Submitted:
05 October 2024
Posted:
07 October 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Normal Function of IDH & the Cancer-Associated Accumulation of D-2-HG
Epigenetic Alterations
JmjC-KDMs
Histone Deacetylases
TET Enzymes
Prolyl Hydroxylases & Hypoxic Response
RNA Transcript Stability
Patterns of Transcription
Metabolic Reprogramming in IDH Mutant Glioma
Comparison of Wildtype and Mutant IDH Reactions
Mutant IDH Cells Do Not Perform Aerobic Glycolysis
Citric Acid Cycle Rewiring in IDH Mutant Cells
Mutant IDH Drives Changes In Amino Acid Metabolism
Consumption of NADPH by the Mutant IDH Enzyme
Dysregulation of Membrane Lipid Biosynthesis
DNA Damage Repair
Immunological Impact of IDH Mutations in Glioma
Diagnostics
Sequencing
Epigenetic Detection
Amplification-Based Detection
Histological Detection
D-2-HG as a Surrogate Marker
MRI
MRS
Clinical Implications of IDH Mutations
Clinical Classification of Gliomas
Influence of Mutational Status on the Production of D-2-HG
Pharmaceutical Treatment of IDH Mutant Gliomas
Molecular Basis for the Improved Prognosis of IDH Mutant Gliomas
Clinical Trials
Conclusions
Author Contributions
Conflicts of interest
Abbreviation
| Abbreviation | Definition |
| 5-mC | 5-methylcytosine |
| ABH | AlkB homologs |
| ADC | Apparent diffusion coefficient |
| αKG | α-ketoglutarate |
| AML | Acute myeloid leukemia |
| ATF | Activating transcription factor |
| ATM | Ataxia-telangiectasia-mutated |
| ATRX | Alpha thalassemia/mental retardation syndrome X-linked gene |
| BBB | Blood-brain barrier |
| BCAA | Branched-chain amino acids |
| BCAT (1/2) | Branched-chain aminotransferase (1 or 2) |
| BEAMing | Beads, emulsion, amplification, magnetics |
| CPE | Carboxypeptidase |
| CDC20 | Cell division cycle 20 |
| cfDNA | Cell-free circulating DNA |
| CHD (3-5) | Chromodomain helicase DNA binding protein (3-5) |
| CRISPR | Clustered regularly interspaced short palindromic repeats |
| CSF | Cerebral spinal fluid |
| D-2-HG | D-2-Hydroxyglutarate |
| ddPCR | Digital droplet PCR |
| DDR | DNA damage repair |
| DBC1 | Deleted in breast cancer 1 |
| DSB | Double-stranded break |
| DSC | Dynamic susceptibility contrast-enhanced MRI |
| DTI | Diffusion tensor imaging |
| DWI | Diffusion weighted MRI |
| EN2 | Engrailed 2 |
| EGFR | Epidermal growth factor receptor |
| FDA | Food and drug administration |
| FFPE | Formalin-fixed paraffin embedded tissue |
| FRET | Fluorescence resonance energy transfer |
| FTO | Fat mass and obesity-associated protein |
| G-CIMP | Glioma CpG island methylator phenotype |
| GBM | Glioblastoma multiforme |
| GC-MS | Gas chromatography mass-spectroscopy |
| H&E | Hematoxylin and Eosin |
| HDAC | Histone deacetylase |
| HIF | Hypoxia inducible factor |
| IDH | Isocitrate dehydrogenase |
| JmjC | Jumonji-C |
| KDM | Histone lysine demethylase |
| L-2-HG | L-2-Hydroxyglutarate |
| LAMP | Loop-mediated isothermal amplification |
| LC-MS | Liquid chromatography mass-spectroscopy |
| LDH (A/B) | Lactate dehydrogenase A or B |
| LNA | Locked nucleic acid |
| m6A | N6-methyladenosine |
| MAPK | Mitogen-activated protein kinase |
| MCT | Monocarboxylate transporters |
| MGMT | Methyl guanine methyl transferase |
| MRI | Magnetic resonance imaging |
| MRS | Magnetic resonance spectroscopy |
| MTT | Mean transit time |
| NAMPT | Nicotinamide phosphoribosyltransferase |
| nCATS | Nanopore Cas9 targeted sequencing |
| NGS | Next generation sequencing |
| NuRD | Nucleosome remodeling |
| ONT | Oxford nanopore technology |
| PARP | Poly(ADP) ribose |
| PDB | Protein data bank |
| PFKP | Phosphofructokinase platelet |
| PHD | Prolyl hydroxylase |
| PNA | Peptide nucleic acid |
| PWI | Perfusion weighted MRI |
| PYCR1 | Proline 5-carboxylase reductase 1 |
| qRT-PCR | Quantitative real-time PCR |
| rCBF | Relative cerebral blood flow |
| rCBV | Relative cerebral blood volume |
| RNAseq | RNA sequencing |
| SNV | Single nucleotide variant |
| ssRNAseq | Single cell RNA sequencing |
| STAT | Signal transducer and activator of transcription |
| T2-FLAIR | T2 fluid-attenuated inversion recovery |
| TERT | Telomerase reverse transcriptase |
| TET | Ten-eleven translocation |
| TMZ | Temozolomide |
| VAF | Variant allele frequency |
| VIM | Vimentin |
| WHO CNS5 | World Health Organization central nervous system 5 |
| WASF3 | Wiskott-Aldrich syndrome protein family |
| ZMYND8 | Zinc finger MYND-type containing 8 |
References
- Ijzerman-Korevaar, M.; Snijders, T.J.; de Graeff, A.; Teunissen, S.C.C.M.; de Vos, F.Y.F. Prevalence of symptoms in glioma patients throughout the disease trajectory: a systematic review. J. Neuro-Oncology 2018, 140, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Weller, Michael, Wolfgang Wick, Ken Aldape, Michael Brada, Mitchell Berger, Stefan M. Pfister, Ryo Nishikawa, Mark Rosenthal, Patrick Y. Wen, Roger Stupp, and Guido Reifenberger. "Glioma." Nature Reviews Disease Primers 1, no. 1 (2015): 15017.
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Jones, D.T.W.; Sill, M.; Hovestadt, V.; Schrimpf, D.; Sturm, D.; Koelsche, C.; Sahm, F.; Chavez, L.; Reuss, D.E.; et al. DNA methylation-based classification of central nervous system tumours. Nature 2018, 555, 469–474. [Google Scholar] [CrossRef]
- Boisselier, B.; Marie, Y.; Labussière, M.; Ciccarino, P.; Desestret, V.; Wang, X.; Capelle, L.; Delattre, J.-Y.; Sanson, M. COLD PCR HRM: a highly sensitive detection method for IDH1 mutations. Hum. Mutat. 2010, 31, 1360–1365. [Google Scholar] [CrossRef] [PubMed]
- Takano, Shingo, Wei Tian, Masahide Matsuda, Tetsuya Yamamoto, Eiichi Ishikawa, Mika Kato Kaneko, Kentaro Yamazaki, Yukinari Kato, and Akira Matsumura. "Detection of Idh1 Mutation in Human Gliomas: Comparison of Immunohistochemistry and Sequencing." Brain Tumor Pathology 28, no. 2 (2011): 115-23.
- Han, S.; Liu, Y.; Cai, S.J.; Qian, M.; Ding, J.; Larion, M.; Gilbert, M.R.; Yang, C. IDH mutation in glioma: molecular mechanisms and potential therapeutic targets. Br. J. Cancer 2020, 122, 1580–1589. [Google Scholar] [CrossRef] [PubMed]
- Parsons, D.W.; Jones, S.; Zhang, X.; Lin, J.C.-H.; Leary, R.J.; Angenendt, P.; Mankoo, P.; Carter, H.; Siu, I.-M.; Gallia, G.L.; et al. An Integrated Genomic Analysis of Human Glioblastoma Multiforme. Science 2008, 321, 1807–1812. [Google Scholar] [CrossRef]
- Popovici-Muller, J.; Lemieux, R.M.; Artin, E.; Saunders, J.O.; Salituro, F.G.; Travins, J.; Cianchetta, G.; Cai, Z.; Zhou, D.; Cui, D.; et al. Discovery of AG-120 (Ivosidenib): A First-in-Class Mutant IDH1 Inhibitor for the Treatment of IDH1 Mutant Cancers. ACS Med. Chem. Lett. 2018, 9, 300–305. [Google Scholar] [CrossRef]
- Mellinghoff, I.K.; Bent, M.J.v.D.; Blumenthal, D.T.; Touat, M.; Peters, K.B.; Clarke, J.; Mendez, J.; Yust-Katz, S.; Welsh, L.; Mason, W.P.; et al. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. New Engl. J. Med. 2023, 389, 589–601. [Google Scholar] [CrossRef]
- Amary, M.F.; Bacsi, K.; Maggiani, F.; Damato, S.; Halai, D.; Berisha, F.; Pollock, R.; O'Donnell, P.; Grigoriadis, A.; Diss, T.; et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J. Pathol. 2011, 224, 334–343. [Google Scholar] [CrossRef]
- Borger, D. R., L. Goyal, T. Yau, R. T. Poon, M. Ancukiewicz, V. Deshpande, D. C. Christiani, H. M. Liebman, H. Yang, H. Kim, K. Yen, J. E. Faris, A. J. Iafrate, E. L. Kwak, J. W. Clark, J. N. Allen, L. S. Blaszkowsky, J. E. Murphy, S. K. Saha, T. S. Hong, J. Y. Wo, C. R. Ferrone, K. K. Tanabe, N. Bardeesy, K. S. Straley, S. Agresta, D. P. Schenkein, L. W. Ellisen, D. P. Ryan, and A. X. Zhu. "Circulating Oncometabolite 2-Hydroxyglutarate Is a Potential Surrogate Biomarker in Patients with Isocitrate Dehydrogenase-Mutant Intrahepatic Cholangiocarcinoma." Clin Cancer Res 20, no. 7 (2014): 1884-90.
- Fathi, A.T.; Sadrzadeh, H.; Comander, A.H.; Higgins, M.J.; Bardia, A.; Perry, A.; Burke, M.; Silver, R.; Matulis, C.R.; Straley, K.S.; et al. Isocitrate Dehydrogenase 1 (IDH1) Mutation in Breast Adenocarcinoma Is Associated With Elevated Levels of Serum and Urine 2-Hydroxyglutarate. Oncol. 2014, 19, 602–607. [Google Scholar] [CrossRef]
- Dang, L.; White, D.W.; Gross, S.; Bennett, B.D.; Bittinger, M.A.; Driggers, E.M.; Fantin, V.R.; Jang, H.G.; Jin, S.; Keenan, M.C.; et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 2010, 462, 739–744. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, V.; Ciotti, G.; Gottardi, M.; Ciccarese, F. 2-Hydroxyglutarate in Acute Myeloid Leukemia: A Journey from Pathogenesis to Therapies. Biomedicines 2022, 10, 1359. [Google Scholar] [CrossRef]
- Malta, T.M.; de Souza, C.F.; Sabedot, T.S.; Silva, T.C.; Mosella, M.S.; Kalkanis, S.N.; Snyder, J.; Castro, A.V.B.; Noushmehr, H. Glioma CpG island methylator phenotype (G-CIMP): biological and clinical implications. Neuro-Oncology 2017, 20, 608–620. [Google Scholar] [CrossRef]
- Cui, D., J. Ren, J. Shi, L. Feng, K. Wang, T. Zeng, Y. Jin, and L. Gao. "R132h Mutation in Idh1 Gene Reduces Proliferation, Cell Survival and Invasion of Human Glioma by Downregulating Wnt/β-Catenin Signaling." Int J Biochem Cell Biol 73 (2016): 72-81.
- Wang, H.-Y.; Tang, K.; Liang, T.-Y.; Zhang, W.-Z.; Li, J.-Y.; Wang, W.; Hu, H.-M.; Li, M.-Y.; Wang, H.-Q.; He, X.-Z.; et al. The comparison of clinical and biological characteristics between IDH1 and IDH2 mutations in gliomas. J. Exp. Clin. Cancer Res. 2016, 35, 1–9. [Google Scholar] [CrossRef]
- Liu, S.; Cadoux-Hudson, T.; Schofield, C.J. Isocitrate dehydrogenase variants in cancer — Cellular consequences and therapeutic opportunities. Curr. Opin. Chem. Biol. 2020, 57, 122–134. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Flanagan, S.; Li, C.C.; Lee, M.; Shivalingham, B.; Maleki, S.; Wheeler, H.R.; E Buckland, M. Expanding the spectrum of IDH1 mutations in gliomas. Mod. Pathol. 2013, 26, 619–625. [Google Scholar] [CrossRef]
- Haider, A.S.; Ene, C.I.; Palmisciano, P.; Haider, M.; Rao, G.; Ballester, L.Y.; Fuller, G.N. Concurrent IDH1 and IDH2 mutations in glioblastoma: A case report. Front. Oncol. 2023, 13. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Ye, D.; Guan, K.-L.; Xiong, Y. IDH1andIDH2Mutations in Tumorigenesis: Mechanistic Insights and Clinical Perspectives. Clin. Cancer Res. 2012, 18, 5562–5571. [Google Scholar] [CrossRef]
- Gross, S.; Cairns, R.A.; Minden, M.D.; Driggers, E.M.; Bittinger, M.A.; Jang, H.G.; Sasaki, M.; Jin, S.; Schenkein, D.P.; Su, S.M.; et al. Cancer-associated metabolite 2-hydroxyglutarate accumulates in acute myelogenous leukemia with isocitrate dehydrogenase 1 and 2 mutations. J. Exp. Med. 2010, 207, 339–344. [Google Scholar] [CrossRef]
- Dow, Jonathan, and Peter M. Glazer. "Chapter 11 - Oncometabolites, Epigenetic Marks, and DNA Repair." In Epigenetics and DNA Damage, edited by Miriam Galvonas Jasiulionis, 191-202: Academic Press, 2022.
- Myllyharju, J. Prolyl 4-hydroxylases, the key enzymes of collagen biosynthesis. Matrix Biol. 2003, 22, 15–24. [Google Scholar] [CrossRef]
- Chaumeil, M.M.; Larson, P.E.; Woods, S.M.; Cai, L.; Eriksson, P.; Robinson, A.E.; Lupo, J.M.; Vigneron, D.B.; Nelson, S.J.; Pieper, R.O.; et al. Hyperpolarized [1-13C] Glutamate: A Metabolic Imaging Biomarker of IDH1 Mutational Status in Glioma. Cancer Res. 2014, 74, 4247–4257. [Google Scholar] [CrossRef] [PubMed]
- Kickingereder, P.; Sahm, F.; Radbruch, A.; Wick, W.; Heiland, S.; von Deimling, A.; Bendszus, M.; Wiestler, B. IDH mutation status is associated with a distinct hypoxia/angiogenesis transcriptome signature which is non-invasively predictable with rCBV imaging in human glioma. Sci. Rep. 2015, 5, 16238. [Google Scholar] [CrossRef] [PubMed]
- Chou, F.-J.; Liu, Y.; Lang, F.; Yang, C. D-2-Hydroxyglutarate in Glioma Biology. Cells 2021, 10, 2345. [Google Scholar] [CrossRef]
- Turcan, S.; Rohle, D.; Goenka, A.; Walsh, L.A.; Fang, F.; Yilmaz, E.; Campos, C.; Fabius, A.W.M.; Lu, C.; Ward, P.S.; et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 2012, 483, 479–483. [Google Scholar] [CrossRef]
- Sarah, L.; Fujimori, D.G. Recent developments in catalysis and inhibition of the Jumonji histone demethylases. Curr. Opin. Struct. Biol. 2023, 83, 102707–102707. [Google Scholar] [CrossRef] [PubMed]
- Manni, W.; Jianxin, X.; Weiqi, H.; Siyuan, C.; Huashan, S. JMJD family proteins in cancer and inflammation. Signal Transduct. Target. Ther. 2022, 7, 1–22. [Google Scholar] [CrossRef]
- Lee, D.H.; Kim, G.W.; Yoo, J.; Lee, S.W.; Jeon, Y.H.; Kim, S.Y.; Kang, H.G.; Kim, D.-H.; Chun, K.-H.; Choi, J.; et al. Histone demethylase KDM4C controls tumorigenesis of glioblastoma by epigenetically regulating p53 and c-Myc. Cell Death Dis. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.-J.; Zhu, M.-H.; Lu, X.-J.; Liu, Y.-J.; Lu, J.-F.; Leung, C.-H.; Ma, D.-L.; Chen, J. The emerging role of KDM5A in human cancer. J. Hematol. Oncol. 2021, 14, 1–18. [Google Scholar] [CrossRef]
- Losman, J.-A.; Koivunen, P.; Kaelin, W.G. 2-Oxoglutarate-dependent dioxygenases in cancer. Nat. Rev. Cancer 2020, 20, 710–726. [Google Scholar] [CrossRef]
- Zhang, Lan, Yao Chen, Zhijia Li, Congcong Lin, Tongtong Zhang, and Guan Wang. "Development of Jmjc-Domain-Containing Histone Demethylase (Kdm2-7) Inhibitors for Cancer Therapy." Drug Discovery Today 28, no. 5 (2023): 103519.
- Gunn, K., M. Myllykoski, J. Z. Cao, M. Ahmed, B. Huang, B. Rouaisnel, B. H. Diplas, M. M. Levitt, R. Looper, J. G. Doench, K. L. Ligon, H. I. Kornblum, S. K. McBrayer, H. Yan, C. Duy, L. A. Godley, P. Koivunen, and J. A. Losman. "(R)-2-Hydroxyglutarate Inhibits Kdm5 Histone Lysine Demethylases to Drive Transformation in Idh-Mutant Cancers." Cancer Discov 13, no. 6 (2023): 1478-97.
- Guerra-Calderas, L.; González-Barrios, R.; Herrera, L.A.; de León, D.C.; Soto-Reyes, E. The role of the histone demethylase KDM4A in cancer. Cancer Genet. 2015, 208, 215–224. [Google Scholar] [CrossRef]
- Wang, Z.; Cai, H.; Zhao, E.; Cui, H. The Diverse Roles of Histone Demethylase KDM4B in Normal and Cancer Development and Progression. Front. Cell Dev. Biol. 2022, 9, 790129. [Google Scholar] [CrossRef] [PubMed]
- Ota, K.; Tong, K.I.; Goto, K.; Tomida, S.; Komuro, A.; Wang, Z.; Nishio, K.; Okada, H. The H3K27 demethylase, Utx, regulates adipogenesis in a differentiation stage-dependent manner. PLOS ONE 2017, 12, e0173713. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, H.; Liu, Y.; Yang, Y.; Wang, P.; Kim, S.-H.; Ito, S.; Yang, C.; Wang, P.; Xiao, M.-T.; et al. Oncometabolite 2-Hydroxyglutarate Is a Competitive Inhibitor of α-Ketoglutarate-Dependent Dioxygenases. Cancer Cell 2011, 19, 17–30. [Google Scholar] [CrossRef]
- Miller, J.L.; Grant, P.A. The Role of DNA Methylation and Histone Modifications in Transcriptional Regulation in Humans. In Epigenetics: Development and Disease; Kundu, T.K., Ed.; Springer: Dordrecht, The Netherlands, 2013; pp. 289–317. [Google Scholar] [CrossRef]
- Laukka, T.; Myllykoski, M.; Looper, R.E.; Koivunen, P. Cancer-associated 2-oxoglutarate analogues modify histone methylation by inhibiting histone lysine demethylases. J. Mol. Biol. 2018, 430, 3081–3092. [Google Scholar] [CrossRef]
- Chen, H.P.; Zhao, Y.T.; Zhao, T.C. Histone Deacetylases and Mechanisms of Regulation of Gene Expression. Crit. Rev. Oncog. 2015, 20, 35–47. [Google Scholar] [CrossRef]
- Feng, Q. , and Y. Zhang. "The Mecp1 Complex Represses Transcription through Preferential Binding, Remodeling, and Deacetylating Methylated Nucleosomes." Genes Dev 15, no. 7 (2001): 827-32.
- Sears, T.K.; Horbinski, C.M.; Woolard, K.D. IDH1 mutant glioma is preferentially sensitive to the HDAC inhibitor panobinostat. J. Neuro-Oncology 2021, 154, 159–170. [Google Scholar] [CrossRef]
- Garrett, M.C.; Albano, R.; Carnwath, T.; Elahi, L.; Behrmann, C.A.; Pemberton, M.; Woo, D.; O’brien, E.; VanCauwenbergh, B.; Perentesis, J.; et al. HDAC1 and HDAC6 are essential for driving growth in IDH1 mutant glioma. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Zhang, Xinchao, Yue Zhang, Chaofu Wang, and Xu Wang. "Tet (Ten-Eleven Translocation) Family Proteins: Structure, Biological Functions and Applications." Signal Transduction and Targeted Therapy 8, no. 1 (2023): 297.
- Gerecke, C.; Rodrigues, C.E.; Homann, T.; Kleuser, B. The Role of Ten-Eleven Translocation Proteins in Inflammation. Front. Immunol. 2022, 13, 861351. [Google Scholar] [CrossRef] [PubMed]
- Bogdanović, O.; Smits, A.H.; Mustienes, E.d.l.C.; Tena, J.J.; Ford, E.; Williams, R.; Senanayake, U.; Schultz, M.D.; Hontelez, S.; van Kruijsbergen, I.; et al. Active DNA demethylation at enhancers during the vertebrate phylotypic period. Nat. Genet. 2016, 48, 417–426. [Google Scholar] [CrossRef]
- Sanstead, P.J.; Ashwood, B.; Dai, Q.; He, C.; Tokmakoff, A. Oxidized Derivatives of 5-Methylcytosine Alter the Stability and Dehybridization Dynamics of Duplex DNA. J. Phys. Chem. B 2020, 124, 1160–1174. [Google Scholar] [CrossRef]
- Vasanthakumar, A.; Godley, L.A. 5-hydroxymethylcytosine in cancer: significance in diagnosis and therapy. Cancer Genet. 2015, 208, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.C.; Houseman, E.A.; King, J.E.; von Herrmann, K.M.; Fadul, C.E.; Christensen, B.C. 5-Hydroxymethylcytosine localizes to enhancer elements and is associated with survival in glioblastoma patients. Nat. Commun. 2016, 7, 13177. [Google Scholar] [CrossRef] [PubMed]
- Nomura, M.; Saito, K.; Aihara, K.; Nagae, G.; Yamamoto, S.; Tatsuno, K.; Ueda, H.; Fukuda, S.; Umeda, T.; Tanaka, S.; et al. DNA demethylation is associated with malignant progression of lower-grade gliomas. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Chou, S.-C.; Liu, C.-Y.; Chen, C.-Y.; Hou, H.-A.; Kuo, Y.-Y.; Lee, M.-C.; Ko, B.-S.; Tang, J.-L.; Yao, M.; et al. TET2 mutation is an unfavorable prognostic factor in acute myeloid leukemia patients with intermediate-risk cytogenetics. Blood 2011, 118, 3803–3810. [Google Scholar] [CrossRef]
- Bray, J.K.; Dawlaty, M.M.; Verma, A.; Maitra, A. Roles and Regulations of TET Enzymes in Solid Tumors. Trends Cancer 2021, 7, 635–646. [Google Scholar] [CrossRef]
- Figueroa, M. E., O. Abdel-Wahab, C. Lu, P. S. Ward, J. Patel, A. Shih, Y. Li, N. Bhagwat, A. Vasanthakumar, H. F. Fernandez, M. S. Tallman, Z. Sun, K. Wolniak, J. K. Peeters, W. Liu, S. E. Choe, V. R. Fantin, E. Paietta, B. Löwenberg, J. D. Licht, L. A. Godley, R. Delwel, P. J. Valk, C. B. Thompson, R. L. Levine, and A. Melnick. "Leukemic Idh1 and Idh2 Mutations Result in a Hypermethylation Phenotype, Disrupt Tet2 Function, and Impair Hematopoietic Differentiation." Cancer Cell 18, no. 6 (2010): 553-67.
- Huang, Y.; Wei, J.; Huang, X.; Zhou, W.; Xu, Y.; Deng, D.-H.; Cheng, P. Comprehensively analyze the expression and prognostic role for ten-eleven translocations (TETs) in acute myeloid leukemia. Transl. Cancer Res. 2020, 9, 7259–7283. [Google Scholar] [CrossRef]
- Laukka, T.; Mariani, C.J.; Ihantola, T.; Cao, J.Z.; Hokkanen, J.; Kaelin, W.G., Jr.; Godley, L.A.; Koivunen, P. Fumarate and Succinate Regulate Expression of Hypoxia-inducible Genes via TET Enzymes. J. Biol. Chem. 2016, 291, 4256–4265. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Dematteo, R.G.; Venneti, S.; Finley, L.W.; Lu, C.; Judkins, A.R.; Rustenburg, A.S.; Grinaway, P.B.; Chodera, J.D.; Cross, J.R.; et al. Hypoxia Induces Production of L-2-Hydroxyglutarate. Cell Metab. 2015, 22, 304–311. [Google Scholar] [CrossRef]
- Belle, R.; Saraç, H.; Salah, E.; Bhushan, B.; Szykowska, A.; Roper, G.; Tumber, A.; Kriaucionis, S.; Burgess-Brown, N.; Schofield, C.J.; et al. Focused Screening Identifies Different Sensitivities of Human TET Oxygenases to the Oncometabolite 2-Hydroxyglutarate. J. Med. Chem. 2024, 67, 4525–4540. [Google Scholar] [CrossRef]
- Yue, X.; Rao, A. TET family dioxygenases and the TET activator vitamin C in immune responses and cancer. Blood 2020, 136, 1394–1401. [Google Scholar] [CrossRef]
- Gerecke, C.; Schumacher, F.; Berndzen, A.; Homann, T.; Kleuser, B. Vitamin C in combination with inhibition of mutant IDH1 synergistically activates TET enzymes and epigenetically modulates gene silencing in colon cancer cells. Epigenetics 2019, 15, 307–322. [Google Scholar] [CrossRef] [PubMed]
- Kusi, M.; Zand, M.; Lin, L.-L.; Chen, M.; Lopez, A.; Lin, C.-L.; Wang, C.-M.; Lucio, N.D.; Kirma, N.B.; Ruan, J.; et al. 2-Hydroxyglutarate destabilizes chromatin regulatory landscape and lineage fidelity to promote cellular heterogeneity. Cell Rep. 2022, 38, 110220–110220. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, M.; Knobbe, C.B.; Itsumi, M.; Elia, A.J.; Harris, I.S.; Chio, I.I.C.; Cairns, R.A.; McCracken, S.; Wakeham, A.; Haight, J.; et al. D-2-hydroxyglutarate produced by mutant IDH1 perturbs collagen maturation and basement membrane function. Genes Dev. 2012, 26, 2038–2049. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Finley, L.W.S. Metabolic signatures of cancer cells and stem cells. Nat. Metab. 2019, 1, 177–188. [Google Scholar] [CrossRef]
- Frost, J.; Frost, M.; Batie, M.; Jiang, H.; Rocha, S. Roles of HIF and 2-Oxoglutarate-Dependent Dioxygenases in Controlling Gene Expression in Hypoxia. Cancers 2021, 13, 350. [Google Scholar] [CrossRef]
- D'Ignazio, L., M. Batie, and S. Rocha. "Hypoxia and Inflammation in Cancer, Focus on Hif and Nf-κb." Biomedicines 5, no. 2 (2017).
- Rocha, S. Gene regulation under low oxygen: holding your breath for transcription. Trends Biochem. Sci. 2007, 32, 389–397. [Google Scholar] [CrossRef]
- Koivunen, P.; Lee, S.; Duncan, C.G.; Lopez, G.; Lu, G.; Ramkissoon, S.; Losman, J.A.; Joensuu, P.; Bergmann, U.; Gross, S.; et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 2012, 483, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Majmundar, A.J.; Wong, W.J.; Simon, M.C. Hypoxia-Inducible Factors and the Response to Hypoxic Stress. Mol. Cell 2010, 40, 294–309. [Google Scholar] [CrossRef]
- Böttcher, M.; Renner, K.; Berger, R.; Mentz, K.; Thomas, S.; Cardenas-Conejo, Z.E.; Dettmer, K.; Oefner, P.J.; Mackensen, A.; Kreutz, M.; et al. D-2-hydroxyglutarate interferes with HIF-1α stability skewing T-cell metabolism towards oxidative phosphorylation and impairing Th17 polarization. OncoImmunology 2018, 7, e1445454. [Google Scholar] [CrossRef]
- Kuiper, C.; Dachs, G.U.; Currie, M.J.; Vissers, M.C. Intracellular ascorbate enhances hypoxia-inducible factor (HIF)-hydroxylase activity and preferentially suppresses the HIF-1 transcriptional response. Free. Radic. Biol. Med. 2014, 69, 308–317. [Google Scholar] [CrossRef]
- Miles, S.L.; Fischer, A.P.; Joshi, S.J.; Niles, R.M. Ascorbic acid and ascorbate-2-phosphate decrease HIF activity and malignant properties of human melanoma cells. BMC Cancer 2015, 15, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, R.; Yeoh, K.K.; Tian, Y.-M.; Hillringhaus, L.; Bagg, E.A.; Rose, N.R.; Leung, I.K.H.; Li, X.S.; Woon, E.C.Y.; Yang, M.; et al. The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep. 2011, 12, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Lin, Y.; Xu, W.; Jiang, W.; Zha, Z.; Wang, P.; Yu, W.; Li, Z.; Gong, L.; Peng, Y.; et al. Glioma-Derived Mutations in IDH1 Dominantly Inhibit IDH1 Catalytic Activity and Induce HIF-1α. Science 2009, 324, 261–265. [Google Scholar] [CrossRef]
- Selak, M. A., S. M. Armour, E. D. MacKenzie, H. Boulahbel, D. G. Watson, K. D. Mansfield, Y. Pan, M. C. Simon, C. B. Thompson, and E. Gottlieb. "Succinate Links Tca Cycle Dysfunction to Oncogenesis by Inhibiting Hif-Alpha Prolyl Hydroxylase." Cancer Cell 7, no. 1 (2005): 77-85.
- Isaacs, J.S.; Jung, Y.J.; Mole, D.R.; Lee, S.; Torres-Cabala, C.; Chung, Y.-L.; Merino, M.; Trepel, J.; Zbar, B.; Toro, J.; et al. HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: Novel role of fumarate in regulation of HIF stability. Cancer Cell 2005, 8, 143–153. [Google Scholar] [CrossRef]
- Hong, J.; Xu, K.; Lee, J.H. Biological roles of the RNA m6A modification and its implications in cancer. Exp. Mol. Med. 2022, 54, 1822–1832. [Google Scholar] [CrossRef]
- Huang, H.; Weng, H.; Chen, J. m6A Modification in Coding and Non-coding RNAs: Roles and Therapeutic Implications in Cancer. Cancer Cell 2020, 37, 270–288. [Google Scholar] [CrossRef]
- Tang, F.; Pan, Z.; Wang, Y.; Lan, T.; Wang, M.; Li, F.; Quan, W.; Liu, Z.; Wang, Z.; Li, Z. Advances in the Immunotherapeutic Potential of Isocitrate Dehydrogenase Mutations in Glioma. Neurosci. Bull. 2022, 38, 1069–1084. [Google Scholar] [CrossRef]
- Pianka, S.T.; Li, T.; Prins, T.J.; Eldred, B.S.; Kevan, B.M.; Liang, H.; Rinonos, S.Z.; Kornblum, H.I.; Nathanson, D.A.; Pellegrini, M.; et al. D-2-HG Inhibits IDH1mut Glioma Growth via FTO Inhibition and Resultant m6A Hypermethylation. Cancer Res. Commun. 2024, 4, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Ouyang, X.; Zuo, L.; Xiao, Y.; Sun, Y.; Chang, C.; Qin, X.; Yeh, S. R-2HG downregulates ERα to inhibit cholangiocarcinoma via the FTO/m6A-methylated ERα/miR16-5p/YAP1 signal pathway. Mol. Ther. - Oncolytics 2021, 23, 65–81. [Google Scholar] [CrossRef]
- Su, R.; Dong, L.; Li, C.; Nachtergaele, S.; Wunderlich, M.; Qing, Y.; Deng, X.; Wang, Y.; Weng, X.; Hu, C.; et al. R-2HG Exhibits Anti-tumor Activity by Targeting FTO/m6A/MYC/CEBPA Signaling. Cell 2018, 172, 90–105. [Google Scholar] [CrossRef]
- Qing, Y.; Dong, L.; Gao, L.; Li, C.; Li, Y.; Han, L.; Prince, E.; Tan, B.; Deng, X.; Wetzel, C.; et al. R-2-hydroxyglutarate attenuates aerobic glycolysis in leukemia by targeting the FTO/m6A/PFKP/LDHB axis. Mol. Cell 2021, 81, 922–939. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Sidoli, S.; Warneford-Thomson, R.; Tatomer, D.C.; Wilusz, J.E.; Garcia, B.A.; Bonasio, R. High-Resolution Mapping of RNA-Binding Regions in the Nuclear Proteome of Embryonic Stem Cells. Mol. Cell 2016, 64, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Delatte, B.; Wang, F.; Ngoc, L.V.; Collignon, E.; Bonvin, E.; Deplus, R.; Calonne, E.; Hassabi, B.; Putmans, P.; Awe, S.; et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science 2016, 351, 282–285. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Zhang, Q.; Shi, Y.; Shi, Q.; Jiang, Y.; Gu, Y.; Li, Z.; Li, X.; Zhao, K.; Wang, C.; et al. Tet2 promotes pathogen infection-induced myelopoiesis through mRNA oxidation. Nature 2018, 554, 123–127. [Google Scholar] [CrossRef]
- Tran, Paul M. H., Lynn K. H. Tran, John Nechtman, Bruno dos Santos, Sharad Purohit, Khaled Bin Satter, Boying Dun, Ravindra Kolhe, Suash Sharma, Roni Bollag, and Jin-Xiong She. "Comparative Analysis of Transcriptomic Profile, Histology, and Idh Mutation for Classification of Gliomas." Scientific Reports 10, no. 1 (2020): 20651.
- Cheng, W.; Ren, X.; Zhang, C.; Cai, J.; Han, S.; Wu, A. Gene Expression Profiling Stratifies IDH1-Mutant Glioma with Distinct Prognoses. Mol. Neurobiol. 2016, 54, 5996–6005. [Google Scholar] [CrossRef]
- Unruh, D.; Zewde, M.; Buss, A.; Drumm, M.R.; Tran, A.N.; Scholtens, D.M.; Horbinski, C. Methylation and transcription patterns are distinct in IDH mutant gliomas compared to other IDH mutant cancers. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Alzial, G.; Renoult, O.; Paris, F.; Gratas, C.; Clavreul, A.; Pecqueur, C. Wild-type isocitrate dehydrogenase under the spotlight in glioblastoma. Oncogene 2021, 41, 613–621. [Google Scholar] [CrossRef]
- Metallo, C.M.; Gameiro, P.A.; Bell, E.L.; Mattaini, K.R.; Yang, J.; Hiller, K.; Jewell, C.M.; Johnson, Z.R.; Irvine, D.J.; Guarente, L.; et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 2012, 481, 380–384. [Google Scholar] [CrossRef]
- Mullen, A.R.; Wheaton, W.W.; Jin, E.S.; Chen, P.-H.; Sullivan, L.B.; Cheng, T.; Yang, Y.; Linehan, W.M.; Chandel, N.S.; DeBerardinis, R.J. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 2011, 481, 385–388. [Google Scholar] [CrossRef]
- Fan, J.; Teng, X.; Liu, L.; Mattaini, K.R.; Looper, R.E.; Vander Heiden, M.G.; Rabinowitz, J.D. Human Phosphoglycerate Dehydrogenase Produces the Oncometabolite d-2-Hydroxyglutarate. ACS Chem. Biol. 2015, 10, 510–516. [Google Scholar] [CrossRef]
- Zhou, L.; Wang, Z.; Hu, C.; Zhang, C.; Kovatcheva-Datchary, P.; Yu, D.; Liu, S.; Ren, F.; Wang, X.; Li, Y.; et al. Integrated Metabolomics and Lipidomics Analyses Reveal Metabolic Reprogramming in Human Glioma with IDH1 Mutation. J. Proteome Res. 2018, 18, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Fendt, S.-M. 100 years of the Warburg effect: A cancer metabolism endeavor. Cell 2024, 187, 3824–3828. [Google Scholar] [CrossRef] [PubMed]
- Khurshed, M.; Molenaar, R.J.; Lenting, K.; Leenders, W.P.; van Noorden, C.J. In silico gene expression analysis reveals glycolysis and acetate anaplerosis in IDH1 wild-type glioma and lactate and glutamate anaplerosis in IDH1-mutated glioma. Oncotarget 2017, 8, 49165–49177. [Google Scholar] [CrossRef] [PubMed]
- Chesnelong, C.; Chaumeil, M.M.; Blough, M.D.; Al-Najjar, M.; Stechishin, O.D.; Chan, J.A.; Pieper, R.O.; Ronen, S.M.; Weiss, S.; Luchman, H.A.; et al. Lactate dehydrogenase A silencing in IDH mutant gliomas. Neuro-Oncology 2013, 16, 686–695. [Google Scholar] [CrossRef]
- Chaumeil, M.M.; Radoul, M.; Najac, C.; Eriksson, P.; Viswanath, P.; Blough, M.D.; Chesnelong, C.; Luchman, H.A.; Cairncross, J.G.; Ronen, S.M. Hyperpolarized 13 C MR imaging detects no lactate production in mutant IDH1 gliomas: Implications for diagnosis and response monitoring. NeuroImage: Clin. 2016, 12, 180–189. [Google Scholar] [CrossRef]
- Dekker, L.J.M.; Wu, S.; Jurriëns, C.; Mustafa, D.A.N.; Grevers, F.; Burgers, P.C.; Smitt, P.A.E.S.; Kros, J.M.; Luider, T.M. Metabolic changes related to the IDH1 mutation in gliomas preserve TCA-cycle activity: An investigation at the protein level. FASEB J. 2020, 34, 3646–3657. [Google Scholar] [CrossRef]
- Arnold, P.K.; Finley, L.W. Regulation and function of the mammalian tricarboxylic acid cycle. J. Biol. Chem. 2022, 299, 102838. [Google Scholar] [CrossRef]
- Biedermann, J.; Preussler, M.; Conde, M.; Peitzsch, M.; Richter, S.; Wiedemuth, R.; Abou-El-Ardat, K.; Krüger, A.; Meinhardt, M.; Schackert, G.; et al. Mutant IDH1 Differently Affects Redox State and Metabolism in Glial Cells of Normal and Tumor Origin. Cancers 2019, 11, 2028. [Google Scholar] [CrossRef]
- Fack, F., S. Tardito, G. Hochart, A. Oudin, L. Zheng, S. Fritah, A. Golebiewska, P. V. Nazarov, A. Bernard, A. C. Hau, O. Keunen, W. Leenders, M. Lund-Johansen, J. Stauber, E. Gottlieb, R. Bjerkvig, and S. P. Niclou. "Altered Metabolic Landscape in Idh-Mutant Gliomas Affects Phospholipid, Energy, and Oxidative Stress Pathways." EMBO Mol Med 9, no. 12 (2017): 1681-95.
- Izquierdo-Garcia, J.L.; Cai, L.M.; Chaumeil, M.M.; Eriksson, P.; Robinson, A.E.; Pieper, R.O.; Phillips, J.J.; Ronen, S.M. Glioma Cells with the IDH1 Mutation Modulate Metabolic Fractional Flux through Pyruvate Carboxylase. PLOS ONE 2014, 9, e108289. [Google Scholar] [CrossRef]
- Lenting, K.; Khurshed, M.; Peeters, T.H.; Heuvel, C.N.A.M.v.D.; van Lith, S.A.M.; de Bitter, T.; Hendriks, W.; Span, P.N.; Molenaar, R.J.; Botman, D.; et al. Isocitrate dehydrogenase 1–mutated human gliomas depend on lactate and glutamate to alleviate metabolic stress. FASEB J. 2018, 33, 557–571. [Google Scholar] [CrossRef]
- Dekker, L.J.M.; Verheul, C.; Wensveen, N.; Leenders, W.; Lamfers, M.L.M.; Leenstra, S.; Luider, T.M. Effects of the IDH1 R132H Mutation on the Energy Metabolism: A Comparison between Tissue and Corresponding Primary Glioma Cell Cultures. ACS Omega 2022, 7, 3568–3578. [Google Scholar] [CrossRef] [PubMed]
- McBrayer, S.K.; Mayers, J.R.; DiNatale, G.J.; Shi, D.D.; Khanal, J.; Chakraborty, A.A.; Sarosiek, K.A.; Briggs, K.J.; Robbins, A.K.; Sewastianik, T.; et al. Transaminase Inhibition by 2-Hydroxyglutarate Impairs Glutamate Biosynthesis and Redox Homeostasis in Glioma. Cell 2018, 175, 101–116. [Google Scholar] [CrossRef] [PubMed]
- Tonjes, M., S. Barbus, Y. J. Park, W. Wang, M. Schlotter, A. M. Lindroth, S. V. Pleier, A. H. C. Bai, D. Karra, R. M. Piro, J. Felsberg, A. Addington, D. Lemke, I. Weibrecht, V. Hovestadt, C. G. Rolli, B. Campos, S. Turcan, D. Sturm,..., and B. Radlwimmer. "Bcat1 Promotes Cell Proliferation through Amino Acid Catabolism in Gliomas Carrying Wild-Type Idh1." Nat Med 19, no. 7 (2013): 901-08.
- Yang, M.; Vousden, K.H. Serine and one-carbon metabolism in cancer. Nat. Rev. Cancer 2016, 16, 650–662. [Google Scholar] [CrossRef] [PubMed]
- Hollinshead, K. E. R., H. Munford, K. L. Eales, C. Bardella, C. Li, C. Escribano-Gonzalez, A. Thakker, Y. Nonnenmacher, K. Kluckova, M. Jeeves, R. Murren, F. Cuozzo, D. Ye, G. Laurenti, W. Zhu, K. Hiller, D. J. Hodson, W. Hua, I. P. Tomlinson,..., and D. A. Tennant. "Oncogenic Idh1 Mutations Promote Enhanced Proline Synthesis through Pycr1 to Support the Maintenance of Mitochondrial Redox Homeostasis." Cell Rep 22, no. 12 (2018): 3107-14.
- Gelman, S.J.; Naser, F.; Mahieu, N.G.; McKenzie, L.D.; Dunn, G.P.; Chheda, M.G.; Patti, G.J. Consumption of NADPH for 2-HG Synthesis Increases Pentose Phosphate Pathway Flux and Sensitizes Cells to Oxidative Stress. Cell Rep. 2018, 22, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Badur, M.G.; Muthusamy, T.; Parker, S.J.; Ma, S.; McBrayer, S.K.; Cordes, T.; Magana, J.H.; Guan, K.-L.; Metallo, C.M. Oncogenic R132 IDH1 Mutations Limit NADPH for De Novo Lipogenesis through (D)2-Hydroxyglutarate Production in Fibrosarcoma Cells. Cell Rep. 2018, 25, 1680–1680. [Google Scholar] [CrossRef]
- Esmaeili, M.; Hamans, B.C.; Navis, A.C.; van Horssen, R.; Bathen, T.F.; Gribbestad, I.S.; Leenders, W.P.; Heerschap, A. IDH1 R132H Mutation Generates a Distinct Phospholipid Metabolite Profile in Glioma. Cancer Res. 2014, 74, 4898–4907. [Google Scholar] [CrossRef]
- Izquierdo-Garcia, J.L.; Viswanath, P.; Eriksson, P.; Chaumeil, M.M.; Pieper, R.O.; Phillips, J.J.; Ronen, S.M. Metabolic Reprogramming in Mutant IDH1 Glioma Cells. PLOS ONE 2015, 10, e0118781. [Google Scholar] [CrossRef]
- Fedeles, B. I., V. Singh, J. C. Delaney, D. Li, and J. M. Essigmann. "The Alkb Family of Fe(Ii)/α-Ketoglutarate-Dependent Dioxygenases: Repairing Nucleic Acid Alkylation Damage and Beyond." J Biol Chem 290, no. 34 (2015): 20734-42.
- Chen, F.; Bian, K.; Tang, Q.; Fedeles, B.I.; Singh, V.; Humulock, Z.T.; Essigmann, J.M.; Li, D. Oncometabolites d- and l-2-Hydroxyglutarate Inhibit the AlkB Family DNA Repair Enzymes under Physiological Conditions. Chem. Res. Toxicol. 2017, 30, 1102–1110. [Google Scholar] [CrossRef]
- Sim, H. W., R. Nejad, W. Zhang, F. Nassiri, W. Mason, K. D. Aldape, G. Zadeh, and E. X. Chen. "Tissue 2-Hydroxyglutarate as a Biomarker for Isocitrate Dehydrogenase Mutations in Gliomas." Clin Cancer Res 25, no. 11 (2019): 3366-73.
- Wang, P.; Wu, J.; Ma, S.; Zhang, L.; Yao, J.; Hoadley, K.A.; Wilkerson, M.D.; Perou, C.M.; Guan, K.-L.; Ye, D.; et al. Oncometabolite D-2-Hydroxyglutarate Inhibits ALKBH DNA Repair Enzymes and Sensitizes IDH Mutant Cells to Alkylating Agents. Cell Rep. 2015, 13, 2353–2361. [Google Scholar] [CrossRef]
- Lin, L.; Cai, J.; Tan, Z.; Meng, X.; Li, R.; Li, Y.; Jiang, C. Mutant IDH1 Enhances Temozolomide Sensitivity via Regulation of the ATM/CHK2 Pathway in Glioma. Cancer Res. Treat. 2021, 53, 367–377. [Google Scholar] [CrossRef]
- Matsuoka, S.; Rotman, G.; Ogawa, A.; Shiloh, Y.; Tamai, K.; Elledge, S.J. Ataxia telangiectasia-mutated phosphorylates Chk2 in vivo and in vitro. Proc. Natl. Acad. Sci. USA 2000, 97, 10389–10394. [Google Scholar] [CrossRef] [PubMed]
- Inoue, S.; Li, W.Y.; Tseng, A.; Beerman, I.; Elia, A.J.; Bendall, S.C.; Lemonnier, F.; Kron, K.J.; Cescon, D.W.; Hao, Z.; et al. Mutant IDH1 Downregulates ATM and Alters DNA Repair and Sensitivity to DNA Damage Independent of TET2. Cancer Cell 2016, 30, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Carney, S. V., K. Banerjee, A. Mujeeb, B. Zhu, S. Haase, M. L. Varela, P. Kadiyala, C. E. Tronrud, Z. Zhu, D. Mukherji, P. Gorla, Y. Sun, R. Tagett, F. J. Núñez, M. Luo, W. Luo, M. Ljungman, Y. Liu, Z. Xia, A. Schwendeman, T. Qin, M. A. Sartor, J. F. Costello, D. P. Cahill, P. R. Lowenstein, and M. G. Castro. "Zinc Finger Mynd-Type Containing 8 (Zmynd8) Is Epigenetically Regulated in Mutant Isocitrate Dehydrogenase 1 (Idh1) Glioma to Promote Radioresistance." Clin Cancer Res 29, no. 9 (2023): 1763-82.
- Jia, P.; Li, X.; Wang, X.; Yao, L.; Xu, Y.; Hu, Y.; Xu, W.; He, Z.; Zhao, Q.; Deng, Y.; et al. ZMYND8 mediated liquid condensates spatiotemporally decommission the latent super-enhancers during macrophage polarization. Nat. Commun. 2021, 12, 1–17. [Google Scholar] [CrossRef]
- Spruijt, C.G.; Luijsterburg, M.S.; Menafra, R.; Lindeboom, R.G.; Jansen, P.W.; Edupuganti, R.R.; Baltissen, M.P.; Wiegant, W.W.; Voelker-Albert, M.C.; Matarese, F.; et al. ZMYND8 Co-localizes with NuRD on Target Genes and Regulates Poly(ADP-Ribose)-Dependent Recruitment of GATAD2A/NuRD to Sites of DNA Damage. Cell Rep. 2016, 17, 783–798. [Google Scholar] [CrossRef]
- Majd, N.; Yap, T.A.; Yung, W.K.A.; de Groot, J. The Promise of Poly(ADP-Ribose) Polymerase (PARP) Inhibitors in Gliomas. J. Immunother. Precis. Oncol. 2020, 3, 157–164. [Google Scholar] [CrossRef]
- Garrett, M.; Sperry, J.; Braas, D.; Yan, W.; Le, T.M.; Mottahedeh, J.; Ludwig, K.; Eskin, A.; Qin, Y.; Levy, R.; et al. Metabolic characterization of isocitrate dehydrogenase (IDH) mutant and IDH wildtype gliomaspheres uncovers cell type-specific vulnerabilities. Cancer Metab. 2018, 6, 4. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Zhou, H.; Sun, W.; Hou, L.; Wang, Y.; Wang, H.; Lv, Z.; Xue, X. IDH1R132H mutation increases radiotherapy efficacy and a 4-gene radiotherapy-related signature of WHO grade 4 gliomas. Sci. Rep. 2023, 13, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Kessler, J.; Güttler, A.; Wichmann, H.; Rot, S.; Kappler, M.; Bache, M.; Vordermark, D. IDH1R132H mutation causes a less aggressive phenotype and radiosensitizes human malignant glioma cells independent of the oxygenation status. Radiother. Oncol. 2015, 116, 381–387. [Google Scholar] [CrossRef]
- Cohen, A.L.; Holmen, S.L.; Colman, H. IDH1 and IDH2 Mutations in Gliomas. Curr. Neurol. Neurosci. Rep. 2013, 13, 1–7. [Google Scholar] [CrossRef]
- Jiao, Y., P. J. Killela, Z. J. Reitman, A. B. Rasheed, C. M. Heaphy, R. F. de Wilde, F. J. Rodriguez, S. Rosemberg, S. M. Oba-Shinjo, S. K. Nagahashi Marie, C. Bettegowda, N. Agrawal, E. Lipp, C. Pirozzi, G. Lopez, Y. He, H. Friedman, A. H. Friedman, G. J. Riggins, M. Holdhoff, P. Burger, R. McLendon, D. D. Bigner, B. Vogelstein, A. K. Meeker, K. W. Kinzler, N. Papadopoulos, L. A. Diaz, and H. Yan. "Frequent Atrx, Cic, Fubp1 and Idh1 Mutations Refine the Classification of Malignant Gliomas." Oncotarget 3, no. 7 (2012): 709-22.
- Núñez, F.J.; Mendez, F.M.; Kadiyala, P.; Alghamri, M.S.; Savelieff, M.G.; Garcia-Fabiani, M.B.; Haase, S.; Koschmann, C.; Calinescu, A.-A.; Kamran, N.; et al. IDH1-R132H acts as a tumor suppressor in glioma via epigenetic up-regulation of the DNA damage response. Sci. Transl. Med. 2019, 11. [Google Scholar] [CrossRef]
- Cai, M.; Zhao, J.; Ding, Q.; Wei, J. Oncometabolite 2-hydroxyglutarate regulates anti-tumor immunity. Heliyon 2024, 10, e24454. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Bansal, A.; Young, E.; Batchala, P.; Patrie, J.; Lopes, M.; Jain, R.; Fadul, C.; Schiff, D. Extent of Surgical Resection in Lower-Grade Gliomas: Differential Impact Based on Molecular Subtype. Am. J. Neuroradiol. 2019, 40, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Gill, B. J., D. J. Pisapia, H. R. Malone, H. Goldstein, L. Lei, A. Sonabend, J. Yun, J. Samanamud, J. S. Sims, M. Banu, A. Dovas, A. F. Teich, S. A. Sheth, G. M. McKhann, M. B. Sisti, J. N. Bruce, P. A. Sims, and P. Canoll. "Mri-Localized Biopsies Reveal Subtype-Specific Differences in Molecular and Cellular Composition at the Margins of Glioblastoma." Proc Natl Acad Sci U S A 111, no. 34 (2014): 12550-5.
- Sottoriva, A.; Spiteri, I.; Piccirillo, S.G.M.; Touloumis, A.; Collins, V.P.; Marioni, J.C.; Curtis, C.; Watts, C.; Tavaré, S. Intratumor heterogeneity in human glioblastoma reflects cancer evolutionary dynamics. Proc. Natl. Acad. Sci. USA 2013, 110, 4009–4014. [Google Scholar] [CrossRef]
- Blanco-Carmona, E.; Narayanan, A.; Hernandez, I.; Nieto, J.C.; Elosua-Bayes, M.; Sun, X.; Schmidt, C.; Pamir, N.; Özduman, K.; Herold-Mende, C.; et al. Tumor heterogeneity and tumor-microglia interactions in primary and recurrent IDH1-mutant gliomas. Cell Rep. Med. 2023, 4, 101249. [Google Scholar] [CrossRef]
- Pirozzi, C.J.; Yan, H. The implications of IDH mutations for cancer development and therapy. Nat. Rev. Clin. Oncol. 2021, 18, 645–661. [Google Scholar] [CrossRef]
- Śledzińska, P.; Bebyn, M.; Szczerba, E.; Furtak, J.; Harat, M.; Olszewska, N.; Kamińska, K.; Kowalewski, J.; Lewandowska, M.A. Glioma 2021 WHO Classification: The Superiority of NGS Over IHC in Routine Diagnostics. Mol. Diagn. Ther. 2022, 26, 699–713. [Google Scholar] [CrossRef] [PubMed]
- Vij, M.; Yokoda, R.T.; Rashidipour, O.; Tran, I.; Vasudevaraja, V.; Snuderl, M.; Yong, R.L.; Cobb, W.S.; Umphlett, M.; Walker, J.M.; et al. The prognostic impact of subclonal IDH1 mutation in grade 2–4 astrocytomas. Neuro-Oncology Adv. 2023, 5, vdad069. [Google Scholar] [CrossRef]
- Singh, Rajesh R. "Next-Generation Sequencing in High-Sensitive Detection of Mutations in Tumors: Challenges, Advances, and Applications." The Journal of Molecular Diagnostics 22, no. 8 (2020): 994-1007.
- Pei, X.M.; Yeung, M.H.Y.; Wong, A.N.N.; Tsang, H.F.; Yu, A.C.S.; Yim, A.K.Y.; Wong, S.C.C. Targeted Sequencing Approach and Its Clinical Applications for the Molecular Diagnosis of Human Diseases. Cells 2023, 12, 493. [Google Scholar] [CrossRef]
- Qin, D. "Next-Generation Sequencing and Its Clinical Application." Cancer Biol Med 16, no. 1 (2019): 4-10.
- Nikiforova, Marina N., Abigail I. Wald, Melissa A. Melan, Somak Roy, Shan Zhong, Ronald L. Hamilton, Frank S. Lieberman, Jan Drappatz, Nduka M. Amankulor, Ian F. Pollack, Yuri E. Nikiforov, and Craig Horbinski. "Targeted Next-Generation Sequencing Panel (Glioseq) Provides Comprehensive Genetic Profiling of Central Nervous System Tumors." Neuro-Oncology 18, no. 3 (2015): 379-87.
- Zacher, A.; Kaulich, K.; Stepanow, S.; Wolter, M.; Köhrer, K.; Felsberg, J.; Malzkorn, B.; Reifenberger, G. Molecular Diagnostics of Gliomas Using Next Generation Sequencing of a Glioma-Tailored Gene Panel. Brain Pathol. 2016, 27, 146–159. [Google Scholar] [CrossRef]
- Higa, N.; Akahane, T.; Yokoyama, S.; Yonezawa, H.; Uchida, H.; Takajo, T.; Kirishima, M.; Hamada, T.; Matsuo, K.; Fujio, S.; et al. A tailored next-generation sequencing panel identified distinct subtypes of wildtype IDH and TERT promoter glioblastomas. Cancer Sci. 2020, 111, 3902–3911. [Google Scholar] [CrossRef]
- Guarnaccia, M.; Guarnaccia, L.; La Cognata, V.; Navone, S.E.; Campanella, R.; Ampollini, A.; Locatelli, M.; Miozzo, M.; Marfia, G.; Cavallaro, S. A Targeted Next-Generation Sequencing Panel to Genotype Gliomas. Life 2022, 12, 956. [Google Scholar] [CrossRef] [PubMed]
- Tirrò, E.; Massimino, M.; Broggi, G.; Romano, C.; Minasi, S.; Gianno, F.; Antonelli, M.; Motta, G.; Certo, F.; Altieri, R.; et al. A Custom DNA-Based NGS Panel for the Molecular Characterization of Patients With Diffuse Gliomas: Diagnostic and Therapeutic Applications. Front. Oncol. 2022, 12, 861078. [Google Scholar] [CrossRef] [PubMed]
- de Biase, D.; Acquaviva, G.; Visani, M.; Marucci, G.; De Leo, A.; Maloberti, T.; Sanza, V.; Di Oto, E.; Franceschi, E.; Mura, A.; et al. Next-Generation Sequencing Panel for 1p/19q Codeletion and IDH1-IDH2 Mutational Analysis Uncovers Mistaken Overdiagnoses of 1p/19q Codeletion by FISH. J. Mol. Diagn. 2021, 23, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, J.; Rothhammer-Hampl, T.; Zoubaa, S.; Bumes, E.; Pukrop, T.; Kölbl, O.; Corbacioglu, S.; Schmidt, N.O.; Proescholdt, M.; Hau, P.; et al. A comprehensive DNA panel next generation sequencing approach supporting diagnostics and therapy prediction in neurooncology. Acta Neuropathol. Commun. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Yaltirik, Cumhur Kaan, Seda Gulec Yilmaz, Selcuk Ozdogan, Ezel Yaltirik Bilgin, Zerrin Barut, Ugur Ture, and Turgay Isbir. "Determination of ≪Em≫Idh1, Idh2, Mgmt, Tert≪/Em≫ and ≪Em≫Atrx≪/Em≫ Gene Mutations in Glial Tumors." In Vivo 36, no. 4 (2022): 1694.
- Wongsurawat, T.; Jenjaroenpun, P.; Anekwiang, P.; Arigul, T.; Thongrattana, W.; Jamshidi-Parsian, A.; Boysen, G.; Suriyaphol, P.; Suktitipat, B.; Srirabheebhat, P.; et al. Exploiting nanopore sequencing for characterization and grading of IDH-mutant gliomas. Brain Pathol. 2023, 34, e13203. [Google Scholar] [CrossRef]
- Mimosa, Mashiat L., Wafa Al-ameri, Jared T. Simpson, Michael Nakhla, Karel Boissinot, David G. Munoz, Sunit Das, Harriet Feilotter, Ramzi Fattouh, and Rola M. Saleeb. "A Novel Approach to Detect Idh Point Mutations in Gliomas Using Nanopore Sequencing: Test Validation for the Clinical Laboratory." The Journal of Molecular Diagnostics 25, no. 3 (2023): 133-42.
- Patel, Areeba, Helin Dogan, Alexander Payne, Elena Krause, Philipp Sievers, Natalie Schoebe, Daniel Schrimpf, Christina Blume, Damian Stichel, Nadine Holmes, Philipp Euskirchen, Jürgen Hench, Stephan Frank, Violaine Rosenstiel-Goidts, Miriam Ratliff, Nima Etminan, Andreas Unterberg, Christoph Dieterich, Christel Herold-Mende, Stefan M. Pfister, Wolfgang Wick, Matthew Loose, Andreas von Deimling, Martin Sill, David T. W. Jones, Matthias Schlesner, and Felix Sahm. "Rapid-Cns2: Rapid Comprehensive Adaptive Nanopore-Sequencing of Cns Tumors, a Proof-of-Concept Study." Acta Neuropathologica 143, no. 5 (2022): 609-12.
- Shirai, Y.; Ueno, T.; Kojima, S.; Ikeuchi, H.; Kitada, R.; Koyama, T.; Takahashi, F.; Takahashi, K.; Ichimura, K.; Yoshida, A.; et al. The development of a custom RNA-sequencing panel for the identification of predictive and diagnostic biomarkers in glioma. J. Neuro-Oncology 2024, 167, 75–88. [Google Scholar] [CrossRef]
- Byron, S.A.; Van Keuren-Jensen, K.R.; Engelthaler, D.M.; Carpten, J.D.; Craig, D.W. Translating RNA sequencing into clinical diagnostics: opportunities and challenges. Nat. Rev. Genet. 2016, 17, 257–271. [Google Scholar] [CrossRef]
- Couturier, Charles P., Shamini Ayyadhury, Phuong U. Le, Javad Nadaf, Jean Monlong, Gabriele Riva, Redouane Allache, Salma Baig, Xiaohua Yan, Mathieu Bourgey, Changseok Lee, Yu Chang David Wang, V. Wee Yong, Marie-Christine Guiot, Hamed Najafabadi, Bratislav Misic, Jack Antel, Guillaume Bourque, Jiannis Ragoussis, and Kevin Petrecca. "Single-Cell Rna-Seq Reveals That Glioblastoma Recapitulates a Normal Neurodevelopmental Hierarchy." Nature Communications 11, no. 1 (2020): 3406.
- Piwecka, M.; Rajewsky, N.; Rybak-Wolf, A. Single-cell and spatial transcriptomics: deciphering brain complexity in health and disease. Nat. Rev. Neurol. 2023, 19, 346–362. [Google Scholar] [CrossRef] [PubMed]
- Venteicher, A.S.; Tirosh, I.; Hebert, C.; Yizhak, K.; Neftel, C.; Filbin, M.G.; Hovestadt, V.; Escalante, L.E.; Shaw, M.L.; Rodman, C.; et al. Decoupling genetics, lineages, and microenvironment in IDH-mutant gliomas by single-cell RNA-seq. Science 2017, 355, eaai8478. [Google Scholar] [CrossRef]
- Martínez-Ricarte, F.; Mayor, R.; Martínez-Sáez, E.; Rubio-Pérez, C.; Pineda, E.; Cordero, E.; Cicuéndez, M.; Poca, M.A.; López-Bigas, N.; Cajal, S.R.Y.; et al. Molecular Diagnosis of Diffuse Gliomas through Sequencing of Cell-Free Circulating Tumor DNA from Cerebrospinal Fluid. Clin. Cancer Res. 2018, 24, 2812–2819. [Google Scholar] [CrossRef]
- Mair, R.; Mouliere, F. Cell-free DNA technologies for the analysis of brain cancer. Br. J. Cancer 2021, 126, 371–378. [Google Scholar] [CrossRef] [PubMed]
- De Mattos-Arruda, L.; Mayor, R.; Ng, C.K.Y.; Weigelt, B.; Martínez-Ricarte, F.; Torrejon, D.; Oliveira, M.; Arias, A.; Raventos, C.; Tang, J.; et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat. Commun. 2015, 6, 8839–8839. [Google Scholar] [CrossRef] [PubMed]
- Wongsurawat, Thidathip, Piroon Jenjaroenpun, Annick De Loose, Duah Alkam, David W. Ussery, Intawat Nookaew, Yuet-Kin Leung, Shuk-Mei Ho, John D. Day, and Analiz Rodriguez. "A Novel Cas9-Targeted Long-Read Assay for Simultaneous Detection of Idh1/2 Mutations and Clinically Relevant Mgmt Methylation in Fresh Biopsies of Diffuse Glioma." Acta Neuropathologica Communications 8, no. 1 (2020): 87.
- Feng, Z.; Kong, D.; Jin, W.; He, K.; Zhao, J.; Liu, B.; Xu, H.; Yu, X.; Feng, S. Rapid detection of isocitrate dehydrogenase 1 mutation status in glioma based on Crispr-Cas12a. Sci. Rep. 2023, 13, 1–12. [Google Scholar] [CrossRef]
- Yu, D.; Zhong, Q.; Xiao, Y.; Feng, Z.; Tang, F.; Feng, S.; Cai, Y.; Gao, Y.; Lan, T.; Li, M.; et al. Combination of MRI-based prediction and CRISPR/Cas12a-based detection for IDH genotyping in glioma. npj Precis. Oncol. 2024, 8, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, M.; Liu, Y.; Ma, P.; Dang, L.; Meng, Q.; Wan, W.; Ma, X.; Liu, J.; Yang, G.; et al. Rapid and sensitive detection of COVID-19 using CRISPR/Cas12a-based detection with naked eye readout, CRISPR/Cas12a-NER. Sci. Bull. 2020, 65, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Tsou, J.-H.; Leng, Q.; Jiang, F. A CRISPR Test for Rapidly and Sensitively Detecting Circulating EGFR Mutations. Diagnostics 2020, 10, 114. [Google Scholar] [CrossRef]
- Murugan, K., A. S. Seetharam, A. J. Severin, and D. G. Sashital. "Crispr-Cas12a Has Widespread Off-Target and Dsdna-Nicking Effects." J Biol Chem 295, no. 17 (2020): 5538-53.
- Zhou, J.; Chen, P.; Wang, H.; Liu, H.; Li, Y.; Zhang, Y.; Wu, Y.; Paek, C.; Sun, Z.; Lei, J.; et al. Cas12a variants designed for lower genome-wide off-target effect through stringent PAM recognition. Mol. Ther. 2021, 30, 244–255. [Google Scholar] [CrossRef]
- Kloosterhof, N.K.; de Rooi, J.J.; Kros, M.; Eilers, P.H.C.; Smitt, P.A.E.S.; Bent, M.J.v.D.; French, P.J. Molecular subtypes of glioma identified by genome-wide methylation profiling. Genes, Chromosom. Cancer 2013, 52, 665–674. [Google Scholar] [CrossRef]
- Capper, D.; Stichel, D.; Sahm, F.; Jones, D.T.W.; Schrimpf, D.; Sill, M.; Schmid, S.; Hovestadt, V.; Reuss, D.E.; Koelsche, C.; et al. Practical implementation of DNA methylation and copy-number-based CNS tumor diagnostics: the Heidelberg experience. Acta Neuropathol. 2018, 136, 181–210. [Google Scholar] [CrossRef]
- Bai, H.; Harmancı, A.S.; Erson-Omay, E.Z.; Li, J.; Coşkun, S.; Simon, M.; Krischek, B.; Özduman, K.; Omay, S.B.; Sorensen, E.A.; et al. Integrated genomic characterization of IDH1-mutant glioma malignant progression. Nat. Genet. 2016, 48, 59–66. [Google Scholar] [CrossRef]
- Schenkel, Laila C., Joseph Mathew, Hal Hirte, John Provias, Guillaume Paré, Michael Chong, Daria Grafodatskaya, and Elizabeth McCready. "Evaluation of DNA Methylation Array for Glioma Tumor Profiling and Description of a Novel Epi-Signature to Distinguish Idh1/Idh2 Mutant and Wild-Type Tumors." Genes 13, no. 11 (2022): 2075.
- Euskirchen, P.; Bielle, F.; Labreche, K.; Kloosterman, W.P.; Rosenberg, S.; Daniau, M.; Schmitt, C.; Masliah-Planchon, J.; Bourdeaut, F.; Dehais, C.; et al. Same-day genomic and epigenomic diagnosis of brain tumors using real-time nanopore sequencing. Acta Neuropathol. 2017, 134, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Willscher, E.; Hopp, L.; Kreuz, M.; Schmidt, M.; Hakobyan, S.; Arakelyan, A.; Hentschel, B.; Jones, D.T.W.; Pfister, S.M.; Loeffler, M.; et al. High-Resolution Cartography of the Transcriptome and Methylome Landscapes of Diffuse Gliomas. Cancers 2021, 13, 3198. [Google Scholar] [CrossRef] [PubMed]
- Wenger, A.; Carén, H. Methylation Profiling in Diffuse Gliomas: Diagnostic Value and Considerations. Cancers 2022, 14, 5679. [Google Scholar] [CrossRef]
- Djirackor, L.; Halldorsson, S.; Niehusmann, P.; Leske, H.; Capper, D.; Kuschel, L.P.; Pahnke, J.; Due-Tønnessen, B.J.; A Langmoen, I.; Sandberg, C.J.; et al. Intraoperative DNA methylation classification of brain tumors impacts neurosurgical strategy. Neuro-Oncology Adv. 2021, 3, vdab149. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, S.; Xu, J.; Liu, H.; Wan, S. Cancer Biomarkers Discovery of Methylation Modification With Direct High-Throughput Nanopore Sequencing. Front. Genet. 2021, 12. [Google Scholar] [CrossRef]
- Beiko, J.; Suki, D.; Hess, K.R.; Fox, B.D.; Cheung, V.; Cabral, M.; Shonka, N.; Gilbert, M.R.; Sawaya, R.; Prabhu, S.S.; et al. IDH1 mutant malignant astrocytomas are more amenable to surgical resection and have a survival benefit associated with maximal surgical resection. Neuro-Oncology 2013, 16, 81–91. [Google Scholar] [CrossRef]
- Cahill, D. P. "Extent of Resection of Glioblastoma: A Critical Evaluation in the Molecular Era." Neurosurg Clin N Am 32, no. 1 (2021): 23-29.
- Jakola, A.S.; Pedersen, L.K.; Skjulsvik, A.J.; Myrmel, K.; Sjåvik, K.; Solheim, O. The impact of resection in IDH-mutant WHO grade 2 gliomas: a retrospective population-based parallel cohort study. J. Neurosurg. 2022, 137, 1321–1328. [Google Scholar] [CrossRef] [PubMed]
- Wolter, Marietta, Jörg Felsberg, Bastian Malzkorn, Kerstin Kaulich, and Guido Reifenberger. "Droplet Digital Pcr-Based Analyses for Robust, Rapid, and Sensitive Molecular Diagnostics of Gliomas." Acta Neuropathologica Communications 10, no. 1 (2022): 42.
- Wang, J.; Zhao, Y.-Y.; Li, J.-F.; Guo, C.-C.; Chen, F.-R.; Su, H.-K.; Zhao, H.-F.; Long, Y.-K.; Shao, J.-Y.; To, S.-S.T.; et al. IDH1 mutation detection by droplet digital PCR in glioma. Oncotarget 2015, 6, 39651–39660. [Google Scholar] [CrossRef]
- Perizzolo, M., B. Winkfein, S. Hui, W. Krulicki, J. A. Chan, and D. J. Demetrick. "Idh Mutation Detection in Formalin-Fixed Paraffin-Embedded Gliomas Using Multiplex Pcr and Single-Base Extension." Brain Pathol 22, no. 5 (2012): 619-24.
- Chen, W.W.; Balaj, L.; Liau, L.M.; Samuels, M.L.; Kotsopoulos, S.K.; A Maguire, C.; LoGuidice, L.; Soto, H.; Garrett, M.; Zhu, L.D.; et al. BEAMing and Droplet Digital PCR Analysis of Mutant IDH1 mRNA in Glioma Patient Serum and Cerebrospinal Fluid Extracellular Vesicles. Mol. Ther. - Nucleic Acids 2013, 2, e109. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, N.; Lv, J.; Zhu, P.; Pan, X.; Hu, J.; Wu, W.; Li, S.; Li, H. Comparing the performance of conventional PCR, RTQ-PCR, and droplet digital PCR assays in detection of Shigella. Mol. Cell. Probes 2020, 51, 101531. [Google Scholar] [CrossRef]
- Orzan, Francesca, Francesca De Bacco, Elisabetta Lazzarini, Giovanni Crisafulli, Alessandra Gasparini, Angelo Dipasquale, Ludovic Barault, Marco Macagno, Pasquale Persico, Federico Pessina, Beatrice Bono, Laura Giordano, Pietro Zeppa, Antonio Melcarne, Paola Cassoni, Diego Garbossa, Armando Santoro, Paolo M. Comoglio, Stefano Indraccolo, Matteo Simonelli, and Carla Boccaccio. "Liquid Biopsy of Cerebrospinal Fluid Enables Selective Profiling of Glioma Molecular Subtypes at First Clinical Presentation." Clinical Cancer Research 29, no. 7 (2023): 1252-66.
- Crucitta, S.; Pasqualetti, F.; Gonnelli, A.; Ruglioni, M.; Luculli, G.I.; Cantarella, M.; Ortenzi, V.; Scatena, C.; Paiar, F.; Naccarato, A.G.; et al. IDH1 mutation is detectable in plasma cell-free DNA and is associated with survival outcome in glioma patients. BMC Cancer 2024, 24, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jones, Jordan J, Hong Nguyen, Stephen Q Wong, James Whittle, Josie Iaria, Stanley Stylli, James Towner, Thomas Pieters, Frank Gaillard, Andrew H Kaye, Katharine J Drummond, and Andrew P Morokoff. "Plasma Ctdna Liquid Biopsy of Idh1, Tertp, and Egfrviii Mutations in Glioma." Neuro-Oncology Advances 6, no. 1 (2024).
- Husain, A.; Mishra, S.; Siddiqui, M.H.; Husain, N. Detection of IDH1 Mutation in cfDNA and Tissue of Adult Diffuse Glioma with Allele-Specific qPCR. Asian Pac. J. Cancer Prev. 2023, 24, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Solomon, J.P.; Munoz-Zuluaga, C.; Slocum, C.; Dillard, A.; Cong, L.; Wang, J.; Lindeman, N.; Kluk, M.; Liechty, B.; Pisapia, D.; et al. Evaluation of the rapid Idylla IDH1-2 mutation assay in FFPE glioma samples. Diagn. Pathol. 2024, 19, 1–9. [Google Scholar] [CrossRef]
- Choate, K.A.; Raack, E.J.; Line, V.F.; Jennings, M.J.; Belton, R.J.; Winn, R.J.; Mann, P.B. Rapid extraction-free detection of the R132H isocitrate dehydrogenase mutation in glioma using colorimetric peptide nucleic acid-loop mediated isothermal amplification (CPNA-LAMP). PLOS ONE 2023, 18, e0291666. [Google Scholar] [CrossRef]
- Choate, Kristian A, Edward J Raack, Paul B Mann, Evan A Jones, Robert J Winn, and Matthew J Jennings. "Rapid Idh1-R132 Genotyping Panel Utilizing Locked Nucleic Acid Loop-Mediated Isothermal Amplification (Lna-Lamp)." Biology Methods and Protocols (2024).
- Yoshida, A.; Satomi, K.; Ohno, M.; Matsushita, Y.; Takahashi, M.; Miyakita, Y.; Hiraoka, N.; Narita, Y.; Ichimura, K. Frequent false-negative immunohistochemical staining with IDH1 (R132H)-specific H09 antibody on frozen section control slides: a potential pitfall in glioma diagnosis. Histopathology 2018, 74, 350–354. [Google Scholar] [CrossRef] [PubMed]
- Capper, D.; Zentgraf, H.; Balss, J.; Hartmann, C.; von Deimling, A. Monoclonal antibody specific for IDH1 R132H mutation. Acta Neuropathol. 2009, 118, 599–601. [Google Scholar] [CrossRef]
- Kato, Y., G. Jin, C. T. Kuan, R. E. McLendon, H. Yan, and D. D. Bigner. "A Monoclonal Antibody Imab-1 Specifically Recognizes Idh1r132h, the Most Common Glioma-Derived Mutation." Biochem Biophys Res Commun 390, no. 3 (2009): 547-51.
- Kato, Y. "Specific Monoclonal Antibodies against Idh1/2 Mutations as Diagnostic Tools for Gliomas." Brain Tumor Pathol 32, no. 1 (2015): 3-11.
- Copaciu, R., J. Rashidian, J. Lloyd, A. Yahyabeik, J. McClure, K. Cummings, and Q. Su. "Characterization of an Idh1 R132h Rabbit Monoclonal Antibody, Mrq-67, and Its Applications in the Identification of Diffuse Gliomas." Antibodies (Basel) 12, no. 1 (2023).
- Rashidian, J.; Copaciu, R.; Su, Q.; Merritt, B.; Johnson, C.; Yahyabeik, A.; French, E.; Cummings, K. Generation and Performance of R132H Mutant IDH1 Rabbit Monoclonal Antibody. Antibodies 2017, 6, 22. [Google Scholar] [CrossRef]
- Lu, V.M.; McDonald, K.L. Isocitrate dehydrogenase 1 mutation subtypes at site 132 and their translational potential in glioma. CNS Oncol. 2018, 7, 41–50. [Google Scholar] [CrossRef]
- Agarwal, S.; Sharma, M.C.; Jha, P.; Pathak, P.; Suri, V.; Sarkar, C.; Chosdol, K.; Suri, A.; Kale, S.S.; Mahapatra, A.K. Comparative study of IDH1 mutations in gliomas by immunohistochemistry and DNA sequencing. Neuro-Oncology 2013, 15, 718–726. [Google Scholar] [CrossRef]
- Zhang, S.; William, C. Educational Case: Histologic and Molecular Features of Diffuse Gliomas. Acad. Pathol. 2020, 7. [Google Scholar] [CrossRef]
- Nakagaki, R.; Debsarkar, S.S.; Kawanaka, H.; Aronow, B.J.; Prasath, V.S. Deep learning-based IDH1 gene mutation prediction using histopathological imaging and clinical data. Comput. Biol. Med. 2024, 179, 108902. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Shah, Z.; Sav, A.; Russo, C.; Berkovsky, S.; Qian, Y.; Coiera, E.; Di Ieva, A. Isocitrate dehydrogenase (IDH) status prediction in histopathology images of gliomas using deep learning. Sci. Rep. 2020, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Liechty, B.; Xu, Z.; Zhang, Z.; Slocum, C.; Bahadir, C.D.; Sabuncu, M.R.; Pisapia, D.J. Machine learning can aid in prediction of IDH mutation from H&E-stained histology slides in infiltrating gliomas. Sci. Rep. 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Chunduru, P.; Phillips, J.J.; Molinaro, A.M. Prognostic risk stratification of gliomas using deep learning in digital pathology images. Neuro-Oncology Adv. 2022, 4, vdac111. [Google Scholar] [CrossRef]
- Cui, D.; Liu, Y.; Liu, G.; Liu, L. A Multiple-Instance Learning-Based Convolutional Neural Network Model to Detect the IDH1 Mutation in the Histopathology Images of Glioma Tissues. J. Comput. Biol. 2020, 27, 1264–1272. [Google Scholar] [CrossRef]
- Arita, H., Y. Narita, A. Yoshida, N. Hashimoto, T. Yoshimine, and K. Ichimura. "Idh1/2 Mutation Detection in Gliomas." Brain Tumor Pathol 32, no. 2 (2015): 79-89.
- Yang, J.; Zhu, H.; Zhang, T.; Ding, J. Structure, substrate specificity, and catalytic mechanism of human D-2-HGDH and insights into pathogenicity of disease-associated mutations. Cell Discov. 2021, 7, 1–17. [Google Scholar] [CrossRef]
- de Goede, K.E.; Harber, K.J.; Gorki, F.S.; Verberk, S.G.; Groh, L.A.; Keuning, E.D.; Struys, E.A.; van Weeghel, M.; Haschemi, A.; de Winther, M.P.; et al. d-2-Hydroxyglutarate is an anti-inflammatory immunometabolite that accumulates in macrophages after TLR4 activation. Biochim. et Biophys. Acta (BBA) - Mol. Basis Dis. 2022, 1868, 166427. [Google Scholar] [CrossRef]
- Achouri, Y.; Noël, G.; Vertommen, D.; Rider, M.H.; Veiga-Da-Cunha, M.; van Schaftingen, E. Identification of a dehydrogenase acting on D-2-hydroxyglutarate. Biochem. J. 2004, 381, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Struys, E.A. D-2-Hydroxyglutaric aciduria: Unravelling the biochemical pathway and the genetic defect. J. Inherit. Metab. Dis. 2006, 29, 21–29. [Google Scholar] [CrossRef]
- Tuna, G., N. E. Dal-Bekar, A. Akay, M. Rükşen, S. İşlekel, and G. H. İşlekel. "Minimally Invasive Detection of Idh1 Mutation with Cell-Free Circulating Tumor DNA and D-2-Hydroxyglutarate, D/L-2-Hydroxyglutarate Ratio in Gliomas." J Neuropathol Exp Neurol 81, no. 7 (2022): 502-10.
- Kalinina, J., J. Ahn, N. S. Devi, L. Wang, Y. Li, J. J. Olson, M. Glantz, T. Smith, E. L. Kim, A. Giese, R. L. Jensen, C. C. Chen, B. S. Carter, H. Mao, M. He, and E. G. Van Meir. "Selective Detection of the D-Enantiomer of 2-Hydroxyglutarate in the Csf of Glioma Patients with Mutated Isocitrate Dehydrogenase." Clin Cancer Res 22, no. 24 (2016): 6256-65.
- Fujita, Y.; Nunez-Rubiano, L.; Dono, A.; Bellman, A.; Shah, M.; Rodriguez, J.C.; Putluri, V.; Kamal, A.H.M.; Putluri, N.; Riascos, R.F.; et al. IDH1 p.R132H ctDNA and D-2-hydroxyglutarate as CSF biomarkers in patients with IDH-mutant gliomas. J. Neuro-Oncology 2022, 159, 261–270. [Google Scholar] [CrossRef]
- Lee, C. L., G. M. O'Kane, W. P. Mason, W. J. Zhang, P. Spiliopoulou, A. R. Hansen, R. C. Grant, J. J. Knox, T. L. Stockley, G. Zadeh, and E. X. Chen. "Circulating Oncometabolite 2-Hydroxyglutarate as a Potential Biomarker for Isocitrate Dehydrogenase (Idh1/2) Mutant Cholangiocarcinoma." Mol Cancer Ther 23, no. 3 (2024): 394-99.
- Delahousse, J.; Verlingue, L.; Broutin, S.; Legoupil, C.; Touat, M.; Doucet, L.; Ammari, S.; Lacroix, L.; Ducreux, M.; Scoazec, J.-Y.; et al. Circulating oncometabolite D-2-hydroxyglutarate enantiomer is a surrogate marker of isocitrate dehydrogenase-mutated intrahepatic cholangiocarcinomas. Eur. J. Cancer 2018, 90, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Strain, Shinji K., Morris D. Groves, and Mark R. Emmett. "Enantioseparation and Detection of (R)-2-Hydroxyglutarate and (S)-2-Hydroxyglutarate by Chiral Gas Chromatography–Triple Quadrupole Mass Spectrometry." In Metabolomics, edited by Paul L. Wood, 89-100. New York, NY: Springer US, 2021.
- Voelxen, N.F.; Walenta, S.; Proescholdt, M.; Dettmer, K.; Pusch, S.; Mueller-Klieser, W. Quantitative Imaging of D-2-Hydroxyglutarate in Selected Histological Tissue Areas by a Novel Bioluminescence Technique. Front. Oncol. 2016, 6, 46–46. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Galán, E.; Massana, N.; Parra-Robert, M.; Hidalgo, S.; Casals, G.; Esteve, J.; Jiménez, W. Validation of a routine gas chromatography mass spectrometry method for 2-hydroxyglutarate quantification in human serum as a screening tool for detection of idh mutations. J. Chromatogr. B 2018, 1083, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.; Ganji, S.K.; DeBerardinis, R.J.; Hatanpaa, K.J.; Rakheja, D.; Kovacs, Z.; Yang, X.-L.; Mashimo, T.; Raisanen, J.M.; Marin-Valencia, I.; et al. 2-hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 2012, 18, 624–629. [Google Scholar] [CrossRef]
- Balss, J.; Pusch, S.; Beck, A.-C.; Herold-Mende, C.; Krämer, A.; Thiede, C.; Buckel, W.; Langhans, C.-D.; Okun, J.G.; von Deimling, A. Enzymatic assay for quantitative analysis of (d)-2-hydroxyglutarate. Acta Neuropathol. 2012, 124, 883–891. [Google Scholar] [CrossRef]
- Xiao, D.; Xu, X.; Gao, K.; Wang, M.; Zhang, W.; Lü, C.; Wang, X.; Wang, Q.; Xu, P.; Ma, C.; et al. A Förster resonance energy transfer-based d-2-hydroxyglutarate biosensor. Sensors Actuators B: Chem. 2023, 385. [Google Scholar] [CrossRef]
- Choate, Kristian Alissa, Wren W. L. Konickson, Matthew James Jennings, Paul Barrie Mann, Robert James Winn, David O. Kamson, and Evan P. S. Pratt. "A Genetically Encoded Fluorescent Sensor Enables Sensitive and Specific Detection of Idh Mutant Associated Oncometabolite D-2-Hydroxyglutarate." bioRxiv (2024): 2024.09.25.615072.
- Fan, B.; Dai, D.; DiNardo, C.D.; Stein, E.; de Botton, S.; Attar, E.C.; Liu, H.; Liu, G.; Lemieux, I.; Agresta, S.V.; et al. Clinical pharmacokinetics and pharmacodynamics of ivosidenib in patients with advanced hematologic malignancies with an IDH1 mutation. Cancer Chemother. Pharmacol. 2020, 85, 959–968. [Google Scholar] [CrossRef] [PubMed]
- Fan, B., I. K. Mellinghoff, P. Y. Wen, M. A. Lowery, L. Goyal, W. D. Tap, S. S. Pandya, E. Manyak, L. Jiang, G. Liu, T. Nimkar, C. Gliser, M. Prahl Judge, S. Agresta, H. Yang, and D. Dai. "Clinical Pharmacokinetics and Pharmacodynamics of Ivosidenib, an Oral, Targeted Inhibitor of Mutant Idh1, in Patients with Advanced Solid Tumors." Invest New Drugs 38, no. 2 (2020): 433-44.
- Pusch, S.; Schweizer, L.; Beck, A.-C.; Lehmler, J.-M.; Weissert, S.; Balss, J.; Miller, A.K.; von Deimling, A. D-2-Hydroxyglutarate producing neo-enzymatic activity inversely correlates with frequency of the type of isocitrate dehydrogenase 1 mutations found in glioma. Acta Neuropathol. Commun. 2014, 2, 19–19. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, K.; Li, S.; Wang, J.; Ma, J.; Jiang, T.; Dai, J. Patterns of Tumor Contrast Enhancement Predict the Prognosis of Anaplastic Gliomas withIDH1Mutation. Am. J. Neuroradiol. 2015, 36, 2023–2029. [Google Scholar] [CrossRef]
- Carrillo, J. A., A. Lai, P. L. Nghiemphu, H. J. Kim, H. S. Phillips, S. Kharbanda, P. Moftakhar, S. Lalaezari, W. Yong, B. M. Ellingson, T. F. Cloughesy, and W. B. Pope. "Relationship between Tumor Enhancement, Edema, Idh1 Mutational Status, Mgmt Promoter Methylation, and Survival in Glioblastoma." AJNR Am J Neuroradiol 33, no. 7 (2012): 1349-55.
- Xing, Z., X. Yang, D. She, Y. Lin, Y. Zhang, and D. Cao. "Noninvasive Assessment of Idh Mutational Status in World Health Organization Grade Ii and Iii Astrocytomas Using Dwi and Dsc-Pwi Combined with Conventional Mr Imaging." AJNR Am J Neuroradiol 38, no. 6 (2017): 1138-44.
- Li, Y.; Qin, Q.; Zhang, Y.; Cao, Y. Noninvasive Determination of the IDH Status of Gliomas Using MRI and MRI-Based Radiomics: Impact on Diagnosis and Prognosis. Curr. Oncol. 2022, 29, 6893–6907. [Google Scholar] [CrossRef]
- Kates, R.; Atkinson, D.; Brant-Zawadzki, M. Fluid-attenuated inversion recovery (FLAIR): clinical prospectus of current and future applications. . 1996, 8, 389–96. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. H., L. M. Poisson, D. J. Brat, Y. Zhou, L. Cooper, M. Snuderl, C. Thomas, A. M. Franceschi, B. Griffith, A. E. Flanders, J. G. Golfinos, A. S. Chi, and R. Jain. "T2-Flair Mismatch, an Imaging Biomarker for Idh and 1p/19q Status in Lower-Grade Gliomas: A Tcga/Tcia Project." Clin Cancer Res 23, no. 20 (2017): 6078-85.
- Pinto, C.; Noronha, C.; Taipa, R.; Ramos, C. T2-FLAIR mismatch sign: a roadmap of pearls and pitfalls. Br. J. Radiol. 2021, 95, 20210825. [Google Scholar] [CrossRef] [PubMed]
- Jain, R., D. R. Johnson, S. H. Patel, M. Castillo, M. Smits, M. J. van den Bent, A. S. Chi, and D. P. Cahill. ""Real World" Use of a Highly Reliable Imaging Sign: "T2-Flair Mismatch" for Identification of Idh Mutant Astrocytomas." Neuro Oncol 22, no. 7 (2020): 936-43.
- Shoaib, Y.; Nayil, K.; Makhdoomi, R.; Asma, A.; Ramzan, A.; Shaheen, F.; Wani, A. Role of diffusion and perfusion magnetic resonance imaging in predicting the histopathological grade of gliomas - A prospective study. Asian J. Neurosurg. 2019, 14, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Aydin, Sonay, Erdem Fatihoğlu, Pınar Nercis Koşar, and Elif Ergün. "Perfusion and Permeability Mri in Glioma Grading." Egyptian Journal of Radiology and Nuclear Medicine 51, no. 1 (2020): 2.
- Álvarez-Torres, M.d.M.; Fuster-García, E.; Juan-Albarracín, J.; Reynés, G.; Aparici-Robles, F.; Ferrer-Lozano, J.; García-Gómez, J.M. Local detection of microvessels in IDH-wildtype glioblastoma using relative cerebral blood volume: an imaging marker useful for astrocytoma grade 4 classification. BMC Cancer 2022, 22, 1–13. [Google Scholar] [CrossRef]
- Feraco, P.; Bacci, A.; Ferrazza, P.; Hauwe, L.v.D.; Pertile, R.; Girlando, S.; Barbareschi, M.; Gagliardo, C.; Morganti, A.G.; Petralia, B. Magnetic Resonance Imaging Derived Biomarkers of IDH Mutation Status and Overall Survival in Grade III Astrocytomas. Diagnostics 2020, 10, 247. [Google Scholar] [CrossRef]
- Tan, W.L.; Huang, W.Y.; Yin, B.; Xiong, J.; Wu, J.S.; Geng, D.Y. Can Diffusion Tensor Imaging Noninvasively Detect IDH1 Gene Mutations in Astrogliomas? A Retrospective Study of 112 Cases. Am. J. Neuroradiol. 2014, 35, 920–927. [Google Scholar] [CrossRef]
- Kurokawa, R.; Baba, A.; Kurokawa, M.; Capizzano, A.; Ota, Y.; Kim, J.; Srinivasan, A.; Moritani, T. Perfusion and diffusion-weighted imaging parameters: Comparison between pre- and postbiopsy MRI for high-grade glioma. Medicine 2022, 101, e30183. [Google Scholar] [CrossRef]
- Gihr, G.; Horvath-Rizea, D.; Kohlhof-Meinecke, P.; Ganslandt, O.; Henkes, H.; Härtig, W.; Donitza, A.; Skalej, M.; Schob, S. Diffusion Weighted Imaging in Gliomas: A Histogram-Based Approach for Tumor Characterization. Cancers 2022, 14, 3393. [Google Scholar] [CrossRef]
- Cho, N.S.; Hagiwara, A.; Eldred, B.S.C.; Raymond, C.; Wang, C.; Sanvito, F.; Lai, A.; Nghiemphu, P.; Salamon, N.; Steelman, L.; et al. Early volumetric, perfusion, and diffusion MRI changes after mutant isocitrate dehydrogenase (IDH) inhibitor treatment in IDH1-mutant gliomas. Neuro-Oncology Adv. 2022, 4, vdac124. [Google Scholar] [CrossRef]
- Stadlbauer, A.; Nikolic, K.; Oberndorfer, S.; Marhold, F.; Kinfe, T.M.; Meyer-Bäse, A.; Bistrian, D.A.; Schnell, O.; Doerfler, A. Machine Learning-Based Prediction of Glioma IDH Gene Mutation Status Using Physio-Metabolic MRI of Oxygen Metabolism and Neovascularization (A Bicenter Study). Cancers 2024, 16, 1102. [Google Scholar] [CrossRef]
- Yuan, J.; Siakallis, L.; Li, H.B.; Brandner, S.; Zhang, J.; Li, C.; Mancini, L.; Bisdas, S. Structural- and DTI- MRI enable automated prediction of IDH Mutation Status in CNS WHO Grade 2–4 glioma patients: a deep Radiomics Approach. BMC Med Imaging 2024, 24, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Xu, X.; Zhang, W.; Zhang, L.; Wen, M.; Gao, J.; Yang, J.; Kan, Y.; Yang, X.; Wen, Z.; et al. A fusion model integrating magnetic resonance imaging radiomics and deep learning features for predicting alpha-thalassemia X-linked intellectual disability mutation status in isocitrate dehydrogenase–mutant high-grade astrocytoma: a multicenter study. Quant. Imaging Med. Surg. 2024, 14, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.-T.; Su, C.-Q.; Lin, J.; Xia, Z.-W.; Lu, S.-S.; Hong, X.-N. T2-FLAIR mismatch sign and machine learning-based multiparametric MRI radiomics in predicting IDH mutant 1p/19q non-co-deleted diffuse lower-grade gliomas. Clin. Radiol. 2024, 79, e750–e758. [Google Scholar] [CrossRef] [PubMed]
- Santinha, J.; Katsaros, V.; Stranjalis, G.; Liouta, E.; Boskos, C.; Matos, C.; Viegas, C.; Papanikolaou, N. Development of End-to-End AI–Based MRI Image Analysis System for Predicting IDH Mutation Status of Patients with Gliomas: Multicentric Validation. J. Imaging Informatics Med. 2024, 37, 31–44. [Google Scholar] [CrossRef]
- Pasquini, L.; Napolitano, A.; Tagliente, E.; Dellepiane, F.; Lucignani, M.; Vidiri, A.; Ranazzi, G.; Stoppacciaro, A.; Moltoni, G.; Nicolai, M.; et al. Deep Learning Can Differentiate IDH-Mutant from IDH-Wild GBM. J. Pers. Med. 2021, 11, 290. [Google Scholar] [CrossRef]
- Carosi, F.; Broseghini, E.; Fabbri, L.; Corradi, G.; Gili, R.; Forte, V.; Roncarati, R.; Filippini, D.M.; Ferracin, M. Targeting Isocitrate Dehydrogenase (IDH) in Solid Tumors: Current Evidence and Future Perspectives. Cancers 2024, 16, 2752. [Google Scholar] [CrossRef]
- Iwahashi, H.; Nagashima, H.; Tanaka, K.; Uno, T.; Hashiguchi, M.; Maeyama, M.; Somiya, Y.; Komatsu, M.; Hirose, T.; Itoh, T.; et al. 2-Hydroxyglutarate magnetic resonance spectroscopy in adult brainstem glioma. J. Neurosurg. 2023, 139, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Suh, C.H.; Kim, H.S.; Jung, S.C.; Choi, C.G.; Kim, S.J. 2-Hydroxyglutarate MR spectroscopy for prediction of isocitrate dehydrogenase mutant glioma: a systemic review and meta-analysis using individual patient data. Neuro-Oncology 2018, 20, 1573–1583. [Google Scholar] [CrossRef]
- Bhandari, A.; Sharma, C.; Ibrahim, M.; Riggs, M.; Jones, R.; Lasocki, A. The role of 2-hydroxyglutarate magnetic resonance spectroscopy for the determination of isocitrate dehydrogenase status in lower grade gliomas versus glioblastoma: a systematic review and meta-analysis of diagnostic test accuracy. Neuroradiology 2021, 63, 1823–1830. [Google Scholar] [CrossRef]
- Branzoli, F.; Di Stefano, A.L.; Capelle, L.; Ottolenghi, C.; Valabrègue, R.; Deelchand, D.K.; Bielle, F.; Villa, C.; Baussart, B.; Lehéricy, S.; et al. Highly specific determination of IDH status using edited in vivo magnetic resonance spectroscopy. Neuro-Oncology 2017, 20, 907–916. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, Y.; Liao, H.; Rowland, B.C.; Kong, X.; Arvold, N.D.; A Reardon, D.; Wen, P.Y.; Lin, A.P.; Huang, R.Y. Diagnostic accuracy of 2-hydroxyglutarate magnetic resonance spectroscopy in newly diagnosed brain mass and suspected recurrent gliomas. Neuro-Oncology 2018, 20, 1262–1271. [Google Scholar] [CrossRef] [PubMed]
- Tietze, A.; Choi, C.; Mickey, B.; Maher, E.A.; Ulhøi, B.P.; Sangill, R.; Lassen-Ramshad, Y.; Lukacova, S.; Østergaard, L.; von Oettingen, G. Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting. J. Neurosurg. 2018, 128, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Andronesi, O.C.; Rapalino, O.; Gerstner, E.; Chi, A.; Batchelor, T.T.; Cahill, D.P.; Sorensen, A.G.; Rosen, B.R. Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate. J. Clin. Investig. 2013, 123, 3659–3663. [Google Scholar] [CrossRef]
- Andronesi, O.C.; Arrillaga-Romany, I.C.; Ly, K.I.; Bogner, W.; Ratai, E.M.; Reitz, K.; Iafrate, A.J.; Dietrich, J.; Gerstner, E.R.; Chi, A.S.; et al. Pharmacodynamics of mutant-IDH1 inhibitors in glioma patients probed by in vivo 3D MRS imaging of 2-hydroxyglutarate. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Lv, X.; Lu, C.; Ye, X.; Chen, X.; Fu, J.; Luo, C.; Zhao, Y. Prognostic factors of patients with Gliomas – an analysis on 335 patients with Glioblastoma and other forms of Gliomas. BMC Cancer 2020, 20, 1–7. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro-Oncology 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Patil, N.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2013–2017. Neuro-Oncology, v96. [CrossRef]
- Shirahata, M., T. Ono, D. Stichel, D. Schrimpf, D. E. Reuss, F. Sahm, C. Koelsche, A. Wefers, A. Reinhardt, K. Huang, P. Sievers, H. Shimizu, H. Nanjo, Y. Kobayashi, Y. Miyake, T. Suzuki, J. I. Adachi, K. Mishima, A. Sasaki,..., and A. von Deimling. "Novel, Improved Grading System(S) for Idh-Mutant Astrocytic Gliomas." Acta Neuropathol 136, no. 1 (2018): 153-66.
- Parsons, D. W., S. Jones, X. Zhang, J. C. Lin, R. J. Leary, P. Angenendt, P. Mankoo, H. Carter, I. M. Siu, G. L. Gallia, A. Olivi, R. McLendon, B. A. Rasheed, S. Keir, T. Nikolskaya, Y. Nikolsky, D. A. Busam, H. Tekleab, L. A. Diaz, Jr.,..., and K. W. Kinzler. "An Integrated Genomic Analysis of Human Glioblastoma Multiforme." Science 321, no. 5897 (2008): 1807-12.
- Yan, H., D. W. Parsons, G. Jin, R. McLendon, B. A. Rasheed, W. Yuan, I. Kos, I. Batinic-Haberle, S. Jones, G. J. Riggins, H. Friedman, A. Friedman, D. Reardon, J. Herndon, K. W. Kinzler, V. E. Velculescu, B. Vogelstein, and D. D. Bigner. "Idh1 and Idh2 Mutations in Gliomas." N Engl J Med 360, no. 8 (2009): 765-73.
- Lopez-Gines, C.; Cerda-Nicolas, M.; Gil-Benso, R.; Pellin, A.; A Lopez-Guerrero, J.; Callaghan, R.; Benito, R.; Roldan, P.; Piquer, J.; Llacer, J.; et al. Association of chromosome 7, chromosome 10 and EGFR gene amplification in glioblastoma multiforme. . 2005, 24, 209–18. [Google Scholar]
- Eckel-Passow, Jeanette E., Daniel H. Lachance, Annette M. Molinaro, Kyle M. Walsh, Paul A. Decker, Hugues Sicotte, Melike Pekmezci, Terri Rice, Matt L. Kosel, Ivan V. Smirnov, Gobinda Sarkar, Alissa A. Caron, Thomas M. Kollmeyer, Corinne E. Praska, Anisha R. Chada, Chandralekha Halder, Helen M. Hansen, Lucie S. McCoy, Paige M. Bracci,..., and Robert B. Jenkins. "Glioma Groups Based on 1p/19q, Idh, and Tert Promoter Mutations in Tumors." New England Journal of Medicine 372, no. 26 (2015): 2499-508.
- Killela, P. J., Z. J. Reitman, Y. Jiao, C. Bettegowda, N. Agrawal, L. A. Diaz, Jr., A. H. Friedman, H. Friedman, G. L. Gallia, B. C. Giovanella, A. P. Grollman, T. C. He, Y. He, R. H. Hruban, G. I. Jallo, N. Mandahl, A. K. Meeker, F. Mertens, G. J. Netto,..., and H. Yan. "Tert Promoter Mutations Occur Frequently in Gliomas and a Subset of Tumors Derived from Cells with Low Rates of Self-Renewal." Proc Natl Acad Sci U S A 110, no. 15 (2013): 6021-6.
- Spiegl-Kreinecker, S.; Lötsch, D.; Ghanim, B.; Pirker, C.; Mohr, T.; Laaber, M.; Weis, S.; Olschowski, A.; Webersinke, G.; Pichler, J.; et al. Prognostic quality of activating TERT promoter mutations in glioblastoma: interaction with the rs2853669 polymorphism and patient age at diagnosis. Neuro-Oncology 2015, 17, 1231–1240. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Skardelly, M.; Bonzheim, I.; Ott, I.; Mühleisen, H.; Eckert, F.; Tabatabai, G.; Schittenhelm, J. ATRX immunostaining predicts IDH and H3F3A status in gliomas. Acta Neuropathol. Commun. 2016, 4, 60. [Google Scholar] [CrossRef]
- Weller, Michael, Patrick Y. Wen, Susan M. Chang, Linda Dirven, Michael Lim, Michelle Monje, and Guido Reifenberger. "Glioma." Nature Reviews Disease Primers 10, no. 1 (2024): 33.
- Pusch, S.; Schweizer, L.; Beck, A.-C.; Lehmler, J.-M.; Weissert, S.; Balss, J.; Miller, A.K.; von Deimling, A. D-2-Hydroxyglutarate producing neo-enzymatic activity inversely correlates with frequency of the type of isocitrate dehydrogenase 1 mutations found in glioma. Acta Neuropathol. Commun. 2014, 2, 19–19. [Google Scholar] [CrossRef]
- Natsumeda, M., H. Igarashi, T. Nomura, R. Ogura, Y. Tsukamoto, T. Kobayashi, H. Aoki, K. Okamoto, A. Kakita, H. Takahashi, T. Nakada, and Y. Fujii. "Accumulation of 2-Hydroxyglutarate in Gliomas Correlates with Survival: A Study by 3.0-Tesla Magnetic Resonance Spectroscopy." Acta Neuropathol Commun 2 (2014): 158.
- Brennan, C. W., R. G. Verhaak, A. McKenna, B. Campos, H. Noushmehr, S. R. Salama, S. Zheng, D. Chakravarty, J. Z. Sanborn, S. H. Berman, R. Beroukhim, B. Bernard, C. J. Wu, G. Genovese, I. Shmulevich, J. Barnholtz-Sloan, L. Zou, R. Vegesna, S. A. Shukla,..., and L. Chin. "The Somatic Genomic Landscape of Glioblastoma." Cell 155, no. 2 (2013): 462-77.
- Ceccarelli, M., F. P. Barthel, T. M. Malta, T. S. Sabedot, S. R. Salama, B. A. Murray, O. Morozova, Y. Newton, A. Radenbaugh, S. M. Pagnotta, S. Anjum, J. Wang, G. Manyam, P. Zoppoli, S. Ling, A. A. Rao, M. Grifford, A. D. Cherniack, H. Zhang,..., and R. G. Verhaak. "Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma." Cell 164, no. 3 (2016): 550-63.
- Aoki, Kosuke, Hideo Nakamura, Hiromichi Suzuki, Keitaro Matsuo, Keisuke Kataoka, Teppei Shimamura, Kazuya Motomura, Fumiharu Ohka, Satoshi Shiina, Takashi Yamamoto, Yasunobu Nagata, Tetsuichi Yoshizato, Masahiro Mizoguchi, Tatsuya Abe, Yasutomo Momii, Yoshihiro Muragaki, Reiko Watanabe, Ichiro Ito, Masashi Sanada,..., and Atsushi Natsume. "Prognostic Relevance of Genetic Alterations in Diffuse Lower-Grade Gliomas." Neuro-Oncology 20, no. 1 (2017): 66-77.
- Yang, R. R., Z. F. Shi, Z. Y. Zhang, A. K. Chan, A. Aibaidula, W. W. Wang, J. S. H. Kwan, W. S. Poon, H. Chen, W. C. Li, N. Y. Chung, G. Punchhi, W. C. Chu, I. S. Chan, X. Z. Liu, Y. Mao, K. K. Li, and H. K. Ng. "Idh Mutant Lower Grade (Who Grades Ii/Iii) Astrocytomas Can Be Stratified for Risk by Cdkn2a, Cdk4 and Pdgfra Copy Number Alterations." Brain Pathol 30, no. 3 (2020): 541-53.
- Johnson, B. E., T. Mazor, C. Hong, M. Barnes, K. Aihara, C. Y. McLean, S. D. Fouse, S. Yamamoto, H. Ueda, K. Tatsuno, S. Asthana, L. E. Jalbert, S. J. Nelson, A. W. Bollen, W. C. Gustafson, E. Charron, W. A. Weiss, I. V. Smirnov, J. S. Song,..., and J. F. Costello. "Mutational Analysis Reveals the Origin and Therapy-Driven Evolution of Recurrent Glioma." Science 343, no. 6167 (2014): 189-93.
- Suzuki, H., K. Aoki, K. Chiba, Y. Sato, Y. Shiozawa, Y. Shiraishi, T. Shimamura, A. Niida, K. Motomura, F. Ohka, T. Yamamoto, K. Tanahashi, M. Ranjit, T. Wakabayashi, T. Yoshizato, K. Kataoka, K. Yoshida, Y. Nagata, A. Sato-Otsubo,..., and S. Ogawa. "Mutational Landscape and Clonal Architecture in Grade Ii and Iii Gliomas." Nat Genet 47, no. 5 (2015): 458-68.
- Favero, F., N. McGranahan, M. Salm, N. J. Birkbak, J. Z. Sanborn, S. C. Benz, J. Becq, J. F. Peden, Z. Kingsbury, R. J. Grocok, S. Humphray, D. Bentley, B. Spencer-Dene, A. Gutteridge, M. Brada, S. Roger, P. Y. Dietrich, T. Forshew, M. Gerlinger,..., and C. Swanton. "Glioblastoma Adaptation Traced through Decline of an Idh1 Clonal Driver and Macro-Evolution of a Double-Minute Chromosome." Ann Oncol 26, no. 5 (2015): 880-87.
- Lopez, G. Y., N. A. Oberheim Bush, J. J. Phillips, J. P. Bouffard, Y. A. Moshel, K. Jaeckle, B. K. Kleinschmidt-DeMasters, M. K. Rosenblum, A. Perry, and D. A. Solomon. "Diffuse Midline Gliomas with Subclonal H3f3a K27m Mutation and Mosaic H3.3 K27m Mutant Protein Expression." Acta Neuropathol 134, no. 6 (2017): 961-63.
- Luo, S.; Zhu, S.; Liao, J.; Zhang, Y.; Hou, X.; Luo, T.; Zhao, E.; Xu, J.; Pang, L.; Liang, X.; et al. IDH clonal heterogeneity segregates a subgroup of non-1p/19q codeleted gliomas with unfavourable clinical outcome. Neuropathol. Appl. Neurobiol. 2020, 47, 394–405. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, J.G.; Fine, H.A. Diffuse Glioma Heterogeneity and Its Therapeutic Implications. Cancer Discov. 2021, 11, 575–590. [Google Scholar] [CrossRef]
- Morgan, K.M.; Danish, S.; Xiong, Z. Diffuse astrocytoma with mosaic IDH1-R132H-mutant immuno-phenotype and low subclonal allele frequency. Intractable Rare Dis. Res. 2022, 11, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Bai, M.; Wang, X.; Zhang, H.; Wang, J.; Lyaysan, G.; Xu, S.; Tian, K.; Wang, T.; Li, J.; Wang, N.; et al. Dissecting and analyzing the Subclonal Mutations Associated with Poor Prognosis in Diffuse Glioma. BioMed Res. Int. 2022, 2022, 1–19. [Google Scholar] [CrossRef]
- Voisin, M.R.; Gui, C.; Patil, V.; Gao, A.F.; Zadeh, G. Methylation Profiling Identifies Stability of Isocitrate Dehydrogenase Mutation Over Time. Can. J. Neurol. Sci. / J. Can. des Sci. Neurol. 2023, 51, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Senft, C.; Behrens, M.; Lortz, I.; Wenger, K.; Filipski, K.; Seifert, V.; Forster, M.-T. The ability to return to work: a patient-centered outcome parameter following glioma surgery. J. Neuro-Oncology 2020, 149, 403–411. [Google Scholar] [CrossRef]
- Loon, E.; Ernens, W.; Heijenbrok-Kal, M.; Horemans, H.; Ribbers, G.; Bent, M. Long-term employment status and the association with fatigue in patients with grade II glioma. J. Rehabilitation Med. 2021, 53, jrm00198. [Google Scholar] [CrossRef]
- Birendra, K.; DiNardo, C.D. Evidence for Clinical Differentiation and Differentiation Syndrome in Patients With Acute Myeloid Leukemia and IDH1 Mutations Treated With the Targeted Mutant IDH1 Inhibitor, AG-120. Clin. Lymphoma Myeloma Leuk. 2016, 16, 460–465. [Google Scholar] [CrossRef]
- Fujii, T.; Khawaja, M.R.; DiNardo, C.D.; Atkins, J.T.; Janku, F. Targeting Isocitrate Dehydrogenase (IDH) in Cancer. 2016, 21, 373–380.
- Mellinghoff, I. K., M. J. van den Bent, D. T. Blumenthal, M. Touat, K. B. Peters, J. Clarke, J. Mendez, S. Yust-Katz, L. Welsh, W. P. Mason, F. Ducray, Y. Umemura, B. Nabors, M. Holdhoff, A. F. Hottinger, Y. Arakawa, J. M. Sepulveda, W. Wick, R. Soffietti,..., and Indigo Trial Investigators. "Vorasidenib in Idh1- or Idh2-Mutant Low-Grade Glioma." N Engl J Med 389, no. 7 (2023): 589-601.
- Wen, F.; Gui, G.; Wang, X.; Qin, A.; Ma, T.; Chen, H.; Li, C.; Zha, X. Discovery of Novel Dual Inhibitors Targeting Mutant IDH1 and NAMPT for the Treatment of Glioma with IDH1Mutation. J. Med. Chem. 2024, 67, 8667–8692. [Google Scholar] [CrossRef]
- Moore, D. Panobinostat (Farydak): A Novel Option for the Treatment of Relapsed Or Relapsed and Refractory Multiple Myeloma. Pharm. Ther. 2016, 41, 296–300. [Google Scholar]
- Pan, D.; Mouhieddine, T.H.; Upadhyay, R.; Casasanta, N.; Lee, A.; Zubizarreta, N.; Moshier, E.; Richter, J. Outcomes with panobinostat in heavily pretreated multiple myeloma patients. Semin. Oncol. 2023, 50, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Gunn, Kathryn, Matti Myllykoski, John Z. Cao, Manna Ahmed, Bofu Huang, Betty Rouaisnel, Bill H. Diplas, Michael M. Levitt, Ryan Looper, John G. Doench, Keith L. Ligon, Harley I. Kornblum, Samuel K. McBrayer, Hai Yan, Cihangir Duy, Lucy A. Godley, Peppi Koivunen, and Julie-Aurore Losman. "(R)-2-Hydroxyglutarate Inhibits Kdm5 Histone Lysine Demethylases to Drive Transformation in Idh-Mutant Cancers." Cancer Discovery 13, no. 6 (2023): 1478-97.
- Nie, Z.; Shi, L.; Lai, C.; O'Connell, S.M.; Xu, J.; Stansfield, R.K.; Hosfield, D.J.; Veal, J.M.; Stafford, J.A. Structure-based design and discovery of potent and selective KDM5 inhibitors. Bioorganic Med. Chem. Lett. 2018, 28, 1490–1494. [Google Scholar] [CrossRef] [PubMed]
- Rautajoki, K. J., S. Jaatinen, A. Hartewig, A. M. Tiihonen, M. Annala, I. Salonen, M. Valkonen, V. Simola, E. M. Vuorinen, A. Kivinen, M. J. Rauhala, R. Nurminen, K. K. Maass, S. L. Lahtela, A. Jukkola, O. Yli-Harja, P. Helén, K. W. Pajtler, P. Ruusuvuori,..., and M. Nykter. "Genomic Characterization of Idh-Mutant Astrocytoma Progression to Grade 4 in the Treatment Setting." Acta Neuropathol Commun 11, no. 1 (2023): 176.
- Weller, M.; Felsberg, J.; Hentschel, B.; Gramatzki, D.; Kubon, N.; Wolter, M.; Reusche, M.; Roth, P.; Krex, D.; Herrlinger, U.; et al. Improved prognostic stratification of patients with isocitrate dehydrogenase-mutant astrocytoma. Acta Neuropathol. 2024, 147, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Mallela, A.N.; Shi, D.D.; Tang, L.W.; Abou-Al-Shaar, H.; Gersey, Z.C.; Zhang, X.; McBrayer, S.K.; Abdullah, K.G. Isocitrate dehydrogenase mutations in gliomas: A review of current understanding and trials. Neuro-Oncology Adv. 2023, 5, vdad053. [Google Scholar] [CrossRef]
- Kayabolen, A., E. Yilmaz, and T. Bagci-Onder. "Idh Mutations in Glioma: Double-Edged Sword in Clinical Applications?" Biomedicines 9, no. 7 (2021).
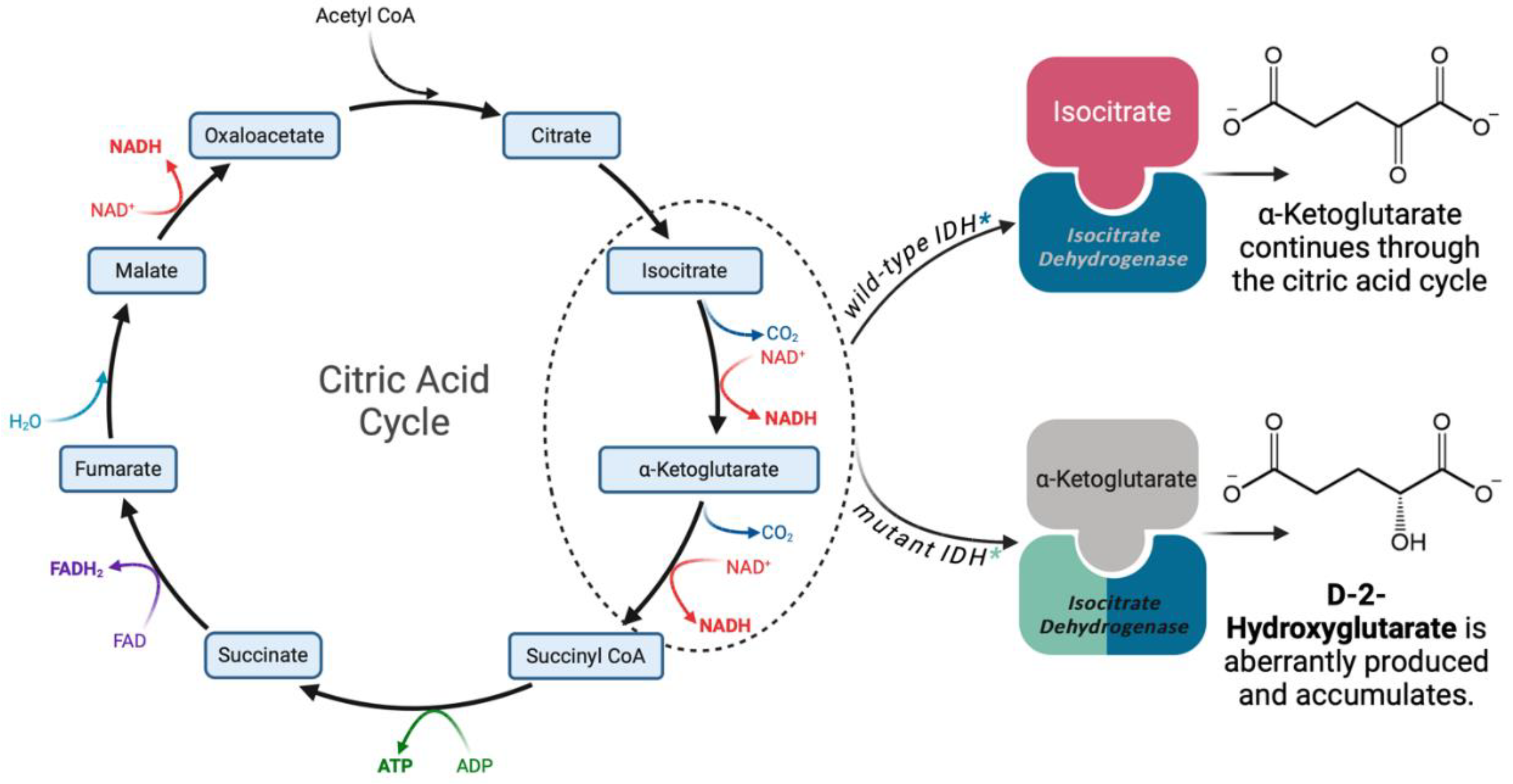
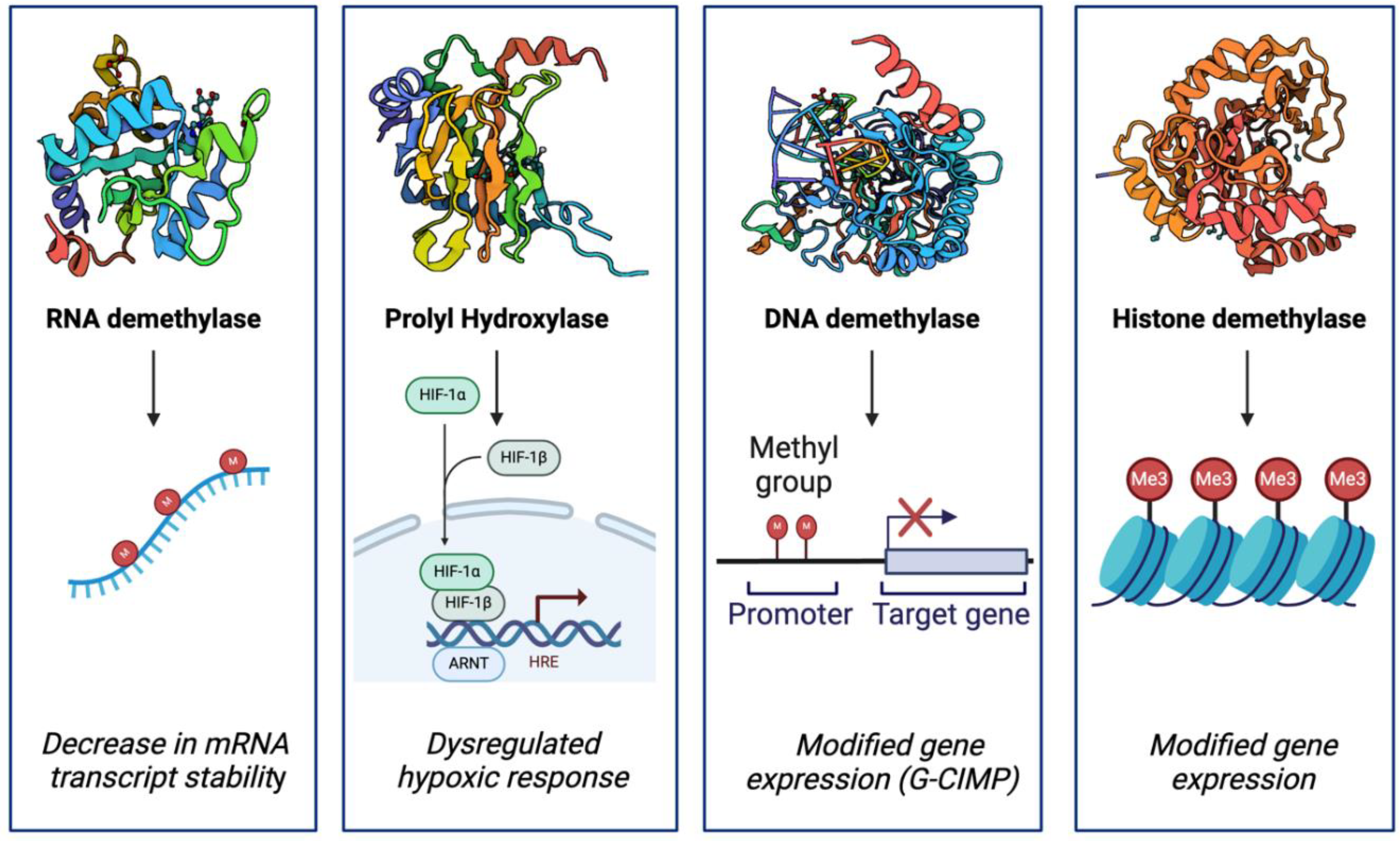
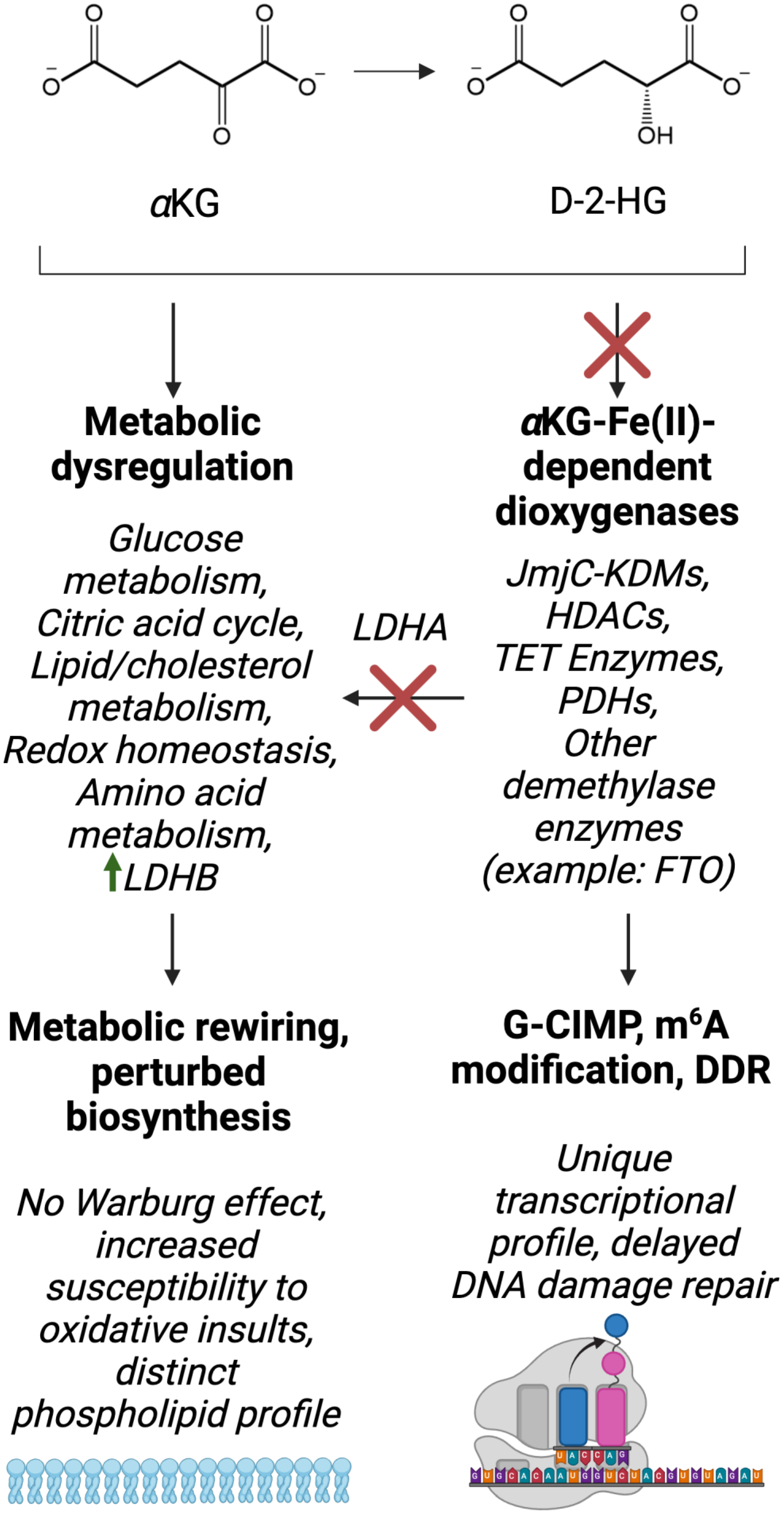
| IDH variant | Frequency in low-grade glioma (%) |
|---|---|
| IDH1-R132H | 62.0-93.0 |
| IDH1-R132C | 2.9-4.3 |
| IDH1-R132G | 1.0-2.5 |
| IDH1-R132S | 1.1-2.2 |
| IDH1-R132L | 0.2-0.6 |
| IDH2-R172K | 2.8-3.0 |
| IDH2-R172W | 0.6 |
| IDH2-R172M | 0.8 |
| IDH2-R172S | 0.2 |
| IDH1-R132PIDH1-R132VIDH2-R172T | Extremely rare |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




