1. Introduction
Beginning in December 2019 and continuing through February 2023, a novel coronavirus, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) caused a worldwide pandemic [
1]. Infection caused by SARS-CoV-2 is referred to as Coronavirus Infectious Disease 2019 (COVID-19) [
2]. Lack of herd immunity and high infectivity rates at the beginning of the pandemic resulted in rapid spread of the virus, mandating a coordinated Public Health response. In the US, pandemic mitigation efforts including travel restrictions, quarantine of new arrivals, social distancing, masking and lockdowns were implemented [
3]. These measures were aimed at reducing the opportunity for the virus to circulate in a susceptible population and may have slowed the spread of the virus [
4,
5], reducing demand on healthcare systems and allowing time for development of vaccines and therapeutics [
6]; however, they had unanticipated adverse impacts on pediatric patients. These included decrease in scheduled health maintenance visits [
5,
7,
8], decrease in vaccination rates [
9,
10,
11], increase in rates of obesity [
12] and reduction in access to emergency [
13,
14] and subspecialty services [
14,
15,
16,
17,
18,
19,
20]. Significant adverse effects on psychosocial health and wellbeing have also been documented [
11,
21,
22]. In New York, for example, pediatric social determinants of health (SDOH) including access to food, housing, legal and public health services were all adversely affected by lockdowns, resulting in significantly worsening emotional and behavioral health concerns [
23].
A substantial amount of literature addresses the clinical manifestations of COVID-19 in adults [
24,
25] and in pediatric patients with chronic illness [
14,
20,
26,
27,
28]; however, considerably fewer studies have addressed its clinical manifestations in previously healthy children [
29,
30]. These studies, along with aggregated data from the CDC suggest that as many as 40 – 50% of infections in children may be asymptomatic, while fewer than 1% have severe illness and an even smaller percentage experience long term sequalae [
25,
29].
The PediCenter and Niles Children’s Clinic provide pediatric primary and urgent care services to an underserved population in central California. We remained open throughout the COVID-19 pandemic, providing scheduled well child-care and sick visits. Beginning in September 2020, we implemented a COVID-19 screening program to facilitate the safe delivery of patient care. Screening was performed on all patients presenting for care and was also made available upon request for patients requiring testing for any purpose. Herein, we provide results from this screening program and describe the clinical manifestations of acute infection in this patient population.
Although our screening encompassed a large number of pediatric patients, it is important to note that the study participants were specifically those who visited our facilities for care during the study period. Consequently, our findings reflect the characteristics of this group and may not necessarily extend to the general population. This focus allows us to offer detailed insights into the infection dynamics and clinical presentations within a specific, potentially unique community setting.
2. Materials and Methods
Following implementation of the routine screening program in September 2020, all patients presenting for care or requesting testing for any reason were asked to complete a questionnaire identifying symptoms and signs of possible COVID-19 infection. Patient vital signs including temperature and oxygen saturation were obtained and a nasal swab for rapid COVID-19 antigen testing was obtained using a commercially available antigen testing kit [Diatrust
TM, Celltrion, USA]. Rapid antigen testing has a sensitivity of >86% and specificity of >99% (diatrustcovid19.com). This is consistent with other reported results in symptomatic adults and children [
31,
32]. All results were recorded in the patient’s electronic medical record (EMR).
Results of testing were made available to providers prior to patient contact. Patients presenting with symptoms or those who tested positive were placed in isolation. All providers were provided with personal protective equipment (PPE) and universal precautions were employed. Patient rooms were sanitized between patient encounters.
Data presented here were obtained from retrospective chart review of electronic medical record (EMR) for all encounters occurring between November 1st, 2020, and December 31st, 2022. Study protocol was reviewed by institutional human subjects review committee (HSRC). Since only anonymous “off the shelf” data from retrospective chart review were used and no additional patient contact was planned, requirement for written informed consent was waived.
Inclusion criteria were patient age less than or equal to 17 years and COVID-19 antigen test results available. To ensure patient anonymity, all encounters were assigned a unique patient encounter number (UPEN) and all other patient identifiers were removed prior to data export. Data exported included patient age (years), sex, date of service, reason for visit, final diagnosis (ICD10 code), temperature (Fahrenheit), pulse oximetry (percent saturation), body mass index (BMI) and COVID antigen test result.
In our study, patients were classified as having COVID-19 if the antigen test was positive. We employed the severity of illness index recommended by the Centers for Disease Control (CDC) [National Institutes of Health, COVID-19 Treatment Guidelines [
https://www.covid19treatmentguidelines.nih.gov/] as follows:
1) asymptomatic/presymptomatic; patient reported no symptoms or signs at time of testing and vital signs were normal.
2) mild disease; patient exhibited symptoms or signs of respiratory or gastrointestinal illness with or without fever but with no evidence of lower respiratory tract infection.
3) moderate disease; patient had evidence of lower respiratory tract infection.
4) severe illness; patient had oxygen saturation level less than 94%.
5) critical illness patient required transfer to emergency department or hospital admission for higher level of care.
In addition, we created a seasonal index based on the date of each patient’s visit. This index assigned a value of 1 to any patient who visited during Fall 2020, 2 for Winter 2021, and so on, through nine seasonal periods ending in Fall 2022 (1-Fall 2020, 2-Winter 2021, 3-Spring 2021, 4-Summer 2021, 5-Fall 2021, 6-Winter 2022, 7-Spring 2022, 8-Summer 2022, 9-Fall 2022). The seasonal index was used as a proxy to explain temporal variations in the pandemic’s progression. Although not strain-specific, this approach allowed us to account for general changes over time, reflecting potential shifts in virus strains or public health measures. This method was necessary given that rapid antigen tests, while effective at detecting infection, did not provide data on specific strains.
Subset analysis was performed on the following groups:
1) all patients who tested positive.
2) all patients who were asymptomatic regardless of test status.
3) all patients who were symptomatic regardless of test status.
The primary variables of interest in this study were symptomatic status, test result, age, gender, BMI, seasonal index, and severity level of illness for patients. We considered a p-value smaller than 0.05 as statistically significant for all analyses. All statistical analyses were performed using R (R Core Team, 2022).
A preliminary univariate analysis was conducted among patients who tested positive to investigate potential associations between severity of illness and patient age, gender, time from onset of pandemic and BMI. A chi-square test found no significant association between gender and severity of illness ( 8.68, df=8, p-value =0.37). For the purpose of a chi-square test, to evaluate the effect of age, we classified patients into three qualitative age groups: infants (birth to 1 year), children (1 year through 12 years), and adolescents (13 years to 17 years). A chi-square test found a significant association between these age groups and severity of illness ( 38.602, df=8, p-value <.001). To assess the effect of BMI, we categorized patients into three groups: healthy (BMI <28), mildly obese (BMI from 28 to 34), and obese (BMI greater than 35). We were not able to confirm a statistically significant association between BMI and severity of illness; however, there was a trend towards increasing severity in obese patients. ( 18.558, df=12, p-value =0.10).
A multivariate analysis was conducted using two ordinal logistic regression model to assesses the following issues regarding severity levels:
Model 1: Among patients who tested positive, are levels of severity associated with a patient’s age, gender, and seasonal index?
Model 2: Among all symptomatic patients, do levels of severity differ among the positive and negative patients while accounting for variables that may significantly affect the level of severity (age, gender, seasonal index)
Note that the dependent variable, level of severity, follows an ordinal distribution with distinct levels representing four different levels of severity (asymptomatic, mild, moderate, and severe). Ordinal logistic regression allows one to investigate the likelihood of each severity level occurring based on a set of independent variables. In this analysis, we model the cumulative odds of each severity level using the logit transformation. Specifically, we examine how the log-odds of the cumulative probability of a specific severity level are linearly related to the independent variables. This approach enables one to explore the factors associated with different severity levels (Faraway, 2006).
To determine which variables significantly affect the likelihood of each severity level occurring, forward model selection is used (Kutner et al., 2005). This method was used to build each model, using a likelihood ratio test as the selection criterion (McCullagh & Nelder, 1989). The estimated model coefficients, standard errors, two-sided p-values, the exponentiated model coefficients (OR), and 95% confidence intervals (CIs) for the exponentiated estimated regression coefficients are given in Table 3 and Table 4 for Model 1 and 2, respectively.
3. Results
A total of 11,649 COVID-19 antigen screening tests were performed. Patients ranged in age from 0.1 to 17.0 years, (mean 8.7y, SD 4.5y). A total of 1,560 pts. (13.4%) tested positive (Group 1). Among these, 665 (43%) were asymptomatic, 560 (36%) had mild disease, 318 (20%) had moderate disease, and 17 (1%) had severe disease.
There were 4,446 patients who reported no symptoms and presented for screening only (Group 2), 15% of whom tested positive.
There were 7,203 patients who reported symptoms consistent with COVID-19 at the time of testing (Group 3). Among these, 87.6% tested negative and 12.4% tested positive.
Brief descriptions of all variables used in this study are provided in
Table 1.
The first quartile, median, mean, and third quartile of the quantitative data, as well as the frequency counts and percentage for levels of the observed qualitative data, for the primary variables of interest are presented in
Table 2.
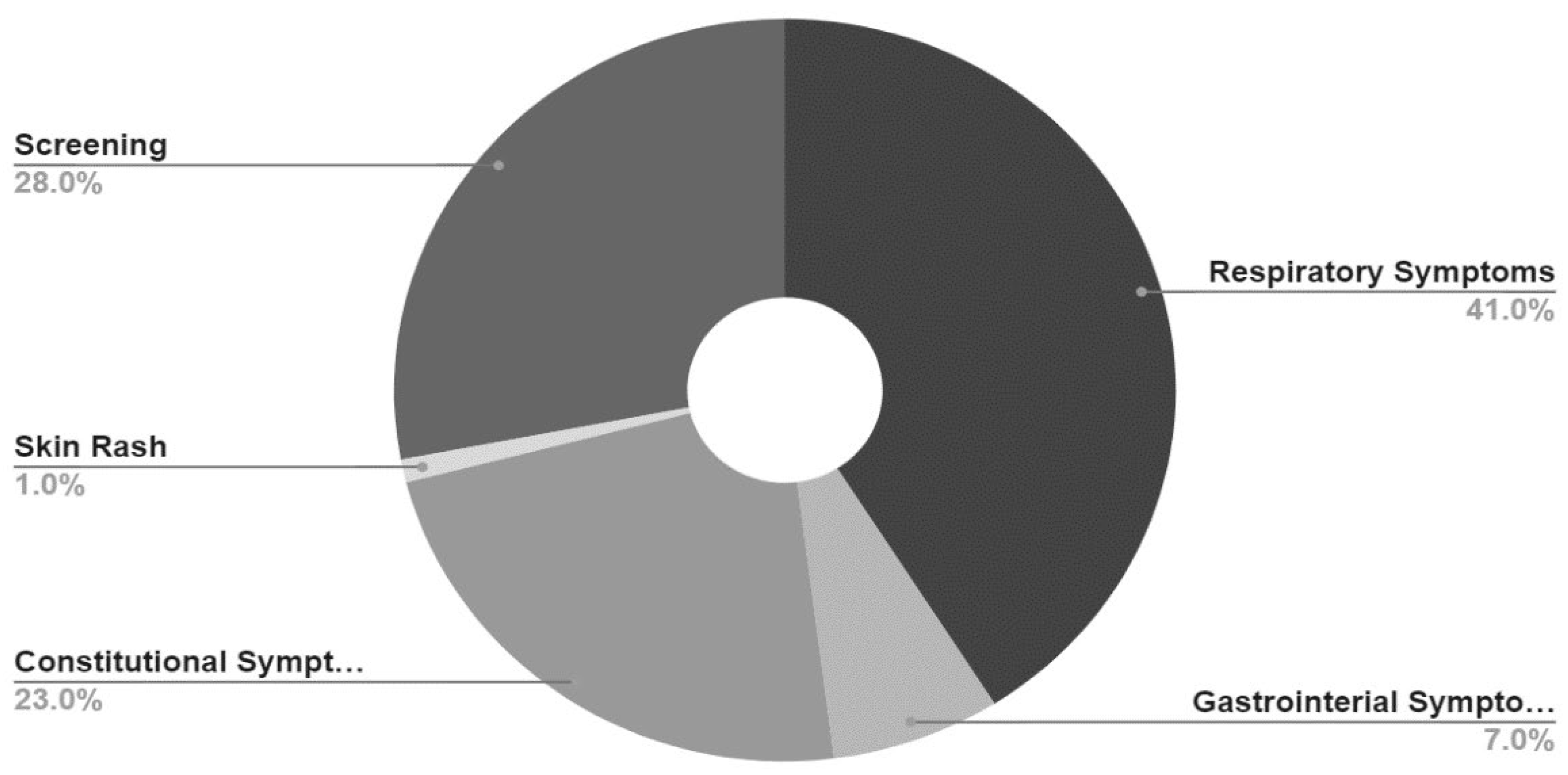
Reason For Visit (Chief Complaint) of study participants is presented in
Table 3 and
Figure 1. Reason for visit was recorded verbatim in a text field in the EMR; therefore, for ease and convenience of presentation of data, chief complaints were categorized as predominantly respiratory symptoms (nasopharyngitis, cough, wheezing, chest pain or shortness of breath), predominantly gastrointestinal symptoms (nausea, vomiting, diarrhea, abdominal pain or loss of appetite), constitutional symptoms (fever, headache, body aches, fatigue without respiratory or gastrointestinal symptoms), skin rash with no other symptoms and screening only (patients who presented without symptoms, requesting testing for any purpose).
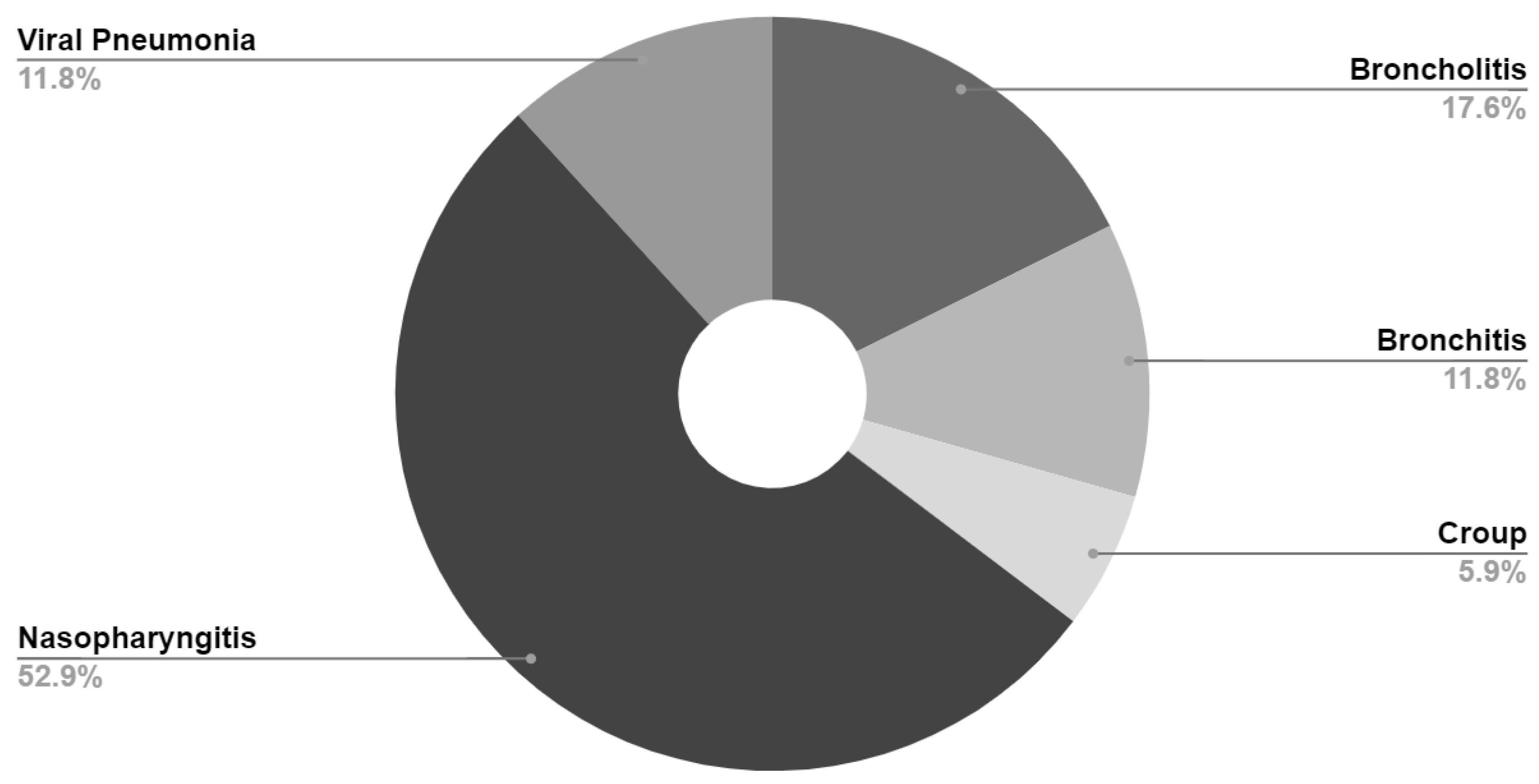
Final diagnosis for patients with severe disease (those with oxygen saturation <94% on presentation) are presented in
Table 4 and
Figure 2. Final diagnosis was based on ICD-10 code assigned by healthcare provider at time of visit.
We evaluated the impact of gender, age, BMI and seasonal index on severity of illness in patients who tested positive (Model 1). In addition, we compared the severity of illness in symptomatic patients who tested positive against those who tested negative (Model 2). For a given model result, any estimated regression coefficient is interpreted when holding the other variables in the model constant. To identify the most significant predictors for our models, we employed an AIC forward stepwise regression approach, optimizing for the best balance between model complexity and predictive accuracy. Following the initial selection via AIC, we further refined our model using Maximum Likelihood Estimation tests to ensure that only statistically significant variables were retained.
Table 5 displays the results from Model 1 analysis, which evaluated the impact of age and seasonal index on severity of illness among patients who tested positive for COVID-19. We found that gender and BMI were not significant predictors in this model. The effect of the seasonal index conveys that the odds of being in a higher severity category increase by 35% (95% CI: 25.9% to 44.6%) for each progression from one season to the next. The effect of patient’s age suggests that for every additional year of age, the odds of being in a higher severity category decrease by 5% (95% CI: 3.5% to 7.0%).
Results for model 2 are displayed in
Table 6. In this model, negative test results serve as the reference level against positive test results. Similarly, the gender female is the reference level against which the other levels (male, other) are compared.
The estimated regression coefficient for when the testing result is positive shows that for patients positive for Covid-19, the odds of being in a higher severity level are 34% (95% CI: 19.6% to 49.8%) higher than the odds for a patient who tested negative. The estimated seasonal index effect suggests that as a season progresses from one season to the next, the odds of being in a higher severity level increase by 41% (95% CI: 37.6% to 44.8%). The estimated regression coefficient for a patient’s age suggests that for every additional year of age, the odds of being in a higher severity category decrease by about 7% (95% CI: 6.4% to 8.0%). In our model, estimated effect for patients who identify as male is not significant, suggesting that the difference in severity levels between males and females is not significant. The estimated effect for patients who identify as other shows that the odds of these patients being in a higher severity level are 39% (95% CI: 18.6% to 53.9%) lower compared to the odds for female patients.
Model 2 considered all symptomatic patients to assess whether levels of severity differ among those who tested positive and negative for COVID-19, while accounting for variables that may significantly affect the level of severity of a patient. The results showed that patients who tested positive for COVID-19 have 33.20% higher odds (95% CI: 19.00% to 48.96%) of being in a higher severity level compared to those who tested negative. Additionally, for each additional year of a patient’s age, the odds of being in a higher severity level decrease by about 7.22% (95% CI: 6.46% to 7.98%). As we progress from one season to the next, the odds of being in a higher severity level increase by 41.42% (95% CI: 37.86% to 45.06%). These findings provide evidence at significance level 0.05 that the severity differs among those who tested positive and negative for COVID-19, even after accounting for factors that significantly affect the level of severity.
4. Discussion
SARS-CoV-2 infects cells by adhering to cell membrane angiotensin-converting enzyme 2 (ACE-2) receptor [
24]. The ACE-2 receptor is widely distributed on endothelial cells, renal epithelium and respiratory epithelial cells, particularly in the lower respiratory tract [
33]. Children are known to have a significantly reduced concentration of ACE-2 receptors on their lower respiratory tract epithelial cells and would therefore be predicted to have a lower severity of illness than older adults [
34]. This may, in part, explain the mild symptom severity in the majority of our patients. Our findings are consistent with reports from CDC and other investigators [
11,
30] which suggest that for most previously healthy pediatric patients, COVID-19 is a very mild infection with a significant percentage having no symptoms at time of diagnosis.
We note that our findings from Model 1 show an unexpected association between age and severity of illness in that for every additional year of age, the odds of a patient being in a higher severity category decrease by 5%. Although the odds ratio is close to 1, research has demonstrated that even small effect sizes can have substantial public health implications when applied to large populations (Carey et al., 2023). This unique observation suggests that the relationship between age and severity in pediatric patients may differ from that in older populations and this warrants further investigation. Recent studies have suggested that SARS-CoV-2 can cause bronchiolitis in infants [
35,
36]. A final diagnosis of bronchiolitis was recorded in 320 (2.7%) of our total study population and in 11.8% of our patients with severe disease. This may, in part, explain our finding of increased risk of severe disease in younger patients. Additional studies are planned to further investigate this association.
It should be noted that 52.9% of our patients with severe infection had a final diagnosis of nasopharyngitis (common cold). Obtaining accurate oxygen saturation by pulse oximetry can be difficult in children, especially younger children who are often crying and non-compliant. Our statistical analysis plan did not allow re-assignment of severity of illness after data extraction; therefore, our finding of 1% of patients with severe infection is likely a significant over estimation.
While we observed a trend towards increasing severity of illness in obese patients (p=0.1), the association did not achieve statistical significance. Note that our analysis did not find evidence of a significant relationship at our chosen significance level between BMI and the severity of illness in Model 1; however, our analysis was constrained by the limited availability of BMI data, which were lacking for approximately 86% of patients, perhaps impacting the ability of our study to confirm an association as reported by other authors [
37,
38,
39,
40].
Our testing consisted solely of rapid antigen detection by immunofluorescent assay, which does not identify viral strain. For that reason, we included an analysis of time from the onset of pandemic to time of testing which was intended as a surrogate marker for the impact of the dominant circulating strain on the severity of illness. Our data show a statistically significant association between time from onset of pandemic and severity of illness (seasonal index), with patients experiencing more severe symptoms as time from the beginning of the pandemic increased. This is most likely explained by the fact that the original Sars-CoV-2 variant, the alpha variant, was displaced as the predominant strain beginning in December of 2020 by the Delta variant and Delta was displaced as the predominant strain in about December of 2021 by the Omicron variant, both of which were documented to be more contagious and to cause more severe illness than the original Alpha variant [
41,
42]. Our data show a statistically significant association between time from onset of pandemic and severity of illness (seasonal index), supporting the notion of these strains being more contagious and severe. Screening proved effective in identifying patients with asymptomatic/ presymptomatic infection, facilitating safe continuation of outpatient services, when combined with appropriate isolation and universal precautions. The deleterious effects of restricted access to health care are now well documented, and we hope our experience may help guide policy decisions during future pandemics. Screening, universal precautions and PPE can be safely and effectively utilized in a primary care setting to facilitate continued provision of care.
Since SARS-CoV-2 is transmitted by droplets, it is unclear whether asymptomatic patients can transmit the infection [
43,
44]; notwithstanding, asymptomatic patients may be a vector for infection [
43]. For that reason, all patients who tested positive regardless of symptoms, were placed in isolation rooms in clinic and were instructed to isolate at home and family contacts were instructed to quarantine per CDC guidelines.
Amongst patients who reported symptoms at time of diagnosis, those who tested negative and were assumed to have an infection other than COVID-19 did not differ significantly demographically from patients who tested positive for COVID-19. Although an infection was not documented or confirmed in all patients who tested negative, reasons for visit and final diagnoses were similar in the 2 groups. Results from Model 2 suggest that the severity of illness score for symptomatic patients who tested positive for COVID-19 was higher than for symptomatic patients who tested negative. An obvious criticism of this analysis is that patients who were asymptomatic/ presymptomatic and who tested positive were excluded from this subset analysis, skewing severity of illness towards a higher level. Not-withstanding, symptomatic patients with COVID-19 need to be evaluated and monitored carefully for severe disease.
A limitation of our data is that it relied on self-reporting of symptoms; therefore, it is possible that patients presenting for screening who required clearance to participate in school or other activities may have underrepresented their symptoms, skewing severity of illness index towards milder infections. This concern is partially offset by the fact that to be designated asymptomatic, patients also needed to have normal vital signs and normal oxygen saturation.
Our study only evaluated patients at time of encounter for screening and we do not have follow-up data to address what percentage of patients went on to develop symptoms. Likewise, we do not have long-term follow-up to evaluate risk of long COVID [
45] or complications including multisystem inflammatory syndrome associated with COVID (MIS-C) [
46]. Additional studies are currently planned to address these issues.
5. Conclusions
Our purpose in conducting this study and presenting our findings is to demonstrate that adherence to well established universal precaution, along with use of PPE and routine screening as implemented in our outpatient practice, can be easily accomplished and can be effective in facilitating the safe continuation of office practice during future pandemics, a desirable outcome if the adverse impacts of lockdowns, as previously enumerated, are to be avoided or mitigated. Further, our study indicates that clinical research can be conducted in a non-academic, outpatient setting and that such research can contribute to the state of knowledge regarding illnesses that impact the general pediatric population, the majority of which is cared for in the community, outside of university affiliated hospitals and clinics.
Overall, our analysis provides valuable insights into the association of variables such as age, gender, seasonal index, and BMI with the severity of illness. These data suggest that COVID-19 in children is generally a mild infection with a substantial proportion reporting no symptoms at the time of diagnosis, a fact that was not well known or anticipated at the onset of the pandemic. Only 1% had severe illness per CDC severity rating and no patient required transfer to ER or hospital admission. Younger patient age was a statistically significant risk factor for increasing severity of illness, as was time from onset of pandemic. While there was no significant association found between BMI and severity in our dataset, this may warrant further investigation given the limited availability of BMI data in our study.
Despite the generally mild severity of COVID-19 in children, the disease burden including school and work absenteism, hospital admission, long term sequelae and even death, are still substantial, so in our practice, we continue to advocate for COVID-19 vaccination in all children older than 6 months of age [
47].
Author Contributions
All authors contributed significantly to study design, data analysis, data interpretation and manuscript preparation.
Funding
No external funding was received for this study.
Institutional Review Board Statement
Universal Urgent Care and Pain Management HSRC study protocol 2021-001.
Informed Consent Statement
Requirement for written informed consent was waived by HSRC because the study used only retrospective data obtained by review of medical records, with all patient identifiers removed prior to data export with no additional patient contact required.
Data Availability Statement
Original Data Tables can be made available upon request to corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Burki, T. WHO ends the COVID-19 public health emergency. Lancet Respir Med 2023, 11 (7), 588. [CrossRef]
- Bellino, S.; Punzo, O.; Rota, M. C.; Del Manso, M.; Urdiales, A. M.; Andrianou, X.; Fabiani, M.; Boros, S.; Vescio, F.; Riccardo, F.; et al. COVID-19 Disease Severity Risk Factors for Pediatric Patients in Italy. Pediatrics 2020, 146. [Google Scholar] [CrossRef] [PubMed]
- Schuchat, A.; Team, C. C.-R. Public Health Response to the Initiation and Spread of Pandemic COVID-19 in the United States, February 24-April 21, 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Bellino, S.; Rota, M. C.; Riccardo, F.; Andrianou, X.; Mateo Urdiales, A.; Del Manso, M.; Punzo, O.; Bella, A.; Villani, A.; Pezzotti, P.; et al. Pediatric COVID-19 Cases Prelockdown and Postlockdown in Italy. Pediatrics 2021, 147. [Google Scholar] [CrossRef]
- Vogel, M.; Beger, C.; Gausche, R.; Jurkutat, A.; Pfaeffle, R.; Korner, A.; Meigen, C.; Poulain, T.; Kiess, W. COVID-19 pandemic and families’ utilization of well-child clinics and pediatric practices attendance in Germany. BMC Res Notes 2021, 14, 140. [Google Scholar] [CrossRef]
- Ciotti, M.; Benedetti, F.; Zella, D.; Angeletti, S.; Ciccozzi, M.; Bernardini, S. SARS-CoV-2 Infection and the COVID-19 Pandemic Emergency: The Importance of Diagnostic Methods. Chemotherapy 2021, 66, 17–23. [Google Scholar] [CrossRef]
- Macy, M. L.; Huetteman, P.; Kan, K. Changes in Primary Care Visits in the 24 Weeks After COVID-19 Stay-at-Home Orders Relative to the Comparable Time Period in 2019 in Metropolitan Chicago and Northern Illinois. J Prim Care Community Health 2020, 11, 2150132720969557. [Google Scholar] [CrossRef]
- Saunders, N.; Guttmann, A.; Brownell, M.; Cohen, E.; Fu, L.; Guan, J.; Sarkar, J.; Mahar, A.; Gandhi, S.; Fiksenbaum, L.; et al. Pediatric primary care in Ontario and Manitoba after the onset of the COVID-19 pandemic: a population-based study. CMAJ Open 2021, 9, E1149–E1158. [Google Scholar] [CrossRef]
- Santoli, J. M.; Lindley, M. C.; DeSilva, M. B.; Kharbanda, E. O.; Daley, M. F.; Galloway, L.; Gee, J.; Glover, M.; Herring, B.; Kang, Y.; et al. Effects of the COVID-19 Pandemic on Routine Pediatric Vaccine Ordering and Administration - United States, 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 591–593. [Google Scholar] [CrossRef]
- Vogt, T. M.; Zhang, F.; Banks, M.; Black, C.; Arthur, B.; Kang, Y.; Lucas, P.; Lamont, B. Provision of Pediatric Immunization Services During the COVID-19 Pandemic: an Assessment of Capacity Among Pediatric Immunization Providers Participating in the Vaccines for Children Program - United States, May 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 859–863. [Google Scholar] [CrossRef]
- Poppe, M.; Aguiar, B.; Sousa, R.; Oom, P. The Impact of the COVID-19 Pandemic on Children’s Health in Portugal: The Parental Perspective. Acta Med Port 2021, 34, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, I.; Delle Cave, F.; Guarracino, C.; De Filippo, M.; Votto, M.; Licari, A.; Pistone, C.; Tondina, E. Obesity and COVID-19 in children and adolescents: a double pandemic. Acta Biomed 2022, 93, e2022195. [Google Scholar] [CrossRef] [PubMed]
- Cozzi, G.; Zanchi, C.; Giangreco, M.; Rabach, I.; Calligaris, L.; Giorgi, R.; Conte, M.; Moressa, V.; Delise, A.; Poropat, F. The impact of the COVID-19 lockdown in Italy on a paediatric emergency setting. Acta Paediatr 2020, 109, 2157–2159. [Google Scholar] [CrossRef] [PubMed]
- Moratilla Lapena, L.; Delgado-Miguel, C.; Sarmiento Caldas, M. C.; Estefania, K.; Velayos, M.; Munoz-Serrano, A.; De Ceano-Vivas, M.; Lopez-Santamaria, M.; Martinez, L. Impact of SARS-CoV-2 pandemic on emergency department activity at the pediatric surgery unit of a third-level hospital. Cir Pediatr 2021, 34, 85–89. [Google Scholar] [PubMed]
- Montalva, L.; Haffreingue, A.; Ali, L.; Clariot, S.; Julien-Marsollier, F.; Ghoneimi, A. E.; Peycelon, M.; Bonnard, A. The role of a pediatric tertiary care center in avoiding collateral damage for children with acute appendicitis during the COVID-19 outbreak. Pediatr Surg Int 2020, 36, 1397–1405. [Google Scholar] [CrossRef]
- Aly, A.; Pettorini, B. COVID-19 lockdown presented a chance to evaluate emergency referrals to paediatric neurosurgical unit: a prospective cohort study. Childs Nerv Syst 2021, 37, 729–732. [Google Scholar] [CrossRef]
- Gibbard, M.; Ponton, E.; Sidhu, B. V.; Farrell, S.; Bone, J. N.; Wu, L. A.; Schaeffer, E.; Cooper, A.; Aroojis, A.; Mulpuri, K.; et al. Survey of the Impact of COVID-19 on Pediatric Orthopaedic Surgeons Globally. J Pediatr Orthop 2021, 41, e692–e697. [Google Scholar] [CrossRef]
- Liguoro, I.; Pilotto, C.; Vergine, M.; Pusiol, A.; Vidal, E.; Cogo, P. The impact of COVID-19 on a tertiary care pediatric emergency department. Eur J Pediatr 2021, 180, 1497–1504. [Google Scholar] [CrossRef]
- Mahat, N.; Zubaidi, S. A.; Soe, H. H. K.; Nah, S. A. Paediatric surgical response to an ‘adult’ COVID-19 pandemic. Med J Malaysia 2021, 76, 284–290. [Google Scholar]
- Burayzat, S. M.; Kilani, M.; Al-Ghawanmeh, R.; Odeh, M.; Assi, O.; Alzoubi, K. H. Evaluation of the Effect Coronavirus Lockdown had on Chronic Disease Management Care in Pediatrics: A Survey of Jordanian Pediatricians. Int J Clin Pract 2022, 2022, 8710176. [Google Scholar] [CrossRef]
- Hefferon, C.; Taylor, C.; Bennett, D.; Falconer, C.; Campbell, M.; Williams, J. G.; Schwartz, D.; Kipping, R.; Taylor-Robinson, D. Priorities for the child public health response to the COVID-19 pandemic recovery in England. Arch Dis Child 2021, 106, 533–538. [Google Scholar] [CrossRef] [PubMed]
- Long, E.; Patterson, S.; Maxwell, K.; Blake, C.; Boso Perez, R.; Lewis, R.; McCann, M.; Riddell, J.; Skivington, K.; Wilson-Lowe, R.; et al. COVID-19 pandemic and its impact on social relationships and health. J Epidemiol Community Health 2022, 76, 128–132. [Google Scholar] [CrossRef] [PubMed]
- Lax, Y.; Cuno, K.; Keller, K.; Kogan, J.; Silver, M.; Avner, J. R. Social Determinants of Health and Pediatric Mental Health Before and During COVID-19 in New York City Primary Care Pediatrics. Popul Health Manag 2022, 25, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Guest, P. C.; Kesharwani, P.; Butler, A. E.; Sahebkar, A. The COVID-19 Pandemic: SARS-CoV-2 Structure, Infection, Transmission, Symptomology, and Variants of Concern. Adv Exp Med Biol 2023, 1412, 3–26. [Google Scholar] [CrossRef] [PubMed]
- Stokes, E. K.; Zambrano, L. D.; Anderson, K. N.; Marder, E. P.; Raz, K. M.; El Burai Felix, S.; Tie, Y.; Fullerton, K. E. Coronavirus Disease 2019 Case Surveillance - United States, January 22-May 30, 2020. MMWR Morb Mortal Wkly Rep 2020, 69, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Punzo, O.; Bellino, S.; Palmieri, L.; Lo Noce, C.; Giuliano, M.; Meli, P.; Boros, S.; Bella, A.; Riccardo, F.; Pezzotti, P.; et al. Clinical characteristics of individuals under 40 years of age who died with COVID-19 in Italy. J Med Virol 2021, 93, 1932–1936. [Google Scholar] [CrossRef]
- Khairwa, A.; Jat, K. R. Autopsy findings of COVID-19 in children: a systematic review and meta-analysis. Forensic Sci Med Pathol 2022, 18, 516–529. [Google Scholar] [CrossRef]
- Calderwood, S.; Sabir, A.; Rao, L.; Baker, B.; Balasa, V.; Sathi, B. K. SARS-CoV-2 Infection Presenting as Acute Chest Syndrome in a Child With Hemoglobin SD-Los Angeles Disease: A Case Report and Review of Literature. J Pediatr Hematol Oncol 2023, 45, 82–87. [Google Scholar] [CrossRef]
- de Souza, T. H.; Nadal, J. A.; Nogueira, R. J. N.; Pereira, R. M.; Brandao, M. B. Clinical manifestations of children with COVID-19: A systematic review. Pediatr Pulmonol 2020, 55, 1892–1899. [Google Scholar] [CrossRef]
- Rankin, D. A.; Talj, R.; Howard, L. M.; Halasa, N. B. Epidemiologic trends and characteristics of SARS-CoV-2 infections among children in the United States. Curr Opin Pediatr 2021, 33, 114–121. [Google Scholar] [CrossRef]
- Fekete, T. In adults and children, a rapid POC antigen test for COVID-19 (LumiraDx) had >/=97% sensitivity and specificity vs. RT-PCR. Ann Intern Med 2021, 174, JC82. [Google Scholar] [CrossRef] [PubMed]
- Sampson, C. Rapid antigen test had up to 98% sensitivity and 100% specificity for detecting COVID-19 in persons with mild symptoms. Ann Intern Med 2021, 174, JC71. [Google Scholar] [CrossRef] [PubMed]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin Immunol 2020, 215, 108427. [Google Scholar] [CrossRef] [PubMed]
- Dhochak, N.; Singhal, T.; Kabra, S. K.; Lodha, R. Pathophysiology of COVID-19: Why Children Fare Better than Adults? Indian J Pediatr 2020, 87, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Andre, M. C.; Patzug, K.; Bielicki, J.; Gualco, G.; Busi, I.; Hammer, J. Can SARS-CoV-2 cause life-threatening bronchiolitis in infants? Pediatr Pulmonol 2020, 55, 2842–2843. [Google Scholar] [CrossRef]
- Cozzi, G.; Sovtic, A.; Garelli, D.; Krivec, U.; Silvagni, D.; Corsini, I.; Colombo, M.; Giangreco, M.; Giannattasio, A.; Milani, G. P.; et al. SARS-CoV-2-related bronchiolitis: a multicentre international study. Arch Dis Child 2023, 108, e15. [Google Scholar] [CrossRef]
- Tolopka, T.; Kuehne, J.; Mainali, K.; Beebe, M.; Garcia, M.; Salameh, M.; Ocampo, R.; Bhalala, U. The Role of Childhood Obesity in Acute Presentations and Outcomes of Hospitalized COVID-19 Patients. Cureus 2022, 14, e28911. [Google Scholar] [CrossRef]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger Than 60 Years Is a Risk Factor for COVID-19 Hospital Admission. Clin Infect Dis 2020, 71, 896–897. [Google Scholar] [CrossRef]
- Choi, J. H.; Choi, S. H.; Yun, K. W. Risk Factors for Severe COVID-19 in Children: A Systematic Review and Meta-Analysis. J Korean Med Sci 2022, 37, e35. [Google Scholar] [CrossRef]
- Farrar, D. S.; Drouin, O.; Moore Hepburn, C.; Baerg, K.; Chan, K.; Cyr, C.; Donner, E. J.; Embree, J. E.; Farrell, C.; Forgie, S.; et al. Risk factors for severe COVID-19 in hospitalized children in Canada: A national prospective study from March 2020-May 2021. Lancet Reg Health Am 2022, 15, 100337. [Google Scholar] [CrossRef]
- Jorgensen, S. B.; Nygard, K.; Kacelnik, O.; Telle, K. Secondary Attack Rates for Omicron and Delta Variants of SARS-CoV-2 in Norwegian Households. JAMA 2022, 327, 1610–1611. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, K.; Mannell, M.; Naqvi, O.; Matson, D.; Stone, J. SARS-CoV-2 B.1.617.2 (Delta) Variant COVID-19 Outbreak Associated with a Gymnastics Facility - Oklahoma, April-May 2021. MMWR Morb Mortal Wkly Rep 2021, 70, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Sayampanathan, A. A.; Heng, C. S.; Pin, P. H.; Pang, J.; Leong, T. Y.; Lee, V. J. Infectivity of asymptomatic versus symptomatic COVID-19. Lancet 2021, 397, 93–94. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J. F. Children are unlikely to be the main drivers of the COVID-19 pandemic - A systematic review. Acta Paediatr 2020, 109, 1525–1530. [Google Scholar] [CrossRef] [PubMed]
- Brugler Yonts, A. Pediatric Long-COVID: A Review of the Definition, Epidemiology, Presentation, and Pathophysiology. Pediatr Ann 2022, 51, e416–e420. [Google Scholar] [CrossRef]
- Lee, S.; Erdem, G.; Yasuhara, J. Multisystem inflammatory syndrome in children associated with COVID-19: from pathophysiology to clinical management and outcomes. Minerva Pediatr (Torino) 2023. [Google Scholar] [CrossRef]
- Panagiotakopoulos, L.; Moulia, D. L.; Godfrey, M.; Link-Gelles, R.; Roper, L.; Havers, F. P.; Taylor, C. A.; Stokley, S.; Talbot, H. K.; Schechter, R.; et al. Use of COVID-19 Vaccines for Persons Aged >/=6 Months: Recommendations of the Advisory Committee on Immunization Practices - United States, 2024-2025. MMWR Morb Mortal Wkly Rep 2024, 73, 819–824. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).