Submitted:
05 October 2024
Posted:
07 October 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
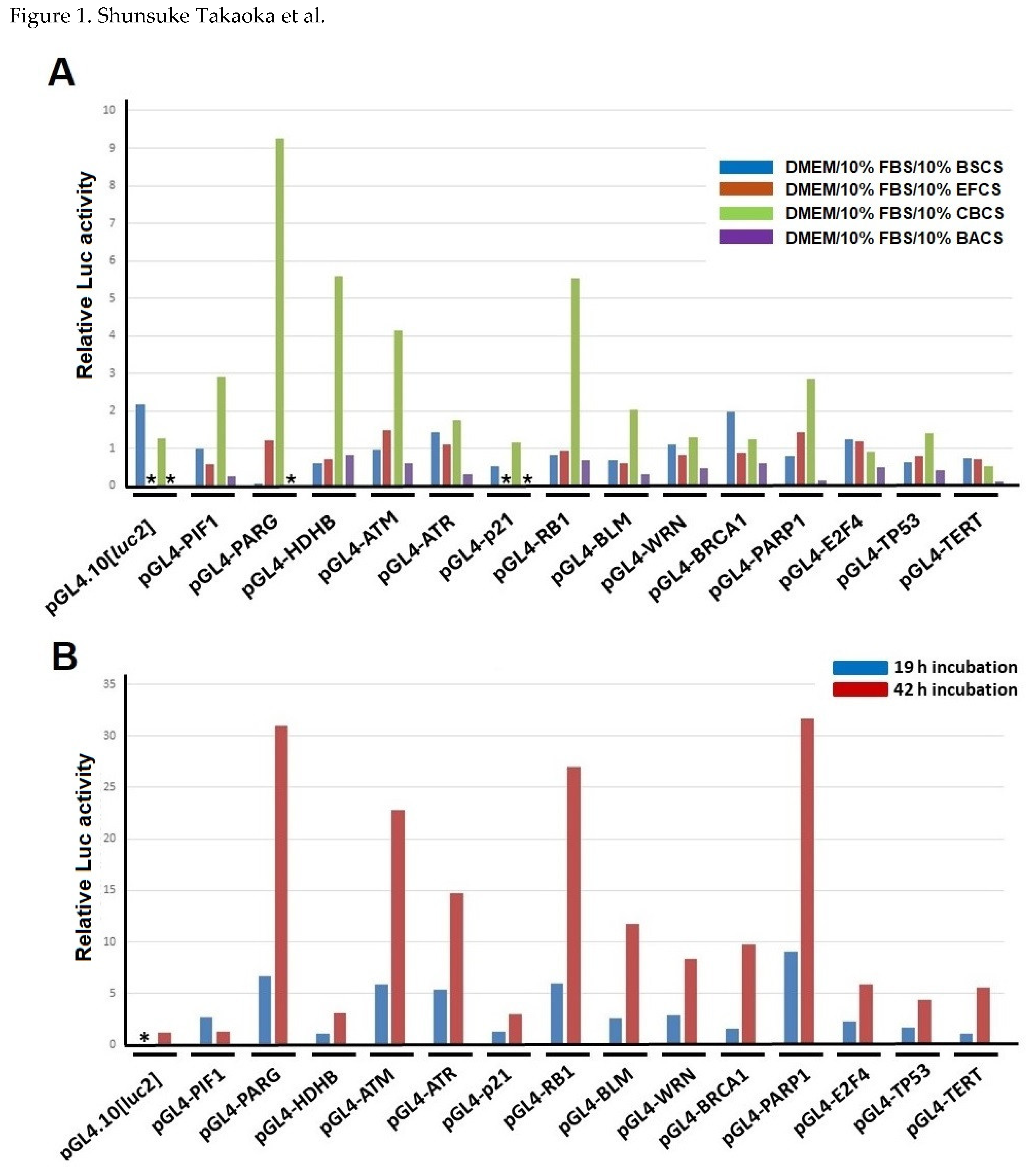
2.1. Screening of Probiotic Bacterial Culture Supernatant That Affects Promoter Activities of Human DNA Repair Factor-Encoding Genes
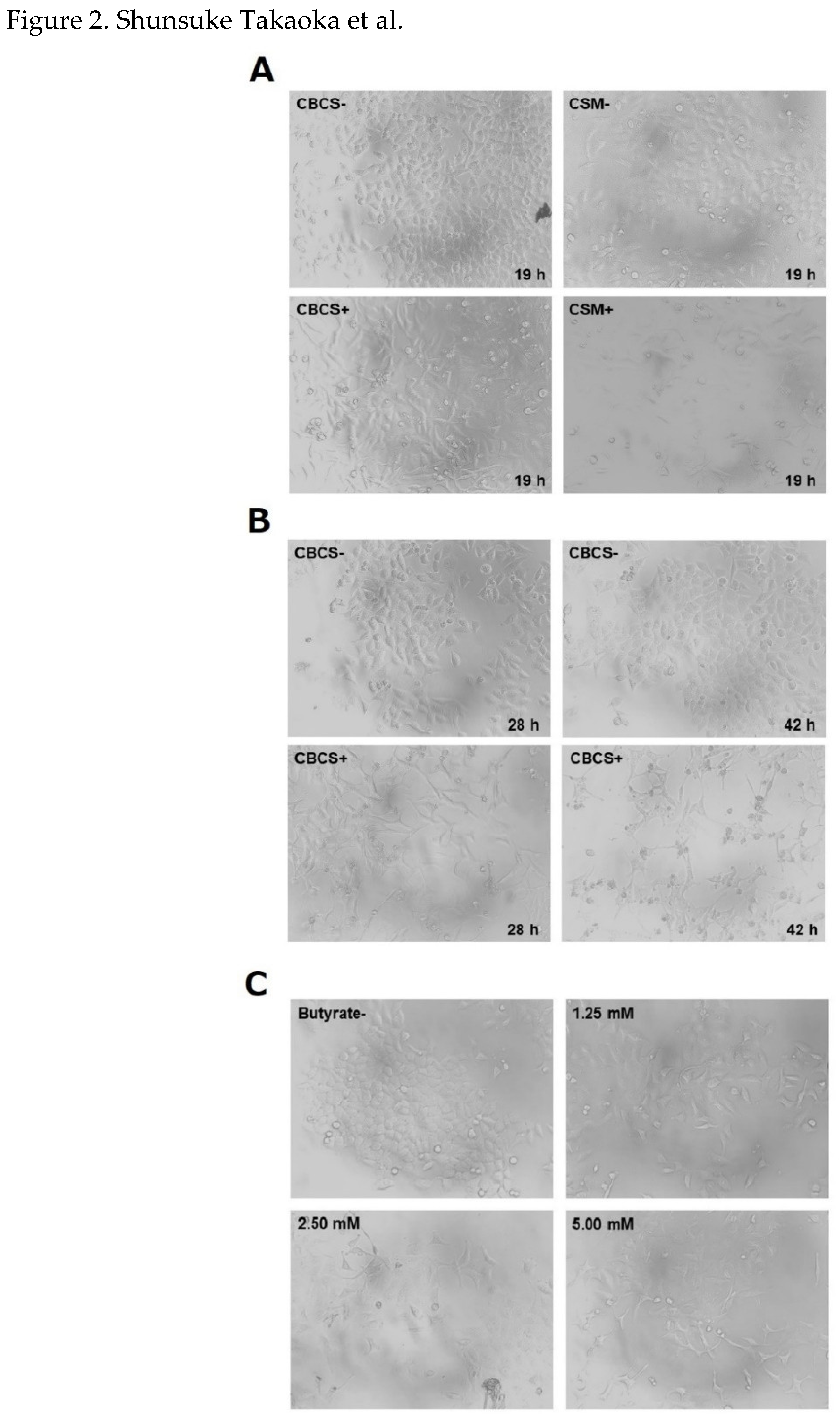
2.2. Morphological Changes and Survival of HeLa S3 CELLS after addition of Probiotic Bacterial Cultivated Supernatants
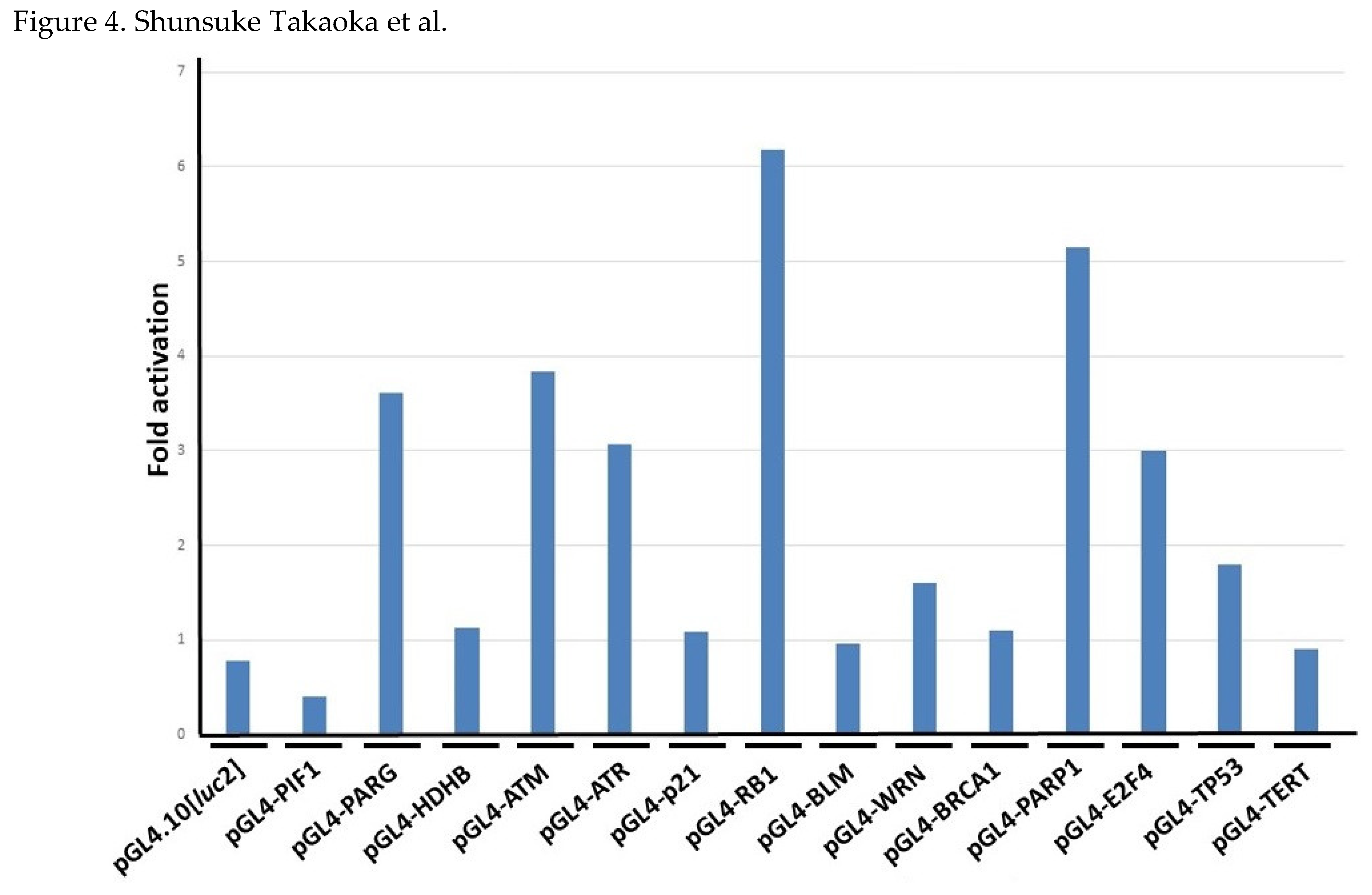
2.3. Responses of Human DNA-Repair Factor-Encoding Gene Promoters to n-Butyric Acid
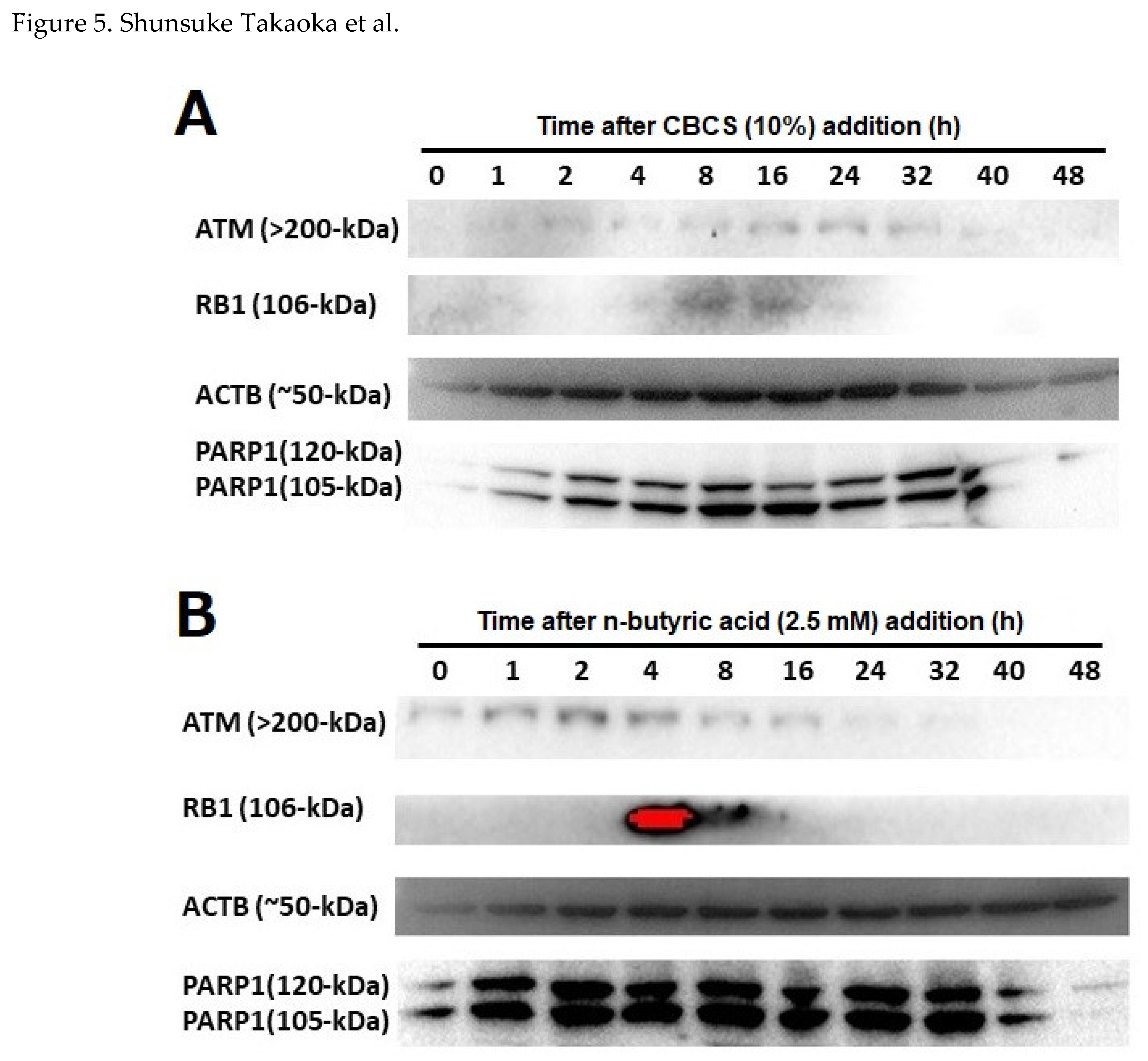
2.4. Amounts of ATM, PARP1, and RB1 in HeLa S3 Cells after CBCS Treatment
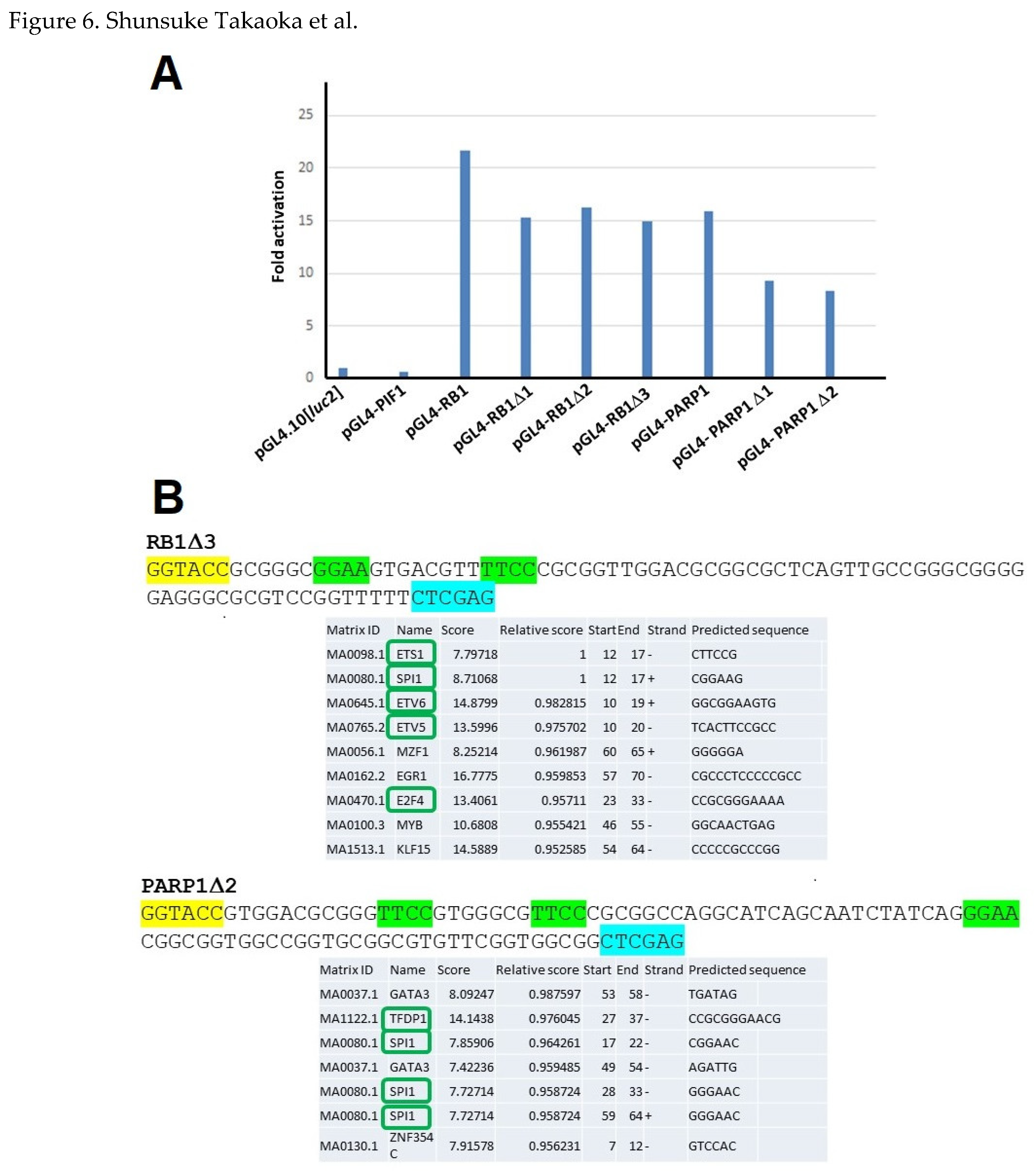
2.5. n-Butyric Acid-Response Elements in the Human RB1 and PARP1 Gene Promoters
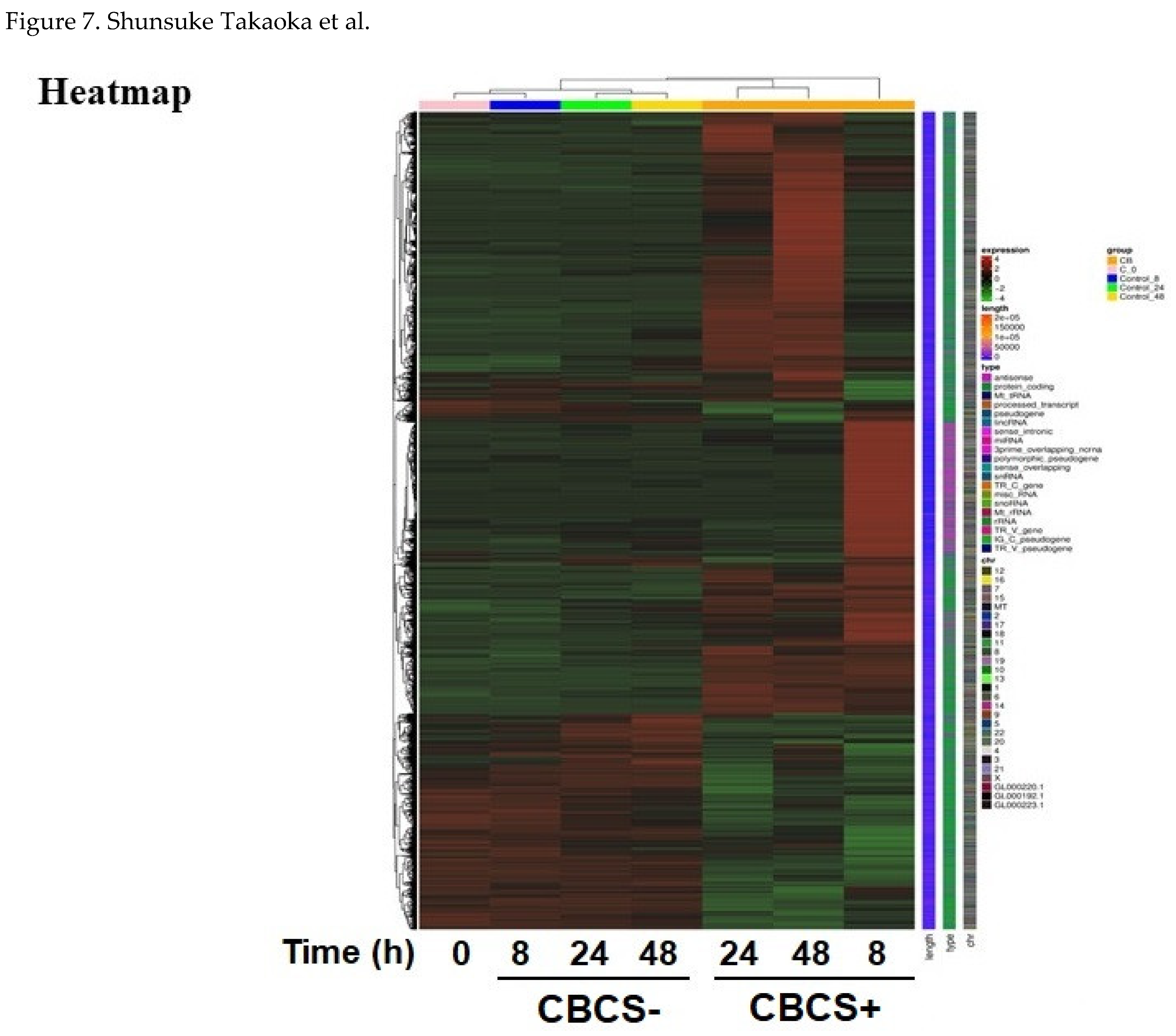
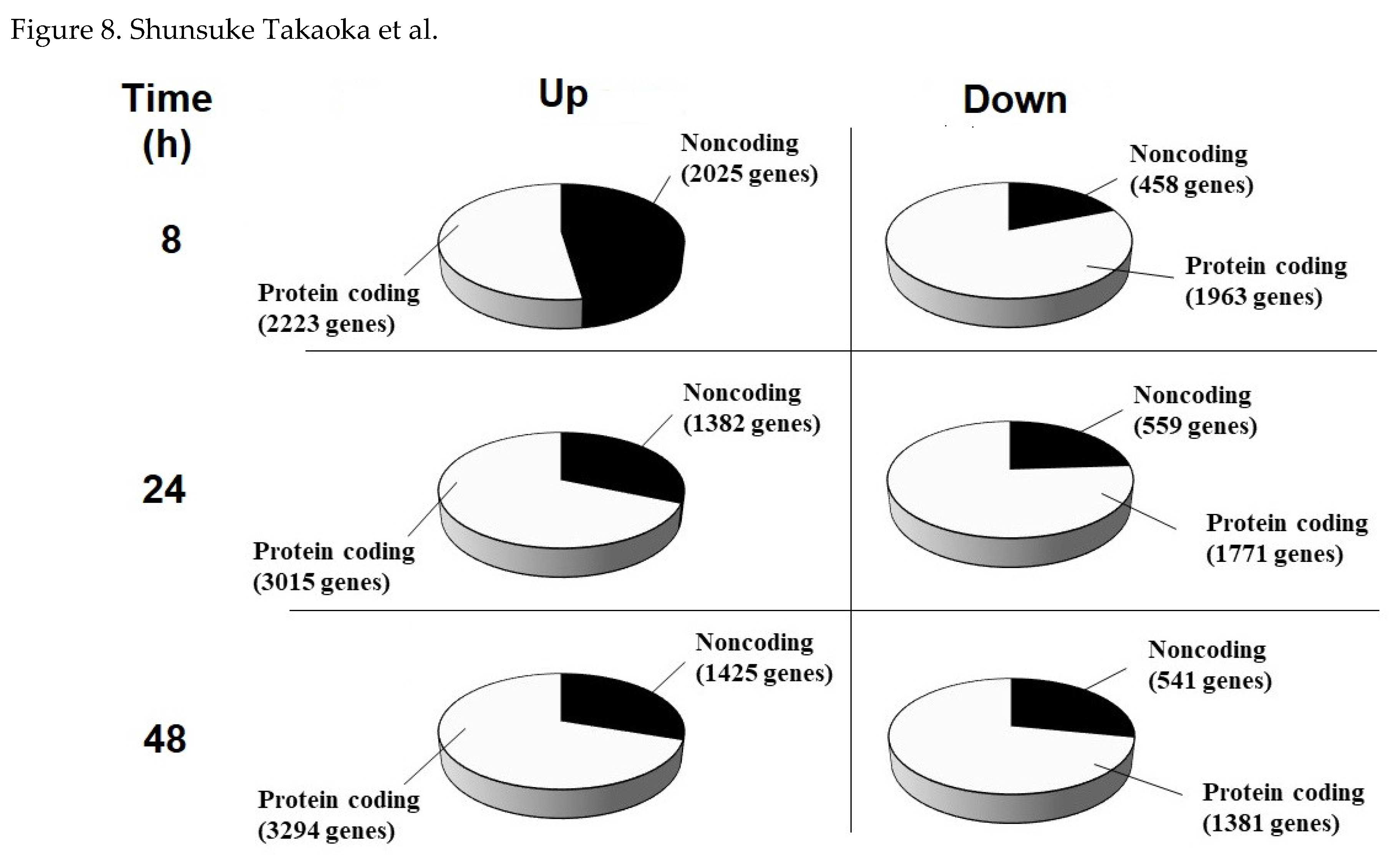
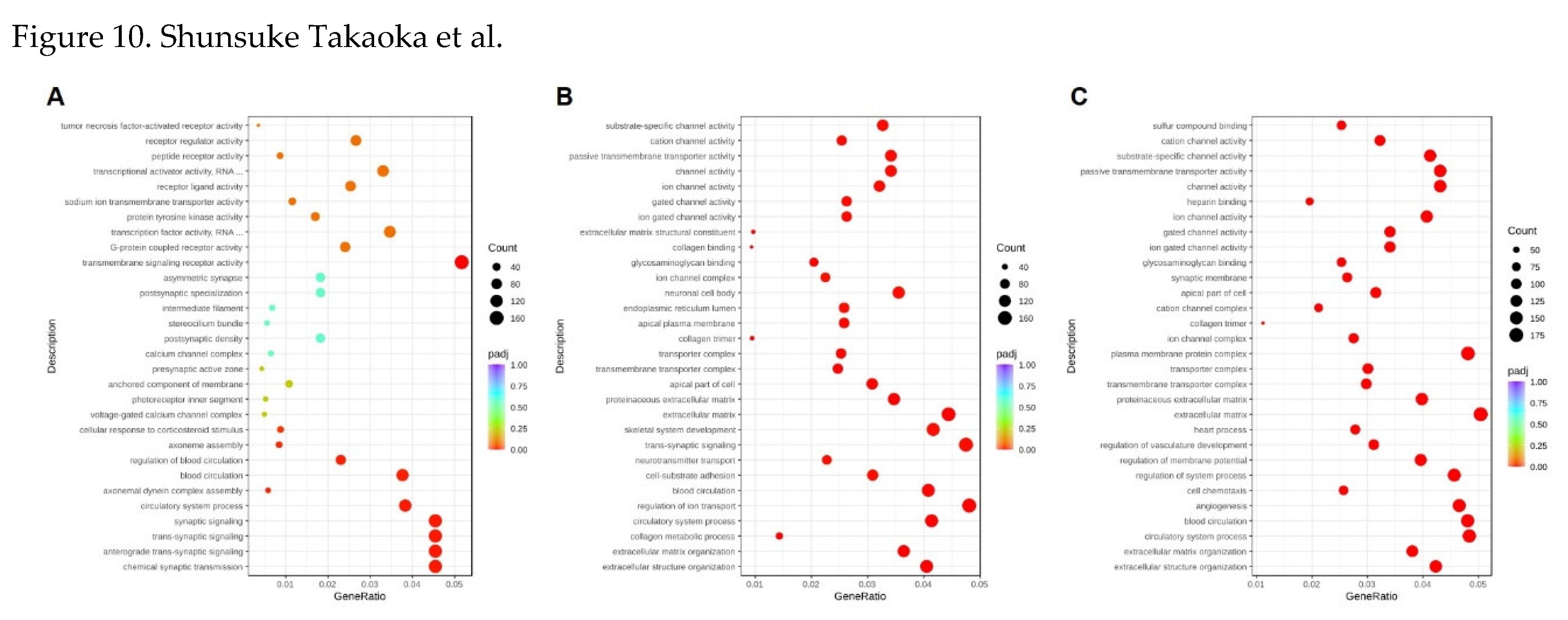
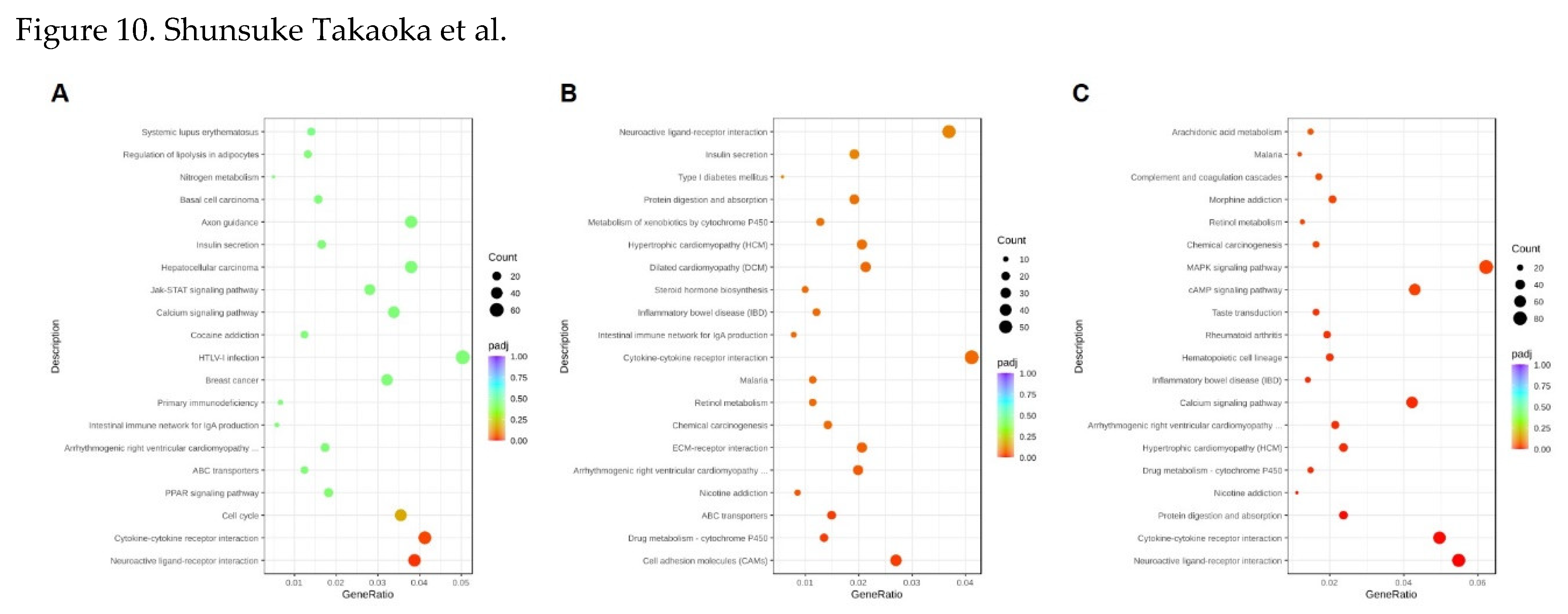
2.6. RNA Sequence (RNAseq) Analysis of HeLa S3 Cells after Cultivation with CBCS
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Probiotic Bacterial Culture Supernatants
4.3. Cells and Cell Culture
4.4. Construction of Luciferase (Luc) Reporter Plasmids
4.5. Transient Transfection and Luciferase (Luc) Assay
4.6. Western Blot Analysis
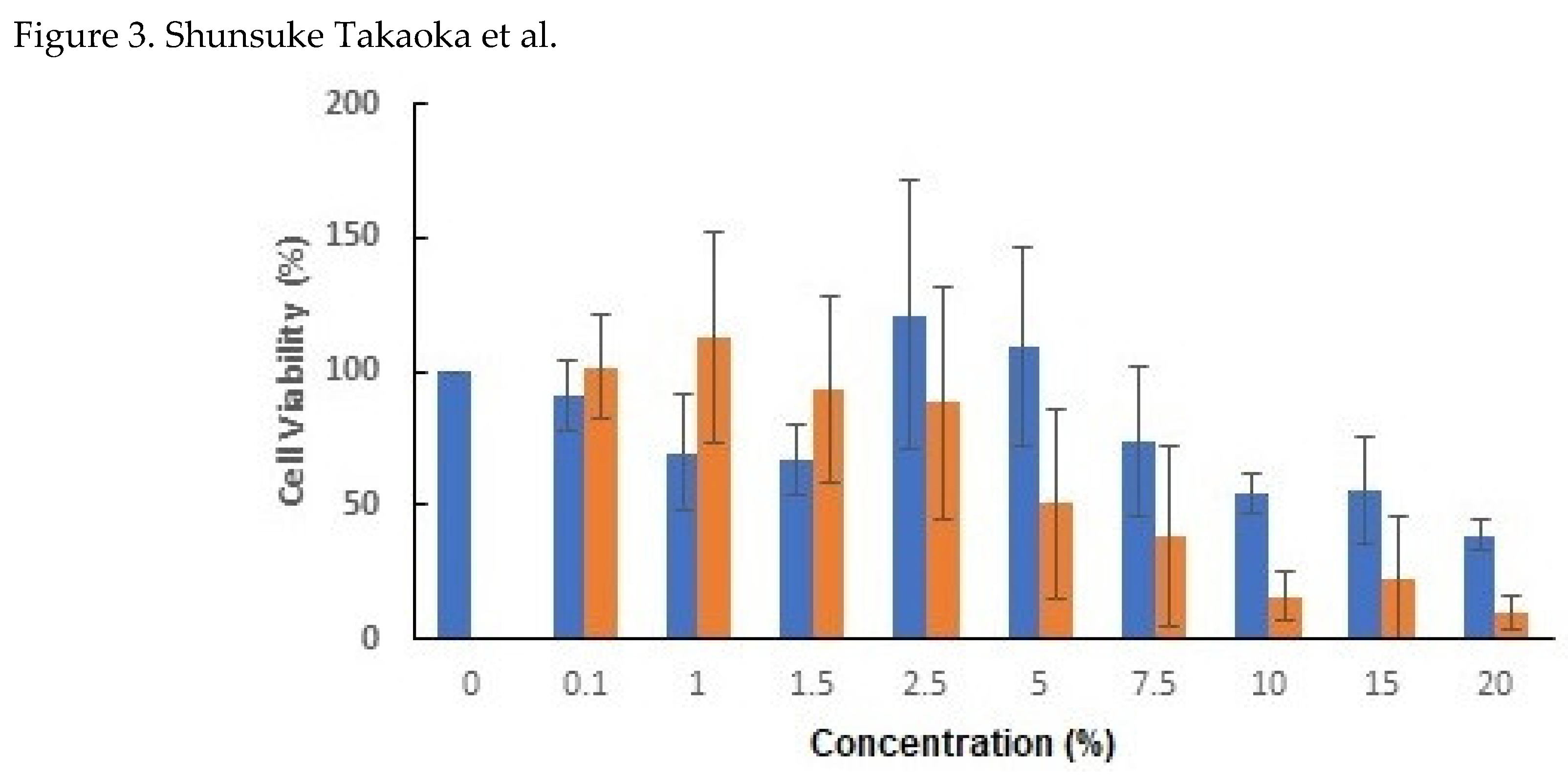
4.7. Cell Viability Assay (CCK-8 Assay)
4.8. Statistical Analysis
4.9. Preparation of Total RNAs from HeLa S3 cells and RNAseq Analysis
4.10. Identification of Differentially Expressed Genes (DEGs)
4.11. Functional Enrichment Analysis
5. Conclusion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
References
- Clarke, T.L.; Mostoslavsky, R. DNA repair as a shared hallmark in cancer and aging. Mol. Oncol. 2022, 16, 3352–3379. [Google Scholar] [CrossRef] [PubMed]
- Uchiumi, F.; Larsen, S.; Tanuma, S. Application of DEAE-dextran to an efficient gene transfer system. In Dextran: Chemical Structure, Application and Potential Side Effects. Figgs, G.P. (ed.) Nova Science Publishers, Inc., Hauppauge, NY, 2014, pp.143-156.
- Takihara, Y.; Sudo, D.; Arakawa, J.; Takahashi, M.; Sato, A.; Tanuma, S.; Uchiumi, F. Nicotinamide adenine dinucleotide (NAD+) and cell aging. In: New Research on Cell Aging and Death. Strakoš, R. and Lorens, B. (eds.) Nova Science Publishers, Inc., Hauppauge, NY, 2018, pp.131-158.
- Uchiumi, F.; Shoji, K.; Sasaki, Y.; Sasaki, M.; Sasaki, Y.; Oyama, K.; Sugisawa, S.; Tanuma, S. Characterization of the 5′-flanking region of the human TP53 gene and its response to the natural compound, Resveratrol. J. Biochem. 2016, 159, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Uchiumi, F.; Arakawa, J.; Iwakoshi, S.; Ishibashi, S.; Tanuma, S. Characterization of the 5′-flanking region of the human DNA helicase B (HELB) gene and its response to trans-resveratrol. Sci. Rep. 2016, 6, 24510. [Google Scholar] [CrossRef] [PubMed]
- Uchiumi, F.; Katsuda, C.; Akui, M.; Kusaka, M.; Tanaka, M.; Asai, M.; Tanuma, S. Effect of the natural compound trans-resveratrol on human MCM4 gene transcription. Oncol. Rep. 2020, 44, 283–292. [Google Scholar] [CrossRef]
- Rooks, M.G.; Garrett, W.S. Gut microbiota, metabolites and host immunity. Nat. Rev. Immunol. 2016, 16, 341–352. [Google Scholar] [CrossRef]
- Strandwitz, P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Arai, E.N.; Yoneda, S.; Yoneda, N.; Ito, M.; Tsuda, S.; Shiozaki, A.; Nohira, T.; Hyodo, H.; Kumazawa, K.; Suzuki, T.; et al. Probiotics including Clostridium butyricum, Enterococcus faecium, and Bacillus subtilis may prevent recurrent spontaneous preterm delivery. J. Obstet. Gynaecol. Res. 2022, 48, 688–693. [Google Scholar] [CrossRef]
- Saito, R.; Sato, N.; Okino, Y.; Wang, D.S.; Seo, G. Bacillus subtilis TO-A extends the lifespan of Caenorhabditis elegans. Biosci. Microbiota Food Health 2023, 42, 124–130. [Google Scholar] [CrossRef]
- Mansour, N.M.; Heine, H.; Abdou, S.M.; Shenana, M.E.; Zakaria, M.K.; El-Diwany, A. Isolation of Enterococcus faecium NM113, Enterococcus faecium NM213 and Lactobacillus casei NM512 as novel probiotics with immunomodulatory properties. Microbiol. Immunol. 2014, 58, 559–569. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef]
- Ginsburg, E.; Salomon, D.; Sreevalsan, T.; Freese, E. Growth inhibition and morphological changes caused by lipophilic acids in mammalian cells. Proc. Nat. Acad. Sci. USA 1973, 70, 2457–2461. [Google Scholar] [CrossRef] [PubMed]
- Waby, J.S.; Chirakkal, H.; Yu, C.; Griffiths, G.J.; Benson, R.S.P.; Bingle, C.D.; Corfe, B.M. Sp1 acetylation is associated with loss of DNA binding at promoters associated with cell cycle arrest and cell death in a colon cell line. Mol. Cancer 2010, 9, 275. [Google Scholar] [CrossRef] [PubMed]
- Uchiumi, F.; Miyazaki, S.; Tanuma, S. The possible functions of duplicated ets (GGAA) motifs located near transcription start sites of various human genes. Cell. Mol. Life Sci. 2011, 68, 2039–2051. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, J.; Kondoh, H.; Matsushita, T.; Ogino, Y.; Asai, M.; Tanuma, S.; Uchiumi, F. Induction of the human CDC45 gene promoter activity by natural compound trans-resveratrol. Mol. Med. Rep. 2024, 29, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wei, G.H.; Badis, G.; Berger, M.F.; Kivioja, T.; Palin, K.; Enge, M.; Bonke, M.; Jolma, A.; Varjosalo, M.; Gehrke, A.R.; Yan, J.; Talukder, S.; Turunen, M.; Taipale, M.; Stunnenberg, H.G.; Ukkonen, E.; Hughes, T.R.; Bulyk, M.L.; Taipale, J. Genome-wide analysis of ETS-family DNA-binding in vitro and in vivo. EMBO J. 2010, 29, 2147–2160. [Google Scholar] [CrossRef]
- Wieratra, I. Sp1: Emerging roles-Beyond constitutive activation of TATA-less housekeeping genes. Biochem. Biophys. Res. Commun. 2008, 372, 1–13. [Google Scholar] [CrossRef]
- Hodny, Z.; Li, R.; Barath, P.; Nelson, B.D. Sp1 and chromatin environment are important contributors to the formation of repressive chromatin structures on the transfected human adenine nucleotide translocase-2 promoter. Biochem. J. 2000, 346, 93–97. [Google Scholar] [CrossRef]
- Bohan, C.A.; Robinson, R.A.; Luciw, P.A.; Srinivasan, A. Mutational analysis of sodium butyrate inducible elements in the human immunodeficiency virus type I long terminal repeat. Virology 1989, 172, 573–583. [Google Scholar] [CrossRef]
- McNabney, S.M.; Henagan, T.M. Short chain fatty acids in the colon and peripheral tissues: A focus on butyrate, colon cancer, obesity and insulin resistance. Nutrients 2017, 9, 1348. [Google Scholar] [CrossRef]
- Bridgeman, S.; Ellison, G.; Newsholme, P.; Mamotte, C. The HDAC inhibitor butyrate impairs b cell function and activates the disallowed gene Hexokinase I. Int. J. Mol. Sci. 2021, 22, 13330. [Google Scholar] [CrossRef]
- Mathis, D.J.; Oudet, P.; Wasylyk, B.; Chambon, P. Effect of histone acetylation on structure and in vitro transcription of chromatin. Nucleic Acids. Res. 1978, 5, 3523–3547. [Google Scholar] [CrossRef] [PubMed]
- Annunziato, A.T.; Frado, L.L.Y.; Seale, R.L.; Woodcock, C.L.F. Treatment with sodium butyrate inhibits the complete condensation of interphase chromatin. Chromosoma 1988, 96, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Darnell, R.B. Independent regulation by sodium butyrate of gonadotropin alpha gene expression and cell cycle progression in HeLa cells. Mol. Cell Biol. 1984, 4, 829–839. [Google Scholar] [PubMed]
- Chou, J.Y.; Takahashi, S. Control of placental alkaline phosphatase gene expression in HeLa cells: Induction of synthesis by prednisolone and sodium butyrate. Biochemistry 1987, 26, 3596–3602. [Google Scholar] [CrossRef]
- Yaginuma, Y.; Westphal, H. Analysis of the p53 gene in human uterine carcinoma cell lines. Cancer Res. 1991, 51, 6506–6509. [Google Scholar]
- Negrini, S.; Gorgoulis, V.G.; Halazonetis, T.D. Genomic instability—An evolving hallmark of cancer. Nat. Rev. Mol. Cell Biol. 2010, 11, 220–228. [Google Scholar] [CrossRef]
- Lührs, H.; Gerke, T.; Boxberger, F.; Backhaus, K.; Melcher, R.; Scheppach, W.; Menzel, T. Butyrate inhibits interleukin-1-mediated nuclear factor-kappa B activation in human epithelial cells. Dig. Dis. Sci. 2001, 46, 1968–1973. [Google Scholar] [CrossRef]
- Jia, J.; Nie, L.; Liu, Yi. Butyrate alleviates inflammatory response and NF-kB activation in human degenerated intervertebral disk tissues. Int. Immunopharmacol., 2020, 78, 2106004. [Google Scholar] [CrossRef]
- Ying, X.D.; Wei, G.; An, H. Sodium butyrate relieves lung ischemia-reperfusion injury by inhibiting NF-kB and JAK2/STAT3 signaling pathways. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 413–422. [Google Scholar]
- Pedersen, S.S.; Prause, M.; Williams, K.; Barrès, R.; Billestrup, N. Butyrate inhibits IL-1b-induced inflammatory gene expression by suppression of NF-kB activity in pancreatic beta cells. J. Biol. Chem. 2022, 298, 102312. [Google Scholar] [CrossRef]
- Uchiumi, F. Chapter 6 Dysregulation of transcription and human diseases. In Bidirectional Gene Promoters-Transcription System and Chromosomal Structure, 1st ed.; Uchiumi, F., Ed.; Elsevier: USA, 2022; Volume 101, p. 122. ISBN 978-0-12-818787-6. [Google Scholar] [CrossRef]
- Kress, H.; Tönjes, R.; Doenecke, D. Butyrate induced accumulation of a 2.3 kb polyadenylated H1(0) histone mRNA in HeLa cells. Nucleic Acids. Res. 1986, 14, 7189–7197. [Google Scholar] [CrossRef] [PubMed]
- Vettese-Dadey, M.; Grant, P.A.; Hebbes, T.R.; Crane-Robinson, C.; Allis, C.D.; Workman, J.L. Acetylation of histone H4 plays a primary role in enhancing transcription factor binding to nucleosomal DNA in vitro. EMBO J. 1996, 15, 2508–2518. [Google Scholar] [CrossRef] [PubMed]
- Egan, P.A.; Levy-Wilson, B. Structure of transcriptionally active and inactive nucleosomes from butyrate-treated and control HeLa cells. Biochemistry 1981, 20, 3695–3702. [Google Scholar] [CrossRef] [PubMed]
- Boffa, L.C.; Mariani, M.R.; Parker, M.I. Selective hypermethylation transcribed nucleosomal DNA by sodium butyrate. Exp. Cell Res. 1994, 211, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sun, Y.; Liu, J.; Wu, X.; Chen, L.; Ma, L.; Wu, P. Histone deacetylase inhibitor sodium butyrate suppresses DNA double strand break repair induced by etoposide more effectively in MCF-7 cells than in HEK293 cells. BMC Biochem. 2015, 16, 2. [Google Scholar] [CrossRef]
- Uchiumi, F.; Sakakibara, G.; Sato, J.; Tanuma, S. Characterization of the promoter region of the human PARG gene and its response to PU.1 during differentiation of HL-60 cells. Genes Cells 2008, 13, 1229–1248. [Google Scholar] [CrossRef]
- Uchiumi, F.; Watanabe, T.; Hasegawa, S.; Hoshi, T.; Higami, Y.; Tanuma, S. The effect of Resveratrol on the Werner Syndrome RecQ helicase gene and telomerase activity. Curr. Aging Sci. 2011, 4, 1–7. [Google Scholar] [CrossRef]
- Uchiumi, F.; Watanabe, T.; Tanuma, S. Characterization of various promoter regions of the human DNA helicase-encoding genes and identification of duplicated ets (GGAA) motifs as an essential transcription regulatory element. Exp. Cell Res. 2010, 316, 1523–1534. [Google Scholar] [CrossRef]
- Uchiumi, F.; Watanabe, T.; Ohta, R.; Abe, H.; Tanuma, S. PARP1 gene expression is downregulated by knockdown of PARG gene. Oncol. Rep. 2013, 29, 1683–1688. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Stoeva, M.K.; Garcia-So, J.; Justice, N.; Myers, J.; Tyagi, S.; Nemchek, M.; McMurdie, P.J.; Kolterman, O.; Eid, J. Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease. Gut Microbes 2021, 13, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Luo, H.; Zhang, J.; Li, K.; Lee, M.H. Therapeutic potential of Clostridium butyricum anticancer effects in colorectal cancer. Gut Microbes 2023, 15, 2186114. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W.; Liu, Y.; Jiang, R.; Piao, M.; Wang, B.; Cao, H. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Steliou, K.; Boosalis, M.S.; Perrine, S.P.; Sangerman, J.; Faller, D.V. Butyrate histone deacetylase inhibitors. Biores. Open Access 2012, 1, 192–198. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.Z. Gut microbial metabolite butyrate and its therapeutic role in inflammatory bowel disease: A literature review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Shadan, F.F.; Villarreal, L.P. n-Butyrate mediated inhibition of papovavirus DNA replication in vivo and in cell culture: A mechanistic approach. Virus Genes 2000, 20, 209–216. [Google Scholar] [CrossRef]
- Pant, K.; Mishra, A.K.; Pradhan, S.M.; Nayak, B.; Das, P.; Shalimar, D.; Saraya, A.; Venugopal, S.K. Butyrate inhibits HBV replication and HBV-induced hepatoma cell proliferation via modulating SIRT-1/Ac-p53 regulatory axis. Mol. Carcinog. 2019, 58, 524–532. [Google Scholar] [CrossRef]
- Shadan, F.F.; Cowsert, L.M.; Villarreal, L.P. n-Butyrate, a cell cycle blocker, inhibits the replication of polyomaviruses and papillomaviruses but not that of adenoviruses and herpesviruses. J. Virol. 1994, 68, 4785–4796. [Google Scholar] [CrossRef]
- Qu, R.; Zhang, Y.; Ma, Y.; Zhou, X.; Sun, L.; Jiang, C.; Zhang, Z.; Fu, W. Role of the gut microbiota and its metabolites in tumorigenesis or development of colorectal cancer. Adv. Sci. (Weinh) 2023, 10, e2205563. [Google Scholar] [CrossRef]
- Palazzo, A.F.; Koonin, E.V. Functional long non-coding RNAs evolve from junk transcripts. Cell 2020, 183, 1151–1161. [Google Scholar] [CrossRef]
- Yan, H.; Bu, P. Non-coding RNA in cancer. Essays Biochem. 2021, 65, 625–639. [Google Scholar] [PubMed]
- Kopp, F.; Mendell, J.T. Functional classification and experimental dissection of long noncoding RNAs. Cell 2018, 172, 393–407. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Dai, X.; Li, K.; Gui, G.; Liu, J.; Yang, H. Clostridium butyricum partially regulates the development of colitis-associated cancer through miR-200c. Cell Mol. Biol. (Noisy-le-grand) 2017, 63, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Lyu, W.; Song, Y.; Chen, K.; Lv, L.; Yang, H.; Wang, W.; Xiao, Y. Anti-inflammatory effect of Clostridium butyricum-derived extracellular vesicles in ulcerative colitis: Impact on host microRNAs expressions and gut microbiome profiles. Mol. Nutr. Food Res. 2023, 67, e2200884. [Google Scholar] [CrossRef]
| Time (h) | 8 | 24 | 48 | |||||||
| Gene | CBCS | FPKM | fold | p-value | FPKM | fold | p-value | FPKM | fold | p-value |
| PIF1 | - | 4.243928 | 0.896654 | 0.604697 | 5.52694 | 0.123736 | 4.59E-11 | 4.295748 | 0.137542 | 5.07E-10 |
| + | 3.805336 | 0.683883 | 0.590845 | |||||||
| PARG | - | 8.055117 | 0.494794 | 0.013937 | 6.682184 | 1.014106 | 0.902802 | 7.401787 | 0.886953 | 0.547691 |
| + | 3.985623 | 6.776441 | 6.565034 | |||||||
| HELB | - | 1.034974 | 0.921202 | 0.748394 | 0.934543 | 0.471383 | 0.017126 | 0.861079 | 0.743293 | 0.290985 |
| + | 0.95342 | 0.440527 | 0.640034 | |||||||
| ATM | - | 3.14527 | 0.736635 | 0.218432 | 3.311033 | 1.050696 | 0.998052 | 3.869949 | 0.891405 | 0.553431 |
| + | 2.316915 | 3.47889 | 3.449691 | |||||||
| ATR | - | 6.495218 | 0.579147 | 0.040087 | 5.301323 | 0.712684 | 0.176728 | 5.465636 | 0.687106 | 0.13576 |
| + | 3.761683 | 3.77817 | 3.75547 | |||||||
| CDKN2A | - | 40.86149 | 0.66904 | 0.113326 | 44.3764 | 0.30763 | 2.88E-05 | 35.56736 | 0.781209 | 0.291049 |
| + | 27.33799 | 13.65149 | 27.78555 | |||||||
| RB1 | - | 18.129 | 0.875097 | 0.515848 | 16.76921 | 0.967857 | 0.771565 | 20.02231 | 1.222334 | 0.611419 |
| + | 15.86463 | 16.23019 | 24.47395 | |||||||
| BLM | - | 9.167653 | 1.289576 | 0.494121 | 6.728003 | 1.382269 | 0.344308 | 6.201939 | 1.918412 | 0.039864 |
| + | 11.82238 | 9.299909 | 11.89788 | |||||||
| WRN | - | 7.16739 | 0.877522 | 0.533055 | 5.909568 | 0.799329 | 0.344161 | 5.718025 | 0.748446 | 0.237544 |
| + | 6.289544 | 4.723689 | 4.279635 | |||||||
| BRCA1 | - | 17.60425 | 0.705496 | 0.162193 | 15.94288 | 1.06099 | 0.977051 | 15.8065 | 0.998662 | 0.843619 |
| + | 12.41973 | 16.91523 | 15.78536 | |||||||
| PARP1 | - | 61.14282 | 0.895026 | 0.563022 | 47.50302 | 1.018003 | 0.908481 | 45.8812 | 0.970177 | 0.764114 |
| + | 54.72441 | 48.35821 | 44.51287 | |||||||
| E2F4 | - | 11.49784 | 1.340332 | 0.414959 | 13.36074 | 0.680938 | 0.133485 | 10.49973 | 0.691647 | 0.14477 |
| + | 15.41092 | 9.097842 | 7.262107 | |||||||
| TP53 | - | 16.52675 | 0.492513 | 0.009924 | 21.79782 | 0.203597 | 6.00E-08 | 20.15492 | 0.292592 | 1.56E-05 |
| + | 8.139646 | 4.437968 | 5.897179 | |||||||
| TERT | - | 0.647083 | 1.291257 | 0.635664 | 0.362969 | 1.783149 | 0.142159 | 0.187431 | 1.122226 | 1 |
| + | 0.835551 | 0.647228 | 0.21034 | |||||||
| 8 h | Up | Down | ||||
| ATP1A3 | ATP1B2 | ATP2A1 | ATF1 | DNMT1 | DNMT3A | |
| ATP6V1C2 | FOSB | HIST1H2AC | EP300 | ELK4 | ETS2 | |
| HIST1H2AG | HIST1H2AG | HIST1H2AI | LIG3 | MCM2 | MCM3 | |
| HIST1H2BD | HIST1H2BJ | HIST1H3J | MCM5 | MCM7 | NFKB1 | |
| HIST1H4H | HIST2H2A4 | HIST2H3C | PARP2 | PARP3 | PCNA | |
| HIST2H2BF | HIST2H3A | HIST2H3D | POLA1 | POLE | POLE2 | |
| POLF | RFC3 | TP53 | ||||
| XBP1 | ||||||
| 24 h | Up | Down | ||||
| ATP1A3 | ATP1B2 | CDKN1A | CDKN2A | ETS2 | ERBB2 | |
| FOS | GATA3 | IL1A | LDHA | HK2 | NEIL1 | |
| IL1B | IL6 | IL8 | NFKB1 | PARP3 | POLE3 | |
| IL21R | JUN | NFATC1 | STAT5A | STAT5B | STAT6 | |
| POLD4 | STAT3 | STAT4 | TP53 | XBP1 | ||
| 48 h | Up | Down | ||||
| BDNF | EGF | FGF18 | APP3 | LIG1 | LIG3 | |
| IL1A | IL1B | IL6 | NEIL1 | NFKB1 | TP53 | |
| IL8 | IL12A | IL12B | ||||
| IL18 | IL21R | IL24 | ||||
| JUN | NFATC1 | RELB | ||||
| Luc plasmid | Primer | Sequence (5′ to 3′) |
| pGL4-RB1-D1 | hRB1-3590 | TTCGGTACCCACGCCAGGTTTCCCAG |
| AhRB1-3787 | AATCTCGAGAAAAACCGGACGCGCCCTC | |
| pGL4-RB1-D2 | hRB1-3649 | TTCGGTACCAGCGCCCCAGTTCCCCAC |
| AhRB1-3787 | AATCTCGAGAAAAACCGGACGCGCCCTC | |
| pGL4-RB1-D3 | hRB1-3707 | TTCGGTACCGCGGGCGGAAGTGACG |
| AhRB1-3787 | AATCTCGAGAAAAACCGGACGCGCCCTC | |
| pGL4-PARP1-D1 | hPARP1-8202 | TTCGGTACCCGGCAGGCGCCCGGGAAACTC |
| AhPARP1-8041 | AATCTCGAGCCGCCACCGAACACGCCGC | |
| pGL4-PARP1-D2 | hPARP1-8135 | TTCGGTACCGTGGACGCGGGTTCCGTGGGC |
| AhPARP1-8041 | AATCTCGAGCCGCCACCGAACACGCCGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





