Pimpa, and K.E. Yamauchi, Effects of dietary bamboo vinegar supplementation on performance, eggshell quality, ileal microflora composition, and intestinal villus morphology of laying hens in the late phase of production. Anim Sci J, 2018. 89(11): p. 1572-1580. [CrossRef]
- Nirmala, C., Bisht, M.S., Bajwa, H.K., Santosh, O.,, Bamboo: A Rich Source of Natural Antioxidants and its Applications in the Food and Pharmaceutical Industry. Trends in Food Science & Technology, 2018. [CrossRef]
- Pei, R., X. Liu, and B. Bolling, Flavonoids and gut health. Curr Opin Biotechnol, 2020. 61: p. 153-159. [CrossRef]
- Kwon, J.H., S.Y. Hwang, and J.S. Han, Bamboo (Phyllostachys bambusoides) leaf extracts inhibit adipogenesis by regulating adipogenic transcription factors and enzymes in 3T3-L1 adipocytes. Food Sci Biotechnol, 2017. 26(4): p. 1037-1044. [CrossRef]
- Van Hoyweghen, L., et al., Phenolic compounds and anti-oxidant capacity of twelve morphologically heterogeneous bamboo species. Phytochem Anal, 2012. 23(5): p. 433-43. [CrossRef]
- Rattanawut, J., et al., Effects of bamboo charcoal powder, bamboo vinegar, and their combination in laying hens on performance, egg quality, relative organ weights, and intestinal bacterial populations. Trop Anim Health Prod, 2021. 53(1): p. 83. [CrossRef]
- Huang, Y., et al., Effects of Fermented Bamboo Shoot Processing Waste on Growth Performance, Serum Parameters, and Gut Microbiota of Weaned Piglets. Animals (Basel), 2022. 12(20). [CrossRef]
- Xiao, Z., et al., Structural characterization, antioxidant and antimicrobial activity of water-soluble polysaccharides from bamboo (Phyllostachys pubescens Mazel) leaves. Int J Biol Macromol, 2020. 142: p. 432-442. [CrossRef]
- Liu, Z., et al., Effects of Fermented Bamboo Powder Supplementation on Serum Biochemical Parameters, Immune Indices, and Fecal Microbial Composition in Growing-Finishing Pigs. Animals (Basel), 2022. 12(22). [CrossRef]
- Fotiadis, D., Y. Kanai, and M. Palacin, The SLC3 and SLC7 families of amino acid transporters. Mol Aspects Med, 2013. 34(2-3): p. 139-58. [CrossRef]
- Roder, P.V., et al., The role of SGLT1 and GLUT2 in intestinal glucose transport and sensing. PLoS One, 2014. 9(2): p. e89977. [CrossRef]
- Malyar, R.M., et al., Fermented Bamboo Powder Activates Gut Odorant Receptors, and Promotes Intestinal Health and Growth Performance of Dwarf Yellow-Feathered Broiler Chickens. Poultry Science, 2024. [CrossRef]
- Nain, S., et al., Effect of metabolic efficiency and intestinal morphology on variability in n-3 polyunsaturated fatty acid enrichment of eggs. 2012. 91(4): p. 888-898.
- Jimenez-Moreno, E., et al., Inclusion of insoluble fiber sources in mash or pellet diets for young broilers. 1. Effects on growth performance and water intake. Poult Sci, 2016. 95(1): p. 41-52. [CrossRef]
- Jimenez-Moreno, E., et al., Insoluble fiber sources in mash or pellets diets for young broilers. 2. Effects on gastrointestinal tract development and nutrient digestibility1. Poult Sci, 2019. 98(6): p. 2531-2547. [CrossRef]
- Pourazadi, Z., et al., Effect of particle size of insoluble fibre on growth performance, apparent ileal digestibility and caecal microbial population in broiler chickens fed barley-containing diets. Br Poult Sci, 2020. 61(6): p. 734-745. [CrossRef]
- Tejeda, O.J. and W.K. Kim, Effects of fiber type, particle size, and inclusion level on the growth performance, digestive organ growth, intestinal morphology, intestinal viscosity, and gene expression of broilers. Poult Sci, 2021. 100(10): p. 101397. [CrossRef]
- Berrocoso, J.D., et al., The effect of added oat hulls or sugar beet pulp to diets containing rapidly or slowly digestible protein sources on broiler growth performance from 0 to 36 days of age. Poult Sci, 2020. 99(12): p. 6859-6866. [CrossRef]
- Ramakrishnan, M., et al., Genetics and genomics of moso bamboo (Phyllostachys edulis): Current status, future challenges, and biotechnological opportunities toward a sustainable bamboo industry. Food and Energy Security, 2020. 9(4). [CrossRef]
- Dai, F., et al., Effects of micronized bamboo powder on growth performance, serum biochemical indexes, cecal chyme microflora and metabolism of broilers aged 1-22 days. Trop Anim Health Prod, 2022. 54(3): p. 166. [CrossRef]
- Dai, F., et al., Effects of micronised bamboo powder on growth performance, intestinal development, caecal chyme microflora and metabolic pathway of broilers aged 24–45 days. 2023. [CrossRef]
- Ogbuewu, I.P., O.O. Emenalom, and I.C. Okoli, Alternative feedstuffs and their effects on blood chemistry and haematology of rabbits and chickens: a review. Comparative Clinical Pathology, 2015. 26(2): p. 277-286. [CrossRef]
- Weingartner, O., et al., Alterations in cholesterol homeostasis are associated with coronary heart disease in patients with aortic stenosis. Coron Artery Dis, 2009. 20(6): p. 376-82. [CrossRef]
- Lee, E.J., et al., Proteasome inhibition protects against diet-induced gallstone formation through modulation of cholesterol and bile acid homeostasis. Int J Mol Med, 2018. 41(3): p. 1715-1723. [CrossRef]
- Oloruntola, O.D., et al., Neem, pawpaw and bamboo leaf meal dietary supplementation in broiler chickens: Effect on performance and health status. 2019. 43(2): p. e12723.
- Ge, Q., et al., Investigation of physicochemical properties and antioxidant activity of ultrafine bamboo leaf powder prepared by ball milling. Journal of Food Processing and Preservation, 2020. 44(7). [CrossRef]
- Luo, X., et al., Hydration properties and binding capacities of dietary fibers from bamboo shoot shell and its hypolipidemic effects in mice. Food Chem Toxicol, 2017. 109(Pt 2): p. 1003-1009. [CrossRef]
- Kim, J.S., et al., Effects of energy levels of diet and β-mannanase supplementation on growth performance, apparent total tract digestibility and blood metabolites in growing pigs. Animal Feed Science and Technology, 2013. 186(1-2): p. 64-70. [CrossRef]
- Wang, H., et al., Effects of replacing soybean meal by detoxified Jatropha curcas kernel meal in the diet of growing pigs on their growth, serum biochemical parameters and visceral organs. Animal Feed Science and Technology, 2011. 170(1-2): p. 141-146. [CrossRef]
- Kelly E. Heim, A.R.T., Dennis J. Bobilya, Flavonoid antioxidants: chemistry, metabolism and structure-activity relationships. Journal of Nutritional Biochemistry, 2002(13): p. 572–584. [CrossRef]
- Niu, Y., et al., Effect of supplemental fermented Ginkgo biloba leaves at different levels on growth performance, meat quality, and antioxidant status of breast and thigh muscles in broiler chickens. Poult Sci, 2017. 96(4): p. 869-877. [CrossRef]
- Wan, X.L., et al., Evaluation of enzymatically treated Artemisia annua L. on growth performance, meat quality, and oxidative stability of breast and thigh muscles in broilers. Poult Sci, 2017. 96(4): p. 844-850. [CrossRef]
- Shen, M., et al., Effect of Bamboo Leaf Extract on Antioxidant Status and Cholesterol Metabolism in Broiler Chickens. Animals (Basel), 2019. 9(9). [CrossRef]
- Montagne, L., J.R. Pluske, and D.J. Hampson, A review of interactions between dietary fibre and the intestinal mucosa, and their consequences on digestive health in young non-ruminant animals. Animal Feed Science and Technology, 2003. 108(1-4): p. 95-117. [CrossRef]
- Xie, Z., et al., Effects of bamboo leaf extract on energy metabolism, antioxidant capacity, and biogenesis of small intestine mitochondria in broilers. 2023. 101: p. skac391. [CrossRef]
- Li, Q., et al., Retarding effect of dietary fibers from bamboo shoot (Phyllostachys edulis) in hyperlipidemic rats induced by a high-fat diet. Food Funct, 2021. 12(10): p. 4696-4706. [CrossRef]
- Kaminski, N.A. and E.A. Wong, Differential mRNA expression of nutrient transporters in male and female chickens. Poult Sci, 2018. 97(1): p. 313-318. [CrossRef]
- Wright, E.M. and E. Turk, The sodium/glucose cotransport family SLC5. Pflugers Arch, 2004. 447(5): p. 510-8. [CrossRef]
- Daniel, H., Molecular and integrative physiology of intestinal peptide transport. Annu Rev Physiol, 2004. 66: p. 361-84. [CrossRef]
- Kheravii, S.K., et al., Upregulation of genes encoding digestive enzymes and nutrient transporters in the digestive system of broiler chickens by dietary supplementation of fiber and inclusion of coarse particle size corn. BMC Genomics, 2018. 19(1): p. 208. [CrossRef]
| Item | Starter phase 1-22 | Grower phase 23-45 |
|---|---|---|
| Corn | 406.6 | 297.3 |
| Wheat | 200 | 400 |
| Soybean meal | 223.1 | 57.3 |
| Sunflower | 30 | 50 |
| Rapeseed meal | 30 | 40 |
| Palm kernel meal | 0 | 20 |
| Corn gluten meal | 40 | 50 |
| Rice husk oil | 23.1 | 42.2 |
| Calcium bisphosphate | 14 | 10.3 |
| Limestone | 11.8 | 11.2 |
| Liquid methionine (88%) | 1.4 | 2.3 |
| Premix | 20 | 20 |
| Total | 1000 | 1000 |
| Calculation of nutrients | ||
| Metabolizable energy (MJ/kg) | 2956 | 3008 |
| Crude protein | 211.4 | 208.7 |
| Crude fat | 40.48 | 44.1 |
| Methionine | 4.86 | 7.64 |
| Lysine | 10.97 | 13.65 |
| Calcium | 9.89 | 9.62 |
| Available phosphorus | 4.85 | 5.1 |
| Item | FBP Composition (%) |
|---|---|
| Moisture | 11.21 |
| Crude protein | 17.07 |
| Coarse fiber | 17.66 |
| Crude fat | 3.48 |
| Coarse Ash content | 9.21 |
| Acid soluble protein | 7.13 |
| Acid washing lignin | 3.41 |
| Calcium (%) | 0.12 |
| Total phosphorus (%) | 0.02 |
| Gene | Reverse primer (5′-3′) | Amplicon Size (bp) |
Accession no |
|---|---|---|---|
| β-actin | F/ GCCCTCTTCCAGCCATCTTT R/CAATGGAGGGTCCGGATTCA | 107bp | NM_205518.2 |
| GLUT1 | F/ATGGGCTTCCAGTACATTGC R/TTTGTCTCCGGCACCTTGA |
110 bp | NM_205209.2 |
| GLUT2 | F/ GTTCCTGGCTGGTCTGATGG R/ TGGCGACCATGCTGACATAA |
107 bp | NM_207178.2 |
| CAT1 | F/ GCAAAGCGACTTTCCGGACT R/GCCTGTAAGAAACTCTGAGAAACC |
132 bp | NM_001398060.1 |
| CAT2 | F/TTGCTACATTGGTGGTGTCCT R/ TGAAACCAAGTGCCATCCAG |
198 bp | XM_040699004.2 |
| LAT1 | F/ TGCGTTACAAGAAGCCGGAG R/ CGATCCCGCATTCCTTTGGT |
129 bp | XM_046911929.1 |
| PepT1 | F/CCTTATCGTGGCTGGAGCAT R/TGGGCTTCAACCTCATTTGGA |
144 bp | NM_204365.2 |
| PepT2 | F/ TAGGTCATCCAACCTGCTCCT R/TGCCTGGAGGAGAAAGAACAC |
109 bp | NM_001319028.3 |
| Parameters | CON | FBP | SEM | p-value |
|---|---|---|---|---|
| Initial BW(g) | 51.98 | 52.35 | 0.53 | 0.477 |
| 1-22 day(1% FBP) | ||||
| BW (g) | 424.83 | 466.88*** | 4.95 | <0.001 |
| BWG(g) | 364.53 | 448.30*** | 7.23 | <0.001 |
| ADFI (g) | 49.21 | 46.00 | 2.96 | 0.334 |
| ADG (g) | 17.36 | 21.35*** | 0.35 | <0.001 |
| FCR | 2.83 | 2.15 | 0.34 | 0.086 |
| Mortality rate % | 1.33 | 1.17 | 0.54 | 0.766 |
| 23-45 day(2%FBP) | ||||
| BW (g) | 1154.33 | 1288.67*** | 21.52 | <0.001 |
| BWG (g) | 728.33 | 813.07*** | 20.93 | <0.001 |
| ADFI (g) | 78.18 | 73.73 | 4.03 | 0.332 |
| ADG (g) | 31.67 | 35.35*** | 0.91 | <0.001 |
| FCR | 2.47 | 2.09* | 0.19 | 0.053 |
| Mortality rate % | 1.25 | 1.00 | 0.32 | 0.470 |
| 1-45 day | ||||
| BW (g) | 1154.33 | 1288.67*** | 21.52 | <0.001 |
| BWG (g) | 1141.03 | 1275.15*** | 26.05 | <0.001 |
| ADFI (g) | 70.71 | 66.57 | 3.72 | 0.330 |
| ADG (g) | 25.93 | 28.98*** | 0.59 | <0.001 |
| FCR | 2.73 | 2.30* | 0.22 | 0.054 |
| Mortality rate % | 2.58 | 2.08 | 0.23 | 0.069 |
| Item | CON | FBP | SEM | p-value |
|---|---|---|---|---|
| duodenum | ||||
| VH(mm) | 0.58 | 0.67** | 0.02 | 0.003 |
| CD (mm) | 0.10 | 0.17 | 0.04 | 0.096 |
| VCR | 6.63 | 4.81 | 1.08 | 0.153 |
| VA (mm2) | 1.66 | 1.92 | 0.21 | 0.263 |
| jejunum | ||||
| VH (mm) | 0.46 | 0.59** | 0.03 | 0.007 |
| CD (mm) | 0.09 | 0.13** | 0.01 | 0.007 |
| VCR | 5.12 | 4.66 | 0.45 | 0.347 |
| VA (mm2) | 1.80 | 2.01 | 0.27 | 0.474 |
| ileum | ||||
| VH (mm) | 0.60 | 0.74* | 0.05 | 0.038 |
| CD (mm) | 0.34 | 0.49* | 0.04 | 0.021 |
| VCR | 1.85 | 1.52 | 0.20 | 0.142 |
| VA (mm2) | 1.55 | 2.86* | 0.50 | 0.048 |
| Parameters | Dietary treatment | SEM | p-value | |
|---|---|---|---|---|
| CON FBP | ||||
| TC(mmol/L) | 3.38 | 2.78 | 0.54 | 0.323 |
| TG(mmol/L) | 0.80 | 0.57* | 0.07 | 0.025 |
| GLU(mmol/L) | 7.25 | 6.55 | 0.70 | 0.079 |
| LDL(mmol/L) | 1.14 | 0.81 | 0.32 | 0.406 |
| HDL(mmol/L) | 1.87 | 2.25 | 0.16 | 0.133 |
| TP(g/L) | 23.80 | 26.35 | 1.24 | 0.108 |
| ALB(g/L) | 13.40 | 14.57 | 1.06 | 0.385 |
| GLB(g/L) | 11.63 | 12.77 | 1.57 | 0.510 |
| sCr (umol/L) | 10.73 | 8.47 | 3.14 | 0.511 |
| UREA(mmol/L) | 1.22 | 0.59 ⃰ | 0.14 | 0.011 |
| AST(U/L) | 303.30 | 259.83 | 29.87 | 0.277 |
| ALT(U/L) | 3.33 | 2.30 ⃰ | 0.31 | 0.028 |
| Item | CON | FBP | SEM | p-value |
|---|---|---|---|---|
| SOD (U/ml) | 48.79 | 122.90** | 5.28 | 0.005 |
| GSH-PX (U/mg) | 119.64 | 136.63 | 7.31 | 0.146 |
| CAT(nmol/mg prot) | 40.73 | 116.38** | 3.84 | 0.003 |
| MAD(nmol/mg prot) | 2.29 | 0.49** | 0.19 | 0.011 |
| HO-1(pg/ml) | 255.37 | 243.03 | 22.01 | 0.631 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





