1. Introduction
Osteoarthritis (OA) is a chronic degenerative inflammatory joint disease with a high prevalence and with a negative impact on quality of life and a high economic burden [
1]. The most common form of OA is that involving the hands, which affects females three times more often. OA of the base of the first finger is present in 21% of the population over 40 years of age and is more frequently related to pain and disability than OA of the interphalangeal joint [
2]. In addition to pain, it can cause deformity, stiffness, reduced mobility and strength, resulting in difficulty performing common activities such as opening vessels, carrying weights and writing [
1].
OA of the base of the first finger is mainly treated conservatively, while surgical treatment is reserved for those whose debilitating symptoms persist despite adequate conservative management [
3]. Surgical management, however, is associated with a number of complications, including tendon rupture, sensory changes, and wound infection [
4]. The traditional treatment involves a period of immobilization with a brace with splint of the first finger, associated with physiotherapy treatment [
5]. The use of the brace reduces pain, preserves first web space, stabilizes the base of the first metacarpal during pinching, prevents adduction of the head of the first metacarpal into the palm of the hand and dorsal subluxation of the base of the metacarpal trapezius [
6]. A program of specific exercises for the thenar muscles (traditionally pinching exercises) allows you to rebalance the deforming force of the trapezoid-metacarpal joint, in which a strong traction by the adductor muscle of the thumb occurs, combined with the weakness of the intrinsic muscles thenar [
5]. High-quality evidence shows that unimodal and multimodal physical therapy treatments can bring clinically useful improvements in pain and function for patients with thumb base OA [
7]. The European League Against Rheumatism (EULAR) guidelines [
8] recommend that a thorough evaluation of physical treatments, such as ultrasound, laser, analgesic currents and local application of heat, is necessary in patients suffering from rhizarthrosis. The first preliminary experiences relating to high-energy laser therapy have demonstrated effectiveness in reducing pain at 12 weeks [
9]. As regards shock wave therapy, so far, only one work has advanced its potential for the treatment of rhizoarthrosis [
10].
The aim of this study is to compare the effects of a shock wave treatment compared to a standard treatment with exercises in patients suffering from arthritis of the first finger. Both treatments were associated with the use of a brace.
2. Materials and Methods
The present study is a prospective randomized clinical trial, to evaluate clinical and functional outcomes and satisfaction in patients with osteoarthritis of the first finger of the hand. The Territorial Ethics Committee of the “Consorziale Policlinico” University Hospital authorized the study (Interregional Ethics Committee, approval no. 7814, meeting of 12/20/2023). Participants gave their written informed consent. The trial was registered at
www.clinical.trial.gov with the trial registration number NCT06056765.
The patients were examined in the outpatient clinic of the Orthopedics and Traumatology Unit of the Policlinico di Bari, Italy. All eligible patients suffering from pain on the radial side of the carpus, which suggested arthrosis of the trapezoid-metacarpal joint, were examined by a hand surgeon. If the patient fell within the following stated criteria, they were selected for the study. The inclusion criteria were: trapeziometacarpal arthrosis with stage 1 or 2 of the Eaton-Littler radiographic classification and pain (recent radiograph within 6 months previously) [
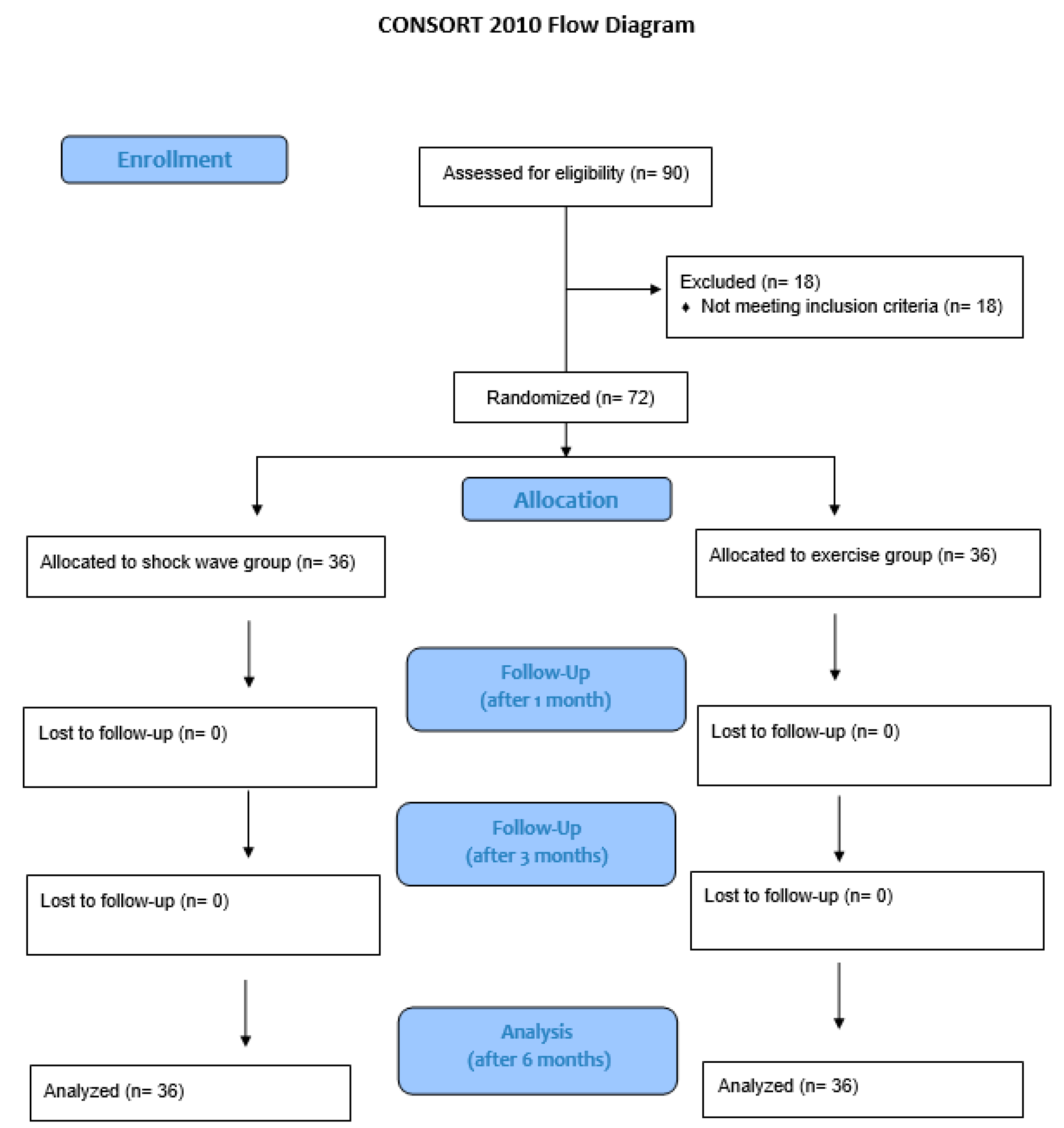
11]; clinical picture that has been occurring for at least 6 months; pain, counted with Visual Analogue Scale (VAS), of at least 4/10. The exclusion criteria are: rheumatoid arthritis or results of trauma in the affected area; contra-indications to treatment with Extracorporeal Shock Wave Treatment (ESWT) (neoplasia, pregnancy, thrombocytopenia, epilepsy, uncompensated heart disease or arrhythmia, pacemaker, local infections); corticosteroid infiltration or physical therapy in the previous 4 weeks. Seventy-two consecutive patients were recruited. Patients were randomized to two types of treatment: shock wave therapy (36 patients) (shock wave group) or therapeutic exercise (36 patients) (exercise group). All patients used a 1-finger splint.
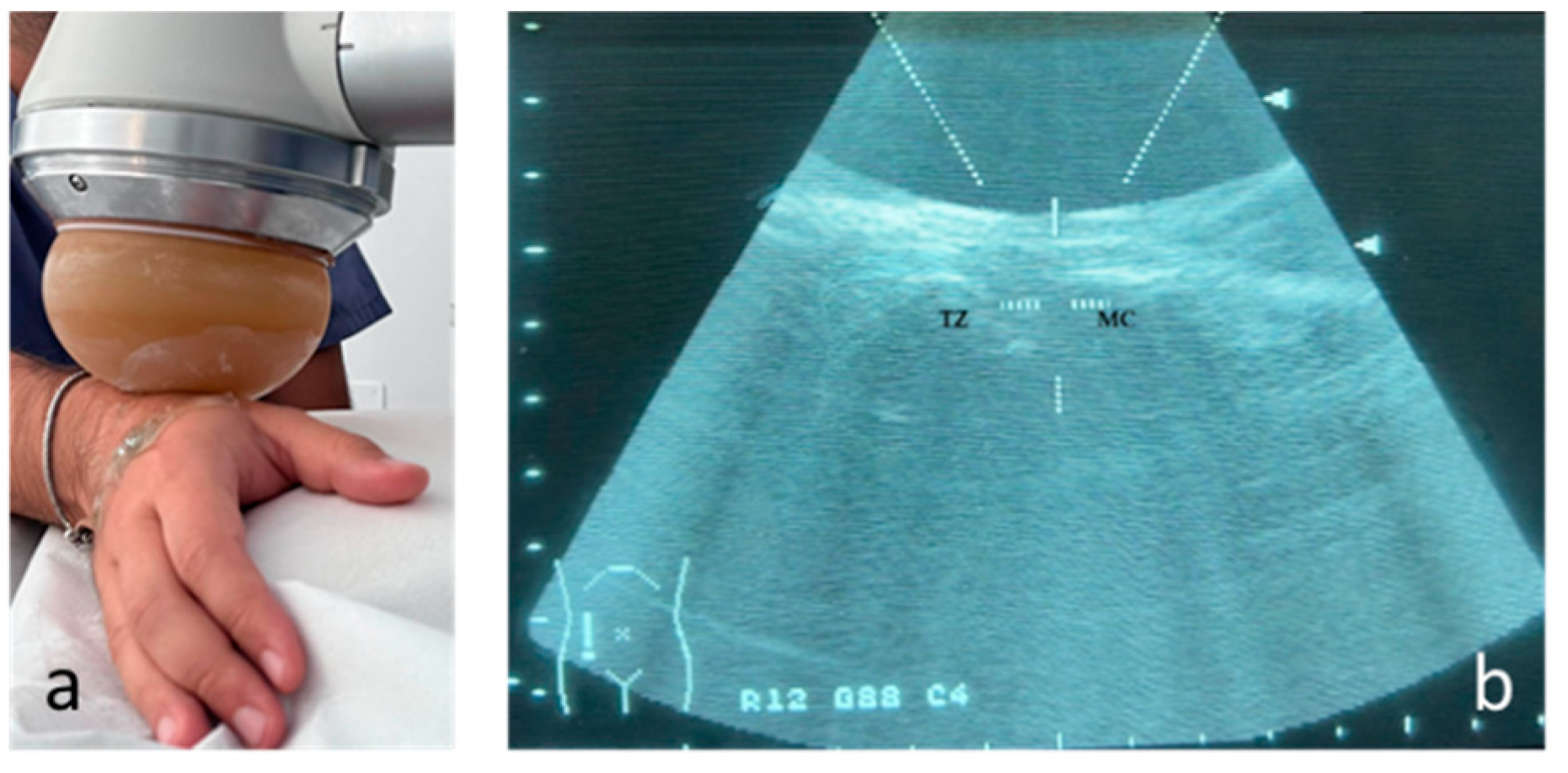
The therapy was applied using a focused shock wave device (Minilith, Storz, Swiss) at the trapezius metacarpal joint, under ultrasound guidance (
Figure 1). The shock wave therapy was performed with the patient's hand in intermediate prono-supination and was administered once a week, for 3 sessions. For each treatment session, 2000 pulses were applied with an average energy flux density (EDF) between 0.03 and 0.08 mJ/mm2 and a frequency of 4 pulses per second (4 Hz). Gel was used between the probe and the skin during applications to ensure conductivity. No local anesthetic was used. The protocol was defined in accordance with the literature [
12].
Patients were instructed in a stretching, stabilization and strengthening program for the thumbs in the 4 weeks following recruitment [
13]. These exercises were aimed at stabilizing the muscles of the thumb, the first dorsal interosseous, the abductor pollicis and flexor pollicis brevis (FPB) muscles). The exercise was initially presented as active movement and, if tolerated, resistance was added. After initial instruction on the exercise program in the office, subjects were instructed to continue performing the exercises at home, 2-3 times per day, every day. Each set of exercises took approximately 10 minutes to perform. At recruitment, patients were instructed on how to perform the exercises. The first treatment session was conducted by an expert physiotherapist in order to demonstrate the exercise program. Once a week, the method and frequency of carrying out the exercises were checked.
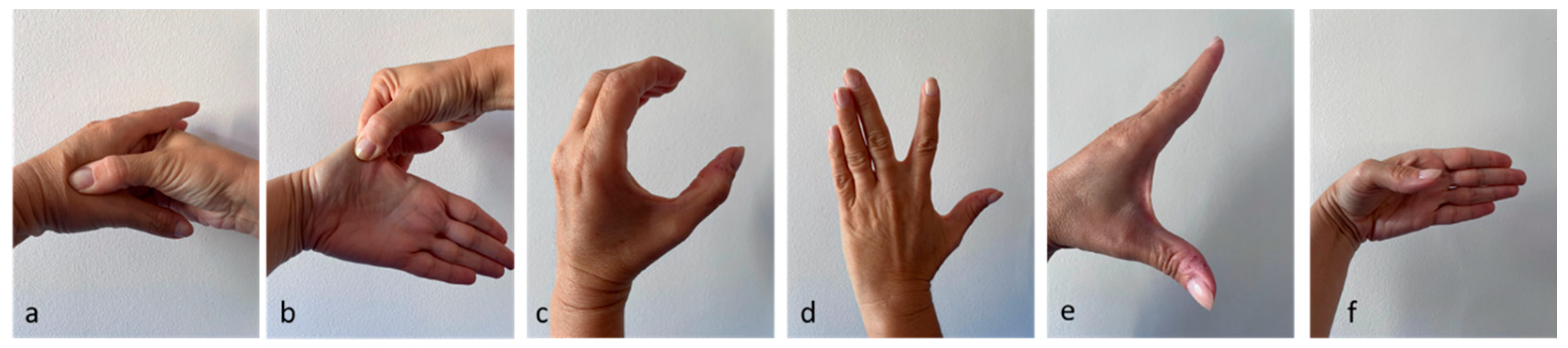
The exercises proposed were the following (
Figure 2):
1. Massage on the space between 1st and 2nd finger (thenar eminence) for 3 minutes (a) [
14,
15].
2. Extend the space between 1st and 2nd finger, maintaining for 30 seconds and perform 4 times per session (b) [
14].
3. "C" contraction: position the first finger and the remaining 4 fingers as if one wanted to form a "C"; maintain for 30 seconds and perform 4 times per session (c) [
14,
15].
4. Active range of motion of the first dorsal interosseous: perform an active radial deviation of the index finger, with the hand resting on the table, for ten repetitions [
14,
15,
16]. If the subject was able to complete 10 repetitions without pain, at the next session the exercise was performed with resistance (manual or through the use of an elastic band). The subject was asked to gradually increase the size and/or strength of the rubber band as much as possible. If this exercise was painful, they were asked to return to active movement(d).
5. Active thumb abduction: active thumb abduction to maintain and/or increase the space between 1 and 2 fingers, for ten repetitions [
14,
15,
17,
18]. If the subject was able to complete 10 repetitions without pain, at the next session he was asked to perform this exercise with resistance (manually or through the use of an elastic band). The subject was asked to gradually increase the size and/or strength of the rubber band as much as possible. If this resistance exercise was painful, it was suggested to return to active movement only (e).
6. Active flexion of the first finger: perform a flexion of the trapezium-metacarpal joint, for ten repetitions [
15,
19]. If the subject was able to complete 10 repetitions with good technique, resistance was added manually or with rubber bands in the next session. If this exercise was painful, they are asked to return to active movement only (f).
Patients in both groups were instructed to maintain full-time splinting for four weeks to avoid mechanical stress on the trapezio-metacarpal joint to promote the resolution of inflammation and pain [
20,
21]. After this period, they were instructed regarding manual activities that should be avoided in order to prevent recurrence of pain in the metacarpal trapezoid joint; these included the exclusion of a strong grip, an imbalance between joint movement and rest, exposure of the finger joints to vibration, and using the joint on an unstable plane [
22].
The assessments were conducted at the beginning of treatment (T0), at 1 month (T1), at 3 months (T2) and at 6 months (T3). At each time point, VAS, Functional index for hand osteoarthritis (FIHOA) and Disabilities of the Arm, Shoulder and Hand (DASH) were administered. At T1, T2 and T3 the Roles and Maudsley Score was assessed.
Pain was measured with the VAS scale, which consists of a 10 cm horizontal line (with 0 cm corresponding to no pain and 10 cm corresponding to worst pain ever experienced) [
23].
Hand functionality was studied with the Functional Index for Hand Osteoarthritis (FIHOA), which is a 10-item questionnaire, with 4 possible answers for each question (0=possible without difficulty, 1=possible slight with difficulty, 2=possible with important difficulty, 3=impossible); the score is between 0 (no limitation) and 30 (maximum limitation) [
24].
The disability is analyzed with the Disabilities of the Arm, Shoulder and Hand (DASH): 11 questions with 5 possible answers each (1=No difficulty, 2=Mild difficulty, 3=Moderate difficulty, 4=Severe difficulty, 5 =Unable) and a total score ranging from 11 (no difficulty) to 55 (inability) [
25].
The Roles and Maudsley Score evaluates the patient's perception of improvement, from 1 (excellent result with no symptoms following treatment) to 4 (poor, symptoms identical or worse than pre-treatment) [
26].
Statistical Analysis
Continuous variables will be presented as mean ± standard deviation and range, while categorical variables will be expressed as proportions. To compare continuous variables between groups, either the independent t-test or the Wilcoxon rank-sum test will be utilized, depending on the data distribution. For the comparison of categorical variables between groups, the chi-square test will be employed.
To estimate the sample size, we considered a VAS value of 7.5 at enrollment (T0) for both groups, with an average reduction at the primary endpoint (6 months) of 4.8 in the control group [
13] and 3.8 in the treatment group [
10], with a standard deviation of 1.4 for both groups. Sample size estimation was conducted using a t-test, with a significance level (alpha) set at 0.05 and a test power of 80%. The estimated sample size was 64 subjects, and to account for a potential 15% loss at follow-up, we aimed to recruit 72 subjects (36 per group). This effect was selected as the smallest effect that would be important to detect, in the sense that any smaller effect would not be of clinical or substantive significance.
The database was built via Microsoft Excel® 2019. The assignment to the groups was performed through randomization, ensuring homogeneity between the two groups for covariates such as sex and age. Randomization was conducted using Stata MP17® software.®. All calculations were performed via Stata MP17® software.®.
3. Results
Population characteristics
The study enrolled 72 subjects, evenly distributed between the study group and control group (
Figure 3).
The population’s characteristics are resumed in
Table 1 and
Table 2. The mean age at diagnosis was 65.40 years (±8.49 years), while the mean Body Mass Index (BMI) was 25.02 kg/m2 (±4.14 kg/m2). The mean time since the beginning of arthritic symptoms was 9.18 months (±2.41 months).
The VAS, FIHOA, DASH and Roles and Maudsley score values over time are described in
Table 3. Normality was proven for the distribution of the VAS values over time. All other scores showed non-normal distribution in the study population. Therefore, the latter were studied via the Wilcoxon signed-rank test.
The mean VAS score showed a significant decrease from T0 to T3 in both the shock wave group (t: 12.80; p-value <0.001) and the exercise group (t: 15.29; p-value <0.001), with a mean reduction of 4.81 (±2.25) in the shock wave group and 2.75 (±1.08) in the exercise group. When considering the intermediate checkpoints, both groups showed a significant reduction of the VAS score from T0 to T1 and from T1 to T2. For the shock wave group, in particular, the T0-T1 interval highlighted a mean 3.39 (±1.76) decrease (t: 11.55; p-value <0.001) and the T1-T2 interval had a mean decrease of 0.72 (±1.14) (t: 3.81; p-value <0.001). For the exercise group, the T0-T1 interval’s VAS score reduction was 1.83 (±0.74) (t: 14.93; p-value <0.001), while the T1-T2 interval had a mean 1.28 (±1.00) decrease (t: 7.64; p-value <0.001). However, when the last interval was considered, a significant 0.69 (±1.01) decrease of the VAS score was highlighted in the shock wave group only (t: 4.13; p-value <0.001), while the exercise group showed a slight, yet significant 0.36 (±1.25) increase of this score (t: -1.74; p-value: 0.045).
The FIHOA score showed a significant reduction over time in both groups. The decrease was 7.08 (±6.03) in the shock waves group (z: 5.12; p-value <0.001) and 5.28 (±3.82) in the exercise group (z: 5.14; p-value <0.001). Analyzing the single checkpoints, a consistently significant reduction was identified in the shock wave group for both the T0-T1 (mean: 5.75 ±5.23; z: 5.03; p-value <0.001) and T1-T2 interval (mean: 0.83 ±1.71; z: 3.23; p-value: 0.001) and in the exercise group for the same intervals (T0-T1 mean: 4.64 ±3.36; z: 5.22; p-value <0.001; T1-T2 mean: 1.19 ±1.51; z: 4.32; p-value <0.001). However, both groups showed no significant difference between the T2 and T3 values of the FIHOA score; the shock wave group, in particular, had a non-significant 0.5 (±1.95) decrease (z: 1.90; p-value: 0.06), while the exercise group had a non-significant 0.56 (±2.21) increase (z: -1.42; p-value: 0.15).
A significant reduction of the DASH score was observed from T0 to T3. In the shock wave group, it was a 13.78 (±7.52) decrease (z: 5.24; p-value <0.001), while in the exercise group it was a 10.03 (±4.35) decrease (z: 5.23; p-value <0.001). When breaking the analysis down to the intermediate checkpoints, the study group showed a significant 11.69 (±7.11) reduction of the DASH score from T0 to T1 (z: 5.23; p-value), the T1-T2 interval showed a significant 0.89 (±3.97) decrease (z: 3.01; p-value <0.01), and the T2-T3 interval highlighted a significant 1.19 (±2.42) reduction (z: 3.40; p-value <0.001). The exercise group, on the other hand, showed a significant 6.56 (±3.42) decrease of the DASH score (z: 5.23; p-value <0.001) from T0 to T1, followed by a still significant 4.58 (±2.68) T1-T2 decrease (z: 5.15; p-value <0.001); after the T2-T3 interval, however, a significant 1.11 (±3.03) increase of the DASH score was observed (z: -2.07; p-value: 0.04).
The VAS modification over time was significantly different between the shock wave and exercise group (t: -4.94; p-value <0.001). The DASH score reduction was also significantly different between the two groups (z: -2.70; p-value <0.01). On the other hand, the FIHOA score changes did not significantly differ between the shock wave and exercise group (z: -0.98; p-value: 0.324). As far as the Roles and Maudlsey score is concerned, the shock wave group showed a consistently lower score than the exercise one. The difference was significant at both T1 (z: 3.69; p-value <0.001), T2 (z: 3.32; p-value <0.001) and T3 (z: 4.48; p-value <0.001).
The VAS variation over time showed to be significantly impacted by the use of shock wave therapy, with greater decrease in the shock wave group than in the exercise one (aOR: 2.14; 95%CI: 1.20 – 3.08; p-value <0.001). The starting value of VAS was also directly associated with the score’s reduction over time (aOR: 0.44; 95%CI: 0.07 – 0.81; p-value: 0.019). Both associations were confirmed by univariable regression (aOR for shock wave therapy: 2.06; 95%CI: 1.22 – 2.89; p-value <0.001; aOR for VAS at T0: 0.66; 95%CI: 0.31 – 1.01; p-value <0.001).
The reduction of the FIHOA score was not significantly impacted by the use of shock wave therapy (aOR: 1.93; 95%CI: -0.06 – 3.92; p-value: 0.057). However, a significant direct association was shown for this score’s value at T0 (aOR: 0.59; 95%CI: 0.40 – 0.78; p-value <0.001), and a reverse association was highlighted for the Ray-X stage (aOR: -2.18; 95%CI: -4.29 – -0.07; p-value: 0.044). The impact of the T0 FIHOA score was further proved by the univariable regression (aOR: 0.63; 95%CI: 0.46 – 0.80; p-value <0.001); the Ray-X stage, on the contrary, was not confirmed to be significant impactful on FIHOA score changes over time (aOR: -2.10; 95%CI: -4.75 – 0.54; p-value: 0.117).
The use of shock wave therapy showed a significant association with the DASH score decrease over time (aOR: 3.47; 95%CI: 0.98 – 5.96; p-value <0.01). The DASH value at T0 was also significantly associated with the score’s decrease (aOR: 0.74; 95%CI: 0.52 – 0.96; p-value <0.001). Both results were confirmed by the univariable regression (aOR for shock wave therapy: 3.75; 95%CI: 0.86 – 6.64; p-value: 0.012; aOR for DASH score at T0: 0.73; 95%CI: 0.53 – 0.93; p-value <0.001).
Finally, the Roles and Maudlsey score showed to be mainly influenced by the use of shock wave therapy. The shock wave group, in fact, showed a consistently lower Roles and Maudlsey score at all checkpoints (aOR at T1: -0.69; 95%CI: -1.04 – -0.34; p-value <0.001; aOR at T2: -0.66; 95%CI: -1.02 – -0.31; p-value <0.001; aOR at T3: -1.01; 95%CI: -1.39 – -0.62; p-value <0.001). Moreover, the score was significantly higher in subjects whose symptoms had started since a longer period of time both at T2 (aOR: 0.83; 95%CI: 0.01 – 0.16; p-value: 0.029) and T3 (aOR: 0.08; 95%CI: 0.01 – 0.16; p-value: 0.041). However, univariable regression did not confirm the impact of time since symptom onset on the Roles and Maudsley score (aOR at T2: 0.03; 95%CI: -0.04 – 0.10; p-value: 0.376; aOR at T3: 0.02; 95%CI: -0.07 – 0.10; p-value: 0.681). On the contrary, the reverse association of shock wave therapy with Roles and Mausdley score at T1 (aOR: -0.64; 95%CI: -0.94 – -0.34; p-value <0.001), T2 (aOR: -0.58; 95%CI: -0.90 – -0.26; p-value: 0.001) and T3 (aOR: -0.89; 95%CI: -1.24 – -0.53; p-value <0.001) was confirmed by univariable regression.
4. Discussion
This study prospectively compared the benefits of conservative treatment with shock waves and bracing versus exercise and bracing, in patients with early stages of arthritis of the trapeziometacarpal joint (Eaton stages 1-2) [
11]. In both groups there was a significant improvement in the assessed parameters of pain (VAS), function (FIHOA) and disability (DASH), in the comparison between recruitment and 6-month follow-up. In the two groups these improvements were also confirmed in the comparison between recruitment and 1 month and between 1 month and 3 months for all scores administered; regarding the comparison between 3 and 6 months, the improvement was maintained only in the shock wave group for pain (VAS) and disability (DASH). In both groups the FIHOA did not present statistically significant improvements between 3 and 6 months. The Roles and Maudlsey score presented statistically lower values in the shock wave group than in the exercise group at the three examination times.
In recent years, shock wave therapy has found application in many musculoskeletal pathologies [
27]. Shock wave therapy is a physical therapy that exploits the biological effects of an acoustic wave that is focused in a small treatment area and causes cavitation effects [
28]. This therapy has proven to be very effective in reducing pain and functional recovery of tendon pathologies, such as calcific cuff tendinopathy, tennis elbow syndrome, plantar fasciitis, tendon disease; it also allows the healing of fracture nonunions, bone edema and complex regional pain syndrome [
28]. A recent meta-analysis demonstrated that the application of ESWT in patients suffering from knee osteoarthritis leads to statistically significant improvement in pain and functional recovery compared to other conservative therapy options [
12]. The physical stimulus of shock waves determines an up regulation of various growth factors, modulation of inflammatory cytokines, chemotaxis of stem cells, proliferative effect and revascularizing action [
29,
30]. When shock waves are applied to the subchondral bone and articular cartilage, neovascularization, osteogenesis and chondrogenesis would occur [
30,
31,
32,
33,
34,
35,
36]. On arthritic joints in an animal model, shock waves were able to determine motor recovery [
37,
38]. To date, only one clinical experience of the application of shock waves in the treatment of rhizoarthrosis has been published [
10]. Ioppolo and colleagues [
10] conducted a clinical study in which they compared the effects of shock waves (SW) vs hyaluronic acid infiltration in the treatment of rhizoarthrosis and the results were evaluated at the end of treatment and at 3 and 6 months. Pain reduction and functional recovery were found in both groups. In particular, as regards primary end points, pain, measured with the VAS scale, was significantly reduced by follow up in the two groups, with results in favor of shock waves at the end of treatment and at 6 months. As regards the Duruoz Hand Index functional scale, a functional recovery was recorded in both groups at the different FUs, without significant differences between the two treatments. As regards the secondary end points, in the shock wave group significant improvements were recorded in finger pinch strength at the end of the treatment and in hand grip strength at 6 months, without significant differences between the two groups The authors conclude that in the treatment of rhizoarthrosis, ESWT could have superior effects to that of hyaluronic acid infiltration with regards to pain reduction, while it would appear to have similar effects with regards to functional and strength recovery. These results would be consistent with the analgesic and anti-inflammatory effects of ESWT and viscosupplementation of intra-articular HA injections.
In our experience the effect of shock waves has been compared with therapeutic exercise. Both therapies behave like mechanical stimuli, with biological effects on tissues. In particular, joint mobilization would determine beneficial effects both through biomechanical responses and neurophysiological effects [
39,
40]. In fact, movement determines the release of endorphins and substance P with inhibition of the nociceptive pathways. Therefore, the combined treatment of exercise and bracing provides good results in terms of clinical-functional recovery and stabilization of rhizoarthrosis [
14,
16,
41,
42,
43]. Studies demonstrate that the trapezoid-metacarpal joint receives its stability from the opposing thumb muscle, abductor pollicis, and the first interosseous [
14,
16,
41,
42,
43].
Previous randomized clinical trials have shown that effective treatment must be continued for at least 2-4 weeks [
43,
44,
45]. Pisano and colleagues [
13] randomized 190 patients suffering from rhizoarthrosis to a standard treatment or associated with home exercises and found clinical and functional improvement at 12 months, without statistically significant differences between the two groups. The results made it possible to confirm that the exercises allow the metacarpal trapezius joint to be stabilized, reducing pain and disability. On the other hand, increasing frequency with the addition of home exercises did not bring additional improvement.
The strength of the study is that it is the first study to investigate shock wave treatment in patients suffering from rhizoarthrosis in comparison with therapeutic exercise. This non-invasive therapeutic option has proven to be equivalent and could be a better alternative to exercise in patients with initial arthritis of the first finger, in particular with greater persistence of results at 6 months. Longer-term monitoring may allow us to verify the persistence of the benefits, as well as the opportunity to repeat a second cycle of shock waves and/or integration with other conservative treatments.
There are some limitations of the study that need to be considered. We did not use instrumental follow-up controls (X-ray, ultrasound, MRI), which could have provided an indication of the effects of the treatment on the cartilage, bone and muscle-tendon of the trapezium-metacarpal joint. Furthermore, the use of the brace may have been under-used or over-used by the patient. Furthermore, in relation to the type of treatment, the blinding of patients and the professionals is lacking. There is a lack of a control group to distinguish the effects of the application of the orthosis from the effects of other therapies. Moreover, the continuous application of an orthosis for the reduction of painful symptoms is known to be effective. We cannot eliminate the possibility that this could influence the results. We did not measure grip strength, hypothesizing that the variations in this parameter could be more relevant to the control group, which performed exercises Another limitation of this study is that, despite periodic checks, it was not possible to be certain how often or for how long the patients in the exercise group performed their exercises. Furthermore, a placebo group is missing and the number may be relatively small.
5. Conclusions
In conclusion, this study offers a new treatment approach for rhizarthrosis in the early stage. Subsequent studies will be able to evaluate the effectiveness of a combined shock wave treatment, brace and therapeutic exercise. Longer-term monitoring will allow us to identify the persistence times of the benefits found.
Author Contributions
Conceptualization, A.N., I.C., and B.M.; methodology, A.N. and I.C.; formal analysis, A.D.L.; investigation, I.C.; data curation A.P, F.S and F.R.; writing—original draft preparation, A.N.; writing—review and editing, A.N., S.D.G., and G.S.; supervision, G.S. and B.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Territorial Ethics Committee of the University Hospital Consortium of the Polyclinic of Bari (protocol code 7814, 20 December 2023). The study is registered at ClinicalTrials.gov (NCT06056765).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request due to privacy.
Acknowledgments
We gratefully acknowledge the colleagues and patients who enabled us to write this manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Batra, S.; Kanvinde, R. Osteoarthritis of the thumb trapeziometacarpal joint. Curr Orthop 2007, 21, 135-144. [CrossRef]
- Wu, J.C.; Calandruccio, J.H.; Weller, W.J.; Henning, P.R.; Swigler, C.W. Arthritis of the Thumb Interphalangeal and Finger Distal Interphalangeal Joint. Orthop Clin North Am 2019, 50(4), 489-496. [CrossRef]
- Berger, A.J.; Meals, R.A. Management of osteoarthrosis of the thumb joints. J Hand Surg Am 2015, 40(4), 843-850. [CrossRef]
- Herren, D.B. Basal thumb arthritis surgery: complications and its management. J Hand Surg Eur Vol 2024, 49(2), 188-200. [CrossRef]
- Poole, J.U.; Pellegrini, V.D. Jr. Arthritis of the thumb basal joint complex. J Hand Ther 2000, 13(2), 91-107. [CrossRef]
- Wajon, A.; Ada, L. No difference between two splint and exercise regimens for people with osteoarthritis of the thumb: a randomised controlled trial. Aust J Physiother 2005, 51(4), 245-249. [CrossRef]
- Ahern, M.; Skyllas, J.; Wajon, A.; Hush, J. The effectiveness of physical therapies for patients with base of thumb osteoarthritis: Systematic review and meta-analysis. Musculoskelet Sci Pract 2018, 35, 46-54. [CrossRef]
- Zhang, W.; Doherty, M.; Leeb, B.F.; Alekseeva, L.; Arden, N.K.; Bijlsma, J.W.; Dinçer, F.; Dziedzic, K.; Häuselmann, H.J.; Herrero-Beaumont, G.; Kaklamanis, P.; Lohmander, S.; Maheu, E.; Martín-Mola, E.; Pavelka, K.; Punzi, L.; Reiter, S.; Sautner, J.; Smolen, J.; Verbruggen, G.; Zimmermann-Górska, I. EULAR evidence based recommendations for the management of hand osteoarthritis: report of a Task Force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007, 66, 377–388. [CrossRef]
- Cantero-Téllez, R.; Villafañe, J.H.; Valdes, K.; García-Orza, S.; Bishop, M.D.; Medina-Porqueres, I. Effects of High-Intensity Laser Therapy on Pain Sensitivity and Motor Performance in Patients with Thumb Carpometacarpal Joint Osteoarthritis: A Randomized Controlled Trial. Pain Med 2020, 21(10), 2357-2365. [CrossRef]
- Ioppolo, F.; Saracino, F.; Rizzo, R.S.; Monacelli, G.; Lanni, D.; Di Sante, L.; Cacchio, A.; Santilli, V.; Venditto, T. Comparison Between Extracorporeal Shock Wave Therapy and Intra-articular Hyaluronic Acid Injections in the Treatment of First Carpometacarpal Joint Osteoarthritis. Ann Rehabil Med 2018, 42(1), 92-100. [CrossRef]
- Zarb, R.M.; Sasor, S.E. Physical Examination and Radiographic Staging of Thumb Carpometacarpal Arthritis. Hand Clin 2022, 38(2), 141-148. [CrossRef]
- Chen, L.; Ye, L.; Liu, H.; Yang, P.; Yang, B. Extracorporeal Shock Wave Therapy for the Treatment of Osteoarthritis: A Systematic Review and Meta-Analysis. Biomed Res Int 2020, 2020, 1907821. [CrossRef]
- Pisano, K.; Wolfe, T.; Lubahn, J.; Cooney, T. Effect of a stabilization exercise program versus standard treatment for thumb carpometacarpal osteoarthritis: A randomized trial. J Hand Ther 2023, 36(3), 546-559. [CrossRef]
- O’Brien, V.H.; Giveans, M.R. Effects of a dynamic stability approach in conservative intervention of the carpometacarpal joint of the thumb: a retrospective study. J Hand Ther 2013, 26, 44–51, quiz 52. [CrossRef]
- Albrect, J. Caring for the Painful Thumb: More Than a Splint. North Mankato, MN: Corporate Graphics, 2008.
- Mobargha, N.; Esplugas, M.; Garcia-Elias, M.; Lluch, A.; Megerle, K.; Hagert, E. The effect of individual isometric muscle loading on the alignment of the base of the thumb metacarpal: a cadaveric study. J Hand Surg Eur 2016, 41, 374–379. [CrossRef]
- Valdes, K.; von der Heyde, R. An exercise program for carpometacarpal osteoarthritis based on biomechanical principles. J Hand Ther 2012, 25(3), 251-62, quiz 263.
- Pellegrini, V.D. Osteoarthritis at the base of the thumb. Orthop Clin North Am 1992, 23, 83–102. [CrossRef]
- Moulton, M.J.; Parentis, M.A.; Kelly, M.J.; Jacobs, C.; Naidu, S.H.; Pellegrini, V.D. Influence of metacarpophalangeal joint position on basal joint-loading in the thumb. J Bone Joint Surg Am 2001, 83, 709–716. [CrossRef]
- Hochberg, M.C.; Altman, R.D., April, K.T.; Benkhalti, M.; Guyatt, G.; McGowan, J.; Towheed, T.; Welch, V.; Wells, G.; Tugwell, P.; American College of Rheumatology. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2012, 64, 465–474. [CrossRef]
- Kjeken, I.; Smedslund, G.; Moe, R.H.; Slatkowsky-Christensen, B.; Uhlig, T.; Hagen, KB. Systematic review of design and effects of splints and exercise programs in hand osteoarthritis. Arthritis Care Res (Hoboken) 2011, 63, 834–848. [CrossRef]
- Stamm, T.A.; Machold, K.P.; Smolen, S.; Fischer, S.; Redlich, K.; Graninger, W.; Ebner, W.; Erlacher, L. Joint protection and home exercises improve hand function in patients with hand osteoarthritis: a randomized controlled trial. Arthritis Rheum 2002, 47, 44-49. [CrossRef]
- Reed, M.D.; Van Nostran, W. Assessing pain intensity with the visual analog scale: a plea for uniformity. J Clin Pharmacol 2014, 54(3), 241–244.
- Dreiser, R.L.; Maheu, E.; Guillou, G.B.; Caspard, H.; Grouin, J.M. Validation of an algofunctional index for osteoarthritis of the hand. Rev Rhum Engl Ed 1995, 62(6 Suppl 1), 43S–53S.
- Hudak ,P.L.; Amadio, P.C.; Bombardier, C. Development of an upper extremity outcome measure: the DASH (disabilities of the arm, shoulder and hand) [corrected]. The Upper Extremity Collaborative Group (UECG). Am J Ind Med 1996, 29(6), 602-608.
- Roles, N.C.; Maudsley, R.H. Radial tunnel syndrome: resistant tennis elbow as a nerve entrapment. J Bone Joint Surg Br 1972, 54, 499-508.
- Notarnicola, A.; Moretti, B. The biological effects of extracorporeal shock wave therapy (eswt) on tendon tissue. Muscles Ligaments Tendons J 2012, 2(1), 33-37.
- D Agostino, M.C.; Frairia, R.; Romeo, P.; Amelio, E.; Berta, L.; Bosco, V.; Gigliotti, S.; Guerra, C.; Messina, S.; Messuri, L.; Moretti, B.; Notarnicola, A.; Maccagnano, G.; Russo, S.; Saggini, R.; Vulpiani, M.C.; Buselli, P. Extracorporeal shockwaves as regenerative therapy in orthopedic traumatology: a narrative review from basic research to clinical practice. J Biol Regul Homeost Agents 2016, 30(2), 323-332.
- Iannone, F.; Moretti, B.; Notarnicola, A.; Moretti, L.; Patella, S.; Patella, V.; Lapadula, G. Extracorporeal shock waves increase interleukin-10 expression by human osteoarthritic and healthy osteoblasts in vitro. Clin Exp Rheumatol 2009, 27(5), 794-799.
- Moretti, B.; Iannone, F.; Notarnicola, A.; Lapadula, G.; Moretti, L.; Patella, V.; Garofalo, R. Extracorporeal shock waves down-regulate the expression of interleukin-10 and tumor necrosis factor-alpha in osteoarthritic chondrocytes. BMC Musculoskelet Disord 2008, 31,9:16. [CrossRef]
- Cheng, J.H.; Wang, C.J. Biological mechanism of shockwave in bone. Int J Surg 2015, 24(Pt B),143-146. [CrossRef]
- Wang, C.J.; Cheng, J.H.; Chou, W.Y.; Hsu, S.L.; Chen, J.H.; Huang, C.Y. Changes of articular cartilage and subchondral bone after extracorporeal shockwave therapy in osteoarthritis of the knee. Int J Med Sci 2017, 14(3), 213-223. [CrossRef]
- Ji, Q.; He, C. Extracorporeal shockwave therapy promotes chondrogenesis in cartilage tissue engineering: A hypothesis based on previous evidence. Med Hypotheses 2016, 91, 9-15. [CrossRef]
- Kim, J.H.; Kim, J.Y.; Choi, C.M.; Lee, J.K.; Kee, H.S.; Jung, K.I.; Yoon, S.R. The dose-related effects of extracorporeal shock wave therapy for knee osteoarthritis. Ann Rehabil Med 2015, 39, 616-623. [CrossRef]
- Notarnicola, A.; Iannone, F.; Maccagnano, G.; Lacarpia, N.; Bizzoca, D.; Moretti B. Chondrocytes treated with different shock wave devices. Muscles Ligaments Tendons J 2017, 7(1),152-156. [CrossRef]
- Tamma, R.; dell'Endice, S.; Notarnicola, A.; Moretti, L.; Patella, S.; Patella, V.; Zallone, A.; Moretti, B. Extracorporeal shock waves stimulate osteoblast activities. Ultrasound Med Biol 2009, 35(12), 2093-2100. [CrossRef]
- Dahlberg, J.; Fitch, G.; Evans, R.B.; McClure, S.R.; Conzemius, M. The evaluation of extracorporeal shockwave therapy in naturally occurring osteoarthritis of the stifle joint in dogs. Vet Comp Orthop Traumatol 2005, 18, 147-152. [CrossRef]
- Revenaugh, M.S. Extracorporeal shock wave therapy for treatment of osteoarthritis in the horse: clinical appli cations. Vet Clin North Am Equine Pract 2005, 21, 609- 625.
- Bialosky, J.E.; Bishop, M.D.; Price, D.D.; Robinson, M.E.; George, S.Z. The mechanisms of manual therapy in the treatment of musculoskeletal pain: a comprehensive model. Man Ther 2009, 14, 531-538. [CrossRef]
- Bialosky, J.E.; George, S.Z.; Bishop, M.D. How spinal manipulative therapy works: why ask why? J Orthop Sports Phys Ther 2008, 38, 293-295.
- McGee, C.; O’Brien, V.; Van Nortwick, S.; Adams, J.; Van Heest, A. First dorsal interosseous muscle contraction results in radiographic reduction of healthy thumb carpometacarpal joint. J Hand Ther 2015, 28, 375–380 quiz 381. [CrossRef]
- Adams, J.E.; O’Brien, V.; Magnusson, E.; Rosenstein, B.; Nuckley, D.J. Radiographic analysis of simulated first dorsal interosseous and opponens pollicis loading upon thumb CMC joint subluxation: a cadaver study. Hand 2018, 13, 40–44. [CrossRef]
- Neumann, D.A; Bielefeld, T. The carpometacarpal joint of the thumb: stability, deformity, and therapeutic intervention. J Orthop Sports Phys Ther 2003, 33, 386– 399. [CrossRef]
- Villafañe, J.H.; Silva, G.B.; Chiarotto, A. Effects of passive upper extremity joint mobilization on pain sensitivity and function in participants with secondary carpometacarpal osteoarthritis: a case series. J Manipulative Physiol Ther 2012, 35, 735- 742. [CrossRef]
- Villafañe, J.H.; Silva, G.B.; Diaz-Parreño, S.A.; Fernandez-Carnero, J. Hypoalgesic and motor effects of Kaltenborn mobilization on elderly patients with secondary thumb carpometacarpal osteoarthritis: a randomized controlled trial. J Manipulative Physiol Ther 2011, 34, 547-556. [CrossRef]
- Villafañe, J.H.; Silva, G.B.; Fernandez-Carnero, J. Effect of thumb joint mobilization on pressure pain threshold in elderly patients with thumb carpometacarpal osteoarthritis. J Manipulative Physiol Ther 2012, 35, 110-120. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).