1. Introduction
Reactive plasma spraying (RPS) is a widely used method for creating surface coatings with unique properties that play a crucial role in various industrial fields, from mechanical engineering to electronics [
1,
2,
3,
4]. However, the efficiency of this process directly depends on the properties of the starting powder materials. In this context, mechanical activation, as a pretreatment, represents a key factor that can significantly modify the structure and characteristics of powders, thus optimising the RPS process.
Mechanical activation is an important process in materials science to modify the properties of powders to improve their reactivity [
5,
6]. This paper deals with the mechanical activation of powders in an Emax grinding machine, which is highly efficient due to the use of centrifugal forces. In this paper, the aim of the study is to analyse the effect of activation parameters on the physical and mechanical properties of powders. Mechanical activation is a process in which solids are subjected to intense mechanical action, resulting in changes in their structure and properties. This process is often used to increase the reactivity of materials, especially powders, by creating defects in the crystal lattice, reducing the particle size and increasing the specific surface area, there is the initiation of mechanochemical reactions in the solid phase.
Mechanical activation in the processing of materials plays a crucial role, influencing various aspects of their properties and structure. It respects the moderate activity of powders, which favourably influences the processes of synthesis of new materials, catalysis and solid state structure formation. In addition, these processes can lead to the formation of amorphous structures in materials, which can be important for applications. Improving the performance of components of powder systems offers the creation of composite materials [
7,
8,
9]. Mechanical activation also increases the diffusion rate in materials, which accelerates processes that require mass transfer. In addition, high-tech powders are better prepared for industrial processes such as pressing, sintering and extrusion. Thus, mechanical activation plays a key role in modern materials processing technologies, contributing to products with innovativeness and enhanced functionality.
Transition metal nitride and carbonitride coatings such as TiN, TiC and Ti(CN) are widely used due to their high hardness and excellent wear resistance. However, their inherent disadvantages such as low oxidation resistance and brittleness limit their further application. Therefore, a number of methods have been proposed to improve the hardness against oxidation, one of which is the incorporation of a second phase into the coating. Si, which is a potential alloying element, can enhance thermal stability and hardness. Among such coatings, TiSiN [
10,
11] [
1,
2,
3] and TiSiCN [
12,
13] [
4,
5] are the most promising candidates for challenging tribological applications due to their high hardness, good oxidation resistance and thermal stability. In addition, TiSiCN coatings are promising materials for marine applications due to their high hardness and low coefficient of friction as well as excellent wear, oxidation and corrosion resistance [
14,
15]. The combination of these properties makes TiSiCN coatings potential candidates for protective coatings in the aerospace, automotive and petroleum industries.
Of particular interest is the study of the effect of mechanical activation on the structural evolution of the TiSiCN powder system, which is one of the promising materials for reactive plasma spraying. Understanding these changes will not only allow optimisation of the spraying process but will also provide opportunities to create new materials with improved properties, which may be of significant importance for various applications of this technology.
2. Materials and Methods
As materials we used fine Ti powder (PTOM-1), particle size 30 μm, fine grinding powder SiC, particle size 20 μm, powdered TiCN, each with a purity of 99.99% produced in Russia, as well as powders produced in China TiCN, Si, TiC, Si3N4 with particle sizes 15-45 μm. A total of 16 samples were prepared, differing in powder composition, country of production and activation time. The samples were divided into four groups: Ti + SiC, TiCN + SiC, TiC + Si₃N₄ and TiCN + Si, with activation times of 0, 30, 60 and 90 min at a rotation speed of 1500 rpm. The samples were fabricated using powders of Russian and Chinese manufacture. The powders were weighed according to the required coating composition calculated from the preservation of stoichiometric atomic-mass ratios of the powders.
Mechanical milling of powders was carried out on a high-energy ball mill Emax (Retsch, Germany) with water cooling for 2 hours at a temperature regime of 23-27°C. The mass ratio of the balls to the mass of the loading (powder) was 10:1. The powders were mixed at a ball mill speed of 1500 rpm for 30, 60, 90 min.
Phase analysis of the synthesised powders was carried out on an X'Per PRO diffractometer using CuKα irradiation.
The microstructure and elemental composition of the synthesised powders were investigated on a TESCAN MIRA3 scanning electron microscope.
For Ti, SiC, TiCN, Si, TiC, Si3N4 powder samples, the surface microstructure and powder size were determined using a Tescan MIRA 3 scanning electron microscope (SEM).
Thermogravimetric analysis (TGA): The studies were carried out in the temperature range of 20-950°C at a heating rate of 10°C/min, using nitrogen in a unit (TGA-1250) as the flushing gas. The samples were weighed on a microbalance and crimped in a ceramic cuvette. The thermogram, i.e. a plot of percentage as a function of temperature, was used to study the variation in the heat resistance of the powder. The error in recording thermograms was ±2°C.
3. Results
This section may be divided by subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.1. SEM Analysis of Powders
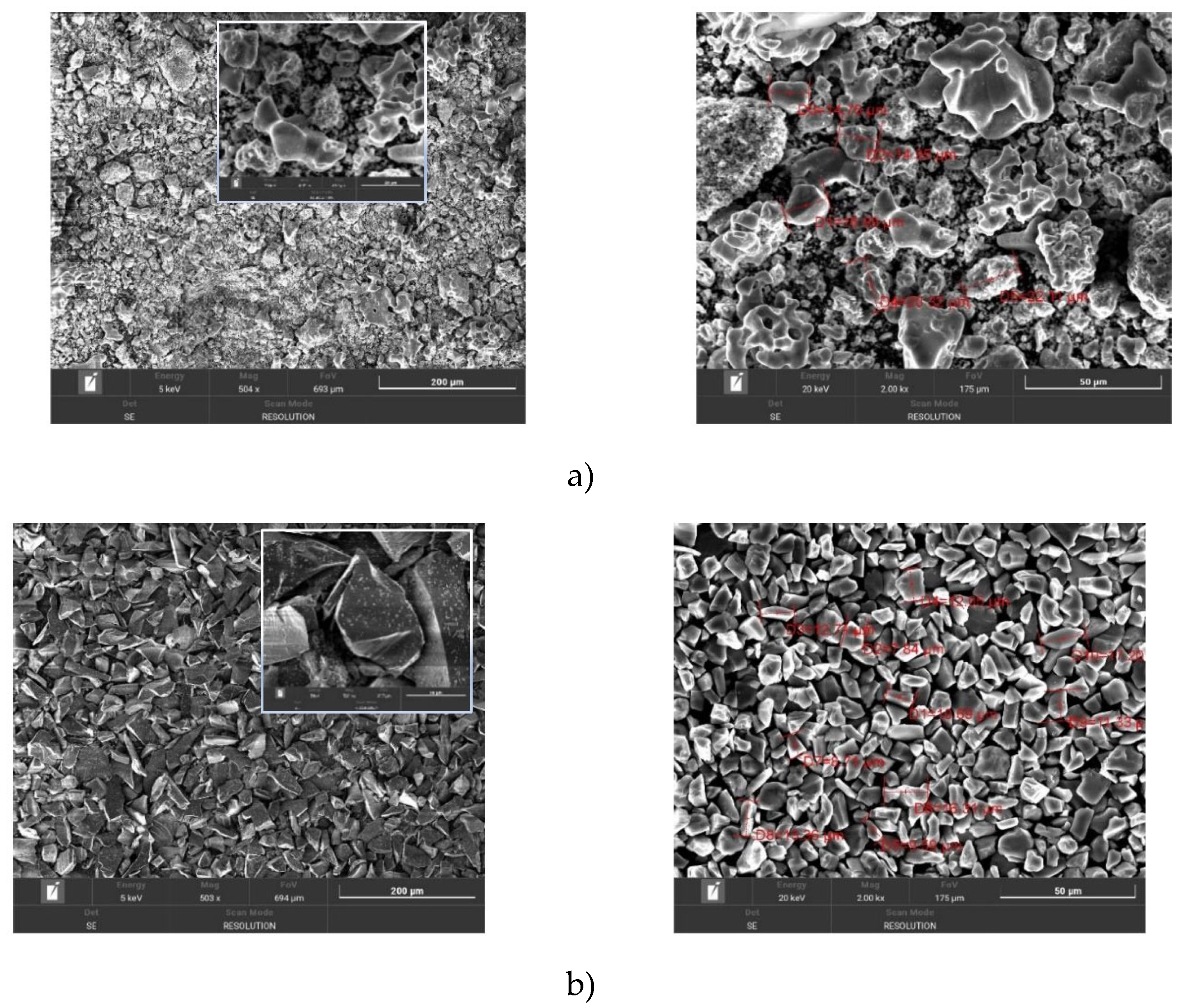
Figure 1 shows the SEM images of the initial titanium (Ti) and silicon carbide (SiC) powders at different scales and the insets show the enlarged fragment. The
Figure 1a shows the porous structure of Ti powder with many small particles and fragments. Some areas appear smooth while others have a rough surface. The
Figure 1 shows a more detailed material structure with porous and rough surfaces. Dimensions are shown for the different objects and pores, which helps to determine their scale and compare them with each other. Also, the porous and uneven structure of the material can be seen in the
Figure 1, consisting of many small particles and fragments. Some areas appear as large pores or voids, which may be the result of natural processes or technological procedures. These pores and irregularities can affect the properties of the material such as its strength, porosity, and adsorption capacity. In
Figure 1b, SiC powders exhibit distinct, angular particles with rough surfaces. The particle size distribution varies, but the particles are mostly tens of micrometres in size. The particles appear sharper and well-defined, with visible edges and faces. The surface of the particles is rough and granular. The particles are angular and irregularly shaped with a rough surface texture, indicating a high degree of heterogeneity
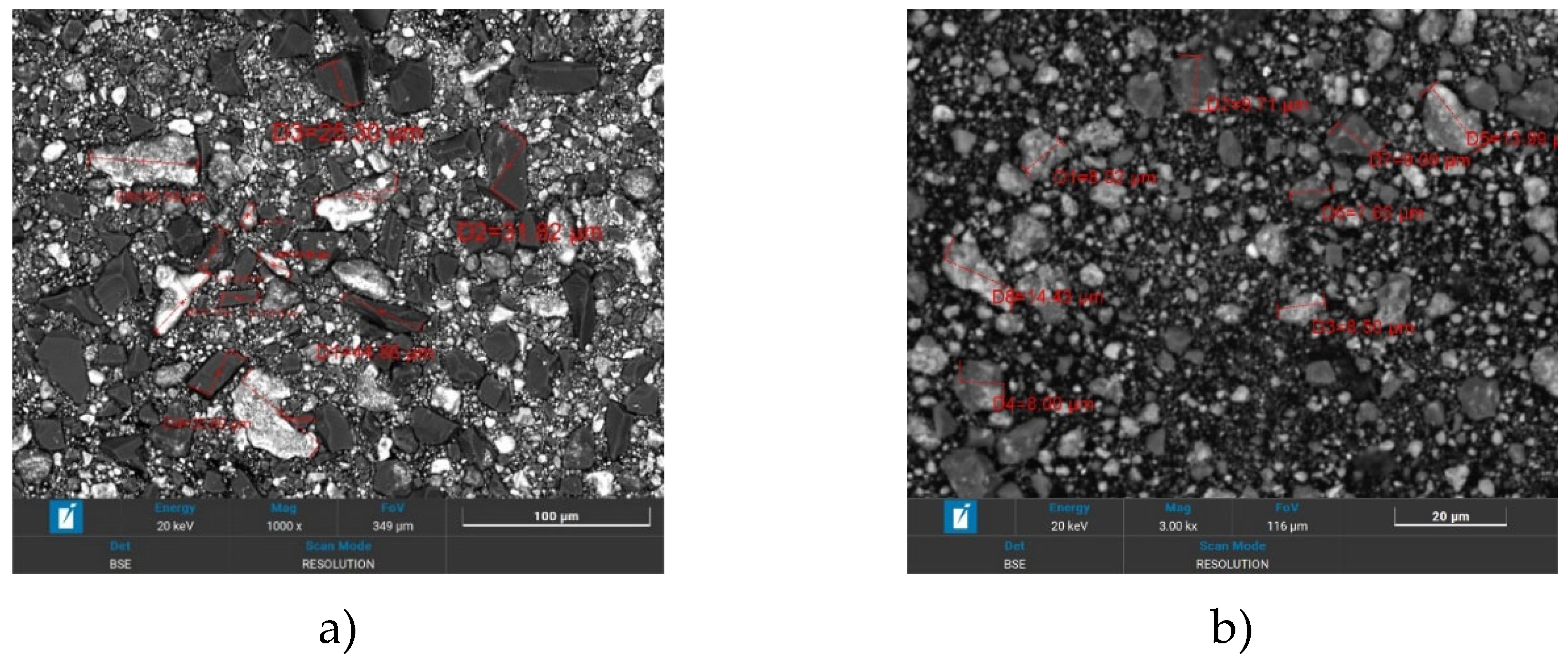
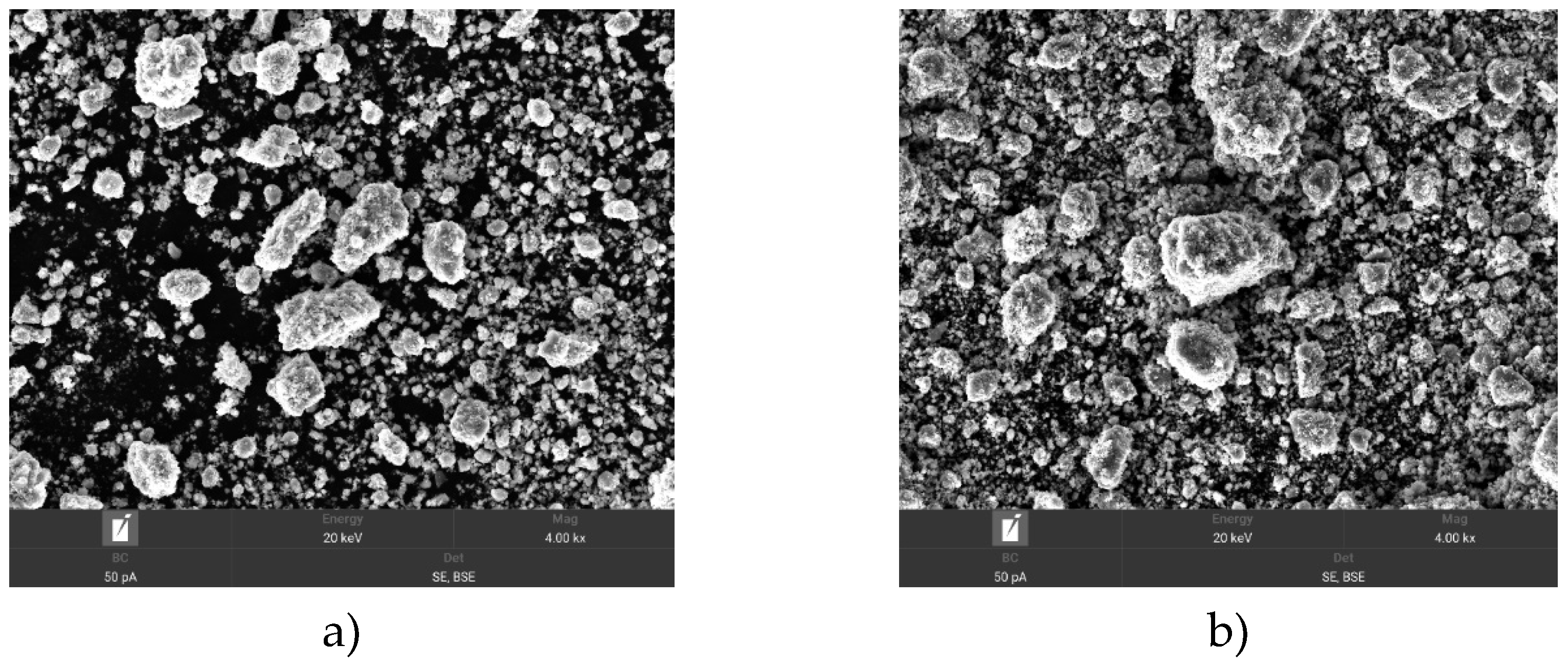
Figure 2 shows SEM images of the powders after Ti+SiC activation.
Figure 2a shows a similar heterogeneous mixture of Ti and SiC particles with 30 min activation and Ti+SiC powder size, where the average particle size is 31.8 μm. However, the particles appear less sintered and more clearly separated from each other compared to the 60 min activation. The texture shows more obvious boundaries between particles and the surface texture shows a combination of smooth and rough areas.
Figure 1b shows the heterogeneous mixture of Ti and SiC particles after 60 min activation of Ti+SiC, where the average particle size is 9.9 μm. Texture-wise, the particles appear more compact with a combination of smooth and rough areas, indicating partial sintering or fusion during activation. The scale bar shows 20 µm, indicating that the mixture consists of smaller particles, possibly down to submicron levels.
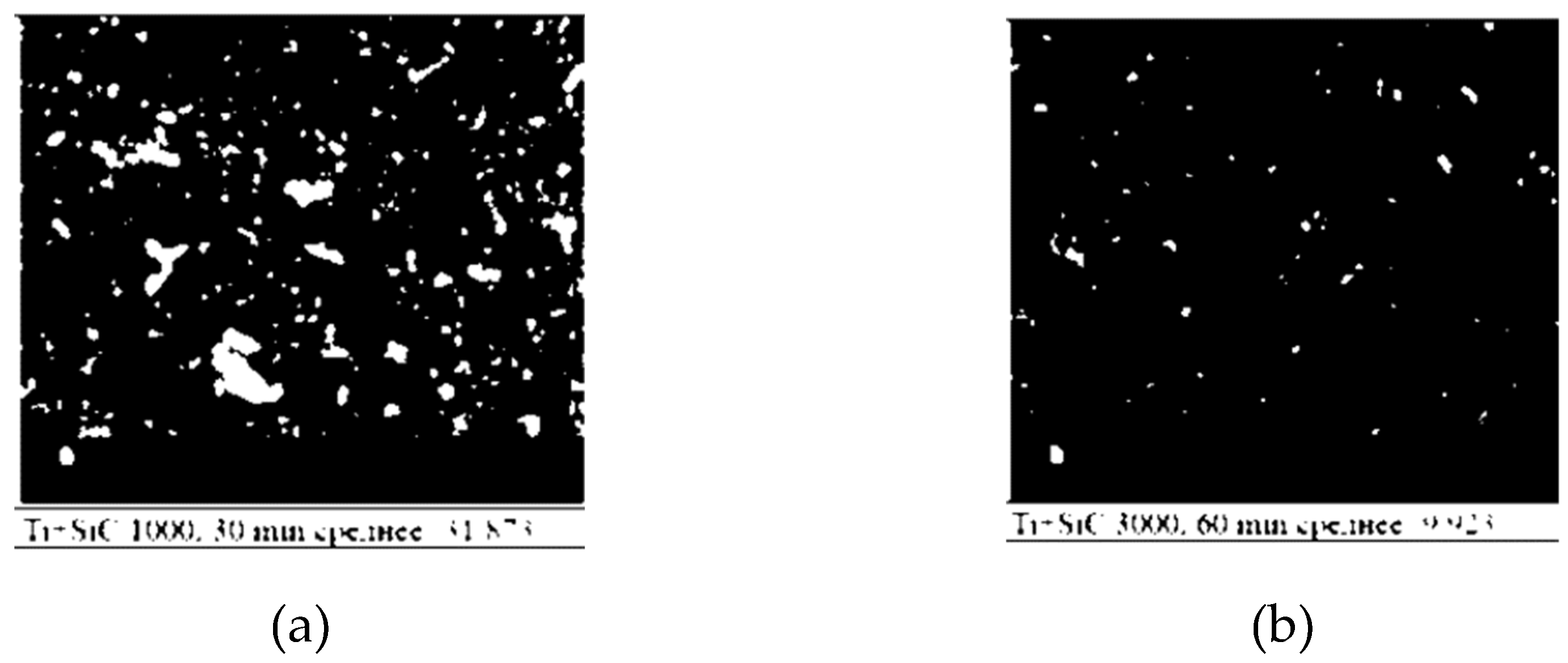
So, the mixed Ti+SiC powders show changes in morphology as a function of activation time. By processing and analysing the SEM image, the porosity analysis of the heterogeneous mixture of Ti+SiC powders after activation is presented in
Figure 3. Based on the analysis, the Ti+SiC mixture exhibits a significant porosity of approximately 68.97%, indicating a significant presence of pores in the material. The 60 min activation results in more compact and possibly sintered particles, whereas the 30 min activation retains more obvious boundaries between particles.
The particles of the Ti+SiC mixture after activation show a heterogeneous structure with different particle sizes and shapes. This morphology can improve the mechanical properties of the coating, such as hardness and wear resistance, but can also lead to non-uniform material distribution during spraying.
So, the Ti+SiC mixture may be suitable for reactive plasma spraying due to its high temperature stability and the possibility of creating coatings with good adhesion and thermal insulation properties. However, its high porosity and heterogeneous structure may require additional steps to optimise the spraying parameters and subsequent coating processing to achieve the desired characteristics.
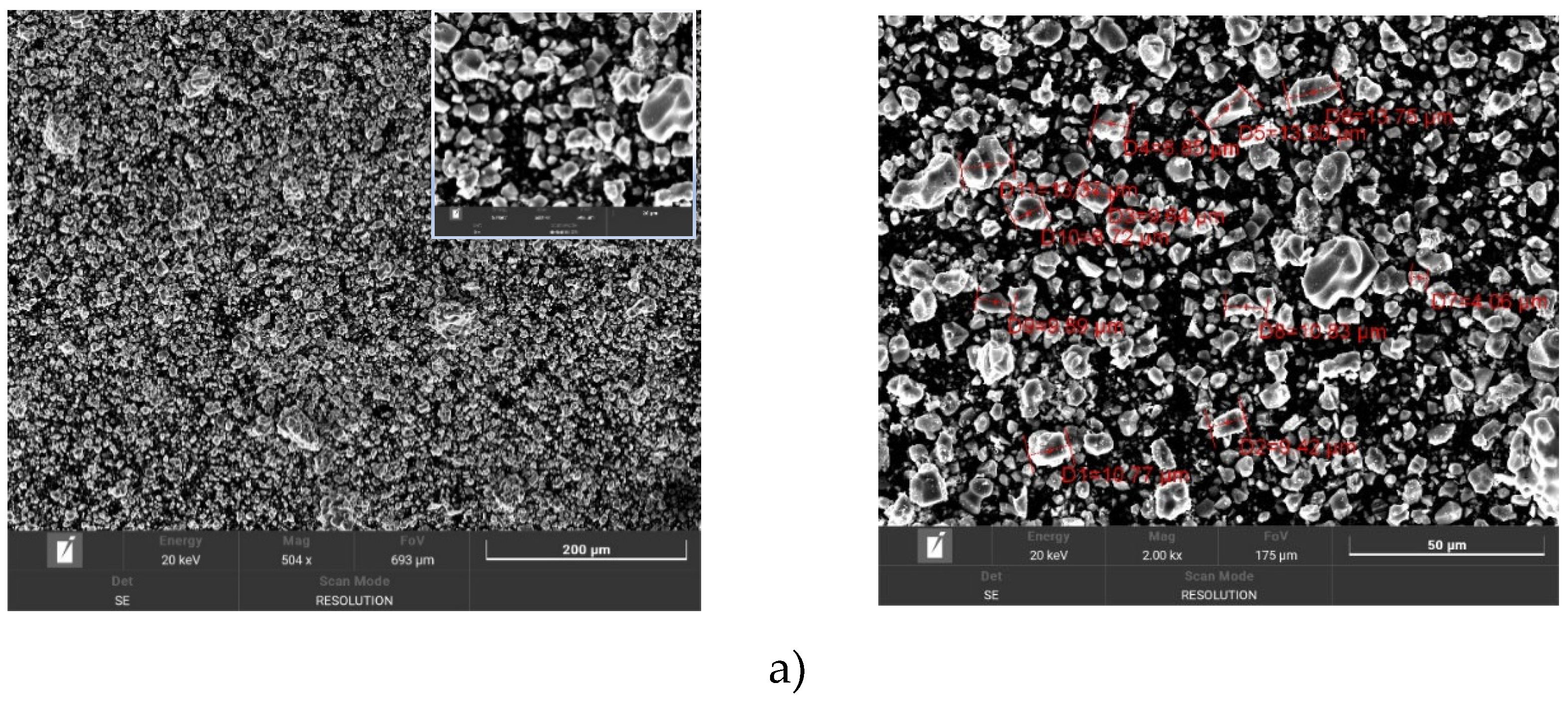
The next composition that was studied with different activation times is TiCN+SiC. The results are presented in
Figure 4a, which shows images of the TiCN powder where the average particle size is 10.25 μm. The image shows the structure of the powder with many small, uniform particles in morphology. The particles are rounded in shape and uniformly distributed on the surface with no obvious large pores or voids. Small pores between the particles are visible on the surface and their crystalline structure is expressed by sharp facets.
Figure 4b shows a SiC powder where the average particle size is 39.85 μm. This image shows the structure of the powder with particles of different shapes and sizes. The SiC particles have an angular shape and sharp edges, which gives them a highly heterogeneous structure. The distribution of the particles seems to be more chaotic compared to TiCN. Large pores and voids between the particles are also visible
The SEM images taken at different magnifications clearly show the effect of mechanical activation on the particle morphology of TiCN and SiC powders. The activation was carried out for 30 min.
Figure 5 shows the image of TiCN+SiC powders after activation for 30, 60 min.
Figure 5a shows the SEM image of TiCN and SiC powders after activation for 30 min. Agglomerates and individual small particles can be seen in more detail. The average particle size is approximately 5-10 μm. The largest agglomerates are composed of smaller particles that are interconnected. It is likely that partial sintering of the smaller particles to form larger agglomerates occurred during the mechanical activation process. Thus, mechanical activation of TiCN and SiC powders for 30 min leads to significant particle size reduction and agglomerate formation.
Figure 5b shows the changes in particle morphology of TiCN and SiC powders after mechanical activation for 60 min. Compared to the samples activated for 30 min, the following features are observed: the particles have a smoother surface compared to the samples activated for 30 min. This may be due to the additional grinding and levelling of the particles. The average particle size varies between 5-8 µm, which is in line with expectations for long term mechanical activation. The surface of the particles becomes more uniform and the particles themselves show clearer boundaries. This indicates effective pulverisation and removal of large defects, which is important for improving coating quality.
Activation for 60 min is more preferable for reactive plasma spraying. This is because 60 min activation provides a more uniform particle size distribution, reduces agglomeration and improves the surface properties of the particles.
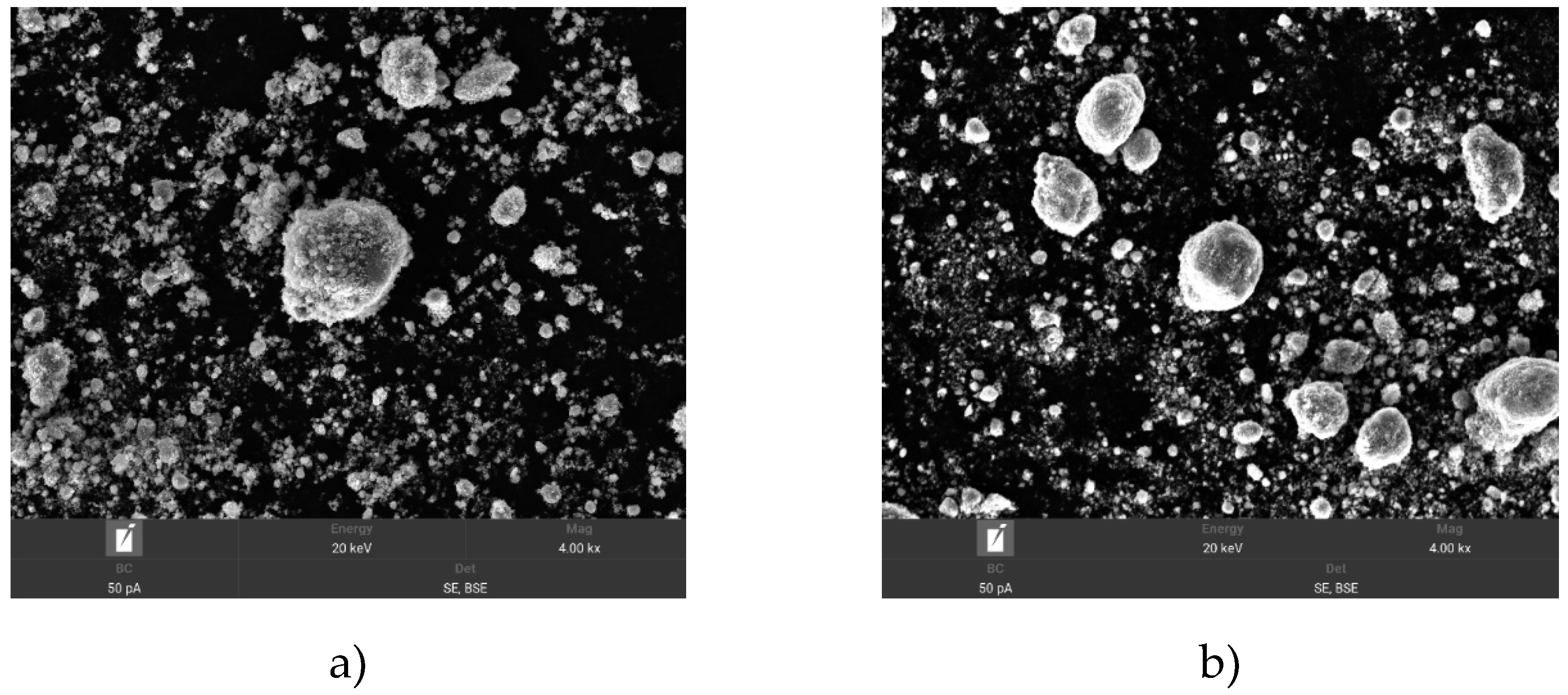
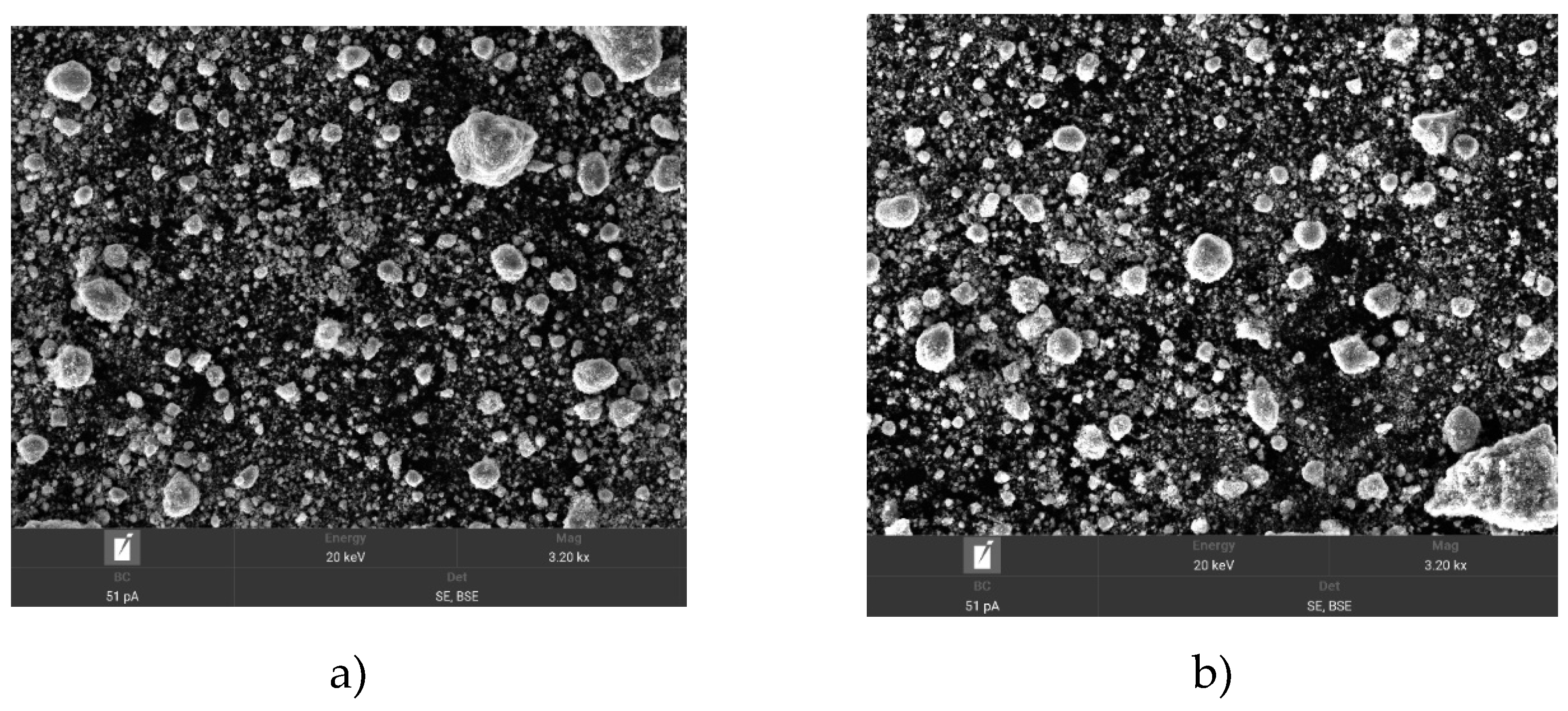
The next composition was TiCN and Si, its SEM imaging showed that the structure of TiCN powder where the surface of the material appears relatively homogeneous with fine grains and the average particle size is 12.59 μm. The average particle size is 21.42 μm. The surface of TiCN particles is rougher and more inhomogeneous compared to the smooth and homogeneous surface of Si particles.
Figure 6 shows the SEM images of TiCN+Si powders after activation at 30 and 60 min of activation. The images in
Figure 6a show a more detailed picture of agglomerates and individual particles. Mechanical activation of TiCN and Si powders for 30 min results in significant changes in particle morphology. As a result of activation, TiCN particles become more homogeneous and smoother, while Si particles remain larger and coarser. The agglomeration of particles, especially Si, can cause inhomogeneity in the coating during spraying, but their slight interaction with TiCN particles can improve the adhesion and physicochemical properties of the final coating.
Figure 6 b, further particle size reduction is observed compared to activation for 30 min. The particle sizes become more homogeneous, and the number of large agglomerates decreases. The TiCN and Si particles are better mixed and distributed, which contributes to a more uniform coating during plasma spraying. The particle size remains in the range of 2 to 10 µm, which is suitable for efficient use in the reactive plasma process.
So, by comparing
Figure 6a-b we find that activation for 60 min is more optimal for the use of TiCN and Si powders in reactive plasma spraying process. Prolonged activation leads to improved particle morphology, reduced agglomeration and particle size levelling, thus achieving higher coating quality.
Figure 7 shows SEM images of TiC and Si3N4 powders before activation. In
Figure 7a, the surface appears dense and homogeneous with fine grains. The particles have irregular shape and angular edges. The TiC particles are of different sizes and most of them are angular and irregularly shaped. On average, the particles are fine and have a wide range of sizes, the average particle size is 10.25 µm. In
Figure 7b, the surface of Si3N4 powder looks rough and inhomogeneous. The particles are of different sizes and shapes, many of them have rough and inhomogeneous edges, and the average particle size is 19.49 μm. The Si3N4 particles also vary in size and shape, but on average they are larger than TiC particles.
Figure 8 shows SEM images of TiC and Si3N4 powders after mechanical activation for 30 min. The image in
Figure 9a allows a closer look at the particle structure. The TiC particles have a relatively smooth surface and more uniform size compared to the Si3N4 particles, which show a greater tendency to agglomerate. This agglomeration can affect the uniformity of the coating during the spraying process. TiC particles show better uniformity and less tendency to agglomerate compared to Si3N4 particles, which remain coarser and prone to form large agglomerates.
Figure 8b shows SEM images of the same powders after mechanical activation for 60 min. Mechanical activation of TiC and Si3N4 powders for 60 min significantly improves their morphological characteristics compared to activation for 30 min.
Based on the SEM studies, it can be concluded that mechanical activation for 60 min is optimal for the preparation of powders for RPS. Longer activation leads to an improvement in the morphology of particles and their surface properties, which favours the formation of coatings with high performance characteristics such as increased hardness, wear resistance and adhesion. And in terms of powder composition, TiC with SiN, TiCN with SiC and TiC with Si3N4 shows better results after 60 min of activation.
3.2. Results of Thermogravimetric Analysis
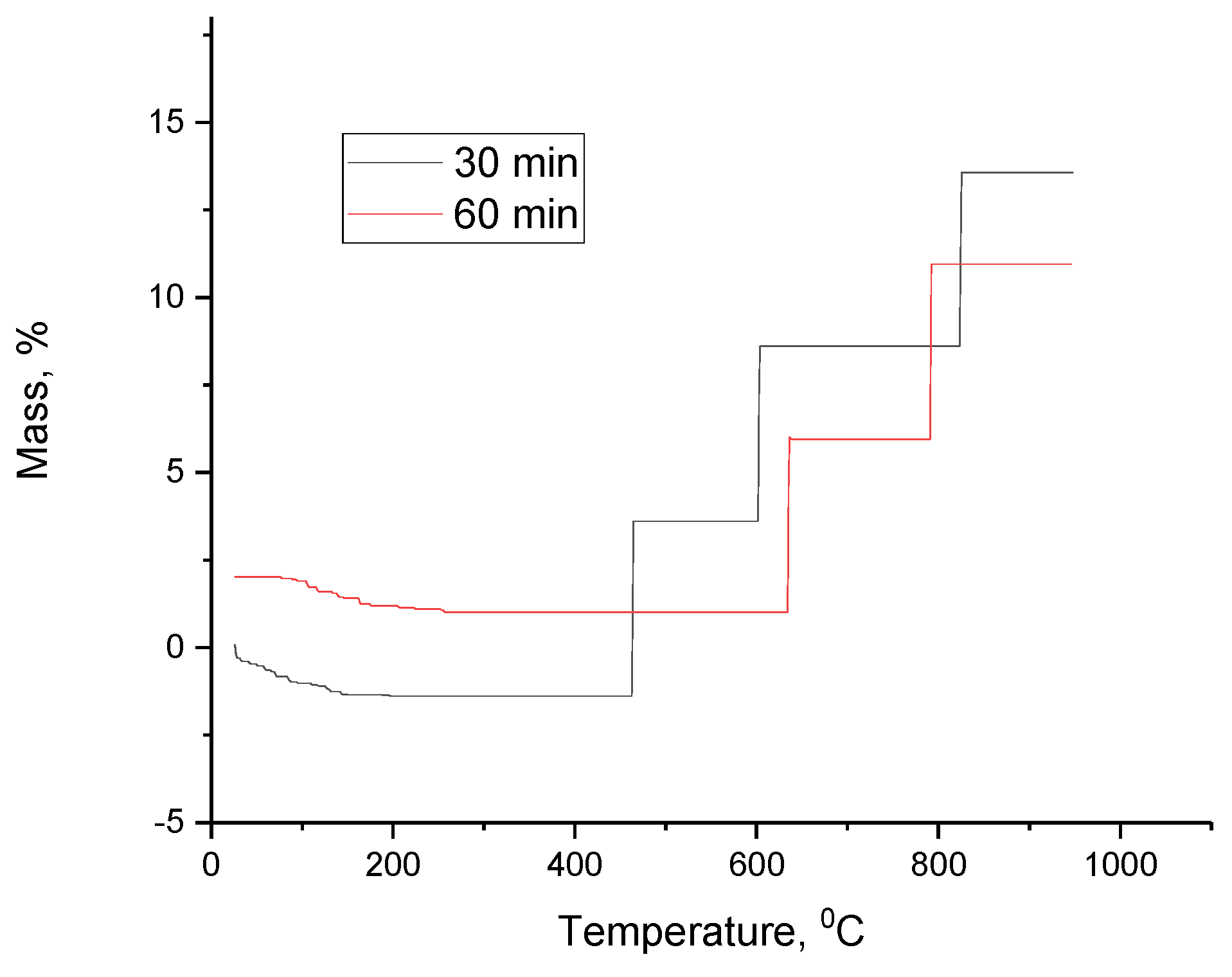
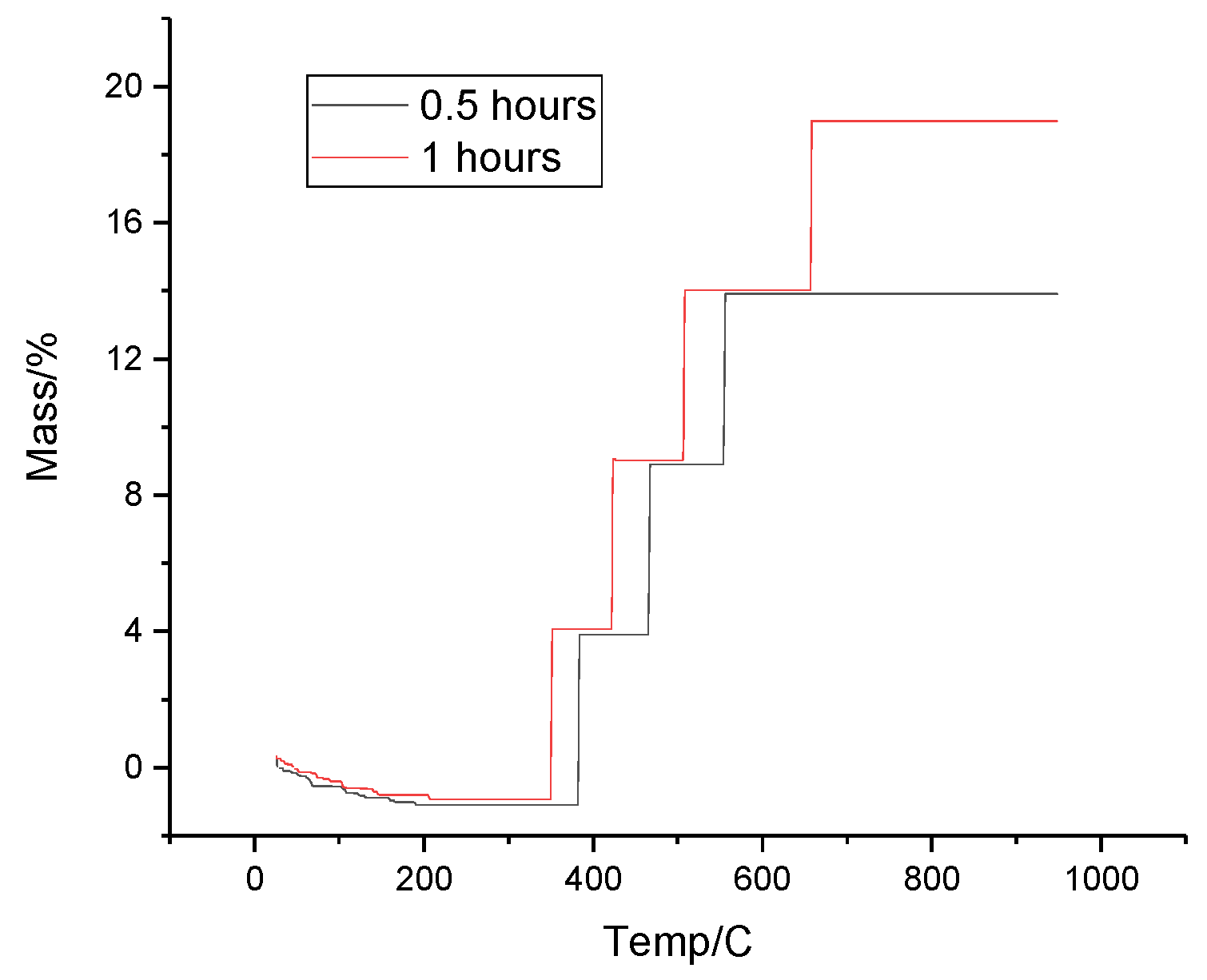
The TGA curves of Ti+SiC multicomponent system, are shown in
Figure 9. From the
Figure 9, it was observed that the plateau areas show that the mass of the sample remains stable in a certain range of temperature or time. And mass decreases (peaks downwards) where mass loss of the sample occurs may be due to degradation, oxidation, vaporisation of components or other thermal processes. Also, stabilisation after mass loss indicates that the process is complete and the mass is no longer changing within a given temperature or time range.
So, let us consider a sample where the activation was 30 min, its initial mass was 3.12 mg. In the first 10 min there was a slight change in mass due to initial stabilisation of the system and sample and removal of surface moisture and volatile components. Then up to 6700C the mass of the sample decreased due to evaporation of adsorbed moisture, depending on the temperature, decomposition of volatile components may begin.
Then after at 47th min at 6700C the sample enters another phase of stability. The mass of the sample stabilises, indicating the completion of the decomposition process. The temperature is maintained at 6700C at a certain level, this indicates a temporary stabilisation of the mass.
By the end of the analysis, the sample reaches a state of stability, representing the residual products after heat treatment.
Figure 10 shows the TGA results for the Ti+SiC multicomponent system at different activation durations: 30 min (black line) and 60 min (red line). In the initial stage of the process (0-200°C), a slight mass change is noticed for both curves. This is due to the removal of adsorbed gases and moisture from the surface of the powders. The black line shows a slight decrease in mass, which is due to degradation or oxidation of the surface. The increase in mass is gradual, indicating several reaction steps. The black line (30 min of activation) shows a more marked increase in mass in the initial stages, which may indicate a more active reaction. The red line (60 min of activation) shows a smoother mass increase, which may indicate a more uniform reaction or that some of the active components have already reacted in the initial stages.
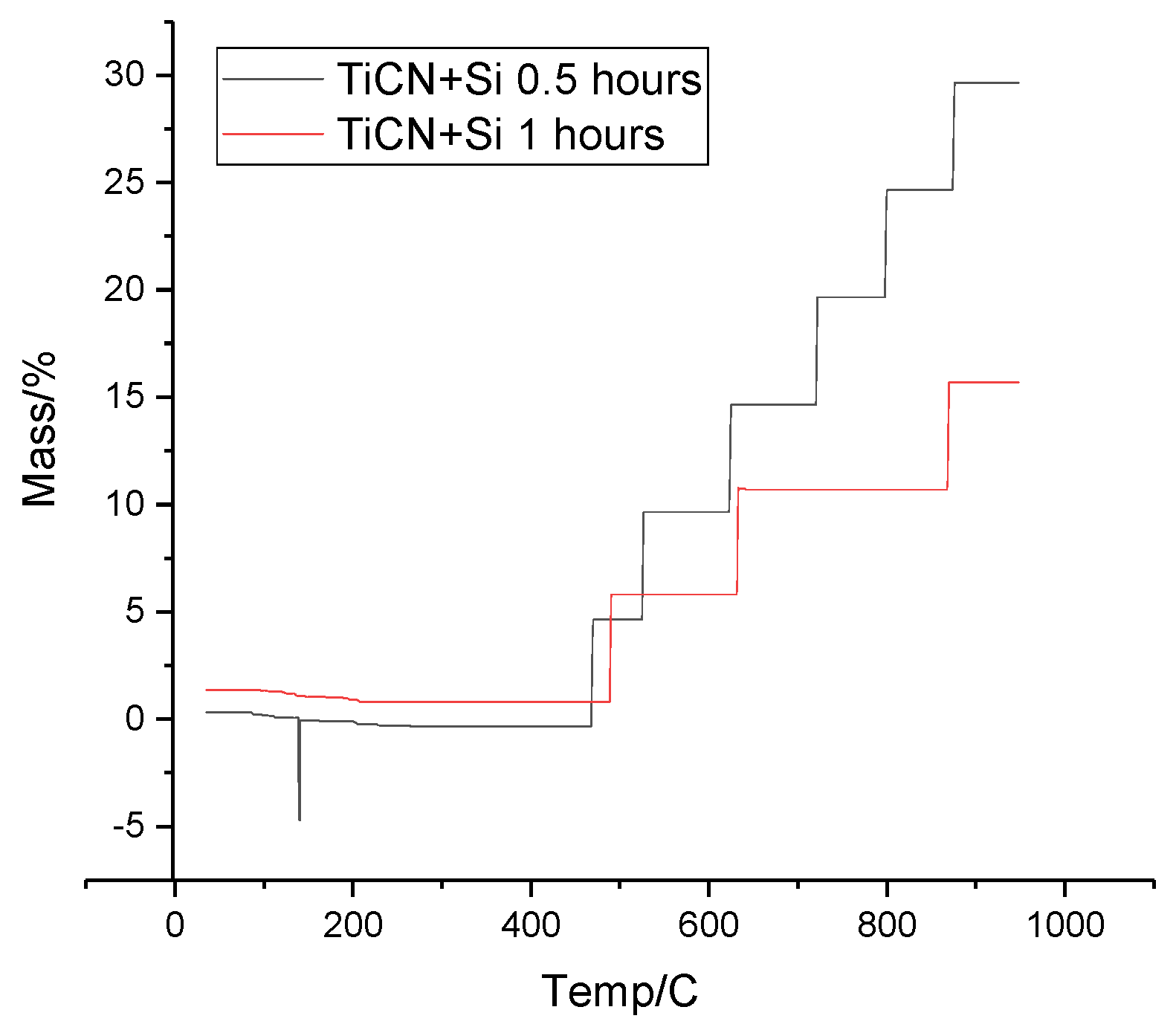
Figure 11 shows the thermogravimetric analysis curves that demonstrate the change in mass of TiCN+SiC powder samples as a function of temperature after activation.
At around 100°C, a slight decrease in mass is observed, which is due to the removal of moisture or other volatile components. At temperatures around 400°C, 600°C and 800°C, sharp mass jumps are observed, indicating phase transitions or reactions between powder components. For the sample activated for 1 hour (red curve), mass changes occur at slightly lower temperatures compared to the sample activated for 0.5 hour (black curve). This may indicate more reactions or phase transitions in the longer activated sample.
From the thermogravimetric analysis carried out for three different compositions and activation times, it can be concluded that the activation time significantly affects the thermal stability and reactivity of the powders.
3.2. Results of X-ray Diffraction Analysis
From the XRD analysis of the samples consisting of TiC, Si₃N₄ and TiCN based systems, important data on the crystal structure and phase composition were obtained.
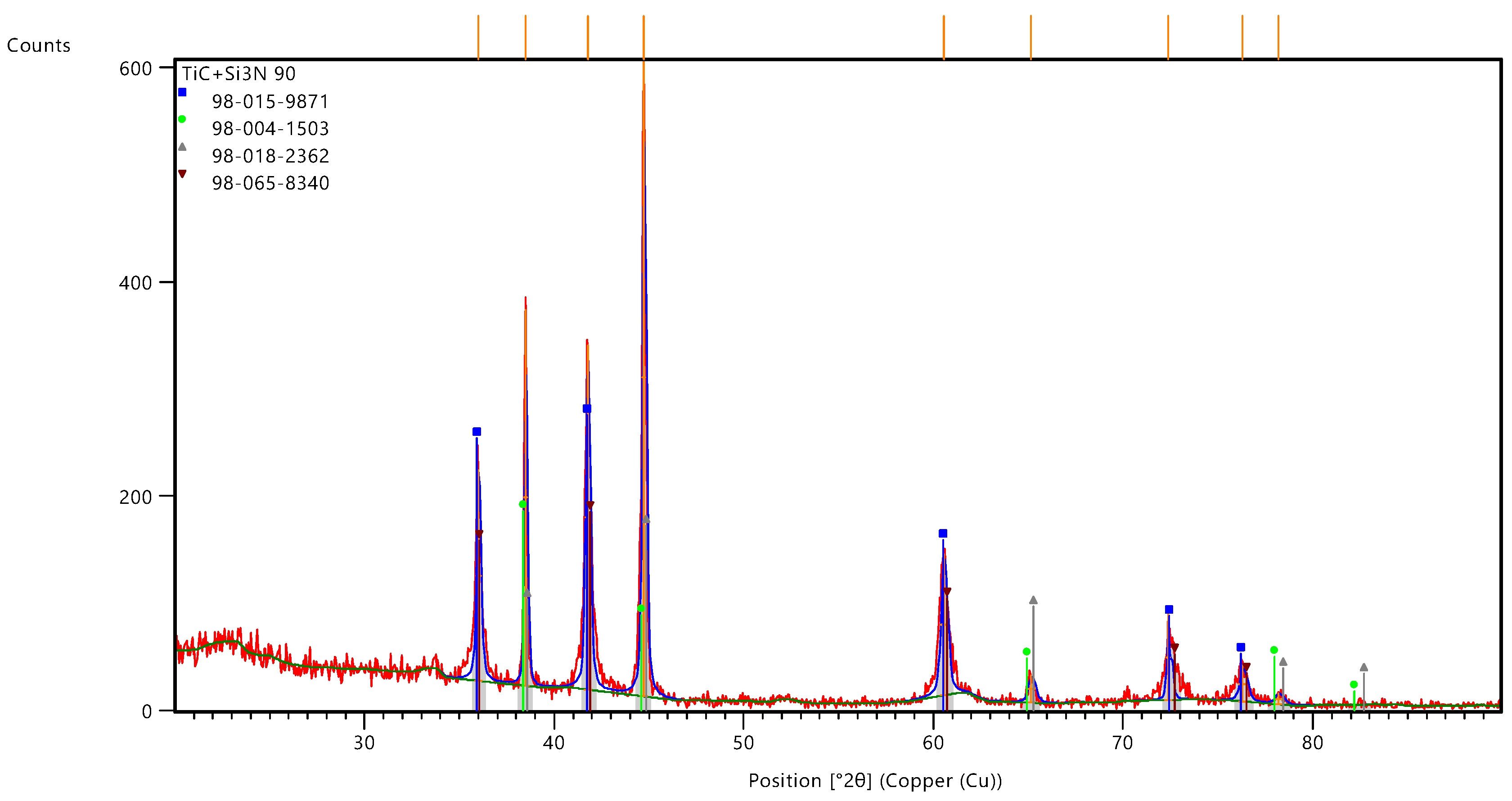
Figure 12 shows the results of the phase composition study of titanium carbide (TiC) and silicon nitride (Si₃N₄) based powder by X-ray diffraction (XRD), which was in activation for 90 min. The diffraction data was analysed using the ICCD PDF phase map database to identify the phases present in the sample. The diffractogram shows intense peaks corresponding to the different phases of the composite material. The obtained presence of the diffractogram showed several distinct diffraction peaks which represented groups with known phases TiC, SiC and Ti2CN. The main phase is titanium carbide (TiC), which provides a low level of the corresponding peaks. The peak width at half height (FWHM) for the TiC phase is quite small (e.g. 0.1968° for the peak at 2θ = 44.7317°), indicating good crystallinity of the material. However, broader peaks (e.g., FWHM = 0.3936° for the peak at 2θ = 72.3816°) may indicate the presence of defects or finely dispersed nanocrystals.
From
Table 1, the peaks and phase composition can be interpreted: The most intense peak at 44°, peaks at 38.4985° and 41.7615° respectively with high manifestation indicate a significant Si₃N₄ content, confirming the addition of this material to improve thermal and performance properties. Small peaks, especially those located at higher 2θ angles such as 72.3816°, 76.3055° and 78.2050°, may indicate the presence of finely dispersed or amorphous phases that may affect structural integrity. The broadness of peaks, such as that at 65.1571° with a FWHM of 0.3936°, may be indicative of fine grain size or high levels of internal stresses in Si₃N₄ crystals. This could be rapid cooling during production or changes in the synthesis process. The high rotation and narrow width of the main TiC peak indicates good crystallinity and minimal lattice strain
The presence of SiC and Ti2CN phases indicates the structural structure and the provision of a multiphase state, which may favour the mechanical and thermal properties of the composite. Further investigation involving a number of phase analyses and microstructural studies may provide additional information on the distributed phases and their influence on the material properties.
According to
Table 2, the titanium carbide (TiC) phase with cubic crystal structure and Fm-3m spatial structure is the main phase in the composite, which ensures the presence of several intense peaks characteristic of this compound. TiC phase and is the most intense in the diffractogram, indicating the predominance of this phase in the continuation of the composite. And titanium nitride-carbide (Ti2CN) phase, which is also present in the material providing a complex multi-component composition of the composite. The combination of TiC, SiC and Ti2CN phases indicates the possibility of stresses due to textures in thermal expansion coefficients and structural incompatibilities between the phases. This may relate to the mechanical properties of the material such as strength and hardness.
The TiC and Si3N4 based sample possesses highly orientated and coherently discontinuous phases, which ensures the formation of a transformative and highly stable coating. The epitaxial orientation of the phases and their coherent nature provide excellent mechanical and thermal properties, making this material suitable for extreme temperature and temperature applications.
So, the TiC and Si3N4 based multi-component powder possesses highly orientated and coherently discontinuous phases, which ensures the formation of a transformative and highly stable coating. The epitaxial orientation of the phases and their coherent nature provide excellent mechanical and thermal properties, making this material suitable for extreme temperature applications.
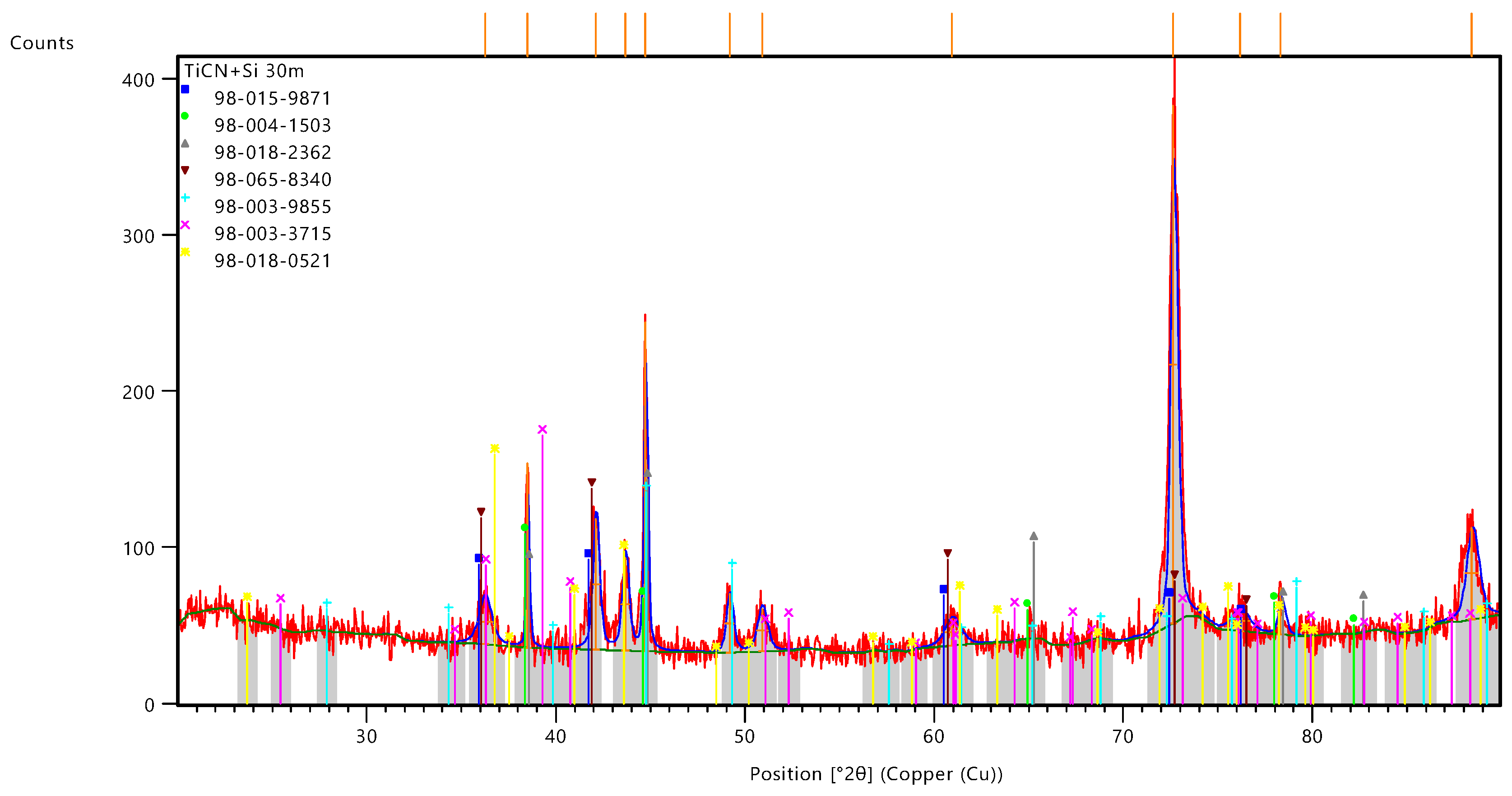
Figure 13 shows the X-ray diffraction pattern of TiCN+Si powder, where the peak intensities are plotted as a function of diffraction angles (positions 2θ2\theta2θ).
During the X-ray diffraction analysis of TiCN+Si powder, data were obtained that allow us to draw important conclusions about the phase composition and crystal structure of the investigated material. The results showed that the sample consists of several phases, each of which plays a different role in the overall composition of the material
The XRD analysis confirmed the presence of multiple phases including titanium carbides and nitrides (TiC and TiN) as well as silicon carbides (SiC). The main phases, such as TiC and TiN, have cubic and tetragonal crystal lattices typical of these compounds. The high intensity of the peaks corresponding to these phases indicates their predominance in the investigated material. In addition, peaks associated with the TiCN phase were detected in the XRD, which confirms the presence of carbide-nitride structures characteristic of materials derived from titanium and carbon.
The high intensity of some peaks indicates the predominance of certain phases (e.g., SiC). The complex superposition of peaks of different phases makes the analysis complex, but confirms the multiphase nature of the sample.
Table 3 contains the results of identification of the phases present in the investigated TiCN+Si sample based on XRD analysis. The following phases were identified during the XRD analysis of the TiCN+Si powder: the high proportion of titanium carbide and nitride may indicate the predominance of thermodynamically stable phases formed at high temperatures. At the same time, the presence of SiC in the structure suggests the participation of silicon in the reactions occurring during synthesis, which could indicate the need for further optimisation of process parameters to control the ratio of phases.
Based on the data obtained, it can be concluded that the TiCN+Si material has unique properties that make it suitable for use in highly stressed environments such as cutting tools or armour elements. However, further research is needed to understand in detail the relationship between the structural features of the material and its performance characteristics. In particular, more research is needed to investigate the influence of different phases on the tribological and thermal properties of the material, which will allow for a wider range of applications.
Titanium carbide (TiC) is present in the sample in small quantities. This phase, with its high hardness and durability, plays an important role in improving the mechanism. Pure titanium (Ti) is found with high compliance score indicating the presence of the phase in the material. A scaling factor of 0.175 indicates a significant amount of Ti in the sample which may affect the overall material properties such as strength and corrosion resistance. Silicon carbide (SiC) in α-form (Rs type) is present in significant amount. The high scale factor indicates that this phase is the only one in the image, resulting in hardness and thermal stability of the material. Titanium Carbide-Nitride (TiCN) Detected in a sample with a high compliance score and significant scale factor. Mechanical and quality materials are used at this stage, considering high durability and resistance to aggressive environments. Silicon carbide in γ-form (SiC - Gamma) is also present in significant amounts. A scaling factor of 0.246 indicates its major role in the manifestation of the material, especially in the ninefold improvement in mechanical properties and temperature resistance. Titanium nitride (Ti₂N) is found in moderate amounts in the materials. Despite the relatively low compliance rating, a high scale factor of 0.328 indicates the presence of this phase, making it important for improving hardness and coding resistance
5. Conclusions
Scanning electron microscopy (SEM), thermogravimetric analysis (TGA) and X-ray diffraction (XRD) analyses demonstrated a significant effect of mechanical activation on the structure and properties of powders of TiC, Si3N4 and TiCN based systems. The SEM study revealed that activation for 60 min leads to particle size reduction, reduced agglomeration and structure levelling, which improves the mechanical properties of the materials such as hardness and strength. The TGA results confirmed these observations, showing an improvement in the thermal stability of the powders, due to the reduction of volatile compounds and improved particle structure. This indicates that the activated powders are able to retain their properties at high temperatures, making them suitable for high thermal stress applications.
X-ray analysis confirmed the presence of a multiphase structure in the investigated materials, where the main phases are TiC, SiC and Ti₂CN. These phases are characterised by high crystallinity and minimal defects. Mechanical activation improved the crystalline structure of the powders, which directly affected their thermal and mechanical stability.
Thus, the combined results of SEM, TGA and XRD show that mechanical activation for 60 min is optimal for improving the microstructure, thermal stability and crystalline structure of the investigated systems. These materials exhibit high performance characteristics including strength, thermal stability and decomposition resistance, which makes them promising for use in extreme environments such as high temperatures and stresses.
Based on a comprehensive analysis of SEM, TGA and X-ray diffraction, the best composition for reactive plasma spraying was determined to be TiC+ Si3N4 based composition as it showed the best structure uniformity, minimal defects and high particle density after 60 min activation. This composition showed high crystallinity as confirmed by XRD analysis and excellent thermal stability as revealed by TGA. It has a high potential for high temperature and extreme mechanical stress applications.
However, the Ti+SiC and TiCN+SiC compositions also performed well in the studies. They show resistance to thermal decomposition and improved microstructure after activation. These materials may be promising for various applications, depending on the specific operating conditions. Further investigation of all three formulations is recommended to evaluate their applicability under different conditions and for different purposes, such as the development of durable coatings resistant to wear and high temperatures.
Thus, all three formulations (TiC+ Si3N4, Ti+SiC, TiCN+SiC) can be considered for industrial applications, with the final choice depending on the specific coating requirements and operating conditions.
Author Contributions
For research articles with multiple authors, a brief paragraph indicating their individual contributions should be provided. The following statements should be used: “Conceptualization, R.B.K.; methodology, K.A.B; validation, R.B.K.; formal analysis, B.L.S.; investigation, B.L.S.; resources, K.R.; data curation, K.A.K.; writing – original draft, B.L.S.; writing – review and editing, B.L.S.; visualization, K.R.; supervision, R.B.K.; project administration, B.L.S.; acquisition of funding, B.L.S. All authors have read and agreed to the published version of the manuscript.”
Funding
This research was funded by Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan, grant number AP19175967.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Dataset available on request from the authors.
Acknowledgments
This research is funded by the Committee of Science of the Ministry of Science and Higher Education of the Republic of Kazakhstan (Grant No. AP19175967).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Baimoldanova, L.S. , Rakhadilov B.K., Kenesbekov A., Kusaynov A., Maulit A. BRIEF REVIEW OF METHODS FOR PRODUCING TiSiCN COATINGS AND THE METHOD OF REACTIVE PLASMA SPRAYING. Bulletin of the NNC RK. 2024;(1):13-23. [CrossRef]
- Dong Yanchun, Yan Dianran, He Jining, Zhang Jianxin, Xiao Lisong, and Li Xiangzhi. Studies on Nanocrystalline TiN Coatings Prepared by Reactive Plasma Spraying. Nanomechanics and Nanostructured Multifunctional Materials: Experiments, Theories, and Simulations. - Vol. 2008, - Article ID 690951. [CrossRef]
- D. Yanchun, Y. D. Yanchun, Y. Dianran, H. Jining, Z. Jianxin, X. Lisong, L. Xiangzhi, Studies on nanocrystalline TiN coatings prepared by Reactive Plasma Spraying, J. Nanomater. (2008) Article ID 690951. [CrossRef]
- Feng W, Yan D, He J et al. Microhardness and toughness of the TiN coating prepared by reactive plasma spraying. Appl Surf Sci, 2005, 243(1-4):204-213. [CrossRef]
- Gaffet, E. , Bernard F. Mechanically activated powder metallurgy processing: A versatile way towards nanomaterials synthesis //Annales de Chimie Science des Matériaux. – No longer published by Elsevier, 2002. – Т. 27. – №. 6. – С. 47-59.
- Mucsi, G. – Csőke, B. – Gál, A. – Szabó, M. (2009): Mechanical activation of lignite fly ash and brown coal fly ash and their use as constituents in binders: Mechanische Aktivierung von Lignit- und Braunkohlenflugasche und ihre Verwendungals Bindemittel. Cement International, Vol. 7, No. 4, pp. 76-85.
- F. Movassagh-Alanagh, A. F. Movassagh-Alanagh, A. Abdollah-zadeh, M. Aliofkhazraei, M. Abedi, Improving the wear and corrosion resistance of Ti–6Al–4V alloy by deposition of TiSiN nanocomposite coating with pulsed-DC PACVD, Wear 390 (2017) 93–103. [CrossRef]
- S. Abraham, E.Y. S. Abraham, E.Y. Choi, N. Kang, K.H. Kim, Microstructure and mechanical properties of Ti-Si-CN films synthesized by plasma-enhanced chemical vapor deposition, Surf. Coat. Technol. 202 (2007) 915–919.
- Yeheyis, M. B. – Shang, J. Q. – Yanful, E. K. (2009): Chemical and Mineralogical Transformations of Coal Fly Ash after Landfilling. World of Coal Ash Conference (WOCA), -7, 2009. Lexington, Kentucky, USA. 4 May.
- Y. Cheng, T. Y. Cheng, T. Browne, B. Heckerman, E. Meletis, Mechanical and tribological properties of nanocomposite TiSiN coatings, Surf. Coat. Technol. 204 (2010) 2123–2129. [CrossRef]
- Pogrebnjak, A. , Buranich V., Ivashchenko V., Baimoldanova L., Rokosz K., Raaen S., Zukowski P., Opielak M., Rakhadilov B., Beresnev V., Erdybaevaba N. The Effect of Substrate Treatment on the Properties of TiAlSiYN/CrN Nanocomposite Coatings // Surfaces and Interfaces.-Vol.30, 22, 101902. (Quartile -Q1, IF=5,85, Scopus percentile–69). 20 June.
- Smyrnova, K.V. , Bondar O.V., Borba-Pogrebnjak S.O., Kravchenko Ya.O., Beresnev V.M., Zhollybekov B., Baimoldanova L. The microstructure and mechanical properties of (TiAlSiY)N nanostructured coatings // 2017 IEEE 7th International Conference on Nanomaterials: Applications & Properties (NAP 2017). – Vol.2. - P. 01FNC13-1-01FNC13-4.
- S. Abraham, E.Y. S. Abraham, E.Y. Choi, N. Kang, K.H. Kim, Microstructure and mechanical properties of Ti-Si-CN films synthesized by plasma-enhanced chemical vapor deposition, Surf. Coat. Technol. 202 (2007) 915–919. [CrossRef]
- Endler, M. Höhn, J. Schmidt, S. Scholz, M. Herrmann, M. Knaut, Ternary and quarternary TiSiN and TiSiCN nanocomposite coatings obtained by chemical vapor deposition, Surf. Coat. Technol. 215 (2013) 133–140. [CrossRef]
- Y. Wang, J. Y. Wang, J. Li, C. Dang, Y. Wang, Y. Zhu, Influence of bias voltage on structure and tribocorrosion properties of TiSiCN coating in artificial seawater, Mater. Char. 2017. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).