1. Introduction
The Avian Influenza Virus (AIV) is having a significant global impact, particularly on the poultry farming industry. Since 1990, industrial poultry production has more than doubled, but the losses from AIV outbreaks continue to rise. For instance, there are approximately 27 billion chickens in hatcheries at any given time (Statista, 2024), although meanwhile, incurring substantial damage from AIV infections.
According to the UN Food and Agriculture Organization, while pork is the most widely consumed meat globally, poultry ranks second at 33% worldwide but is the top choice in both the U.S. and Canada (Usesilo.com, 2024). This makes the protection of the poultry industry critically important. For years, the U.S. poultry sector has faced challenges from highly virulent strains of AIV, commonly known as “bird flu”. The virus has driven up prices for poultry, eggs, and turkey and has spread beyond birds to various mammalian species, including sea lions, mice, quails, guinea fowls, ducks, cats, and even humans (Accessed on Jul 26, 2024 (
https://www.vox.com/future-perfect/362527/bird-flu-100-million-chickens-dead-risk-humans))). The list of affected species continues to grow, now including dairy cows as well.
The Center for Disease Control and Prevention (CDC) continues to address the public health challenge posed by multistate outbreaks of the AIV (H5N1) virus, commonly known as “H5N1 bird flu” in the United States (accessed Jul 26, 2024,
https://www.cdc.gov/bird). This highly contagious viral disease has caused significant economic ramifications; for instance, a single outbreak in British Columbia in 2004 resulted in an estimated
$400 million loss to Canada’s economy (Steckle, 2005). Additionally, to mitigate the spread of the virus, entire flocks of laying hens have been culled wherein during 2014 and 2015, over 1.3 million chickens and turkeys were destroyed, and between 2022 and 2023, nearly 60 million birds were killed in the U.S. alone (Awaidy et al., 2024).
Wild, aquatic migratory birds are the primary reservoir of most AI virus subtypes and can be asymptomatic carriers of AIV. In the last 20 years there have been increasing reports of mortality in wild birds which necessitated gaining awareness of the timing of arrival of wild and infected migrating birds into specific regions, involving control measures, to minimize the spread of the virus. The result is that options to identify the arrival of migrating birds carrying bird flu (H5N1) are of great interest. Parts of North America, Europe, Asia and Africa are currently encountering extensive bird flu outbreaks making it very important to be apprised for early awareness of migratory birds arriving in the vicinity of barns for chickens.
AIV primarily spreads among birds through respiratory secretions and fecal material, and it can also be transmitted indirectly via contaminated food, water, and surfaces. Therefore, when AIV is detected in an area, it’s crucial to bring free-range chickens back into barns. Additionally, workers must take extensive precautions to prevent spreading the virus, such as ensuring their boots do not bring the contamination into barns. Identifying the arrival times of infected birds is essential, as they shed the virus in saliva, nasal secretions, and feces, which can contaminate water sources. Wild waterfowl can carry the virus asymptomatically, posing risks to domestic birds that come into contact with contaminated water. Hence, monitoring water sources and enforcing biosecurity measures are vital to preventing the spread of avian influenza among poultry.
The importance of knowing when migratory birds are infected with AIV and are arriving or present in a particular area, the primary method of identifying their presence has currently involved teams of field personnel moving around areas where birds are nesting/sleeping in the area. When there is suspicion of the presence of AIV-carrying birds, the personnel capture alive birds (or retrieve dead birds) and insert a tube up the anus of the captured birds to enable lab testing whether the captured birds are infected. Hence, the current procedure involves fieldwork in the middle of the night, to capture birds, collect the fluids, followed transport to professional laboratories (Blais-Savoie et al., 2024).
Public health agencies monitor outbreaks closely, emphasizing the importance of vaccination for poultry and surveillance of wild bird populations. While the risk to humans has continued to remain low at present, vigilance is essential to prevent potential zoonotic transmission. Of further concern, as climate change alters migratory patterns and habitats, the risk of new outbreaks may increase, making ongoing research and preparedness critical in managing AIV threats.
The research described herein represents the results using an alternative strategy to identify the presence of the AIV using ‘torpedoes’, an alternative procedure which has the ability to identify the presence/absence of AIV from ambient water in a lake, rather than directly from birds. The torpedoes approach, by its nature, develops samples over a large geographical lake, having been field-deployed by dragging the torpedo along behind either an airboat or a drone, rather than the current strategy involving the testing of migratory birds. The intent herein, is to describe the results of this important novel method that is efficient and cost-effective. This paper demonstrates some aspects of the two alternative procedures for identifying the presence of AIV, thereby allowing a comparison of alternative methodologies in terms of information gained.
2. Background
Brief descriptions of both technologies are provided below:
- (i)
Capture of birds and extract a sample from both alive and dead birds
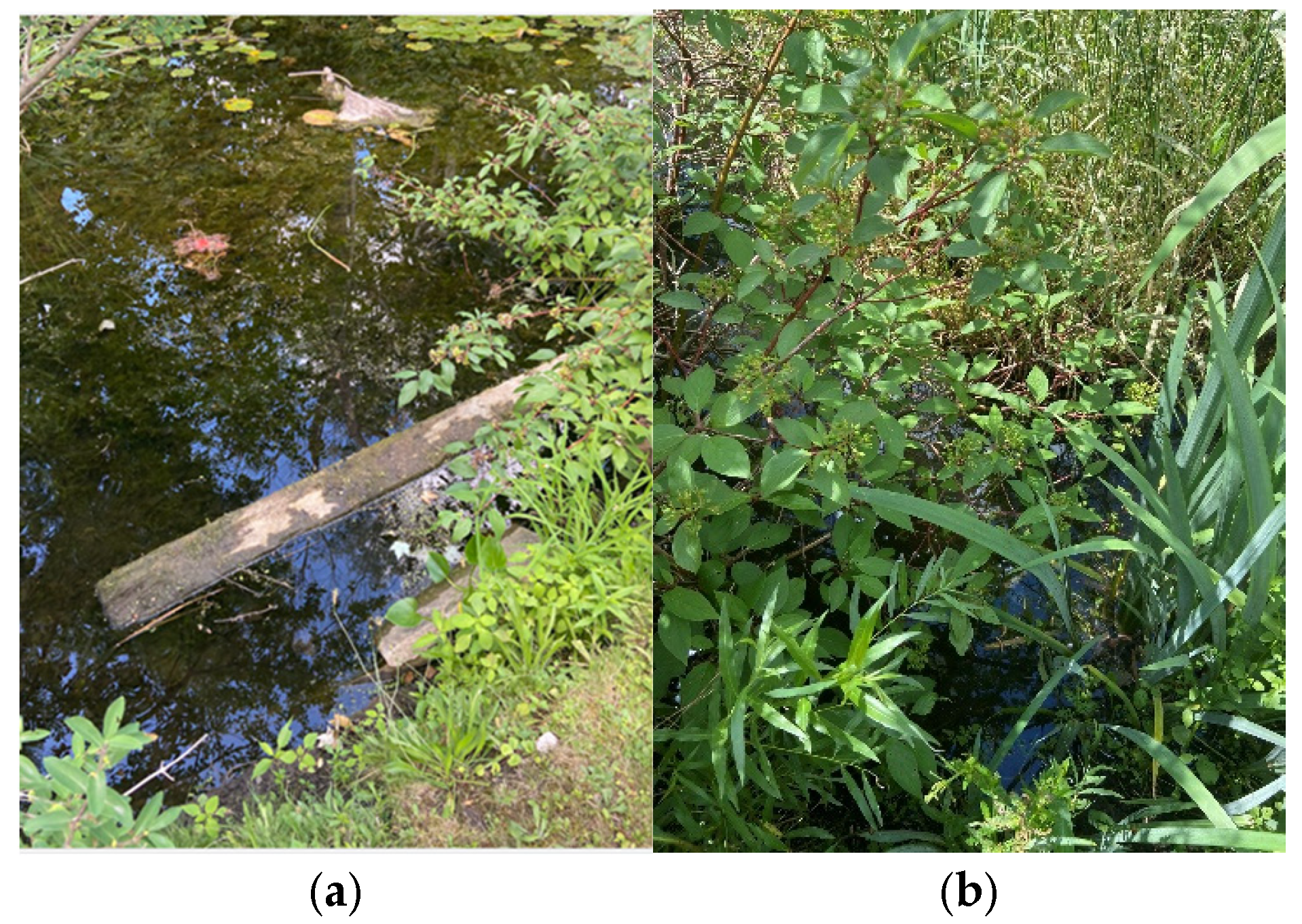
The current strategy for identifying the degree of presence of AIV-infected birds is to capture live birds at night while they are resting in the underbrush in the lakes and/or utilize dead birds, by collecting fluids which can then be tested for presence of AIV. An indication of the procedure is depicted in
Figure 1. In birds, the main site of replication for AIV is the gastrointestinal tract. While the content of the photo shows the strategy, the challenges of collecting many birds in the middle of the night are quite evident.
This approach has limitations due to the time, resources, and accessibility of an airboat, etc, in the vicinity of poultry farms. This is the strategy used in Ontario, Canada, as well as by USDA (USDA 2024).
- (ii)
The procedure being investigated in this research is to ‘drag a torpedo’ through portions of a lake by pulling the ‘torpedo’ through areas to test for presence of AIV
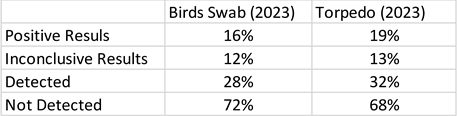
By pulling a torpedo through a suspected portion of a lake, this allows sorption of the AIV to materials contained within the torpedo, to sorb the AIV passing through the torpedo. The ‘torpedo’ device involves a strategy using a 3D printer to create the ‘housing’ which in turn contains the sorbent materials, as depicted in
Figure 2. The sorbent materials then can be removed from the 3D printer torpedo, placed in a plastic Ziploc bag, and then transported to a laboratory for testing for AIV in a professional laboratory.
The torpedo technology is essentially the same as the procedure used for testing wastewater for COVID-19 during the pandemic for SARS CoV-2 in municipal wastewater (e.g., see Habtewold et al., 2022; Jiang et al., 2022). Selection of different sorption materials remains a decision to be made for AIV, but clearly, the procedure allows passage of water through the torpedo, and thereby allowing sorption of the target AIV as the water moves through the torpedo. The difference between the two applications (for COVID and AIV) of the torpedo technology is that for the application in wastewater, the water moves by gravity in a sewer, whereas for the AIV application in a lake (which is relatively stagnant), the torpedo is dragged through the lake water by an airboat or a drone.
It is noted that if the water to be tested for presence of AIV is in a small creek and thus carrying water from a lake, the torpedo technology could be used in virtually an identical strategy wherein the torpedo would be attached to vegetation, allowing the torpedo to remain stationary while the water in the flowing creek passes through the torpedo.
In both applications (for COVID-19 and for AIV), once utilized by dragging the torpedo, the torpedo is taken to the laboratory for analysis where the sorbents are removed followed by analytical extraction/testing for the target viruses. Selection of the most appropriate sorbent materials is still underway although two of the sorbents used for COVID sampling are so far, proving ‘very promising’ to be rapid and appropriate for AIV.
It is noted that the term ‘torpedo’ is due to the shape of the monitoring device. The capture ability of the torpedo has already shown some initial promise (Blais-Savoie et al. (2023, 2024(a, b)). However, the ability to use the torpedo to identify the presence of H5N1 is challenging with some dimensions requiring a ‘learning curve’. Some learning metrics as evaluated/tested during 2022 and 2023 (see Schryer et al., 2022) as utilized in field-tests in southwest Ontario are mentioned herein and warrant influencing how the technology can be most effectively utilized.
3. Strategy for Testing of the Torpedo Versus Current Approach of Sampling Directly Using Birds
The strategies include:
- (i)
Large numbers of wild waterfowl congregate at breeding and resting sites and disseminate the viruses along their major flyways to alternative sites, indicating priority areas where dragging the torpedo through the water will be most effective. However, issues such as the variability in the water depth, and the presence of twigs/logs/vegetation, etc. are widely evident in these areas and entangle the torpedo (for examples see
Figure 3a,b).
- (ii)
As well, if the torpedoes are pulled too quickly through the water, this makes the torpedo rise to the water surface, whereas the sampling field of interest is below the water surface. Weights on the pull-cord are needed to keep the front portion of torpedo from coming to the surface.
The virus’s survival in water varies based on factors such as temperature, pH, and the presence of organic matter. Generally, AIV can remain infectious for days to weeks; colder temperatures prolong its viability, while warmer conditions decrease it. Organic material, such as feces, can create a more favorable environment for the virus (Alwaidy et al., 2024). Notably, the H5N1 strain, the most significant subtype of AIV, can survive for extended periods, with infected birds shedding the virus in saliva and feces for up to 10 days (see
https://www.healthline.com for symptoms, causes, and risk factors). These findings indicate the virus is potentially able to reside in the environment for sufficient periods of time as bird flu viruses can remain infectious for months (
https://www.the-scientist.com › news-opinion ›.
During the eve of the 2010-11 influenza flu season, scientists and engineers identified the environmental conditions and surfaces that could enable a highly pathogenic bird flu virus to survive for prolonged periods of time (at least two weeks and up to two months, as reported in Science Daily (Wood et al., 2010).
Complicating matters also may exist. Using a combination of lab and field experiments, researchers at the USGS showed that viruses shed by wild ducks were still viable after more than 209 days in-situ, suggesting that these areas could act as environmental reservoirs for the pathogens while birds overwinter in areas further south (Ramey et al. 2020). Further, AIV were reported as remaining infectious for more than seven months in northern wetlands of North America, (Ramey et al., 2020).
Hence, adapting the Torpedo approach for AIV surveillance in water samples allows targeting high risk areas and improves the ability to provide an early warning for AIV incursions into these areas, allowing producers to implement enhanced biosecurity protocols. This approach also offers the ability to monitor a substantial area (including water in a flowing creek, or within the nearshore area of a small lake) with a single passive sample. This technique could therefore be utilized in a series of locations very rapidly and with limited labour, to allow a comprehensive evaluation of the presence and strains of AIV present in the ambient environment.
4. Procedure Setup (Results from Field-Testing of Torpedo in Lakes in Ontario)
4.1. Description of Field Tests in 2023
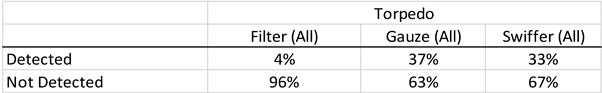
For field deployment, the water is drawn through the torpedo, past the sorbent materials inside the passive sampler, allowing the sorption to take place as the water passes through and exits the passive sampler. The sorbent materials are collected and stored in sealed specimen bags, followed by delivery of the sealed bag to the laboratory for the PCR analyses. For the field testing, each torpedo sampler contained three sampling materials: (i) polycarbonate membrane filter, (ii) Swiffer dry sweeping cloth, and (iii) Cellulose filter support pad.
Torpedo sampling during the research trials was undertaken in the same general vicinity as the traditional sampling (capture of the birds) for each of the three lakes sampled (although the areas being sampled are different, since the traditional sampling is point-specific reliant upon the location of the captured birds, while the torpedo technology involves the dragging through a portion of a lake, as depicted in
Figure 4 and
Figure 5).
Use of different pathways through the area(s) of concern at depths of approximately 10 cm were employed where there is potential for presence of AIV. This facilitated more certainty of finding the AIV (in a manner similar to COVID wastewater, where torpedoes were commonly left for periods of 24 hours or longer. See Habtewold et al. (2022)).
Laboratory results from the two approaches for evaluating AIV presence (i.e., from wild birds and the passive sampler technology) are summarized for the findings as determined during the 2023 sampling efforts are summarized in
Table 1,
Table 2 and
Table 3. An airboat was utilized at wetland sites, and torpedo samples were collected by tying the torpedo device to the back of the airboat and dragging it in the water while maintaining the depth of the torpedo was ~ 10cm beneath the surface.
The following results indicate that the torpedo method has merit for the purpose intended. The degrees of terrain with variable depths of water, extensive vegetation, depths, etc. do result in there being a significant challenge in deployment.
Designing and utilizing a passive sampling device such as the torpedo for detection of environmental AIV would increase the surveillance coverage of environments and wildlife populations of interest. This could reduce sampling costs and simplify logistics while increasing the geographical area investigators would be able to capture. This surveillance could provide a wider picture of the prevalence of these potential zoonotic viruses in environments such as those with high human-wildlife interaction.
Sampling was conducted at six locations in Ontario wetlands from 17 August to 20 September 2023. The focus was below-surface travel, with water samples taken ~10 cm below the surface where possible.
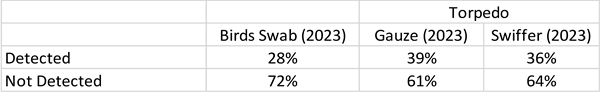
In 2023, a total of 200 samples were taken via Bird Swab and a total of 72 samples using torpedoes in the water testing. Two tables compared the results of samples for 2023. A cycle threshold value (Ct) <40 was considered a positive result for influenza RNA detection and (Ct) =>40 was taken that influenza RNA was detected and hence, called ‘inconclusive’ results. The Ct is a proxy for the amount of viral RNA in the sample, with lower Ct values indicating more viral RNA.
Torpedo samples yielded 19% positive results, while Bird Swab samples showed 16%. Inconclusive results were found in 13% of Torpedo samples and 12% of Bird Swabs. Overall, Torpedo samples detected 32% of cases, compared to 28% for Bird Swabs. (It is noted that while the findings are similar, the two technologies are using different types of testing (bird swabs versus presence in the water).
The results using the filter material were less consistent, suggesting that future torpedoes include only Swiffer and Gauze.
As reported in Blais-Savoie et al. (2024), all AIV samples were tested by qPCR with universal influenza A matrix gene and H5 gene targets. Samples that were matrix gene-positive and H5-negative underwent whole-genome sequencing using the Illumina MiniSeq platform. H5-positives were sent to the National Centre for Foreign Animal Disease for further analysis (Blais-Savoie et al., 2024). The results from the field tests indicate that the torpedo environmental sampling method is capable of detecting AIV in water in a field setting.
Adapting this approach for AIV surveillance will allow the targeting of high risk areas and improve ability to provide early warnings for AIV incursions into these areas. Early warning of AIV incursions will provide important information to plan their enhanced biosecurity protocols. This approach also provides the ability to monitor sizable areas within lakes with a single passive sample. If successful, this technique could be rapidly utilized in a series of locations and with limited labour, to allow a comprehensive evaluation of the presence and strains of AIV present in the environment.
5. Conclusions
The results from these tests indicate that passive water sampling using a torpedo device provides opportunity to quite rapidly implement widespread sampling of large areas, becoming aware of where bird flu is present. The torpedo is a method for sampling for AIV water sources such as wetlands, allows for sampling geographically more challenging areas, and serves to minimize animal-human contact, as well as allows wider areas of geography to be sampled. The torpedo approach is less expensive and provides the opportunity for greater accessibility and produces more transportable sample materials, accomplished using either an airboat or a drone.
The Torpedo for detection of avian influenza viruses has the potential to increase the surveillance coverage of different environments and wildlife populations. There is need for more testing to demonstrate efficiency of the torpedo to identify H5N1, but no reason that the torpedo is incapable of detecting H5N1, using Swiffer dry sweeping cloths and electronegative polycarbonate membrane filters. The potential appears to be large as the longevity of the virus in water is supportive of the potential for use.
Acknowledgements
Research funding provided by Ontario Agri-Food Innovation Alliance (through OAHN) and NSERC 400677 and 401757 are gratefully acknowledged. The authors acknowledge the contributions of Drs. Mubareka and Jadine providing laboratory activities and Larissa Nituch of MNR, and Juliette Blais-Savoie, Renee Schryer, Larissa Nituch, Ayden Sherritt, and Emily Chien.
References
- American Chemical Society. “Highly pathogenic bird flu virus can survive months on steel or glass at cooler temperatures.” ScienceDaily. ScienceDaily, 14 October 2010. <www.sciencedaily.com/releases/2010/10/101013124334.htm>.
- Awaidy, S.,T., Ashghar, R.J., Zaraket,H., “How Concerned Should We Be About the Recent Avian Influenza Outbreaks”, Oman Medical Journal, Vol. 39, No. 2, 2024.
- Blais-Savoie, J., Sherritt, A., Schryer, R., Tashnimul, Schryer, R., Chien, E., Yim, M., Yip, L., Signore, A., Berhene, Y., Ojkic, D., Nituch, L., McBean, E., Mubareka, S., Jardine, C., “Launching the Novel ‘Torpedo’ Sampling Method for Avian Influenza Viruses in Wetlands”, Emerging and Pandemic Infections Consortium (EPIC), University of Toronto, ON, Jun 2024 (b).
- Blais-Savoie, J., Sherritt, A., Schryer, R., Tashnimul, M. H., Chien, E., Yim, W., Yip, L., Mubareka, L., Nituch, L., McBean, E., Jardine, C., “Launching a Novel ‘Torpedo’ Sampling Method for Avian Influenza Virus Surveillance in Ontario Wetlands”, presented at Canadian Society for Virology and Institute for Pandemics & Canadian Society for Virology Symposium, Toronto, ON, April 16, 2024(a).
- Blais-Savoie, J., Sherritt, A., Schryer, R., Tashnimul, M. H., Chien, E., Yim, W., Yip, L., Mubareka, L., Nituch, L., McBean, E., Jardine, C., “Launching a Novel ‘Torpedo’ Sampling Method for Avian Influenza Virus Surveillance in Ontario Wetlands”, presented at Canadian Society for Virology and Institute for Pandemics, Toronto, ON April 2023.
- Habtewold, J., McCarthy, D., McBean, E., Law, I., Goodridge, L., Habash, M., Murphy, H., 2022. “Passive Sampling, a Practical Method for Wastewater-Based Surveillance of SARS-CoV-2”, Environmental Research, 204-112058. [CrossRef]
- Jiang, A., Nian, F., Chen, H., McBean, E., 2022. “Passive Samplers, an Important Tool for Understanding the COVID-19 Pandemic—with Insights to the outbreak in Nanjing of July 2021”, Environmental Science and Pollution Research. [CrossRef]
- McBean, E., 2022. “Use of Artificial Intelligence and Sampling Methodologies to Investigate the Occurrence and Severity of COVID-19 Outbreaks”, Universal Journal of Computational Analysis, European Union Academy of Sciences (EUAS), pp. 18-24.
- McBean, E., “Wastewater-Based Epidemiology—A Valuable Tool for Monitoring During Pandemics”, Distinguished Presentation at Gastro-Hepato 2023, 4th Global Congress on Advances in Gastroenterology and Hepatology, Barcelona, Spain, October 25-26, 2023.
- Ramey, A.M., Reeves, A.B., Drexler, J.Z., Ackerman, J.T., De La Cruz, S., Lang, A.S., Leyson, C., Link, P., ,Prosser, D.J., Robertson, G.J., Wight, J., Sungsu Youk, S., Erica Spackman, E., 2020, Proc. Royal Society, B 287: 20201680. [CrossRef]
- Salemi, A., and Schmidt, T., “Recent Advances and Applications of Passive Sampling Devices”, 2023, Advances in Sample Preparation, Volume 41, Issue s11, Pages: 22–24. [CrossRef]
- Schryer, R., Yip, L., Yim, W., Chien, E., McBean, E., Jardine, C., and Mubareka, S., “Conducting a Preliminary Investigation to Evaluate a Novel Passive Sampling Design for Detection of Avian Influenza RNA in Ontario Wetlands”, LMPSURE Poster, LMP Summer Undergraduate Research Experience, 2022.
- Statista, “Number of Chickens Worldwide” accessed Jul 29 2024 https://www.statista.com/statistics/263962/Usesilo.com (https://usesilo.com/blog/7-most-popular-meats-in-america), accessed Jul 29 2024.
- Wood, J.P., Young, W. Choi, W., Chappie, D.J., Rogers, J.V., Kaye, J.V., “Environmental Persistence of a Highly Pathogenic Avian Influenza (H5N1) Virus”, Environmental Science & Technology, 2010, No. 19, p. 7515-7520, American Chemical Society (ACS). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).